Abstract
Collagen XVIII is a component of the highly specialized extracellular matrix associated with basement membranes of epithelia and endothelia. In the normal kidney, collagen XVIII is distributed throughout glomerular and tubular basement membranes, mesangial matrix, and Bowman's capsule. Proteolytic cleavage within its C-terminal domain releases the fragment endostatin, which has antiangiogenic properties. Because damage to the glomerular basement membrane (GBM) accompanies immune-mediated renal injury, we investigated the role of collagen XVIII/endostatin in this disorder. We induced anti-GBM glomerulonephritis in collagen XVIII α1-null and wild-type mice and compared the resulting matrix accumulation, inflammation, and capillary rarefaction. Anti-GBM disease upregulated collagen XVIII/endostatin expression within the GBM and Bowman's capsule of wild-type mice. Collagen XVIII/endostatin-deficient mice developed more severe glomerular and tubulointerstitial injury than wild-type mice. Collagen XVIII/endostatin deficiency altered matrix remodeling, enhanced the inflammatory response, and promoted capillary rarefaction and vascular endothelial cell damage, but did not affect endothelial proliferation. Supplementing collagen XVIII-deficient mice with exogenous endostatin did not affect the progression of anti-GBM disease. Taken together, these results suggest that collagen XVIII/endostatin preserves the integrity of the extracellular matrix and capillaries in the kidney, protecting against progressive glomerulonephritis.
The major constituents of all basement membranes (BMs) are predominantly laminins, nidogen/entactin, collagen IV, and heparan sulfate proteoglycans (HSPGs).1,2 HSPGs are a class of biomolecules that consist of a core protein with covalently attached heparan sulfate sugar side chains. HSPGs are involved in biologic processes such as glomerular filtration, cell adhesion, migration, proliferation, and differentiation,3–5 which are mediated by the binding of chemokines, cytokines, enzymes, growth factors, or other bioactive molecules.6 Collagen XVIII (Col 18) is a HSPG associated with BMs of almost all epithelia and endothelia. This collagen contains an N-terminal noncollagenous domain (NC-11), 10 collagenous domains alternating with 9 noncollagenous repeats, and a C-terminal noncollagenous domain (NC-1).7 In the normal kidney, Col 18 is distributed throughout glomerular and tubular BMs, mesangial matrix, and Bowman's capsule in both humans and mice.8,9
Inactivating mutations in the human gene for Col 18, COL18A1, have been identified in patients with Knobloch syndrome, which is an autosomal recessive disorder characterized by the occurrence of vitreoretinal degeneration with retinal detachment, high myopia, macular degeneration, occipital encephalocele, and minor renal abnormalities.10,11 The kidney of Col 18/endostatin-null mice exhibits no abnormalities on light microscopy, where expansion of the mesangial matrix and thickened proximal tubular BM are observed on electron microscopy.7,8 The study of Utriainen et al.8 suggested that Col 18/endostatin may have a role in maintaining the structural integrity of the extracellular matrix in the normal kidney, whereas its role in susceptibility and progression of inflammatory glomerular diseases remains to be clarified.
Proteolytic cleavage within NC-1 of Col 18 releases a fragment termed endostatin, which has been shown to have anti-angiogenesis activity in vitro and in vivo.1,12–16 Endostatin is an endogenous angiogenesis inhibitor and is detected in the circulation at a physiologic level of 20 to 50 ng/ml in serum.1,7,17–19 Col 18/endostatin-null mice display enhanced tumor growth when implanted with tumor cells that are unable to produce Col 18.18 In contrast, overexpression of circulating endostatin in the transgenic mice leads to reduced tumor growth and vascularization.18 Nonetheless, it is still unclear whether endostatin plays a role in renal disease as an endogenous angiogenesis inhibitor.
As a major component of the ultrafiltration apparatus in the kidney, the glomerular basement membrane (GBM) is constantly exposed to serum flow and pressure and thus needs to be functionally sound and to stringently maintain its structural integrity. Mouse anti-GBM glomerulonephritis (GN) is characterized by damage of GBM followed by invasion of inflammatory cells, accumulation of mesangial matrix, and destruction of the glomerular capillary network, finally resulting in the development of glomerular sclerosis.20–22
In this study, we induced anti-GBM disease in Col 18/endostatin-null mice to test the hypothesis that Col 18/endostatin is critical for maintaining the integrity of the GBM and capillaries in the glomerulus. We demonstrate that Col 18/endostatin-deficient mice with anti-GBM disease have altered distribution of extracellular matrix, accelerated inflammatory responses, and more severe endothelial cell (EC) damage compared with wild-type (WT) mice with this disease. These results suggest that Col 18/endostatin may play an important role in preserving the integrity of extracellular matrixes and capillary vessels in the kidneys of patients with immune complex glomerulonephritis.
Results
Col 18/Endostatin Expression Was Upregulated in the GBM and Bowman's Capsule in WT Mice with Anti-GBM Disease
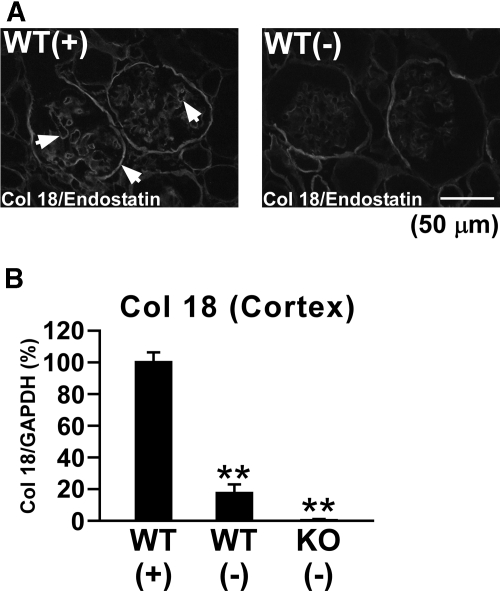
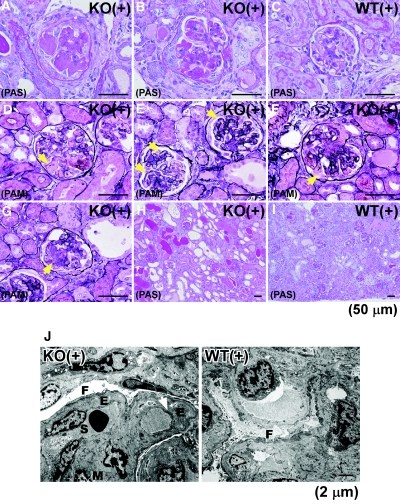
In control Col 18/endostatin-null mice, no renal histologic abnormalities were seen by light microscopy compared with WT mice (Figure 3, A and B). The renal cortex of the control WT mice showed faint Col 18/endostatin staining within glomerular and tubular BMs and Bowman's capsule (Figure 1). Upon induction of anti-GBM GN in WT mice, Col 18/endostatin protein expression was clearly upregulated within the GBM and Bowman's capsule and Col 18 mRNA expression was significantly elevated in the renal cortex on day 6 (Figure 1, A arrows and B).
