Abstract
The recent increase in fructose consumption in industrialized nations mirrors the rise in the prevalence of hypertension, but epidemiologic studies have inconsistently linked these observations. We investigated whether increased fructose intake from added sugars associates with an increased risk for higher BP levels in US adults without a history of hypertension. We conducted a cross-sectional analysis using the data collected from the National Health and Nutrition Examination Survey (NHANES 2003 to 2006) involving 4528 adults without a history of hypertension. Median fructose intake was 74 g/d, corresponding to 2.5 sugary soft drinks each day. After adjustment for demographics; comorbidities; physical activity; total kilocalorie intake; and dietary confounders such as total carbohydrate, alcohol, salt, and vitamin C intake, an increased fructose intake of ≥74 g/d independently and significantly associated with higher odds of elevated BP levels: It led to a 26, 30, and 77% higher risk for BP cutoffs of ≥135/85, ≥140/90, and ≥160/100 mmHg, respectively. These results suggest that high fructose intake, in the form of added sugar, independently associates with higher BP levels among US adults without a history of hypertension.
Hypertension is the most common chronic disease in developed countries.1 Studies have documented a progressive increase in the prevalence of hypertension in the United States, from a frequency of approximately 5 to 10% of the adult population at the turn of the 20th century to approximately 31% of the adult population today.2 In addition to an alarming increase in the prevalence of hypertension in the adult population, essential hypertension is becoming increasingly common in adolescents.3 This has grave implications considering that it is a major risk factor for coronary artery disease, congestive heart failure, stroke,4 and chronic kidney disease5 and that it is estimated to account for 7.1 million deaths per year worldwide.6 Despite the presence of effective antihypertensive agents, two thirds of these patients remain either untreated or treated ineffectively7; therefore, it is important to identify potentially modifiable factors that contribute to the increased number of cases of hypertension in the United States and worldwide.
The rise in the incidence and prevalence of hypertension has been linked to adaptation of Western diets and culture. In particular, fructose consumption has increased dramatically in industrialized nations including the United States since the 1900s.8,9 Fructose is a simple sugar that is a key component in table sugar (sucrose) and high-fructose corn syrup. Although fructose also occurs naturally in fruits, the noted increase in fructose consumption in the United States is mainly due to an increase in the intake of added sugars in processed drinks and foods such as sugary soft drinks and grain (bakery) products.10 Studies in rats suggested that fructose can raise BP.11 Few epidemiologic studies have examined the association between fructose intake and BP levels, the results of which have been conflicting.12–14 This conflicting evidence is underlined by a recent statement by the American Heart Association that highlights the controversy over whether increased intake of fructose in the form of added sugar causes hypertension.9
The National Health and Nutrition Examination Survey (NHANES 2003 to 2006) involved a large cross-sectional evaluation of healthy adults from whom direct BP measurements were obtained and for whom fructose intake could be calculated from a self-administered diet questionnaire. Using this population, we determined the median intake of fructose from foods high in added sugar in a nationally representative population, and we examined the hypothesis that excessive fructose intake from added sugars is associated with higher BP levels in participants with no history of hypertension.
Results
Clinical Characteristics of Participants by Systolic BP Categories
Of the 4528 eligible individuals, 2774 had normal systolic BP (SBP) readings defined as an SBP <120 mmHg. Of the remaining individuals, 1369 had BP levels consistent with prehypertension and 355 had stage 1 (n = 261) and stage 2 (n = 94) hypertension according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) guidelines,15 in which stage 1 hypertension is defined as SBP 140 to 159 mmHg or diastolic BP (DBP) 90 to 99 mmHg and stage 2 hypertension is defined as SBP ≥160 mmHg or DBP ≥100 mmHg. Table 1 describes the distribution of the cohort's baseline characteristics by SBP category. There was a trend toward greater fructose intake with the higher BP levels that achieved borderline statistical significance. Individuals with prehypertension and hypertension were older and more likely to be male. In addition, the individuals with higher BP levels had a larger prevalence of diabetes, greater waist circumferences, and higher serum glucose and uric acid levels.
Table 1.
