Abstract
Adult newts have the remarkable ability to regenerate their spinal cords after a complete transection injury. In order to understand this process, we have developed a method for visualizing the cellular and molecular events during regeneration in whole-mount preparations using fluorescent probes (streptavidins and antibodies) and confocal microscopy. This method was optimized by varying parameters associated with fixation, tissue trimming, fluorescent probe penetration, and clearing and represents a significant advance in our ability to observe the intact and regenerating newt spinal cord. These methods should also be widely applicable to the study of other newt tissues and adult tissues from other model systems.
Keywords: spinal cord regeneration, newt, whole-mount, immunohistofluorescence, confocal imaging
Introduction
Often, the first step in understanding a complex biological process is to carefully observe the process, to identify the structural, cellular or molecular players and watch their behaviors. Ideally, this is best done in a way that maintains three-dimensional (3D) relationships. While 3D relationships can be reconstructed from images of serial sections, this method is time consuming and introduces artifacts. Individual sections can be warped or torn, and this can make it difficult to faithfully reconstruct the 3D image. A better and more efficient way to observe 3D relationships is to image tissues in whole-mount on a confocal microscope. For this method, the tissue must be fluorescently labeled, optically clear, and not more than about 300 μm thick. Thus, zebrafish embryos, which are clear and small, are ideal for confocal microscopy and can even be imaged live if fluorescent labels are introduced in vivo. Many specimens, however, must be trimmed, fluorescently labeled ex vivo, and cleared after an animal has been sacrificed and its tissues fixed. While methods for performing each of these steps have been established for many developmental model organisms (Klymkowsky and Hanken, 1991; Kardon, 1998), these methods have not been optimized for use in many adult tissues, whi ch are larger and less permeable, or for use in many less common model organisms, such as the newt.
Newts are remarkable for their ability to regenerate body parts after they are lost or damaged. A newt can regenerate its limbs (Brockes, 1997), tail (Iten and Bryant, 1976), jaw (Ghosh et al., 1994), retina (Mitashov, 1996), lens (Eguchi et al., 1974), portions of its heart (Oberpriller and Oberpriller, 1974) and even its spinal cord (Piatt, 1955). After a complete transection injury to the spinal cord, the newt can regenerate its spinal cord and regain the use of its hindlimbs and tail in as little as 4 weeks (Davis et al., 1990). To begin to understand how newts are able to do this, we sought to develop methods that would allow us to visualize with unprecedented clarity the cellular and molecular events after a spinal cord transection injury in the adult newt.
Here we report a method for obtaining 3D images of intact and regenerating spinal cords of adult newts via confocal microscopy that was optimized by varying parameters associated with fixation, tissue trimming, fluorescent labeling, and clearing. This method will be invaluable for future studies of spinal cord regeneration in the newt, as well as for the development of methods for obtaining 3D images of other newt or adult tissues.
Results and Discussion
The sequential steps to our method are as follows: fixation, bone decalcification (to prepare for trimming), bleaching (part of clearing), embedding and vibratome sectioning (to trim the tissue), permeabilization and incubation with fluorescent probes, and clearing (Table 1). Parameters tested for each step were evaluated on 4 mm segments of spinal cord from the lower trunk region of the animal. Spinal cords were intact or regenerating after a transection injury. In some animals, the axon tracer, biotinylated dextran amine (BDA), was applied in vivo and visualized with streptavidin-Cy5. The method was first optimized for the streptavidin probe because of its smaller size (about 60 kDa) and was then later modified to work with antibodies, which are much larger (150 kDa for IgG, 900 kDa for IgM). Spinal cords were imaged on a confocal microscope through the dorsal-ventral axis, such that each confocal plane is a longitudinal section. Table 1 shows the parameters that were tried for each step and identifies the preferred conditions for streptavidin and antibody labeling.
Table 1. Sequential steps, parameters tested for each step and preferred conditions for streptavidin and antibody labeling.
| Sequential Steps | Parameters Tested | Preferred Conditions for: | |
|---|---|---|---|
| Streptavidin Labeling | Antibody Labeling | ||
| fixation | PLP with 0.5% PFA PLP with 2%PFA 2% glutaraldehyde/2%PFA perfusion vs non-perfusion |
perfuse & post-fix PLP with 0.5% PFA |
post-fix without perfusion PLP with 0.5% PFA |
| decalcification | Morse's solution | Morse's solution | Morse's solution |
| bleaching | modified Dent's bleach formamide bleach |
formamide bleach | formamide bleach |
| embedding | 3%, 4%, and 6% agarose | 4% agarose | 4% agarose |
| sectioning | 100 – 400 um razor blades, feather blades |
400 um feather blades |
350-375 um feather blades |
| permeabilization | 20% DMSO with one of: 0.5 – 2% Triton X-100 (non-ionic) 1% Tween-20 (weaker non-ionic) 1% NP-40 (stronger non-ionic) 1% CHAPS (zwitterionic) 1% SDS in pre-treatment (ionic) |
20% DMSO 0.5% Triton X-100 |
20% DMSO 2% Triton X-100 |
| incubation | 1 – 10 days 96 well plate, small centrifuge tubes |
7 days 96 well plate or centrifuge tubes |
10 days 1° 10 days 2° centrifuge tubes |
| clearing | glycerol BABB |
BABB | BABB |
Fixation
We sought a fixative that would preserve cellular morphology and molecular antigenicity and still allow fluorescent probes to penetrate fully into the newt spinal cord. Fixatives tried included glutaraldehyde (GA), paraformaldehyde (PFA), and periodate lysine paraformaldehyde (PLP). GA is commonly used for electron microscopy because it preserves morphology very well, but because it interacts strongly with proteins, it tends to disrupt antigen-antibody interactions. PFA does not preserve morphology as well as GA, but it is more commonly used for immunohistochemistry because it does not cross-link proteins and disrupt antigenicity as much as GA. PFA, however, tends to increase autofluorescence to the already highly autofluorescent newt tissues. Finally, PLP was tried because it was found to preserve morphology as well as GA (McLean and Nakane, 1974), and because it can be effective with lower concentrations of PFA, it may reduce autofluorescence and allow fluorescent probes to more readily enter the tissue, while still preserving antigenicity. Alcohol-based fixatives were not tried because they tend to denature antigens, and molecules that are soluble in alcohol are lost. (McLean and Nakane, 1974; Klymkowsky and Hanken, 1991; Rogers, 1999).
