Abstract
The impact of subclinical viral infection on chronic allograft injury in the pediatric renal transplant population is not well defined. We prospectively assessed cytomegalovirus (CMV) and Epstein-Barr virus (EBV) DNAemia by monthly PCR in 55 pediatric renal transplant recipients for the first 2 years after transplantation. Subclinical CMV and EBV infection occurred in 22 and 36%, respectively. Multivariable linear regression analysis suggested that both subclinical CMV and EBV infection independently associate with significant declines in GFR during the first 2 years after transplantation. CMV seronegativity associated with a significantly greater decline in GFR than seropositivity (P < 0.01). Subclinical CMV infection and subclinical EBV infection each associated with approximately fourfold greater odds of histologic evidence of chronic allograft injury (odds ratio 4.61 [95% confidence interval 1.18 to 18.07] and odds ratio 4.33 [95% confidence interval 1.34 to 14.00], respectively). An increase in viral load of CMV or EBV also associated with increased risk for moderate to severe chronic allograft injury. Taken together, these results demonstrate an association between subclinical CMV and EBV infections, which occur despite standard antiviral prophylaxis, and chronic allograft injury in pediatric renal transplant recipients.
Chronic allograft injury has replaced acute rejection as the major cause of graft loss in renal transplantation. The more potent immunosuppressive therapy that has successfully reduced the incidence of acute rejection has also resulted in a higher incidence of viral infection.1 In the pediatric renal transplant population, infections have replaced rejection as the leading cause of hospitalization.2
Viral disease has been implicated in the pathogenesis of chronic allograft injury and represents a potential modifiable risk factor. Epidemiologic studies, primarily in adult transplant recipients, have identified an association between cytomegalovirus (CMV) disease and chronic allograft injury.3–6 In animal models, the evidence that CMV contributes to allograft dysfunction is well established. The mechanisms for this include increased expression of alloantigens, adhesion molecule expression by endothelial cells, and cytokine release.7–10 Importantly, the augmented intragraft responses can be prevented by treatment with ganciclovir but not with nonspecific enhancement of the immune system.8,11,12
Increasingly sensitive molecular methods allow for the detection of subclinical viral infection after transplantation, but its role in the pathogenesis of chronic allograft injury is not well established. We present a study that prospectively monitored pediatric renal transplant recipients for subclinical Epstein-Barr virus (EBV) and CMV viremia in the first 2 years after transplantation to evaluate the association between subclinical viral infection and chronic allograft injury.
Results
Study Population
A total of 61 consecutive patients were initially screened for inclusion in the study. Six patients were excluded from the analyses after being lost to follow-up as a result of moving out of the region or transitioning to adult care. In total, 55 patients completed the 2-year surveillance study. Patient characteristics are shown overall and by subclinical infection status in Table 1. Patients in the study ranged in age from 2.3 to 20.4 years, with a mean age of 11.2 (SD 5.83); 55% were female, 85% were white, 76% were CMV seronegative, and 55% were EBV seronegative. Patients were primarily treated with both IL-2 receptor antagonist (94%) and calcineurin inhibitors (96%). Donors for the majority of patients in this study were deceased (62%), CMV seropositive (67%), and EBV seropositive (87%). There was no significant difference in the number of HLA mismatches (A, B, and DR) or mean cold ischemia time among the infected versus noninfected group.
Table 1.
Characteristics of the study population
| Characteristic | All Patients (n = 55) | Subclinical CMV Infection (n = 12a) | No Subclinical CMV Infection (n = 43) | Subclinical EBV Infection (n = 20a) | No Subclinical EBV Infection (n = 35) | No Subclinical EBV or CMV Infection (n = 29a) |
