Abstract
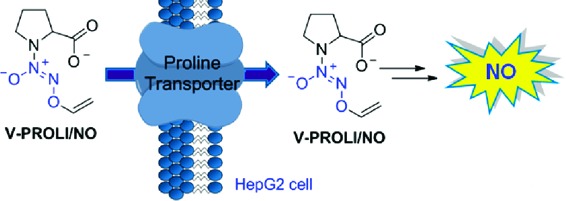
V-PYRRO/NO is a well-studied nitric oxide (NO) prodrug that has been shown to protect human liver cells from arsenic, acetaminophen, and other toxic assaults in vivo. Its proline-based analogue, V-PROLI/NO, was designed to be a more biocompatible form that decomposes to the naturally occurring metabolites of proline, NO, and glycolaldehyde. Like V-PYRRO/NO, this cytochrome P450-activated prodrug was previously assumed to passively diffuse through the cellular membrane. Using 14C-labeled proline in a competition assay, we show that V-PROLI/NO is transported through proline transporters into multiple cell lines. A fluorescent NO-sensitive dye (DAF-FM diacetate) and nitrite excretion indicated elevated intracellular NO release after metabolism over V-PYRRO/NO. These results also allowed us to predict and design a more permeable analogue, V-SARCO/NO. We report a proline transporter-based strategy for the selective transport of NO prodrugs that may have enhanced efficacy and aid in the development of further NO prodrugs with increased permeability.
Keywords: Nitric oxide, prodrug, proline, transporter, PROLI/NO, V-PROLI/NO
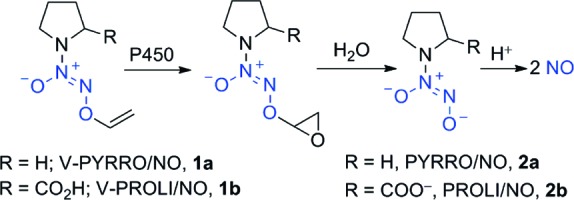
Site-directed delivery of therapeutic nitric oxide (NO) is challenging.1−8 Among the numerous approaches, diazeniumdiolate-based NO prodrugs have shown promise. For example, V-PYRRO/NO (1a), a liver-selective NO prodrug, is a hepatoprotective agent in a number of in vitro and in vivo studies (Scheme 1).9−26 V-PYRRO/NO (1a) was found to be metabolized by several cytochrome P450 (CYP) isoforms;23 the proposed mechanism for NO release is olefin epoxidation followed by hydrolytic cleavage to generate PYRRO/NO (2a), a spontaneously NO-releasing diazeniumdiolate ion (Scheme 1). V-PROLI/NO (1b), a proline-based analogue of V-PYRRO/NO, was recently reported to be metabolized by two isoforms of cytochrome P450 and was shown to protect human liver HepG2 cells against arsenic-induced toxic effects (Scheme 1).27,28
Scheme 1. Metabolism of NO Prodrugs V-PYRRO/NO (1a) and V-PROLI/NO (1b) Is Proposed To Be Initiated by Olefin Epoxidation by Cytochrome P450 Followed by Hydrolysis To Generate Diazeniumdiolate Anions Such as PYRRO/NO (2a) and PROLI/NO (2b), Which Spontaneously Decompose under Physiological Conditions to Generate NO.
The ability of these prodrugs to generate NO intracellularly was probed using a NO-sensitive fluorescent probe, 4-amino-5-methylaminofluorescein diacetate (DAF-FM DA).29,30 Human liver HepG2 cells that were preloaded with DAF-FM DA were treated with V-PYRRO/NO (1a) or V-PROLI/NO (1b) at various concentrations. Fluorescence measurements were carried out 1 h post-treatment; the fluorescence values relative to DMSO-treated cells are reported.29,30 Under the assay conditions, no significant increase in relative fluorescence at 100 μM V-PYRRO/NO (1a) was observed (Figure 1A).
Figure 1.
