Abstract
The safety of prescribing digoxin in ESRD is unknown. Hypokalemia, which frequently occurs among dialysis patients, may enhance the toxicity of digoxin. Here, we analyzed the association between digoxin prescription and survival in a retrospective cohort using covariate- and propensity score–adjusted Cox models to minimize the potential for confounding by indication. Among 120,864 incident hemodialysis patients, digoxin use associated with a 28% increased risk for death (hazard ratio [HR] 1.28; 95% confidence interval 1.25 to 1.31). Increasing serum digoxin level was also significantly associated with mortality (HR 1.19 per ng/ml increase; 95% confidence interval 1.05 to 1.35). This increased mortality risk with level was most pronounced in patients with lower predialysis serum potassium (K) levels (HR 2.53 [P = 0.01] for K <4.3 mEq/L versus HR 0.86 [P = 0.35] for K >4.6 mEq/L). In conclusion, digoxin use among patients who are on hemodialysis associates with increased mortality, especially among those with low predialysis K concentrations.
Digoxin has been used for decades for the treatment of cardiovascular disease (CVD). In 1997, the US Food and Drug Administration officially approved digoxin for the treatment of heart failure and atrial fibrillation. The decision was mostly based on the Digitalis Investigation Group (DIG) trial, which reported a 28% reduction in hospitalization for congestive heart failure (CHF) from digoxin without any effect on mortality in patients without kidney failure.1 On the basis of that and other studies, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI)2 also included digoxin in its ESRD CVD guidelines for the treatment of cardiomyopathy and atrial fibrillation; however, few studies have been conducted to verify the drug is safe in hemodialysis (HD) patients, who are prone to considerable intracellular–extracellular shifts in potassium (K) during dialysis that may directly mediate the efficacy and toxicity of the drug. Exactly how cardiac glycosides should be properly managed and monitored in patients who are on long-term renal replacement therapy (RRT) remains unanswered. We conducted an observational cohort analysis of a large and diverse population of incident dialysis patients to determine the association between mortality and digoxin in relation to the pharmacodynamic parameters of the drug (e.g., prescribed digoxin dosage, serum digoxin level) and predialysis serum K level.
Results
Population Characteristics
During a 6-year period, we identified 120,864 incident HD patients, 4549 of whom were digoxin users (4% digoxin prevalence on the 90th day of long-term HD); the median prescribed dosage was 62.5 μg/d (125 μg every other day). Within this cohort of digoxin users, 44% of the patients received serum digoxin level monitoring at an average of 2.7 times in the first 90 days of dialysis. The median serum level was 1.0 ng/ml (mean 0.8 ng/ml). A total of 3.5% of patients had high serum levels (>2.2 ng/ml), whereas 53% had a serum level ≥1.0 ng/ml. Only 49% of patients with an “abnormal” serum digoxin level (<0.6 or >2.2 ng/ml) had their prescribed digoxin dosage adjusted within the subsequent 30 days. A highly significant correlation existed between the change in serum digoxin level and the change in the prescribed dosage (R = 0.20, P < 0.0001); however, no relationship between the serum digoxin level and the prescribed digoxin dosage (R = 0.004, P = 0.86) could be demonstrated, suggesting large interpatient variability in “digoxin responsiveness” or in the volume of distribution.
Association Between Digoxin Use and Mortality
As expected, patients who were on digoxin (versus not on digoxin) were generally “sicker” with increased age, comorbidity, and medication use with lower BP readings at baseline (Table 1). In survival analysis (Figure 1), digoxin use (versus nonuse) was statistically and clinically significantly associated with increased mortality in both unadjusted (hazard ratio [HR] 1.89; 95% confidence interval [CI] 1.18 to 1.97) and adjusted models (HR 1.28; 95% CI 1.25 to 1.31). The increased risk for death remained in subanalyses that compared digoxin patients with those with a comparable indication but not on the drug (Table 2).
Table 1.
Baseline patient characteristics of incident HD patients with digoxin use versus no digoxin use
| Characteristic | No Digoxin Use | Digoxin Use | P |
|---|---|---|---|
| N | 116,315 | 4549 | |
| Demographic | |||
| age (years) | 62.50 (0.04) | 69.40 (0.10) | <0.0001 |
| male gender (%) | 53.5 (0.1) | 59.6 (0.1) | <0.0001 |
| race (%) | |||
| black | 31.90 (0.10) | 23.10 (0.10) | <0.0001 |
| other | 9.50 (0.08) | 7.40 (0.07) | |
| white | 58.50 (0.10) | 69.50 (0.10) | |
| Access (%) | |||
| AVF | 22.00 (0.10) | 19.60 (0.10) | 0.001 |
| catheter | 55.30 (0.10) | 57.00 (0.10) | |
| graft | 17.90 (0.10) | 18.80 (0.10) | |
| unknown | 4.80 (0.06) | 4.70 (0.06) | |
| Hemodynamic | |||
| SBP (mmHg) | 143.400 (0.050) | 137.400 (0.200) | <0.0001 |
| DBP (mmHg) | 73.500 (0.030) | 67.700 (0.100) | <0.0001 |
| interdialytic weight gain (kg) | 2.300 (0.003) | 2.400 (0.010) | <0.0001 |
| weight (kg) | 77.300 (0.060) | 74.900 (0.200) | <0.0001 |
| Comorbidity | |||
| Charleson index | 4.700 (0.006) | 5.200 (0.020) | <0.0001 |
| diabetes (%) | 63.4 (0.1) | 69.6 (0.1) | <0.0001 |
| Laboratory | |||
| hemoglobin (g/dl) | 11.400 (0.004) | 11.400 (0.010) | 0.0009 |
| albumin (g/dl) | 3.500 (0.001) | 3.500 (0.006) | <0.0001 |
| potassium (mEq/L) | 8.800 (0.002) | 8.800 (0.009) | 0.01 |
| calcium (mg/dl) | 4.500 (0.002) | 4.500 (0.008) | 0.60 |
| creatinine (mg/dl) | 6.600 (0.008) | 5.300 (0.030) | <0.0001 |
| Treatment parameters | |||
| time (hours) | 3.600 (0.001) | 3.600 (0.006) | <0.0001 |
| dialysate potassium (mEq/L) | 2.300 (0.002) | 2.400 (0.008) | <0.0001 |
| dialysate calcium (mEq/L) | 2.600 (0.001) | 2.600 (0.005) | <0.0001 |
| Medications | |||
| EPO (1000 U per session) | 7.20 (0.02) | 7.50 (0.10) | 0.004 |
| antiplatelet (%) | 34.3 (0.10) | 44.2 (0.10) | <0.0001 |
| statin (%) | 33.0 (0.10) | 37.1 (0.10) | <0.0001 |
| β blocker (%) | 51.0 (0.10) | 59.9 (0.10) | <0.0001 |
| ACEI or ARB (%) | 40.9 (0.10) | 45.0 (0.10) | <0.0001 |
Data are means (SE). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVF, arteriovenous fistula; DBP, diastolic blood pressure; SBP, systolic blood pressure; EPO, Epogen.
