Abstract
Animal and human studies suggest that aging is associated with increased formation of kynurenine (KYN) from tryptophan (TRY). The rate-limiting factors of TRY–KYN metabolism are transmembrane transport of TRY, and activity of enzyme, TRY 2,3-dioxygenase (TDO2). Eye-color mutants, white (w1118) (impaired TRY transport) and vermilion (v48a and v2) (deficient TDO activity), were compared with wild-type Oregon-R (Ore-R) strain of Drosophila melanogaster. Female 1-day-old adult flies maintained on a standard medium, and acclimatized to 12-h light:12-h dark cycle were collected, and then regularly transferred to fresh medium every 3–4 days. The number of dead flies was recorded at the time of transfer. Forty flies were studied in each experimental group. The life span of w1118 (mean = 45.5 days), and v48a (mean = 47.6 days) and v2 (mean = 43.8 days), were significantly longer than of wild-type Ore-R flies (27.1 days) (p < 0.001, Logrank test). There were no differences in life span between w1118 and v48a and v2 mutants. Present results suggest that prolongation of life span may be associated with slow rate of KYN formation from TRY.
Keywords: Drosophila, Aging, Kynurenines
Introduction
Tryptophan (TRY) is an amino acid participating in biosynthesis of proteins and methoxyindoles (serotonin and melatonin). The major non-protein route of TRY metabolism is the cleavage of its indole ring with the formation of kynurenine (KYN) (Oxenkrug 2007).
Animal and human studies suggest that aging is associated with upregulation of TRY–KYN metabolism. KYN/TRY ratio was found to be increased with aging in humans when comparing three age groups (34–60, 61–71, and 72–93 years) (Frick et al. 2004) and nonagenarians with 45-year-old subjects (Pertovaara et al. 2006). Increased formation of KYN derivative, kynurenic acid, was observed in aged rat brain (Moroni et al. 1988; Gramsbergen et al. 1992) and in human serum (Urbanska et al. 2006). The higher rate of TRY conversion into KYN at the entry into the study was predictive of higher mortality in 10-year prospective study of nonagenarians (Pertovaara et al. 2006). Association between TRY–KYN metabolism and aging might be further supported by observation of the increased rate of TRY conversion into KYN in obesity, diabetes, atherosclerosis, menopause and other components of aging-associated metabolic syndrome (Oxenkrug 2009).
Drosophila is well suited for studies of the effect of TRY–KYN metabolism on aging since TRY–KYN pathway and related genes were described in Drosophila melanogaster (Savvateeva-Popova et al. 2003). However, genes impacting TRY–KYN pathways have not been considered among genetic pathways influencing longevity in Drosophila (Seroude 2002). There is only one study evaluating the life span of Drosophila mutants with impaired kynurenine pathway of TRY metabolism (Kamyshev 1980). TRY conversion into KYN occurs in pigmented eyes of Drosophila (Tearle 1991). The rate-limiting enzyme of this reaction in Drosophila, as in the other species, is tryptophan 2,3-dioxygenase (TDO2). The X-linked vermilion (v) mutants have deficient TDO activity (Beadle and Ephrussi 1936). Life spans of v flies were 33% longer than of wild-type Canton-S flies (Kamyshev 1980). This data in line with the above mentioned observation of high mortality in humans with increased KYN/TRY ratio (Pertovaara et al. 2006). Therefore, prolongation of life span might be associated with the slow rate of KYN formation from TRY. Since TDO is an intracellular enzyme (Kudo et al. 2001), TRY must enter the pigment cell to be available as a substrate for KYN formation. Thus, besides TDO2, transmembrane transport of TRY is another rate-limiting factor of TRY conversion into KYN. The deficient transmembrane transport of TRY underlines impaired formation of KYN from TRY in white (w) mutant of D. melanogaster (Sullivan and Sullivan 1975).