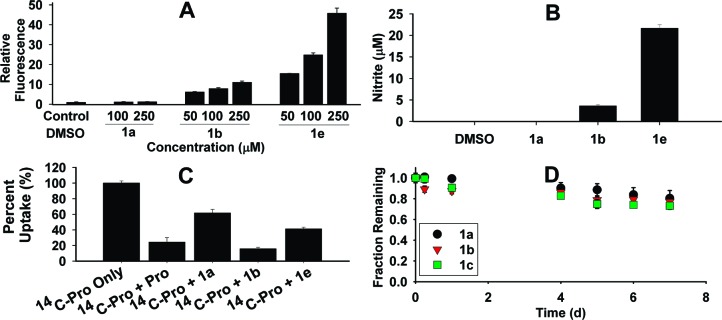
(A) Intracellular NO release in HepG2 cells as measured by DAF-FM DA assay. Cells that were preloaded with DAF-FM DA dye were treated with DMSO (control), V-PYRRO/NO, or V-PROLI/NO at various concentrations. Fluorescence measurements were carried out after 1 h. Values reported are averages of triplicate measurements. (B) Extracellular nitrite in HepG2 cells as measured by chemiluminescence. Cells were treated with a 250 μM concentration of the compound; nitrite levels were measured after 6 h. (C) l-[14C]Proline (14C-Pro) uptake. HepG2 cells were treated with a 5 nM concentration of 14C-Pro and a 5 mM concentration of the compounds. Data were normalized to 14C-Pro only uptake. (D) Decomposition in pH 7.4 Hank's balanced salt solution (HBSS) at 37 °C over 7 days. The decomposition profile was measured by HPLC.
However, V-PROLI/NO (1b) showed elevated fluorescence levels relative to control not just at 100 μM, but even at 50 μM (Figure 1A). The formation of extracellular nitrite (a product of aerobic oxidation of NO) upon treatment of HepG2 cells with these compounds is indicative of V-PYRRO/NO (1a) and V-PROLI/NO (1b) metabolism to release NO. At comparable concentrations (250 μM), V-PROLI/NO (1b) formed much higher levels of nitrite (3 μM) in comparison with those of V-PYRRO/NO (not detectable, <0.6 μM) after 6 h (Figure 1B). This finding was similar with literature values.28,31
V-PROLI/NO (1b) and V-PYRRO/NO (1a) were stable in pH 7.4 buffer at 37 °C for 7 days (see the Supporting Information) and had comparable CYP2E1-mediated metabolism profiles (roughly 30% of prodrug metabolized in 1 h).23,27 Taken together, these results suggest no major differences in decomposition profiles of V-PROLI/NO and V-PYRRO/NO (1a).
Recently, O2-(2,4-dinitrophenyl)diazeniumdiolates with a free carboxylic acid were reported as poor sources of intracellular NO and showed diminished antiproliferative activity against human leukemia HL-60 cells.32 Their carboxylic acid ester prodrugs, however, were superior to their carboxylic acid counterparts in both their ability to permeate cells to release NO and their antiproliferative activity. Thus, in an attempt to improve cell permeability, 1c (Chart 1) was prepared from V-PROLI/NO (1b) using a reported procedure.33
Chart 1.
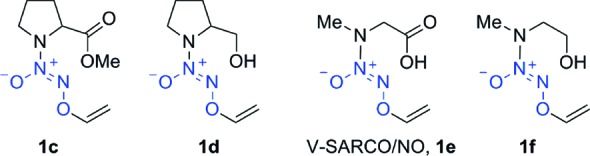
Surprisingly, in the DAF-FM DA assay, 1c, the methyl ester of V-PROLI/NO, did not show a very significant increase in fluorescence and was comparable with the DMSO control (see the Supporting Information). Compounds 1a, 1b, 1c, and the prolinol derivative 1d were also included (Chart 1). Again, we found that among the five-membered ring analogues tested, V-PROLI/NO (1b) was the most active (see the Supporting Information). Taken together, these results suggest that V-PROLI/NO is a far superior source of intracellular NO in comparison with V-PYRRO/NO and its closed ring structural analogues prepared in this study.
Our data suggested preferential entry for V-PROLI/NO over V-PYRRO/NO and other analogues. The literature is replete with transporters for proline and related peptides,34−39 and the affinity of several such transporters appears to be sensitive to structural modifications.40 For example, the proton-coupled amino acid transporter has considerably less affinity for substrates such as pyrrolidine, proline methyl ester, and prolinol than l-proline or even sarcosine.40 This structure−affinity pattern (Table 1) is consistent with our observations of cell permeability in this study.
