Abstract
Mutations in PKD1, which encodes polycystin-1 (PC1), contribute to >85% of cases of autosomal dominant polycystic kidney disease (ADPKD). The planar cell polarity (PCP) pathway is necessary for the oriented cell division and convergent extension that establishes and maintains the structure of kidney tubules, but the role of this pathway in the pathophysiology of ADPKD is incompletely understood. Here, we show that inactivation of Pkd1 in postnatal developing mouse kidneys leads to a defect in oriented cell division in precystic kidney tubules. We also observed this defect in precystic Pkd1-inactivated mature kidneys subjected to ischemia-reperfusion injury as a “third hit.” Cystic kidneys exhibited striking upregulation and activation of Frizzled 3 (Fz3), a regulator of PCP, and its downstream effector, CDC42. Precystic kidneys demonstrated upregulation of CDC42, but the localization of the polarity proteins Par3 and Par6 was similar to control. Fz3 was expressed on the cilia of cystic kidneys but barely detected on the cilia of normal kidneys. In vitro, PC1 and Fz3 antagonized each other to control CDC42 expression and the rate of cell migration in HEK293T cells. Taken together, our data suggest that PC1 controls oriented cell division and that aberrant PCP signaling contributes to cystogenesis.
Polycystic kidney disease (PKD) is a common genetic disorder, affecting one in 500 individuals in the United States. This disease is identified by the growth of numerous cysts in the kidneys, which eventually lead to ESRD necessitating dialysis and kidney transplantation.1 Autosomal dominant PKD (ADPKD) is the most common inherited form, caused by mutations in PKD1 and PKD2 in 85 and 15% of the cases, respectively. ADPKD also affects other tissues, resulting in hepatic and pancreatic cysts, intracranial aneurysms, and heart valve defects.1 Overexpression or downregulation of polycystin-1 (PC1) and -2 (PC2), gene products of PKD1 and PKD2, leads to uncontrolled tubule lumen size.2 The molecular mechanism of tubule lumen size restriction, however, is still unclear.
Most of our major organs, including lung, kidney, mammary gland, and vasculature, are composed primarily, sometimes exclusively, of tubules.3 During tubule growth, cell polarity must be precisely controlled and cellular adherens junctions need to be continuously remodeled without losing cell–cell contacts. This is a complex two-step process in which cells depolarize and migrate away to form elongated tubules and repolarize once they have reached their new position.4 At least in postnatal kidneys, maturation of tubules requires substantial elongation, involving an intense proliferation phase. This type of tubule elongation is associated with oriented cell division in which mitotic cells are oriented along the tubular axis. This process determines the diameter of a tubule and likely requires intrinsic planar cell polarity (PCP) signaling,5 a noncanonical Wnt signaling pathway. PCP was first described in Drosophila and is defined as the process in which epithelial cells become polarized in the plane of a tissue, perpendicular to the apical-basal axis.6 The core PCP components Frizzled (Fz), Dishevelled (Dvl), Prickle, Van Gogh, Diego, and Flamingo were first identified in Drosophila, where they function in regulating tissue organization.6,7 In mammals, PCP signaling also regulates convergent extension movements that are required for neural tube closure and lengthening of embryos and kidney tubules.8,9 Recently, a group of PCP genes, Fat, Dachsous, and Four-jointed (Fj), were identified in Drosophila as an upstream cassette of transmembrane proteins, providing an initial cue at the cell surface to induce asymmetrical localization of the core PCP components. Interestingly, loss of the vertebrate Fat homolog, Fat4, disrupts oriented cell division and tubule elongation during kidney development, causing tubule dilation. This cystic phenotype in Fat4 mutants is enhanced by loss of the core PCP component Vangl2 as well as loss of the Fj ortholog, Fjx1.10
In this study, we demonstrated that Pkd1 inactivation affects oriented cell division in precystic Pkd1-inactivated developing and injured kidneys. Moreover, the PCP components Fz3 and CDC42 are significantly upregulated and activated in cystic kidneys. Interestingly, we found that Fz3 localizes to the cilia and the centrosomes in renal tubules. We also showed that Fz3 and PC1 antagonize each other to regulate CDC42 expression and cell migration in kidney cells, suggesting that the polycystin pathway interacts with the Fz3 pathway.
Results
Tubular Cell Circumference in Normal and Pkd1-Inactivated Postnatal Mouse Kidneys
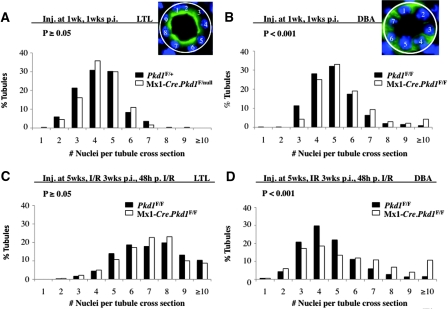
The number of cells surrounding a tubule, namely tubular cell circumference, in postnatal developing or adult mouse kidneys is not known. We used lotus tetragonolobus lectin (LTL) to mark proximal tubules and dolichos biflorus agglutinin (DBA) for collecting tubules/ducts to determine the tubular cell circumference in these tubular segments. To ensure that only cross-sections of a tubule were evaluated (Figure 1, A and B, insets), we excluded tubules that varied significantly from being perfect circles. We found that average tubular cell circumference for proximal tubules increased by approximately 1.6-fold in 6-week-old (data not shown) and by approximately 1.7-fold in 8-week-old kidneys compared with 2-week-old kidneys (Supplemental Figure S1A). By contrast, a slight decrease in average tubular cell circumference was seen in DBA+ tubules (Supplemental Figure S1A). It is noteworthy that the rate of cell proliferation is high at 2 weeks and low at 8 weeks but is comparable between proximal tubules and connecting tubules/ducts (data not shown). This suggests that the increase of tubular cell circumference in the proximal tubule may be due to the absence or lesser degree of convergent extension during postnatal development in this segment of the tubule.
Figure 1.
Tubular cell circumference (TCC) distribution is altered in distal precystic Pkd1-inactivated tubules. (A through D) Graphs represent the distribution of TCC in proximal tubules (LTL; A and C) and distal tubules (DBA; B and D) in 2-week-old developing Mx1-Cre.Pkd1F/null or Mx1-Cre.Pkd1F/F precystic (LTL n = 310, DBA n = 225, four mice) and Pkd1F/+ or Pkd1F/F control (LTL n = 169, DBA n = 458, three mice) kidneys (A and B) and in 8-week-old injured Mx1-Cre.Pkd1F/F precystic (LTL n = 459, DBA n = 470, three mice) and Pkd1F/F control (LTL n = 359, DBA n = 511, three mice) kidneys (C and D). (A and C) Note that there is no difference in TCC distribution in normal versus precystic proximal tubules. The TCC distribution in precystic collecting tubules shifts to the right. Insets are shown to demonstrate that only perfect epithelial cross-sections were evaluated.