|---|---|---|---|---|---|---|
| Age at transplantation (years; mean ± SD) | 11.2 ± 5.8 | 13.2 ± 5.3 | 12.4 ± 5.2 | 11.3 ± 6.2 | 12.1 ± 5.8 | 10.7 ± 5.8 |
| Baseline creatinine (mg/dl; mean ± SD) | 0.85 ± 0.38 | 0.91 ± 0.33 | 0.88 ± 0.31 | 0.89 ± 0.45 | 0.90 ± 0.39 | 0.84 ± 0.35 |
| Baseline GFR (ml/min per 1.73 m2; mean ± SD) | 70 ± 21 | 69 ± 19 | 70 ± 20 | 70 ± 18 | 71 ± 20 | 69 ± 22 |
| Male gender (n [%]) | 25 (45) | 6 (50) | 19 (44) | 12 (63) | 13 (37) | 11 (38) |
| White race (n [%]) | 47 (85) | 11 (92) | 36 (84) | 18 (95) | 29 (83) | 23 (79) |
| Deceased donor (n [%]) | 34 (62) | 9 (75) | 25 (58) | 13 (68) | 21 (60) | 17 (59) |
| CMV status (n [%]) | ||||||
| CMV D+/R− | 25 (45) | 8 (67) | 17 (40) | 10 (53) | 15 (43) | 11 (38) |
| CMV D−/R− | 17 (31) | 1 (8) | 16 (37) | 5 (26) | 12 (34) | 11 (38) |
| CMV D+/R+ | 12 (22) | 3 (25) | 9 (21) | 4 (21) | 8 (67) | 6 (21) |
| CMV D−/R+ | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 1 (3) |
| EBV status (n [%]) | ||||||
| EBV D+/R− | 26 (47) | 6 (50) | 20 (47) | 13 (68) | 13 (37) | 11 (38) |
| EBV D−/R− | 4 (7) | 0 (0) | 4 (9) | 1 (5) | 3 (9) | 3 (10) |
| EBV D+/R+ | 22 (40) | 6 (50) | 16 (37) | 5 (26) | 17 (49) | 12 (41) |
| EBV D−/R+ | 3 (5) | 0 (0) | 3 (7) | 0 (0) | 3 (9) | 3 (10) |
| IL-2 receptor antagonist (n [%]) | 52 (94) | 11 (95) | 41 (95) | 20 (100) | 32 (91) | 27 (93) |
| Calcineurin inhibitor (n [%]) | 53 (96) | 12 (100) | 41 (95) | 20 (100) | 33 (94) | 27 (93) |
| Acute rejection (n [%]) | 16 (29) | 4 (33) | 12 (28) | 5 (25) | 11 (31) | 9 (31) |
| 12 months of antiviral therapy (n [%]) | 25 (46) | 8 (67) | 17 (40) | 6 (30) | 19 (54) | 15 (52) |
aNumbers do not add up to 55 because six patients had both CMV and EBV infection.
There were a total of 16 (29%) cases of biopsy-proven acute rejection. Of these, nine were detected on surveillance biopsy and seven were detected on standard-of-care biopsy performed for elevated creatinine levels from baseline. The classification of rejection on the basis of Banff '07 classification of renal allograft pathology was as follows: Borderline changes in seven (44%) of 16, Banff IA in four (25%) of 16, Banff IB in three (19%) of 16, and Banff IIA in two (13%) of 16.13 Two cases also had evidence of Banff II antibody-mediated rejection with positive C4d staining and the presence of circulating anti-HLA antibodies. There was no significant difference in the incidence of biopsy-proven acute rejection among patients with subclinical infection compared with those without.
Incidence of Subclinical Viremia and Viral Disease
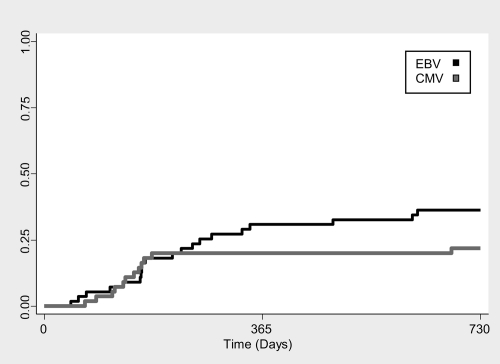
Fifty-five patients had a total of 1176 samples (88% of monthly scheduled) evaluated for EBV and CMV by PCR. The cumulative incidence of subclinical CMV and EBV infection is shown in Figure 1. Subclinical CMV infection occurred in 12 (22%; 95% confidence interval [CI] 12 to 35%) patients at a median of 143 days after transplantation (range 68 to 682 days). Median peak CMV level was 725 copies/ml (range 100 to 12,000 copies/ml), with infection lasting for a median duration of 37 days (range 10 to 98 days). Subclinical EBV infection occurred in 20 (36%; 95% CI 24 to 50%) patients at a median of 192 days after transplantation (range 44 to 625 days) with a median peak level of 350 copies/ml (range 120 to 14,000 copies/ml) and lasted for a median duration of 44 days (range 14 to 365 days). Six (11%) patients developed both subclinical CMV and EBV infection. Four (67%) of six patients were EBV D+/R− and CMV D+/R− and two (33%) of six were EBV D+/R+ and CMV D+/R+.
Figure 1.
Time posttransplant to onset of subclinical CMV and EBV viremia.
CMV disease occurred in two patients CMV D+/R− (e.g., fever, malaise, leucopenia, associated with mild elevation of transaminases in one and gastrointestinal disease in the other) at 221 and 125 days after transplantation. Six patients had primary EBV disease, four of whom developed posttransplantation lymphoproliferative disease at a mean of 211 days after transplantation.
