Abstract
Atherosclerosis is a vascular disease that involves lesion formation at sites of disturbed flow under the influence of genetic and environmental factors. Endothelial expression of adhesion molecules that enable infiltration of immune cells is important for lesion development. Platelet/Endothelial Cell Adhesion Molecule-1 (PECAM-1; CD31) is an adhesion and signaling receptor expressed by many cells involved in atherosclerotic lesion development. PECAM-1 transduces signals required for pro-inflammatory adhesion molecule expression at atherosusceptible sites; thus, it is predicted to be pro-atherosclerotic. PECAM-1 also inhibits inflammatory responses, on which basis it is predicted to be athero-protective. We evaluated herein the effect of PECAM-1 deficiency on development of atherosclerosis in LDL receptor-deficient mice. We found that PECAM-1 has both pro-atherosclerotic and atheroprotective effects, but that the former dominate in the inner curvature of the aortic arch whereas the latter dominate in the aortic sinus, branching arteries, and descending aorta. Endothelial cell expression of PECAM-1 was sufficient for its atheroprotective effects in the aortic sinus but not in the descending aorta, where PECAM-1’s atheroprotective effects also required its expression on bone marrow-derived cells. We conclude that PECAM-1 influences initiation and progression of atherosclerosis both positively and negatively, and that it does so in a site-specific manner.
Introduction
Atherosclerosis is a chronic inflammatory disease that involves formation, at discrete regions within the vasculature, of lesions characterized by the presence of inflammatory cells, lipid deposits and extracellular matrix deposition.1 Atherosclerotic lesions form preferentially at sites of vessel branching or high vessel curvature, which are exposed to low shear stresses and/or oscillatory or turbulent flow; however, genetic and environmental factors that determine circulating lipid levels, gender and immune status strongly influence lesion development in atherosusceptible regions.2 Endothelial cells respond to low shear stress in atherosusceptible regions with increased expression of adhesion molecules that enable recruitment and infiltration of immune cells that contribute to lesion development.3 Nevertheless, the roles of individual adhesion molecules in regulating atherosclerotic lesion development are not completely understood.
PECAM-1 is expressed on the surfaces of many cells involved in atherosclerotic lesion development, including monocytes, lymphocytes, platelets and endothelial cells.4 PECAM-1 expression is equally distributed over the entire surface of the aorta, including advanced lesions, and does not appear to be affected by hemodynamic forces or lipid levels.5,6 PECAM-1 engages in homophilic7 and heterophilic8 interactions at sites of cell-cell contact. On the basis of its role as a critical component of a mechanotransducing complex that is required for expression of pro-inflammatory adhesion molecules at atherosusceptible sites,9 PECAM-1 has been predicted to have pro-atherosclerotic properties.3 However, PECAM-1 also functions to inhibit both systemic and organ-specific inflammatory responses10-18 and the oxidative environment in the vasculature,19,20 on the basis of which PECAM-1 might be predicted to be athero-protective.
The purpose of this study was to determine whether the pro-atherosclerotic or athero-protective effects of PECAM-1 dominate in an in vivo situation. We therefore evaluated the effect of PECAM-1 deficiency on development of atherosclerosis in LDL receptor-deficient mice. We found that PECAM-1 has both pro-atherosclerotic and atheroprotective effects in LDLR-deficient mice, but that the former dominate in the inner curvature of the aortic arch whereas the latter dominate in the aortic sinus, branching arteries, and descending aorta. On the basis of these findings, we conclude that PECAM-1 influences initiation and progression of atherosclerosis both positively and negatively, and that it does so in a site-specific manner.
Methods
Animals and Diet
Mice were maintained in a facility free of well-defined pathogens under the supervision of the Biological Resource Center at the Medical College of Wisconsin (MCW). Animal protocols were approved by the MCW Institutional Animal Care and Use Committee. Mice were housed in groups of four per cage, maintained under alternating 12-h light and dark cycles and had free access to food and water. PECAM-1-deficient (pecam-1−/−) mice,21 backcrossed for more than ten generations onto a C57BL/6J background, were crossbred with LDL receptor–deficient (ldlr−/−) mice (Jackson Laboratory). Subsequently, pecam-1+/−ldlr−/− offspring were bred to obtain pecam-1+/+ldlr−/− and pecam-1−/−ldlr−/− mice. The resulting genetic background was 98.6% C57BL/6 and 1.4% 129Sv. Six- to eight-week-old male and female pecam-1+/+ldlr−/− and pecam-1−/−ldlr−/− were placed on a high-fat diet (HFD; Western Type Diet TD 88137; Harlan Teklad) containing 42% of calories derived from fat. Age- and gender-matched pecam-1+/+ldlr−/− relative to pecam-1−/−ldlr−/− mice gained similar amounts of weight over 24 weeks on the HFD (Table 1).
Table 1.
Plasma lipid analysis and weight gain of mice after 24 weeks on the HFD.
| Variable |
pecam-1+/+ldlr−/− Male |
pecam-1−/−ldlr−/− Male |
pecam-1+/+ldlr−/− Female |
pecam-1−/−ldlr−/− Female |
|---|---|---|---|---|
| Cholesterol (mg/dL) | 1335 ± 280 (n = 6) |
1605 ± 167 (n = 8) |
1048 ± 120 (n = 9) |
1291 ± 152 (n = 9) |
| Triglycerides (mg/dL) | 302 ± 97 (n = 5) |
354 ± 62 (n = 8) |
120 ± 25 (n = 9) |
184 ± 19 (n = 9) |
| HDL Cholesterol (mg/dL) | 335 ± 36 (n = 7) |
374 ± 29 (n = 7) |
340 ± 25 (n = 9) |
367 ± 16 (n = 9) |
| Body Weight (g) | 37 ± 2.8 (n = 10) |
44 ± 3.8 (n = 8) |
33 ± 1.6 (n = 14) |
34 ± 1.7 (n = 11) |
Results are expressed as mean ± S.E. No statistically significant differences were found between the groups.
