Abstract
The central role of the multifunctional protein nephrin within the macromolecular complex forming the glomerular slit diaphragm is well established, but the mechanisms linking the slit diaphragm to the cytoskeleton and to the signaling pathways involved in maintaining the integrity of the glomerular filter remain incompletely understood. Here, we report that nephrin interacts with the bicarbonate/chloride transporter kidney anion exchanger 1 (kAE1), detected by yeast two-hybrid assay and confirmed by immunoprecipitation and co-localization studies. We confirmed low-level glomerular expression of kAE1 in human and mouse kidneys by immunoblotting and immunofluorescence microscopy. We observed less kAE1 in human glomeruli homozygous for the NPHS1FinMaj nephrin mutation, whereas kAE1 expression remained unchanged in the collecting duct. We could not detect endogenous kAE1 expression in NPHS1FinMaj podocytes in primary culture, but heterologous re-introduction of wild-type nephrin into these podocytes rescued kAE1 expression. In kidneys of Ae1−/− mice, nephrin abundance was normal but its distribution was altered along with the reported kAE1-binding protein integrin-linked kinase (ILK). Ae1−/− mice had increased albuminuria with glomerular enlargement, mesangial expansion, mesangiosclerosis, and expansion of the glomerular basement membrane. Glomeruli with ILK-deficient podocytes also demonstrated altered AE1 and nephrin expression, further supporting the functional interdependence of these proteins. These data suggest that the podocyte protein kAE1 interacts with nephrin and ILK to maintain the structure and function of the glomerular basement membrane.
Anion exchanger 1 (AE1; SLC4A1), an SLC4 bicarbonate transporter family member, is transcribed as an erythroid isoform (eAE1) and a truncated kidney isoform (kAE1) lacking amino acids 1 through 65 in humans.1 eAE1 comprises the core of the multiprotein complex of integral and peripheral membrane proteins essential to the structural integrity of the red cell membrane, and its bicarbonate/chloride activity is required for gas transport (see reviews2,3). In the kidney, kAE1 is localized to the basolateral membrane of collecting duct type A intercalated cells. Normal terminal urinary acidification by these cells requires kAE1-mediated bicarbonate reabsorption into the blood. Specific mutations in AE1 usually cause either autosomal dominant hereditary ovalo-spherocytosis or distal renal tubular acidosis (dRTA).4 In rare cases of homozygous recessive or compound heterozygous AE1 mutations, both the erythroid and renal phenotypes can manifest in the same individuals.5–7 In addition to these established roles, low-level AE1 expression has been detected in the glomerulus,8,9 but its potential function or interactions in the glomerulus are unknown.
AE1 possesses a long cytoplasmic N-terminus, a 12- to 14-span transmembrane transporter domain, and a short C-terminal cytoplasmic tail (Supplemental Figure 1). Both the N- and C-terminal domains of kAE1 contain tyrosine residues critical for basolateral targeting,10,11 which is likely regulated by phosphorylation.11 The N-terminus of kAE1 interacts with integrin-linked kinase (ILK),12 a protein that binds the cytoplasmic domains of β-integrins and cytoskeleton-associated proteins.13,14 The kAE1/ILK interaction enhanced kAE1 trafficking to the plasma membrane in HEK293 cells,12 but deletion of the majority of the ILK-interacting region in kAE1 did not affect its polarized trafficking in MDCK cells.11 Thus, the physiologic importance of the kAE1–ILK interaction in the kidney remains unclear. We searched for proteins that interact with the C-terminus of kAE1, using a yeast two-hybrid screen of a human kidney cDNA library, and identified a novel interaction between kAE1 and nephrin.
Nephrin is a single-spanning transmembrane Ig superfamily protein (Supplemental Figure 1) and an integral component of the podocyte slit diaphragm (SD), a structure critical to the glomerular selectivity filter.15 Mutations or gene targeting of nephrin results in congenital nephrotic syndrome.16,17 The nephrin extracellular domain contributes to the structural framework of the SD via homo- and heterodimeric interactions with neighboring nephrin polypeptides and nephrin-like homologs Neph1 and Neph2.15,18–20 The intracellular domain of nephrin contains multiple tyrosine phosphorylation sites and interacts with podocin,21,22 CD2-associated protein,23,24 Nck proteins,25–27 the ion channel TRPC6,28,29 and adherens junction proteins.30,31 These interactions anchor the SD complex to the underlying cytoskeleton and participate in signal transduction. Nephrin also forms a multicomponent ternary complex with ILK.32 The proteinuric phenotype of mice with podocyte-specific deletions of ILK and other components of the basally situated ILK/integrin complex32–35 suggests that SD and basal domain signaling complexes of podocytes cooperate to maintain integrity of the glomerular filtration barrier.
In view of the direct associations of ILK with kAE112 and nephrin,32 we investigated the physiologic significance of the nephrin/kAE1 interaction. Our studies demonstrate the importance of nephrin for stable kAE1 expression in podocytes and the in vivo interdependence of levels and subcellular localization among kAE1, nephrin, and ILK in podocytes, suggesting a novel role of kAE1 in glomerular function.
Results
Nephrin Interacts with kAE1 in the Yeast Two-Hybrid Assay
A yeast two-hybrid screen of a human kidney cDNA library using the C-terminus (residues 877 to 911) of human kAE1 revealed an interaction with two clones identical to nephrin (GenBank accession number AF035835). This cDNA was subcloned, and subsequent directed two-hybrid assay confirmed the interaction between the C-terminal domains of kAE1 and nephrin.
kAE1 Binds to Nephrin in HEK293T Cells, but Its Transport Activity Is Unaffected by Nephrin Coexpression in Xenopus Oocytes
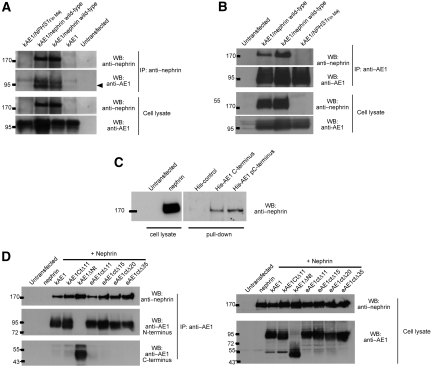
Co-immunoprecipitation studies confirmed the interaction between kAE1 and nephrin (Figure 1). kAE1 was transiently expressed in HEK293T cells alone or in combination with either wild-type nephrin or the most prevalent nephrin mutant linked with congenital nephrotic syndrome of the Finnish type, a frameshift leading to a 90-residue C-terminal truncation (NPHS1Fin Maj). kAE1 was co-immunoprecipitated from the co-transfected HEK293T cells with wild-type but not mutant nephrin using an antibody against N-terminus of nephrin (Figure 1A). In the reciprocal experiment, wild-type nephrin was co-immunoprecipitated with kAE1 using an antibody against the N-terminus of kAE1 (Figure 1B). These findings indicate that kAE1 and nephrin can interact to form a protein complex.