Clinical characteristics of participants by SBP categories (n = 4528)
| Characteristic | SBP Categorya |
P for Trend | |||
|---|---|---|---|---|---|
| Normal SBP (<120 mmHg; n = 2774) | Prehypertension (120 to 139 mmHg; n = 1369) | Stage 1 (140 to 159 mmHg; n = 261) | Stage 2 (≥160 mmHg; n = 94) | ||
| Fructose intake (g/d; mean ± SD) | 71 ± 93 | 76 ± 96 | 86 ± 81 | 98 ± 85 | 0.05 |
| Age (years; mean ± SD) | 37 ± 13 | 44 ± 16 | 58 ± 15 | 66 ± 12 | <0.0001 |
| Gender (%) | <0.0001 | ||||
| male | 38 | 40 | 47 | 68 | |
| female | 62 | 60 | 53 | 32 | |
| Ethnicity | <0.07 | ||||
| non-Hispanic white | 52 | 52 | 56 | 48 | |
| non-Hispanic black | 18 | 21 | 20 | 27 | |
| Mexican American | 26 | 23 | 20 | 21 | |
| other | 4 | 4 | 4 | 5 | |
| Smoking status (%) | <0.0001 | ||||
| never | 53 | 49 | 47 | 59 | |
| former | 18 | 23 | 28 | 27 | |
| current | 23 | 25 | 24 | 14 | |
| unknown | 5 | 3 | 1 | 0 | |
| Diabetes (%) | 2 | 5 | 9 | 8 | <0.0001 |
| Average daily METs exercise (mean ± SD) | 210 ± 356 | 198 ± 256 | 175 ± 229 | 152 ± 199 | 0.02 |
| Waist circumference (in; mean ± SD) | 37 ± 6 | 39 ± 6 | 39 ± 5 | 38 ± 5 | <0.0001 |
| BMI (kg/m2; mean ± SD) | 27 ± 6 | 29 ± 6 | 29 ± 6 | 28 ± 5 | <0.0001 |
| Serum glucose levels (mg/dl; mean ± SD) | 90 ± 23 | 97 ± 31 | 101 ± 27 | 102 ± 30 | <0.0001 |
| Uric acid (mg/dl; mean ± SD) | 5.0 ± 1.3 | 5.4 ± 1.3 | 5.3 ± 1.3 | 5.3 ± 1.1 | <0.0001 |
| Total cholesterol (mg/dl; mean ± SD) | 199 ± 44 | 204 ± 42 | 214 ± 49 | 218 ± 32 | <0.0001 |
| LDL (mg/dl; mean ± SD) | 129 ± 43 | 138 ± 45 | 147 ± 52 | 144 ± 36 | <0.0001 |
| HDL (mg/dl; mean ± SD) | 57 ± 17 | 53 ± 17 | 55 ± 15 | 60 ± 14 | <0.0001 |
| Triglycerides (mg/dl; mean ± SD) | 128 ± 100 | 154 ± 132 | 168 ± 180 | 143 ± 88 | <0.0001 |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 103 ± 26 | 95 ± 23 | 88 ± 20 | 81 ± 18 | <0.0001 |
| Dietary recall (mean ± SD) | |||||
| kilocalories | 2187 ± 868 | 2300 ± 901 | 2052 ± 750 | 1849 ± 679 | 0.21 |
| carbohydrate intake (g/d) | 274 ± 117 | 281 ± 120 | 252 ± 93 | 240 ± 106 | 0.03 |
| sodium (mg/d) | 3466 ± 1529 | 3557 ± 1580 | 3248 ± 1338 | 2863 ± 991 | <0.05 |
| potassium (mg/d) | 2688 ± 1134 | 2776 ± 1103 | 2671 ± 954 | 2399 ± 730 | 0.83 |
| alcohol intake (g/d) | 7 ± 17 | 12 ± 27 | 8 ± 19 | 5 ± 12 | 0.0002 |
| vitamin C (mg/d) | 98 ± 92 | 94 ± 88 | 95 ± 70 | 90 ± 57 | 0.15 |
In accordance with the JNC 7 classification of hypertension.
Factors Independently Associated with Fructose Intake
Factors that were associated with high fructose intake are shown in Table 2. Non-Hispanic black race and total carbohydrate and vitamin C intake were independently and directly correlated with high fructose intake. In contrast, dietary sodium, potassium, and alcohol intake were independently and inversely associated with high fructose intake. Total kilocalorie intake and serum uric acid levels were not associated with high fructose intake in this multivariate analysis.
Table 2.
