Abstract
Belatacept is a first-in-class co-stimulation blocker in development for primary maintenance immunosuppression. A Phase II study comparing belatacept with cyclosporine (CsA) for prevention of acute rejection and protection of renal function in kidney transplant recipients demonstrated similar efficacy and significantly higher measured GFR at 1 year for belatacept, but the incidence of posttransplantation lymphoproliferative disorder was higher. Here, we present the results for the extension of this trial, which aimed to assess long-term safety and efficacy of belatacept. Seventy-eight of 102 patients who were receiving belatacept and the 16 of 26 who were receiving CsA completed the long-term extension period. GFR remained stable in patients who were receiving belatacept for 5 years, and the incidences of death/graft loss or acute rejection were low. The frequencies of serious infections were 16% for belatacept and 27% for CsA, and neoplasms occurred in 12% of each group. No patients who were treated with belatacept and one patient who was treated with CsA developed posttransplantation lymphoproliferative disorder during the follow-up period. Serious gastrointestinal disorders occurred more frequently with belatacept (12% belatacept versus 8% CsA), and serious cardiac disorders occurred more frequently with CsA (2% belatacept versus 12% CsA). Pharmacokinetic analyses showed consistent exposure to belatacept over time. CD86 receptor saturation was higher in patients who were receiving belatacept every 4 weeks (74%) compared with every 8 weeks (56%). In conclusion, this study demonstrated high patient persistence with intravenous belatacept, stable renal function, predictable pharmacokinetics, and good safety with belatacept over 5 years.
Current immunosuppressive therapies for kidney transplants provide excellent 1-year rates of graft and patient survival, but these rates are not maintained long term.1,2 Chronic allograft nephropathy (CAN) and death with a functioning graft as a result of cardiovascular disease are the leading causes of late renal graft loss.3 The nonselectivity of standard immunosuppressive therapies can contribute to nephrotoxicity that enhances graft deterioration over time,4 and other off-target effects can promote or exacerbate cardiovascular disease, which may increase the long-term risk for cardiac death.5 New immunosuppressive therapies with reduced renal and cardiovascular toxicities and good overall safety are therefore needed to improve long-term outcomes.
Belatacept is a first-in-class co-stimulation blocker that binds CD80/CD86 on antigen-presenting cells with high avidity and specificity to prevent T cell activation.6 In a Phase II trial, the rate of clinically suspected biopsy-proven acute rejection (CSBPAR) by 6 and 12 months of maintenance immunosuppression with belatacept was comparable to that with cyclosporine (CsA), and the two agents resulted in similar patient/graft survival at 1 year.7 More patients in the belatacept group received treatment for suspected rejection; however, overall biopsy-proven rejection rates were similar.7 Overall, rates of infections and neoplasms were comparable between belatacept and CsA, although three patients who were on high-dosage belatacept and one patient who was on CsA developed posttransplantation lymphoproliferative disorder (PTLD). There was a trend toward a lower incidence of CAN in the belatacept group, which did not reach statistical significance. Renal function was significantly higher at 1 year in each belatacept group compared with CsA-treated patients by measured (iohexol) GFR, done in 37 to 52% of patients. GFR difference was less pronounced using calculated GFR (Modification of Diet in Renal Disease [MDRD]), done in 69 to 83% of patients.7
Because transplant recipients remain on immunosuppressant therapies for the life of the graft, evaluation of long-term efficacy and safety is critical; therefore, the Phase II trial was extended to understand better the long-term safety and efficacy of belatacept therapy and its pharmacokinetic (PK) and immunogenic profile. This report presents data from the long-term extension (LTE) phase of this study. Because of the small number of patients who were on CsA and participated in the LTE, only limited conclusions can be drawn from direct comparisons between the two arms; therefore, this report focuses primarily on the long-term experience with belatacept.
Results
Patient Disposition
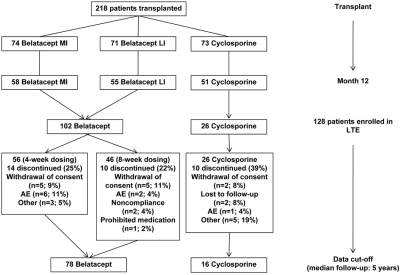
Patient disposition for the original and LTE phases is shown in Figure 1. Overall, 128 patients consented to continue in the LTE phase: 102 (90%) of 113 in the combined belatacept group and 26 (51%) of 51 in the CsA group. These represent the intention-to-treat (ITT) population. Fifty-six belatacept recipients received 4-week dosing, and 46 received 8-week dosing (Figure 1). Seven (7%) belatacept recipients switched to tacrolimus during the study: Five in year 2, one in year 3, and one in year 5. One CsA (4%) recipient switched to tacrolimus in year 4. One (1%) belatacept recipient switched from mycophenolate mofetil (MMF) to sirolimus, and three (3%) belatacept recipients switched from MMF to a combination of MMF (reduced dosage) and sirolimus. Switched patients were included in final analyses.
Figure 1.
High participation and retention of belatacept-treated patients in the LTE.
The data cutoff date occurred 6 years and 7 months after initiation of the original trial. At the time of data cutoff, 76% of LTE belatacept and 62% of LTE CsA recipients remained in the study; all were ≥5 years after transplantation. Twenty-four (24%) patients in the combined belatacept group and 10 (39%) patients in the CsA group withdrew from the LTE (Figure 1). Median follow-up was 5 years after transplantation (range 1 to 7 years).
Patient Demographics
Demographics and pretransplantation disease characteristics were similar among patients who did or did not enter the LTE and similar to patients in the original trial7; however, patients who entered the LTE were a self-selected population who did particularly well during the first phase of the study. The average GFR at LTE entry was higher than in patients who did not enter the LTE: 75.8 versus 69.5 ml/min per 1.73 m2 for belatacept and 74.4 versus 67.4 ml/min per 1.73 m2 for CsA.