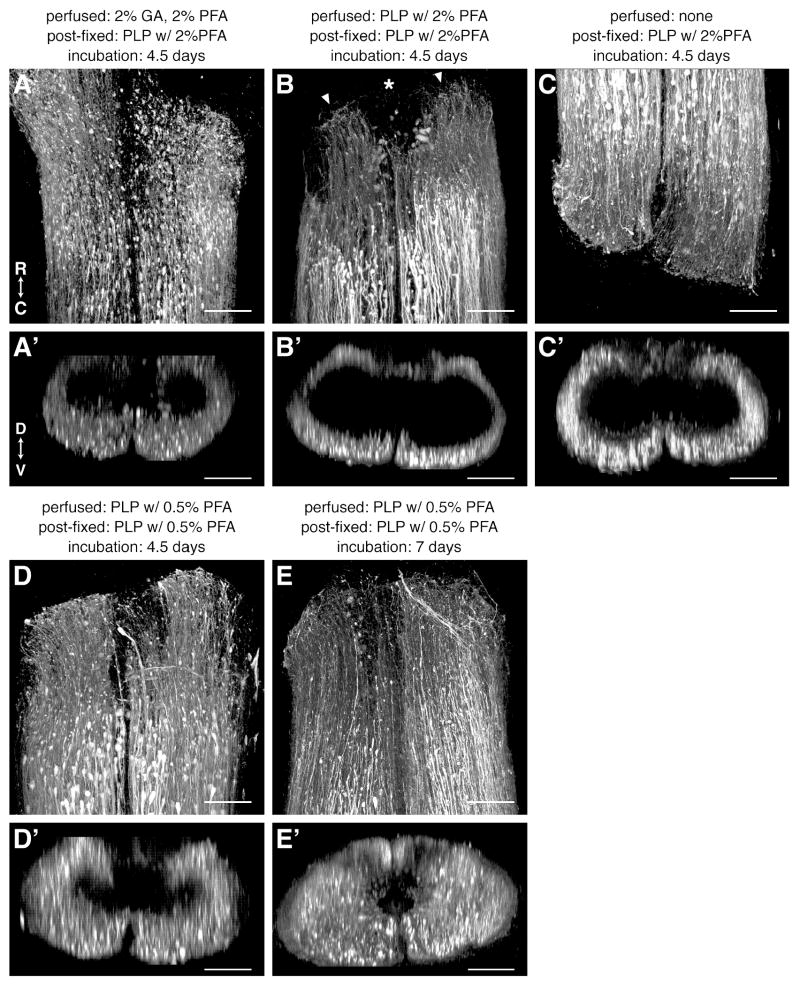
The fixatives were evaluated in newt spinal cords that had regenerated 3d after a spinal cord transection (Figure 1). BDA was applied rostral or caudal to the injury site to label descending or ascending axons, respectively. Animals were perfused with 2% GA/2% PFA, PLP with 2% PFA or PLP with 0.5% PFA, and 4 mm of spinal column containing the injury site was post-fixed in PLP with 2% PFA (after GA/PFA and PLP with 2% PFA perfusion) or PLP with 0.5% PFA (after PLP with 0.5% PFA perfusion). One animal was not perfused, but was only post-fixed with PLP with 2% PFA. All tissues were trimmed to a thickness of 400 μm, except the non-perfused tissue which was trimmed to 300 μm, and incubated with streptavidin-Cy5 for 4.5 days.
Figure 1.
Comparison of different methods of fixation. A-E: Dorsal views of 3d regenerating spinal cords, fixed as indicated above each panel, imaged on a confocal microscope and 3D-rendered in Fluorender. The axon tracer, BDA, was applied rostral (C) or caudal (A,B,D,E) to the injury site (asterisk in B), labeled with streptavidin-Cy5 for the duration indicated above each panel, and is shown in white. Arrowheads in B mark the end of the cut spinal cord. A′-E′: Cross sectional views of the same cords shown in A-E. R, rostral; C, caudal; D, dorsal; V, ventral. Scale bars: 100 μm.
As shown in Figure 1, morphology was well preserved and the amount of autofluorescence was low in all animals, regardless of which fixative was used and whether or not the tissue was perfusion-fixed (Fig. 1A-D). The different fixatives did have an effect, however, on how far the streptavidin probe was able to penetrate into the spinal cord. This is evident when the spinal cords are viewed in cross section (Fig. 1A′-D′). Though not complete after 4.5 days of incubation, penetrance was best when the fixative with the least amount of formaldehyde was used, namely, PLP with 0.5% PFA.
Therefore, we favored the PLP with 0.5% PFA fixative and found that we could improve streptavidin penetration without detrimentally affecting morphology by increasing the incubation duration to 7 days (Fig. 1E,E′). Perfusion fixation does not appear to be necessary to preserve morphology (compare Fig. 1B,C) and it may hinder probe penetrance (compare Fig. 1B′,C′), but because perfusion makes the tissue stiffer and, therefore, easier to section, we preferred to include this step for streptavidin-only labeling. We preferred to omit perfusion, however, on tissue that was labeled with antibodies in order to optimize their penetration into the spinal cord (see below).
