Abstract
The differential effects between cinacalcet and active vitamin D compounds on parathyroid function, mineral metabolism, and skeletal function are incompletely understood. Here, we studied cinacalcet and active vitamin D compounds in mice expressing the null mutation for Cyp27b1, which encodes 25-hydroxyvitamin D-1α-hydroxylase, thereby lacking endogenous 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. Vehicle-treated mice given high dietary calcium had hypocalcemia, hypophosphatemia, and marked secondary hyperparathyroidism. Doxercalciferol and 1,25(OH)2D3 each normalized these parameters and corrected both the abnormal growth plate architecture and the diminished longitudinal bone growth observed in these mice. In contrast, cinacalcet suppressed serum parathyroid hormone (PTH) cyclically and did not correct the skeletal abnormalities and hypocalcemia persisted. Vehicle-treated mice given a “rescue diet” (high calcium and phosphorus, 20% lactose) had normal serum calcium and PTH levels; cinacalcet induced transient hypocalcemia and mild hypercalciuria. The active vitamin D compounds and cinacalcet normalized the increased osteoblast activity observed in mice with secondary hyperparathyroidism; cinacalcet, however, increased the number and activity of osteoclasts. In conclusion, cinacalcet reduces PTH in a cyclical manner, does not eliminate hypocalcemia, and does not correct abnormalities of the growth plate. Doxercalciferol and 1,25(OH)2D3 reduce PTH in a sustained manner, normalize serum calcium, and improve skeletal abnormalities.
Vitamin D and extracellular calcium ([Ca2+]e) play central roles in regulating systemic calcium homeostasis. [Ca2+]e activates a cation-sensing G-protein coupled receptor, the calcium-sensing receptor (CaSR), to modulate the concentrations of circulating parathyroid hormone (PTH) and to regulate renal calcium re-absorption.1 The activated form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D] is synthesized via the action of the enzyme 25-hydroxyvitamin D-1α-hydroxylase [1α(OH)ase], which converts 25-hydroxyvitamin D to 1,25(OH)2D. This activated form of vitamin D acts predominantly on a nuclear vitamin D receptor (VDR) to modulate parathyroid function, mineral metabolism, and skeletal function.2 1,25(OH)2D synthesis is impaired in chronic kidney disease (CKD), at least in part because of suppression of the renal 1α(OH)ase by elevated serum phosphorus and/or serum fibroblast growth factor 23 (FGF-23), and in part because of loss of renal parenchyma. Consequently, low levels of 1,25(OH)2D develop in stage 3 CKD, leading to reduced calcium absorption.3 The resultant hypocalcemia and low 1,25(OH)2D can lead to the development of secondary hyperparathyroidism, mainly because of the fact that extracellular ionized calcium [Ca2+]e and 1,25(OH)2D regulate PTH biosynthesis, with 1,25(OH)2D predominantly suppressing PTH gene transcription4,5 and [Ca2+]e predominantly downregulating PTH mRNA translation.6,7 Both calcium8 and activated vitamin D9,10 coordinately regulate the growth of the parathyroid gland. In addition, increased [Ca2+]e, by activating the CaSR, potently suppresses PTH secretion.1 The ensuing secondary hyperparathyroidism, with disordered skeletal remodeling and mineralization defects, can contribute to the manifestations of renal osteodystrophy.
Active vitamin D compounds including 1,25(OH)2D3 (calcitriol)11 and doxercalciferol [1αOHD2, Hectorol],12 a vitamin D2 analog that undergoes metabolic activation in vivo to form 1,25(OH)2D2, are used clinically to manage elevated PTH in CKD. The calcimimetic cinacalcet, an allosteric modulator of the CaSR, requires the presence of calcium ions to be effective13 and is used clinically to manage secondary hyperparathyroidism.14 Cinacalcet has also been approved for use in primary hyperparathyroidism due to parathyroid carcinoma.15 Recently, calcimimetics have been proposed as a potential adjuvant treatment for familial hypophosphatemic rickets, which is complicated by secondary hyperparathyroidism.16 However, the skeletal impact of calcimimetics is unclear. We therefore compared active vitamin D compounds and the calcimimetic, cinacalcet, in mice with targeted inactivation of Cyp27b1, the gene encoding the 1α(OH)ase enzyme, that lack the capacity to synthesize endogenous 1,25(OH)2D. We exposed these mice [1α(OH)ase null mice] to two different environments: a high-calcium intake on which 1α(OH)ase null mice are known to develop secondary hyperparathyroidism, rickets, and osteomalacia17–19 and a “rescue” diet18 containing high calcium, high phosphorus, and 20% lactose, which is known to normalize serum calcium and phosphate in the absence of either active vitamin D or the vitamin D receptor. We then determined the relative effects of active vitamin D compounds and calcimimetics on parathyoid gland function, mineral homeostasis, and skeletal function.