Figure 3.
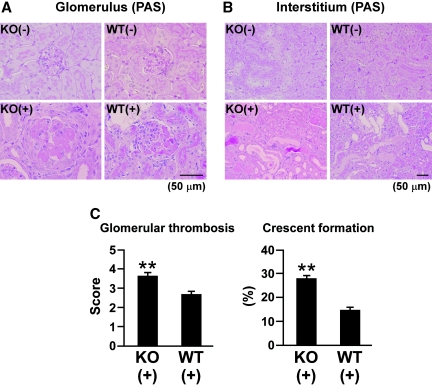
Increased glomerular thrombosis and crescent formation in nephritic Col 18/endostatin-null mice compared to nephritic WT mice. Glomerulus (A) and tubulointerstitium (B) from female control and nephritic Col 18/endostatin-null [KO(−), n = 5; KO(+), n = 8] and WT [WT(−), n = 5; WT(+), n = 10] mice were stained with periodic acid–Schiff reagent. Representative pictures are shown in (A) and (B). The glomeruli were more enlarged with massive thrombosis in the nephritic Col 18/endostatin-null [KO(+)] mice compared with nephritic WT [WT(+)] mice, and degeneration of the tubular epithelium was observed in both groups of mice. Scale bar: 50 μm. (C) Col 18/endostatin deficiency contributed significantly to severe glomerular damage including glomerular thrombosis and crescent formation. The results are shown as the mean ± SEM. **P < 0.01, compared with WT(+) group.
Figure 1.
Col 18/endostatin expression is upregulated predominantly within the GBM in WT mice with anti-GBM disease. (A) Renal sections from female control WT [WT(−), n = 5] and nephritic WT [WT(+), n = 5] mice on day 6 were stained with goat antibody recognizing the C-terminal NC-1 of Col 18 and observed by immunofluorescence microscopy. Nephritic WT [WT(+)] mice were immunized subcutaneously with normal rabbit IgG in complete Freund's adjuvant, followed by intravenous injection of the old NTS. Control WT [WT(−)] mice were immunized subcutaneously with normal rabbit IgG in complete Freund's adjuvant. Representative immunofluorescence staining images are shown in the figure. Scale bar: 50 μm. (B) Expression of Col 18 mRNA was also assessed in the renal cortex of WT(+), WT(−), and female control Col 18/endostatin-null mice [KO(−), n = 5] by real-time RT-PCR. Percent expression to WT(+) group was calculated. The results are shown as the mean ± SEM. **P < 0.01, compared with WT(+) group. Col 18/endostatin protein expression was clearly upregulated within the GBM and Bowman's capsule on day 6 as indicated by the arrows in nephritic WT mice compared with that of control WT mice, which was confirmed by Col 18 mRNA expression.
Col 18/Endostatin-Null Mice Demonstrated Enhanced Renal Injury upon Induction of Anti-GBM Disease
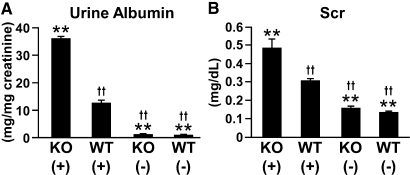
Five days after induction of anti-GBM GN, several Col 18/endostatin-null mice began to die and other mice became very feeble with signs of severe edema and ascites and died within 7 days, whereas all WT mice survived up to day 12. Among the mice with anti-GBM GN, urine protein excretion on day 6 was significantly higher in Col 18/endostatin-null mice than in WT mice (Figure 2A). Renal function deteriorated significantly in nephritic Col 18/endostatin-null mice compared with that in nephritic WT mice as assessed by the blood urea nitrogen (BUN) and serum creatinine (Scr) levels on day 6 (Figure 2B; Supplemental Figure 1). In WT mice, cell proliferation in the glomerulus, glomerular thrombosis, and crescent formation were seen on day 6 after induction of anti-GBM GN (Figure 3, A and C; Supplemental Figure 2A). In nephritic Col 18/endostatin-null mice, the glomeruli were more enlarged (Figure 3A) and the total score of the glomerulus, score of glomerular thrombosis, and crescent formation were significantly higher than those in nephritic WT mice (Figure 3C; Supplemental Figure 2A). However, there were no significant differences in the total score of the interstitium, score of infiltration of mononuclear cells, score of tubular damage, and score of interstitial fibrosis (Figure 3B; Supplemental Figure 2B).
Figure 2.
Urine protein excretion and Scr are significantly higher in Col 18/endostatin-null mice than in WT mice. Urine protein excretion (A) and Scr (B) were estimated in female control and nephritic Col 18/endostatin-null [KO(−), n = 5; KO(+), n = 8] and WT [WT(−), n = 5; WT(+), n = 10] mice. Urine albumin excretion and Scr significantly increased in Col 18/endostatin-null [KO(+)] mice compared with WT [WT(+)] mice during anti-GBM disease. The results are shown as the mean ± SEM. **P < 0.01, compared with WT(+) group. ††P < 0.01, compared with KO(+) group.
Heterologous and Autologous Antibody Responses in Col 18/Endostatin-Null and WT Mice
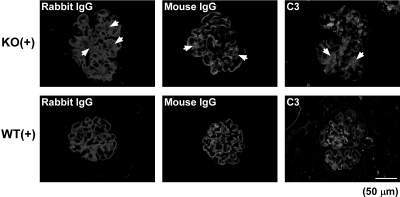
In nephritic Col 18/endostatin-null and WT mice, similar amounts of heterologous and autologous antibodies were deposited on the GBM on day 6, as semiquantitatively assessed by the binding of rabbit anti-GBM antibody and mouse IgG, respectively (Figure 4). In addition, there was no obvious difference in the amount of complement deposited along the GBM on day 6, as semiquantitatively assessed by glomerular C3 staining, between Col 18/endostatin-null and WT mice (Figure 4). Deposition of rabbit and mouse IgG and C3 was detected in the mesangial area of glomeruli of nephritic Col 18/endostatin-null mice, but not in nephritic WT mice (Figure 4, arrows). No deposition of rabbit and mouse IgG and C3 was observed in the GBM and mesangium of glomeruli of control Col 18/endostatin-null and WT mice (data not shown).
Figure 4.
Similar amounts of heterologous and autologous antibodies and C3 are deposited in the GBM of nephritic Col 18/endostatin-null and WT mice. Representative immunofluorescence staining for deposition of rabbit IgG, mouse IgG, and C3 on glomeruli of female nephritic Col 18/endostatin-null [KO(+), n = 5] and WT [WT(+), n = 5] mice. Note that deposition was detected on the mesangium of nephritic Col 18/endostatin-null [KO(+)] glomeruli as indicated by the arrows in the figure, but not on nephritic WT [WT(+)] glomeruli. Scale bar: 50 μm.