To determine whether tubular cell circumference varied between control and precystic kidneys, we measured the number of cells that make up the proximal and collecting tubules/ducts in an Pkd1 inducible knockout (IKO) mouse model that we recently generated.11 We noticed a right shift in tubular cell circumference distribution in DBA+ tubules of IKO mice 1 week after Pkd1 inactivation at 1 week of age (Figure 1B). Average tubular cell circumference was increased approximately 0.49 cells compared with their age-matched control littermates (Supplemental Figure S1A). Because there is no increase in cell proliferation between normal and precystic kidneys,11 this might be due to aberrant cell–cell intercalations or convergent extension movements in the distal tubular segments. A stronger shift to the right (Figure 1D) in distal tubular cell circumference distribution was seen in adult IKO mice with unilateral renal ischemia-reperfusion injury (IRI),12 where the average tubular cell circumference was increased by 1.03 cells. This might be partially due to increased cell proliferation after IRI in IKO kidneys.12 It is noteworthy that there is no change in tubular cell circumference distribution in proximal tubules (Figure 1, A and C), where Cre recombinase is not expressed in this model at either stage.11
Oriented Cell Division Is Randomized in Precystic Pkd1 IKO Mouse Kidneys
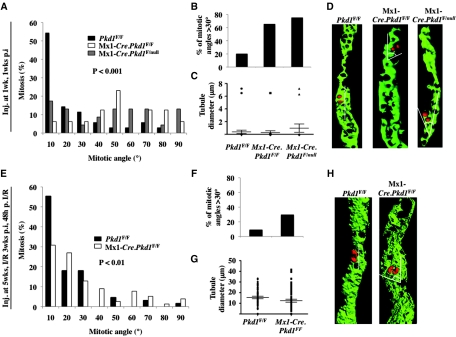
One possible explanation for increased tubular cell circumference in DBA+ tubules of IKO mice is an abnormality in oriented cell division or in the cell intercalation process during tubule elongation. We stained DBA+ tubules with phospho-histone 3 to label condensing chromosomes in dividing cells in 2-week-old precystic Pkd1 IKO kidneys and controls. In control kidneys, the orientation of mitotic angles of dividing tubular cells is mostly in parallel with the tubular axis (Figure 2, A through D), only 20% of dividing cells have the mitotic angles >30° (Figure 2B). By contrast, there is a right shift in the distribution of the measured mitotic angles in IKO kidneys (Figure 2, A through D) with approximately 65.2 and 75.0% of the mitotic angles >30° (Figure 2B), indicating aberrant oriented cell division in precystic kidneys.
Figure 2.
Mitotic angles are randomized in precystic Pkd1 KO kidneys. (A through H) Mitotic orientations of dividing precystic Pkd1-inactivated distal tubules are randomized in 2-week-old developing (A through D) and in 8-week-old injured mouse kidneys (E through H). Graphs demonstrate the distribution of mitotic angles in DBA+ tubules in 2-week-old Mx1-Cre.Pkd1F/F (n = 23, three mice), Mx1-Cre.Pkd1F/null (n = 13, two mice), and Pkd1F/F control kidneys (n = 35, three mice; A) and in 8-week-old Mx1-Cre.Pkd1F/F (n = 67, two mice) precystic and Pkd1F/F control injured kidneys (n = 78, three mice; E). (B and F) Note that there is a significant shift to the right for both 2- and 8-week-old groups, resulting in a significantly higher amount of mitotic angles >30° in Pkd1-inactivated kidneys. (C and G) Note that the diameter of tubules used for measurements of mitotic angles in precystic IKO kidneys is not different from that in control kidneys shown by t test (P > 0.1). (D and H) Three-dimensional reconstruction of the mitotic orientations of dividing control and precystic renal tubular cells.
A recent study showed defects in oriented cell division after toxic tubular injury in renal proximal tubules of a Pkd1 IKO mouse model.13 We looked at the effect of renal IRI on oriented cell division in distal tubular segments. We found an oriented cell division in normal DBA+ tubules after IRI such that only 8.5% of the mitotic angles are >30°. In IKO kidneys, however, 29.49% of mitotic angles are >30° (Figure 2, E through H). In agreement with these data, we observed up to 17-fold higher rate of “out of the plane” division, namely cell division occurs perpendicular to the tubule length in precystic DBA+ tubules (Supplemental Figure S1), which suggests randomization of oriented cell division in IKO IRI kidneys. In addition, we measured the diameters of tubules used for the oriented cell division analyses and observed no difference between the control and IKO kidneys (Figure 2, C and G). These data demonstrate that loss of oriented cell division precedes tubule dilation.
PCP Components Fz3 and CDC42 Are Upregulated in Cystic Kidneys of Pkd1 KO Mice
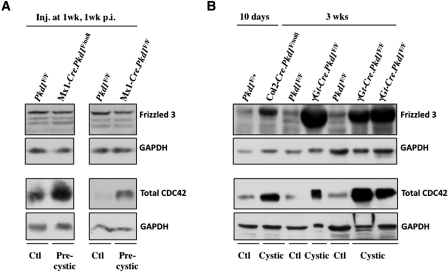
Oriented cell division in tubular cells requires intrinsic PCP.5 Fz is a core PCP component and controls the orientation of asymmetric sense organ precursor cell divisions in Drosophila.14 In vertebrates, Fz3 and Fz6 are considered to be the key Fz receptors regulating PCP signaling. Although Fz3 is not obviously upregulated in precystic IKO kidneys (Figure 3A), its levels are strongly increased in Col2-Cre.Pkd1F/null, γGt-Cre.Pkd1F/F and Mx1-Cre.Pkd1F/F cystic kidneys, compared with control littermate kidneys (Figure 3B; data not shown).
Figure 3.
Fz3 is upregulated in cystic but not in precystic Pkd1-inactivated mouse kidneys. (A and B) Western blots show that CDC42 but not Fz3 protein levels are upregulated in precystic Mx1-Cre.Pkd1F/F kidneys (A). Both Fz3 and CDC42 levels are greatly upregulated in 10-day-old Col2-Cre.Pkd1F/null and 3-week-old γGt-Cre.Pkd1F/F cystic kidneys. GAPDH was used as loading control.
CDC42 is a downstream PCP component, and its depletion causes mitotic spindle misorientation.15 We detected an increase in CDC42 levels in precystic IKO kidneys compared with control kidneys (Figure 3A). This increase was even more pronounced in Col2-Cre.Pkd1F/null, γGt-Cre.Pkd1F/F and Mx1-Cre.Pkd1F/F cystic kidneys (Figure 3B; data not shown). These data suggest that the PCP pathway is upregulated in precystic and cystic kidneys. CDC42 associates with the Par3 and Par6 polarity complex16; however, the expression and localization of the Par3 and the Par6 polarity complex seem to be unchanged in precystic kidneys compared with control littermates (Supplemental Figure S2).