Efficacy
Renal Function
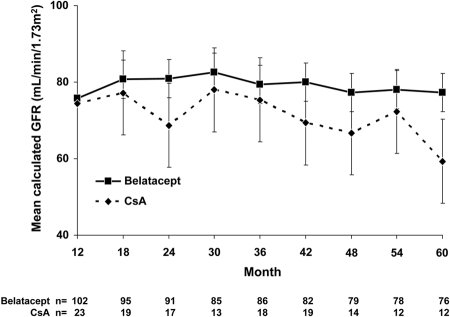
Renal function was stable in belatacept patients over the 5-year period. Average calculated GFR was 75.8 ± 20.1 ml/min per 1.73 m2 in LTE belatacept recipients at 12 months after transplantation and 77.2 ± 22.7 ml/min per 1.73 m2 in patients who reached 60 months after transplantation. No substantive differences in GFR were observed between patients on 4- or 8-week dosing (data not shown). In the CsA group, GFR decreased from 74.4 ± 23.7 ml/min per 1.73 m2 at 12 months after transplantation to 59.3 ± 15.3 ml/min per 1.73 m2 at month 60 (Figure 2).
Figure 2.
Stable renal function in belatacept-treated patients over time. Calculated GFR (based on the MDRD formula) was assessed at 6-month intervals in treated patients who reached the indicated time points. Data are means ± SD.
Death/Graft Loss
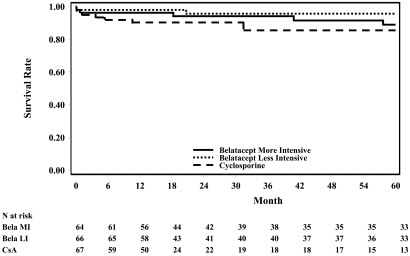
Kaplan-Meier curves for graft loss or death in the ITT population without cancer (skin cancer, organ cancer, and PTLD) are shown in Figure 3. Three (3%) belatacept patients died (two with a functioning graft, one after graft loss), and one (1%) patient had graft loss only. Causes of death in belatacept recipients were myocardial infarction, cardiopulmonary arrest, and pneumonia, and occurred in years 2, 4, and 5 after transplantation, respectively. Graft loss was caused by CAN in one belatacept patient, but the cause was unspecified in the patient who died after graft loss. Two (8%) deaths with a functioning graft occurred in the CsA group; causes were pneumonia and PTLD in years 3 and 5 after transplantation, respectively. No CsA recipients had graft loss.
Figure 3.
Low occurrence of death or graft loss in belatacept-treated patients over time. The Kaplan-Meier plot is for all randomly assigned and transplant recipients in the ITT population without cancer over 60 months. Meddra 11.0 was used for skin and organ cancer; Meddra 10.0 was used for PTLD. Patients with events of cancer including skin, organ cancer, or PTLD were excluded.
Acute Rejection
Six (6%) cases of biopsy-proven acute rejection (BPAR) occurred in the belatacept group. Two (4%) cases occurred in patients who were on 4-week dosing during year 2, and four (9%) cases occurred in patients who were on 8-week dosing in years 2, 3, 4, and 5 (one case each). BPAR cases included two patients with CSBPAR, both on 8-week dosing, in years 4 and 5. All BPAR was of mild or moderate severity: Banff grade IB (n = 1) or IIA (n = 5). Treated acute rejection (AR) was reported for 11 (11%) belatacept recipients: four (7%) patients on 4-week dosing and seven (15%) patients on 8-week dosing. There were no BPAR cases in the CsA group; however, three (12%) patients were treated for AR. Overall, episodes of BPAR, CSBPAR, and treated AR were uncommon in the LTE. No BPAR events in the belatacept group were associated with graft loss.
Cardiovascular Risk Factors
BP remained stable over time in the belatacept group (Table 1). At 60 months after transplantation, 86% of patients in the belatacept group and 89% in the CsA group were taking antihypertensive medications. Both treatment groups had a decline in non-HDL cholesterol during the LTE. Lipid-lowering medications were required by 52% of belatacept recipients and 72% of CsA recipients at month 60. Nine (10%) patients in the belatacept group and two (9%) in the CsA group developed new-onset diabetes after transplantation (NODAT) during the LTE (Table 1).
Table 1.
Cardiovascular risk factors in the LTE
| Parameter | 24 Months | 36 Months | 48 Months | 60 Months |
|---|---|---|---|---|
| Systolic BP (mmHg; mean [SD]) | ||||
| belatacept | 129 (15.3) | 129 (13.4) | 126 (15.6) | 125 (13.9) |
| CsA | 132 (20.2) | 129 (8.9) | 140 (21.0) | 138 (18.9) |
| Diastolic BP (mmHg; mean [SD]) | ||||
| belatacept | 76 (10.5) | 76 (9.5) | 76 (9.9) | 76 (10.1) |
| CsA | 78 (9.3) | 76 (7.5) | 77 (11.9) | 83 (8.9) |
| Non-HDL cholesterol (mg/dl; mean [SD]) | ||||
| belatacept | 150 (35.7) | 144 (37.5) | 138 (38.8) | 128 (37.3) |
| CsA | 140 (44.9) | 130 (31.7) | 131 (38.6) | 119 (29.5) |
| NODAT (n [%])a | ||||
| belatacept | 7 (7) | 8 (9) | 8 (9) | 9 (10) |
| CsA | 2 (9) | 2 (9) | 2 (9) | 2 (9) |
aNew cases that occurred during the LTE (cumulative over time) in patients without pretransplantation diabetes: n = 94 for belatacept; n = 22 for CsA.