Table 1. Inhibition of Proline Uptake by Various Substrates40a.
| entry | compound | l-[3H]proline uptake (%) |
|---|---|---|
| 1 | control | 100 |
| 2 | l-proline | 32 |
| 3 | l-prolinol | 85 |
| 4 | l-proline methyl ester | 63 |
| 5 | pyrrolidine | 82 |
| 6 | sarcosine | 24 |
Uptake of l-[3H]proline (10 nM) in CaCo-2 cells at pH 6 of various compounds (10 mM) as reported by Metzner et al.40.
Next, the ability of V-PROLI/NO to inhibit transport of l-proline was evaluated using a protocol similar to that reported by Metzner and co-workers.40 Under these conditions, radiolabeled proline uptake was inhibited by V-PROLI/NO at a level comparable to l-proline. V-PYRRO/NO showed considerably less inhibition of proline uptake (Figure 1C) but was also comparable to its amine counterpart, pyrrolidine, in its reported inhibition of proline uptake.40
On the basis of the study by Metzner and co-workers, we predicted that the sarcosine analogue, V-SARCO/NO (1e),33 would have a cellular penetrance comparable to that of V-PROLI/NO (1b).40 Indeed, when HepG2 and CaCo-2 cells were independently pretreated with DAF-FM DA, exposed to 100 μM 1a−f, and examined by fluorescence after 1 h, we observed higher fluorescence generated by V-SARCO/NO (1e) in comparison with all other analogues tested including the sarcosinol derivative, 1f (see the Supporting Information). The extracellular nitrite level was also much higher in the case of V-SARCO/NO (Figure 1B).41
Finally, proline transport inhibition by V-SARCO/NO (1e) was somewhat diminished in comparison with V-PROLI/NO, suggesting that V-SARCO/NO cellular uptake may not be restricted to proline transporters (Figure 1C). The conformational flexibility of the open chain of sarcosine may allow V-SARCO/NO (1e) to access transporters other than those accessed by proline. Taken together, these observations may provide us new modes for targeting NO. Future work will focus on elucidating mechanisms of uptake of V-PROLI/NO and the identity of such transporters.
Experimental Procedures
The intracellular level of NO after diazeniumdiolate prodrug treatment was quantified using the NO-sensitive fluorophore DAF-FM DA. Cells were loaded with 2 mL of 2.0 μM DAF-FM DA in HBSS in each well at 37 °C and 5% CO2. HepG2 cells were loaded in a six-well plate at 3 million cells per well and allowed to grow overnight. CaCo-2 cells were loaded in a six-well plate at 500000 cells per well and allowed to grow overnight. After 30 min of incubation, the cells were rinsed with HBSS to remove excess of probe, and 3 mL of HBSS was added. The compounds were made up in DMSO solutions and added to the cells. After 60 min of incubation, each well was scraped to suspend the cells and pipetted into test tubes. The fluorescence of the benzotriazole derivative formed on DAF-FM DA's reaction with NO was analyzed by a fluorescence spectrometer with the excitation source at 495 nm and emission at 515 nm. The mean value of two trials is reported in Tables S1 and S2 (Supporting Information).
Stock solutions (10 mM) of the compounds in DMSO were prepared and diluted in HBSS to a final concentration of 100 μM and incubated at 37 °C in the dark. The mean value of two trials carried out over 7 days is reported (Table S3 in the Supporting Information).
HepG2 cells in DMEM were loaded with 3 mL of 250 μM prodrug solutions. After a 6 h incubation at 37 °C and 5% CO2, the supernatant of each well was removed to be analyzed. A procedure provided by the manufacturer was used where 50 μL of the supernatant was injected into a reducing solution of 1% w/v potassium iodide solution in glacial acetic acid to convert nitrite to NO.42 The results of two trials were averaged. Nitrite levels were interpolated from a standard curve (Table S4 in the Supporting Information). Various concentrations of sodium nitrite in DMEM media were used as standards for the calibration experiment. All assays were performed in duplicate, and data can be found in Table S5 (see the Supporting Information).