Factors associated with high fructose intake in multivariate linear regression analysis
| Covariate | Coefficient | 95% CI | P |
|---|---|---|---|
| Male gender | −13.1 | −20.7 to −5.5 | 0.0009 |
| Non-Hispanic black ethnicity | 19.1 | 11.1 to 27.1 | <0.0001 |
| Dietary kilocalories | 0.02 | 0.01 to 0.03 | 0.07 |
| Dietary carbohydrates | 0.10 | 0.04 to 0.16 | <0.0001 |
| Dietary sodium | −0.005 | −0.009 to −0.001 | 0.0005 |
| Dietary potassium | −0.009 | −0.010 to −0.005 | <0.0001 |
| Dietary alcohol | −8.3 | −13.0 to −3.6 | 0.0006 |
| Dietary vitamin C | 0.0400 | 0.0008 to 0.0800 | 0.03 |
Positive coefficients indicate a direct relationship between the variable and high fructose intake ≥74 g/d.
Cross-sectional Relationship Between High Fructose Intake and BP Levels
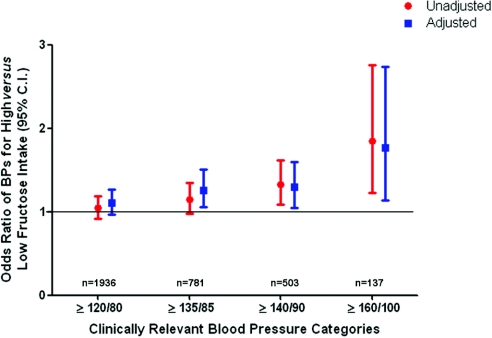
A total of 2253 participants included in the analysis were noted to have high fructose intake defined as ≥74 g/d. Figure 1 shows that a fructose intake of ≥74 g/d is associated, in unadjusted analysis, with higher odds of elevated BP levels. After adjustment for age; gender; race/ethnicity; smoking; diabetes, average daily metabolic equivalents (METs); waist circumference; body mass index (BMI); glucose; uric acid; total cholesterol; HDL cholesterol; triglycerides; LDL cholesterol; estimated GFR (eGFR) by the Modification of Diet in Renal Disease (MDRD) formula; and dietary recalls of total kilocalories, total carbohydrate, sodium, potassium, alcohol, and vitamin C, high fructose intake ≥74 g/d is associated with an increased risk for elevated BP levels. Specifically, increased fructose intake led to a 26, 30, and 77% higher risk for the following clinically relevant BP cutoffs: ≥135/85, ≥140/90, and ≥160/100 mmHg, respectively, in multivariate analysis. Almost identical results were obtained for logistic regression analysis in which fructose intake was entered as a continuous measure (data not shown).
Figure 1.
Cross-sectional association between high fructose intake and clinically relevant BP categories in individuals with no history of hypertension (n = 4528). Data are odds ratios (ORs) and 95% CIs. Adjusted analysis included the following covariates: Age, gender, race/ethnicity, smoking, diabetes, average daily METs, waist circumference, BMI, serum glucose levels, serum uric acid levels, total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, and eGFR, in addition to 24-hour dietary recalls of kilocalories and total carbohydrate, sodium, potassium, alcohol, and vitamin C intake.
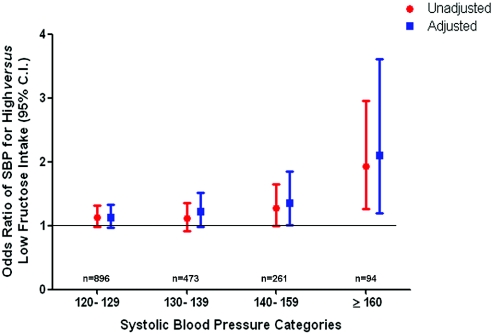
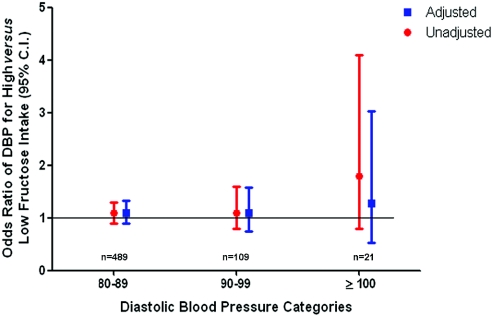
To characterize further the relationship between high fructose intake from added sugars and BP levels, we applied the same multivariate logistic regression model to SBP and DBP categories individually. In Figure 2, we show that increased fructose intake from added sugars is independently associated with SBP. This risk increases with higher SBP: It is 36% greater (multiple adjusted odds ratio 1.36; 95% confidence interval [CI] 1.01 to 1.85) for participants with an SBP of 140 to 159 mmHg and more than double (multiple adjusted odds ratio 2.10; 95% CI 1.20 to 3.61) for participants with an SBP of ≥160 mmHg. We found no association between high fructose intake from added sugars and DBP on unadjusted or adjusted analysis (Figure 3).