Glomerular Inflammation Was Augmented in Col 18/Endostatin-Null Mice with Anti-GBM Disease
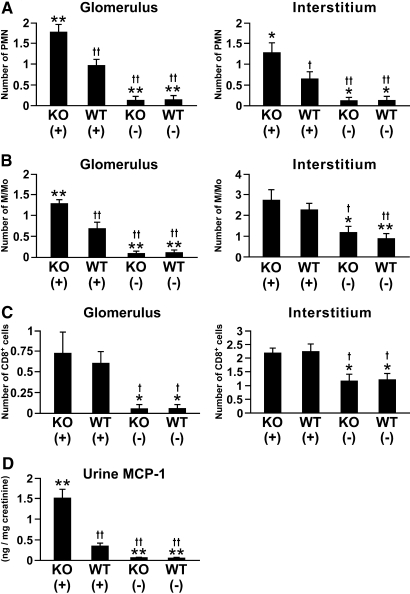
In the renal cortex of WT mice, the influx of polymorphonuclear leukocytes (PMN), macrophages/monocytes (M/Mo), and CD8+ cells significantly increased on day 6 after induction of anti-GBM GN (Figure 5, A through C; Supplemental Figure 3, A through C). The influx of PMN into glomeruli and interstitium and M/Mo into glomeruli were significantly higher in nephritic Col 18/endostatin-null mice than in nephritic WT mice (Figure 5, A and B; Supplemental Figure 3, A and B), whereas similar levels of glomerular and interstitial infiltration of CD8+ cells were observed in the two groups of nephritic mice (Figure 5C; Supplemental Figure 3C). Urine monocyte chemoattractant protein-1 (MCP-1) excretion significantly increased in nephritic Col 18/endostatin-null mice compared with that in nephritic WT mice (Figure 5D). In nephritic Col 18/endostatin-null mice, the elevated influx of M/Mo into the glomeruli was consistent with the increase in renal synthesis of MCP-1.
Figure 5.
Increased influx of PMN into glomeruli and interstitium and M/Mo into glomeruli in nephritic Col 18/endostatin-null mice compared to nephritic WT mice. Frozen kidney sections from female control and nephritic Col 18/endostatin-null [KO(−), n = 5; KO(+), n = 8] and WT [WT(−), n = 5; WT(+), n = 10] mice were stained with antibodies against PMN (A), M/Mo (B), and CD8+ cells (C). The influx of PMN and M/Mo into nephritic Col 18/endostatin-null [KO(+)] glomeruli was significantly increased when compared with that into nephritic WT [WT(+)] glomeruli. The results are shown as the mean ± SEM. (D) Urine MCP-1 concentration was calculated in all groups during anti-GBM nephritis as described in Concise Methods. Each bar represents the mean ± SEM. **P < 0.01, compared with WT(+) group. *P < 0.05, compared with WT(+) group. ††P < 0.01, compared with KO(+) group. †P < 0.05, compared with KO(+) group.
Altered Matrix Deposition Was Observed in Col 18/Endostatin-Null Mice
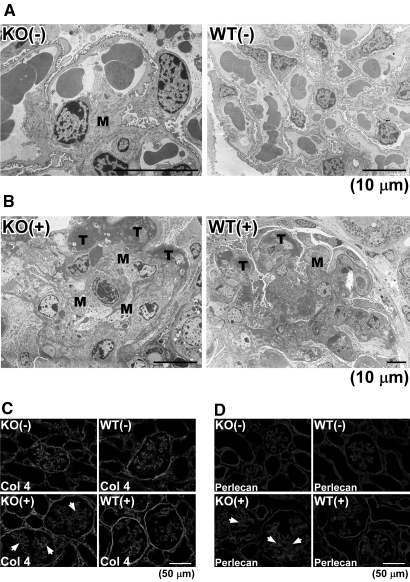
Because Col 18/endostain has also been detected in the mesangial matrix of the normal kidney,8,9 we studied control and nephritic kidneys from Col 18/endostatin-null and WT mice by electron microscopy. Among controls, the mesangial matrix expanded in some of the glomeruli of Col 18/endostatin-null mice compared with that in WT mice, as shown previously (Figure 6A).8 After induction of anti-GBM disease, the mesangial matrix became further expanded and massive fibrinoid thrombi were seen in the capillary loops on day 6 in the glomeruli of Col 18/endostatin-null mice compared with WT mice (Figure 6B). Immunohistochemical analyses showed that deposition of collagen IV and perlecan on the mesangial matrix increased in the glomeruli of nephritic Col 18/endostatin-null mice compared with that in nephritic WT mice (Figure 6, C and D arrows).
Figure 6.
Altered matrix deposition in Col 18/endostatin-null mice. Representative electron micrographs of the glomerulus from female control and nephritic Col 18/endostatin-null [KO(−), n = 5; KO(+), n = 5] and WT [WT(−), n = 5; WT(+), n = 5] mice are shown in (A) and (B). Mesangial expansion (M) was seen in the control Col 18/endostatin-null [KO(−)] glomerulus. Fibrinoid thrombi (T), mesangial expansion (M), and effacement of foot process were observed extensively in the nephritic Col 18/endostatin-null [KO(+)] mice. Scale bar: 10 μm. Deposition of collagen IV (C) and perlecan (D) was observed by immunohistochemistry on the mesangial matrix (arrows) in the nephritic Col 18/endostatin-null [KO(+)] glomerulus. Representative staining is shown in the figure. Scale bar: 50 μm.
Col 18/Endostatin Deficiency Led to Severe Glomerular and Interstitial Capillary Loss
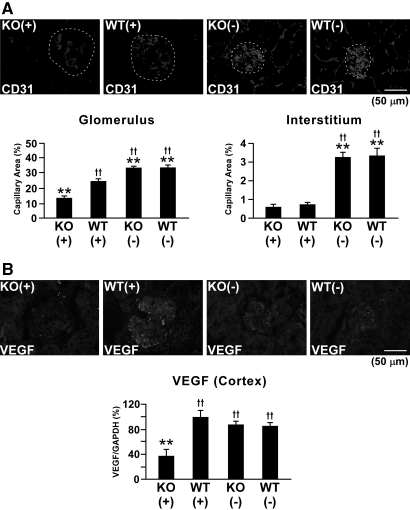
Because endostatin is known to be a potent endogenous angiogenesis inhibitor,7,18,19 we compared the density of glomerular and interstitial capillaries in Col 18/endostatin-null and WT mice. In WT mice, the capillary area as assessed by CD31+ endothelial cells in the glomeruli and interstitium dramatically decreased on day 6 of anti-GBM GN (Figure 7A). Unexpectedly, Col 18/endostatin-null mice with anti-GBM disease showed further progressive loss of glomerular capillaries: severe vascular injury was observed in the glomeruli of Col 18/endostatin-null mice compared with that in WT mice (Figure 7A). In contrast, the density of interstitial capillaries was equal in Col 18/endostatin-null and WT mice on day 6 after the onset of anti-GBM GN (Figure 7A). Whereas vascular endothelial growth factor (VEGF) expression in the glomeruli and cortex of WT mice increased after induction of anti-GBM GN, VEGF expression significantly decreased along with the density of glomerular capillaries in the glomeruli and cortex of Col 18/endostatin-null mice (Figure 7, A and B).