Subclinical CMV and EBV Infection Is Associated with Decreased Renal Function
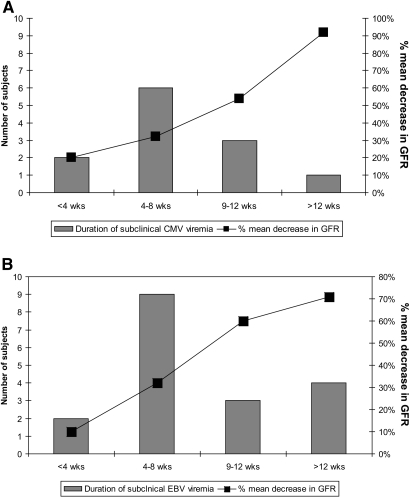
Patients with subclinical CMV and EBV infections experienced a mean decrease of 30% (SD 26%) and 25% (SD 24%), respectively, in GFR during the 2-year period after transplantation, compared with a mean decrease of 6% (SD 14) among patients with no subclinical infection (Table 2), Figure 2). The six patients with both subclinical CMV and subclinical EBV infections experienced a median decrease in GFR of 32% (SD 36%) at 2 years after transplantation.
Table 2.
Subclinical infection and renal function
| Parameter | Subclinical CMV Infection (n = 12) | Subclinical EBV Infection (n = 20) | No Subclinical Infection (n = 29) |
|---|---|---|---|
| Baseline creatinine (mg/dl; mean ± SD) | 0.91 ± 0.33 | 0.89 ± 0.45 | 0.84 ± 0.35 |
| Creatinine at 2 years after transplantation (mg/dl; mean ± SD) | 1.50 ± 0.53 | 1.30 ± 0.48 | 0.97 ± 0.45 |
| % change creatinine (mean ± SD)a | 72 ± 68 | 64 ± 89 | 16 ± 18 |
| Baseline GFR (ml/min per 1.73 m2; mean ± SD) | 69 ± 19 | 70 ± 18 | 69 ± 22 |
| GFR 2 years after transplantation (ml/min per 1.73 m2; mean ± SD) | 47.0 ± 19.2 | 51.0 ± 19.5 | 65.0 ± 22.7 |
| % change GFR (mean ± SD)a | −30 (26) | −25 (24) | −6 (14) |
| Delayed graft function (n [%]) | 0 (0) | 1 (5) | 2 (7) |
| Acute rejection (n [%]) | 4 (33) | 5 (25) | 9 (31) |
| BK viremia (n [%]) | 0 (0) | 1 (5) | 2 (7) |
a% Change defined as the difference between value at 24 months and baseline divided by baseline.
Figure 2.
Longer duration of subclinical CMV and EBV viremia associates with a greater decrease in GFR at 2 years after transplantation. (A) Duration of subclinical CMV viremia and percentage mean decrease in GFR 2 years after transplantation. (B) Duration of subclinical EBV viremia and percentage mean decrease in GFR 2 years after transplantation.
We performed a multivariable linear regression model of the change from baseline GFR to 2 years after transplantation. Both subclinical CMV and EBV infections were independently associated with significant declines in GFR when adjusted for deceased-donor source, delayed graft function, BK viremia (there were no cases of BK virus nephropathy), and acute rejection. Subclinical CMV infection was associated with an additional decrease (from baseline levels) of 11.54 ml/min per 1.73 m2 (95% CI 22.17 to 0.92), and subclinical EBV infection was associated with an additional decrease of 10.55 ml/min per 1.73 m2 (95% CI 19.61 to 1.50).
Serostatus and Renal Function
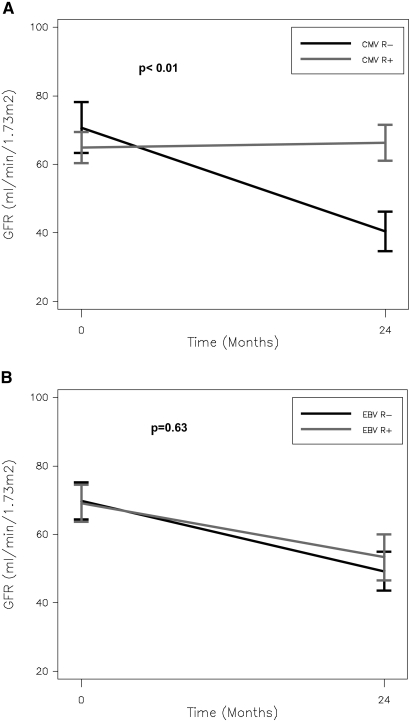
Subclinical infection occurred primarily in seronegative recipients. Among the 12 patients with subclinical CMV infection, eight (67%) were CMV D+/R−, one (8%) was CMV D−/R−, and three (25%) were CMV D+/R+. Among the 20 patients with subclinical EBV infection, 13 (68%) were EBV D+/R−, one (5%) were EBV D−/R−, and five (26%) were EBV D+/R+. We detected a significant interaction between CMV recipient serostatus and the impact of subclinical infection on GFR (P = 0.01). Figure 3A shows that among patients with subclinical infection, there is a greater decline in GFR among CMV-seronegative recipients than seropositive recipients (−30 versus −2 ml/min per 1.73 m2). A similar interaction between recipient serostatus and GFR was not seen with subclinical EBV infection (Figure 3B).
Figure 3.