HepG2 cells were plated in six-well plates at a density of 500000 cells per well and cultured for 48 h. Cells were washed once with PBS and then loaded with 1.0 mL of HBSS containing 0.8 μM 14C-labeled l-proline (4 × 105 DPM mL−1) with either 5 mM l-proline, V-PYRRO/NO, V-PROLI/NO, or V-SARCO/NO. The cells were then incubated at 37 °C and 5% CO2 for 15 min. The cells were then washed three times with ice-cold PBS and lysed in 1 mL of cold RIPA buffer. A 200 μL amount from each sample was then added to 12 mL of scintillation cocktail and analyzed. All samples were performed in triplicate (Table S6 in the Supporting Information). The disintegrations per minute (DPM) were determined using the relationship between counts per minute (CPM) and the scintillation counter's counting efficiency, DPM = (CPM − CPMbackground)/counting efficiency. The average observed background was 85 CPM. The counting efficiency was 92.5%.
Supporting Information Available
General experimental methods, data for fluorescence measurements, HPLC curve areas, nitrite determination, and radiolabeled proline uptake. This material is available free of charge via the Internet at http://pubs.acs.org.
This project has been funded with Federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E, and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Smith D. J.; Chakravarthy D.; Pulfer S.; Simmons M. L.; Hrabie J. A.; Citro M. L.; Saavedra J. E.; Davies K. M.; Hutsell T. C.; Mooradian D. L.; Hanson S. R.; Keefer L. K. Nitric oxide-releasing polymers containing the [N(O)NO]- group. J. Med. Chem. 1996, 39, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Hrabie J. A.; Keefer L. K. Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate (“Nitrosohydroxylamine”) Functional Group and Its Oxygen-Substituted Derivatives. Chem. Rev. 2002, 102, 1135–1154. [DOI] [PubMed] [Google Scholar]
- Pavlos C. M.; Xu H.; Toscano J. P. Controlled photochemical release of nitric oxide from O2-substituted diazeniumdiolates. Free Radical Biol. Med. 2004, 37, 745–752. [DOI] [PubMed] [Google Scholar]
- Scatena R.; Bottoni P.; Martorana G. E.; Giardina B. Nitric oxide donor drugs: an update on pathophysiology and therapeutic potential. Expert Opin. Invest. Drugs 2005, 14, 835–846. [DOI] [PubMed] [Google Scholar]
- Keefer L. K. Nitric Oxide (NO)- and Nitroxyl (HNO)-Generating Diazeniumdiolates (NONOates): Emerging Commercial Opportunities. Curr. Top. Med. Chem. 2005, 5, 625–636. [DOI] [PubMed] [Google Scholar]
- Frost M. C.; Reynolds M. M.; Meyerhoff M. E. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials 2005, 26, 1685–1693. [DOI] [PubMed] [Google Scholar]
- Thatcher G. R. An Introduction to NO-related Therapeutic Agents. Curr. Top. Med. Chem. 2005, 5, 597–601. [DOI] [PubMed] [Google Scholar]
- Stasko N. A.; Schoenfisch M. H. Dendrimers as a scaffold for nitric oxide release. J. Am. Chem. Soc. 2006, 128, 8265–8271. [DOI] [PubMed] [Google Scholar]
- Saavedra J. E.; Billiar T. R.; Williams D. L.; Kim Y.; Watkins S. C.; Keefer L. K. Targeting Nitric Oxide (NO) Delivery in Vivo. Design of a Liver-Selective NO Donor Prodrug That Blocks Tumor Necrosis Factor-a-Induced Apoptosis and Toxicity in the Liver. J. Med. Chem. 1997, 40, 1947–1954. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Kim T.; Chung H.; Talanian R. V.; Yin X.; Billiar T. R. Nitric oxide prevents tumor necrosis factor α-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology 2000, 32, 770–778. [DOI] [PubMed] [Google Scholar]