Figure 7.
Decreased capillary density and VEGF expression in the glomeruli of nephritic Col 18/endostatin-null mice. (A) Capillary density in the female control and nephritic Col 18/endostatin-null [KO(−), n = 5; KO(+), n = 8] and WT [WT(−), n = 5; WT(+), n = 10] mice was assessed by the staining of CD31+ endothelial cells. Nephritic Col 18/endostatin-null [KO(+)] mice showed progressive loss of glomerular capillaries compared with nephritic WT [WT(+)] mice. Representative staining is shown and the glomerulus is enclosed by the dotted line in the figure. Scale bar: 50 μm. The results from semiquantitative analysis are shown as the mean ± SEM. (B) The protein (upper panel) and mRNA (lower panel) expression of VEGF in female nephritic Col 18/endostatin-null [KO(+)] kidney, as assessed by immunohistochemistry and real-time RT-PCR, respectively, was significantly lower than that in nephritic WT [WT(+)] kidney, corresponding with the results on the density of glomerular capillaries. Percentage of expression to WT(+) group was calculated and indicated in the lower panel. The results are shown as the mean ± SEM. Scale bar: 50 μm. **P < 0.01, compared with WT(+) group. ††P < 0.01, compared with KO(+) group.
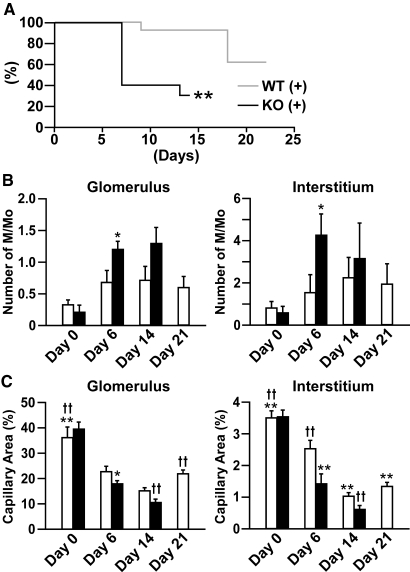
Capillary rarefaction was extensively induced at the late stage in anti-GBM disease models,23,24 and accelerated capillary repair due to the lack of the endogenous angiogenesis inhibitor may be overlooked in the kidney of Col 18/endostatin-null mice. Therefore, we decided to use new rabbit anti-mouse GBM serum (nephrotoxic serum [NTS]), which induces moderate renal injuries. A total of 300 or 500 μl of the new NTS was administered to female and male mice, respectively. On day 6, nephritic WT mice caused by the new NTS had a Scr level (about 0.2 mg/dl) that was about two thirds of that in nephritic WT mice caused by the old NTS and the renal dysfunction progressed on day 14 (Supplemental Figures 4A and 6A). The body weight of WT mice increased on day 6 of anti-GBM GN, after which the weight of WT mice returned to normal on day 21 (Supplemental Figure 7). Capillary rarefaction on days 6 and 14 was followed by capillary repair in the glomerulus and interstitium of WT mice on day 21, which was consistent with the improvement of renal function (Figure 8C; Supplemental Figures 4A and 6A).
Figure 8.
Decrease in survival, increased M/Mo infiltration on day 6 and capillary rarefaction on days 6 and 14 in nephritic Col 18/endostatin-null mice compared with nephritic WT mice. (A) Survival curve depicted a statistically significant decrease in survival in nephritic Col 18/endostatin-null [KO(+)] mice (n = 13, black line) in comparison with that of nephritic WT [WT(+)] mice (n = 20, gray line). All surviving WT(+) were sacrificed on day 21. **P < 0.01, compared with WT(+) group. The influx of M/Mo (B) and capillary density (C) were assessed in the glomerulus and interstitium of female control WT (n = 4, day 0) and Col 18/endostatin-null (n = 4, day 0) mice and nephritic WT [WT(+), white bar] and Col 18/endostatin-null [KO(+), black bar] mice on day 6 [WT(+), n = 4; KO(+), n = 4], day 14 [WT(+), n = 4; KO(+), n = 3], and day 21 [WT(+), n = 4] as described in Concise Methods. Each bar represents the mean ± SEM. **P < 0.01, compared with WT(+) on day 6 group. *P < 0.05, compared with WT(+) on day 6 group. ††P < 0.01, compared with WT(+) on day 14 group.
Although there was no obvious difference in the amount of rabbit and mouse IgG and C3 deposited along the GBM on day 6 between nephritic Col 18/endostatin-null and WT mice, the Col 18/endostatin-null mice caused by the new NTS began to die on day 7 and almost all Col 18/endostatin-null mice died within 14 days (Figure 8A; Supplemental Figure 6B; and data not shown). The body weight of nephritic Col 18/endostatin-null mice was significantly reduced on day 14 (Supplemental Figure 7). Concomitant with the above study with the old NTS, the crescent formation and M/Mo infiltration increased and the density of capillaries decreased on days 6 and 14 in the glomeruli of Col 18/endostatin-null mice compared with WT mice. Severe tubulointerstitial damage, increased M/Mo infiltration on day 6, tubular damage with cast formation and capillary rarefaction on days 6 and 14, and interstitial fibrosis on day 14 were observed in nephritic Col 18/endostatin-null mice compared with that in nephritic WT mice (Figure 8, B and C; Figure 9; Supplemental Figure 4, B and C; and data not shown). Nephritic Col 18/endostatin-heterozygous mice showed a moderate Scr level between the Scr level in nephritic Col 18/endostatin-null mice and that in WT mice and fewer total scores than those of Col 18/endostatin-null mice on day 14, suggesting that the progression of anti-GBM disease was dependent on the dosage of COL18A1 gene (Supplemental Figure 4, A through D). Nephritic Col 18/endostatin-null mice, compared with nephritic WT mice, displayed damage of the central area of the liver related to abnormal hepatic circulation, proliferation of lymphocytes within the splenic red pulp, and influx of PMN into alveolar septa, whereas there were no differences in the heart, testis, and degree of elevation of serum liver enzymes between nephritic Col 18/endostatin-null and WT mice (Supplemental Figure 8; and data not shown).
Figure 9.