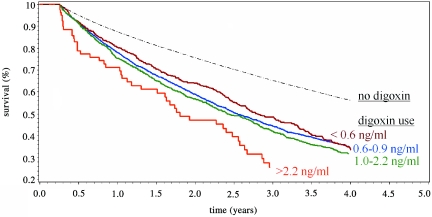
Figure 1.
Crude survival curves show decreased survival with digoxin use. Digoxin users had a statistically significant increased mortality risk when compared with patients who were not prescribed digoxin (P < 0.0001). Among digoxin users, higher serum digoxin levels in the first 90 days of long-term HD were associated with an increased risk for death (P = 0.01, <0.6 versus 0.6 to 0.9 versus 1.0 to 2.2 ng/ml groups).
Table 2.
Mortality HR in digoxin users versus nonusers among incident HD patients
| Parameter | n | Unadjusted Model (HR [95% CI]) | Covariate- and Propensity Score–Adjusted Model (HR [95% CI])a |
|---|---|---|---|
| All incident hemodialysis patients | 120,864 | 1.89 (1.91 to 1.97) | 1.28 (1.25 to 1.31) |
| Incident HD patient with only coexistent afib | 1840 | 1.55 (0.67 to 3.56) | 1.84 (1.55 to 2.18) |
| Incident HD patient with only coexistent CHF | 25,851 | 1.55 (0.67 to 3.56) | 1.18 (1.13 to 1.23) |
| Incident HD patient with both coexistent afib + CHF | 9011 | 1.08 (0.38 to 3.08) | 1.20 (1.12 to 1.28) |
aParameters used as baseline covariates in the Cox models and in the propensity score: Age, gender, race (white, black, other), cause of ESRD (diabetes, hypertension, glomerulonephritis, other), systolic and diastolic BP (pre- and postdialysis readings), weight, interdialytic weight gain, access (fistula, graft, catheter, unknown), dialysis adequacy (eKt/V with Kru), Charleson comorbidity index, diabetes status, coronary heart disease, laboratory values (calcium, phosphorus, albumin, hemoglobin, bicarbonate, white blood cell count, creatinine, parathyroid hormone), dialysate K, dialysate calcium, vitamin D use, EPO dosage, study entry date, facility-standardized mortality ratio, oral medication use (angiotensin receptor blocker, angiotensin-converting enzyme inhibitor, nitroglycerine, clopidogrel, aspirin, warfarin, statin, β blocker), ultrafiltration rate, actual dialysis time, and residual renal function.
Legend: afib, atrial fibrillation; CHF, congestive heart failure.
Association Between Digoxin Dosage and Mortality
Patients who were on higher prescribed dosages of digoxin (μg/d) were more likely to be male and black and have diabetes (Table 3). Patients with higher serum digoxin levels (ng/ml) were more likely to be older and female, with lower levels of albumin, diastolic BP readings, antiplatelet use, dialysis time, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use (Table 4). There were no clinically appreciable differences in the prescribed dialysate K or calcium among the groups. Higher weight patients were on a higher prescribed digoxin dosage but had a lower measured serum digoxin level (consistent with the drug's large volume of distribution). Crude survival curves showed prescribed digoxin dosage was unrelated to the risk for death (P = 0.50), whereas higher serum digoxin levels were associated with increased mortality (P = 0.01; Figure 1). Unadjusted mortality HRs confirmed a significant 22% increased risk for death per 1-ng/ml increase in serum digoxin level. Statistically significant associations with increased mortality were noted when the serum digoxin level was ≥1.0 ng/dl (Table 5) and >1.55 ng/dl when the cohort was examined by quintiles (Table 6). After covariate and propensity score adjustment, death risk still remained poorly related with the prescribed digoxin dosage (P = 0.11, P = 0.42 for trend; Table 5); however, each 1-ng/ml increase in serum digoxin level significantly increased the risk for mortality by 19% (P < 0.006, p = 0.004 for trend; Table 5) after covariate and propensity score adjustment.
Table 3.