Fz3 Translocates to the Apical Membrane of the Cyst-Lining Epithelium in Pkd1 Inactivated Cysts Derived from Distal Tubules
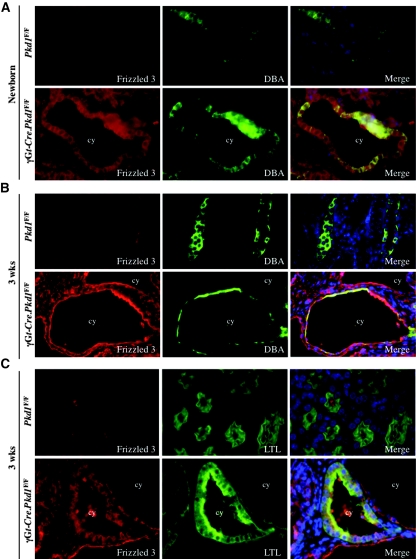
Asymmetric subcellular distribution of core PCP components is critical for correct PCP signaling in Drosophila7; therefore, we double-stained newborn and 3-week-old cystic kidneys from γGt-Cre.Pkd1F/F mice and Cre− control littermates with Fz3 antibodies and tubular markers DBA or LTL. Rabbit IgG was used as control (Supplemental Figure S3A). Consistent with our Western blot data, we detected weak Fz3 signals in controls and prominent signals in the apical membrane of DBA+ cysts in γGt-Cre.Pkd1F/F cystic kidneys. There was no specific asymmetric localization of Fz3 in DBA+ tubules in either newborn or 3-week-old kidneys regardless of their genotype (Figure 4, A and B). Strong apical expression of Fz3 was also observed in Mx1-Cre.Pkd1F/null and in Col2-Cre.Pkd1F/null cystic kidneys (data not shown; Table 1). Some DBA− cysts also showed enhanced apical Fz3 signals (Figure 4B). These cysts are likely derived from the thin ascending loop of Henle, where Pkd1 is also inactivated. In agreement with Western blot results, there was no change in Fz3 expression levels or apical membrane localization in precystic kidneys with Pkd1 KO induced at either 1 week (data not shown) or 5 weeks (Supplemental Figure S3B, Table 1).
Figure 4.
Fz3 is upregulated at the apical cyst-lining epithelium in cysts derived from the DBA+ tubules in 3-week-old mouse kidneys. (A through C) Immunofluorescence micrographs show the Fz3 expression pattern in newborn (A) and 3-week-old (B and C) γGt-Cre.Pkd1F/F cystic and Pkd1F/F control kidneys. Fz3 is upregulated in newborn (A, bottom) as well as in 3-week-old (B and C, bottom) γGt-Cre.Pkd1F/F cystic kidneys. In newborn kidneys, Fz3 localizes diffusely in normal as well as in the cyst-lining DBA+ epithelial cells (A). (B) In 3-week-old normal kidneys, Fz3 is diffusely localized in DBA+ tubules. In γGt-Cre.Pkd1F/F cystic kidneys, strong Fz3 signals are found in the apical membrane of cyst-lining DBA+ epithelial cells. (C) In 3-week-old Pkd1F/F control kidneys, weak apical expression of Fz3 is seen in LTL+ proximal tubules, which become diffused in the cytoplasm of γGt-Cre.Pkd1F/F LTL+ cysts. Pictures are taken with the same exposure time. Magnification, ×60.
Table 1.
Fz3 localization on DBA+ and LTL+ tubules in inducible and conditional Pkd1 KO mice
| Phenotype | Genotype | Fz3 Localization |
Age of Analyses | Age after Injury | Age after IRI | |
|---|---|---|---|---|---|---|
| DBA+ Tubules | LTL+ Tubules | |||||
| WT precystic | Control1 Mx1-Cre.Pkd1F/F | Weak cystosol and apical | Apical | 6 weeks | 1 weeks | |
| WT cystic | Control2 Mx1-Cre.Pkd1F/null | Weak cystosol and apical, intense apical membrane of cysts | Apical | 10 weeks | 5 weeks | |
| WT very cystic | Control2 Mx1-Cre.Pkd1F/null | Weak cystosol and apical, intense apical membrane of cysts | Apical | 25 weeks | 20 weeks | 17 weeks |
| WT cystic | Control1 gGt-Cre.Pkd1F/F | Weak cytosol, slightly increased cytosol | ND | Newborn | ||
| WT very cystic | Control1 Col2/gGt-Cre.Pkd1F/F | Weak cystosol and apical, intense apical membrane of cysts | Weakly apically diffuse | 3 weeks | ||
Control1, Pkd1F/F; Control2, Pkd1F/+.
In LTL+ tubules, weak Fz3 expression was seen in the apical membrane of control kidneys, although it was replaced by stronger cytoplasmic signals in LTL+ cysts in 3-week-old γGt-Cre.Pkd1F/F kidneys (Figure 4C, Table 1). As expected, we did not detect differences in Fz3 localization in LTL+ tubules in IKO cystic kidneys (Table 1), which do not develop cysts in this model.11 These data suggest that Fz3 may have distinct roles in proximal and distal tubular segments, and its activity increases before or coincident with cyst formation.
CDC42 Activation in Cyst-Lining Epithelial Cells in Pkd1-Inactivated Kidneys
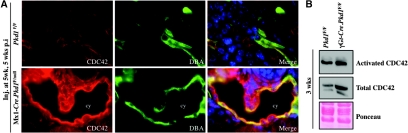
Membrane localized CDC42 seems to be critical for its function.17 Thus, we investigated CDC42 subcellular localization in the kidney. Double-labeling of control and cystic kidneys with CDC42 antibodies and DBA revealed increased CDC42 expression in IKO cystic kidneys compared with their control littermates (Figure 5A). Whereas CDC42 localized diffusely in the cytoplasm of control collecting ducts, it translocated to the apical membrane in DBA+ cyst-lining cells in IKO kidneys (Figure 5A), suggesting its activation. Active CDC42 pull-down assay confirmed CDC42 activation in cystic γGt-Cre.Pkd1F/F kidneys (Figure 5B).
Figure 5.
CDC42 is activated in cystic kidneys. (A) Immunofluorescence micrographs represent the CDC42 expression pattern in Mx1-Cre.Pkd1F/null cystic and Pkd1F/F control kidneys. Note that in Mx1-Cre.Pkd1F/null cystic kidneys, CDC42 localizes particularly to the apical membrane, in contrast to a diffused expression pattern in Pkd1F/F control kidneys. Pictures are taken with the same exposure time. (B) Western blots demonstrate that total as well as activated CDC42 is increased in γGt-Cre.Pkd1F/F cystic kidneys. Ponceau staining is used to show equal loading (bottom). Magnification, ×60.