Adverse Events and Serious Adverse Events
During the LTE, 90% of belatacept and 92% of CsA recipients experienced adverse events (AEs). The most common AEs were nasopharyngitis, urinary tract infection (UTI), diarrhea, and upper respiratory tract infection, each occurring in approximately 30% of belatacept recipients and in 27 to 35% of CsA recipients (Table 2). Forty-six percent of belatacept recipients and 54% of CsA recipients experienced serious AEs (SAEs). The most common SAEs with belatacept were UTI, basal cell carcinoma, and diarrhea, each occurring in 4% of patients. The most common SAEs with CsA were UTI and coronary artery stenosis, occurring in 12 and 8% of patients, respectively (Table 3). There was a low occurrence of serious cardiac disorders with belatacept in the LTE (2%), with cardiac failure and cardiopulmonary arrest occurring in one (1%) patient each. A total of three (12%) CsA recipients had serious cardiac disorders, including two events of coronary artery stenosis and one each of coronary artery disease, myocardial infarction, and atrial fibrillation.
Table 2.
Frequencies and incidence rates of AEs that occurred in ≥10% of patients in any group
| Parameter | Belatacept |
CsA |
||
|---|---|---|---|---|
| % (n = 102) | Incidence Rate/100 patient-years (95% CI) | % (n = 26) | Incidence Rate/100 patient-years (95% CI) | |
| Nasopharyngitis | 30 | 10.0 (6.8 to 14.3) | 27 | 9.0 (3.6 to 18.6) |
| UTI | 30 | 9.8 (6.7 to 13.9) | 31 | 10.7 (4.6 to 21.2) |
| Diarrhea | 30 | 9.7 (6.6 to 13.8) | 35 | 10.5 (4.8 to 20.0) |
| Upper respiratory tract infection | 29 | 9.9 (6.7 to 14.2) | 31 | 10.5 (4.5 to 20.8) |
| Arthralgia | 21 | 6.0 (3.7 to 9.1) | 15 | 4.6 (1.3 to 11.8) |
| Peripheral edema | 18 | 5.1 (3.0 to 8.0) | 15 | 4.3 (1.2 to 11.0) |
| Cough | 17 | 4.8 (2.8 to 7.6) | 12 | 3.3 (0.7 to 9.5) |
| Pyrexia | 16 | 4.3 (2.5 to 7.1) | 8 | 2.1 (0.3 to 7.5) |
| Nausea | 15 | 4.2 (2.4 to 6.9) | 15 | 4.4 (1.2 to 11.3) |
| Pain in extremity | 14 | 4.0 (2.2 to 6.6) | 15 | 4.8 (1.3 to 12.2) |
| Headache | 14 | 3.9 (2.1 to 6.6) | 12 | 3.5 (0.7 to 10.1) |
| Bronchitis | 14 | 3.9 (2.1 to 6.5) | 8 | 2.1 (0.3 to 7.5) |
| Hypertension | 12 | 3.3 (1.7 to 5.7) | 23 | 7.9 (2.9 to 17.2) |
| Influenza | 12 | 3.2 (1.6 to 5.5) | 4 | 1.0 (0.0 to 5.8) |
| Back pain | 11 | 3.0 (1.5 to 5.3) | 15 | 4.5 (1.2 to 11.5) |
| Vomiting | 11 | 3.0 (1.5 to 5.3) | 12 | 3.1 (0.6 to 9.2) |
| Hyperlipidemia | 11 | 3.0 (1.5 to 5.3) | 4 | 1.1 (0.0 to 5.9) |
| Osteopenia | 7 | 1.9 (0.7 to 3.8) | 12 | 3.4 (0.7 to 9.8) |
| Hypotension | 6 | 1.6 (0.6 to 3.4) | 12 | 3.2 (0.7 to 9.4) |
| Anemia | 3 | 0.8 (0.2 to 2.2) | 23 | 6.9 (2.5 to 15.0) |
| Influenza-like illness | 3 | 0.8 (0.2 to 2.2) | 12 | 3.3 (0.7 to 9.5) |
| Atrial fibrillation | 0 | 0.0 (0.0 to 0.9) | 12 | 3.1 (0.6 to 9.1) |
CI, confidence interval.
Table 3.
Frequencies and incidence rates of SAEs that occurred in two or more patients in any group by category
| Parameter | Belatacept |
CsA |
||
|---|---|---|---|---|
| % (n = 102) | Incidence Rate/100 patient-years (95% CI) | % (n = 26) | Incidence Rate/100 patient-years (95% CI) | |
| Infections to total | 16 | 4.0 (2.3 to 6.5) | 27 | 7.1 (2.8 to 14.6) |
| UTI | 4 | 1.0 (0.3 to 2.6) | 12 | 3.4 (0.7 to 9.9) |
| pneumonia | 3 | 0.8 (0.2 to 2.2) | 0 | 0.0 (0.0 to 3.7) |
| pyelonephritis | 2 | 0.5 (0.1 to 1.8) | 4 | 1.0 (0.0 to 5.7) |
| gastroenteritis | 2 | 0.5 (0.1 to 1.8) | 4 | 1.1 (0.0 to 5.9) |
| Neoplasms to total | 12 | 3.0 (1.5 to 5.2) | 12 | 3.0 (0.6 to 8.9) |
| basal cell carcinoma | 4 | 1.0 (0.3 to 2.6) | 4 | 1.0 (0.0 to 5.8) |
| squamous cell carcinoma | 2 | 0.5 (0.1 to 1.8) | 4 | 1.0 (0.0 to 5.7) |
| breast cancer | 2 | 0.5 (0.1 to 1.8) | 0 | 0.0 (0.0 to 3.7) |
| Gastrointestinal disorders to total | 12 | 3.0 (1.5 to 5.2) | 8 | 2.0 (0.2 to 7.3) |
| diarrhea | 4 | 1.0 (0.3 to 2.6) | 4 | 1.0 (0.0 to 5.8) |
| abdominal pain | 2 | 0.5 (0.1 to 1.8) | 0 | 0.0 (0.0 to 3.7) |
| pancreatitis | 2 | 0.5 (0.1 to 1.8) | 0 | 0.0 (0.0 to 3.7) |
| General disorders to total | 3 | 0.7 (0.2 to 2.2) | 4 | 1.0 (0.0 to 5.6) |
| pyrexia | 2 | 0.5 (0.1 to 1.8) | 4 | 1.0 (0.0 to 5.8) |
| Cardiac disorders to total | 2 | 0.5 (0.1 to 1.8) | 12 | 3.0 (0.6 to 8.9) |
| coronary artery stenosis | 0 | 0.0 (0.0 to 0.9) | 8 | 2.1 (0.3 to 7.5) |
CI, confidence interval.