- Ricciardi R.; Foley D. P.; Quarfordt S. H.; Saavedra J. E.; Keefer L. K.; Wheeler S. M.; Donohue S. E.; Callery M. P.; Meyers W. C. V-PYRRO/NO: an hepato-selective nitric oxide donor improves porcine liver hemodynamics and function after ischemia reperfusion. Transplantation 2001, 71, 193–198. [DOI] [PubMed] [Google Scholar]
- Shokolenko I.; Oberyszyn T. M.; D'Ambrosio S. M.; Saavedra J. E.; Keefer L. K.; LeDoux S. P.; Wilson G. L.; Robertson F. M. Protection of Human Keratinocyte mtDNA by Low-Level Nitric Oxide. Nitric Oxide Biol. Chem. 2001, 5, 555–560. [DOI] [PubMed] [Google Scholar]
- Liu J.; Saavedra J. E.; Lu T.; Song J.; Clark J.; Waalkes M. P.; Keefer L. K. O 2-Vinyl 1-(Pyrrolidin-1-yl)diazen-1-ium-1,2-diolate Protection Againstd-Galactosamine/Endotoxin-Induced Hepatotoxicity in Mice: Genomic Analysis Using Microarrays. J. Pharmacol. Exp. Ther. 2002, 300, 18–25. [DOI] [PubMed] [Google Scholar]
- Stinson S. F.; House T.; Bramhall C.; Saavedra J. E.; Keefer L. K.; Nims R. W. Plasma pharmacokinetics of a liver-selective nitric oxide-donating diazeniumdiolate in the male C57BL/6 mouse. Xenobiotica 2002, 32, 339–347. [DOI] [PubMed] [Google Scholar]
- Li C.; Liu J.; Saavedra J. E.; Keefer L. K.; Waalkes M. P. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology 2003, 189, 173–180. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li C.; Waalkes M. P.; Clark J.; Myers P.; Saavedra J. E.; Keefer L. K. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology 2003, 37, 324–333. [DOI] [PubMed] [Google Scholar]
- Gong P.; Cederbaum A. I.; Nieto N. The Liver-Selective Nitric Oxide Donor O2-Vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) Protects HepG2 Cells against Cytochrome P450 2E1-Dependent Toxicity. Mol. Pharmacol. 2004, 65, 130–138. [DOI] [PubMed] [Google Scholar]
- Liu J.; Qu W.; Saavedra J. E.; Waalkes M. P. The Nitric Oxide Donor, O2-Vinyl 1-(Pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO), Protects against Cadmium-Induced Hepatotoxicity in Mice. J. Pharmacol. Exp. Ther. 2004, 310, 18–24. [DOI] [PubMed] [Google Scholar]
- Balogh G. T.; Dalmadi B.; Bielik A.; Keseru G. M. Identification of Nitric Oxide Donors by Biomimetic HTS Application. Comb. Chem. High Throughput Screening 2005, 8, 347–352. [DOI] [PubMed] [Google Scholar]
- Liu J.; He Y.; Chignell C. F.; Clark J.; Myers P.; Saavedra J. E.; Waalkes M. P. Limited protective role of V-PYRRO/NO against cholestasis produced by alpha-naphthylisothiocyanate in mice. Biochem. Pharmacol. 2005, 70, 144–151. [DOI] [PubMed] [Google Scholar]
- Liu J.; Waalkes M. P. Nitric oxide and chemically induced hepatotoxicity: beneficial effects of the liver-selective nitric oxide donor, V-PYRRO/NO. Toxicology 2005, 208, 289–297. [DOI] [PubMed] [Google Scholar]
- Qu W.; Liu J.; Fuquay R.; Shimoda R.; Sakurai T.; Saavedra J. E.; Keefer L. K.; Waalkes M. P. The nitric oxide prodrug, V-PYRRO/NO, protects against cadmium toxicity and apoptosis at the cellular level. Nitric Oxide Biol. Chem. 2005, 12, 114–120. [DOI] [PubMed] [Google Scholar]
- Inami K.; Nims R. W.; Srinivasan A.; Citro M. L.; Saavedra J. E.; Cederbaum A. I.; Keefer L. K. Metabolism of a liver-selective nitric oxide-releasing agent, V-PYRRO/NO, by human microsomal cytochromes P450. Nitric Oxide Biol. Chem. 2006, 14, 309–315. [DOI] [PubMed] [Google Scholar]
- Qu W.; Liu J.; Fuquay R.; Saavedra J. E.; Keefer L. K.; Waalkes M. P. The nitric oxide prodrug, V-PYRRO/NO, mitigates arsenic-induced liver cell toxicity and apoptosis. Cancer Lett. 2007, 256, 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.; Feng H.; Reynolds C.; Mao L.; Rockey D. C. Effect of the nitric oxide donor V-PYRRO/NO on portal pressure and sinusoidal dynamics in normal and cirrhotic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1311–G1317. [DOI] [PubMed] [Google Scholar]