Plasma Lipid Analyses
Plasma aliquots (100 μL) of blood collected by cardiac puncture from anesthetized mice were stored at −80°C. Levels of total cholesterol, HDL cholesterol and triglycerides, which were determined in individual aliquots by the clinical laboratory at Children’s Hospital of Wisconsin using the Vitros 5.1 FS Chemistry System (Ortho-Clinical Diagnostics), did not differ significantly between age- and gender-matched pecam-1+/+ldlr−/− and pecam-1−/−ldlr−/− mice after 24 weeks on the HFD (Table 1).
Preparation of Mouse Aortas and Quantification of Atherosclerosis
The heart and aorta of each animal were perfused, dissected out, and subjected to quantification of atherosclerosis as previously described22, 23. Briefly, to quantitate atherosclerosis in the aortic sinus, the heart was embedded in optimal cutting temperature (OCT) medium and frozen, after which serial sections (10 μm) were taken from the aortic sinus and valve region.22 Images were obtained of the sections following staining with oil red O (neutral lipid; counterstaining with hematoxylin) and lesion area was quantified using SPOT image analysis software (Diagnostic Instruments). The percent lesion area in each section was calculated as follows: lesion area/total area surrounded by aortic wall × 100. The mean percent atherosclerotic lesion area for each animal was determined by averaging 4-6 sections from each mouse, using 60-80 μm intervals between sections. To quantify atherosclerosis in the aorta by the en face method, the entire aortic tree was dissected free of fat and other tissue. The aorta was stained with oil red O and digitally scanned. Lesion area was assessed using Adobe Photoshop software and expressed as a percent of the total surface area encompassed by the aortic arch, inner curvature of the arch, thoracic aorta, abdominal aorta or total aorta as indicated.
Immunohistochemistry
Serial cryostat sections of aortic sinus adjacent to oil red O–stained sections were stained with Sirius Red to detect collagen or anti-mouse CD68 (1:100, AbD Serotec) and a tyramide amplification plus kit (Perkin Elmer) to identify macrophages. Sections stained with anti-CD68 were counterstained with DAPI to visualize nuclei. Quantification of macrophage and collagen content was determined by computer-assisted image analysis and expressed as percentage of lesion area.
Ex-vivo Micro-CT Imaging and Quantification of Plaque Volume
Mouse aortic specimens were dissected free of fat and incubated overnight with 2% osmium tetroxide (OT).24,25 Prepared aortic samples were placed in a 1.5ml plastic microcentrifuge tube and subjected to computed tomography (CT) scanning using a custom Keck microfocal X-ray imaging system at the Zablocki VA Medical Center, Milwaukee, WI. Details of the micro-focal x-ray CT system and CT reconstruction methods were described previously.26 Briefly, the imaging system comprises a 3-μm focal-spot cone-bean X-ray source, precise specimen manipulation stage, and image intensifier coupled to a CCD camera. Planar images (360, in 1° increments) of the aortic samples were acquired with source-to-specimen distance of 4.6 cm, source-to-detector distance of 71.5 cm, source current of 222 μA, and source voltage of 36 kV. A Feldkamp algorithm was used to produce isotropic (4973 pixels) 3-D reconstructions with 12.8 μm/pixel resolution. Floating-point grayscale voxel values were scaled to 8-bit [0(air)-255(punctate accumulations of OT)] for normalization and reduction of memory and processing requirements. Data were visualized and morphometrics (based on regional lesion volume) performed using multidimensional imaging software Analyze8.0.26,27 A single observer defined threshold-based images of the vessel wall (threshold 52) and plaque (threshold 93). Lesion volumes were quantified in defined areas of the aortic arch (24.1 ± 2.5 mm2) and proximal parts of the three major branching arteries, including the innominate (13.1 ± 0.5 mm2), left common carotid (4.8 ± 0.2 mm2), and left subclavian (3.6 ± 0.2 mm2) arteries.
Creation of radiation chimeras
Bone marrow cells were collected from the femur and tibia of pecam-1+/+ldlr−/− or pecam-1−/−ldlr−/− donor mice and the small mononuclear cell-enriched fraction was injected into irradiated, 4- to 6-week-old pecam-1+/+ldlr−/− or pecam-1−/−ldlr−/− recipient mice as previously described.13 Transplant recipients were fed a normal chow diet for 4 weeks, after which they were maintained on the HFD for an additional 24 weeks. Flow cytometry was performed on whole blood obtained from recipients at 12 weeks after transplantation to ensure that full engraftment had taken place, with both CD3-positive leukocytes and platelets examined for PECAM-1 expression (Figure S1).
Statistical analysis
Data are presented for each individual animal or as mean ± SEM. Differences between means were analyzed using two-tailed unpaired Student’s t-test or 2-way ANOVA followed by Bonferroni post-hoc testing using GraphPad Prism 4 software (GraphPad Software Inc.).