Figure 1.
kAE1 interacts with nephrin. Nephrin and/or kAE1 was expressed in HEK293T cells as indicated. (A) kAE1 co-immunoprecipitates with wild-type nephrin but not the NPHS1Fin Maj mutant nephrin, using an N-terminal nephrin antibody. Immunoprecipitated kAE1 runs as a doublet band in SDS-PAGE gel, representing core- and complex-glycosylated forms. (B) The reciprocal experiment demonstrates the co-immunoprecipitation of wild-type nephrin with kAE1 using BRIC 170. Representative blots of at least three independent experiments are shown. These results confirm that kAE1 binds to the full-length nephrin. (C) kAE1 C-terminal pulldown experiments on HEK293T cells transfected with wild-type nephrin. Synthetic histidine-tagged full-length C-terminal peptide with or without phosphorylation of tyrosine 904 (His-AE1 pCT and His-AE1 CT, respectively) interacts specifically and equally with nephrin. This confirms the interaction between kAE1 C-terminus and nephrin. (D) Co-immunoprecipitations conducted on HEK293T cells coexpressing nephrin and a panel of N- or C-terminally truncated eAE1/kAE1 constructs. The kAE1–nephrin association persists after deletion of the kAE1 cytoplasmic N-terminal domain (AE1 amino acids 66 through 360) or its entire C-terminal cytoplasmic domain (amino acids 876 through 911). This indicates that interaction sites in addition to the kAE1 C-terminus exist between nephrin and kAE1.
We next used kAE1 C-terminal synthetic peptides to pull down nephrin from the lysates of HEK293T cells overexpressing nephrin (Figure 1C). A synthetic peptide corresponding to either full-length (33 amino acids; RNVELQCLDADAKATFDEEEGRDEYDEVAMPV) or an identical C-terminal peptide with phosphorylated tyrosine Y904 specifically interacted with nephrin. Tyrosine phosphorylation of Y904 had no effect on the binding of the isolated kAE1 full-length C-terminus to nephrin.
Additional co-immunoprecipitation experiments in HEK293T cells coexpressing nephrin and a panel of N- or C-terminally truncated eAE1/kAE1 constructs (Figure 1D) demonstrated that the binding of kAE1 to nephrin persisted even after deletion of the majority of the kAE1 cytoplasmic N-terminal domain (amino acids 66 through 360; containing the ILK-binding site) or the entire kAE1 C-terminal cytoplasmic domain (amino acids 876 to 911). Taken together, these results indicate the existence of nephrin-binding sites in kAE1 in addition to that in its cytosolic C-terminus. These sites may occur within the kAE1 transmembrane, juxtamembrane, or extracellular domains, akin to the multiple AE1-binding sites in glycophorin A (GPA).4,5,36 The existence of multiple kAE1–nephrin interaction sites suggests a close association between the two membrane proteins, but further work is needed to characterize these additional sites.
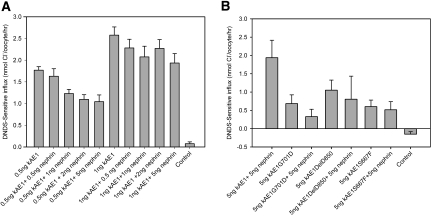
Several proteins that interact with eAE1 in red cells, including GPA, enhance AE1 trafficking and/or activity when coexpressed in heterologous expression systems7,12,36–39; however, nephrin coexpression with kAE1 had no obvious effect on kAE1 subcellular localization in HEK293T as observed by confocal microscopy (data not shown). We therefore investigated the effect of nephrin coexpression on kAE1 transport activity in Xenopus oocytes (Figure 2). In vitro–transcribed kAE1 and nephrin cRNAs were co-injected into Xenopus oocytes and the kAE1-specific anion transport estimated as stilbene-sensitive chloride uptake induced in the oocytes. The chloride influx did not increase when kAE1 was coexpressed with nephrin. Rather, at low concentrations of injected kAE1 cRNA, nephrin coexpression slightly reduced kAE1 transport activity, probably as a result of competition between cRNAs for the translational machinery, as has been previously observed in some other types of coexpression experiments.38 Nephrin did not rescue activity of any tested dRTA- or hereditary spherocytosis–associated kAE1 mutants. These findings suggest that nephrin is not a nonspecific chaperonin protein for kAE1.
Figure 2.
Nephrin coexpression does not enhance normal kAE1 or mutant kAE1 activity in oocytes. Xenopus oocytes are administered an injection of kAE1 cRNA with or without wild-type nephrin cRNAs as indicated. DNDS-sensitive chloride uptake by the oocytes (10 to 15 per group) over a 20-minute period is measured 24 hours after injection. Results are means ± SEM. (A) Comparative effects of nephrin cRNA (0.5 to 5.0 ng) coexpression on kAE1 activity (0.5 or 1.0 ng of kAE1 cRNA). Nephrin does not increase kAE1 activity. (B) Nephrin does not rescue the activities of the kAE1G701D or kAE1DelV850 (dRTA mutants) or kAE1S667F (hereditary spherocytosis mutant) previously shown to be rescued partially by GPA.5,7,69
kAE1 Is Endogenously Expressed and Co-localized with Nephrin in the Human Glomerulus
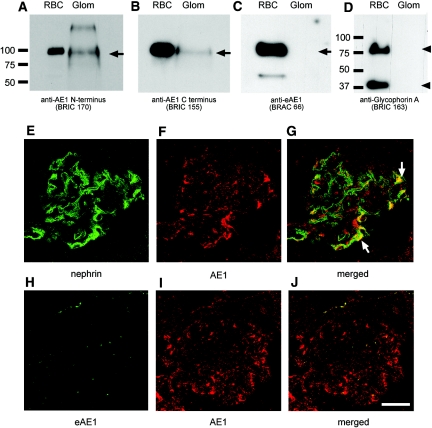
The endogenous glomerular expression of kAE1 was confirmed by Western blot analysis of glomerular lysate and by immunofluorescence confocal microscopy (Figure 3). In the absence of available immunocytology-competent antibodies specific for the kidney AE1 isoform, the presence of kAE1 protein in human glomerular lysate was deduced as follows (Figure 3, A through D): AE1 was detected at a very low level of expression in the lysate by two monoclonal AE1 antibodies that recognized distinct regions of AE1 that are common to both isoforms; in contrast, neither the erythroid isoform, eAE1, nor its subunit, GPA, which is present only in the red cells, was detectable in the glomerular lysate with specific anti-eAE1 and anti-GPA antibodies. These results indicate specific detection of kAE1 in the glomerular lysate and argue against the presence of eAE1 associated with residual red cells in the lysate preparation. The low level of kAE1 expression in glomeruli and in podocyte cell lines (see Nephrin is Required for Stable Endogenous Expression of kAE1 in Cultured Podocytes section below) has so far precluded confirmation of the interaction between kAE1 and nephrin by endogenous co-immunoprecipitation.
Figure 3.
kAE1 is expressed in human glomerulus. (Top) A low level of AE1 protein (approximately 95 kD) is detected in human glomerular lysate by Western blot analysis using two monoclonal AE1 antibodies (BRIC 170 and BRIC 155) that detect both kidney (kAE1) and erythroid (eAE1) isoforms (A and B). The erythroid-specific eAE1 mAb BRAC 66 and the erythrocyte GPA antibody BRIC 163 (monomer and dimer, arrowhead) reacted only with the red blood cell membranes and not with glomerular lysate (C and D). Thus, kAE1 is the isoform expressed in the glomerulus and this is not eAE1 (100 kD) from erythrocyte contamination. (Middle) Confocal immunofluorescence microscopy of nephrin and kAE1 in normal human kidney sections. (E) Nephrin (7C1 mAb) exhibits the characteristic ribbon-like pattern of the glomerular capillary loops, consistent with podocyte staining. (F and G) kAE1 (rabAE1Ct1) displays a more diffuse capillary loop pattern in the glomerulus (f), which partially overlaps with nephrin staining (arrows, g). (Bottom) Absence of glomerular immunostaining using the eAE1-specific antibody (BRAC 66; H) is consistent with the glomerular AE1 staining (rabAE1Ct1; I) being the kAE1 isoform. Bar = 150 μm.