To further assess the effect of impaired conversion of TRY into KYN on life span, the present study compared life spans of the w mutant of D. melanogaster with the TDO2-deficient v mutants and wild-type Oregon-R flies.
Materials and methods
Wild-type Oregon-R (Ore-R), and eye-color mutants with impaired transmembrane transport of TRY (w1118) and deficient TDO2 activity [v48a and hypomorph v2 (Searles et al. 1990)] of D. melanogaster were obtained from the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu). Flies were raised at 25°C, 30% humidity at 12-h light:12-h dark (LD 12:12) cycle on a standard Drosophila medium consisting of cornmeal, agar, brewers yeast, dextrose, sucrose and wheat germ. One-day-old adult flies were collected, and then regularly transferred to fresh medium every 3–4 days. The number of dead flies was recorded at the time of transfer. Forty flies were studied in each experimental group. The study was carried out between January and March.
Results
Ore-R had statistically significant shorter survival time (mean = 27.1 days) than v2 (mean = 43.8 days), v48a (mean = 47.6 days) and w1118 (mean = 45.5 days) (Logrank test) (Fig. 1).
Fig. 1.
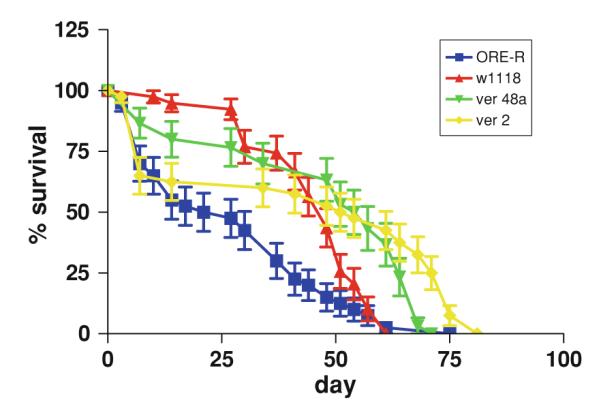
Survival time of Drosophila melanogaster mutants with impaired formation of kynurenine. ORE-R Oregon-R, w1118 white, ver 48a vermilion, ver2 vermilion hypomorph
There were no statistically significant differences in survival time of w1118, v2 and v48a flies.
Discussion
The results of the present study confirm the earlier observation of prolonged life span of TDO2-deficient v mutants in comparison with wild-type Canton-S D. melanogaster (Kamyshev 1980). In addition, the present study evaluated the impact of impaired transmembrane transport of TRY on life span of Drosophila. Conversion of TRY into KYN in D. melanogaster is an initial step of biosynthesis of brown eye pigment, ommochrome (Tearle 1991). The red pigments, drosopterins, (synthesized from guanine) contribute to pigmentation of the eye of D. melanogaster as well (Mackenzie et al. 1999). The w1118 mutant of D. melanogaster is deficient in transport of both TRY, the initial substrate for biosynthesis of brown pigments, ommochromes, and guanine, the initial substrate for biosynthesis of red pigments, drosopterins, in pigment cells (Sullivan et al. 1980). The present study revealed that deficient transmembrane transport of TRY is associated with prolonged survival time of w1118 mutant in comparison with wild-type Ore-R strain of D. melanogaster. Since white mutants have deficient transport of guanine, the initial substrate for red eye pigment, their prolonged survival time might depend not only on TRY but guanine transport deficiency as well. While such a possibility could not be ruled out based on available data, some other factors might affect life span of white mutant, the parent strain of Methuselah Drosophila (Borycz et al. 2008; Petrosyan et al. 2007).