Results
Serum Biochemistry and Parathyroid Gland Histology
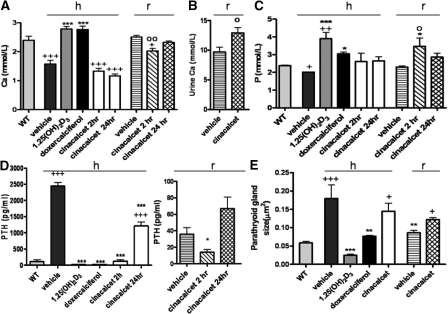
Although vehicle-treated 1α(OH)ase null mice on the high-calcium intake were hypocalcemic and hypophosphatemic and had markedly increased serum PTH (Figure 1), doxercalciferol normalized serum calcium and serum phosphorus. 1,25(OH)2D3 similarly increased serum calcium (Figure 1A) but increased serum phosphorus more markedly than doxercalciferol (Figure 1C). Doxercalciferol and 1,25(OH)2D3 each dramatically reduced circulating concentrations of PTH (Figure 1D). With cinacalcet treatment, serum calcium remained low at 2 and 24 hours after dose administration (Figure 1A), and cyclic PTH fluctuations were observed (Figure 1D): that is, PTH levels decreased at 2 hours after dose administration, but rebounded by 24 hours, although not to vehicle-treated levels (Figure 1D). 1,25(OH)2D3 and doxercalciferol both decreased the size of the parathyroid glands below that seen in the vehicle-treated 1α(OH)ase null mice on a high-calcium diet (Figure 1E); parathyroid glands remained enlarged however in the cinacalcet-treated mice (Figure 1, D and E).
Figure 1.
Active vitamin D compounds and cinacalcet affect calcium, phosphorus, and parathyroid homeostasis differently. Serum and urine biochemistry and histomorphometry of the parathyroid glands are shown: in wild-type (WT) mice; in 1α(OH)ase null mice [1α(OH)ase−/−] on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, cinacalcet for 2 hours (hr), and cinacalcet for 24 hours; and in 1α(OH)ase null mice on a rescue diet (r) treated with vehicle or cinacalcet for 24 hours. Each value is the mean ± SEM. (A) Number of replicates for serum calcium from left to right are as follows: 4 for WT, 6 for vehicle, 5 for 1,25(OH)2D3,7 for doxercalciferol, 3 for cinacalcet 2 hours, 3 for cinacalcet 24 hours, 7 for vehicle, 5 for cinacalcet 2 hours, and 5 for cinacalcet 24 hours. (B) Number of replicates for 24-hour urine calcium are as follows: 4 for vehicle and 4 for cinacalcet. (C) Number of replicates for serum phosphorus from left to right are as follows: 3 for WT, 4 for vehicle, 4 for 1,25(OH)2D3, 6 for doxercalciferol, 3 for cinacalcet 2 hours, 3 for cinacalcet 24 hours, 6 for vehicle, 4 for cinacalcet 2 hours, and 5 for cinacalcet 24 hours. (D) Number of replicates for serum PTH from left to right are as follows: 3 for WT, 7 for vehicle, 5 for 1,25(OH)2D3, 7 for doxercalciferol, 3 for cinacalcet 2 hours, 3 for cinacalcet 24 hours, 4 for vehicle, 4 for cinacalcet 2 hours, and 5 for cinacalcet 2 hours. (E) Number of replicates for parathyroid, gland size done by histomorphometry from left to right are as follows: 3 for WT, 3 for vehicle, 3 for 1,25(OH)2D3, 3 for doxercalciferol, 3 for cinacalcet, 6 for vehicle, and 6 for cinacalcet. +P < 0.05 compared with WT mice; ++P < 0.01 compared with WT mice; +++P < 0.001 compared with WT mice; *P < 0.05 compared with vehicle-treated 1α(OH)ase null mice on a high-calcium intake; **P < 0.01 compared with vehicle-treated 1α(OH)ase null mice on a high-calcium intake; ***P < 0.001 compared with vehicle-treated 1α(OH)ase null mice on a high-calcium intake. ○P < 0.05 compared with vehicle-treated 1α(OH)ase null mice on a rescue diet; ○○P < 0.01 compared with vehicle-treated 1α(OH)ase null mice on a rescue diet.