Infections of Special Interest
During the LTE, one (1%) case each of cytomegalovirus (CMV) colitis and CMV infection were reported in the belatacept group. One (4%) patient in the CsA group developed CMV infection. Two (2%) belatacept recipients and no CsA recipients developed human BK polyomavirus infection. Herpes was reported in 21 (21%) belatacept recipients and in three (12%) CsA recipients. Two (2%) belatacept and one (4%) CsA recipient experienced multiple herpes infections. In the belatacept group, no herpes cases were serious. Influenza was reported in 12 (12%) belatacept recipients and one (4%) CsA recipient.
Neoplasms
Twelve percent of patients in both the belatacept and CsA groups developed neoplasms in the LTE, most commonly nonmelanoma skin cancer (Table 4). Most neoplasms occurred in years 3 to 5 after transplantation. One LTE CsA recipient developed PTLD in year 4 after transplantation, whereas no belatacept recipient developed PTLD during the LTE (Table 4). In the original study, three cases of PTLD occurred between 3 and 13 months after transplantation in belatacept recipients, all in patients who were receiving the more intensive regimen.7 Taking into account these patients, the overall incidence of PTLD from time of transplantation were three (2.1%) of 143 in the belatacept arms and one (1.4%) of 73 in the CsA arm.
Table 4.
Neoplasms in the LTE
| Neoplasm (n [%]) | Belatacept (n = 102) | CsA (n = 26) |
|---|---|---|
| Nonmelanoma skin cancers | 9 (9)a | 2 (8) |
| Kaposi sarcoma | 1 (1) | 0 (0) |
| Breast cancer | 2 (2) | 0 (0) |
| Prostate cancer | 1 (1) | 0 (0) |
| Malignant melanoma | 1 (1) | 0 (0) |
| PTLD | 0 (0) | 1 (4) |
aOne patient had both squamous cell carcinoma and malignant melanoma, and one patient had squamous cell carcinoma and two occurrences of basal cell carcinoma.
Pharmacokinetics
Preinfusion (trough) blood samples were collected from all belatacept-treated patients at prespecified intervals. The mean concentrations of belatacept in these samples were achieved as predicted by dosage and consistently maintained in both dosing schedules over time (Table 5)
Table 5.
Trough concentrations of belatacept
| 4-Week Schedule |
8-Week Schedule |
||||
|---|---|---|---|---|---|
| Months after Transplantation | n | Trough Concentration (μg/ml) Geometric Mean (CV%) | Months after Transplantation | n | Trough Concentration (μg/ml) Geometric Mean (CV%) |
| 13 | 49 | 3.43 (55) | 13 | 24 | 0.13 (128) |
| 24 | 46 | 3.95 (52) | 25 | 38 | 0.14 (97) |
| 36 | 39 | 4.76 (43) | 37 | 30 | 0.19 (193) |
| 48 | 24 | 4.43 (52) | 49 | 20 | 0.21 (150) |
| 60 | 15 | 4.85 (36) | 59 | 15 | 0.17 (93) |
Patients received a 5-mg/kg infusion 4 or 8 weeks before sample collection. n = number of individual patient samples available for analysis at database lock.
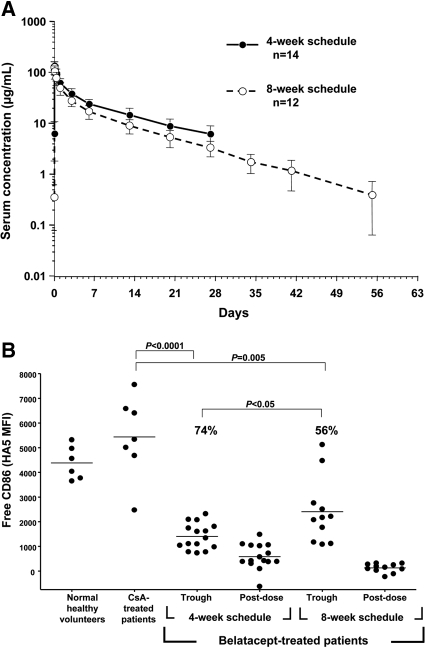
PK studies were performed in a subset of belatacept recipients from both dosing groups in the LTE. After intravenous infusion of 5 mg/kg belatacept, the area under the plasma concentration-time curve from time 0 to the last measurable concentration for patients on the 4-week schedule was slightly higher (1.3-fold) than for patients on the 8-week schedule (Table 6). Mean half-life of belatacept was approximately 8 days. There was a predictable decline in belatacept levels over time with little interpatient variability (Figure 4A).
Table 6.