PC1 and Fz3 Have Antagonizing Effects in Stable YFP-PC1-myc–Inducible HEK293T Cells
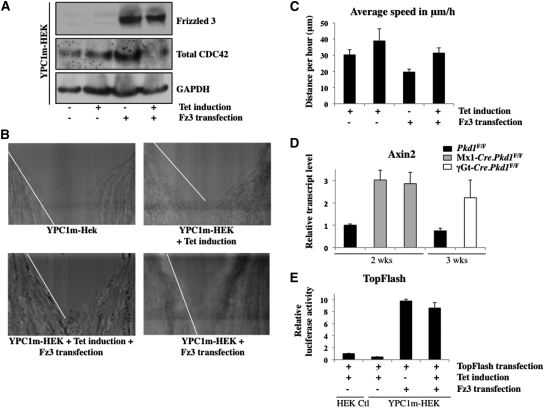
Because Fz3 and PC1 both are heterotrimeric G-protein–coupled transmembrane receptors, we hypothesized that they may act in concert to regulate downstream signaling. We transiently expressed Fz3 in an inducible HEK293T cell line stably expressing PC1 (YPC1m-HEK) and assayed the expression of CDC42. Expression of Fz3 induced CDC42 expression when compared with parental cells (Figure 6A). Coexpression of PC1 and Fz3, however, caused a reduction in the levels of total CDC42 (Figure 6A). Induction of PC1 expression alone did not increase total CDC42 expression (Figure 6A, lane 2). To evaluate further the antagonizing effect of PC1 and Fz3, we subjected the same cells to a wound-healing assay and observed the rate of cell migration toward the wound by time-lapse live-cell imaging for 20 hours for each experiment (Figure 6B). We found that cells with induced PC1 expression moved faster than control cells (Figure 6, B and C), as previously reported.18 By contrast, Fz3-expressing cells moved slower than control cells (Figure 6, B and C). When both Fz3 and PC1 were present, the cells moved at the same rate as the control cells (Figure 6, B and C). These data suggest that Fz3 antagonizes PC1 effects on cell migration.
Figure 6.
Fz3 and PC1 antagonize each other in an inducible PC1-expressing cell line, and Axin2 expression is increased in precystic and cystic Pkd1 KO kidneys. (A) Western blots show that total CDC42 expression is not increased after PC1 induction (compare first and second lanes with GAPDH) but strongly after Fz3 overexpression (compare first and third lanes). Note that Fz3 and PC1 coexpression decreases total CDC42 expression levels (compare first and last lanes). GAPDH is used as loading control. (B) Chymographs demonstrate the distance traveled by YPC1m-HEK cells during a time-lapse videomicroscopy recording of wound-healing assays. Pictures are taken every 6 minutes during 20 hours. A white line is drawn next to the edge of the wound; a steeper white line indicates a slower closure. PC1 induction increases the speed, demonstrated by a gentle slope; Fz3 decreases the speed, showed by a steeper slope. Note that the slope in the first, control, and in the last, Fz3-transfected and PC1-induced chymograph, is the same. (C) Graph represents the quantification of the speed of migration of YPC1m-HEK cells in different fields in B. This confirms that cells transfected with Fz3 migrate slower, whereas cells transfected with Fz3 and induced with PC1 move with a similar speed as their counterpart controls. (D) Graph demonstrates the upregulation of Axin2 via quantitative reverse transcriptase–PCR in precystic Mx1-Cre.Pkd1F/F and Mx1-Cre.Pkd1F/null and in cystic γGt-Cre.Pkd1F/F kidneys compared with their control littermates. All samples are normalized to the 2-week-old control kidney, which was set as 1. (E) Graph represents the relative luciferase activity in HEK control and YPC1m-HEK cells with or without Fz3 transfection and tetracycline induction. PC1 induction slightly reduces luciferase activity. All samples were normalized to the HEK control column, which was set as 1. Bars indicate SEs. All experiments were repeated at least three times. Magnification, ×10.
Activation of the Canonical Wnt Signaling Pathway in Pkd1 Mutants Is Likely Independent of Fz3-CDC42
Fz3 homodimerization and CDC42 both have been shown to promote β-catenin–dependent transcription.19,20 In addition, canonical Wnt signaling was shown to be upregulated in ADPKD.13 To investigate whether upregulation of Fz3 and CDC42 corresponds to an increase in β-catenin–dependent gene transcription, we measured the mRNA levels of Axin2 as readout for the canonical Wnt signaling pathway in precystic and cystic kidneys. By quantitative reverse transcriptase–PCR, we observed an increase in Axin2 transcript levels in cystic kidneys (Figure 6D, compare the right two columns), where both Fz3 and CDC42 are upregulated (Figure 3B), as well as in precystic kidneys (Figure 6D, compare the left three columns), where only CDC42 is upregulated (Figure 3A). Similar results were obtained for the canonical Wnt target gene Cyclin D (data not shown). To determine whether this β-catenin–dependent transcription in precystic kidneys might be CDC42 dependent, we examined β-catenin–dependent transcription in Fz3 and PC1 coexpressing cells with very low CDC42 expression (Figure 6A). TopFlash reporter assays in induced YPC1m-HEK cells transiently expressing Fz3 failed to show significant reduction of the relative luciferase activity (Figure 6E), suggesting that β-catenin–dependent transcription in Pkd1 mutants is likely independent of Fz3-CDC42.
Aberrant Regulation of Fz3-CDC42 in Human ADPKD Kidneys
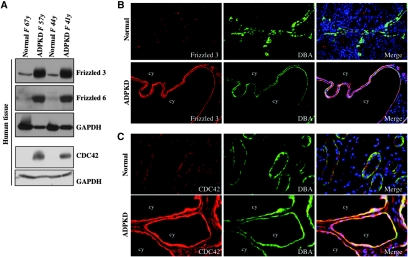
To determine whether Fz3 and CDC42 upregulation also occurs in humans, we performed Western blot analyses on kidneys from four unrelated patients with ADPKD and four normal individuals. Strong Fz3 and CDC42 upregulation was detected in patients with ADPKD, compared with normal kidneys (Figure 7A). Interestingly, Fz6 expression was also upregulated in kidneys of patients with ADPKD (Figure 7A).
Figure 7.
Fz3 is upregulated in human ADPKD kidneys. (A) Western blots represent increased Fz3, Fz6, and CDC42 protein levels in human ADPKD kidneys (lanes 2 and 4) compared with normal kidneys (lanes 1 and 3). GAPDH is used as a loading control (bottom). (B and C) Immunofluorescence micrographs demonstrate Fz3 (B) and CDC42 (C) localization in normal (top) and ADPKD (bottom) kidneys. Note in DBA+ tubules of normal kidneys, Fz3 localizes weakly at the apical membrane, whereas CDC42 localizes diffusely in the cytosol. In ADPKD kidneys, both Fz3 and CDC42 are strongly upregulated at the apical membrane of cyst-lining epithelial cells in distal cysts. Pictures are taken with the same exposure time. Magnification, ×20 in B; ×40 in C.