Results
PECAM-1 deficiency renders LDLR-deficient mice more susceptible to development of atherosclerotic lesions in the aortic sinus
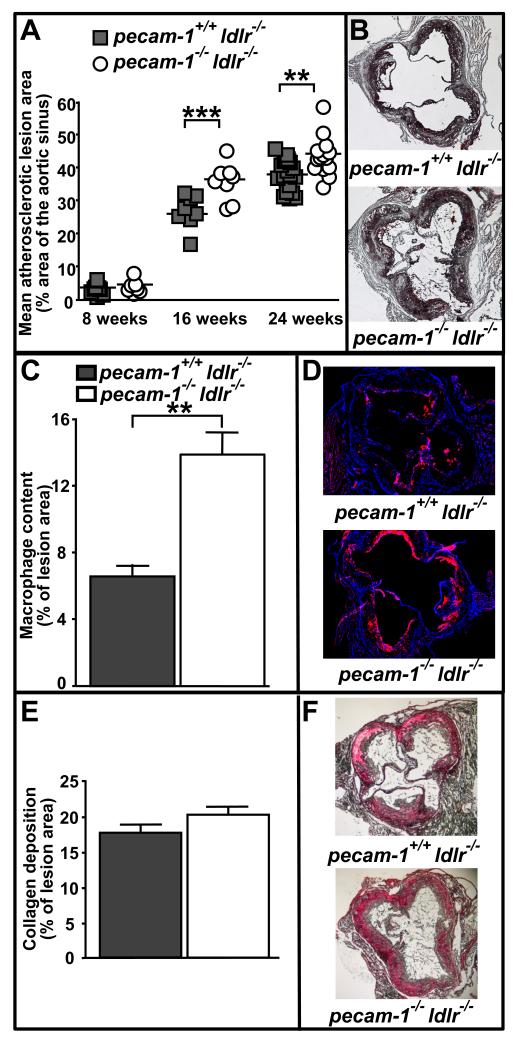
To determine whether the mechanosensory or anti-inflammatory role of PECAM-1 dominates in atherosclerosis, we tested the effect of PECAM-1 deficiency on development and progression of atherosclerosis in LDL receptor-deficient mice.28 We first evaluated the time course over which atherosclerotic lesions develop in the aortic sinus (Figure 1A and B). Both pecam-1−/−ldlr−/− and pecam-1+/+ldlr−/− mice developed similarly small atherosclerotic lesions in the aortic sinus after 8 weeks on the HFD (fractional lesion areas (FLA) - 4.6±0.7% (n=7) and 3.7±0.5% (n=8), respectively). However, after 16 weeks on the HFD, atherosclerotic lesions in the aortic sinuses of pecam-1−/−ldlr−/− mice were significantly larger than were those in pecam-1+/+ldlr−/− mice (FLA - 36.4±2.0% (n=8) and 25.9±1.7% (n=8), respectively) and this difference persisted through 24 weeks on the HFD (FLA - 41.8±1.2% (n=14) and 36.3±1.6% (n=20), respectively). Differences in aortic sinus lesion areas between pecam-1−/−ldlr−/− and pecam-1+/+ldlr−/− mice were statistically significant when both genders were evaluated together and when each gender was evaluated separately (data not shown). Because the composition of atherosclerotic lesions, along with lesion size, is critically important in atherogenesis, cell and matrix components of the lesions were also characterized by analyzing macrophage and collagen content, respectively. After 24 weeks on the HFD, macrophage content in atherosclerotic lesions of the aortic sinus was significantly greater in pecam-1−/−ldlr−/− mice than in pecam-1+/+ldlr−/− mice (Figure 1C and 1D), whereas collagen deposition was similar between the two groups of mice (Figure 1E and 1F). These results indicate that PECAM-1 inhibits development of, and macrophage accumulation in, atherosclerotic lesions in the aortic sinus of LDLR-deficient mice.
Figure 1. PECAM-1 suppresses atherosclerotic lesion development in the aortic sinuses of LDLR-deficient mice.
A) Each symbol represents the mean percent atherosclerotic lesion area calculated from 4-6 oil red O-stained sections spanning the aortic sinus of an individual pecam-1+/+ldlr−/− (filled squares) or pecam-1−/−ldlr−/− (open circles) mouse maintained on a HFD for the indicated time. Horizontal lines indicate mean percent lesion area for each group of animals. Asterisks denote statistically significant differences between groups (**p < 0.01, ***p< 0.001). B) Representative oil red O-stained aortic sinus sections from pecam-1+/+ldlr−/− (top) and pecam-1−/−ldlr−/− (bottom) mice fed a HFD for 24 weeks. C) Quantitative analysis of macrophage content in aortic sinus lesions of pecam-1+/+ldlr−/− (filled bars) or pecam-1−/−ldlr−/− (open bars) mice fed a HFD for 24 weeks. Results are expressed as the mean percent of atherosclerotic lesion area occupied by CD68+ macrophages ± S.E. Asterisks denote statistically significant differences between groups (**p < 0.01). D) Representative anti-CD68-stained aortic sinus sections from pecam-1+/+ldlr−/− (top) and pecam-1−/−ldlr−/− (bottom) mice. E) Quantitative analysis of collagen deposition in aortic sinus lesions of pecam-1+/+ldlr−/− (filled bars) or pecam-1−/−ldlr−/− (open bars) mice fed a HFD for 24 weeks. Results are expressed as the mean percent of atherosclerotic lesion area containing collagen, as identified by staining with Sirius Red, ± S.E. F) Representative Sirius Red-stained aortic sinus sections from pecam-1+/+ldlr−/− (top) and pecam-1−/−ldlr−/− (bottom) mice.