Figure 2.
Cross-sectional association between high fructose intake and SBP in individuals with no history of hypertension (n = 4528). Data are ORs and 95% CIs. Adjusted analysis included the following covariates: Age, gender, race/ethnicity, smoking, diabetes, average daily METs, waist circumference, BMI, serum glucose levels, serum uric acid levels, total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, and eGFR, in addition to 24-hour dietary recalls of kilocalories and total carbohydrate, sodium, potassium, alcohol, and vitamin C intake.
Figure 3.
Cross-sectional association between high fructose intake and DBP in individuals with no history of hypertension (n = 4528). Data are ORs and 95% CIs. Adjusted analysis included the following covariates: Age, gender, race/ethnicity, smoking, diabetes, average daily METs, waist circumference, BMI, serum glucose levels, serum uric acid levels, total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, and eGFR, in addition to 24-hour dietary recalls of kilocalories and total carbohydrate, sodium, potassium, alcohol, and vitamin C intake.
When we examined the association between fructose intake and SBP and DBP levels all modeled as continuous variables, we found that fructose intake from added sugars directly correlated with SBP (adjusted correlation coefficient 1.70; 95% CI 0.72 to 2.68; P = 0.0007). We found no correlation between fructose intake and DBP.
DISCUSSION
We report an independent association between high fructose intake from added sugars and higher BP levels of the participating individuals in a large sample representative of the US adult population independent of demographics; comorbidities; physical activity; total kilocalorie intake; and dietary confounders such as total carbohydrate, alcohol, salt, and potassium intake. Fructose intake, based on the US Department of Agriculture (USDA) fructose content of various foods, was calculated and applied to the NHANES dietary questionnaire, and the analysis excluded individuals without a history of hypertension. We show that the median fructose intake from added sugars for the NHANES population is 74 g/d (the equivalent of 2.5 soft drinks per day). The large amount of fructose ingested from added sugar by this population could potentially explain why our study has identified an association between fructose intake and elevated BP levels whereas other studies have not.12,13
Dhingra et al.13 explored the relationship between soft drink consumption and metabolic syndrome in the Framingham Heart Study population. In their study, individuals who consumed ≥1 soft drink per day had a 25 to 32% higher adjusted risk for incidence of each individual metabolic trait (obesity, increased waist circumference, increased fasting glucose, high triglycerides, and lower HDL cholesterol), except for hypertension. According to the USDA, only approximately one third of ingested added sugars come from soft drinks; the rest comes from bakery products, fruit drinks, dairy desserts, and candies, and analyses exclusive to soft drinks likely underestimate the actual sugar intake of the participating individuals.16 Forman et al.12 studied the association between fructose and hypertension prospectively in three large cohort studies: The Nurses Health Study I (NHSI), The Nurses Health Study II (NHSII), and the Health Professionals Follow up Study (HPFS). The investigators found no association between fructose intake and hypertension; however, all of the participants in those cohorts were health care professionals and had a high intake of fruits that were included in the fructose intake scale (e.g., 23.6% of total fructose intake in NHSI was from fruits). This pattern of dietary intake does not mirror that of the general US population, in which the majority of the fructose ingested comes from added sugars rather than from fruits.16
Recently, Nguyen et al.14 showed an association among sugar-sweetened beverages, serum uric acid levels, and SBP in a cross-sectional analysis of 4867 adolescents who were between the ages of 12 and 18 years and had participated in the NHANES (1999 to 2004). Studies in animals suggested that fructose may raise BP via several mechanisms, including stimulation of uric acid,17–19 inhibition of endothelial nitric oxide synthase system,20,21 and stimulation of the sympathetic nervous system,22 or by directly increasing sodium absorption in the gut.23 Although uric acid has been shown to mediate fructose-induced hypertension in rats, we could not demonstrate a relationship between high fructose intake and serum uric acid levels in this study. In our study, serum uric acid levels were not associated with increased fructose intake; neither were triglycerides, BMI, waste circumference, or diabetes. One potential explanation is that the individuals with these characteristics of the metabolic syndrome ingested smaller amounts of fructose-rich foods in an attempt to control their weight or their blood glucose levels. Alternatively, other mechanisms, such as discussed already, may underlie our findings.