Dual immunofluorescence of normal human kidneys with Fz3 or CDC42 antibodies and DBA revealed weak Fz3 expression in the apical membrane of DBA+ tubules (Figure 7B), whereas CDC42 was diffusely localized in the cytoplasm (Figure 7C). In ADPKD kidneys, both Fz3 and CDC42 expression was elevated, and strong signals delineated the apical membrane of the cyst-lining epithelium in DBA+ cysts (Figure 7, B and C). We therefore believe that Fz3-CDC42 activation is not limited to Pkd1 KO mouse models.
Fz3 Localizes to the Primary Cilia
Studies have suggested that PCP signaling in vertebrates may be controlled and modulated by primary cilia, organelles that emerge from the apical membrane of most cell types.21–24 Core PCP components Fat4 and Vangl2 also localize at the base of primary cilia.10 To explore a possible role for Fz3 on cilia, we investigated whether Fz3 localizes to the cilia in tissues and epithelial cell lines from human and mouse kidneys with or without defects in PC1.
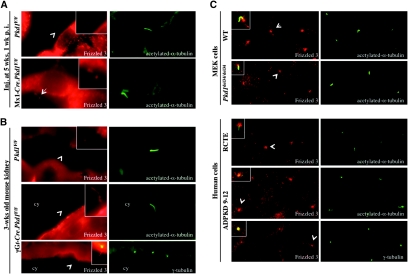
Using acetylated α-tubulin as a cilium marker, we found that Fz3 localizes to the primary cilia, although its signal seems to be stronger on cilia in precystic tubules in Mx1-Cre.Pkd1F/F mice (Figure 8 A) and cyst-lining epithelia in γGt-Cre.Pkd1F/F mouse kidneys (Figure 8B) than in tubules of control kidneys. Similar results were obtained from mouse Mx1-Cre.Pkd1F/null cystic kidneys and human ADPKD kidneys (data not shown). Ciliary localization for Fz3 was confirmed by double labeling of Fz3 and γ-tubulin (Figure 8B) to exclude the possibility of nonspecific binding of the Fz3 antibody to the acetylated α-tubulin antibody. In cultured wild-type and Pkd1del34/del34 mouse embryonic kidney cells and in human cyst-lining epithelial cells 9-12 immortalized from human ADPKD kidneys and control renal cortical tubular epithelial cells immortalized from normal human kidneys25 (Figure 8C), Fz3 is localized at the base of the cilia. These data suggest that PC1 mutation does not affect Fz3 ciliary localization.
Figure 8.
Fz3 localizes to the cilia. (A through C) Dual immunofluorescence of Fz3 and acetylated α-tubulin or γ-tubulin show Fz3 localization on cilia in mouse Pkd1F/F control and Mx1-Cre.Pkd1F/F precystic kidneys (A) and mouse Pkd1F/F control and γGt-Cre.Pkd1F/F cystic kidneys (B) and Fz3 localization at the base of the cilia in WT (top), Pkd1del34/del34 (second panel) mouse embryonic kidney cells, human retinal cortical tubular epithelial cells (third panel), and ADPKD 9-12 cells (bottom; C). Arrowheads point to cilia/centrosomes shown in the insets. Magnification, ×60.
Discussion
In this study, we showed that tubular cell circumference increases significantly in proximal tubules of normal kidneys during postnatal weeks 2 through 8, in contrast to a slight decrease in DBA+ tubules. In embryonic kidneys, tubule formation involves convergent extension movements, which increase tubular length and decrease tubule diameter.26 We speculate that in postnatal kidneys, convergent extension movements may also occur in collecting tubules, whereas the absence or decrease of these movements in proximal tubules may contribute to this change in tubular cell circumference, given the similar rate of cell proliferation in these tubular segments during this period.11
Polarized cell divisions have been proposed to maintain tubule lumen size.26 We found that Pkd1-inactivated precystic developing kidneys or injured adult kidneys display randomized mitotic orientation (Figure 2), which may contribute to loss of lumen size control in PKD. In addition, we observed that oriented cell division occurs before tubule dilation (Figure 2, C and G), in contrast to what was recently reported.27 The difference in the developmental stages of kidneys used for the measurements of oriented cell division between this study (2- and 8-week; Figure 2) and postnatal days 7 through 10 described by Nishio et al.27 may account for the discrepancy between the data. An alternative possibility is that the precystic tubules used in the measurements of oriented cell division in that study may not have complete loss of PC1. The extensiveness of oriented cell division defects in 2-week-old precystic homozygous Pkd1 IKO kidneys is more striking than that recently reported in 2-day-old Pkd1+/− kidneys,28 probably because of the mutation in both Pkd1 alleles in our model. The larger number of cells dividing “out of the plane” in precystic IKO kidneys provides additional evidence that oriented cell division is defective in Pkd1 KO mouse models. Defects in oriented cell division may result in disorganized intercalation of the daughter cells and increase tubule diameter instead of lengthening the tubule. The slower and scattered cell migration we observed in Pkd1del34/del34 mouse embryonic kidney cells (data not shown) may also contribute to abnormal cell–cell intercalations required to lengthen the tubule as part of the convergent extension movements in Pkd1 inactivated tubules, which ultimately lead to a cyst.
Convergent extension movements and oriented cell division are dependent on PCP signaling. We therefore hypothesized that aberrant PCP signaling may underlie PKD. There are several homologs of the core PCP components in mammals: 3 Dvl, 2 Vangl (Van Gogh homolog), 3 Celsr (Flamingo homolog), 2 Prickle, 1 ANKRD6 (Diego homolog), and 10 Fz genes.9 Among the 10 Fz genes, only Fz3 and Fz6 have been demonstrated to be important for PCP signaling in mammals.29,30 Surprisingly, we did not detect significant increase in protein levels for Fz3 in precystic kidneys, although its expression on the cilia in precystic tubules seems to be increased and its downstream component CDC42 is upregulated (Figure 3A). Normal Fz3 protein levels are very low, so a slight change, unnoticeable by Western blot, might be sufficient to disrupt normal PCP signaling. Alternatively, other Fz family members may mediate PCP signaling upstream of CDC42 in precystic kidneys.