Tissue Trimming
In order to minimize the amount of tissue the confocal laser and fluorescent probes must penetrate, we used a vibratome to remove tissue dorsal and ventral to the spinal cord. We preferred to do this rather than dissect the cord out of the spinal column because dissection would disrupt the 3D relationships of a spinal cord injury site.
To prepare for vibratome sectioning, segments of spinal column were decalcified with Morse's solution and embedded in agarose. The agarose served as a support for the tissue during sectioning and in order for it to provide sufficient support, it needed to have a density similar to that of the spinal column. This is a challenge because spinal column tissues have heterogeneous densities: the spinal cord is very soft compared to the muscle and decalcified bone around it. We tried 3%, 4%, and 6% agarose and found that, when sections are cut with thin, sharp blades, 4% worked best and enabled us to reliably obtain good sections. Agarose-embedded tissue blocks were glued onto vibratome chucks, ventral side down, and the dorsal tissue was trimmed until the spinal cord was exposed. A 350 to 400 μm thick section was then cut which contained the entire spinal cord. The ventral side of the spinal cord was not always exposed in a 400 μm thick cut, but nonetheless this was adequate for streptavidin labeling. For antibody labeling, however, we preferred to risk losing a little of the ventral spinal cord to ensure that both sides were exposed and, therefore, cut 350 – 375 μm thick sections. It was not possible to obtain sections much thinner than about 100 μm because of the heterogeneous nature of the spinal column.
Fluorescent Labeling
Specific probes, such as antibodies and streptavidin, bind with high affinity to their specific binding sites and with low affinity to non-specific sites. To obtain efficient, specific and even labeling of whole-mount preparations with such probes, specific binding sites must be made accessible throughout the tissue. Methods for making the tissue permeable to probes include the use of detergents, heat, enzymes, and carrier agents such as dimethyl sulfoxide (DMSO). At the same time, conditions for non-specific binding must be made unfavorable either with blocking reagents or by incubation at lower temperatures or both.
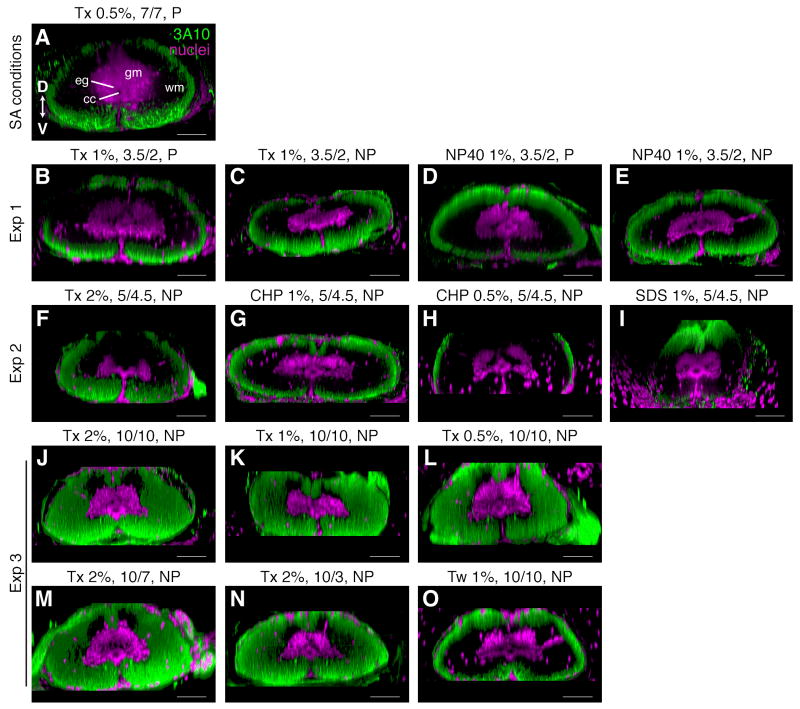
In initial experiments, we used phosphate buffered saline (PBS) with 0.5% of the detergent Triton X-100 and 20% of the carrier agent DMSO to permeabilize the newt spinal cord and incubated the sections at room temperature (RT) for 7 days. To block non-specific binding, we used 1% BSA and 0.1% fish gelatin. This was sufficient to allow the streptavidin probe to penetrate fully into a 400 μm section and label the axon tracer specifically with minimal background signal (Fig. 1E). This was not sufficient, however, for antibody labeling. Using these same parameters and a 7d incubation with primary antibody followed by a 7d incubation with secondary antibody at RT, there were regions in the middle of the spinal cord that were not labeled with the 3A10 antibody, which is an axonal marker (Fig. 2A).
Figure 2.
Comparison of detergents and incubation times. A-O: Longitudinal sections of intact spinal cords were imaged through the dorsal-ventral axis, 3D-rendered in Fluorender and rotated to reveal cross sectional morphology. Dorsal is up. Axons were labeled with the 3A10 antibody (green) and nuclei with SYTOX green (magenta). Headings above each panel indicate the amount and type of detergent used to permeabilize the section (Tx, Triton X-100; NP40, NP-40; CHP, CHAPS; SDS, SDS; Tw, Tween-20), the incubation times with primary and secondary antibodies (10/7 indicates 10 days with primary and 7 days with secondary), and whether the tissue was perfused (P) or not perfused (NP). Headings to left of each row group images into their respective experiments. Details of each experiment are summarized in Table 2. SA, streptavidin; cc, central canal; eg, ependymoglia; gm, grey matter; wm, white matter; D, dorsal; V, ventral. Scale bars: 100 μm.