Among patients with subclinical CMV infection, there is a greater decline in GFR among CMV-seronegative recipients than seropositive recipients. (A) Impact of recipient CMV serostatus on GFR among children who develop subclinical CMV infection. (B) Impact of recipient EBV serostatus on GFR among children who develop subclinical EBV infection.
Subclinical Infection and Interstitial Fibrosis and Tubular Atrophy
A total of 23 (52%), 19 (35%), and two (5%) patients developed mild, moderate, or severe interstitial fibrosis and tubular atrophy (IF/TA) at 2 years after transplantation, respectively. Subclinical CMV infection (odds ratio [OR] 4.61; 95% CI 1.18 to 18.07) and subclinical EBV infection (OR 4.33; 95% CI 1.34 to 14.00) were found to have significant associations with development of moderate to severe IF/TA after adjustment for age, gender, acute rejection status, and deceased-donor source (Table 3.
Table 3.
Association between patient characteristics and development of moderate or severe IF/TA
| Variable | Effect | Unadjusted OR (95% CI) | IF/TA Final Model |
|
|---|---|---|---|---|
| Adjusted OR (95% CI) | Pa | |||
| EBV subclinical infection | 12 versus 3 months | 4.33 (1.34 to 14.00) | 4.71 (1.21 to 18.38) | 0.025 |
| CMV subclinical infection | + versus − | 4.61 (1.18 to 18.07) | 6.76 (1.38 to 33.22) | 0.019 |
| Acute rejection | + versus − | 0.65 (0.19 to 2.25) | 0.74 (0.18 to 3.10) | 0.684 |
| Deceased donor | + versus − | 1.01 (0.33 to 3.08) | 0.75 (0.20 to 2.82) | 0.675 |
| Age (years) | per 1 year | 0.94 (0.85 to 1.03) | 0.89 (0.79 to 1.01) | 0.675 |
| Male gender | 1.57 (0.53 to 4.70) | 1.29 (0.35 to 4.77) | 0.700 | |
| EBV donor status | D+versus D− | 4.28 (0.48 to 38.41) | — | |
| EBV recipient status | R+versus R− | 0.84 (0.28 to 2.52) | — | |
| CMV donor status | D+versus D− | 1.98 (0.59 to 6.70) | — | |
| CMV recipient status | R+versus R− | 0.65 (0.17 to 2.47) | — | |
| Antiviral use | 12 versus 3 months | 0.84 (0.28 to 2.52) | — | |
| Caucasian | + versus − | 1.03 (0.22 to 4.86) | — | |
| Results from exploratory analysis of role of the impact of viral exposure | ||||
| log(AUC EBV)b | 1.2 (1.04 to 1.35) | 0.0109 | ||
| log(AUC CMV)b | 1.2 (1.06 to 1.46) | 0.0069 | ||
AUC, area under the curve.
aP values are from multivariate model.
bExploratory analysis of the role of overall viral exposure was explored in the context of the final model. ORs and P values reflect adjustment for acute rejection, deceased donor status, recipient age, and recipient gender.
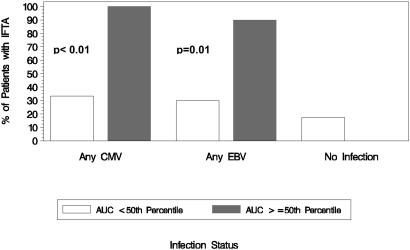
Exploratory analyses found significant associations between overall CMV and EBV exposure and the risk for developing moderate to severe IF/TA (Table 3, Figure 4). A 1-unit increase in CMV viral load measured as log area under the curve (OR 1.2; 95% CI 1.06 to 1.46; P = 0.007) and EBV viral load (OR 1.2; 95% CI 1.04 to 1.35; P = 0.0109) were associated with increased risk for moderate to severe IF/TA at 2 years after transplantation (Table 3). This model suggests that having both subclinical CMV and EBV infection is associated with a greater risk for chronic allograft injury than having subclinical CMV or EBV infection alone.
Figure 4.
Significant associations between overall CMV and EBV exposure and the risk for developing moderate to severe IF/TA. Incidence of IF/TA at 2 years after transplantation by subclinical viremia status measured by area under the curve.
Analysis of Virus in Renal Biopsy
A total of 44 (80%) renal allograft biopsies performed at 2 years after transplantation were reviewed independently by a renal pathologist who was blinded to the clinical status of the patient. In all cases, light microscopic examination was negative for the presence of inclusion bodies or viral cytopathic effect. Analysis by PCR of renal allograft biopsy tissue revealed CMV in zero patients and EBV in four (9%) patients (mean 111 copies/ml; SD 296 copies/ml).