Immunofluorescence confocal microscopy of normal human kidney sections demonstrated low levels of kAE1 staining in the glomeruli, some of which was in a linear capillary loop pattern similar to that of nephrin but at a much lower level of expression than in distal nephron. Merging of the kAE1 and nephrin images indicated partial co-localization of the two proteins in the glomerulus (Figure 3, E through G). In contrast, labeling with a specific anti-eAE1 antibody revealed absence of glomerular signal except for occasional punctate staining corresponding to red cells (Figure 3, H and I). In addition, kAE1 staining was adjacent to but not overlapping with that of the endothelial marker platelet-endothelial cell adhesion molecule 1 or with that of the mesangial marker α-smooth muscle actin (data not shown). These observations are consistent with the suggestion that the kAE1 isoform is expressed endogenously in podocytes and co-localizes with nephrin.
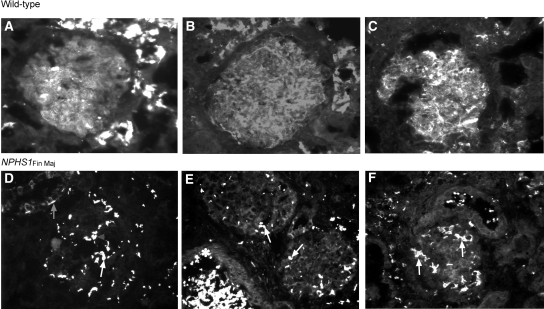
Expression of kAE1 in NPHS1Fin Maj Human Kidney Sections Is Diminished
We further examined the endogenous relationship between kAE1 and nephrin by immunofluorescence staining of kAE1 in nephrectomy specimens without glomerular pathology and in specimens from a patient homozygous for the NPHS1Fin Maj mutation (Figure 4). In the normal kidney, kAE1 staining was evident in glomerular capillary loops, with staining in the periglomerular tuft. In contrast, kAE1 expression was altered in the patient's kidney. The punctate clusters of AE1 staining throughout the patient's glomeruli overlapped with the erythrocyte-specific protein GPA, identifying them as red cell aggregates (Supplemental Figure 2) Of note, kAE1 staining in the connecting tubules and collecting ducts, which do not express nephrin, remained undisturbed in the NPHS1Fin Maj mutant kidney sections. These observations suggest that diminution and altered distribution of kAE1 expression within the glomerulus was due to the loss of wild-type nephrin and not secondary to nonspecific renal parenchymal damage. Hence, normal glomerular expression of kAE1 seems to be dependent on the presence of intact nephrin.
Figure 4.
kAE1 expression is disrupted in NPHS1Fin Maj human glomeruli. (A through F) A panel of anti-AE1 antibodies (BRIC 170 [A and D], rabAE1Ct1 [B and E], and rabAE1Ct2 [C and F]) are used. Under wide-field microscopy, glomerular kAE1 distribution in the wild-type kidney (top) displays a diffuse capillary loop pattern, consistent with podocyte staining. In the mutant kidney (bottom), the normal kAE1 distribution is lost and the intensely stained clusters throughout the glomeruli (white arrows) correspond to eAE1 staining in clumps of red cells (see Supplemental Figure 2). Intense staining of kAE1 is seen in the connecting tubules surrounding the glomeruli in the top panel (deliberately oversaturated to detect the low glomerular expression of kAE1), whereas its basolateral position in the type A intercalated cells is best appreciated in D (gray arrow). kAE1 staining in the distal tubule, where nephrin is not expressed, is undisturbed in the mutant kidney sections. (E) Red blood cells, which stain positively for eAE1, can be seen in a vessel lumen (*). Magnification, ×40.
Nephrin Is Required for Stable Endogenous Expression of kAE1 in Cultured Podocytes
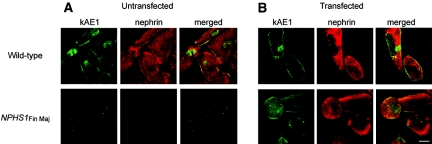
The dependence of kAE1 expression on nephrin was confirmed in immunofluorescence studies with conditionally immortalized human wild-type and mutant nephrin (NPHS1Fin Maj) podocytes (Figure 5). In wild-type podocytes, endogenous kAE1 expression was detected at the cell surface with some faint cytoplasmic staining, a pattern coincident with that of nephrin staining. In contrast, the absence of nephrin expression in the mutant podocytes was accompanied by concomitant loss of detectable plasma membrane kAE1 and diminished intracellular kAE1 expression (Figure 5A). Furthermore, transfection of wild-type nephrin into mutant podocytes restored the endogenous expression of kAE1 (Figure 5B). Immunoprecipitation experiments confirmed the presence of kAE1 in the wild-type podocytes (both untransfected and those transfected with wild-type nephrin) and the absence of detectable kAE1 in the mutant podocytes. The low and variable efficiency of nephrin transfection into the cultured podocytes prevented reproducible demonstration by immunoprecipitation of nephrin rescue of kAE1 expression in the mutant podocytes (Supplemental Figure 3); however, this rescue was consistently observed by immunofluorescence imaging of nephrin-transfected cells. These results strongly suggest that stable endogenous kAE1 expression in podocytes requires expression of wild-type nephrin.
Figure 5.
Stable endogenous expression of kAE1 in cultured podocytes is dependent on the presence of wild-type nephrin. Immunofluorescence staining of kAE1 (rabAE1Ct1, green) and nephrin (7C1, red) in immortalized podocytes. (A) In wild-type cultured podocytes (top), low levels of endogenous kAE1 are detectable at the cell surface along with faint cytoplasmic and nuclear staining. Nephrin staining is visible throughout the cells and at the plasma membrane. Both kAE1 and nephrin expression are absent in NPHS1Fin Maj mutation podocytes (bottom). (B) Transfection of wild-type nephrin into the wild-type podocytes had little effect on the kAE1 subcellular expression (top), whereas the re-introduction of wild-type nephrin into the mutant podocytes rescues the expression of kAE1 at the cell surface and in the cytoplasm (bottom). As previously noted,5 rabAE1Ct1 gives a faint nuclear stain in the absence of kAE1 (bottom left panel of A). Bar = 30 μm.
Diminished Glomerular Expression of Nephrin in Ae1−/− Mice Is Associated with Albuminuria
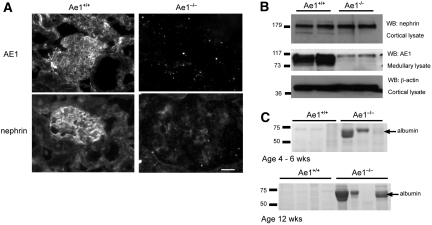
To examine whether nephrin expression was reciprocally dependent on kAE1, we examined glomerular expression of nephrin in 12-week-old Ae1−/− and Ae1+/+ mouse littermates by immunofluorescence staining and Western blotting (Figure 6). kAE1 was present in the glomeruli and distal nephron tubules in Ae1+/+ mice but absent in the kidneys from Ae1−/− animals. Nephrin staining in the glomerulus was more diffusely localized and reduced in intensity in Ae1−/− mice (Figure 6A), whose glomeruli were significantly enlarged compared with their wild-type counterparts (Ae1−/− glomeruli 147 ± 8; Ae1+/+ glomeruli 69 ± 2; mean cross-sectional diameter μm ± SEM; P < 0.0001; n = 18). Immunoblotting of glomeruli-enriched cortical lysates revealed comparable abundance of nephrin protein in 12-week-old Ae1−/− and Ae1+/+ littermates (Figure 6B). However, urine from Ae1−/− and Ae1+/+ littermates aged 4 to 6 weeks or 12 weeks demonstrated by SDS-PAGE abundant 70-kD protein corresponding to albumin in two of the three younger and three of the four older Ae1−/− mice but in none of the Ae1+/+ animals (Figure 6C). Thus, although the absence of kAE1 is not associated with detectable change in total nephrin protein levels, it does result in altered glomerular distribution of nephrin and variably increased albuminuria.