- Hong S. Y.; Saavedra J. E.; Keefer L. K.; Chakrapani H. Improved synthesis of V-PYRRO/NO, a liver-selective nitric oxide prodrug, and analogues. Tetrahedron Lett. 2009, 50, 2069–2071. [Google Scholar]
- Chakrapani H.; Showalter B. M.; Kong L.; Keefer L. K.; Saavedra J. E. V-PROLI/NO, a Prodrug of the Nitric Oxide Donor, PROLI/NO. Org. Lett. 2007, 9, 3409–3412. [DOI] [PubMed] [Google Scholar]
- Qu W.; Liu J.; Dill A. L.; Saavedra J. E.; Keefer L. K.; Waalkes M. P. V-PROLI/NO, a nitric oxide donor prodrug, protects liver cells from arsenic-induced toxicity. Cancer Sci. 2009, 100, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H.; Urano Y.; Kikuchi K.; Higuchi T.; Hirata Y.; Nagano T. Fluorescent Indicators for Imaging Nitric Oxide Production. Angew. Chem., Int. Ed. 1999, 38, 3209–3212. [DOI] [PubMed] [Google Scholar]
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radical Biol. Med. 2007, 43, 995–1022. [DOI] [PubMed] [Google Scholar]
- Gong P.; Cederbaum A. I.; Nieto N. The liver-selective nitric oxide donor O2-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) protects HepG2 cells against cytochrome P450 2E1-dependent toxicity. Mol. Pharmacol. 2004, 65, 130–138. [DOI] [PubMed] [Google Scholar]
- Chakrapani H.; Maciag A. E.; Citro M. L.; Keefer L. K.; Saavedra J. E. Cell-Permeable Esters of Diazeniumdiolate-Based Nitric Oxide Prodrugs. Org. Lett. 2008, 10, 5155–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. Y.; Nandurdikar R. S.; Keefer L. K.; Saavedra J. E.; Chakrapani H. An improved synthesis of V-PROLI/NO, a cytochrome P450-activated nitric oxide prodrug. Tetrahedron Lett. 2009, 50, 4545–4548. [Google Scholar]
- Herrera-Ruiz D.; Knipp G. T. Current perspectives on established and putative mammalian oligopeptide transporters. J. Pharm. Sci. 2003, 92, 691–714. [DOI] [PubMed] [Google Scholar]
- Daniel H. Molecular and Integrative Physiology of Intestinal Peptide Transport. Annu. Rev. Physiol. 2004, 66, 361–384. [DOI] [PubMed] [Google Scholar]
- Brandsch M. Transport of L-proline, L-proline-containing peptides and related drugs at mammalian epithelial cell membranes. Amino Acids 2006, 31, 119–136. [DOI] [PubMed] [Google Scholar]
- Bröer S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [DOI] [PubMed] [Google Scholar]
- Bröer S. Apical Transporters for Neutral Amino Acids: Physiology and Pathophysiology. Physiology 2008, 23, 95–103. [DOI] [PubMed] [Google Scholar]
- Brandsch M.; Knütter I.; Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J. Pharm. Pharmacol. 2008, 60, 543–585. [DOI] [PubMed] [Google Scholar]
- Metzner L.; Neubert K.; Brandsch M. Substrate specificity of the amino acid transporter PAT1. Amino Acids 2006, 31, 111–117. [DOI] [PubMed] [Google Scholar]
- The half-lives of PYRRO/NO and PROLI/NO are 3 and 1.8 s, respectively,9,43 and are expected to be completely decomposed during 1 h. SARCO/NO is expected to have a half-life comparable to PYRRO/NO and PROLI/NO and should completely decompose within 1 h.
- Nitric Oxide Analyzer NOA 280i Operation and Maintenance Manual, Version 3.0; Sievers Instruments, Inc.: Boulder, CO, 2000. [Google Scholar]
- Saavedra J. E.; Southan G. J.; Davies K. M.; Lundell A.; Markou C.; Hanson S. R.; Adrie C.; Hurford W. E.; Zapol W. M.; Keefer L. K. Localizing antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J. Med. Chem. 1996, 39, 4361–4365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