Duration of Antiviral Therapy
As a result of a change in our center's standard of care during the study, 30 (55%) patients received 3 months of antiviral prophylaxis and 25 (45%) patients received 12 months of therapy. There was no significant difference in the incidence of subclinical CMV (P = 0.51, Fisher exact test), time of CMV onset (P = 0.23, Wilcoxon test), or peak CMV load (P = 0.44, Wilcoxon test) between those who were treated with 3 and 12 months of antiviral therapy. Similarly, there was no significant difference in the incidence of subclinical EBV infection (P = 0.09, Fisher exact test), time of EBV onset (P = 0.93, Wilcoxon test), or peak EBV load (P = 0.51, Wilcoxon test) between the 3- and 12-month groups. There was a trend toward a smaller decline in GFR in the patients who received 12 months of antiviral prophylaxis compared with the 3-month prophylaxis group (−7.5 versus −14.9 ml/min per 1.73 m2; P = 0.06, t test).
Discussion
The results of our 2-year prospective study of pediatric renal transplant recipients demonstrate that subclinical CMV and EBV viremia are common in the early posttransplantation period despite antiviral prophylaxis. We observed a relationship between the degree of viral exposure (viral load and number of viruses) and the degree of chronic allograft injury. In addition, primary subclinical infection was associated with a greater degree of allograft injury than reactivation subclinical infection. Thus, subclinical viremia represents a potentially modifiable risk factor for chronic allograft injury in pediatric renal transplantation.
Antiviral prophylaxis has been effective in decreasing the incidence of clinically symptomatic CMV disease, but, as seen in our cohort, subclinical infection remains relatively common. Limited data from prospective studies exist on the incidence and natural history of subclinical CMV and EBV in pediatric renal transplant recipients. In our study, among 55 pediatric renal transplant recipients, subclinical CMV infection occurred in 22% and subclinical EBV infection occurred in 36%, despite antiviral prophylaxis. These rates of subclinical infection are consistent with other studies of pediatric and adult renal transplant recipients.14,15
We demonstrate an association between subclinical CMV and EBV infection and histologic evidence of moderate to severe chronic allograft injury at 2 years after transplantation. An association with subclinical CMV infection and chronic allograft dysfunction has been reported in the heart, lung, and adult kidney transplant population.16–20 In pediatric renal transplant recipients, Li et al.15 demonstrated an association between CMV and/or EBV viremia and decreased renal function in patients who were younger than 5 years. In contrast, we demonstrated an association between subclinical CMV and EBV viral infection and decreased renal function in pediatric patients of all ages. In addition, analysis of renal biopsy tissue revealed significantly higher grades of IF/TA at 2 years after transplantation in patients with subclinical viral infection with CMV and EBV.
The virologic dynamics of subclinical infection and allograft injury remains poorly described, and this study is the first to report a dose-response relationship between subclinical CMV and EBV infection and chronic allograft injury defined by both renal function and histologic examination. Importantly, our results revealed that the risk for developing moderate to severe chronic allograft injury increases with an increase in viral load. The findings also suggest that the effect of EBV and CMV subclinical infections is additive—that is, patients with both subclinical CMV and EBV infections are at a higher risk for chronic allograft injury than patients with only CMV or only EBV infection. In addition, CMV seronegative status of the recipient was identified as a risk factor for a greater decline in GFR, suggesting that primary infection—even when subclinical—may be more likely to lead to complications than reactivation infection. This is similar to the higher morbidity associated with primary EBV infection and the increased risk for posttransplantation lymphoproliferative disease in the EBV-seronegative recipient.21–24
The impact of antiviral prophylaxis on subclinical infection and associated allograft dysfunction is not well established, but there is evidence that prolonged therapy may improve outcomes in adult heart transplant recipients.16 In our cohort, patients received either 3 or 12 months of antiviral prophylaxis because of a change in our center's standard of care. The incidence, onset, and peak viral load were not different between the two groups, but we did observe a trend toward a smaller decline in GFR at 2 years in the 12-months compared with the 3-month prophylaxis group. Results of this subanalysis must be interpreted with caution, however, because of the small number of patients.
Unlike for viral disease, the mechanism of chronic allograft injury associated with subclinical viral infection is not well established. Our study did not identify evidence of significant viral replication in the renal allograft at 2 years after transplantation, suggesting that graft dysfunction is not related to chronic tissue infection. Thus, whether direct viral cytopathic effects,25–28 indirect inflammatory effects,25 or a combination of multiple mechanisms leads to allograft injury remains a key question for future studies.
The main strength of this prospective study of subclinical viral infection is the frequency of the viral monitoring and clinical assessments in a single center. We acknowledge potential confounders that may have affected the observed association between subclinical viremia and chronic allograft injury, including other viral infections and immunosuppressive medication regimens. Although BK viremia and BK virus nephropathy are known risk factors for allograft injury, they were not identified as risk factors in our analysis, potentially because of the low incidence of this complication in our cohort. Both over- and underimmunosuppression may contribute to allograft injury; however, our study was performed at a single center with the same target drug levels, and immunosuppression was not reduced below target levels in response to detected viremia. In addition, there were no significant differences in the rates of acute cellular/antibody-mediated rejection or prevalence of anti-HLA antibodies between patients with and without subclinical viremia. Finally, the use of plasma, rather than whole blood, may have underestimated the measured viremia, especially for EBV; however, we chose to focus on lytic replication of virus and avoid quantifying virus in latently infected white blood cells.