Baseline patient characteristics by the daily prescribed dosage of digoxin on the 90th day of long-term dialysis
| Characteristic | Dosage of Digoxin (μg/d) |
P | |||
|---|---|---|---|---|---|
| <55.0 | 55.0 to 62.5 | 62.6 to 120.0 | >120.0 | ||
| N | 1450 | 1238 | 273 | 1446 | |
| Demographic | |||||
| age (years) | 70.2 (0.3) | 70.6 (0.3) | 71.1 (0.6) | 67.3 (0.3) | <0.0001 |
| male gender (%) | 56.8 (0.7) | 59.6 (0.7) | 62.9 (0.7) | 61.8 (0.7) | 0.03 |
| race (%) | |||||
| black | 22.3 (0.6) | 21.3 (0.6) | 14.3 (0.5) | 27.2 (0.6) | <0.0001 |
| other | 6.2 (0.3) | 7.0 (0.3) | 7.3 (0.3) | 7.6 (0.3) | |
| white | 71.4 (0.6) | 71.6 (0.6) | 78.4 (0.6) | 65.2 (0.7) | |
| Access (%) | |||||
| AVF | 20.0 (0.6) | 19.4 (0.5) | 20.1 (0.6) | 19.4 (0.5) | 0.52 |
| catheter | 54.8 (0.7) | 58.6 (0.7) | 59.0 (0.7) | 57.0 (0.7) | |
| graft | 19.9 (0.6) | 18.2 (0.5) | 17.2 (0.5) | 18.7 (0.5) | |
| unknown | 5.4 (0.3) | 3.8 (0.2) | 3.7 (0.2) | 4.9 (0.3) | |
| Hemodynamic | |||||
| SBP (mmHg) | 136.1 (0.5) | 137.4 (0.5) | 137.0 (1.1) | 138.8 (0.4) | 0.002 |
| DBP (mmHg) | 67.1 (0.2) | 67.3 (0.2) | 66.9 (0.5) | 68.8 (0.2) | <0.0001 |
| interdialytic weight gain (kg) | 2.40 (0.02) | 2.40 (0.02) | 2.40 (0.10) | 2.50 (0.02) | 0.03 |
| weight (kg) | 72.8 (0.5) | 74.5 (0.6) | 73.7 (1.0) | 77.6 (0.6) | <0.0001 |
| Comorbidity | |||||
| Charleson index | 5.20 (0.05) | 5.10 (0.05) | 5.10 (0.10) | 5.20 (0.04) | 0.30 |
| diabetes (%) | 67.5 (0.7) | 68.5 (0.6) | 67.0 (0.7) | 72.6 (0.6) | 0.01 |
| Laboratory | |||||
| hemoglobin (g/dl) | 11.40 (0.03) | 11.40 (0.03) | 11.50 (0.07) | 11.30 (0.03) | 0.18 |
| albumin (g/dl) | 3.50 (0.01) | 3.50 (0.01) | 3.50 (0.02) | 3.50 (0.01) | 0.52 |
| potassium (mEq/L) | 4.50 (0.01) | 4.50 (0.01) | 4.50 (0.03) | 4.50 (0.01) | 0.09 |
| calcium (mg/dl) | 8.80 (0.02) | 8.80 (0.02) | 8.80 (0.04) | 8.80 (0.02) | 0.03 |
| creatinine (mg/dl) | 5.30 (0.06) | 5.40 (0.06) | 5.30 (0.10) | 5.40 (0.06) | 0.56 |
| Treatment parameters | |||||
| time (hours) | 3.50 (0.01) | 3.60 (0.01) | 3.60 (0.03) | 3.60 (0.01) | 0.64 |
| dialysate potassium (mEq/L) | 2.43 (0.02) | 2.40 (0.02) | 2.45 (0.03) | 2.37 (0.01) | 0.006 |
| dialysate calcium (mEq/L) | 2.60 (0.01) | 2.60 (0.01) | 2.60 (0.02) | 2.60 (0.01) | 0.41 |
| Medications | |||||
| EPO (1000 U per sessions) | 7.6 (0.1) | 7.4 (0.1) | 7.4 (0.4) | 7.5 (0.1) | 0.91 |
| antiplatelet (%) | 42.8 (0.7) | 46.7 (0.7) | 48.7 (0.7) | 42.4 (0.7) | 0.03 |
| statin (%) | 35.0 (0.7) | 38.2 (0.7) | 39.9 (0.7) | 37.7 (0.7) | 0.21 |
| β blocker (%) | 60.2 (0.7) | 59.2 (0.7) | 59.3 (0.7) | 60.6 (0.7) | 0.89 |
| ACEI or ARB (%) | 46.1 (0.7) | 45.3 (0.7) | 43.6 (0.7) | 44.5 (0.7) | 0.79 |
Data are means (SE). A total of 142 patients who were on digoxin did not have a dosage documented.
Legend: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVF, arteriovenous fistula; DBP, diastolic blood pressure; SBP, systolic blood pressure; EPO, Epogen.
Table 4.