PECAM-1 deficiency renders LDLR-deficient mice more susceptible to development of atherosclerotic lesions in the descending aorta
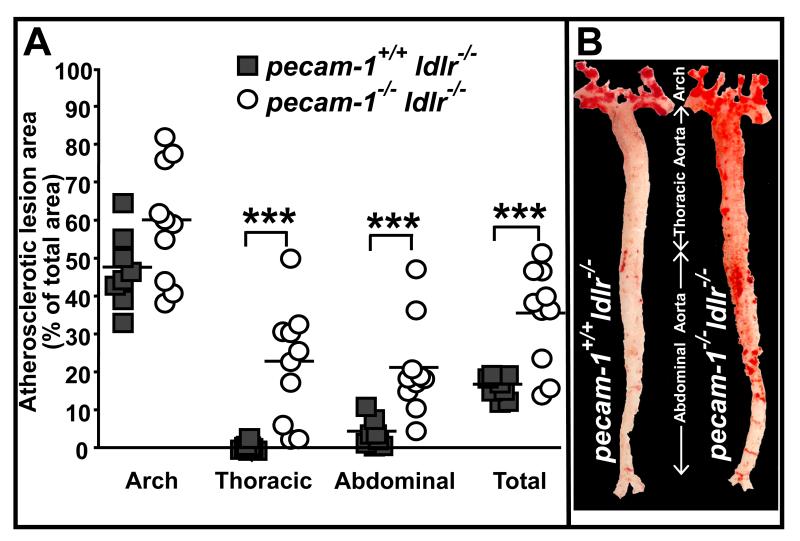
We next assessed the effect of PECAM-1 deficiency on development of atherosclerotic lesions in the aorta of LDLR-deficient mice by en face staining with oil red O. We found that lesion area in the aortic arch as a whole did not differ significantly between pecam-1+/+ldlr−/− and pecam-1−/−ldlr−/− mice after 24 weeks on the HFD (Figure 2). However, lesion areas in both the thoracic and abdominal aorta, and thus the total aorta, were significantly greater in pecam-1−/−ldlr−/− relative to pecam-1+/+ldlr−/− mice (Figure 2). These results reveal that, as it does in the aortic sinus, PECAM-1 inhibits development of atherosclerotic lesions in the descending aorta of LDLR-deficient mice.
Figure 2. PECAM-1 suppresses atherosclerosis in the descending aorta.
A) Each symbol represents the percent of total area of the indicated section of aorta that stained positively for oil red O in individual pecam-1+/+ldlr−/− (filled squares) or pecam-1−/−ldlr−/− (open circles) mice maintained on a HFD for 24 weeks. Horizontal lines indicate mean percent lesion area in the indicated aortic section, including the aortic arch, thoracic aorta, abdominal aorta and total aorta (aortic arch + thoracic aorta + abdominal aorta), for each group of animals. Asterisks denote statistically significant differences between groups (***p< 0.001). B) Representative oil red O-stained aortas from pecam-1+/+ldlr−/− (left) and pecam-1−/−ldlr−/− (right) mice maintained on a HFD for 24 weeks.
PECAM-1 affects atherosclerotic lesion development in the aortic arch in a site-specific manner
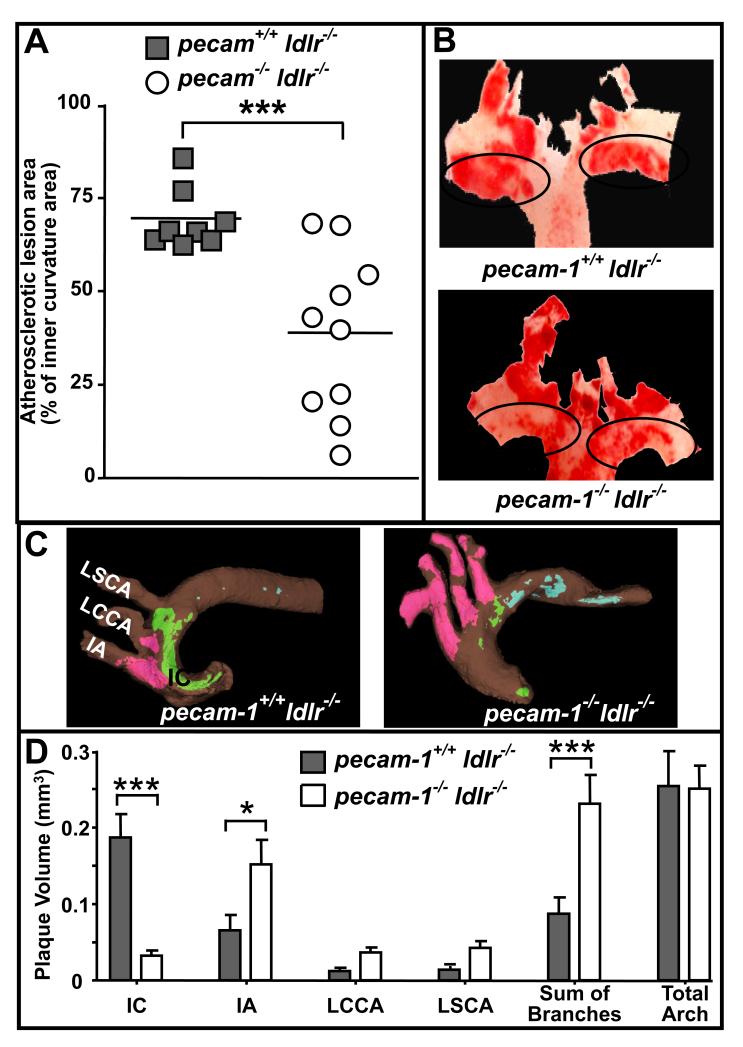
We observed a distinct pattern of plaque development in aortic arches of pecam-1+/+ldlr−/− relative to pecam-1−/−ldlr−/− mice by en face staining with oil red O. The pecam-1+/+ldlr−/− mice had significantly larger lesions along the inner curvature of the arch but smaller lesions in branching arteries compared to the pecam-1−/−ldlr−/− mice (Figure 3A and 3B). We therefore decided to evaluate the effect of PECAM-1 deficiency on development of atherosclerotic lesions in the aortic arch in more detail using another method. We utilized micro-computed tomography (micro-CT), which is a powerful imaging technique for visualization of small specimens.24 Micro-CT has been recently used to visualize the artery wall and early atherosclerotic lesions both with25 and without29 OT as a contrast agent. Micro-CT images revealed striking differences in plaque localization in pecam-1+/+ldlr−/− relative to pecam-1−/−ldlr−/−mice. Specifically, in pecam-1+/+ldlr−/− mice, lesions were less extensive in the aortic branches and more extensive along the inner curvature of the aortic arch (Figure 3C). In contrast, pecam-1−/−ldlr−/− mice developed substantial atherosclerosis in branching arteries but few lesions along the inner curvature of the arch (Figure 3). By visualizing the specimen in three-dimensions, it is apparent that plaque was extensively distributed along the inner curvature of the arch and only minimally present along the lateral wall of the innominate artery (IA) and left common carotid artery (LCCA) of pecam-1+/+ldlr−/− mice (Figure S2), whereas plaque was broadly distributed along the lateral walls of all three branching arteries and restricted to small patches along the inner curvature of the arch in pecam-1−/−ldlr−/− mice (Figure S3). We also utilized OT in micro-CT to quantify plaque volume over defined areas of vessel wall within discrete regions of the aortic arch. We found that pecam-1+/+ldlr−/− mice had significantly more plaque volume than did pecam-1−/−ldlr−/− mice along the inner curvature of the arch; however, this was offset by the presence of significantly greater plaque volume in the branching arteries of pecam-1−/−ldlr−/− relative to pecam-1+/+ldlr−/− mice, such that plaque burden in the total aortic arch as a whole was similar in pecam-1+/+ldlr−/− and pecam-1−/−ldlr−/− mice (Figure 3D). Overall, these results indicate that PECAM-1 expression in LDLR-deficient mice is pro-atherosclerotic along the inner curvature of the aortic arch but athero-protective in aortic branches.