Results of a pharmacokinetic substudy of the LTE
| PK Parameter | 4-Week Schedule(n = 14) | 8-Week Schedule(n = 12) |
|---|---|---|
| Cmax (μg/ml; geometric mean [CV%]) | 136.3 (20) | 124.6 (27) |
| AUC0–τ (μg · h per ml; geometric mean [CV%]) | 13,587.1 (27) | 10,106.5 (27) |
| AUC∞ (μg · h per ml; geometric mean [CV%]) | NR | 10,219.7 (28) |
| Tmax (h; median [min, max]) | 0.50 (0.48, 2.03) | 0.55 (0.50, 1.92) |
| t1/2 (h; mean [SD]) | NR | 196.6 (57.2) |
| CLT (ml/h per kg; mean [SD]) | NR | 0.51 (0.14) |
| VSS (L/kg; mean [SD]) | NR | 0.12 (0.03) |
| Cmin (μg/ml; mean [SD]) | 6.32 (2.83) | NR |
Steady-state PK parameters were calculated for patients in the 4-week schedule; single-dose PK parameters were calculated for patients in the 8-week schedule. AUC0–τ, area under the plasma concentration-time curve from time 0 to the last measurable concentration; AUC∞, area under the serum concentration-time curve from time 0 extrapolated to infinity; CLT, total body clearance; Cmax, maximum serum concentration; Cmin, trough serum concentration; NR, not reported; t1/2, serum half-life; Tmax, time of maximum observed serum concentration; VSS, volume of distribution at steady state.
Figure 4.
Higher exposure and CD86 receptor saturation with 4-week belatacept dosing. (A) Belatacept serum concentration was measured in subsets of LTE long-term extension patients on 4- and 8-week schedules during one dosing interval (5 mg/kg at 0 hours). Concentration of belatacept is indicated on the y axis (semilog scale). Data are means ± SD. (B) The graph shows the amount of free CD86 (HA5 binding) on monocytes from whole blood. Each dot represents an individual sample; some patients contributed more than one sample.
Anti-belatacept Antibodies
Anti-belatacept antibodies were detected in six (12%) patients who were receiving 4-week dosing and in 10 (23%) patients who were receiving 8-week dosing; however, at the time of data cutoff, only eight patients were positive at their last visit (two in the 4-week group and six in the 8-week group). Overall, two belatacept-treated patients (both in the 8-week regimen) developed neutralizing antibodies; both are continuing treatment with belatacept. No seropositive patient, including those who had neutralizing antibodies, died, had graft loss or AR, or experienced serious infusion-related or autoimmune adverse events.
CD86 Receptor Saturation
Trough samples from both belatacept groups showed significantly higher CD86 receptor saturation than negative control samples from CsA-treated patients or normal healthy volunteers, indicating persistent drug activity (Figure 4B); however, trough samples from patients who were receiving 4-week belatacept showed significantly higher saturation of CD86 receptors than samples from patients who were receiving 8-week belatacept (74 versus 56%; P < 0.05).
Discussion
In a Phase II trial versus CsA, belatacept demonstrated comparable rates of AR, a trend toward reduced CAN, significantly higher measured GFR, and excellent patient/graft survival by 12 months.7 This report on the LTE phase demonstrates continued efficacy and safety of belatacept for long-term maintenance immunosuppression. In belatacept-treated patients, renal function remained stable; death, graft loss, and AR were infrequent; and there were low rates of serious infections or malignancies during 5 years median follow-up. Six cases of BPAR were reported during the follow-up period in the belatacept group, but none resulted in death or graft loss.
Although there was a higher incidence of PTLD with belatacept versus CsA during the initial 12-month study, no belatacept recipients developed PTLD during the LTE, and the overall incidence of PTLD from transplantation through 5 years median follow-up was comparable in the two treatment groups; however, pooled data from all Phase II and III studies in the belatacept renal transplant program suggest that the risk for PTLD, particularly central nervous system PTLD, may be increased with belatacept versus CsA.8 PTLD occurrence with belatacept is clustered around the traditional known risk factors of Epstein-Barr virus–negative status, CMV disease, and use of T cell–depleting therapies. Steps can be taken to reduce this risk by avoiding belatacept use in Epstein-Barr virus–negative patients, judicious use of T cell–depleting therapies in the management of rejection, and appropriate CMV prophylaxis.
The LTE study demonstrated high patient persistence with intravenous belatacept therapy over the long term. Most (90%) belatacept recipients chose to enter the LTE, compared with only approximately half of CsA recipients. Furthermore, a higher percentage of belatacept recipients remained in the LTE study at the time of data cutoff: 76 versus 62% for CsA. At the time of data cutoff, all continuing patients were ≥5 years after transplantation, with the longest followed belatacept patients >6.5 years after transplantation. The low rate of discontinuation during long-term treatment suggests that intravenous administration may not be a challenge to persistence.
An important finding in our study was that renal function in belatacept recipients remained stable over 5 years. This is in contrast to the decline of 1 to 3 ml/min per 1.73 m2/yr typically observed with standard-dosage calcineurin inhibitors (CNIs)9,10 and is consistent with the renal benefit seen with belatacept in the original study at 1 year.7 It should be noted that in our study, the GFR at the beginning of the LTE was similar in the belatacept and CsA groups, despite a difference in favor of belatacept at the end of the original study. This likely reflects the self-selected nature of the study population, whereby only patients who did especially well during the first year entered the LTE, and a high proportion (49%) of CsA recipients did not progress into the LTE. Chronic graft dysfunction usually precedes graft failure, and the severity of renal dysfunction and rate of decline are independent predictors of graft loss in renal transplant recipients11,12; therefore, the maintenance of stable renal function during long-term follow-up in the belatacept cohort is likely to predict good outcomes. Our study demonstrated that belatacept had a good long-term efficacy profile overall, with few late rejections, graft losses, or deaths.
There was a trend toward improved cardiovascular risk factors with belatacept in the initial 12-month study.7 In the LTE, BP remained stable, and non-HDL cholesterol levels decreased in belatacept recipients over time. The percentages of patients who required antihypertensive and lipid-lowering medications at 5 years were similar to those reported for CNI-treated patients at 5 years.13 Although the incidence of NODAT was 10% with belatacept, this was similar to the incidence among patients who were taking CsA, and patients in both groups were on maintenance steroid therapy, which is a known risk factor for NODAT.