Baseline patient characteristics by mean serum digoxin level in the first 90 days of long-term dialysis
| Serum Digoxin Level (ng/ml) |
P | ||||
|---|---|---|---|---|---|
| <0.60 | 0.60 to 0.99 | 1.00 to 2.20 | >2.20 | ||
| N | 381 | 552 | 973 | 79 | |
| Demographic | |||||
| age (years) | 67.1 (0.6) | 70.2 (0.5) | 71.2 (0.3) | 71.9 (1.3) | <0.0001 |
| male gender (%) | 66.7 (1.1) | 63.6 (1.1) | 56.3 (1.1) | 51.9 (1.1) | 0.0005 |
| race (%) | |||||
| black | 24.9 (0.9) | 20.1 (0.9) | 18.8 (0.8) | 22.8 (0.9) | 0.13 |
| other | 7.3 (0.5) | 6.0 (0.5) | 6.4 (0.5) | 2.5 (0.3) | |
| white | 67.7 (1.0) | 73.9 (0.9) | 74.8 (0.9) | 74.7 (0.9) | |
| Access (%) | |||||
| AVF | 20.5 (0.9) | 22.6 (0.9) | 19.9 (0.8) | 19.0 (0.8) | 0.66 |
| catheter | 53.3 (1.1) | 53.3 (1.1) | 56.4 (1.1) | 53.2 (1.1) | |
| graft | 21.0 (0.9) | 20.1 (0.9) | 19.2 (0.8) | 19.0 (0.8) | |
| unknown | 5.2 (0.5) | 4.0 (0.4) | 4.4 (0.4) | 8.9 (0.6) | |
| Hemodynamic | |||||
| SBP (mmHg) | 137.2 (0.9) | 136.6 (0.7) | 136.4 (0.6) | 134.2 (2.2) | 0.62 |
| DBP (mmHg) | 69.0 (0.4) | 67.5 (0.4) | 66.1 (0.3) | 64.6 (0.9) | <0.0001 |
| interdialytic weight gain (kg) | 2.50 (0.05) | 2.40 (0.04) | 2.40 (0.03) | 2.30 (0.10) | 0.09 |
| weight (kg) | 77.8 (1.0) | 75.2 (0.9) | 73.8 (0.7) | 73.4 (3.0) | 0.01 |
| Comorbidity | |||||
| Charleson index | 5.40 (0.09) | 5.20 (0.08) | 5.30 (0.06) | 5.10 (0.20) | 0.44 |
| diabetes (%) | 73.8 (0.9) | 66.8 (1.1) | 69.0 (1.0) | 62.0 (1.1) | 0.07 |
| Laboratory | |||||
| hemoglobin (g/dl) | 11.30 (0.06) | 11.40 (0.05) | 11.50 (0.03) | 11.30 (0.10) | 0.17 |
| albumin (g/dl) | 3.60 (0.02) | 3.60 (0.01) | 3.50 (0.01) | 3.40 (0.04) | 0.005 |
| potassium (mEq/L) | 4.50 (0.02) | 4.50 (0.02) | 4.50 (0.01) | 4.60 (0.06) | 0.30 |
| calcium (mg/dl) | 8.80 (0.03) | 8.80 (0.03) | 8.80 (0.02) | 8.80 (0.08) | 0.80 |
| creatinine (mg/dl) | 5.60 (0.11) | 5.30 (0.08) | 5.20 (0.06) | 5.70 (0.31) | 0.009 |
| Treatment parameters | |||||
| time (hours) | 3.60 (0.02) | 3.60 (0.02) | 3.50 (0.01) | 3.40 (0.04) | <0.0001 |
| dialysate potassium (mEq/L) | 2.39 (0.03) | 2.42 (0.02) | 2.42 (0.02) | 2.37 (0.06) | 0.65 |
| dialysate calcium (mEq/L) | 2.60 (0.02) | 2.60 (0.02) | 2.60 (0.01) | 2.60 (0.03) | 0.18 |
| Medications | |||||
| EPO (1000 U per sessions) | 7.4 (0.3) | 7.4 (0.3) | 7.1 (0.2) | 8.0 (0.7) | 0.56 |
| antiplatelet (%) | 45.7 (1.1) | 48.4 (1.1) | 44.6 (1.1) | 30.4 (1.0) | 0.02 |
| statin (%) | 35.4 (1.1) | 39.1 (1.1) | 37.3 (1.1) | 32.9 (1.1) | 0.57 |
| β blocker (%) | 59.3 (1.1) | 60.0 (1.1) | 60.4 (1.1) | 57.0 (1.1) | 0.92 |
| ACEI or ARB (%) | 51.4 (1.1) | 46.4 (1.1) | 42.0 (1.1) | 44.3 (1.1) | 0.01 |
Data are means (SE). A total of 2564 (56%) patients did not have in-center digoxin level monitoring in the first 90 days of dialysis.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVF, arteriovenous fistula; DBP, diastolic blood pressure; SBP, systolic blood pressure; EPO, Epogen.
Table 5.
Mortality HR by dosage in digoxin users
| Parameter | N | Unadjusted Model (HR [95% CI]) | Covariate- and Propensity Score–Adjusted Model (HR [95% CI])a |
|---|---|---|---|
| Mortality risk by prescribed daily digoxin dosage | |||
| per 62.5-μg/d increase | 4407 | 0.96 (0.90 to 1.02) | 1.05 (0.99 to 1.12) |
| no documented digoxin dosage | 142 | 1.18 (0.93 to 1.51) | 1.05 (0.80 to 1.38) |
| <55.0 μg/d | 1466 | 1.00 (reference) | 1.00 (reference) |
| 55.0 to 62.5 μg/d | 273 | 0.99 (0.89 to 1.10) | 0.96 (0.85 to 1.07) |
| 62.6 to 120.0 μg/d | 1238 | 0.97 (0.81 to 1.16) | 1.00 (0.83 to 1.21) |
| >120.0 μg/d | 1450 | 0.93 (0.84 to 1.03) | 1.02 (0.91 to 1.14) |
| P for trend | 0.13 | 0.42 | |
| Mortality risk by baseline serum digoxin level | |||
| per 1-ng/ml increase | 1985 | 1.22 (1.10 to 1.35) | 1.19 (1.05 to 1.35) |
| no digoxin level monitoring | 2564 | 1.12 (0.96 to 1.31) | 1.05 (0.89 to 1.24) |
| <0.6 ng/ml | 381 | 1.00 (reference) | 1.00 (reference) |
| 0.6 to 0.9 ng/ml | 552 | 1.05 (0.87 to 1.26) | 1.01 (0.83 to 1.23) |
| 1.0 to 2.2 ng/ml | 973 | 1.19 (1.01 to 1.41) | 1.13 (0.95 to 1.35) |
| >2.2 ng/ml | 79 | 1.59 (1.15 to 2.19) | 1.76 (1.26 to 2.47) |
| P for trend | 0.001 | 0.004 |
aParameters used as baseline covariates in the Cox models and in the propensity score: Age, gender, race (white, black, other), cause of ESRD (diabetes, hypertension, glomerulonephritis, other), systolic and diastolic BP (pre- and postdialysis readings), weight, interdialytic weight gain, access (fistula, graft, catheter, unknown), dialysis adequacy (eKt/V with Kru), Charleson comorbidity index, diabetes status, coronary heart disease, laboratory values (calcium, phosphorus, albumin, hemoglobin, bicarbonate, white blood cell count, creatinine, parathyroid hormone), dialysate K, dialysate calcium, vitamin D use, EPO dosage, study entry date, facility-standardized mortality ratio, oral medication use (angiotensin receptor blocker, angiotensin-converting enzyme inhibitor, nitroglycerine, clopidogrel, aspirin, warfarin, statin, β blocker), ultrafiltration rate, actual dialysis time, and residual renal function.