In summary, we have demonstrated an independent association between the duration and severity of subclinical CMV and EBV infection and chronic allograft injury in pediatric renal transplant recipients that occurs despite standard antiviral prophylaxis. These findings suggest that prospective monitoring for subclinical EBV and CMV infections may potentially identify a subset of patients for whom targeted intervention may be warranted. Future studies to confirm these results and further elucidate the mechanism of injury as a means to develop novel interventions should be performed.
Concise Methods
Study Design
This prospective study was conducted at a single pediatric renal transplant center between 2000 and 2005. The inclusion criteria were ability to give informed consent, age 1 to 21 years, and being <1 month after transplantation. Exclusion criteria were inability to provide consent/assent and greater >1 month after transplantation. All patients who met study inclusion criteria were offered participation, and written informed consent was obtained from study participants. The study was approved by the institutional review board of Seattle Children's Hospital.
Study Procedures
Viral Surveillance
CMV and EBV serology was performed on all recipients and living donors within 1 month before transplantation. Plasma was evaluated for CMV and EBV by real-time quantitative PCR monthly for 2 years after transplantation.29–31 In addition, CMV and EBV PCRs were performed as part of standard of care when patients had clinical signs of illness (e.g., fever, malaise) and at the time of renal biopsy. All positive PCR values were repeated for confirmation within 1 week and then repeated weekly until negative for 2 consecutive weeks.
Subclinical CMV or EBV infection was defined as asymptomatic DNAemia. CMV disease was defined as CMV syndrome (one or more of the following: Fever, malaise, leukopenia, atypical lymphocytosis, thrombocytopenia, and elevated hepatic transaminases), pneumonia, gastrointestinal disease, hepatitis, central nervous system disease, retinitis, or other tissue-invasive disease.32 EBV disease was defined as EBV DNAemia in association with fever, malaise, leukopenia, and/or organ involvement. Organ involvement included physical examination abnormalities and biochemical and/or radiographic evidence of organ dysfunction (e.g., hepatitis, pneumonitis). The diagnosis of posttransplantation lymphoproliferative disease was based on the presence of both lymphoid proliferation and EBV confirmed by Epstein-Barr early RNA in the tissue samples.
Renal Biopsy
Surveillance renal biopsies were performed at 3 to 6, 12, and 24 months after transplantation. In addition, standard-of-care biopsies were performed for clinical indications such as increased creatinine from baseline. All biopsies were reviewed by a pathologist using Banff '07 classification of renal allograft pathology.13 Acute rejection was treated with a course of methylprednisolone (20 to 25 mg/kg for 3 days, maximum dosage 1 g/d). Acute antibody-mediated rejection was treated with 3 days of plasmapheresis, intravenous Ig, and rituximab.
IF/TA was evaluated and graded as follows: Grade 1, mild (<25% of cortical area); grade 2, moderate (26 to 50% of cortical area); and grade 3, severe (>50% cortical area).
Sign and Symptom Assessment
Assessments of clinical status were performed monthly by telephone interview, clinic visit, and medical record review. In addition, sign and symptom assessments were performed weekly from the time of the first positive viral result until negative for 2 weeks.
Renal Function
Kidney function was measured using two methods: Creatinine and estimated GFR on the basis of the Schwartz method.33 Baseline kidney function was defined as the nadir creatinine reached on two occasions at least 1 week apart.
CMV Prophylaxis
All patients received intravenous ganciclovir in the immediate postoperative period at a dosage of 5 mg/kg per d for GFR >50 ml/min per 1.73 m2, 2.5 mg/kg per d for GFR 25 to 49 ml/min per 1.73 m2, 1.25 mg/kg per d for GFR 10 to 24 ml/min per 1.73 m2, and 1.25 mg/kg three times per week after dialysis for GFR <10 ml/min per 1.73 m2. Upon hospital discharge, intravenous ganciclovir was replaced with oral ganciclovir or valganciclovir. The dosage of ganciclovir was 40 mg/kg per dose (up to 1 g/dose) three times per day. The dosage of oral valganciclovir in patients with GFR >60 ml/min per 1.73 m2 was 10 to 12 mg/kg once daily for children weighing <30 kg, 450 mg/d (30 to 50 kg), and 900 mg/d (>50 kg). Duration of antiviral prophylaxis was 3 months for those who underwent transplantation between 2000 and 2003 and 12 months for those who underwent transplantation thereafter.