To improve antibody penetration, therefore, we tested whether increasing the amount or strength of the detergent could improve antibody labeling, compared to 0.5% Triton X-100, without detrimentally affecting morphology. We did not consider enzyme treatments, such as trypsin or collagenase, because we were interested in studying the expression of extracellular matrix proteins. Detergents tested, in order of increasing strength, were Tween-20 (weaker non-ionic), Triton X-100 (non-ionic), NP-40 (stronger non-ionic), CHAPS (zwitterionic), and SDS (ionic) (Bhairi and Mohan, 2007). SDS was only used during the pre-treatment period since it is a denaturing detergent. The detergents were evaluated in intact newt spinal cords that were labeled with the 3A10 antibody and an antibody against glial fibrillary acidic protein (GFAP), which is an ependymoglial and astrocytic marker in the newt. None of the other detergents out-performed Triton X-100. CHAPS and NP-40 worked about as well as similar concentrations of Triton X-100 (compare Fig. 2B,C with D,E; and F with G) and, as expected, Tween-20 did not work as well (compare Fig. 2K with O). SDS disrupted the morphology of the spinal cord, in that the cord appeared stretched and warped, without improving antibody penetration (Fig 2I).
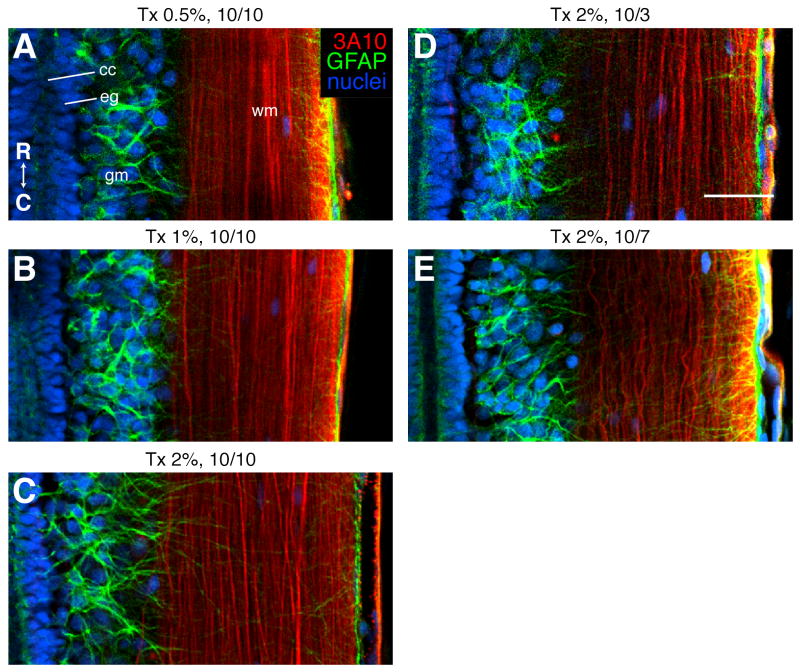
Thus, Triton X-100 remained our detergent of choice and we were able to modestly improve antibody penetration by increasing the amount of Triton X-100 to 2% (compare Fig. 2A and F). This, however, was still not sufficient. In order to get labeling throughout the spinal cord, we increased incubation times to 10 days with primary and 10 days with secondary antibodies and improved the ability of the antibody solution to mix with the sections by incubating sections in higher volumes of antibody solution and in small centrifuge tubes, rather than in 96-well plates (Fig. 2J-L). In fact, these adjustments appeared to have a greater effect on antibody penetrance than the amount of detergent, for these conditions enabled full penetrance with even 0.5% Triton X-100 (Fig. 2L). The quality of labeling, though, was best with 2% Triton X-100. The fine GFAP positive processes emanating from ependymoglia and astrocytes are most clearly labeled in sections permeabilized with 2% Triton X-100 (compare Fig. 3A-C).
Figure 3.
Comparison of the quality of antibody labeling in experiment 3. A-E: Single confocal planes, representing longitudinal sections through intact spinal cords at the level of the central canal (cc). Rostral is up. Axons were labeled with the 3A10 antibody (red), astrocytes and ependymoglia (eg) with a GFAP antibody (green), and nuclei with SYTOX green (blue). gm, grey matter; wm, white matter. Headings above each panel indicate the amount of Triton X-100 (Tx) detergent used to permeabilize the section and the incubation times with primary and secondary antibodies (10/7 indicates 10 days with primary and 7 days with secondary). Details of experiment 3 are summarized in Table 2. R, rostral; D, dorsal. Scale bar: 100 μm (all images are the same scale).
Given that secondary antibodies often require less incubation time than primary antibodies, we tested whether this might be true in our sections. We permeabilized sections with 2% Triton X-100 and incubated them with primary antibody for 10 days and compared secondary incubation times of 3, 7 and 10 days. While antibody penetration was complete for all three time points (Fig. 2J,M,N), the quality of labeling was best when incubation with secondary antibody was 10 days (compare GFAP labeling in Fig. 3C,D,E).
These results are summarized in Table 2. We optimized antibody labeling by increasing the amount of Triton X-100 detergent to 2%, increasing the incubation times to 10 days each with primary and secondary antibody, and improving the ability of the antibody solution to mix with the sections. This increased signal without adding background noise and without detrimental effects to morphology. Permeabilizing the tissue at a higher temperature (37°C) before the addition of antibodies did not appear to have a large effect on the efficiency of antibody penetration. Other factors that likely promote antibody labeling included decreasing the section thickness to 350 – 375 μm to ensure that the spinal cord was exposed on both sides of the section and omitting the perfusion fixation step and fixing the tissue after harvest only.