Table 6.
Mortality HR by quintiles of serum digoxin level
| Quintile | Serum Digoxin Level (ng/ml) | n | HR (95% CI) |
|---|---|---|---|
| 1 | <0.60 | 381 | 1.00 (reference) |
| 2 | 0.60 to 0.87 | 416 | 0.97 (0.78 to 1.20) |
| 3 | 0.88 to 1.19 | 385 | 1.11 (0.90 to 1.37) |
| 4 | 1.20 to 1.55 | 410 | 1.04 (0.84 to 1.28) |
| 5 | >1.55 | 393 | 1.43 (1.17 to 1.76) |
Sensitivity Analysis: Serum Digoxin Level Associates with Increased Mortality
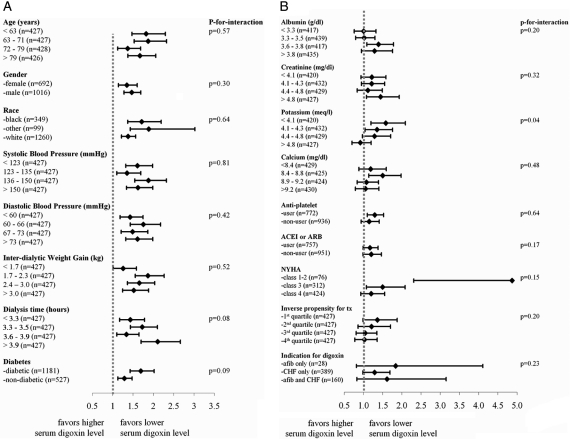
Stratified analyses demonstrated persistent increased mortality associated with higher serum digoxin levels in 53 of 58 subgroups (Figure 2). Effect modification was noted between serum digoxin and predialysis K level (P = 0.04 for interaction; Figure 2B). For example, the mortality risk with a high digoxin level (digoxin >2.2 versus <0.6 ng/ml) was much greater when the predialysis K was low (HR 2.53 when K <4.3 mEq/L; Figure 3) than when the K was high (HR 0.86 when K >4.6 mEq/L; Figure 3). Increasing serum digoxin levels were associated with increased mortality when the serum K was ≤4.6 mEq/L but reversed to decrease mortality when the serum K was >4.6 mEq/L (Figure 3).
Figure 2.
Mortality HRs per 1-ng/ml increase in serum digoxin level stratified by patient characteristics demonstrated a persistent mortality trend in digoxin users. A statistically significant interaction effect existed between K and digoxin level. Models were covariate and propensity score adjusted. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 3.
The mortality effect associated with a higher serum digoxin level is magnified with decreasing serum K level. Mortality HRs were covariate and propensity score adjusted.
The effect of functional status was also explored. Covariate- and propensity-adjusted Cox models, which adjusted for New York Heart Association (NYHA) functional class, still demonstrated increased mortality with increasing serum levels of digoxin (HR 1.19; 95% CI 1.06 to 1.34). In fact, the mortality effect of digoxin was six times greater (Figure 2B) when the drug was prescribed to patients with good exercise tolerance (NYHA ≤2) instead of patients with the poorest exercise capacity (NYHA = 4). Under a new digoxin user design, which minimized the potential for survivorship bias, the risk for death remained significantly greater with higher serum digoxin levels (HR 1.24 per ng/ml increase; 95% CI 1.06 to 1.45).
Results from time-varying analyses accounted for longitudinal changes in serum digoxin level at 90-day intervals and were concordant with the primary findings (Table 7). Each 1-ng/ml increase in serum digoxin was associated with an 11% increase in mortality. In matched analyses, increasing serum digoxin level remained associated with an increased risk for death in subcohorts that balanced for unmeasured confounding from the facility, the treating physician, and the propensity for digoxin level (Table 7).
Table 7.
Sensitivity analyses by serum digoxin level
| Parameter | N | Unadjusted Model (HR [95% CI]) | Covariate- and Propensity Score–Adjusted Model (HR [95% CI])a |
|---|---|---|---|
| Mortality risk by time-varying serum digoxin level | |||
| per 1-ng/ml increase | 3313b | 1.22 (1.11 to 1.34) | 1.11 (1.00 to 1.24) |
| no digoxin level monitoring | 40.7%c | 1.16 (1.02 to 1.32) | 1.14 (0.99 to 1.32) |
| <0.6 ng/ml | 23.8% | 1.00 (reference) | 1.00 (reference) |
| 0.6 to 0.9 ng/ml | 12.6% | 1.05 (0.89 to 1.24) | 1.06 (0.87 to 1.29) |
| 1.0 to 2.2 ng/ml | 20.3% | 1.33 (1.16 to 1.53) | 1.19 (0.98 to 1.44) |
| >2.2 ng/ml | 2.6% | 1.59 (1.14 to 2.23) | 1.14 (1.01 to 1.30) |
| P for trend | 0.0007 | 0.14 | |
| Mortality risk after covariate matching | |||
| matched on facility | 928 | 1.22 (1.03 to 1.43) | 1.05 (0.88 to 1.27) |
| matched on physician | 840 | 1.27 (1.08 to 1.50) | 1.18 (1.01 to 1.38) |
| matched on propensity scored | 1626 | 1.22 (1.09 to 1.37) | 1.55 (1.05 to 2.27) |
aParameters used in the propensity score were the same as the primary analysis (see Table 2 footnote); covariates used in the Cox model were the same as the primary analysis with the exception of serum digoxin level, K, calcium, dialysate potassium, dialysate calcium, albumin, hemoglobin, systolic and diastolic BP (pre- and postdialysis readings), weight, interdialytic weight gain, and Charleson comorbidity index, which were modeled as time-dependent parameters (90-day cycles).