Immunosuppression
Induction therapy included anti-lymphocyte preparations (antithymocyte globulin) or IL-2 receptor antagonists (basiliximab or daclizumab). Maintenance immunosuppressive regimens included cyclosporine or tacrolimus, mycophenolate mofetil or sirolimus, and prednisone. Target trough levels for cyclosporine were 200 to 250 ng/ml (days 1 through 14), 150 to 200 ng/ml (days 15 through 90), 100 to 150 ng/ml (days 91 through 365), and then 50 to 100 ng/ml. Target tacrolimus levels were 15 to 18 ng/ml (days 1 through 17), 10 to 15 ng/ml (days 18 through 90), 7 to 10 ng/ml (days 181 through 365), and 3 to 5 ng/ml thereafter. Target trough levels for sirolimus were 10 to 20 ng/ml.
Statistical Analysis
Descriptive statistics were used to summarize cohort characteristics overall and by subclinical infection status (e.g., no infection, any CMV, any EBV [any CMV and any EBV categories are not mutually exclusive]). Exact binomial CIs were calculated for the proportion of patients who developed subclinical CMV and EBV infections and IF/TA during the study period. Plots of the cumulative incidence of first EBV and CMV subclinical infection were generated to explore the time elapsed between transplantation and initial onset of EBV and CMV subclinical infection.
The change and percentage change (calculated by dividing the difference between 2-year and baseline values by the baseline value) in GFR that occurred during the 2-year period were summarized by subclinical infection status. Linear regression modeling was used to evaluate associations between clinical factors and change in GFR. A priori, it was determined that age (in years), EBV subclinical infection, CMV subclinical infection, deceased donor status, delayed graft function, and acute rejection status would be included in the final model. Estimates from the linear model (adjusted only for baseline GFR) of the effect of each potential individual risk factor on change from baseline GFR were computed. Donor and recipient EBV/CMV serostatus, gender, antiviral treatment, BK viremia, and race were considered for inclusion in the final model but ultimately excluded from in the final model because of lack of a significant univariate association (e.g., P < 0.05).
For exploration of potential interactions between EBV/CMV recipient serostatus and the impact of each type of subclinical infection, two additional models were fit (one for CMV and one for EBV), including recipient serostatus and the interaction between recipient serostatus and subclinical infection on change in GFR.
Logistic regression was used to evaluate whether subclinical infection has a significant impact on the risk for developing moderate to severe IF/TA after accounting for the effect of other known risk factors. A priori, the following risk factors were selected for inclusion in the model: Any CMV subclinical infection, any EBV subclinical infection, patient age, acute rejection, deceased-donor source, and gender. We also explored EBV/CMV donor and recipient status, duration of antiviral use (3 versus 12 months), BK viremia, and race but excluded them after they failed to show significant association (P > 0.05) with development of moderate to severe IF/TA.
To explore whether a dose-response relationship exists between quantitative measures of CMV/EBV infection and risk for developing moderate to severe IF/TA, we explored inclusion of covariates for overall CMV and EBV viral exposure (duration × intensity of infection) in place of the dichotomous indicators of CMV infection and EBV infection in the logistic regression model for the risk for developing moderate to severe IF/TA. Overall CMV and overall EBV viral exposure were estimated as the area under curve from the start of subclinical infection to the last measurable viral concentration for each patient using the linear trapezoidal rule. Because of the nature of the viral exposure variable, which has a right-skewed distribution, we chose to log-transform these variables before entering them into the model.
Disclosures
None.
Acknowledgments
We gratefully acknowledge Dr. Ajit Limaye for his valuable contributions to this work. This work was supported by K23 DK068204 from the National Institute of Diabetes and Digestive and Kidney Diseases and Clinical and Translational Science Awards (CTSA) grant 1 ULI RR025014-02 from the National Center for Research Resources (NCRR).