Belatacept was generally safe over the course of the study. Because of the small numbers of CsA recipients in this study, it is useful to examine the belatacept data in the context of other follow-up studies among CNI-treated patients. Serious infections occurred in 16% of belatacept recipients, comparable to recent reports of 10 to 13% with CNIs at 3 years14; five (5%) belatacept recipients developed malignancies versus 13% reported with CNIs at 5 years;13 and the 2% rate of serious cardiac events with belatacept was within the estimated range of 1 to 5% for major adverse cardiac events after transplantation.15,16 Overall, infection rates with belatacept were typical for long-term renal transplant recipients, with the possible exception of herpes, which occurred at a higher rate than in previous reports in renal transplant recipients: 21 versus 7%.17 UTI was the most common late serious infection, as was reported previously,18 and CMV and BK polyomavirus infection rates with belatacept were similar to published results in CNI-treated patients.19,20
Our study was not powered to detect a difference in outcomes between the 4- and 8-week dosing groups; therefore, safety and efficacy were primarily analyzed using pooled data from both groups. There were no substantive differences in GFR or death/graft loss between the two dosing groups, however, there was a higher incidence of AR with the 8-week dosing. No differences in safety between the two schedules were apparent. The small number of patients in this study preclude accurate determination of which patients could potentially benefit from 8-week dosing. Four-week dosing was chosen for the Phase III clinical trial program with belatacept,21,22 and the results of the LTE support this decision, because the 8-week group had lower trough drug levels, lower saturation of CD86 receptors, and a higher prevalence of anti-belatacept antibodies.
Exposure to belatacept had relatively low interpatient variability. Trough concentrations of belatacept were achieved as predicted by dosage and consistently maintained between years 2 and 5 after transplantation for both dosing groups. The lower trough levels for the 8-week schedule are consistent with the longer dosing interval. These data suggest that therapeutic drug monitoring for belatacept is not necessary from PK perspectives. In total, anti-belatacept antibodies developed in 16 (17%) patients in the LTE, two of whom developed neutralizing antibodies. Although a seroconversion rate of 17% would seem high, this rate accrued over a 5-year period, which, expressed on an annualized basis, would be approximately 3% per year. Furthermore, the antibodies were not truly persistent, because half of the formerly positive patients were no longer positive at their last assessment, suggesting that belatacept is not highly immunogenic. Although no apparent adverse outcomes were associated with the presence of anti-belatacept antibodies, the impact of anti-belatacept antibodies, including neutralizing antibodies, on clinical outcomes needs further assessment in large-scale clinical trials.
Pharmacodynamic (PD) studies showed significant saturation of the CD86 receptor in both 4- and 8-week dosing regimens, suggesting that CD86 receptor binding by belatacept remains effective over many years of administration. On the basis of previously published in vitro data, trough levels achieved with 4-wk dosing (reported in Table 5) would be expected to provide 84 to 85% CD86 receptor saturation, and trough levels achieved with 8-week dosing would be expected to provide 53 to 59%, consistent with what was seen in this study: 74 and 56%, respectively.23 Although belatacept binds to both the CD86 and CD80 receptors, our assessment focused on CD86 receptors because only CD86 is constitutively expressed on monocytes, and saturation of these receptors more closely reflects inhibition of the alloresponse.23
A limitation of this study is that our cohort was a nonrandomized, self-selected group of patients who did particularly well during the first year. LTE enrollees had fewer occurrences of AR in the first year, compared with all patients in the original study. Also, average 1-year GFR was approximately 3 ml/min per 1.73 m2 higher for belatacept recipients and 6 ml/min per 1.73 m2 higher for CsA recipients who entered the LTE than all belatacept or CsA recipients in the original study. Similarly, the LTE portion of this Phase II study was underpowered to detect meaningful differences between the treatment arms, particularly when some patients chose not to continue in the LTE or withdrew during long-term follow-up. This particularly affected the CsA group, resulting in a small comparator arm, and the lengths of follow-up were not identical between the belatacept and CsA groups, limiting meaningful data comparisons. Although our study compared belatacept with CsA, and tacrolimus is now the more widely used CNI for maintenance immunosuppression,24 preliminary data from a randomized study suggest that belatacept may also have a renal function advantage over tacrolimus in the first 6 months after transplantation.25
In summary, this study demonstrates feasibility, persistence, and tolerability of long-term intravenous maintenance immunosuppression with belatacept. In particular, our data show no instance of late PTLD in belatacept groups and no need for therapeutic drug monitoring. Belatacept may provide an efficacious immunosuppressive regimen for kidney transplant recipients, with a good safety profile and stable renal function over the long term.
Concise Methods
Study Design
The original Phase II study was an open-label, partially blinded, randomized, multicenter study initiated in March 2001.7 The LTE began in March 2002, and this report includes data gathered from then until October 2007. The study protocol and amendments were reviewed and approved by the institutional review board/independent ethics committee for each site. The original study has been described in detail elsewhere,7 but, briefly, 218 patients were randomly assigned in equal numbers to receive a more intensive regimen of belatacept, a less intensive regimen of belatacept, or CsA (Neoral; Novartis) for primary maintenance immunosuppression. All patients received induction therapy with basiliximab (Simulect; Novartis) and adjuvant therapy with MMF (CellCept; Roche) and corticosteroids. Belatacept patients were randomly assigned again at 3 or 6 months to receive 5 mg/kg belatacept at either 4- or 8-week intervals for the maintenance phase.