Nephritic Col 18/endostatin-null mice on day 14 exhibit severe EC damage as well as necrotizing lesions, cellular crescent formation, and rupture of the GBM in the glomeruli. (A through I) Glomerulus and tubulointerstitium from female nephritic Col 18/endostatin-null [KO(+), n = 3] and WT [WT(+), n = 7] mice on day 14 were stained with periodic acid–Schiff and periodic acid–methenamine silver reagents. Necrotizing lesions (A), thrombosis, cellular crescent formation (B), detachment of ECs (D), thickening of capillary walls (E), rupture of the GBM (F), and mesangial cell proliferation (G) were extensively observed in the glomeruli from KO(+), compared with those in WT(+) (C). (H and I) Degeneration of the tubular epithelium with cast formation was extensively observed in KO(+). Representative pictures are shown. Scale bar: 50 μm. (J) In KO(+) glomeruli, narrowing and loss of capillary lumens were evident with swelling of ECs (S) and loss of fenestra (arrow), and the subendothelial space was expanded with thickening of the GBM (E). Mesangial matrix also accumulated (M) and diffuse effacement of foot process in podocytes was noted (F). In contrast, in WT(+) glomeruli the glomerular capillaries were well preserved and ECs were present along the GBM, whereas mild swelling of ECs was noted with well-preserved fenestra of ECs. Focal effacement of foot process in podocytes was also noted (F). Scale bar: 2 μm.
In addition, nephritic Col 18/endostatin-null mice on day 14 exhibited severe EC damage, detachment and disappearance of ECs, and thickening of capillary walls with prominent double outlines in the glomeruli, and also necrotizing lesions, thrombosis, cellular crescent formation, and rupture of the GBM, some of which were also detected in the glomeruli of WT mice (Figures 9, A through I). These results were confirmed by electron microscopy. Narrowing and loss of capillary lumens were evident with EC swelling and loss of fenestra, and the subendothelial space was expanded with thickening of the GBM in nephritic Col 18/endostatin-null mice (Figure 9J).
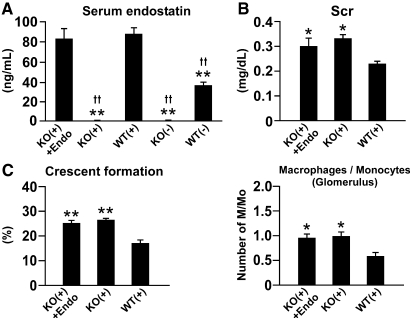
We also administered mouse endostatin protein or peptide (mP1) to nephritic Col 18/endostatin-null mice to study the role of endogenous circulating endostatin on the progression of anti-GBM GN. The antiangiogenic effects of the endostatin protein and mP1 were demonstrated in the growth factor–supplemented Matrigel plug assay (Supplemental Figure 9). Supplementation with the protein and peptide did not affect the Scr level nor the total scores, although the serum endostatin concentration in treated Col 18/endostatin-null mice was similar to that in nontreated nephritic WT mice (Figure 10, A through C; Supplemental Figures 5 and 10). No positive staining of Col 18/endostatin was detected within the renal section of treated Col 18/endostatin-null mice by immunohistochemical staining (data not shown). These results suggest that circulating soluble endostatin has little effect on the progression of anti-GBM disease.
Figure 10.
Intravenous administration of mouse endostatin protein has no effect on the progression of anti-GBM disease. Mouse endostatin protein or saline as a control was intravenously injected into male Col 18/endostatin-null mice during anti-GBM GN (Exp II) for 8 days. (A) Analysis of circulating endostatin levels in the sera of nephritic WT [WT(+), n = 6], Col 18/endostatin-null [KO(+), n = 4], and endostatin-supplemented KO(+) [KO(+) + Endo, n = 5] mice and control WT [WT(−), n = 6] and KO [KO(−), n = 4] mice on day 6. Endostatin was not detected in the sera of KO(+) mice, whereas KO(+) + Endo mice had a similar circulating endostatin level to that in WT(+) mice. Each bar represents the mean ± SEM. **P < 0.01, compared with WT(+) group. ††P < 0.01, compared with KO(+) + Endo group. (B and C) Scr, the percentage of crescent formation, and the influx of M/Mo in the glomerulus were evaluated. There were no differences in Scr, the percentage of crescent formation, nor the influx of M/Mo between KO(+) and KO(+) + Endo mice. Each bar represents the mean ± SEM. **P < 0.01, compared with WT(+) group. *P < 0.05, compared with WT(+) group.
Discussion
In this study, we focused on Col 18/endostatin because its antiangiogenic properties have been extensively characterized.1,12–16,19,25–27 Whereas several studies on tumor progression, atherosclerosis, cutaneous wound healing, and visual function have demonstrated that endogenous Col 18/endostatin is a potent factor in the inhibition of angiogenesis and in matrix remodeling,7,8,10,18,28,29 little attention has been paid to its potential function in a model of inflammatory renal disease.
The first major finding of this study was that Col 18/endostatin expression in the GBM and Bowman's capsule is upregulated during anti-GBM GN and that its absence in Col 18/endostatin-null mice enhanced inflammation, matrix accumulation, and capillary rarefaction in the injured kidney. These results suggest that increased deposition of Col 18/endostatin in the GBM and Bowman's capsule may be associated with protective effects against renal disease.
Col 18/endostatin deficiency led to increased glomerular influx of PMN and M/Mo, but not CD8+ cells, demonstrating that Col 18/endostatin deficiency could, by itself, accelerate the progression of anti-GBM disease at the early stage.5,30–33 In nephritic Col 18/endostatin-null mice, deposition of heterologous and antologous antibodies, C3, collagen IV, and perlecan was observed in the mesangium, where additional immunological responses including influx of PMN and M/Mo into the glomeruli may enhance early progression of anti-GBM disease. Col 18/endostatin may have an anchoring function that prevents loosening of the GBM; that is, it may serve as a scaffold on which other BM proteins interact. Previous studies demonstrated that endostatin bound to other BM components such as perlecan and laminin.7–9,34 Lack of or altered interaction between endostatin and laminin has also been suggested to contribute to the other phenotypes of Col 18/endostatin-null mice and patients with Knobloch syndrome.7,35 Lack of interaction between endostatin and perlecan may contribute to the augmentation of renal damage in nephritic Col 18/endostatin-null mice because mutations in human perlecan were related to renal abnormalities including thickening of glomerular and tubular BMs and expansion of mesangium,36 which were shown in Col 18/endostatin-null mice. However, the precise role of Col 18/endostatin in the human kidney remains unclear.