On the rescue diet, serum calcium, phosphorus, and PTH were normalized in null mice (Figure 1, A, C, and D, respectively). Cinacalcet treatment significantly reduced serum calcium after 2 hours [although not to the levels seen in the vehicle-treated 1α(OH)ase null mice on the high-calcium intake], but not after 24 hours (Figure 1A). Cinacalcet also increased 24-hour urine calcium (Figure 1B). Serum phosphorus levels were higher than those in the vehicle-treated controls (Figure 1C) 2 hours after cinacalcet administration but were not significantly elevated by 24 hours. Serum PTH levels fell at 2 hours, but were not significantly different from vehicle-treated levels after 24 hours (Figure 1D). Parathyroid glands also remained slightly increased in the cinacalcet-treated mice on a rescue diet (Figure 1E).
Mineralization of Bone and Cartilage
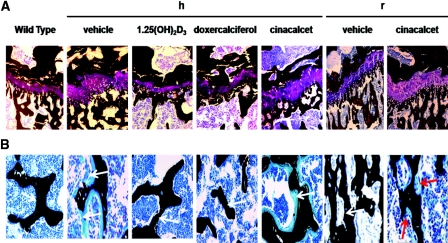
On the high-calcium intake, vehicle-treated 1α(OH)ase null mice had widened and distorted growth plates which were poorly mineralized (Figure 2A). Excess osteoid was also seen in the bone of the metaphysis (Figure 2B). Both 1,25(OH)2D3 and doxercalciferol normalized the size, structure, and mineralization of the growth plate, and reduced osteoid was seen (Figure 2). In contrast, widened and poorly mineralized growth plates were seen after cinacalcet treatment along with abundant osteoid lining trabeculae (Figure 2). On the rescue diet, the width of the growth plate in the 1α(OH)ase null mice was largely restored in vehicle-treated mice and mineralization of bone was normalized (Figure 2). After cinacalcet treatment, the growth plate remained widened; however, mineralization of bone markedly improved (Figure 2).
Figure 2.
Active vitamin D compounds but not cinacalcet normalize an unmineralized growth plate and unmineralized bone. Representative von Kossa stains of (A) the growth plates and (B) the metaphyses are shown: of the tibiae of wild-type mice; of 1α(OH)ase null mice [1α(OH)ase−/−] on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, and cinacalcet; and of 1α(OH)ase null mice [1α(OH)ase−/−] on a rescue diet (r) treated with vehicle, and cinacalcet. Black staining denotes mineralization; purple staining depicts the collagen in the growth plates. The white arrows on the black mineralized trabeculae denote the absence or presence of unmineralized bone, which stains light blue. Red arrows denote osteoclasts lining the bone of cinacalcet-treated null mice on a rescue diet. Magnification: ×100 in A; ×200 in B.