Our study has several limitations. First, this is was cross-sectional analysis, the results of which cannot be used to deduce a cause-and-effect relationship. Second, the exact intake of fructose was not measured in the participants; by definition, we relied on self-reporting. That may have led to misclassification of individuals into either category of fructose intake. Third, we calculated fructose intake from sources that are rich in added sugars and include fructose and glucose in almost equal amounts. Although we did adjust for total carbohydrate intake in an attempt to control for glucose intake, we cannot exclude the possibility that the high glucose content of the included foods might have contributed to our findings.
Despite these limitations, our study has several strengths. First, it includes a large nationally representative population of adults in the United States. Second, we took advantage of the availability of actual BP measurements for the participating individuals. Third, although we were unable to take into account all of the possible confounding dietary factors for which 24-hour dietary recall was unavailable (e.g., animal protein), we did account for a large number for potentially confounding variables, including the most important risk factors for elevated BP: Sodium intake and body weight. In addition, our reported results are independent of total kilocalorie and total carbohydrate intake.
In conclusion, our findings suggest that a high intake of fructose of ≥74 g/d (corresponding to 2.5 sugary soft drinks per day), in the form of table sugar or high-fructose corn syrup, is independently associated with a greater risk for elevated SBP levels in the adult US population with no history of hypertension. These findings support the hypothesis that increased intake of fructose may result in hypertension through a variety of mechanisms. Limiting fructose intake is readily feasible, and, in light of our results, prospective studies are needed to assess whether decreased intake of fructose from added sugars will reduce the incidence of hypertension and the burden of cardiovascular disease in the US adult population.
CONCISE METHODS
Study Population and Sample
Data for this analysis were obtained from the NHANES (2003 to 2006), a cross-sectional survey conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. A stratified, multistage sampling design was used, with oversampling of non-Hispanic black individuals, Mexican-American individuals, and individuals aged ≥60 years. Data collection consisted of a home interview, during which the medical history of the participants was obtained via a standardized questionnaire administered by the interviewer, and of a detailed physical examination. In addition, the home visit included the collection of blood specimens at a mobile examination center or at the participant's home.24–26
The analysis was restricted to adult participants who were aged ≥18 years and had complete dietary data (n = 6653) and no history of hypertension. Participants who had been told by a physician that they have high BP or were taking antihypertensive medications were excluded from this analysis (n = 1824). Participants who did not have hypertension or BP data (n = 301) were also excluded from the analysis. Thus, the final sample size used in this analysis was 4528 participants.
Primary Predictor and Outcome
The independent variable used in this analysis was fructose intake from added sugars, and it was calculated from the NHANES (2003 to 2006) dietary questionnaire that was self-administered to the participating individuals and was based on their intake during the previous month to the home interview.27 Food frequency questionnaire assessments are a valid and reproducible method for assessing average dietary consumption.28,29 Briefly, each food item was assigned grams of fructose per portion size on the basis of the documented USDA fructose content of various foods, supplemented with information from manufacturers. Only foods with high fructose content from added sugars were included, such as fruit juices and punch; sugar-sweetened soft drinks and beverages; and bakery products such as pies, cakes, strudels, doughnuts, and cookies, in addition to dairy dessert, chocolate, candy, and dried fruits. Natural fruits were excluded from the calculation, because although they do contain fructose, they also have a high content of potassium, ascorbate, and other antioxidants. These nutrients have been shown to counter the effects of fructose in animal models, and controlling for these factors can be difficult.30,31 Contrary to natural fruits, because dried fruits are deprived of their natural content of vitamins and antioxidants and yet maintain a high fructose content when processed, they were included in the analysis. For each questionnaire, the total intake of grams of fructose per day was determined. We found the median fructose intake for the included participants was 74 g/d (interquartile range 40 to 133 g/d). An intake of 74 g/d fructose is the equivalent of 2.5 soft drinks per day. Previous reports of the NHANES have associated the consumption of two or more soft drinks with an increased risk for adverse events, namely albuminuria.32 Hence, we decided to dichotomize the exposure variable on the basis of the median fructose intake; therefore, participants with an average daily fructose intake of ≥74 g were labeled as having high fructose intake, and participants with a daily intake of <74 g were considered to have low fructose intake.