MCP-1 has been demonstrated to be the dominant chemokine involved in M/Mo recruitment in anti-GBM disease and the binding properties of MCP-1 to HSPGs have been well defined in several reports.21,24,31,37 In particular, Col 18 plays a role in enhancing renal inflammation because Col 18 bound to locally produced MCP-1 and presented it to infiltrated monocytes, leading to their adhesion to vascular cell adhesion molecule-1.37–39 However, the amount of MCP-1 and influx of M/Mo in the kidney were greater in Col 18/endostatin-null mice than in WT mice during anti-GBM nephritis. The reason remains unclear, but a deficiency in Col 18 may be compensated by other HSPGs such as agrin and perlecan,37 and additional inflammatory responses in the mesangium may overcome a decrease in renal MCP-1 likely influenced by Col 18-deficiency.5,39
The second major finding of our study was that Col 18/endostatin deficiency augmented the glomerular and interstitial capillary loss caused by anti-GBM disease and that supplementation with endostatin had no effect on the progression of anti-GBM disease. The importance of the microvasculature after renal injury has been demonstrated by several studies.22,40 In particular, the progression of glomerular sclerosis during anti-GBM nephritis was associated with destruction of the glomerular capillary network and subsequently impairment of the repair process.24 In parallel with the inflammatory and matrix-remolding responses, Col 18/endostatin deficiency in mice with anti-GBM disease led to severe damage of the glomerular and interstitial capillaries, and progressive capillary loss without any recovery could be associated with fatal renal failure at the late phase. Indeed, the electron microscopic study on day 14 revealed extensive, severe EC damage in glomeruli of nephritic Col 18/endostatin-null mice. In addition, intravenous or subcutaneous supplementation of endostatin had no effect on the progression of anti-GBM disease, demonstrating that soluble endostatin plays a minimal role as an antiangiogenic factor in the circulation during anti-GBM disease. The endostatin domain at the C-terminus of Col 18 could stabilize BMs by connecting Col 18 to the BM scaffolds through its interaction with perlecan and laminin,7–9,34 thereby preserving the integrity of the GBM and vascular BM, and could protect renal capillaries in the progressive stage of anti-GBM disease. Further studies are ongoing to identify the effect of Col 18 on the function of vascular ECs in anti-GBM disease.
The present study showed that, after induction of anti-GBM GN, VEGF expression increased in nephritic WT glomeruli but not in nephritic Col 18/endostatin-null glomeruli and a decrease in VEGF-mediated EC survival may also lead to enhanced progression of anti-GBM disease. The study of Shimizu et al.24 suggested that systemic administration of VEGF induced glomerular capillary repair in a rat anti-GBM GN model. In normal glomeruli, podocytes express VEGF. In diseased glomeruli, VEGF was released by endothelial cells, activated mesangial cells, and infiltrating leukocytes as well as podocytes.24,40,41 The loss of VEGF expression in Col 18/endostatin-null glomeruli may have been due to loss of or severe injury to the resident cells by necrotizing glomerular destruction and crescent formation. Also, Col 18 affects adhesive properties between ECs and BMs and may play a role in supplying VEGF to glomerular ECs.29,39
In conclusion, the lack of Col 18/endostatin augmented several phenotypic responses in the renal injury of anti-GBM nephritis. The augmentation of renal injury may potentially be mediated by altered distribution of matrix accumulation, enhanced inflammation, and capillary rarefaction. Furthermore, endostatin deficiency did not prevent glomerular and interstitial capillary loss that occurred in this renal disease model. These results indicate that Col 18/endostatin plays an important role in preserving the integrity of the extracellular matrix and capillary vessels in the kidneys and inhibiting the progression of anti-GBM disease.
Concise Methods
Mice and Reagents
Heterozygous Col 18/endostatin-deficient mice were backcrossed to the C57BL/6J mouse strain (Jackson Laboratory, Bar Harbor, ME) for more than 20 generations to produce an inbred C57BL/6J −/− line. The resulting line was maintained by means of Col 18/endostatin −/− mating. The C57BL/6J mice from Jackson Laboratory were used as controls. We paid close attention to making the background of the control WT mice correspond with that of the Col 18/endostatin-null mice because a different background of Col 18/endostatin-null mice exhibited different phenotype such as the emergence of hydrocephalus.7,8 There were no differences in the appearance and life span between Col 18/endostatin-null and WT mice (data not shown). The mouse genotypes were confirmed by PCR as described previously.18 Col 18/endostatin-null mice were originally described by Fukai et al.28 and were a gift from Dr. B. Olsen (Harvard Medical School, Boston, Massachusetts). All mouse studies were reviewed and approved by the Animal Care and Use Committee of Chiba University. Recombinant mouse endostatin protein was purchased from Alpha Diagnostic (San Antonio, TX) and diluted in PBS for use in animal experiments. mP1 (amino acid sequence: H-HTHQDFQPVLHLVALNTPLSGGMRGIR-OH), encompassing the 1-27 amino acid portion of mouse endostatin, was synthesized and purified by HPLC (Sigma, St. Louis, MO).42 The purity of mP1 peptide was more than 95% and it was dissolved in PBS for use.
Induction of Accelerated Anti-GBM Glomerulonephritis in Mice
Rabbit anti-GBM antiserum was prepared as described previously.21 Seven- to nine-week-old, female or male Col 18/endostatin-null, heterozygous and wild-type mice were immunized subcutaneously with 250 μg of normal rabbit IgG in complete Freund's adjuvant, followed by intravenous injection of a total of 120 μl of old NTS in the first experiment (Exp I) and a total of 300 or 500 μl of new NTS in the second experiment (Exp II) 5 and 6 days later. Control mice were immunized subcutaneously with 250 μg of normal rabbit IgG in complete Freund's adjuvant. On day 6 in Exp I and days 6, 14, and 21 after the second injection of NTS in Exp II, serum and urine samples were collected to evaluate renal function, and mice were sacrificed for histologic assessment. As to the administration of recombinant mouse endostatin protein and mP1 peptide, nephritic Col 18/endostatin-null mice were administered mouse endostatin protein (50 μg/kg per intravenous injection, one injection per day), mP1 peptide (3 μg/kg per subcutaneous injection, two injections per day) or saline for 8 days after the first injection of NTS and then sacrificed. In Exp I, three independent experiments were performed and representative data are shown in the figures. In Exp I and Exp II, the number of mice used in each group is indicated in the figure legends.
Hematologic Evaluation and Determination of Albuminuria
The levels of Scr and BUN were determined by standard methods using the following kits: CRTNase-POD (Kainos, Tokyo, Japan) for Scr and Urease-GLDH (Kyowa, Tokyo, Japan) for BUN. Spot urine was collected when mice were sacrificed. Albumin (Sigma, St. Louis, MO) and creatinine (Cayman Chemical, Ann Arbor, MI) concentrations in the urine were estimated using a colorimetric assay according to the manufacturer's recommendations. Urine albumin excretion was estimated as the quotient of urine albumin and urine creatinine.43
Histologic Analysis
The kidney, liver, heart, spleen, lung, and testis were fixed in 10% buffered formalin and embedded in paraffin. The paraffin sections were stained with periodic acid–Schiff, periodic acid–methenamine silver, Masson's trichrome, or hematoxylin and eosin and evaluated by light microscopy. Morphologic changes in the glomeruli or tubulointerstitial area were scored on a scale of 0 to 5: 0, none of the glomeruli or no tubulointerstitial area was affected by morphologic changes; 1, <20%; 2, <40%; 3, <60%; 4, <80%; 5, full. In the analysis of glomeruli, scores were assigned for intraglomerular cell proliferation, glomerular thrombosis, and extracapillary cellular proliferation (crescent formation), and the total score was the sum of the three scores. Crescent formation was quantified by calculation of the percentage of affected glomeruli. In the analysis of interstitium, scores were assigned for infiltration of mononuclear cells, damage of tubules, and interstitial fibrosis, and the total score was the sum of the three scores. Fifty glomeruli per mouse were assessed for glomerular damage and at least 20 separate 200× fields per mouse were assessed for tubulointerstitial damage. The scoring was performed by two independent observers on blinded sections.