Table 2. Amount of antibody penetrance obtained with various detergents and incubation times.
| exp | perfuse | detergent | thick-ness | 1° Ab time | 2° Ab time | penetrance | quality of labeling |
|---|---|---|---|---|---|---|---|
| initial | yes | 0.5% Tx | 400 μm | 7d | 7d | fair | – |
| 1 | no | 1% Tx | 300 μm | 3.5d | 2d | fair | – |
| 1 | yes | 1% Tx | 300 μm | 3.5d | 2d | fair | – |
| 1 | no | 1% NP40 | 400 μm | 3.5d | 2d | fair | – |
| 1 | yes | 1% NP40 | 400 μm | 3.5d | 2d | fair | – |
| 2 | no | 0.5% Chp | 350 μm | 5d | 4.5d | poor | – |
| 2 | no | 1% Chp | 350 μm | 5d | 4.5d | fair | – |
| 2 | no | 1% SDS in pre-tr, then 2% Tx | 350 μm | 5d | 4.5d | fair | poor morphology |
| 2 | no | 2% Tx | 300 μm | 5d | 4.5d | fair | – |
| 3 | no | 0.5% Tw | 350 μm | 10d | 10d | fair | – |
| 3 | no | 0.5% Tx | 350 μm | 10d | 10d | complete | ok |
| 3 | no | 1% Tx | 350 μm | 10d | 10d | complete | good |
| 3 | no | 2% Tx | 350 μm | 10d | 3d | complete | ok |
| 3 | no | 2% Tx | 350 μm | 10d | 7d | complete | good |
| 3 | no | 2% Tx | 350 μm | 10d | 10d | complete | best |
Conditions for each experiment (exp) are listed below.
initial: permeabilize 2 hrs, RT; incubate in 250 μl in 96-well plate
exp 1: permeabilize 2 hrs, 37°C; incubate in 250 μl in 96-well plate
exp 2: permeabilize 5 hrs, 37°C; incubate in 250 μl in 96-well plate
exp 3: permeabilize 4.5 hrs, RT; incubate in 400 μl in small centrifuge tubes
Ab, antibody; tr, treatment; Tx, Triton X-100; Tw, Tween-20; Chp, CHAPS
Clearing
Whole-mount specimens must be free of light scattering pigment and optically clear before high-quality confocal images through the entire specimen can be obtained. For this, bleaching and clearing agents are used (Klymkowsky and Hanken, 1991).
We tried two types of bleach, a modified Dent's bleach containing 15% hydrogen peroxide and a formamide bleach containing 6% hydrogen peroxide and 3% formamide. The formamide bleach was superior. All pigment was completely gone after just 2 hours in the formamide bleach while the Dent's bleach was unable to remove all pigment even after a 2 day incubation period.
To clear our whole-mounts, we tried glycerol and BABB. Despite the disadvantages to working with BABB, namely that it is a caustic solution and makes the tissue brittle, we found it to work exceptionally well and preferred to use it over glycerol. Care was taken to always wear gloves and avoid the use of plastics when working with BABB. Sections were transferred to glass vials before they were cleared. Cleared sections were transferred to metal slides for imaging and transferred back to the glass vials containing BABB for storage at 4°C. Sections stored in this way have remained well preserved for up to 2 years.
Viewing 3D Relationships
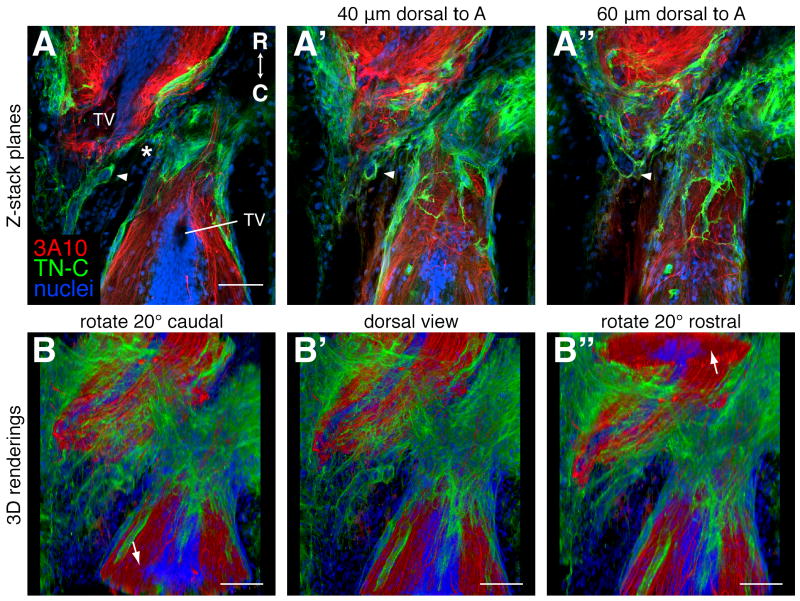
As mentioned above, spinal cords were imaged on a confocal microscope, an Olympus FV300, in 2 to 5 μm steps through the dorsal-ventral axis. 3D relationships were then visualized with ImageJ or Fluorender software. This is demonstrated on a 3 week regenerating spinal cord that was prepared with the optimized method outlined in Table 1 and labeled with the 3A10 antibody and an antibody against the extracellular matrix protein, tenascin C (TN-C). In ImageJ, the planes of the confocal z-stack can be viewed individually in rapid succession to allow one to visualize the detail in each confocal plane in relationship to the whole stack (Fig. 4A-A″, supplemental movie 1). Using this view, one can appreciate that some of the TN-C in this injured spinal cord lines tube-like structures that are connected (arrowheads in Fig. 4A-A″). This type of 3D structure would not be as well appreciated if it were viewed in a single 2D image. In Fluorender, the whole volume is rendered in 3D and it can be rotated to reveal, for example, cross-sectional morphologies and 3D shapes (Fig. 4B-B″, supplemental movie 2).