Correct PCP signaling in Drosophila requires asymmetric subcellular localization of PCP components.7 So far, the only evidence for asymmetric localization of PCP components in vertebrates is in the inner ear.30–32 We did not detect asymmetric Fz3 localization in normal, precystic, or cystic kidneys (Figure 4, Supplemental Figure S3, Table 1). This might be due to the low protein level of Fz3 in normal kidneys and that the organization of kidney tubules (either cross- or sagittal-section) does not allow one to distinguish the localization of a protein on one or the other lateral surface of a tubular epithelial cell. Another possibility is that Fz3 asymmetric localization is not essential for correct PCP signaling in the kidneys. We did observe, however, strong upregulation of Fz3 protein levels in cystic kidneys from Pkd1 KO mice and patients with ADPKD (Figures 3B and 7A). Strong Fz3 expression is seen on the apical membrane of cyst-lining epithelia of collecting duct origin (Figures 4 and 7B, Table 1), albeit not of proximal tubule origin, suggesting increased Fz3 expression correlates with tubular segment-specific activation of Pkd1. Activation and apical localization of CDC42, a downstream effector of Fz3 signaling, at the membrane of cyst-lining DBA+ cysts (Figure 5) also support that abnormal PCP signaling mediates cyst enlargement, in addition to its possible role in initiating tubule dilation by affecting oriented cell division. Previously, we showed that collecting tubule/duct cysts grow faster and larger than proximal cysts in mice as well as in patients.33 It is possible that aberrant activation of Fz3-CDC42 signaling in the distal tubular segments contributes to the different rate of cyst development. Interestingly, recent studies demonstrated Fz3 upregulation and increased apical localization in dilated tubules 3 days after urinary tract obstruction,34 suggesting that Fz3 upregulation occurs in different cystic models.
Fz3 plays a dual role in canonical and noncanonical (PCP) Wnt signaling, depending on cellular context. Fz3 homodimerization is known to stimulate canonical Wnt signaling in Xenopus laevis embryos.19 Thus, it is possible that increased Fz3 expression in human and mouse cystic kidneys, reported in this study, promotes its homodimerization and stimulates canonical Wnt signaling. In agreement with this, we observed increased Axin2 transcript levels in cystic kidneys (Figure 6D); however, similar results were observed in precystic kidneys (Figure 6D, compare left three columns), where Fz3 expression is not upregulated (Figure 3A). This suggests that the upregulation of canonical Wnt signaling in precystic and cystic kidneys is independent of Fz3. In addition, the activation of CDC42 that is also seen in cystic kidneys supports a role for Fz3 in the noncanonical Wnt signaling pathway. CDC42 has been suggested to regulate β-catenin–dependent transcription.20 Because cells with reduced CDC42 levels continue to have high levels of β-catenin–dependent transcription in the TopFlash reporter assays (Figure 6E), we think CDC42 is unlikely to be responsible for the activation of canonical Wnt signaling in precystic kidneys. Future experiments on reducing CDC42 expression in Pkd1 KO mice are required to clarify this.
CDC42 and the Par protein polarity complex associate with each other and act together in a range of cell types for polarized cell growth, cell migrations, and cell positioning.16,35 To investigate whether there is a defect in localization of the Par3 and Par6 polarity complex along with CDC42 overexpression, we examined Par3 and Par6 in precystic kidneys by immunohistochemistry. We did not observe aberrant expression or localization of the Par3 and the Par6 proteins in precystic kidneys (Supplemental Figure S2), thus excluding CDC42 overexpression in precystic kidneys as a result of apical-basal polarity defects.
Flow sensing by the primary cilia of the kidney epithelial cells is implicated in cyst formation in PKD. Loss of either PC1 or PC2 does not affect cilia formation but disables the primary cilia to function as a flow mechanosensor.36 PCP signaling might be controlled and modulated by primary cilia.21–24 Mutant mice that contain shortened or no cilia display aberrant oriented cell division and develop cysts.37 Aberrant expression of several PCP components results in loss or disruption of cilia.38,39 Loss of ciliary Fat4 does not affect cilia formation but causes renal tubule dilation.10 The ciliary localization of Fz3 in normal tubules and the possible increase in the cilia of precystic tubules and cyst-lining epithelial cells suggest that Fz3 could mediate primary cilium-dependent PCP signaling. Together with the antagonistic effects of PC1 and Fz3 on cell migration and CDC42 activation, it is possible that loss of PC1 results in the loss of inhibition of Fz3 function on the primary cilia, which in turn results in increased CDC42 activation and contributes to cyst formation. Fz3 is also seen on the mother centriole in several renal epithelial cell lines (Figure 8), where it may also antagonize the function of Diversin, a distant homolog of the fly PCP mediator Diego, whose centrosomal localization is necessary for its function in inhibiting canonical Wnt signaling.40
Germline Fz3−/− mice die within 30 minutes after birth as a result of neural defects,41 which precludes the use of these mice to genetically remove Fz3 expression in our Pkd1 IKO mice. Inactivating one allele of Fz3 is likely insufficient to overcome the strong Fz3 upregulation in Pkd1 KO mice. We had the opportunity to examine embryonic and newborn Fz3−/− kidneys, but we did not detect any cysts (Dr. Jeremy Nathans, Johns Hopkins University School of Medicine, Baltimore, MD, personal communication, June 2009). This may be explained by the fact that Fz3 and Fz6 are functionally redundant.9 Most PCP phenotypes, such as auditory hair cell orientation, neural tube closure, eyelid closure, and convergent extension, are observed only in Fz3−/−/Fz6−/− double mutants.9,30 Consistently, we detected upregulation of both Fz3 and Fz6 in ADPKD kidneys (Figure 7A). Thus, it would be of specific interest to investigate whether Fz3-overexpressing mice display cysts and mimic Pkd1 loss of function.
Previously, we showed that loss of PC1 causes spina bifida occulta.42 In Xenopus, inhibition of convergent extension can also prevent neural tube closure, causing a “spina bifida–like” phenotype.43 Via mutational analyses, several of the genes, such as Vangl2, involved in the PCP pathway have been shown to be necessary for neural tube closure in the mouse,30,43–45 validating that abnormal PCP can cause spina bifida.46 In this study, we showed, via a scratch wound–healing assay, that overexpression of Fz3 caused a reduction in cell migration rate in kidney cells (Figure 6, B and C). It is noteworthy that overexpression of Vangl2 in HT-1080 fibrosarcoma cells also results in slower cell migration in a wound-healing assay.47 The reduced speed caused by Fz3 overexpression during wound healing could be rescued by induction of PC1 (Figure 6, B and C). Whether Fz is also upregulated in neural crest cells as in the kidney in Pkd1 KO mice and whether the reduced cell migration rate correlates with aberrant convergent extension movements and consequently contributes to the spina bifida occulta phenotype deserves future study.