Figure 6.
Nephrin expression is altered with associated albuminuria in Ae1−/− mice. (A) AE1 immunostaining is seen in the glomeruli and distal tubules of Ae1+/+ mice and is absent in Ae1−/− animals. Nephrin staining appears in a capillary loop pattern in the Ae1+/+ glomeruli and is reduced in intensity and more diffuse in the Ae1−/− glomeruli, which are also enlarged compared with wild-type glomeruli. Bar = 20 μm. (B) Western analysis demonstrates comparable levels of nephrin protein in the cortical lysates from Ae1−/− and Ae1+/+ animals. As expected, kAE1 protein is present in the medullary lysate from the Ae1+/+ mice but absent from those of the Ae1−/− animals, in which only nonspecific background bands are observed. Cortical and medullary samples are chosen because these regions are enriched in nephrin and AE1 protein expression, respectively. Seventy-five micrograms of total protein was loaded per lane, and β-actin staining confirmed equal loading. WB, Western blotting. (C) SDS-PAGE of urine samples demonstrates the presence of a protein band corresponding to albumin (approximately 70 kD) in two of the three 4- to 6-week-old and three of four 12-week-old male Ae1−/− mice. This band is absent in wild-type littermates. Urine loading is normalized to creatinine excretion, 5 mg/dl loaded per lane.
Morphologic Alterations in Glomeruli of Ae1−/− Mice
We examined kidney sections from four pairs of 12-week-old Ae1−/− and Ae1+/+ mice by light and electron microscopy for structural changes in the glomerulus. Light microscopy (Supplemental Figure 4) revealed in Ae1−/− mice glomerular enlargement, moderate to marked mesangial expansion with increased matrix deposition, mesangiosclerosis, and mesangiolysis. Preservation of podocytes was confirmed in the Ae1−/− glomeruli by immunofluorescence staining with an additional podocyte marker (synaptapodin) and was accompanied by increased mesangial cell staining and fibronectin deposition (Supplemental Figure 5).
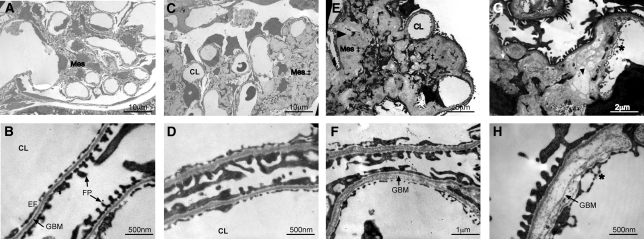
Transmission electron microscopy (Figure 7) further confirmed marked mesangial expansion and sclerosis with collagen fibrils and hyaline deposition in all four Ae1−/− mice examined. At the ultrastructural level, a spectrum of changes was observed in the glomerular basement membrane (GBM) of the knockout animals, ranging from mild to marked, and irregular GBM thickening up to 10-fold greater than that of Ae1+/+ mice. Splitting and lamination of the GBM were also frequently observed in Ae1−/− mice. The podocyte foot processes, filtration slits, and endothelial fenestrations remained largely intact, with only focal areas of effacement. There was no podocyte detachment from the GBM.
Figure 7.
Ae1−/− mice have mesangial expansion and sclerosis and glomerular basement membrane defects. (A through H) Representative transmission electron micrographs from four pairs of 12-week-old Ae1+/+ (A and B) and Ae1−/− mice (C through H) are shown. The mesangium (Mes) was abnormal in all Ae1−/− mice examined, with marked expansion, increased matrix accumulation (‡), mesangiosclerosis (black arrowhead), and deposition of hyaline and collagen fibrils (white arrow; C, E, and G). Varying degrees of GBM abnormalities are seen in three of the four mice, ranging from mild uniform (D) to marked thickening (H) up to 10-fold increase in thickness compared with the wild-type (B), with a lamellar appearance and splitting of the lamina densa clearly seen in F and H. In contrast, the podocyte foot processes (FP) and endothelial fenestrations (EF) in Ae1−/− animals were largely intact, with only focal areas of foot process effacement, subendothelial expansion, and endothelial cell arcade formation (*, g and H). CL, capillary lumen.
Nephrin, ILK, and AE1 Immunofluorescence Staining Is Altered in Ae1−/− and Podo-Ilk−/− Mice
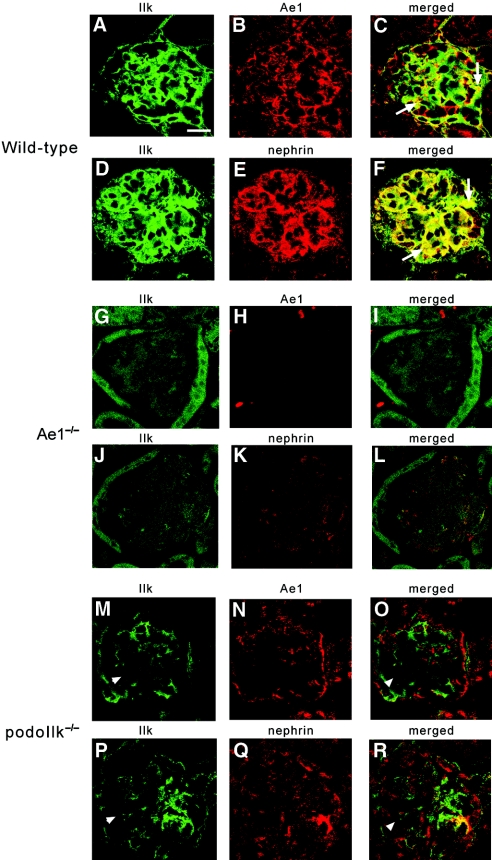
In view of the associations of ILK with kAE112 and with nephrin32 and considering the phenotype of mice with podocyte-specific Ilk deletion (podo-Ilk−/− mice),32–34 we examined the possible in vivo interrelationship among the three proteins by confocal immunofluorescence microscopy (Figure 8). In wild-type mouse glomeruli (Figure 8, A through F), both nephrin and AE1 demonstrated linear patterns of staining along the GBM, consistent with podocyte staining, and each co-localized with ILK. In the Ae1−/− mouse (Figure 8, g through L), AE1 staining was absent and staining of both ILK and nephrin was altered. ILK staining appeared diffuse and reduced in intensity. Nephrin staining was also more diffuse, consistent with the findings in Figure 6A. In podo-Ilk−/− mice (Figure 8, M through R), glomerular AE1 and nephrin both exhibited altered staining patterns with reduced intensity and patchy distribution throughout the glomerulus. These observations strongly suggest interdependence among ILK, AE1, and nephrin such that deletion of any one polypeptide alters expression and/or distribution of the others.
Figure 8.