Figure 4.
Visualizing 3D relationships. A 3 week regenerating spinal cord was prepared for antibody labeling as outlined in Table 1 and longitudinal planes through the dorsal-ventral axis were imaged on a confocal microscope. Axons were labeled with the 3A10 antibody (red), the extracellular matrix with a TN-C antibody (green), and nuclei with SYTOX green (blue). Rostral is up. A-A″: Single confocal planes through the level of the terminal vesicles (TV, A), and 40 μm (A′) and 60 μm (A″) dorsal to the TVs. The arrowheads mark TN-C lined tube-like structures that can be seen to be connected when the whole z-stack is viewed (see supplemental movie 1). The asterisk marks the injury site. B-B″: Snapshots of a movie of the entire z-stack rendered in 3D with Fluorender and rotated around the horizontal axis (supplemental movie 2). B: A dorsal view rotated 20° to reveal a cross section of the spinal cord caudal to the injury (arrow). B′: A flat dorsal view. B″: A dorsal view rotated 20° to reveal a cross section of the spinal cord rostral to the injury (arrow). Scale bars: 100 μm (A-A″ are the same scale).
This method of visualizing the cellular and molecular structure of the regenerating newt spinal cord represents a significant advance in the field of newt regeneration biology and will be a useful tool for future studies into the mechanisms of spinal cord regeneration. These methods should also be widely adaptable to anyone wishing to study 3D relationships in adult tissues.
Detailed Methods
Animals
Adult newts, Notophthalmus viridescens, were purchased from Charles D. Sullivan Co. Inc., (Nashville, TN), housed at 22°C in glass aquariums equipped with water filters, and fed live blackworms, Lumbriculus varietgatus (Eastern Aquatics, PA). All animal protocols were approved by the University of Utah Institutional Animal Care and Use Committee.
Spinal Cord Injury Surgery
Transect the spinal cord and allow it to regenerate for the desired length of time, if applicable. Perform transection injuries as described in Davis et al (1989; 1990). Anesthetize newts in Tricaine solution for 10 min and then place in ice for 30 min. Place the animal prone on a sylgard dish with its trunk region propped up slightly. Cover the head and tail with moist kimwipes. Swab the skin with 10% providone iodine and make a deep incision between two vertebral bones about 1 cm rostral to the hindlimbs. Using forceps, gently separate the vertebrae and remove the dorsal lamina of the caudal-most vertebra to expose the spinal cord. Cut the cord completely with fine spring scissors. Irrigate blood with sterile 85% PBS, if necessary. Place newts on ice for 15 min after the surgery and allow them to recover in bins that are tilted to produce a shallow area of water. Clean bins every day or two during the recovery period and replace the water with fresh de-chlorinated tap water.
Tissue Preparation
Day 1 (Apply axon tracer, harvest tissue, and fix)
-
1
Apply the axon tracer to the spinal cord and allow it to travel for 6 hours, if applicable. Our procedure was modified from that used in zebrafish (Becker et al., 1997; Becker and Becker, 2001). Prepare tracer-soaked pieces of gel foam ahead of time: soak a small piece of gel foam in 1.5 μl of a 10% solution of biotinylated dextran amine (BDA, 3kD, lysine fixable, Invitrogen, D7135) and let it air dry in a tissue culture hood for 40 min to further concentrate the solution. Add fast green dye (Fisher, F-99) to the tracer solution at 0.05 mg/ml to make it visible in vivo, if desired. To apply the tracer, anesthetize newts as above and place in ice for 30 min. Transect the spinal cord as above one segment rostral or caudal to the original injury site (or targeted injury site if the cord is still intact) and insert a piece of tracer-soaked gel foam into the wound. Control excessive bleeding before adding the tracer, if necessary, by inserting a piece of fresh gel foam into the wound and allowing the animal to sit on ice for a few minutes. Minimize use of this technique as it decreases the efficiency of tracer uptake.
-
2
Once the tracer dye has labeled the spinal cord, perfuse the newts through the heart with 1.5 ml 85% PBS followed by 3 ml PLP with 0.5% PFA (omit if labeling with antibodies). We use a micromanipulator to control the needle and an infusion pump set to run at about 1 ml per 1.5 min. Anesthetize newts in Tricaine solution for 10 min and then place on ice for 15 min. To perfuse, place the newt supine on a sylgard dish and pin the arms apart. Make a dorsal-ventral incision between the forelimbs up to the jaw/neck to expose the heart. Position the perfusion needle near the heart, turn on the perfusion pump to run 85% PBS, nick the sinus venosus and insert the needle into the ventricle. After 1.5 ml of 85% PBS has run through the animal, switch the pump to the fixative and run 3 ml of it through the animal. The liver should be cleared of blood and the animal should feel stiff after perfusion.
-
3
Harvest about 4 mm of lower trunk spinal column. If the animal has not been perfused, then sacrifice via decapitation after the animal is fully anesthetized. Use a microtome blade (extremely sharp; Ted Pella, 27237, Accu-Edge blades) to separate about 4 mm of the lower trunk spinal column from the rest of the spinal column and scissors to remove the 4 mm long segment from the rest of the animal. Trim away tissue lateral to the spinal column so that the specimen is about 2 mm wide. Always use a very sharp blade when cutting or trimming the spinal column so that you do not pull the very soft spinal cord out of the spinal column.
Note: All incubations are at RT and with rocking unless otherwise specified.
-
4
Post-fix in PLP with 0.5% PFA for 2 hours. Use 3 ml in a small vial if fixing 1 or 2 segments together. Use 10 ml in 15 ml conical tube if fixing 3 to 8 segments together.