Immunohistochemistry
Immunohistochemical staining was performed as described previously.44 Briefly, frozen kidney sections were cut at a thickness of 4 μm and fixed in cold (−20°C) 100% acetone for 3 minutes. For the immunofluorescence study, cryostat sections were stained with Alexa Fluor 568–conjugated goat anti-rabbit IgG (1:200), Alexa Fluor 488–conjugated rat anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA) or rat anti-mouse C3 antibodies (1:50; MP Biomedicals, Solon, OH) for heterologous antibodies, autologous antibodies, and complement, respectively. In addition, the sections were stained with the following primary and Alexa Fluor 488– and Alexa Fluor 568–conjugated secondary antibodies. For primary antibodies, we used goat anti-mouse collagen type IV (1:50; Millipore, Billerica, MA), goat anti-mouse Col 18 (1:25; R&D Systems, Minneapolis, MN) for the C-terminal NC-1 of Col 18, rat anti-mouse CD31 (1:50; BD PharMingen, San Diego, CA) for vascular endothelial cells, goat anti-mouse VEGF antibodies (1:25; R&D Systems, Minneapolis, MN), and rat anti-mouse perlecan (1:50; Fitzgerald Industries, Concord, MA). Vectashield (Vector Laboratories, App Imaging, Burlingame, CA) antifade mounting medium was applied and imaged by immunofluorescence microscopy (BX51; Olympus, Japan). As for the immunohistochemical study, the cryostat sections were quenched with intrinsic peroxidase by H2O2 and incubated with rat anti-mouse neutrophil (clone 7/4) (1:20; Immunotech, Praha, Czech Republic) for PMN, rat anti-mouse CD11b (1:50; BD PharMingen, San Diego, CA) for M/Mo, or rat anti-mouse CD8a antibodies (1:50, BD PharMingen, San Diego, CA) and horseradish peroxidase–conjugated antibodies. For visualization, 3-3′-diaminobenzidine was used as the substrate. Fifty glomeruli per mouse were assessed for glomerular damage and at least 15 separate 200× fields per mouse were assessed for tubulointerstitial changes. In each group, the stained cells were counted and the stained area was quantified using NIH Image J, software for image processing and analysis developed by the National Institutes of Health (http://www.nih.gov), and averaged.
Electron Microscopy
Kidneys from Col 18/endostatin-null and WT mice were minced into 1-mm2 pieces and were fixed in 2.5% glutaraldehyde in 0.05 M sodium phosphate (pH 7.4) overnight followed by postfixation in 1% osmium tetroxide in 0.05 M sodium phosphate (pH 7.4) for 1.5 hours. Subsequently, the tissue were dehydrated in a graded series of ethanol and propylene oxide and embedded in an epoxy resin. Tissue sections were cut at 80-nm thickness and stained with uranyl acetate and lead citrate. Specimens were imaged using an electron microscope (JEM-1010; JEOL, Japan).
Measurement of Urine MCP-1 and Serum Endostatin Concentrations
MCP-1 concentrations in the spot urine were estimated using ELISA according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA). Urine MCP-1 excretion as a marker of glomerular MCP-1 production was estimated as the quotient of urine MCP-1 and urine creatinine. Serum endostatin concentrations were measured as described previously.18
RNA Isolation and Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from frozen renal cortex using RNeasy Mini Kit (Qiagen, Tokyo, Japan). Reverse transcription of 300 ng RNA was performed with PrimeScript RT Reagent Kit (Takara, Shiga, Japan) and cDNA was used as template in RT-PCR using SYBR Premix Ex Taq II (Takara, Shiga, Japan) with gene-specific primers [Col 18; (forward) GTG CCC ATC GTC AAC CTG AA (reverse) AGT TGA CCC TGG GAG CCA GA, VEGF; (forward) CTG GAT ATG TTT GAC TGC TGT GGA (reverse) GTT TCT GGA AGT GAG CCA ATG TG, glyceraldehyde-3-phosphate dehydrogenase (GAPDH); (forward) ATG GGG TGA GGC CGG TGC TG (reverse) CTT GAT GTC ATC ATA CTT GG] on the Mini Opticon RT-PCR system (Bio-Rad, Hercules, CA). Expression of the housekeeping gene GAPDH was measured to quantify gene expression using the relative-expression method with the standards graph.
Matrigel Plug Assay
In vivo Matrigel plug assay was performed as described previously.45 Briefly, Matrigel (BD Biosciences, San Jose, CA) was mixed with 20 units/ml of heparin, 50 ng/ml of VEGF (R&D Systems, Minneapolis, MN), and 0.5 μM mouse endostatin or 10 μM mP1 or PBS. The Matrigel mixture was injected subcutaneously into the back of WT mice. Six days after the injection, all mice were sacrificed. The Matrigel plugs were removed and fixed with 4% paraformaldehyde and 10% formalin. They were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Sections were examined using 30 different fields by light microscopy, and the number of blood vessels at 400× magnification were counted and averaged. All sections were coded and observed by an investigator who was blinded for study protocols.