Patients who completed 12 months on the original study arms were eligible for the LTE at the investigators' and patients' discretion. Continuing patients signed a new consent form and remained on the original maintenance treatment arms: CsA or belatacept (5 mg/kg at 4- or 8-week intervals). Treatments were administered in an unblinded manner. Switching from CsA to tacrolimus (Prograf®; Astellas) was permitted at the discretion of the investigator; switched patients were permitted to continue in the study. Patients who did not tolerate MMF could be switched to sirolimus. Corticosteroid weaning and withdrawal was permitted, although all patients continued on corticosteroids; the mean daily dosage ranged from 5.0 to 8.5 mg/d.
Study Objectives
The primary objective of the LTE was to assess the safety and tolerability of long-term belatacept administration in kidney transplant recipients. The secondary objective was to assess the efficacy of belatacept as a long-term maintenance immunosuppressant. PK and PD (CD86 receptor saturation) properties of belatacept and anti-belatacept antibody generation were also investigated.
Patient Population
The original patient population has been described previously.7 Adult recipients of a de novo renal allograft from a non–HLA-identical living or deceased donor were eligible for inclusion in the original study. The main exclusion criterion for participation in the LTE was development of a malignancy during the initial 12-month study.
Safety and Efficacy End Points
All patients were monitored for AEs and SAEs. Key efficacy measures were renal function (calculated GFR using the Modification of Diet in Renal Disease formula), CSBPAR, BPAR, treated AR, and patient and graft survival. CSBPAR was defined as an increase in serum creatinine of >0.5 mg/dl over baseline in the absence of factors that are known to affect renal function, with histologic confirmation by a central pathologist using Banff '97 criteria and blinded to treatment group. BPAR was defined as a histologically confirmed AR by the central pathologist, regardless of the reason for biopsy. Treated AR was defined as treatment for an episode of suspected AR, regardless of whether the protocol-defined suspicion criteria were satisfied, with or without histologic confirmation. Additional efficacy variables included the proportion of patients with hypertension, dyslipidemia, and NODAT. Non-HDL cholesterol was defined as total cholesterol minus HDL cholesterol. NODAT was defined as the need for treatment of hyperglycemia for a total of >4 weeks or hemoglobin A1c >7% in a patient who did not have diabetes before transplantation.
Pharmacokinetics
Blood samples for PK analyses were obtained from belatacept-treated patients before belatacept infusion at prespecified time points. In addition, samples for a more detailed PK substudy were collected from a subset of patients 3 to 5 years after transplantation during one dosing interval as follows: Predose; 0.5 (end of infusion), 2, 8, 24, and 72 hours; and days 7, 14, 21, and 28 for the 4- and 8-week schedules. Samples on days 35, 42, and 56 were also collected for the 8-week schedule. A sensitive, validated, ELISA was used to measure concentrations of belatacept in serum. Steady-state PK parameters were calculated for patients on the 4-week schedule, and single-dose PK parameters were calculated for patients on the 8-week schedule. Individual patient PK parameters of belatacept were derived from serum concentration versus time data using a noncompartmental method, using Kinetica 4.4.1 (Thermo Electron Corp., Philadelphia, PA).
Anti-belatacept Antibodies
Blood samples for anti-belatacept antibody analyses were obtained from belatacept-treated patients before belatacept infusion at least every 12 months. A sensitive, validated, electrochemiluminescence immunoassay was used to detect antibodies directed against the whole molecule or modified CTLA-4 portion of belatacept.
CD86 Receptor Saturation
Whole blood was collected from a subset of belatacept patients 4 to 5 years after transplantation on both 4-week (n = 7) and 8-week (n = 7) dosing. Control samples were collected from CsA-treated patients (n = 7) and normal healthy volunteers (n = 6). For belatacept recipients, samples were collected before infusion (trough) and 30 minutes after infusion (postdose). The CD86 receptor saturation by belatacept on whole-blood CD14+ monocytes was determined using FACS. The degree of saturation was determined by comparing the belatacept-treated patient data and the CsA-treated patient data. The mean fluorescence intensity of HA5 antibody binding was used for this comparison: HA5 competes with belatacept for binding to CD86.23
Data Analysis: Efficacy and Safety
All patients who enrolled in the LTE (defined as the ITT population) were evaluated for efficacy and safety. Patients who switched medications but continued in the study were analyzed on the basis of their original ITT designation. Patients who discontinued the study were not followed for efficacy after discontinuation but were followed for 8 weeks after the last dose for safety. No pretrial statistical power calculation was undertaken for the PK substudy, but the number of patients in each treatment schedule group (n = 16) would provide 90% confidence that the estimate of the geometric mean maximum serum concentration would be within 12% of the true value.
Efficacy and safety data were analyzed for all belatacept-treated patients combined or on the basis of 4- or 8-week dosing and were summarized from entry into the LTE. The study was not designed to perform formal statistical testing for safety and efficacy end points. Death/graft loss and AR data are presented as the number and percentage of patients who met the end point criteria. GFR data were collected every 6 months, and cardiovascular/metabolic parameter data were collected at yearly intervals. GFR and cardiovascular/metabolic parameters were calculated on the basis of the number of patients who had reached the designated time points. No imputation of missing data was performed on any efficacy end point. AEs and SAEs are reported as both frequencies and incidence rates per 100 patient-years of exposure (95% confidence interval). Frequencies were used to facilitate comparison with historical data but may be less accurate because of the small numbers and different lengths of follow-up in this study. Incidence rates were undertaken to normalize for the different lengths of follow-up and may be more precise estimates of event occurrence. PK and PD data were analyzed on the basis of 4- and 8-week dosing schedules. PD data were analyzed by t test.