We observed that Fz3 and PC1 have antagonizing effects (Figure 6), indicating that these two receptor molecules somehow modulate each other's function. Our efforts on testing an interaction between these two proteins by co-immunoprecipitation were not successful (data not shown), although we cannot exclude a transient or weak interaction. Fz3 overexpression in YPC1m-HEK cells increased total CDC42 protein levels, consistent with what we observed in cystic Pkd1 KO kidneys (Figures 6A and 3B). In normal tissue and cells, Fz3 and PC1 may cooperatively modulate CDC42 expression and activation. That induction of PC1 expression in Fz3-overexpressing HEK293T cells lowered CDC42 expression and loss of PC1 has an opposite effect suggest a fine balance of PC1 is necessary to maintain normal CDC42 levels.
In summary, we demonstrate, for the first time, that there is activation of the PCP pathway, exemplified by Fz3-CDC42, in cystic kidneys in orthologous mouse models of human ADPKD as well as in human patients with ADPKD. The Fz-CDC42 pathway may represent a new therapeutic target for this devastating disease.
Concise Methods
Mouse Models and IRI
The γGt-Cre.Pkd1F/F mouse was previously described,33 the Col2-Cre.Pkd1F/null mouse was previously described,48 and Mx1-Cre.Pkd1F/null and Mx1-Cre.Pkd1F/F mice were previously described.11 This model uses the Cre/loxP system and an IFN-inducible Mx1 promoter driving Cre expression in the distal tubular segments, which include the ascending loop of Henle, distal tubule, and collecting duct. Briefly, mice were administered an intraperitoneal injection of 250 μg of IFN inducer pI:pC (Sigma) for 5 consecutive days at 1 or 5 weeks of age to induce the expression of Cre recombinase. IRI was performed at 8 weeks of age, 3 weeks after Pkd1 inactivation. Kidneys were analyzed 48 hours after IRI. Mouse and renal IRI studies were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Harvard University and as described by Takakura et al.12
Molecular Biology
The pRK5-Fz3 expression construct was provided by Dr. Jeremy Nathans (Johns Hopkins University School of Medicine, Baltimore, MD). YPC1m-HEK cells transfected and/or induced with 10 ng of tetracycline were also used to detect active CDC42 with the active CDC42 pull-down and detection kit (cat. no. 89857; Thermo-Scientific, Waltham, MA). The kit was used as described by the manufacturers. Quantitative reverse transcriptase–PCR was performed on total RNA from kidneys of Mx1-Cre.Pkd1F/null, Mx1-Cre.Pkd1F/F, and γGt-Cre.Pkd1F/F mice and their littermate controls as described previously.33 Briefly, RT-PCR conditions were as follows: 95°C for 15 minutes; cycling: melt 94°C for 15 seconds; annealing: 55°C for 8 seconds; extension: 72°C for 8 seconds. The primers used for Axin2 were as follows: Forward 5′-CTC CCC ACC TTG AAT GAA GA-3′ and reverse 5′-ACA TAG CCG GAA CCT ACG TG-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. GAPDH primers used are as described previously.33
Cell Lines
HEK293T cells originated from American Type Culture Collection (Manassas, VA) and were routinely cultured in DMEM1x supplemented with 10% FBS (Invitrogen, Carlsbad, CA). Human immortalized DBA selected renal cortical tubular epithelial cells and ADPKD 9 to 12 cells were described25 and cultured in DMEM1x supplemented with 10% FBS. A pCDNA4 expression construct containing both N- and C-terminally tagged PC1 was stably transfected in HEK293TRex cells (Su et al., manuscript in preparation). WT and Pkd1del34/del34 mouse embryonic kidney cells derived from the distal tubules were described previously.36
Immunofluorescence
For microscopic analyses, cells were plated on glass coverslips in six-well plates (Corning Inc., Corning, NY), fixed with 4% paraformaldehyde, permeabilized with 0.5% (vol/vol) Triton-X and blocked with 5% BSA-PBS. Paraffin-embedded sections (4 μm), derived from perfused kidneys, were dewaxed, rehydrated through graded alcohols, and boiled in 10 mM citrate (pH 6.0; Vector Laboratories, Burlingame, CA) for 30 minutes. The sections were then placed in the staining dish at room temperature, allowed to cool for 1 to 2 hours, and blocked with 5% BSA-PBS for 30 minutes. Sections and cells were stained with anti-acetylated α-tubulin (cat. no. T6793; Sigma Aldrich, St. Louis, MO) 1:20,000 dilution to detect the cilia, anti-Fz3 antibodies (cat. no. AF1001; R&D Systems, Minneapolis, MN) 1:40 dilution, anti-CDC42 (cat. no. sc-87; Santa Cruz Biotechnology, Santa Cruz, CA) 1:100 dilution, anti-Par6 (cat. no. ab45394; Abcam, Cambridge, MA) 1:100 dilution, or anti-Par3 (provided by Dr. Ian Macara) 1:50 dilution and appropriate Alexa-conjugated secondary antibodies (Invitrogen) 1:500 dilution. Fluorescein DBA (cat. no. FL-1031; Vector Laboratories) 1:500 dilution or fluorescein LTL (cat. no. FL-1321; Vector Laboratories) 1:500 dilution was used to detect, respectively, collecting tubules/ducts or proximal tubules. Slides were mounted with ProLong Gold antifade reagent with DAPI (cat. no. P36935; Invitrogen). Images were collected with Nikon-1000 epifluorescence microscope (Nikon, Tokyo, Japan).
Wound-Healing Assay
YPC1m-HEK cells were plated at confluence on six-well plates, transfected with an expression construct for Fz3 by homemade transfection reagent PEI and induced or not with 10 ng of tetracycline. After 24 hours of culture, cells were scratched and imaged every 6 minutes for 20 hours with Nikon-TE1000 live-cell imaging set up with Slidebook software (Intelligent Imaging Innovations, Denver, CO) to study cell movement. The distance traveled in 20 hours was measured at three different spots per scratch, and average speed per hour was calculated.
Western Blotting
Cells and kidneys were extracted in RIPA buffer (cat. no. 20-188; Millipore, Billerca, MA) supplemented with protease inhibitors (cat. no. 11 836 145 001; Roche, Basel, Switzerland). Protein samples (50 μg) were separated on 7.5% SDS-PAGE for Fz3 detection and on 12% SDS-PAGE for CDC42 detection. The proteins were then transferred on a nitrocellulose membrane (GE Healthcare, Piscataway, NJ). The membranes were blocked in 5% milk in PBS-0.5%Tween and incubated with anti-Fz3 antibodies (provided by Dr. Jeremy Nathans) 1:2000 dilution, anti-Fz6 antibodies (provided by Dr. Jeremy Nathans) 1:2000 dilution, anti-CDC42 antibodies (cat. no. sc-81; Santa Cruz Biotechnology) 1:200 dilution, or anti-GAPDH antibodies (cat. no. sc-25778; Santa Cruz Biotechnology) 1:500 dilution. The secondary antibodies used were goat anti-rabbit IgG–horseradish peroxidase (Amersham Biosciences, Piscataway, NJ) 1:5000 dilution or donkey anti-goat IgG–horseradish peroxidase (cat. no. sc-2020; Santa Cruz Biotechnology) 1:2000 dilution. Signals were visualized via ECL detection.