Statistical Analysis
The number of mice used for each experiment is provided in the figure legends. The significance of differences between two groups was assessed by t test or Mann-Whitney test. Statistical analyses among more than two groups were performed using ANOVA. As needed, additional analysis was carried out using the t test with Bonferroni correction to identify significant differences. Kaplan-Meier curves were used for survival analysis, and the log-rank (Mantel-Cox) test was used to determine statistical significance. P < 0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Dr. Bjorn R. Olsen of Harvard Medical School (Boston, MA) for providing the Col 18 α1-null mice. We are also grateful to Dr. Akira Shimizu from Nippon Medical School for evaluating the histopathological findings, to Mr. Takuya Okamura and Mr. Mutsuo Jinnai from Dokkyo Medical University Koshigaya Hospital for technical assistance with electron microscopy, to Dr. Hiromichi Yoshida from San-ai Memorial Hospital for discussion, and to Mr. Takahiro Aoki, Mr. Hajime Morita, and Mr. Yuki Tokui for technical assistance. R.K. was supported by National Institutes of Health grant DK55001 and research funds of Division of Matrix Biology at Beth Israel Deaconess Medical Center. Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Kalluri R: Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Timpl R: Structure and biological activity of basement membrane proteins. Eur J Biochem 180: 487–502, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Raats CJ, Van Den Born J, Berden JH: Glomerular heparan sulfate alterations: Mechanisms and relevance for proteinuria. Kidney Int 57: 385–400, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Iozzo RV: Matrix proteoglycans: From molecular design to cellular function. Annu Rev Biochem 67: 609–652, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Rops AL, Gotte M, Baselmans MH, van den Hoven MJ, Steenbergen EJ, Lensen JF, Wijnhoven TJ, Cevikbas F, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J: Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int 72: 1204–1215, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Jackson RL, Busch SJ, Cardin AD: Glycosaminoglycans: Molecular properties, protein interactions, and role in physiological processes. Physiol Rev 71: 481–539, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Marneros AG, Olsen BR: Physiological role of collagen XVIII and endostatin. FASEB J 19: 716–728, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T: Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet 13: 2089–2099, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Miosge N, Simniok T, Sprysch P, Herken R: The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem 51: 285–296, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Marneros AG, Keene DR, Hansen U, Fukai N, Moulton K, Goletz PL, Moiseyev G, Pawlyk BS, Halfter W, Dong S, Shibata M, Li T, Crouch RK, Bruckner P, Olsen BR: Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J 23: 89–99, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Passos-Bueno MR, Suzuki OT, Armelin-Correa LM, Sertie AL, Errera FI, Bagatini K, Kok F, Leite KR: Mutations in collagen 18A1 and their relevance to the human phenotype. An Acad Bras Cienc 78: 123–131, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Boehm T, Folkman J, Browder T, O'Reilly MS: Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390: 404–407, 1997 [DOI] [PubMed] [Google Scholar]
- 13. O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Standker L, Schrader M, Kanse SM, Jurgens M, Forssmann WG, Preissner KT: Isolation and characterization of the circulating form of human endostatin. FEBS Lett 420: 129–133, 1997. 9459295 [Google Scholar]
- 15. Marneros AG, Olsen BR: The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol 20: 337–345, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP: Endostatin induces endothelial cell apoptosis. J Biol Chem 274: 11721–11726, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkila P, Rehn M, Sorsa T, Salo T, Pihlajaniemi T: Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res 307: 292–304, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R: Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci U S A 102: 2934–2939, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seppinen L, Sormunen R, Soini Y, Elamaa H, Heljasvaara R, Pihlajaniemi T: Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol 27: 535–546, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ: The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med 4: 575–580, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Park SY, Ueda S, Ohno H, Hamano Y, Tanaka M, Shiratori T, Yamazaki T, Arase H, Arase N, Karasawa A, Sato S, Ledermann B, Kondo Y, Okumura K, Ra C, Saito T: Resistance of Fc receptor-deficient mice to fatal glomerulonephritis. J Clin Invest 102: 1229–1238, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimizu A, Kitamura H, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N: Rare glomerular capillary regeneration and subsequent capillary regression with endothelial cell apoptosis in progressive glomerulonephritis. Am J Pathol 151: 1231–1239, 1997 [PMC free article] [PubMed] [Google Scholar]
- 23. Daniel C, Amann K, Hohenstein B, Bornstein P, Hugo C: Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in experimental glomerulonephritis in mice. J Am Soc Nephrol 18: 788–798, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Shimizu A, Masuda Y, Mori T, Kitamura H, Ishizaki M, Sugisaki Y, Fukuda Y: Vascular endothelial growth factor165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol 15: 2655–2665, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R: Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci U S A 100: 4766–4771, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26. Hanai J, Gloy J, Karumanchi SA, Kale S, Tang J, Hu G, Chan B, Ramchandran R, Jha V, Sukhatme VP, Sokol S: Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol 158: 529–539, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X: Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 110: 1330–1336, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR: Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J 21: 1535–1544, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, Olsen BR: Increased angiogenic response in aortic explants of collagen XVIII/endostatin-null mice. Am J Pathol 165: 415–424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii T, Hamano Y, Ueda S, Akikusa B, Yamasaki S, Ogawa M, Saisho H, Verbeek JS, Taki S, Saito T: Predominant role of FcγRIII in the induction of accelerated nephrotoxic glomerulonephritis. Kidney Int 64: 1406–1416, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Isome M, Fujinaka H, Adhikary LP, Kovalenko P, El-Shemi AG, Yoshida Y, Yaoita E, Takeishi T, Takeya M, Naito M, Suzuki H, Yamamoto T: Important role for macrophages in induction of crescentic anti-GBM glomerulonephritis in WKY rats. Nephrol Dial Transplant 19: 2997–3004, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schrijver G, Bogman MJ, Assmann KJ, de Waal RM, Robben HC, van Gasteren H, Koene RA: Anti-GBM nephritis in the mouse: Role of granulocytes in the heterologous phase. Kidney Int 38: 86–95, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Feith GW, Assmann KJ, Bogman MJ, van Gompel AP, Schalkwijk J, Koene RA: Lack of albuminuria in the early heterologous phase of anti-GBM nephritis in beige mice. Kidney Int 43: 824–827, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Javaherian K, Park SY, Pickl WF, LaMontagne KR, Sjin RT, Gillies S, Lo KM: Laminin modulates morphogenic properties of the collagen XVIII endostatin domain. J Biol Chem 277: 45211–45218, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR: Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum Mol Genet 9: 2051–2058, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Hansen PM, Chowdhury T, Deckert T, Hellgren A, Bain SC, Pociot F: Genetic variation of the heparan sulfate proteoglycan gene (perlecan gene). Association with urinary albumin excretion in IDDM patients. Diabetes 46: 1658–1659, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, Drager AM, Zweegman S, Kessler FL, Beelen RH, Florquin S, Aten J, van den Born J: Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol 170: 1865–1878, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawashima H, Watanabe N, Hirose M, Sun X, Atarashi K, Kimura T, Shikata K, Matsuda M, Ogawa D, Heljasvaara R, Rehn M, Pihlajaniemi T, Miyasaka M: Collagen XVIII, a basement membrane heparan sulfate proteoglycan, interacts with L-selectin and monocyte chemoattractant protein-1. J Biol Chem 278: 13069–13076, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Rops AL, van der Vlag J, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH: Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int 65: 768–785, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ: Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol 147: 1715–1727, 1995 [PMC free article] [PubMed] [Google Scholar]
- 41. Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Tjin Tham Sjin RM, Satchi-Fainaro R, Birsner AE, Ramanujam VM, Folkman J, Javaherian K: A 27-amino-acid synthetic peptide corresponding to the NH2-terminal zinc-binding domain of endostatin is responsible for its antitumor activity. Cancer Res 65: 3656–3663, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R: Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Hamano Y, Grunkemeyer JA, Sudhakar A, Zeisberg M, Cosgrove D, Morello R, Lee B, Sugimoto H, Kalluri R: Determinants of vascular permeability in the kidney glomerulus. J Biol Chem 277: 31154–31162, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R: Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem 275: 21340–21348, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.