The dependent variables of interest were BP levels. For the purpose of this analysis, results are presented according to clinically relevant BP categories: ≥120/80, ≥135/85, ≥140/90, and ≥160/100 mmHg. In addition, we examined the association between high fructose intake from added sugars and SBP and DBP categories. SBP was stratified as follows: 120 to 129, 130 to 139, 140 to 159, and ≥160 mmHg. DBP was similarly stratified: <80, 80 to 89, 90 to 99, and ≥100 mmHg. BP was measured at the mobile examination centers by physicians with mercury sphygmomanometers using a standard protocol. Up to three measurements were collected from each participant while in the sitting position; when more than two measurements were collected, the last two were averaged. SBP was defined as the point at which the first Korotkoff sound was heard; the DBP was the level of mercury 2 mm below where the last sound was heard.
Other Measurements
Potential confounding factors were chosen on the basis of previous studies or of their biologic plausibility. The following covariates were included in the analysis: Age, gender, race/ethnicity, smoking, diabetes, physical activity (average daily METs), waist circumference, BMI, glucose, uric acid, total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, and eGFR. In addition, we adjusted for the intake of multiple dietary factors that have been reported to influence BP, such as total kilocalories and total carbohydrate, sodium, potassium, alcohol, and vitamin C intake. Adjustment for potentially confounding dietary factors was based on a 24-hour dietary recall questionnaire rather than on the 1-month NHANES dietary questionnaire that was used to calculate fructose intake.
Age was modeled as a continuous variable. Race/ethnicity was classified into four categories: Non-Hispanic white, non-Hispanic black, Mexican American, and other. Smoking status was classified as never, former, and current. Participants were considered to have diabetes when they had been told by a physician that they have diabetes, were taking hypoglycemic medications, or had a fasting glucose concentration of ≥126 mg/dl. Physical activity was measured as average METs exercised per day and was based on the average duration of the activity reported by the participant and the known MET score per activity. (METs are a simplified system for classifying physical activities: 1 MET is equal to the resting oxygen consumption, which is approximately 3.5 ml/kg per min oxygen). BMI was calculated as weight in kilograms divided by the square of height in meters. Average METs and BMI both were modeled as continuous variables.
Serum uric acid levels were measured by oxidization with the specific enzyme uricase to form allantoin and H2O2 (Hitachi model 737 Multichannel Analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). Fasting levels of HDL cholesterol and triglycerides were measured enzymatically with a Hitachi 704 analyzer (Boehringer Mannheim Diagnostics). LDL cholesterol concentration was calculated using the Friedewald equation (LDL cholesterol = total cholesterol − HDL cholesterol −triglycerides/5), except for those with triglycerides of >400 mg/dl.33 GFR was estimated from the abbreviated MDRD study formula34 as follows: eGFR = 186.3 × (serum creatinine mg/dl)−1.154 × age−0.203 × 0.742 (if female) × 1.21 (if black). As recommended by NHANES analytic guidelines,27 NHANES serum creatinine values in 2005 through 2006 were adjusted to ensure comparability with standard creatinine using the following formula: Standard creatinine (mg/dl) = −0.016 + 0.978 × (NHANES 2005 through 2006 uncalibrated serum creatinine [mg/dl]). No adjustment was needed for serum creatinine levels measured in 2003 through 2004.35 Total kilocalorie consumption and total carbohydrate, sodium, potassium, alcohol, and vitamin C intake were based on the self-reported values from the 24-hour dietary recall.