Nephrin, ILK, and AE1 expression is disrupted in both Ae1−/− and podoILK−/− glomeruli, suggesting an interdependency among the three proteins. (A through R) Confocal immunofluorescence microscopy demonstrating the distribution of ILK, AE1, and nephrin in the glomeruli of the wild-type (6 weeks of age; A through F), Ae1−/− (12 weeks of age; G through L), and podo-Ilk−/− mice (6 weeks; M through R). In the wild-type animals, AE1 and nephrin appear in a capillary loop pattern, and the arrows in the merged images indicate partial co-localization of ILK with AE1 (C) and nephrin (F), respectively, in the podocytes. In the Ae1−/− mouse (confirmed by the absence of AE1 signal in H), staining of ILK (G and J) and nephrin (K) is diffuse and reduced in intensity, and nephrin co-localization with ILK is lost (L). (N and Q) In the podo-Ilk−/− mice, both AE1 and nephrin staining exhibit patchy distribution throughout the glomerulus. The arrowheads denote areas of ILK-deficient podocytes in which AE1 and nephrin expression is lost. Bar = 15 μm in A through C and P through R; 17.24 μm in D through F and M through O; 30 μm in G through L.
Discussion
This study reports several novel aspects of glomerular biology. It reveals that kAE1 is expressed at low levels in glomerular podocytes and is dependent on the SD protein nephrin. Expression and localization of nephrin, as well as of ILK, which forms part of the integrin complex, are similarly dependent on the presence of kAE1 in the glomerulus. These three proteins seem to be functionally interconnected and may contribute to the novel role of glomerular kAE1 in maintaining the glomerular filtration barrier to prevent or minimize proteinuria.
We first tested the hypothesis that nephrin may act as a chaperonin for kAE1 in the kidney. Although no enhancement of nephrin on kAE1 transport activity in Xenopus oocytes was detected, we did observe a dependence of kAE1 expression on nephrin expression in podocytes, both in immortalized cell lines and in intact kidney. We do not know which aspect of kAE1 trafficking or stabilization in podocytes is dependent on nephrin. In the absence of nephrin, kAE1 may be incorrectly targeted or once at the plasma membrane may be unstable, leading to increased degradation.
Strong evidence for the physiologic significance of the kAE1/nephrin interaction comes from the abnormal histology and albuminuria observed in Ae1−/− mice. These mice exhibit severe hemolytic anemia and dRTA, but glomerular abnormalities had not been previously reported.40–42 Ae1−/− mice showed mesangiosclerosis and GBM abnormalities but no obvious podocyte ultrastructural changes. It is possible that the mesangial and GBM defects might be secondary to a functional podocyte defect, but this will need future studies. The marked alterations in nephrin expression in the Ae1−/− mouse are unlikely to be simply secondary to the hemolytic anemia per se, because our analysis of two mouse models of hemolysis (sickle cell disease and β-Thalassemia) with glomerular disease phenotypes43,44 demonstrated significant nephrin and AE1 expression (Supplemental Figure 6); however, expression and distribution of both kAE1 and nephrin were substantially altered in glomeruli of proteinuric CD2AP−/− mice (Supplemental Figure 7). CD2AP binds nephrin in the SD23,24 and may link it to the cytoskeleton.45 The loss of this important attachment site may destabilize nephrin localization and indirectly affect kAE1 expression. We as yet have not investigated the possibility of a direct interaction of kAE1 with CD2AP in podocytes.
Importantly, we observed that localization of both ILK and nephrin was altered in Ae1−/− mice. Localization of AE1 and nephrin was similarly altered in podo-Ilk−/− mice, which develop GBM defects within 3 to 4 weeks of birth, coinciding with the onset of albuminuria and preceding the development of foot process effacement and glomerulosclerosis,32–34 and in which altered nephrin localization has been previously reported.32 The α3β1-integrin–ILK complex located at the basal domain of the podocyte foot process links the actin cytoskeleton to the extracellular matrix, mediating podocyte attachment to the GBM, cytoskeleton modulation, and cell–matrix signaling.34 The functional links between the SD complex and the integrin–ILK complex are increasingly appreciated as being important for the functional integrity of the glomerular filter.
Our findings of disrupted glomerular localization of nephrin, ILK, and AE1 in the Ae1−/− and podo-Ilk−/− mice, together with previously reported interactions of ILK with AE112 and nephrin,32 strongly suggest that the three molecules may exist in a multiprotein complex. Within this complex, kAE1 may act as a “functional bridge” connecting the signal complexes of the SD with those of the basal domain, by simultaneously binding ILK12 and nephrin, via its N- and C-terminal domains, respectively. Although some kAE1 may exist in alternative complexes, we speculate that the multiprotein complex that does contain kAE1, nephrin, and ILK, may include other SD and cytoskeletal proteins (e.g., CD2AP, Nck, Neph1 and Neph2, spectrin), extrapolating from reported interactions,19,20,23–26 and by analogy with the interactions of eAE1 with multiple erythroid cytoskeleton proteins that modulate erythrocyte mechanical and morphologic properties.2 Of note, stable kAE1 overexpression in MDCK cells alters paracellular permeability and may involve changes in the actin cytoskeleton–linked epithelial tight junctions.10 It is tempting to speculate that kAE1 in podocytes, rather than being required for its transport activity, may play a role in the organization and/or function of the SD, which has features of both adherens and tight junctions.46,47 Both kAE1 and nephrin are endocytosed from the cell surface when tyrosine phosphorylated,11,48 suggesting a potential mechanism of co-regulation of kAE1 and nephrin during SD remodeling. Alternatively, the presence of kAE1 may establish pH microdomains in the SD that may potentially modulate protein activity or signaling (e.g., TRPC channels). Thus, the potential interactions and role of kAE1 alongside other SD and adaptor proteins merit further study.
Finally, the potential effects of dRTA-associated AE1 mutations on glomerular function in humans should be considered. Proteinuria is not a characteristic feature of sporadic dRTA or of dRTA associated with dominant or recessive kAE1 mutations in the absence of (nominally) secondary chronic renal insufficiency or nephrocalcinosis. The proposed underlying mechanism of dominant dRTA is hetero-oligomerization of the mutant with the wild-type kAE1 protein, resulting in mislocalization of the wild-type protein in the type A intercalated cells, where high levels of basolateral kAE1 are needed for bicarbonate absorption.10,49,50 Podocytes represent a different cellular context with distinct interacting proteins (including nephrin) likely involved in kAE1 targeting/stability. In dRTA, sufficient levels of podocyte kAE1 may be physiologically targeted to allow normal function. Albuminuria may nonetheless be present in some patients with dRTA.51
In conclusion, we have identified kAE1 as a physiologically significant protein in the podocyte, whose newly identified interaction with nephrin defines a previously unknown role for kAE1 in the function of the glomerular filtration barrier. kAE1 may play a role in the organization or stability of the SD and ILK–integrin complexes and in facilitating cross-talk between subdomains of the foot process to maintain normal architecture under constantly changing glomerular filtration pressures and for repair of the GBM in response to injury.