-
5
Rinse with PBS briefly, then for 30 min (3 times), then overnight at 4°C.
Day 2 (Decalcify)
-
6
Decalcify with Morse's solution for 24 hrs.
Day3 (Bleach, embed, section, permeabilize)
-
7
Rinse with PBS briefly, then for 30 min (3 times).
-
8
Bleach with formamide bleach for 2 hrs or until all pigment is gone.
-
9
Rinse with PBS briefly, then for 30 min (3 times).
-
10
Embed in 4% agarose for longitudinal sections. Dissolve agarose in PBS by heating in a microwave and stabilize the temperature to 60°C in an oven prior to embedding. Keep agarose liquefied during embedding by putting it on a hot plate at the bench. Dab tissue on a kimwipe, fill the mold with agarose, quickly position the tissue in the mold, and place the mold on ice to let the agarose congeal.
-
11
Use a vibratome to obtain thick sections containing the entire spinal cord. Fill the collection bath with pre-chilled PBS and keep it cold with PBS ice cubes. Use feather thin blades (Ted Pella, 121-6, Double Edge, Breakable Style) rather than razor blades to section and always use a fresh part of the blade for each block. Remove agarose block from the mold, trim, and use Super Glue to glue the block to the chuck so that the ventral side of the spinal column is down. We used a Leica VT1000S vibratome and set the speed to 6.5 (out of 10) and the amplitude to 9 (out of 10). Trim the dorsal tissue until the spinal cord is exposed. Set the machine to cut a 400 μm (for streptavidin only labeling) or a 350-375 μm (for antibody labeling) thick section that contains the whole spinal cord. The spinal cord should be exposed on both sides for antibody labeling. Trim agarose away from the sides of the section and transfer the section to a small centrifuge tube containing PBS.
Fluorescent Labeling
Note: Use at least 400 μl per small centrifuge tube. PBS-TxD or PBS-TxDB solutions should be made fresh and have 0.5% Triton X-100 for streptavidin staining and 2% Triton X-100 for antibody staining. If labeling with both streptavidin and antibodies, then use the parameters for antibody labeling.
-
12
Permeabilize with PBS-TxD for 4 hrs to overnight.
-
13
Incubate in streptavidin-Cy5 or primary antibody diluted in PBS-TxDB for 7 or 10 days, respectively.
-
14
Rinse with PBS-Tx briefly, then for 30 min (5 times).
-
15
Incubate in secondary antibody diluted in PBS-TxDB for 10 days.
-
16
Rinse with PBS-Tx briefly, then for 30 min (4 times). Then rinse with TNT for 30 min.
-
17
Incubate in SYTOX green solution for 2-3 hours to stain nuclei.
Dehydration and Clearing
-
18
Rinse with TN for 10 min (3 times) and transfer sections to glass vials.
-
19
Dehydrate through a methanol (MeOH)/TN series: 20%, 50%, 75%, 100% MeOH, 15 min each. These times are critical. Once dehydrated, move sections into the clearing agent as soon as possible.
-
20
Clear with BABB (1 part benzyl alcohol, 2 parts benyzl benzoate) and store in BABB at 4°C. When working with BABB, wear gloves and do not use plastics. Dispose of used BABB via incineration.
Confocal Imaging and Image Analysis
The metal slides made for imaging are 77 × 26 mm2 and 1 mm thick and have a 13 mm diameter hole drilled through the center. A glass coverslip covers the hole on one side to create a well and is glued in place with clear nail polish. Before imaging, dump a cleared section and the BABB in which it is stored into a glass petri dish, and use forceps to transfer the section to the well in the metal slide. Use a glass pipet to fill the well with BABB, and cover the well with another coverslip. Transfer the remaining BABB back into the glass vial. After imaging, remove the top coverslip and transfer the section back to the BABB in the glass vial for storage at 4°C.
Spinal cords were imaged on an Olympus FV300 laser scanning confocal microscope using a 20× air objective. Images were processed with ImageJ, version 1.40g (Rasban; ;http://rsb.info.nih.gov/ij;NIH, Bethesda, MD), Fluorender, version 2.5.0 (Otsuna and Wan; ;http://www.sci.utah.edu/software/13-software/127-fluorender.html; University of Utah, Salt Lake City, UT) and Adobe Photoshop CS2. Levels were adjusted in ImageJ and/or Photoshop to maximize the signal to noise ratio.
Solutions
Tricaine solution: 0.1% w/v Tricaine (Ethyl 3-aminobenzoate methanesulfonate salt, Sigma, A5040) in 20 mM Tris-HCl, pH 7.5.
PBS: 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.15 mM KH2PO4, pH 7.4.
PLP fixative: 75 mM lysine, 0.5-2% PFA, 10 mM NaIO4 in 85% PBS. Prepare by mixing 3 parts 0.1 M lysine stock with 1 part 4× PFA, then add solid NaIO4. Make fresh and use within 2 hrs. Make stock solutions ahead of time and store at -20°C: 0.1 M lysine in 85% PBS; 16% PFA in 85% PBS, pH 7.4.
GA/PFA fixative: 2% GA, 2% PFA in 85% PBS, pH 7.4.
Morse's solution: 22.5% formic acid, 10% sodium citrate.
Formamide bleach: 6% H2O2, 3% formamide, 0.1% Triton X-100 in PBS.
Modified Dent's bleach: 15% H2O2, 40% MeOH, 10% DMSO.
PBS-TxD: PBS with 0.5% or 2% Triton X-100 and 20% DMSO.
PBS-TxDB: PBS-TxD with the blocking reagents 1% BSA (EMD, OmniPur, Fraction V, Heat Shock Isolation, 2960) and 0.1% fish skin gelatin (Sigma G7765, 45% solution).