Figure 3. PECAM-1 influences the site of atherosclerotic lesion development in the aortic arch.
A) Each symbol represents the percent of inner curvature area of the aortic arch that stained positively for oil red O in individual pecam-1+/+ldlr−/− (filled squares) or pecam-1−/−ldlr−/− (open circles) mice maintained on a HFD for 24 weeks. Horizontal lines indicate mean percent lesion area for each group of animals. Asterisks denote statistically significant differences between groups (***p< 0.001). B) Representative oil red O-stained aortas from pecam-1+/+ldlr−/− (left) and pecam-1−/−ldlr−/− (right) mice maintained on a HFD for 24 weeks. C) Representative false-color micro-CT images of osmium tetroxide-stained aortas from pecam-1+/+ldlr−/− (left) and pecam-1−/− ldlr−/− (right) mice maintained on a HFD for 24 weeks. Lesions along the inner curvature (IC) of the aortic arch, green, lesions in the three major branches emanating from the aortic arch (innominate artery (IA), left common carotid artery (LCCA), and left subclavian artery (LSCA)), magenta, and lesions in the descending aorta, blue. D) Quantitative analysis of atherosclerotic plaque volume in the IC of the aortic arch, the proximal IA, LCCA, and LSCA, the sum of branches (IA + LCCA + LSCA) or the total aorta (sum of branches + IC of aortic arch) of pecam-1+/+ldlr−/− (filled bars; n=6) vs. pecam-1−/−ldlr−/− (open bars; n=5) mice maintained on a HFD for 24 weeks. Results are expressed as mean plaque volume ± S.E. Asterisks denote statistically significant differences between groups (*p < 0.05, ***p< 0.001).
Endothelial cell PECAM-1 expression protects against development of atherosclerosis in the aortic sinuses of LDLR-deficient mice
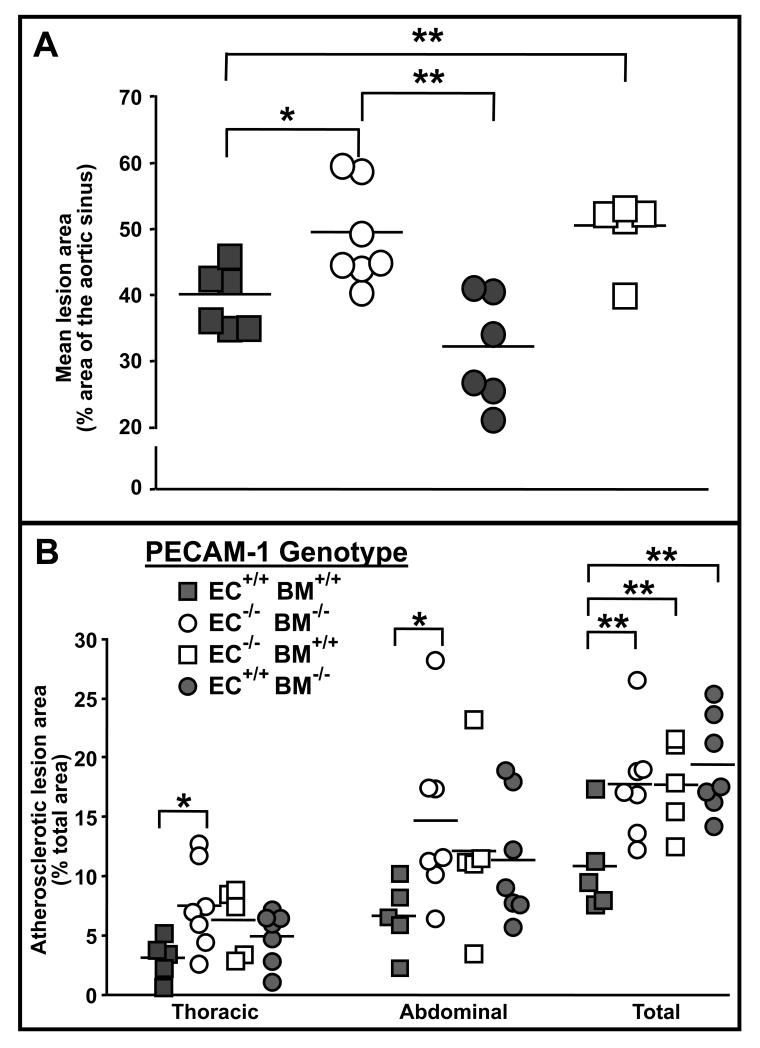
PECAM-1 is expressed on both endothelial cells and bone marrow-derived leukocytes and platelets, and is capable of transducing signals that affect the function of cells from both of these compartments30. It was therefore important to determine whether the effects of PECAM-1 on atherosclerosis required its expression on endothelial cells, bone marrow-derived cells, or both. We created bone marrow chimeric ldlr−/− mice that selectively expressed PECAM-1 either on endothelium or bone marrow-derived cells (Figure S1), and measured atherosclerotic lesion sizes after feeding these mice a HFD for 24 weeks. Unfortunately, the bone marrow transplantation experiments did not recapitulate decreased lesion formation in the lesser curvature of the arch in pecam-1−/− mice. The lesion area along the inner curvature of the arch of ldlr−/− mice was comparable in pecam-1+/+ recipients of pecam-1+/+ marrow and pecam-1−/− recipients of pecam-1−/− marrow (Figure S4); therefore, we could not use bone marrow chimeric mice to determine whether endothelial or bone marrow-derived leukocyte or platelet expression of PECAM-1 enhances lesion development in this region. However, like untransplanted pecam-1−/−ldlr−/− relative to pecam-1+/+ldlr−/− mice, pecam-1−/− recipients of pecam-1−/− bone marrow developed larger lesions in the aortic sinus (Figure 4A) and total aorta (Figure 4B) relative to pecam-1+/+ recipients of pecam-1+/+ marrow. Thus, bone marrow chimeric mice could be used to determine the effects of endothelial vs. hematopoietic cell expression of PECAM-1 on lesion development in the aortic sinus and total aorta of ldlr−/− mice.