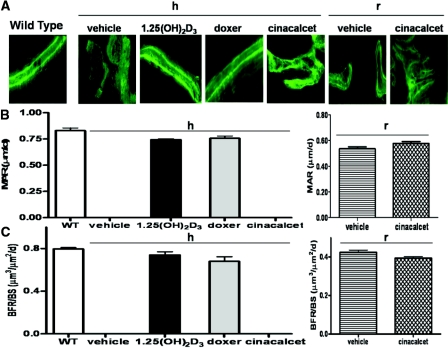
Compared with the double labeling seen in wild-type mice, altered deposition of calcein, a fluorochrome that binds newly calcified matrix, was seen in the bone of vehicle-treated 1α(OH)ase null mice on a high-calcium intake compared with the double labeling seen in wild-type mice (Figure 3A). Calcein labeling was similarly but somewhat less markedly abnormal in cinacalcet-treated null animals (Figure 3A). In contrast, discrete double labels were easily evident in trabecular bone of 1,25(OH)2D3 and doxercalciferol-treated 1α(OH)ase null mice, similar to that observed in wild-type mice (Figure 3A), and the mineral apposition rate (MAR) (Figure 3C) and bone formation rate (BFR/BS) (Figure 3C) in these groups was not significantly different from those of wild-type mice (Figure 3B). In null mice on a rescue diet, the MAR was markedly improved in the vehicle-treated and cincalcet-treated mice (Figure 3B) and the BFR/BS was not significantly different between the vehicle-treated and cinacalcet-treated mice (Figure 3C).
Figure 3.
Treatment of osteomalacic null mice with active vitamin D compounds but not with cinacalcet normalizes MAR and BFR/BS. Dynamic histomorphometry results are shown. (A) Photomicrographs of fluorescence double calcein labeling of bone: in wild-type (WT) mice; in 1α(OH)ase null mice [1α(OH)ase−/−] on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, and cinacalcet; in 1α(OH)ase null mice on a rescue diet (r) treated with vehicle, and cinacalcet. Magnification, ×200. (B) MARs, calculated from double calcein labeling as described in Concise Methods: in wild-type mice; in 1α(OH)ase null mice on a high-calcium intake (hours) treated with 1,25(OH)2D3 and doxercalciferol; in 1α(OH)ase null mice on a rescue diet treated with vehicle, and cinacalcet. Each value is the mean ± SEM. (C) BFR/BS, calculated as described in Concise Methods: in WT mice; in 1α(OH)ase null mice on a high-calcium intake (hours) treated with 1,25(OH)2D3 and doxercalciferol; in 1α(OH)ase null mice on a rescue diet treated with vehicle, and cinacalcet. Each value is the mean ± SEM. Number of replicates for MAR and BFR/BS from left to right are as follows: 3 for WT; 4 for 1,25(OH)2D3, and 5 for doxercalciferol for 1α(OH)ase−/− mice on a high-calcium intake; 8 for vehicle, and 8 for cinacalcet on a rescue diet. MAR and BFR could not be calculated for vehicle-treated and cinacalcet-treated 1α(OH)ase null mice on a high-calcium intake (h) in view of the indistinct calcein labeling in the osteomalacic bone of these mice, which precluded calculation of interlabel width. Nevertheless, the results of these treatment arms are denoted in Figure 3, B and C, as zero.
Assessment of Bone Turnover
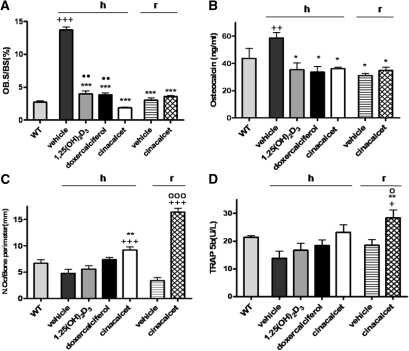
Osteoblast surfaces of bone and serum osteocalcin levels were increased in vehicle-treated null mice on a high-calcium intake, compared with those in wild-type mice (Figure 4, A and B). Osteoblast surfaces and osteoblast activity as indicated by serum osteocalcin were reduced to normal in all treated animals after secondary hyperparathyroidism was reduced or eliminated (Figure 4, A and B). In null mice on a rescue diet, elimination of the secondary hyperparathyroidism also resulted in a normalized osteoblast surface, and osteocalcin levels were not different from those in wild-type mice (Figure 4, A and B).
Figure 4.