bA total of 1237 of 4550 patients were censored at death because no in-center serum digoxin level monitoring was done.
cResults are reported as a percentage of time in each serum digoxin ordinal given patients had changing serum digoxin levels during the follow-up period.
dStandardized difference = 0.4% and variance ratio = 0.02% for the propensity score between the groups after matching.
Discussion
In this observational cohort of 120,864 incident HD patients, the use of digoxin was associated with an increased risk for death. The mortality effect was significantly potentiated by high serum digoxin and low serum K levels, which seemed independent of patient characteristics, propensity for treatment, the facility, the physician, disease severity, or time.
Clinically, digoxin has been used for many years for the treatment of CVD.3 On the basis of several trials in the general population, the drug is sometimes used for the treatment of heart failure1 and atrial fibrillation,4 given the unique inotropic5 and chronotropic6 properties of digoxin. The purported benefits of digoxin may initially seem favorable to the comorbidity profile of dialysis patients, given the high prevalence of atrial fibrillation7–9 and hospitalization10–12; however, almost no trials have been conducted to examine whether the hospitalization efficacy, rate control properties, and safety of digoxin translate to patients who are undergoing long-term renal replacement therapy (RRT).
Digoxin directly inhibits the Na+/K+-ATPase pump in the membrane of the cardiac myocyte, which causes intracellular increases in sodium and a consequent rise in calcium through the sodium-calcium exchanger.13,14 Ultimately, a rise in local calcium levels directly prolongs the cardiac action potential and results in a decreased heart rate and an increased binding with troponin C to promote cardiac contractility.
Serum K level and its rapid decline during dialysis may play an important role in the therapeutic effects and toxicity of the drug, because both K and digoxin compete for the same ATPase-binding site. Hyperkalemia may decrease the effectiveness of digoxin, whereas hypokalemia can potentiate toxicity. This study reported a statistically significant interaction between a low predialysis serum K and a high serum digoxin level on all-cause mortality (Figures 2B and 3) consistent with the inhibition of the Na+/K+-ATPase pump for the biological mechanism of action of digoxin.
Overall, we suggest caution for digoxin use in the general HD population, given its prescription was associated with increased mortality in this study. Since the publication of the DIG study in 1997,1 data from CHF registries and trials have demonstrated the progressive decline of digoxin use15 in the setting of observational studies that raise potential safety concerns for cardiac glycosides. Most notable, post hoc analyses of the original DIG study reported a significant 4.2% absolute increase in mortality for women16 and a decreased risk for death when serum levels were maintained at lower, “therapeutic” concentrations.17–19 Consequently, digoxin has been progressively reclassified from a class I recommendation in 200120 to a class IIa recommendation in 2005,21 with explicit cautions in the 2009 American College of Cardiology/American Heart Association guideline that recommend a target serum range of 0.5 to 1.0 ng/dl.22
Among patients who were on digoxin in this retrospective study, the risk for death significantly increased with serum digoxin levels but not with the prescribed digoxin dosage. Digoxin is a drug with a narrow therapeutic/toxic ratio whereby patient-level pharmacokinetics, metabolism, and clearance factors aggregate to introduce variability in drug responsiveness. For example, digoxin distributes extensively to peripheral tissue (average volume of distribution of 785 L)23; heavier patients had higher prescribed dosages and lower serum levels (Tables 3 and 4). Consequently, targeted serum-level therapy with frequent serum-level monitoring seems prudent as a means to achieve the very narrow therapeutic window for the drug.
Several characteristics inherent to the dialysis treatment may explain the study's finding. The narrow therapeutic index and proarrhythmic qualities of the drug24 have the potential to limit the overall effectiveness of digoxin in the ESRD population.1,4 Patients who are on long-term RRT are subject to recurrent fluid/electrolyte shifts, hypoalbuminuria, and end-organ damage, which may predispose patients with ESRD to adverse reactions from digoxin. For example, (1) plasma K concentration drops by approximately 40% during HD and then “rebounds” in the postdialytic period (i.e., transient hypokalemia),25 (2) ultrafiltration may temporarily increase serum digoxin level through its concentration,26 and (3) uremia has been associated with altered digitoxin metabolism27 with a 35% decrease in digitoxin binding reported during active HD treatment.28 Furthermore, responses to dosage corrections are slow because of the extended half-life of the drug. Overall, the cumulative effect of these factors likely complicates the maintenance of stable/subtoxic digoxin levels and may increase the risk–benefit ratio of digoxin use in the ESRD population.
There are some important limitations of this study. Potential unmeasured confounders may provide for study bias. For example, dry weight remains an inexact and uncharted patient characteristic that could affect mortality; structural cardiac parameters, such as ejection fraction and left ventricular dimension, were unavailable for covariate adjustment; digoxin prescription (versus nonuse) and higher digoxin levels may have been targeted in the more severely ill patients; and residual confounding from the facility and physician may remain despite case-control matching. Information bias from misclassification could also be present through the review of medical records as opposed to prospective data collection and adjudication under a research protocol.