Statistical Analysis
Univariate modeling was first performed to determine whether a directional trend existed between variables and ordered stages of hypertension in accordance with JNC 7 classification. Categorical variables were examined with a Cochran-Armitage trend test, and continuous variables were examined with one-way ANOVA. The independent relationship of high fructose intake on BP levels, stratified into clinically relevant BP categories (≥120/80, ≥135/85, ≥140/90, and ≥160/100 mmHg), was investigated using logistic regression models, adjusting simultaneously for several potential confounders. The listed clinically relevant BP categories each were referenced to the group <120/80 mmHg. Logistic regression analysis was also used to examine the relationship of fructose intake to SBP categories (120 to 129, 130 to 139, 140 to 159, and ≥160 mmHg) and to DBP categories (80 to 89, 90 to 99, and ≥100 mmHg). The listed categories each were referenced to <120 and <80 mmHg for the SBP and DBP categories, respectively.
In our analysis, fructose intake was modeled as a categorical variable derived from the median value of grams per day of fructose intake reported by participants during the previous month to the home interview (<74 versus ≥74 g/d) or as a continuous variable. Because of skewness of the distribution, fructose intake was logarithmically transformed for statistical analyses. In all regression models, observations were weighted to reflect the general US population as of early 2000s, using weights calculated for that purpose by the National Center for Health Statistics. Analyses were conducted using SAS 9.1.3 (Research Triangle Institute, Research Triangle Park, NC). P < 0.05 was considered statistically significant.
D.I.J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
DISCLOSURES
R.J.J. is listed as an inventor on several patent applications on lowering uric acid as it relates to BP and metabolic syndrome; in addition, he is author of The Sugar Fix (2008, Rodale Inc.; 2009, Simon and Schuster).
Acknowledgments
This work was supported by National Institutes of Health grants DK-5121, HL-68607, 1R01 DK081473-01A1, and 1R01DK078112-01A2.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Dietary Fructose and Elevated Levels of Blood Pressure,” on pages 1416–1418.
REFERENCES
- 1. Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290: 199–206, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG: Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P: The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension 44: 398–404, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D: Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345: 1291–1297, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 6. World Health Report 2002: Reducing Risks, Promoting Healthy Life, Geneva, World Health Organization, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, Bakris GL: Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 121: 332–340, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Jabara C, Valdes A: World sugar policies and developing countries. In: The Economics and Politics of World Sugar Policies, edited by Marks SV, Maskus KE, Ann Arbor, University of Michigan Press, 1993, pp 135–162 [Google Scholar]
- 9. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J, American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention : Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 120: 1011–1020, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Marriott BP, Cole N, Lee E: National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139: 1228S–1235S, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M: Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 292: F423–F429, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Forman JP, Choi H, Curhan GC: Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol 20: 863–871, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS: Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 116: 480–488, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen S, Choi HK, Lustig RH, Hsu CY: Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 154: 807–813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Seventh Report of the Joint National Committee in Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, Bethesda, US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, 2004 [PubMed] [Google Scholar]
- 16. Bowman SA: Family Economics and Nutrition Review, Washington, DC, USDA, 2004 [Google Scholar]
- 17. Fox IH, Kelley WN: Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 21: 713–721, 1972 [DOI] [PubMed] [Google Scholar]
- 18. Fox IH, Kelley WN: Studies on the mechanism of fructose-induced hyperuricemia in man. Adv Exp Med Biol 41: 463–470, 1974 [DOI] [PubMed] [Google Scholar]
- 19. Feig DI, Soletsky B, Johnson RJ: Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 300: 924–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, Sanchez-Lozada LG, Johnson RJ, Nakagawa T: Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 19: 1712–1720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW: Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J Pharmacol Exp Ther 328: 610–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brito JO, Ponciano K, Figueroa D, Bernardes N, Sanches IC, Irigoyen MC, De Angelis K: Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz J Med Biol Res 41: 804–808, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M: Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int 74: 438–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Health and Nutrition Examination Survey (NHANES) Analytical Guidelines. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm Accessed November 12, 2008
- 25. Survey Operations Manuals, Brochures, and Consent Documents: 1999-current NHANES. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/current_nhanes_03_04.htm Accessed November 12, 2008
- 26. Plan and Operation of the Third National Health and Nutrition Examination Survey 1988–1994, Hyattsville, MD, National Center for Health Statistics, 1995 [Google Scholar]
- 27. NHANES Food Questionnaire. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/tq_fpq_c.pdf Accessed November 12, 2008
- 28. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC: Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 69: 243–249, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC: Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 93: 790–796, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T: Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens 17: 470–476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C, Nakagawa T: Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 30: 96–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, Cooper RS: Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS ONE 3: e3431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 34. National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 35. Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]