Active vitamin D compounds and cinacalcet normalize osteoblast activity but cinacalcet, and not active vitamin D compounds, increases osteoclasts. Bone turnover is shown: in wild-type (WT) mice; in 1α(OH)ase null mice [1α(OH)ase−/−] on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, and cinacalcet; in 1α(OH)ase null mice on a rescue diet (r) treated with vehicle, and cinacalcet. (A) Osteoblast surface relative to bone surface (OB.S/BS); (B) serum osteocalcin concentrations; (C) osteoclast numbers relative to bone perimeter [N.Oc/bone perimeter (mm)]; (D) serum concentrations of TRAP5b. Each value is the mean ± SEM. Number of replicates for OB.S/BS from left to right are as follows: 4 for WT, 5 for vehicle, 5 for 1,25(OH)2D3, 5 for doxercalciferol, 5 for cinacalcet, 7 for vehicle, and 6 for cinacalcet. Number of replicates for osteocalcin from left to right are as follows: 5 for WT, 3 for vehicle, 4 for 1,25(OH)2D3, 5 for doxercalciferol, 3 for cinacalcet, 6 for vehicle, and 5 for cinacalcet. Number of replicates for N.Oc/bone perimeter from left to right are as follows: 3 for WT, 4 for vehicle, 7 for 1,25(OH)2D3, 5 for doxercalciferol, 5 for cinacalcet, 8 for vehicle, and 12 for cinacalcet. Number of replicates for serum TRAP5b from left to right are as follows: 4 for WT, 3 for vehicle, 3 for 1,25(OH)2D3, 3 for doxercalciferol, 3 for cinacalcet, 3 for vehicle, and 4 for cinacalcet. +P < 0.05 relative to WT mice; ++P < 0.01 relative to WT mice; +++P < 0.001 relative to WT mice; *P < 0.05 relative to vehicle-treated 1α(OH)ase null mice on a high-calcium intake; **P < 0.01 relative to vehicle-treated 1α(OH)ase null mice on a high-calcium intake; ***P < 0.001 relative to vehicle-treated 1α(OH)ase null mice on a high-calcium intake; ○P < 0.05 relative to vehicle-treated 1α(OH)ase null mice on a rescue diet. ○○○P < 0.001 relative to vehicle-treated 1α(OH)ase null mice on a rescue diet. ●●P < 0.01 relative to cinacalcet-treated 1α(OH)ase null mice on a high-calcium intake.
In 1α(OH)ase null mice on a high-calcium intake, osteoclast numbers were increased after cinacalcet treatment relative to wild-type mice and to the vehicle-treated control. Serum TRAP5b levels generally paralleled these changes (Figure 4, C and D). On a rescue diet, cinacalcet treatment also increased osteoclast numbers significantly compared with vehicle-treated 1α(OH)ase null mice, and compared with wild-type mice. TRAP5b levels were increased in parallel with the increases in osteoclasts in the cinacalcet-treated animals on a rescue diet.
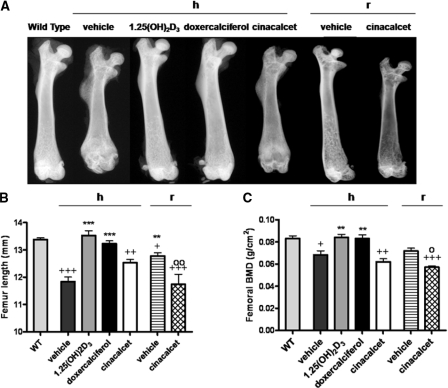
Growth and Mineral Density of Long Bones
On a high-calcium intake, femurs of vehicle-treated 1α(OH)ase null mice showed increased radiolucency and reduced length compared with femurs of wild-type mice (Figure 5, A and B). Both the radiolucency and length of the femurs normalized after 1,25(OH)2D3 and doxercalciferol treatment (Figure 5A). Femurs of cinacalcet-treated mice remained more radiolucent and shorter than those of wild-type mice (Figure 5, A and B). Bone mineral density (BMD) testing confirmed the normalization of mineralization of the bones after 1,25(OH)2D3 and doxercalciferol treatment, but BMD was still reduced after cinacalcet treatment associated with the decreased mineralization (Figure 5C).