Concise Methods
Antibodies
A panel of anti-human AE1 antibodies were used: Mouse monoclonal BRIC 170 and BRIC 155 that recognized epitopes at the N- and extreme C-termini of AE1, respectively10; an eAE1-specific rat monoclonal BRAC 66 antibody that recognized an epitope within 1 to 65 amino acids of eAE152; a mouse monoclonal BRIC 163 antibody against GPA52; and three rabbit polyclonal antibodies raised against residues 881 through 911 of AE1 (rabAE1Ct1; rabAE1Ct2)52 or N-terminal AE1 (65 to 80 amino acids; rabAE1Nt1). The anti-human nephrin antibodies used were mouse monoclonal (7C1) antibody and a rabbit polyclonal (K2737) that recognized intracellular C-terminal domain53 and a rabbit polyclonal antibody (H-300) raised against amino acids 23 through 322 of the extracellular domain of nephrin (Santa Cruz Biotechnology, Santa Cruz, CA). Two additional rabbit polyclonal antibodies were raised against a peptide comprising the last 30 amino acids of nephrin C-terminus (N3 and N4). For the staining of mouse tissues, polyclonal guinea pig and rabbit anti-mouse AE1,42 polyclonal rabbit anti-mouse nephrin,54 mouse monoclonal anti-mouse ILK,33 rabbit polyclonal anti-synaptopodin (Santa Cruz Biotechnology), mouse monoclonal anti–α-smooth muscle actin (Sigma-Aldrich, St. Louis, MO), and rabbit polyclonal anti-fibronectin (Sigma-Aldrich) antibodies were used.
Species-specific horseradish peroxidase–conjugated antibodies (DakoCytomation, Cambridge, UK), FITC- and tetramethylrhodamine-labeled antibodies (Jackson Immunoresearch, Philadelphia, PA), or Alexa fluor 488– and 594–conjugated antibodies (Molecular Probes, Invitrogen, Paisley, UK) were used in Western blotting and immunofluorescence studies, respectively.
Plasmid Constructs
The plasmids containing human kAE1 in the mammalian expression vector pcDNA3.kAE155 and in the Xenopus expression vector BSXG1.kAE137 have been described previously. pcDNA3.1.AE1mem (lacking AE1 N-terminal residues 1 through 360) or C-terminal p3.1eAE1 truncation constructs have been previously published.10,49 The mammalian plasmid pcDNA3.1.nephrin has also been described.56 BSXG.nephrin was constructed by PCR amplification using Expand Taq polymerase and pcDNA3.1.nephrin as a template and primers that incorporated Nhe1 sites. The resulting PCR product was cloned into pCR2.1-TOPO vector (Invitrogen), excised, and ligated into BSXG using compatible enzyme sites. NephrinFin Maj encoding the 2-bp frameshift deletion in NPHS1 (nt121 del 2; NPHS1Fin Maj) was generated using the QuikChange Site-Directed Mutagenesis kit using pcDNA3.1.nephrin or BSXG.nephrin according to the manufacturer's protocol (Strategene, Amsterdam Zuidoost, Netherlands). The yeast two-hybrid bait plasmid GBKT7-AE1Ct, containing the C-terminal residues 877 through 911 of AE1, was PCR amplified from BSXG1.kAE1 using primers that incorporated an EcoR1 and Sal1 sites. The resulting PCR product was cloned into pCR2.1-TOPO vector (Invitrogen), excised using restriction enzyme sites EcoR1 and Sal1, and ligated into the bait vector GBKT7. Automated DNA sequencing was performed to validate all constructs (Geneservice, Department of Biochemistry, Oxford, UK).
Yeast Two-Hybrid Screen
Yeast two-hybrid screening was performed using a pretransformed Matchmaker human kidney cDNA library (BD Biosciences Clontech) and yeast strains according to the manufacturer's protocol. The bait plasmid was transformed into AH109 cells and mated with the pretransformed library strain Y187 cells containing human kidney cDNA in the pACT2 vector. Positive colonies were selected by blue growth on SD/-Ade/-His/-Leu/-Trp/X-β-Gal plates, which also contained 3 mM 3-amino-1,2,4-triazole. Candidate clones were recovered from the yeast and retransformed into strain Y187 in the pGADT7 vector (BD Biosciences Clontech), then mated back to the original AH109 [AE1] bait strains and tested again for growth on SD/-Ade/-His/-Leu/-Trp/X-β-Gal plates. Positive colony inserts were sequenced and identified by a BLAST search (http://ncbi.nlm.gov/blast).
Cell Culture and Transfection
Human embryonic kidney cells (HEK293T) were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin under 5% CO2 at 37°C. Conditionally immortalized human wild-type and mutant podocyte cell lines were cultured as described previously.53,57 The mutant podocytes were derived from a nephrectomy specimen of a patient homozygous for the NPHS1Fin Maj frame-shift mutation (nt121 del 2) encoding a truncated 90-residue nephrin protein. Transient transfection of podocyte cell lines with wild-type nephrin was performed as described previously.56 Podocyte transfection was achieved at the permissive temperature of 33°C, and cells were allowed to differentiate for 14 days after temperature shift. Nephrin-transfected cells were studied at passages 2 to 3.
Human Kidney Tissues
Human kidney tissue was obtained from a specimen of normal adult donor kidney unused for transplantation (with institutional ethical approval, University of Bristol, UK). The cortical tissue was separated and frozen in liquid nitrogen and stored at −80°C until processed for immunohistochemistry. Glomeruli were isolated with the sieving method, as described previously.58
Mouse Kidney Tissues
The generation and characterization of the Ae1−/− mouse model have been described in detail in previous publications.40,42,59 Ae1−/− mice were generated by gene targeting, producing a null mutation with no detectable AE1 peptides in the erythrocyte membrane and in the kidneys. Ae1−/− animals experienced severe hemolytic anemia and growth retardation at birth with high neonatal mortality. The survival rate was improved to approximately 25% by reducing litter sizes after birth and using surviving males to continue breeding.42 The kidney samples used in this study originated from such surviving 12-week-old mice. Detailed renal phenotyping in the surviving adult Ae1−/− mice showed spontaneous hyperchloremic metabolic acidosis, elevated serum urea, but normal serum creatinine concentrations and normal creatinine clearance, suggestive of mild systemic dehydration and renal impairment.42 Cortical and medullary membrane protein extracts and kidney cryosections were prepared as described previously.42 Protein quantification was performed using a modified Lowry assay60 (Bio-Rad DC protein assay; Bio-Rad Laboratories, Hercules, CA), and 75 μg of crude membrane protein was solubilized in Laemmli sample buffer and analyzed by SDS-PAGE and Western blotting.
Kidney specimens from 6-week-old podocyte-specific Ilk knockout (podo-Ilk−/−) mice,33 11- to 12-week-old mouse models of β-Thalassemia44 and sickle cell anemia,43 and 3-week-old CD2AP−/− mice23 were also used for immunohistochemistry.
Co-immunonoprecipitation Studies
Co-immunoprecipitation experiments were performed as described previously.61 HEK293T cells were transfected with kAE1 (pcDNA3.1kAE1 or pcDNA3.1e AE1 truncation mutants; 5 μg) either alone or in combination with the wild-type (pcDNA3.1.nephrin; 5 or 10 μg) or mutant (pcDNA3.1.nephrinFin Maj; 5 μg) nephrin plasmids, using the calcium precipitation.62 After 24 hours of incubation, cells were lysed with immunoprecipitation buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 1 mM PMSF, 10 g/ml antipain, 10 g/ml leupeptin, 100 U/ml aprotinin, 50 mM NaF, 1 mM EDTA, and 1 mM orthovanadate) for 30 minutes on ice and clarified by centrifugation at 15,000 × g for 15 minutes at 4°C. Cell lysates containing equal amounts of total protein were precleared with protein A or G-Sepharose, then incubated for 4 hours at 4°C with either protein A or G-Sepharose beads conjugated with the H-300 or BRIC 170 antibody, respectively. The precipitated complexes were eluted from the beads with SDS Laemmli buffer and analyzed by SDS-PAGE and Western blotting using multiple anti-nephrin and anti-AE1 antibodies, yielding similar results. Additional pulldown experiments were conducted on HEK293T cells transfected with wild-type nephrin, using the indicated hexahistidine-tagged synthetic C-terminal kAE1 peptides (synthesized by University of Bristol Peptide Synthesis facility) in conjunction with His Pur Cobolt beads (Themo Scientific).