In conclusion, digoxin should be prescribed with caution to patients who are on long-term HD. Given the alteration in pharmacokinetic and K shifts seen during dialysis, combined with the potential toxicity and tight therapeutic index of the drug, strict K and digoxin level management is recommended when patients with ESRD remain on digoxin until its overall safety is better evaluated through randomized, controlled trials.
Concise Methods
Overview, Population, and Data Sources
All incident HD patients who were admitted to a Fresenius Medical Care North America (FMCNA) facility (>1800 facilities) during a 6-year period were enrolled in the study when (1) they were admitted as “new to chronic hemodialysis” (i.e., incident patient), and (2) the admission occurred between January 1, 2001, and December 31, 2006. Incident patients were further classified as digoxin “nonusers” when they had no documented prescription of the drug in the first 90 days of HD and “users” when (1) digoxin was on their list of admission oral medications, and (2) the patient remained on digoxin for ≥90 days after the initiation of long-term RRT. Follow-up occurred from after the 90-day baseline period for up to 4 years until patients were deceased, received a transplant, or transferred to a non-FMCNA clinic or on April 1, 2009 (the last day of follow-up). The analysis was intention-to-treat, whereby patients who changed or stopped their digoxin remained in their exposure group and were followed until death or censorship. Patients were excluded (7.7% of patients) when they survived <90 days after the initiation of ESRD therapy.
Deidentified clinical data abstracted from the medical records of FMCNA was used for the analysis. The clinical information system prospectively collects demographic, laboratory, treatment modality, medication, and outcome parameters for patients in all FMCNA clinics. A usual indication for digoxin therapy (atrial fibrillation or CHF) was charted in the FMCNA medical records of 78% of the patients who were on digoxin. Further description of the clinical data system for pharmacoepidemiologic research is provided through previous peer-reviewed publications.7,8,29–31 All laboratory testing was processed through a single accredited laboratory (Spectra Laboratories, Rockleigh, NJ).
Outcomes, Exposures, and Covariates
The primary outcome of the study was mortality. Initially, the risk for death between digoxin users and nonusers was calculated among all incident patients and then subsequently among digoxin users only by the prescribed digoxin dosage (μg/d on the 90th day) and measured serum digoxin level (ng/ml) during the first 90 days of dialysis. Digoxin dosage was modeled both as a continuous and an ordinal variable. Baseline covariates (listed in Table 2) were ascertained during the initial 90 days of HD.
Statistical Analysis
Baseline patient characteristics were tabulated by exposure group and compared using t tests (continuous parameters) or χ2 statistic (categorical parameters). Crude survival curves were initially produced using the Kaplan-Meier method and compared using log-rank tests. Unadjusted Cox regression was then used to determine the relative mortality risk by exposure group. The final adjusted model included 40 baseline covariates with additional weighting by the inverse probability of treatment32,33 (i.e., 1/probability of digoxin dosage) to control for potential confounding by indication. The probability of treatment (i.e., propensity score) was calculated as a function of all baseline covariates (Table 2) using logistic regression for digoxin users versus nonusers and then with generalized logistic regression by four increasing ordinals of digoxin exposure.34 Model overfitting was assessed with the bootstrap resampling method (n = 200) described by Harrell et al.35,36 and was reported only when the R2 was >5% (i.e., overfitting). Potential collinearity between exposure and model covariates was evaluated by the Pearson correlation coefficient. Effect modification was defined as significant when the P value of the interaction term (digoxin*covariate) was ≤0.05. P for trend was determined using the median for each category. Statistical computations were done using SAS 9.1 (SAS Institute, Cary, NC).
Sensitivity Analysis: Serum Digoxin Level Associates with Increased Mortality
Covariate- and propensity-adjusted models were further stratified on nine predetermined patient characteristics (age, gender, race, diabetes status, calcium, K, NYHA functional class, inverse propensity for treatment, and indication for digoxin) and seven variables for which significant baseline differences were noted (BP, interdialytic weight gain, albumin, antiplatelet use, creatinine, dialysis time, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use). Because of statistically significant interaction effects between digoxin and K, adjusted mortality HRs were also calculated after stratification by tertiles of serum K and digoxin level.
To account for disease severity, we repeated the primary analysis with NYHA functional class as an additional covariate added to propensity score and Cox regression models. The prognostic value of NYHA on mortality in ESRD was previously validated by Postorino et al.37
Given the potential for survivorship bias, a “new user” design38,39 was implemented whereby mortality was examined for patients who initiated digoxin during ESRD, instead of patients who continued their digoxin into ESRD. More specifically, patients were enrolled in the secondary study when their first cardiac glycoside prescription occurred after the first 90 days of HD.
The primary analysis was intention-to-treat and did not take into account changing digoxin levels over time; consequently, we also performed a time-varying analysis that accounted for the longitudinal variations in serum digoxin level and 13 patient characteristics (see of Table 7). For example, mortality HRs in the primary analysis were recalculated when serum digoxin level and patient characteristics were modeled as time-varying estimates (90-day cycles).
The potential for residual and nonlinear confounding from the facility, treating physician, and propensity score were also explored in secondary subcohorts that were formed with 1:1 “greedy matching.”40,41 For balancing for possible unmeasured confounders inherent to the treating clinic, each “high” digoxin user (serum level >1.0 ng/ml) was paired with a “low” user (serum level ≤1.0 ng/ml) in the same facility. The procedure was then repeated with matching on physician and propensity score instead.
Disclosures
None.
Acknowledgments
We express our appreciation to the staff in > 1800 Fresenius dialysis clinics who continually make great efforts to ensure the accurate charting of clinical data in the computer system.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Digitalis and Hemodialysis Is a Bad Combination,” on pages 1418–1420.