These data were presented in part at the American Transplant Congress, Boston, May 30 to June 3, 2009.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Husain S, Singh N: The impact of novel immunosuppressive agents on infections in organ transplant recipients and the interactions of these agents with antimicrobials. Clin Infect Dis 35: 53–61, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Dharnidharka VR, Stablein DM, Harmon WE: Post-transplant infections now exceed acute rejection as cause for hospitalization: A report of the NAPRTCS. Am J Transplant 4: 384–389, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Humar A, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, Matas AJ: Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation 68: 1879–1883, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Rubin RH, Tolkoff-Rubin NE, Oliver D, Rota TR, Hamilton J, Betts RF, Pass RF, Hillis W, Szmuness W, Farrell ML: Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation 40: 243–249, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Soderberg-Naucler C, Emery VC: Viral infections and their impact on chronic renal allograft dysfunction. Transplantation 71 [Suppl]:SS24–SS30, 2001 [PubMed] [Google Scholar]
- 6. Tong CY, Bakran A, Peiris JS, Muir P, Herrington CS: The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation 74: 576–578, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Borchers AT, Perez R, Kaysen G, Ansari AA, Gershwin ME: Role of cytomegalovirus infection in allograft rejection: A review of possible mechanisms. Transpl Immunol 7: 75–82, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Koskinen PK, Kallio EA, Tikkanen JM, Sihvola RK, Häyry PJ, Lemström KB: Cytomegalovirus infection and cardiac allograft vasculopathy. Transpl Infect Dis 1: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Lautenschlager I, Soots A, Krogerus L, Kauppinen H, Saarinen O, Bruggeman C, Ahonen J: Effect of cytomegalovirus on an experimental model of chronic renal allograft rejection under triple-drug treatment in the rat. Transplantation 64: 391–398, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Streblow DN, Kreklywich C, Yin Q, De La Melena VT, Corless CL, Smith PA, Brakebill C, Cook JW, Vink C, Bruggeman CA, Nelson JA, Orloff SL: Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J Virol 77: 2182–2194, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemström KB, Koskinen PK, Bruning JH, Bruggeman CA, Lautenschlager IT, Häyry PJ: Effect of ganciclovir prophylaxis on cytomegalovirus-enhanced allograft arteriosclerosis. Transpl Int 7 [Suppl 1]:S383–S384, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Tikkanen JM, Kallio EA, Bruggeman CA, Koskinen PK, Lemström KB: Prevention of cytomegalovirus infection-enhanced experimental obliterative bronchiolitis by antiviral prophylaxis or immunosuppression in rat tracheal allografts. Am J Respir Crit Care Med 164: 672–679, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Kranz B, Vester U, Wingen AM, Nadalin S, Paul A, Broelsch CE, Hoyer PF: Acute rejection episodes in pediatric renal transplant recipients with cytomegalovirus infection. Pediatr Transplant 12: 474–478, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Li L, Chaudhuri A, Weintraub LA, Hsieh F, Shah S, Alexander S, Salvatierra O, Jr, Sarwal MM: Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant 11: 187–195, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Potena L, Holweg CT, Chin C, Luikart H, Weisshaar D, Narasimhan B, Fearon WF, Lewis DB, Cooke JP, Mocarski ES, Valantine HA: Acute rejection and cardiac allograft vascular disease is reduced by suppression of subclinical cytomegalovirus infection. Transplantation 82: 398–405, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Ruttmann E, Geltner C, Bucher B, Ulmer H, Höfer D, Hangler HB, Semsroth S, Margreiter R, Laufer G, Müller LC: Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation 81: 1415–1420, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC: Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation 75: 2064–2068, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degré M, Fauchald P, Rollag H: Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66: 329–337, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Sagedal S, Nordal KP, Hartmann A, Sund S, Scott H, Degré M, Foss A, Leivestad T, Osnes K, Fauchald P, Rollag H: The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2: 850–856, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Cockfield SM, Preiksaitis JK, Jewell LD, Parfrey NA: Post-transplant lymphoproliferative disorder in renal allograft recipients: Clinical experience and risk factor analysis in a single center. Transplantation 56: 88–96, 1993 [DOI] [PubMed] [Google Scholar]
- 22. McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, Wyatt R, Arar M, Liereman D, Bridges N, Harmon W: CCTPT Study Group: Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant 8: 984–989, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, Woodle ES, Kelly SM, Koeppen H, Hart J, Rubin CM, Thistlethwaite JR, Jr: Posttransplant lymphoproliferative disease in pediatric liver transplantation: Interplay Between Epstein-Barr virus infection and immunosuppression. Transplantation 62: 370–375, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Smith JM, Corey L, Healey PJ, Davis CL, McDonald RA: Primary EBV infection in pediatric renal transplantation: Adolescents at higher risk for PTLD. Transplantation 83: 1423–1428, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Reinke P, Prösch S, Kern F, Volk HD: Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl Infect Dis 1: 157–164, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Wu TC, Hruban RH, Ambinder RF, Pizzorno M, Cameron DE, Baumgartner WA, Reitz BA, Hayward GS, Hutchins GM: Demonstration of cytomegalovirus nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol 140: 739–747, 1992 [PMC free article] [PubMed] [Google Scholar]
- 27. Gerstenkorn C, Robertson H, Mohamed MA, O'Donnell M, Ali S, Talbot D: Detection of cytomegalovirus (CMV) antigens in kidney biopsies and transplant nephrectomies as a marker for renal graft dysfunction. Clin Chem Lab Med 38: 1201–1203, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Barzon L, Murer L, Pacenti M, Biasolo MA, Della Vella M, Benetti E, Zanon GF, Palù G: Investigation of intrarenal viral infections in kidney transplant recipients unveils an association between parvovirus B19 and chronic allograft injury. J Infect Dis 199: 372–380, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Lai KK, Cook L, Krantz EM, Corey L, Jerome KR: Calibration curves for real-time PCR. Clin Chem 51: 1132–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, Corey L: Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol 42: 1142–1148, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimura H, Morita M, Yabuta Y, Kuzushima K, Kato K, Kojima S, Matsuyama T, Morishima T: Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol 37: 132–136, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Humar A, Michaels M: American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 6: 262–274, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]