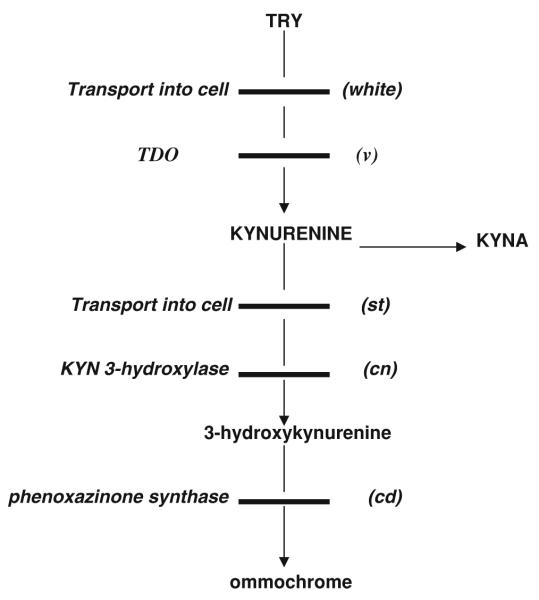
The possible mechanisms of the effect of impaired TRY conversion into KYN on life span are not clear. Since TRY is an initial substrate of melatonin biosynthesis, one might suggest that decreased utilization of TRY for KYN formation might increase the formation of melatonin that is known to prolong life span of flies (Bonilla et al. 2002). The effect of down-regulation of TRY–KYN pathway on life span might be linked with the effects of nicotinamide adenine dinucleotide (NAD+), the final product of TRY–KYN pathway, on sirtuins genes that require NAD+ for their deacetylase or ADP-ribosyl transferase activity (Guarente 2007). The other possibility is the impairment of post-KYN metabolism. Post-KYN metabolism in D. melanogaster consists of two competitive routes: formation of 3-hydroxyKYN (catalyzed by KYN-3-hydroxylase), and, consequently, ommochromes (catalyzed by phenoxazinone synthase); or formation of KYNA (catalyzed by aminotransferases) (Howells et al. 1977; Savvateeva-Popova et al. 2003) (Fig. 2).
Fig. 2.
Kynurenine pathway of tryptophan metabolism in Drosophila melanogaster. TRY tryptophan, TDO TRY 2,3-dioxygenase, KYNA kynurenic acid, v vermilion, st scarlet, cn cinnabar, cd cardinal
Considering the neurotoxic, anti-cholinergic and free radical-generating properties of KYN and its derivatives (summarily called “kynurenines”), their increased production might contribute to initiation and/or maintaining of aging processes (Oxenkrug, 2007). However, there was no difference between life spans of wild-type Canton-S flies and mutants with deficient post-KYN metabolism: scarlet (deficient transmembrane transport of KYN); cinnabar (deficient KYN 3-hydroxylase activity resulted in accumulation of KYN and KYNA), and cardinal (deficient phenoxazinone synthase resulted in excess of 3-hydroxy-kynurenine) in the only one study addressing this issue (Kamyshev 1980).
Therefore, literature data and the results of the present study suggest that prolongation of life span in D. melanogaster is associated with impaired transmembrane transport of TRY resulting in diminished availability of TRY as a substrate for KYN formation (w1118 mutant) or impaired TDO2 activity (v mutants). The present results suggest that D. melanogaster mutants with deficient TRY–KYN metabolism might be considered as an experimental model for studies of certain aspects of aging mechanisms. Drosophila model may have some relevance for humans considering that major components of Drosophila’s TRY– KYN metabolism are conserved in humans (Savvateeva-Popova et al. 2003); that upregulation of TRY–KYN metabolism was associated with aging in rodents and humans (see “Introduction”); and that anti-aging effect of competitive TDO2 inhibitor melatonin (Walsh and Daya 1997) was reported in Drosophila (Bonilla et al. 2002; Anisimov et al. 1997), and rodents and humans (Karasek 2004).
Acknowledgments
Author is very thankful to C. I. Chen for the excellent technical assistance and to Robin Ruthazer for the invaluable statistical expertise.