REFERENCES
- 1. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med 336: 525–533, 1997 [DOI] [PubMed] [Google Scholar]
- 2. K/DOQI clinical practice guidelines on cardiovascular disease in dialysis patients: Overview of the epidemiology of cardiovascular disease. Am J Kidney Dis 45: 8–9, 2005 [PubMed] [Google Scholar]
- 3. Haji SA, Movahed A: Update on digoxin therapy in congestive heart failure. Am Fam Physician 62: 409–416, 2000 [PubMed] [Google Scholar]
- 4. Olshansky B, Rosenfeld LE, Warner AL, Solomon AJ, O'Neill G, Sharma A, Platia E, Feld GK, Akiyama T, Brodsky MA, Greene HL: The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: Approaches to control rate in atrial fibrillation. J Am Coll Cardiol 43: 1201–1208, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Hauptman PJ, Kelly RA: Digitalis. Circulation 99: 1265–1270, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Moe GK, Farah AE: Digitalis and allied cardiac glycosides. In: The Pharmacological Basis of Therapeutics, edited by Goodman L, Gilman A, New York, Macmillan, 1965, p 674 [Google Scholar]
- 7. Chan KE, Lazarus JM, Thadhani R, Hakim RM: Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol 20: 872–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan KE, Lazarus JM, Thadhani R, Hakim RM: Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 20: 2223–2233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott MJ, Zimmerman D, Holden RM: Warfarin anticoagulation in hemodialysis patients: A systematic review of bleeding rates. Am J Kidney Dis 50: 433–440, 2007 [DOI] [PubMed] [Google Scholar]
- 10. US Renal Data System: USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 11. Jencks SF, Williams MV, Coleman EA: Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 360: 1418–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Chan KE, Lazarus JM, Wingard RL, Hakim RM: Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int 76: 331–341, 2009 [DOI] [PubMed] [Google Scholar]
- 13. McMahon WS, Holzgrefe HH, Walker JD, Mukherjee R, Arthur SR, Cavallo MJ, Child MJ, Spinale FG: Cellular basis for improved left ventricular pump function after digoxin therapy in experimental left ventricular failure. J Am Coll Cardiol 28: 495–505, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Smith TW: Digitalis: Mechanisms of action and clinical use. N Engl J Med 318: 358–365, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Gheorghiade M, Braunwald E: Reconsidering the role for digoxin in the management of acute heart failure syndromes. JAMA 302: 2146–2147, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Rathore SS, Wang Y, Krumholz HM: Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 347: 1403–1411, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M: Digoxin and reduction in mortality and hospitalization in heart failure: A comprehensive post hoc analysis of the DIG trial. Eur Heart J 27: 178–186, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM: Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 289: 871–878, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Ahmed A, Pitt B, Rahimtoola SH, Waagstein F, White M, Love TE, Braunwald E: Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: A propensity-matched study of the DIG trial. Int J Cardiol 123: 138–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC, Jr: ACC/AHA Guidelines for the Evaluation, Management of Chronic Heart Failure in the Adult: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration with the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 104: 2996–3007, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B: ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 112: e154–e235, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW: 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: e391–e479, 2009 [DOI] [PubMed] [Google Scholar]
- 23. De Vito JM, Crass RE, Blum RA, Pleasants RA, Schentag JJ: Estimation of the steady-state volume of distribution for digoxin: A comparison of model-independent methods with a two-compartment model in healthy volunteers. Drug Intell Clin Pharm 19: 837–839, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Morrison G, Michelson EL, Brown S, Morganroth J: Mechanism and prevention of cardiac arrhythmias in chronic hemodialysis patients. Kidney Int 17: 811–819, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Feig PU, Shook A, Sterns RH: Effect of potassium removal during hemodialysis on the plasma potassium concentration. Nephron 27: 25–30, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Steiner JF, Robbins LJ, Hammermeister KE, Roth SC, Hammond WS: Incidence of digoxin toxicity in outpatients. West J Med 161: 474–478, 1994 [PMC free article] [PubMed] [Google Scholar]
- 27. Storstein L: Studies on digitalis: XI. Digitoxin metabolism in patients with impaired renal function. Clin Pharmacol Ther 21: 536–546, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Storstein L: Studies on digitalis: V. The influence of impaired renal function, hemodialysis, and drug interaction on serum protein binding of digitoxin and digoxin. Clin Pharmacol Ther 20: 6–14, 1976 [DOI] [PubMed] [Google Scholar]
- 29. Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, Hakim RM: Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 17: 363–371, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Rosenbaum PR: Model-based direct adjustment. J Am Stat Assoc 82: 387–394, 1987 [Google Scholar]
- 33. Hirano KI: Estimation of causal effects using propensity score weighting: An application to data on right heart catheterization. Health Serv Outcomes Res Methodol 2: 259–278, 2001 [Google Scholar]
- 34. Imbens G: The role of propensity score in estimating dose-response in observational studies for causal effect. Biometrika 3: 706–710, 2000 [Google Scholar]
- 35. Harrell FE, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Stocken DD, Hassan AB, Altman DG, Billingham LJ, Bramhall SR, Johnson PJ, Freemantle N: Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 99: 883–893, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Postorino M, Marino C, Tripepi G, Zoccali C: Prognostic value of the New York Heart Association classification in end-stage renal disease. Nephrol Dial Transplant 22: 1377–1382, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA: Antidepressants and the risk of falls among nursing home residents. N Engl J Med 339: 875–882, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Ray WA, Chung CP, Murray KT, Hall K, Stein CM: Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 360: 225–235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kosanke J, Bergstralh E: Gmatch: SAS macro, 2004. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas Accessed December 12, 2008
- 41. Rubin D: Matching to remove bias in observational studies. Biometrics 29: 159–183, 1973 [Google Scholar]