Figure 4. The effect of endothelial cell vs. blood cell PECAM-1 expression on development of atherosclerosis in LDLR-deficient mice.
A) Endothelial cell PECAM-1 protects against development of atherosclerosis in the aortic sinus. Each symbol represents the mean percent atherosclerotic lesion area calculated from 4-6 oil red O-stained sections spanning the aortic sinus of an individual pecam-1+/+ldlr−/− (filled symbols) or pecam-1−/−ldlr−/− (open symbols) recipient mouse transplanted with pecam-1+/+ldlr−/− (squares) or pecam-1−/−ldlr−/− (circles) donor bone marrow and maintained on a HFD for 24 weeks. Horizontal lines indicate the mean percent aortic sinus lesion area for each group of animals. Asterisks denote statistically significant differences between groups (*p < 0.05, **p< 0.01). B) Endothelial cell and blood cell PECAM-1 expression are required for PECAM-1-mediated protection against development of atherosclerosis in the aorta. Each symbol represents the percent of total area of the indicated section of aorta that stained positively for oil red O in an individual pecam-1+/+ldlr−/− (filled symbols) or pecam-1−/−ldlr−/− (open symbols) recipient mouse transplanted with either pecam-1+/+ldlr−/− (squares) or pecam-1−/−ldlr−/− (circles) donor bone marrow and maintained on a HFD for 24 weeks. Horizontal lines indicate mean percent lesion area in the indicated aortic section, including the thoracic aorta, abdominal aorta and total aorta (aortic arch + thoracic aorta + abdominal aorta), for each group of animals. Asterisks denote statistically significant differences between groups (*p < 0.05, **p< 0.01).
In the aortic sinus, lesions that developed in pecam-1+/+ recipients of pecam-1−/− marrow were similar in size to those of pecam-1+/+ recipients of pecam-1+/+ marrow, and significantly smaller than the lesions that developed in pecam-1−/− recipients of pecam-1−/− marrow (Figure 4A). Also, lesions that developed in the aortic sinuses of pecam-1−/− recipients of pecam-1+/+ marrow were similar in size to those of pecam-1−/− recipients of pecam-1−/− marrow and significantly larger than the lesions that developed in pecam-1+/+ recipients of pecam-1+/+ marrow (Figure 4A). These results indicate that endothelial cell expression of PECAM-1 inhibits development of atherosclerotic lesions in the aortic sinuses of ldlr−/− mice.
Interestingly, mice that were missing PECAM-1 from either endothelial cells, bone marrow-derived leukocytes and/or platelets, or both exhibited a trend toward increased lesion sizes in the thoracic and abdominal aorta relative to mice that expressed PECAM-1 on both endothelial and bone marrow-derived cells; however, these differences did not reach statistical significance until lesion areas in the total aorta were compared (Figure 4B). These results indicate that expression of PECAM-1 on both endothelial and bone marrow-derived leukocytes and/or platelets is required for PECAM-1 to inhibit development of atherosclerotic lesions in the aortas of ldlr−/− mice.
Discussion
The major finding of this study is that PECAM-1 expression affects development of atherosclerosis differently at different lesion-prone sites of the vasculature. Specifically, PECAM-1 is pro-atherosclerotic in the inner curvature of the aortic arch, but atheroprotective in the aortic sinus, branching arteries, and descending aorta. The atheroprotective effect of PECAM-1 in the aortic sinus requires PECAM-1 expression only on endothelial cells, whereas PECAM-1 expression on both endothelial cells and bone marrow-derived cells is required for its atheroprotective effects in the descending aorta.