References
- Anisimov VN, Mylnikov SV, Oparina TI, Khavinson VK. Effect of melatonin and pineal peptide preparation epithalamin on life span and free radical oxidation in Drosophila melanogaster. Mech Ageing Dev. 1997;97:81–91. doi: 10.1016/s0047-6374(97)01897-6. [DOI] [PubMed] [Google Scholar]
- Beadle GW, Ephrussi B. Development of eye colors in Drosophila: transplantation experiments with suppressor of vermilion. Proc Natl Acad Sci USA. 1936;22:536–540. doi: 10.1073/pnas.22.9.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E, Medina-Leendertz S, Díaz S. Extensión of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp Gerontol. 2002;37:629–638. doi: 10.1016/s0531-5565(01)00229-7. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211(Pt 21):3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuin genes function as anti-aging genes in yeast, Caenorhabditis elegans, and Drosophila. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- Howells AJ, Summers KM, Ryall RL. Developmental patterns of 3-hydroxykynurenine accumulation in white and various other eye color mutants of Drosophila melanogaster. Biochem Genet. 1977;15:1049–1059. doi: 10.1007/BF00484496. [DOI] [PubMed] [Google Scholar]
- Kamyshev MG. Longevity and its relation to the locomotor activity in tryptophan–xanthommatin metabolic pathway mutant of Drosophila. Dokl Acad Nauk USSR. 1980;253:1476–1480. [Google Scholar]
- Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2, 3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535(Pt 1):207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SM, Brooker MR, Gill TR, Cox GB, Howells AJ, Ewart GD. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colour-ation. Biochim Biophys Acta. 1999;1419:173–185. doi: 10.1016/s0005-2736(99)00064-4. [DOI] [PubMed] [Google Scholar]
- Moroni F, Russi P, Carlá V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1988;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: new target for clinical intervention in vascular dementia, depression and aging. Ann. N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders and dysregulation of tryptophan–kynurenine pathway metabolism. Ann N Y Acad Sci. 2009 doi: 10.1111/j.1749-6632.2009.05356.x. (in press) [DOI] [PubMed] [Google Scholar]
- Pertovaara M, Raitala A, Lehtimäki T. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Petrosyan A, Hsich I-H, Saberi K. Age-dependent stability of sensimotor functions in the late life-extended Drosophila mutant Methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- Savvateeva-Popova EV, Popov AV, Heinemann T, Riederer P. Drosophila mutants of the kynurenine pathway as a model for ageing studies. Adv Exp Med Biol. 2003;527:713–722. doi: 10.1007/978-1-4615-0135-0_84. [DOI] [PubMed] [Google Scholar]
- Searles LL, Ruth RS, Pret AM, Fridell RA, Ali AJ. Structure and transcription of the Drosophila melanogaster vermilion gene and several mutant alleles. Mol Cell Biol. 1990;10:1423–1431. doi: 10.1128/mcb.10.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroude L. Differential gene expression and aging. Sci World J. 2002;2:618–631. doi: 10.1100/tsw.2002.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DT, Sullivan MC. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem Genet. 1975;13:603–613. doi: 10.1007/BF00484918. [DOI] [PubMed] [Google Scholar]
- Sullivan DT, Bell LA, Paton DR, Sullivan MC. Genetic and functional analysis of tryptophan transport in malpighian tubules of Drosophila. Biochem Genet. 1980;18:1109–1130. doi: 10.1007/BF00484342. [DOI] [PubMed] [Google Scholar]
- Tearle R. Tissue specific effects of ommochrome pathway mutations in Drosophila melanogaster. Genet Res. 1991;57:257–266. doi: 10.1017/s0016672300029402. [DOI] [PubMed] [Google Scholar]
- Urbańska EM, Luchowski P, Luchowska E, Pniewski J, Woźniak R, Chodakowska-Zebrowska M, Lazarewicz J. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep. 2006;58:507–511. [PubMed] [Google Scholar]
- Walsh HA, Daya S. Inhibition of hepatic tryptophan-2,3-dioxygenase: superior potency of melatonin over serotonin. J Pineal Res. 1997;23:20–23. doi: 10.1111/j.1600-079x.1997.tb00330.x. [DOI] [PubMed] [Google Scholar]