Measurements of Oriented Cell Division
Paraffin-embedded kidney sections (30 μm) from mice at 2 and 8 weeks of age were stained with anti–phospho-histone H3 (Ser10) antibody (anti-H3pS10; cat. no. ab5176; Abcam) 1:500 dilution and Alexa-594–conjugated goat anti-rabbit secondary antibodies (Invitrogen) 1:500 dilution to detect the separating chromosomes in dividing cells. Sections were counterstained with fluorescein DBA to identify distal tubules. Slides were mounted with ProLong Gold antifade reagent with DAPI (cat. no. P36935; Invitrogen). Confocal imaging (Nikon) using the ×40 oil immersion objective lens was used to make Z-stacks through the tissue at a step size of 0.5 μm. Imaris 3D software (Bitplane, Zurich, Switzerland) was used to measure the mitotic angles by calculating the angle between the separating chromosomes and the longitudinal axis of the tubule and to make three-dimensional reconstructions.
Luciferase Assays for TopFlash
YPC1m-HEK cells were plated on 12-well plates and transfected with a combination of plasmids in the presence or absence of tetracycline induction as described in the legend of Figure 6. The TopFlash luciferase construct was provided by Dr. Randy Moon (University of Washington, Seattle, WA). The renilla luciferase under the control of a cytomegalovirus promoter was used to normalize all values for transfection efficiency. The dual luciferase assay kit (cat. no. E1910; Promega, Madison, WI) was used as described by the manufacturers.
Statistical Analysis
Mann-Whitney U test was used to assess whether the distribution of two sample sets was significantly different.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK51050 and DK40703) to J.Z. X.S. was a recipient of a postdoctoral fellowship from the Polycystic Kidney Disease Foundation.
We thank Dr. I. Macara for providing us with Par3 antibodies; Dr. R. Moon for providing us with the TopFlash reporter construct; and Dr. J. Nathans and Z. Hua for helpful discussion and providing us with Fz antibodies, expression constructs, and Fz3 KO kidneys. We also thank members of the Zhou Lab, in particular Courtney LaPierre for technical assistance, and members of the Harvard Center for Polycystic Kidney Disease Research for scientific discussions and support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Balancing the Wnts in Polycystic Kidney Disease,” on pages 1412–1414.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Zhou J, Pei Y: Autosomal dominant polycystic kidney disease. In: The Molecular and Genetic Basis of Kidney Disease: A Companion to Brenner and Rector's The Kidney, edited by Mount DB, Pollak MR. Philadelphia, Saunders, 2007, pp 85–171 [Google Scholar]
- 2. Zhou J: Polycystins and primary cilia: Primers for cell cycle progression. Annu Rev Physiol 71: 83–113, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE: Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 13: 169–176,2003 [DOI] [PubMed] [Google Scholar]
- 4. Pollack AL, Runyan RB, Mostov KE: Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol 204: 64–79, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Adler PN: Planar signaling and morphogenesis in Drosophila. Dev Cell 2: 525–535, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Strutt DI: The asymmetric subcellular localisation of components of the planar polarity pathway. Semin Cell Dev Biol 13: 225–231, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Nathans J: Tissue/planar cell polarity in vertebrates: New insights and new questions. Development 134: 647–658, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Takakura A, Contrino L, Beck AW, Zhou J: Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol 19: 2351–2363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J: Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ: Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Gho M, Schweisguth F: Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature 393: 178–181, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Jaffe AB, Kaji N, Durgan J, Hall A: Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joberty G, Petersen C, Gao L, Macara IG: The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Burbelo P: CDC42. Nature: The signaling gateway, mini molecule page, 2009. Available at: http://www.signaling-gateway.org/molecule/query%3Bjsessionid=c6ca4bf1229ccbe6c21c9bd4429abb1be4519d0fb772?afcsid=A000605&type=mmpdocs Accessed October 2009
- 18. Boca M, D'Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A: Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell 18: 4050–4061, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carron C, Pascal A, Djiane A, Boucaut JC, Shi DL, Umbhauer M: Frizzled receptor dimerization is sufficient to activate the Wnt/beta-catenin pathway. J Cell Sci 116: 2541–2550, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C: Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev 20: 571–585, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones C, Chen P: Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol 85: 197–224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park TJ, Haigo SL, Wallingford JB: Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38: 303–311, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Wallingford JB: Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet 15: R227–R234, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL: Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Loghman-Adham M, Nauli SM, Soto CE, Kariuki B, Zhou J: Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am J Physiol Renal Physiol 285: F397–F412, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ: Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S: Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21: 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonnet CS, Aldred M, von Ruhland C, Harris R, Sandford R, Cheadle JP: Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum Mol Genet 18: 2166–2176, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Guo N, Hawkins C, Nathans J: Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A 101: 9277–9281, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Guo N, Nathans J: The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci 26: 2147–2156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davies A, Formstone C, Mason I, Lewis J: Planar polarity of hair cells in the chick inner ear is correlated with polarized distribution of c-flamingo-1 protein. Dev Dyn 233: 998–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P: Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet 37: 980–985, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Starremans PG, Li X, Finnerty PE, Guo L, Takakura A, Neilson EG, Zhou J: A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5′ end of Pkd1. Kidney Int 73: 1394–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Li L, Zepeda-Orozco D, Patel V, Truong P, Karner CM, Carroll TJ, Lin F: Aberrant planar cell polarity induced by urinary tract obstruction. Am J Physiol Renal Physiol 297; F1526–F1533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Etienne-Manneville S: Cdc42: The centre of polarity. J Cell Sci 117: 1291–1300, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB: Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benzing T, Simons M, Walz G: Wnt signaling in polycystic kidney disease. J Am Soc Nephrol 18: 1389–1398, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Itoh K, Jenny A, Mlodzik M, Sokol SY: Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci 122: 3791–3798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J: Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci 22: 8563–8573, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J: Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet 10: 2385–2396, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Wallingford JB, Harland RM: Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development 129: 5815–5825, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN: Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13: 1129–1133, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A: Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129: 5827–5838, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Ueno N, Greene ND: Planar cell polarity genes and neural tube closure. Birth Defects Res C Embryo Today 69: 318–324, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Cantrell VA, Jessen JR: The planar cell polarity protein Van Gogh-like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett 287: 54–61, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Kolpakova-Hart E, Nicolae C, Zhou J, Olsen BR: Col2-Cre recombinase is co-expressed with endogenous type II collagen in embryonic renal epithelium and drives development of polycystic kidney disease following inactivation of ciliary genes. Matrix Biol 27: 505–512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.