The pro-atherosclerotic effect of PECAM-1 in the inner curvature of the arch is consistent with its role as part of a mechanostimulatory complex on endothelial cells that activates NF-κB in response to low shear stress and induces expression of adhesion molecules that enable recruitment of inflammatory cells into the lesion9. A mechanostimulatory function for PECAM-1 is supported by the findings in many9,31-33, but not all34, studies that PECAM-1 facilitates responses of cultured endothelial cells to osmotic and fluid shear stresses. Previous studies have established that PECAM-1 is rapidly phosphorylated on cytoplasmic tyrosine residues in cultured endothelial cells exposed to fluid shear or osmotic stress;31-36 however, whether PECAM-1 tyrosine phosphorylation is required for its mechanostimulatory function is not yet known.
The atheroprotective effect of PECAM-1 in the aortic sinus, branching arteries, and descending aorta indicates that PECAM-1 normally inhibits development of atherosclerosis in these regions of the vasculature. Our studies of bone marrow chimeric mice revealed that the cells on which PECAM-1 must be expressed to inhibit lesion development vary by vascular region. Specifically, in both the aortic sinus and descending aorta, the atheroprotective effect of PECAM-1 required its expression on endothelial cells; in the aortic sinus, PECAM-1 expression on endothelial cells alone was sufficient for its inhibitory function. Two functions of the endothelium that impact lesion development in atherosusceptible regions include maintenance of the vascular permeability barrier and insurance of nitric oxide (NO) bioavailability.2 PECAM-1 has been shown to support maintenance of vascular integrity in at least four different models of inflammation, including intradermal injection of histamine,11 autoimmune encephalomyelitis,11 collagen-induced arthritis,12,15 and LPS-induced endotoxemia13,14. PECAM-1 deficiency has also been shown to affect NO bioavailability, either as a consequence of decreased production of NO19 or increased production of reactive oxygen species.20 Thus, either increased vascular permeability or decreased NO bioavailability could contribute to the increased atherosclerosis observed in the aortic sinuses and descending aortas of mice with PECAM-1-deficient relative to PECAM-1-positive endothelium.
The inhibitory effect of PECAM-1 on lesion development in the aorta as a whole, in contrast, required its expression not only on endothelial cells but also on hematopoietic cells. The hematopoietic cells thought to play crucial roles in atherosclerotic lesion development include monocytes, T lymphocytes, and platelets.1,3 There is ample evidence that PECAM-1 inhibits platelet responsiveness30,37 and PECAM-1 is also capable of interfering with both macrophage-mediated phagocytosis of viable cells38,39,40 and T cell receptor-mediated signaling pathways.41 Indeed, loss of PECAM-1 from circulating T cells correlated with occurrence of atherothrombotic plaque complications in humans42 and mice.43 Furthermore, in vivo administration of PECAM-1/IgG fusion proteins reduced lesion sizes in atherosusceptible mice coincident with blunted T cell activation, increased numbers of circulating regulatory T cells, and decreased infiltration of T cells into accumulating plaque.44 Collectively, these studies support the conclusion that interactions between PECAM-1 expressing endothelial cells and either platelets, monocytes or T cells may decrease atherosclerosis in aortas of PECAM-1-positive relative to PECAM-1-negative mice. Studies in which the pecam-1 gene is knocked out in specific types of hematopoietic cells are needed to determine the extent to which PECAM-1 expression by any one of these cell types normally interferes with development of atherosclerosis.
Finally, our findings indicate that PECAM-1 has both pro-atherosclerotic and atheroprotective effects on the vasculature; however, each of these opposing effects dominates in a different region of the vasculature. Thus, the inner curvature of the arch is more strongly influenced by the mechanostimulatory and therefore pro-atherosclerotic function of PECAM-1, whereas other atherosusceptible regions of the vasculature are more strongly influenced by its anti-inflammatory and therefore athero-protective effects. A possible explanation for the differential sensitivity of these regions to PECAM-1’s mechanosensory vs. anti-inflammatory roles is that PECAM-1 might influence the type and/or magnitude of hemodynamic shear stress to which different regions of the vasculature are exposed, which can be addressed by comparing the hemodynamic properties of PECAM-1+/+ vs. PECAM-1−/− aortas.45 Alternatively, PECAM-1 might contribute in different ways to the responses of cells in different regions of the vasculature, even if they are exposed to the same shear stresses. This possibility is consistent with the concept that site-specific responses to systemic factors modulate how atherosclerosis develops in different atherosusceptible regions.2 In either case, by demonstrating that PECAM-1 both promotes and impedes development of atherosclerotic lesions in site-specific ways, our findings provide a more complete understanding of the factors that interact in complex ways to control initiation and progression of atherosclerosis.
Acknowledgements
The authors thank J.D. Smith and M. Febbraio (Cleveland Clinic Foundation) for guidance in quantification of atherosclerosis, D.D. Gutterman, D.R. Harder and Daniel Rowe (Medical College of Wisconsin) for helpful suggestions, and Marjorie Kipp for help with the mouse colony.
Sources of Funding This work was supported by HL-68769 and HL-40926 (PJN and DKN) and an American Heart Association Postdoctoral Fellowship (RG).
Footnotes
Disclosures PJN is a consultant for Novo Nordisk and serves on the scientific advisory board of the New York Blood Center.