Disclosures
F.V. has performed clinical trial research for Roche Pharmaceuticals, Bristol-Myers Squibb, Genentech, Genzyme, and Pfizer and has received a research grant from Novartis; P.F. was a consultant for Bristol-Myers Squibb, has collected honoraria payments from Wyeth and Organ Recovery Systems, and has attended meetings with support from Astellas and Wyeth; P.F.H. has been an ad hoc consultant for Bristol-Myers Squibb, has conducted collaborative research for Stromedix, and has been as consultant for an Astellas advisory board; C.P.L. was a consultant for Bristol-Myers Squibb; B.N. has served on the advisory boards for Pfizer and Bristol-Myers Squibb and has served on both the advisory board and the Speaker's Bureau for Novartis; J.-P.S. is a founder and a stockholder for TC Land Expression; Y.V. has collected speaker's fees from VitroPharma and has accepted a travel grant from an Astellas advisory board; T.W. has collected speaker's fees from Astellas and Wyeth and has received research support from Bristol-Myers Squibb; M.A., S.G., J.S., R.S., and R.T. are employees of Bristol-Myers Squibb.
Acknowledgments
Selected data in this manuscript were previously presented at the annual meeting of the American College of Clinical Pharmacology; September 9 to 11, 2007; San Francisco, CA; and the XXII International Congress of the Transplantation Society; August 10 to 14, 2008; Sydney, Australia (Clin Pharmacol 47(9): 1203, 2007; Muelbacher F, Charpentier B, Larsen C, Agarwal M, Vincenti F: Long-term safety of belatacept: 5 yr results of a Phase II study [Abstract]. Presented at the XXII International Congress of the Transplantation Society; August 10 to 14, 2008; Sydney, Australia).
We acknowledge Britt Anderson, PhD, Chameleon Communications International, for medical writing services funded by Bristol-Myers Squibb.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. The United States Renal Data System 2008 Annual Data Report. Available at: http://www.usrds.org/research_2008.htm Accessed July 15, 2009 [DOI] [PubMed]
- 2. Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB: Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 346: 580–590, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RD: Calcineurin inhibitor nephrotoxicity: Longitudinal assessment by protocol histology. Transplantation 78: 557–565, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Miller LW: Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2: 807–818, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobertm E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ: Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 5: 443–453, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B, Belatacept Study Group : Costimulation blockade with belatacept in renal transplantation. N Engl J Med 353: 770–781, 2005 [DOI] [PubMed] [Google Scholar]
- 8.. Grinyó J, Larsen C, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti FG, Di Russo G, Shi R: One year safety profile of belatacept in kidney transplant patients (BENEFIT and BENEFIT-EXT). Poster presented at the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA [Google Scholar]
- 9. Gill JS, Tonelli M, Mix CH, Pereira BJ: The change in allograft function among long-term kidney transplant recipients. J Am Soc Nephrol 14: 1636–1642, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, Gherardi G, Gotti E, Segoloni G, Salvadore M, Rigotti P, Valente U, Donati D, Sandrini S, Sparacino V, Remuzzi G, Perico N: MY S.S. Study Investigators: Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant 4: 1826–1835, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Marcén R, Pascual J, Tenorio M, Ocaña EJ, Teruel JL, Villafruela JJ, Fernández M, Burgos FJ, Ortuño J: Chronic kidney disease in renal transplant recipients. Transplant Proc 37: 3718–3720, 2005 [DOI] [PubMed] [Google Scholar]
- 12. de Bruijne MH, Sijpkens YW, Paul LC, Westendrop RG, van Houwelingen AC, Zwinderman AH: Predicting kidney graft failure using time-dependent renal function covariates. J Clin Epidemiol 56: 448–455, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J: A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation 73: 775–782, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P: Calcineurin inhibitor minimization in the Symphony Study: Observational results 3 years after transplantation. Am J Transplant 9: 1876, 2009 [DOI] [PubMed] [Google Scholar]
- 15. de Mattos AM, Prather J, Olyaei AJ, Shibagaki Y, Keith DS, Mori M, Norman DJ, Becker T: Cardiovascular events following renal transplantation: Role of traditional and transplant-specific risk factors. Kidney Int 70: 757–764, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LY: Early renal insufficiency and hospitalized heart disease after renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol 14: 2358–2365, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK: Herpes zoster infection following solid organ transplantation: Incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant 4: 108–115, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Abbott KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, Barbour G, Lipnick R, Cruess DF: Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis 44: 353–362, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Becker BN, Becker YT, Leverson GE, Simmons WD, Sollinger HW, Pirsch JD: Reassessing the impact of cytomegalovirus infection in kidney and kidney-pancreas transplantation. Am J Kidney Dis 39: 1088–1095, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Bonvoisin C, Weekers L, Xhignesse P, Grosch S, Milicevic M, Krzesinski JM: Polyomavirus in renal transplantation: a hot problem. Transplantation 85 [Suppl]:S42–S48, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Vincenti F, Charpentier B, Vanrenterghan Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin C-S, Garg P, Larsen CP: A phase III study of belatacept-based immunosuppression regimens vs cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 10: 535–546, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Durrbach, Pestana JM, Pearson T, Vincenti F, Duro Gardia V, Campistol J, del Carmen Rial M, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyó J: A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 10: 547–557, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Latek R, Fleener C, Lamian V, Kulbokas E, 3rd, Davis PM, Suchard SJ, Curran M, Vincenti F, Townsend R: Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation 87: 926–933, 2009 [DOI] [PubMed] [Google Scholar]
- 24. 2008 Annual Report of the U.S. Organ Procurement Network/Scientific Registry of Transplant Recipients: Transplant Data 1998–2007, Rockville, US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2008 [Google Scholar]
- 25. Grinyó J, Marks W, Vincenti F, Kaufman DB, Marder BA, Woodle ES, Citterior F, Agarwal M, Dong Y, Garg P, Ferguson R: Immunosuppression with belatacept-based, CNI-free, steroid-avoiding regimens in kidney transplant recipients: Six month interim results. Presented at the American Transplant Congress; May 30 through June 3, 2009; Boston, MA [Google Scholar]