TN: 100 mM Tris-HCl, pH 7.5, 150 mM NaCl.
TNT: TN with 0.05% Tween-20
SYTOX green solution: Dilute SYTOX green (Invitrogen, S7020) 1:1000 in DMSO to make a stock solution and store at -20°C. Before use, dilute the stock another 1:25 in TNT.
Antibodies and Streptavidin
Primary antibodies and dilutions used are as follows: 3A10 (mouse anti-chick neurofilament associated protein IgG1 monoclonal, DSHB, 3A10) 1:25; GFAP (rabbit anti-cow GFAP polyclonal, Dako, Z0334), 1:200; TN-C (rabbit anti-chick tenascin C polyclonal, Chemicon, AB19013) 1:100. Secondary antibodies (Invitrogen, whole antibodies, 2 mg/ml, conjugated to Alexa 568 or 633) were diluted 1:100. Streptavidin-Cy5 (Invitrogen, SA1011) and Streptavidin-Alexa 633 (Invitrogen, S21375) were diluted to 4 μg/ml. Lyophilized streptavidin-Alexa 633 was reconstituted with PBS to make a 1 mg/ml stock.
Supplementary Material
Movie through whole confocal z-stack of the 3 week regenerate shown in Fig. 4A-A″. It begins on the ventral side of the cord and moves in 2 μm increments through to the dorsal side. Rostral is up. Axons were labeled with the 3A10 antibody (red), the extracellular matrix with a TN-C antibody (green), and nuclei with SYTOX green (blue).
A movie of the Fluorender 3D rendering of the 3 week regenerate shown in Fig. 4B-B″. Axons were labeled with the 3A10 antibody (red), the extracellular matrix with a TN-C antibody (green), and nuclei with SYTOX green (blue). It begins with a dorsal view in which rostral is up. Scale bar: 100 μm.
Acknowledgments
We thank the following individuals for their valuable contributions to this study: Chris Rodesch and the Cell Imaging Core for help with confocal microscopy; Gary Schoenwolf and lab for use of their vibratome; Gabrielle Kardon for advice on the use of DMSO and BABB; George Eisenhoffer for the formamide bleach recipe and advice on permeabilization; and Hideo Otsuna for help with the Fluorender software. The 3A10 antibody was provided by the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Grant Sponsor: Craig H. Neilsen Foundation (S. Odelberg); Grant Number: N/A
Grant Sponsor: NIH Developmental Biology Training Grant (K. Zukor); Grant Number: 5T32 HD07491
Grant Sponsor: NIH Cardiology Fellowship Training Grant (D. Kent); Grant Number: 5T32 HL07576
References
- Becker T, Becker CG. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol. 2001;433:131–147. doi: 10.1002/cne.1131. [DOI] [PubMed] [Google Scholar]
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bhairi SM, Mohan CM. Calbiochem detergents: a guide to the properties and uses of detergents in biology and biochemistry. EMD Biosciences,; Diego, CA.: 2007. http://www.emdchemicals.com/life-science-research/request-literature/ [Google Scholar]
- Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- Davis BM, Ayers JL, Koran L, Carlson J, Anderson MC, Simpson SB., Jr Time course of salamander spinal cord regeneration and recovery of swimming: HRP retrograde pathway tracing and kinematic analysis. Exp Neurol. 1990;108:198–213. doi: 10.1016/0014-4886(90)90124-b. [DOI] [PubMed] [Google Scholar]
- Davis BM, Duffy MT, Simpson SB., Jr Bulbospinal and intraspinal connections in normal and regenerated salamander spinal cord. Exp Neurol. 1989;103:41–51. doi: 10.1016/0014-4886(89)90183-0. [DOI] [PubMed] [Google Scholar]
- Eguchi G, Abe SI, Watanabe K. Differentiation of lens-like structures from newt iris epithelial cells in vitro. Proc Natl Acad Sci U S A. 1974;71:5052–5056. doi: 10.1073/pnas.71.12.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Thorogood P, Ferretti P. Regenerative capability of upper and lower jaws in the newt. Int J Dev Biol. 1994;38:479–490. [PubMed] [Google Scholar]
- Iten LE, Bryant SV. Stages of tail regeneration in the adult newt, Notophthalmus viridescens. J Exp Zool. 1976;196:283–292. doi: 10.1002/jez.1401960303. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Hanken J. Methods in Cell Biology. Academic Press Inc.; 1991. Whole-mount staining of Xenopus and other vertebrates; pp. 419–441. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mitashov VI. Mechanisms of retina regeneration in urodeles. Int J Dev Biol. 1996;40:833–844. [PubMed] [Google Scholar]
- Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- Piatt J. Regeneration of the spinal cord in the salamander. J Exp Zool. 1955;129:177. [Google Scholar]
- Rogers S. Imaging Technology Group, Beckman Institute for Advanced Science and Technology . Cell biological applications of fluorescence microscopy. University of Illinois at Urbana-Champaign; Urbana, IL: 1999. http://www.itg.uiuc.edu/publications/techreports/99-006/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie through whole confocal z-stack of the 3 week regenerate shown in Fig. 4A-A″. It begins on the ventral side of the cord and moves in 2 μm increments through to the dorsal side. Rostral is up. Axons were labeled with the 3A10 antibody (red), the extracellular matrix with a TN-C antibody (green), and nuclei with SYTOX green (blue).
A movie of the Fluorender 3D rendering of the 3 week regenerate shown in Fig. 4B-B″. Axons were labeled with the 3A10 antibody (red), the extracellular matrix with a TN-C antibody (green), and nuclei with SYTOX green (blue). It begins with a dorsal view in which rostral is up. Scale bar: 100 μm.