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 3.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DE. The unfolding tale of PECAM-1. Febs Lett. 2003;540:7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 5.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–7. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–51. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 7.Sun Q-H, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–8. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 8.Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J Biol Chem. 2007;282:23603–12. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 9.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 10.Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–92. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tada Y, Koarada S, Morito F, Ushiyama O, Haruta Y, Kanegae F, Ohta A, Ho A, Mak TW, Nagasawa K. Acceleration of the onset of collagen-induced arthritis by a deficiency of platelet endothelial cell adhesion molecule 1. Arthritis Rheum. 2003;48:3280–90. doi: 10.1002/art.11268. [DOI] [PubMed] [Google Scholar]
- 13.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 14.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–96. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong MX, Hayball JD, Hogarth PM, Jackson DE. The inhibitory co-receptor, PECAM-1 provides a protective effect in suppression of collagen-induced arthritis. J Clin Immunol. 2005;25:19–28. doi: 10.1007/s10875-005-0354-7. [DOI] [PubMed] [Google Scholar]
- 16.Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–8. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- 17.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 18.Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. Eur J Neurol. 2006;13:1284–90. doi: 10.1111/j.1468-1331.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 19.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–5. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R57–R65. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- 21.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, de la Pompa J Luis, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–30. [PubMed] [Google Scholar]
- 22.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–38. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Baglione J, Smith JD. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol Med. 2006;129:83–95. doi: 10.1385/1-59745-213-0:83. [DOI] [PubMed] [Google Scholar]
- 24.Ritman EL. Molecular imaging in small animals--roles for micro-CT. J Cell Biochem Suppl. 2002;39:116–24. doi: 10.1002/jcb.10415. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XY, Bentley MD, Chade AR, Ritman EL, Lerman A, Lerman LO. Early changes in coronary artery wall structure detected by micro computed tomography in experimental hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00362.2007. [DOI] [PubMed] [Google Scholar]
- 26.Molthen RC, Karau KL, Dawson CA. Quantitative models of the rat pulmonary arterial tree morphometry applied to hypoxia-induced arterial remodeling. J Appl Physiol. 2004;97:2372–84. doi: 10.1152/japplphysiol.00454.2004. [DOI] [PubMed] [Google Scholar]
- 27.Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging. 2001;20:854–67. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- 28.Wouters K, Shiri-Sverdlov R, van Gorp PJ, van BM, Hofker MH. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med. 2005;43:470–9. doi: 10.1515/CCLM.2005.085. [DOI] [PubMed] [Google Scholar]
- 29.Langheinrich AC, Bohle RM, Greschus S, Hackstein N, Walker G, Von GS, Rau WS, Holschermann H. Atherosclerotic lesions at micro CT: feasibility for analysis of coronary artery wall in autopsy specimens. Radiology. 2004;231:675–81. doi: 10.1148/radiol.2313021718. [DOI] [PubMed] [Google Scholar]
- 30.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1. New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–64. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 31.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–85. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai LK, Zheng Q, Pan S, Jin ZG, Berk BC. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem. 2005;280:29620–4. doi: 10.1074/jbc.M501243200. [DOI] [PubMed] [Google Scholar]
- 33.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–11. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 34.Sumpio BE, Yun S, Cordova AC, Haga M, Zhang J, Koh Y, Madri JA. MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J Biol Chem. 2005;280:11185–91. doi: 10.1074/jbc.M414631200. [DOI] [PubMed] [Google Scholar]
- 35.Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–37. [PubMed] [Google Scholar]
- 36.Kaufman DA, Albelda SM, Sun J, Davies PF. Role of lateral cell-cell border location and extracellular/transmembrane domains in PECAM/CD31 mechanosensation. Biochem Biophys Res Commun. 2004;320:1076–81. doi: 10.1016/j.bbrc.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 37.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–25. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 38.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–3. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 39.Vernon-Wilson EF, Aurade F, Brown SB. CD31 promotes beta1 integrin-dependent engulfment of apoptotic Jurkat T lymphocytes opsonized for phagocytosis by fibronectin. J Leukoc Biol. 2006;79:1260–7. doi: 10.1189/jlb.1005571. [DOI] [PubMed] [Google Scholar]
- 40.Vernon-Wilson EF, Aurade F, Tian L, Rowe IC, Shipston MJ, Savill J, Brown SB. CD31 delays phagocyte membrane repolarization to promote efficient binding of apoptotic cells. J Leukoc Biol. 2007;82:1278–88. doi: 10.1189/jlb.0507283. [DOI] [PubMed] [Google Scholar]
- 41.Newton-Nash DK, Newman PJ. A new role for Platelet-Endothelial Cell Adhesion Molecule-1 (CD31): Inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–8. [PubMed] [Google Scholar]
- 42.Caligiuri G, Rossignol P, Julia P, Groyer E, Mouradian D, Urbain D, Misra N, Ollivier V, Sapoval M, Boutouyrie P, Kaveri SV, Nicoletti A, Lafont A. Reduced immunoregulatory CD31+ T cells in patients with atherosclerotic abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2006;26:618–23. doi: 10.1161/01.ATV.0000200380.73876.d9. [DOI] [PubMed] [Google Scholar]
- 43.Caligiuri G, Groyer E, Khallou-Laschet J, Al Haj ZA, Sainz J, Urbain D, Gaston AT, Lemitre M, Nicoletti A, Lafont A. Reduced immunoregulatory CD31+ T cells in the blood of atherosclerotic mice with plaque thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1659–64. doi: 10.1161/01.ATV.0000172660.24580.b4. [DOI] [PubMed] [Google Scholar]
- 44.Groyer E, Nicoletti A, it-Oufella H, Khallou-Laschet J, Varthaman A, Gaston AT, Thaunat O, Kaveri SV, Blatny R, Stockinger H, Mallat Z, Caligiuri G. Atheroprotective effect of CD31 receptor globulin through enrichment of circulating regulatory T-cells. J Am Coll Cardiol. 2007;50:344–50. doi: 10.1016/j.jacc.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 45.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–51. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]