Xenopus Oocyte Expression and Chloride Transport
The methods used for the preparation of purified cRNA and expression in oocytes and assay of 36Cl− uptake were as described previously.37
SDS-PAGE and Western Blot Analysis
Protein samples were electrophoresed on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The blots were blocked with 5% milk powder in Tris-buffered saline/0.1% Tween 20 and sequentially incubated with the appropriate primary antibodies and horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature. Antibody binding was detected by enhanced chemiluminescence analysis (Western Lightning; Pierce) before exposure to x-ray film (Hyperfilm; GE Healthcare, Amersham). Urinary protein excretion in Ae1−/− mice was determined by Coomassie blue staining of polyacrylamide gels after urine protein electrophoresis.
Immunofluorescence Microscopy
The immunolabeling of human kidney sections was performed as described previously.63 Briefly, 5-μm cryosections were fixed and permeabilized with 2.5% paraformaldehyde and 0.3% Triton-100 or in methanol/acetone (6:4 vol/vol). Sections were rinsed with PBS and blocked with 4% BSA for 30 minutes before application of primary antibodies for 60 minutes at room temperature. Dual labeling was performed sequentially, and sections were washed extensively after each incubation period. Antigen–antibody complexes were detected with appropriate fluorochrome-conjugated secondary antibodies. Sections were again washed before mounting with Vectashield (Vector Laboratories, Burlingame, CA). Standard wide-field images were obtained via a Leica photomicroscope attached to a Spot 2 slider digital camera (Diagnostic Instruments) and were processed with Adobe Photoshop 5.0 software (Adobe, San Jose, CA). Confocal imaging was conducted using a Leica TCS-NT confocal laser-scanning microscope (Leica-Microsystems, Milton Keynes, UK) using a ×63/1.32 oil immersion objective equipped with a Kr/Ar laser.11
Cultured podocytes were fixed in methanol/acetone, blocked with 4% BSA, serially incubated with rabbit anti-Ct AE1 antibody then with nephrin antibody 7C1, and followed by detection with suitable fluorochrome-conjugated secondary antibodies. Cells were mounted and imaged by confocal laser-scanning microscopy as described already.
Mouse 5-μm cryosections were fixed and labeled as for human sections and viewed using Olympus Cell wide-field imaging system, comprising an IX81 microscope and MT20 illumination unit, CCD camera, and a ×60 1.25 NA oil immersion lens. Confocal images were taken on a Leica TCS-NT confocal laser-scanning microscope (as described already). Images were assembled using Adobe Photoshop 8 software and Adobe Illustrator CS 11 software.
Ae1−/− Kidney Histology and Electron Microscopy
For histologic analysis under light microscopy, sections were stained with periodic acid-Schiff, Masson's, and hematoxylin and eosin stains using standard methods. For transmission electron microscopy, specimens were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer64,65 and processed through to embedding in Araldite epoxy resin.66 Ultrathin sections (100 nm) were cut, mounted on 300 mesh copper grids, and stained with uranyl acetate67 and lead citrate.68 Ultrathin sections were viewed on a Philips CM10, and images were captured via SIS iTEM software.
Statistical Analysis
Statistical analysis was performed using the t test.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by National Health Service Blood and Transplant Career Development Wellcome Trust Fellowship (A.M.T.), Medical Research Council (M.A.S.), National Institutes of Health grants DK43495 and HL000765 (S.L.A.), Swiss National Science Foundation (3100A0-122217/1), and the 6th EU Frame work project EUREGENE (C.A.W.).
Portions of this work were presented at the annual meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
We thank Prof. Matthias Kretzler for providing the podo-Ilk−/− specimens and antibodies to ILK and nephrin, Mario Schiffer for providing the CD2AP−/− samples, and Prof. Dave Anstee for providing mAbs to AE1 and GPA. We also thank Rosey Mushens for culturing mAbs, Debbie Martin for carrying out the cryosectioning, and Emanuelle Cordat and Prof. Reinhart Reithmeier for the pcDNA3.1eAE1 truncation constructs.
Footnotes
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Kollert-Jons A, Wagner CA, Hubner S, Appelhans H, Drenckhahn D: Anion exchanger-1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol 265: F813–F821, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Tanner MJ: Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders. Curr Opin Hematol 9: 133–139, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Alper S: Molecular physiology of SLC4 anion exchangers. Exp Physiol 91: 153–161, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Williamson RC, Toye AM: Glycophorin A: Band 3 aid. Blood Cells Mol Dis 41: 35–43, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Toye A, Williamson R, Khanfar M, Bader-Meunier B, Cynober T, Thibault M, Tchernia G, Déchaux M, Delaunay J, Bruce LJ: Band 3 Courcouronnes (Ser667Phe): A trafficking mutant differentially rescued by wildtype band 3 and glycophorin A. Blood 111: 5380–5389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribeiro ML, Alloisio N, Almeida H, Gomes C, Texier P, Lemos C, Mimoso G, Morle L, Bey-Cabet F, Rudigoz RC, Delaunay J, Tamagnini G: Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood 96: 1602–1604, 2000 [PubMed] [Google Scholar]
- 7. Bruce LJ, Wrong O, Toye AM, Young MT, Ogle G, Ismail Z, Sinha AK, McMaster P, Hwaihwanje I, Nash GB, Hart S, Lavu E, Palmer R, Othman A, Unwin RJ, Tanner MJ: Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: Loss of up to 95% band 3 transport in red cells. Biochem J 350: 41–51, 2000 [PMC free article] [PubMed] [Google Scholar]
- 8. Hazen-Marin D, Pasternack G, Hennigar R, Spicer S, Sens D: Immunocytochemistry of band 3 protein in kidney and other tissues of control and cystic fibrosis patients. Pediatr Res 21: 235–237, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Wainwright S, Tanner M, Martin G, Yendle J, Holmes C: Monoclonal antibodies to the membrane domain of the human erythrocyte anion transport protein. Biochem J 258: 211–220, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toye AM, Banting G, Tanner MJ: Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: Mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 117: 1399–1410, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Williamson RC, Brown AC, Mawby WJ, Toye AM: Human kidney anion exchanger 1 localisation in MDCK cells is controlled by the phosphorylation status of two critical tyrosines. J Cell Sci 121: 3422–3432, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Keskanokwang T, Shandro H, Johnson D, Kittanakom S, Vilas G, Thorner P, Reithmeier R, Akkarapatumwong V, Yenchitsomanus P, Casey J: Interaction of integrin-linked kinase with the kidney chloride/bicarbonate exchanger, kAE1. J Biol Chem 282: 23205–23217, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Wu C, Dedhar S: Integrin-linked kinase (ILK) and its interactors: A new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155: 505–510, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Legate K, Montanez E, Kudlacek O, Fassler R: ILK, PINCH, and parvin: The tIPP of integrin signalling. Nat Rev 7: 20–31, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Ptaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan C, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Wartiovaara J, Ofverstedt L, Khoshnoodi J, Zhang J, Makela E, Sandin S, Ruotsalainen V, Cheng R, Jalanko H, Skoglund U, Tryggvason K: Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerke P, Huber T, Sellin L, Benzing T, Walz G: Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 14: 918–926, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, Walz G: NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol 16: 1693–1702, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Huber T, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Schwarz K, Simons M, Reiser J, Saleem M, Faul C, Kriz W, Shaw A, Holzman L, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shih N, Li J, Karpitskii V, Nguyen A, Dustin M, Kanagawa O, Miner JH, Shaw A: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Shih N, Li J, Cotran R, Mundel P, Miner J, Shaw A: CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol 159: 2303–2308, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman L: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones N, Blasutig I, Eremina V, Ruston J, Bladt F, Li H, Huang H, Larose L, Li S, Takano T, Quaggin S, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T: Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganisation. Mol Biol Cell 28: 2035–2046, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winn M, Conlon P, Lynn K, Farrington M, Creazzo T, Hawkins A, Daskalakis N, Kwan S, Ebersviller S, Burchette J, Pericak-Vance M, Howell D, Vance J, Rosenberg P: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Reiser J, Polu K, Möller C, Kenlan P, Altintas M, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith P, Clapham D, Pollak M: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehtonen E, Ryan J, Kudlicka K, Iino N, Zhou H, Farquhar M: Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A 102: 9814–9819, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar M: Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 165: 923–936, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dai C, Stolz D, Batsacky S, At-Arnauld R, Wu C, Dedhar S, Liu Y: Essential role of the integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006 [DOI] [PubMed] [Google Scholar]
- 33. El-Aouni C, Herbach N, Blattner S, Henger A, Rastaldi M, Jarad G, Miner J, Moeller M, Arnaud R, Dedhar S, Holzman L, Wanke R, Kretzler M: Podocyte-specific deletion of integrin-liked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17: 1334–1344, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee S, Leblue V, Gattone V, Jr, Kalluri R: Integrin β1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 548–593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachs N, Kreft M, van den Bergh Weerman M, Beynon A, Peters T, Weening J, Sonnenberg A: Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young MT, Tanner MJ: Distinct regions of human glycophorin A enhance human red cell anion exchanger (band 3; AE1) transport function and surface trafficking. J Biol Chem 278: 32954–32961, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Groves JD, Tanner MJ: Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J Biol Chem 267: 22163–22170, 1992 [PubMed] [Google Scholar]
- 38. Toye AM, Ghosh S, Young MT, Jones GK, Sessions RB, Ramauge M, Leclerc P, Basu J, Delaunay J, Tanner MJ: Protein-4.2 association with band 3 (AE1, SLCA4) in Xenopus oocytes: Effects of three natural protein-4.2 mutations associated with hemolytic anemia. Blood 105: 4088–4095, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Sterling D, Reithmeier R, Casey J: A transport metabolon: Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276: 47886–47894, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Peters L, Shivdasani R, Hanspal M, John K, Gonzlez J, Brugnara C, Gwynn B, Mohandas N, Alper S, Orkin S, Lux S: Anion exchanger 1(Band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 86: 917–927, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Hassoun H, Wang Y, Vassiliadis J, Lutchman M, Palek J, Aish L, Aish IS, Liu S-C, Chishti AH: Targeted inactivation of murine band 3 (AE1) gene produces a hypercoagulable state causing widespread thrombosis in vivo. Blood 92: 1785–1792, 1998 [PubMed] [Google Scholar]
- 42. Stehberger P, Shmukler B, Stuart-Tilley A, Peters L, Alper S, Wagner C: Distal renal tubular acidosis in mice lacking the AE1 (Band3) Cl−/HCO−3 exchanger (slc4a1). J Am Soc Nephrol 18: 1408–1418, 2007 [DOI] [PubMed] [Google Scholar]
- 43. De Paepe M, Trudel M: The transgenic SAD mouse: A model of human sickle cell glomerulopathy. Kidney Int 46: 1337–1345, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Yang B, Kirby S, Lewis J, Detloff P, Maeda N, Smithies O: A mouse model for β0-thalassemia. Proc Natl Acad Sci U S A 92: 11608–11612, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lehtonen S, Zhao F, Lehtonen E: CD2-associated protein directly interacts with the actin cytoskeleton. Am J Physiol Renal Physiol 283: F734–F743, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Fukasawa H, Bornheimer S, Kudlicka K, Farquhar M: Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20: 1491–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin X-S, Tsukaguchi H, Shono A, Yamamoto A, Hidetake K, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cordat E, Li J, Reithmeier R: Carboxyl-terminal truncations of human anion exchanger impair its trafficking to the plasma membrane. Traffic 4: 642–651, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Walsh S, Turner CM, Toye A, Wagner C, Jaeger P, Laing C, Unwin R: Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant 22: 807–812, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Wrong O: Nephrocalcinosis. In: Oxford Textbook of Clinical Nephrology, 2nd Ed., edited by Davison AM, Cameron JS, Grunfeld JP, Kerr DN, Ritz E, Winearel CG, Oxford, Oxford University Press, 1998, pp 1375–1395 [Google Scholar]
- 52. Beckmann R, Toye AM, Smythe JS, Anstee DJ, Tanner MJ: An N-terminal GFP tag does not alter the functional expression to the plasma membrane of red cell and kidney anion exchanger (AE1) in mammalian cells. Mol Membr Biol 19: 187–200, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Saleem M, O'Hare M, Reiser J, Coward R, Inward C, Farren T, Xing C, Ni L, Mathieson P, Mundel P: A conditionally immortalised human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Holzman L, John P, St., Kovari I, Verma R, Holthofer H, Abrahamson D: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Toye AM, Bruce LJ, Unwin RJ, Wrong O, Tanner MJ: Band 3 Walton, a C-terminal deletion associated with distal renal tubular acidosis, is expressed in the red cell membrane but retained internally in kidney cells. Blood 99: 342–347, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Coward RJ, Welsh GI, Koziell A, Hissain S, Lennon R, Ni L, Tavare JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Coward R, Foster R, Patton D, Ni L, Lennon R, Bates D, Harper S, Mathieson P, Saleem M: Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol 16: 629–637, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Holthofer H, Reivinen J, Miettinen A: Nephron segment and cell-type specific expression of gangliosides in the developing and adult kidney. Kidney Int 45: 123–130, 1994 [DOI] [PubMed] [Google Scholar]
- 59. Akel A, Wagner C, Kovacikova J, Kasinathan R, Kiedaisch V, Koka S, Alper S, Bernhardt I, Wieder T, Huber S, Lang F: Enhanced suicidal death of erythrocytes from gene-targeted mice lacking the Cl−/HCO3− exchanger AE1. Am J Physiol 292: 1759–1767, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Lowry O, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 61. Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Seisuke Hattori S: Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem 283: 9177–9186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Graham F, van der Eb A: A new technique for assay of infectivity of human adenovirus 5 DNA. Virology 52: 456–467, 1973 [DOI] [PubMed] [Google Scholar]
- 63. Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstadt H, Hsu H, Sanday J, Satchell SC, Lennon R, Ni L, Bottinger EP, Mundel P, Mathieson PW: The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sabatini DD, Bensch K, Barrnett RJ: Cytochemistry and electron microscopy: The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol 17: 19–58, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coetzee J, van der Merwe CF: Some characteristics of the buffer vehicle in gluteraldehyde-based fixatives. J Microsc 146: 143–155, 1987 [Google Scholar]
- 66. Glauert AM, Rogers GE, Glauert RH: A new embedding medium for electron microscopy. Nature 178: 803, 1956 [DOI] [PubMed] [Google Scholar]
- 67. Stempac JG, Ward RT: An improved staining method for electron microscopy. J Cell Biol 22: 697–701, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reynolds ES: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL: Novel AE1 mutations in recessive distal renal tubular acidosis: Loss-of-function is rescued by glycophorin A. J Clin Invest 102: 2173–2179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.