Figure 5.
Active vitamin D compounds, but not cinacalcet, normalize femoral length and BMD. Representative contact radiographs (A), quantitation of femur lengths (B), and BMD of femurs (C) are shown: from wild-type (WT) mice; from 1α(OH)ase null mice [1α(OH)ase−/−] on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, and cinacalcet; from 1α(OH)ase null mice on a rescue diet (r) treated with vehicle, and cinacalcet. Each femur length value is the mean ± SEM. Number of replicates for femur length from left to right are as follows: 6 for WT, 5 for vehicle, 5 for 1,25(OH)2D3, 7 for doxercalciferol, 6 for cinacalcet, 5 for vehicle, and 5 for cinacalcet. Each BMD value is the mean ± SEM. Number of replicates for BMD from left to right are as follows: 4 for WT, 8 for vehicle, 5 for 1,25(OH)2D3, 7 for doxercalciferol, 6 for cinacalcet, 6 for vehicle, and 6 for cinacalcet. +P < 0.05 relative to WT mice; ++P < 0.01 relative to WT mice; +++P < 0.001 relative to WT mice; **P < 0.01 relative to vehicle-treated 1α(OH)ase null mice on a high-calcium intake; ***P < 0.001 relative to vehicle-treated 1α(OH)ase null mice on a high-calcium intake. ○P < 0.05 relative to vehicle-treated 1α(OH)ase null mice on a rescue diet; ○○P < 0.01 relative to vehicle-treated 1α(OH)ase null mice on a rescue diet.
On a rescue diet, radiolucency (Figure 5A) and length of bones improved in the vehicle-treated null mice but the femurs remained slightly shorter than the femurs of wild-type mice (Figure 5B). Cinacalcet treatment resulted in more radiolucent and shorter bones (Figure 5, A and B). Bones of cinacalcet-treated 1α(OH)ase null mice on a rescue diet also displayed lower BMD than the vehicle-treated mice and the wild-type mice (Figure 5C), but this appeared to reflect primarily increased bone resorption.
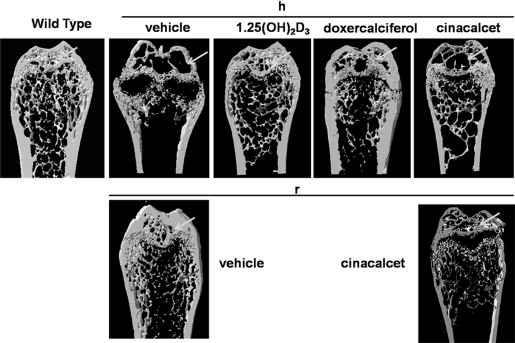
MicroCT images confirmed the reduced mineralization of secondary ossification centers in the epiphyses and the reduction of mineralized trabeculae in the metaphyses of femurs of vehicle-treated 1α(OH)ase null mice on a high-calcium diet (Figure 6). Reduced mineralization of the epiphyses was also seen with cinacalcet but a modest increase in mineralized trabeculae was observed (Figure 6). In contrast, both 1,25(OH)2D3 and doxercalciferol produced marked improvement in mineralization in the epiphyses and an increase in mineralized trabeculae (Figure 6).
Figure 6.
Active vitamin D compounds, but not cinacalcet, improve mineralization of the epiphyses and increase mineralized trabeculae. Representative longitudinal sections of three-dimensional reconstructed distal ends of the femurs are shown: from wild-type mice; from 1α(OH)ase null mice on a high-calcium intake (h) treated with vehicle, 1,25(OH)2D3, doxercalciferol, and cinacalcet; from 1α(OH)ase null mice on a rescue diet (r) treated with vehicle, and cinacalcet. Two-dimensional images of the femora were obtained by micro-CT to generate three-dimensional reconstructions from 20 adjacent images as described in Concise Methods. White arrows denote mineralization in the secondary ossification sites in the epiphyses and grey arrows denote mineralized bony trabeculae in the metaphyses.
In the vehicle-treated and cinacalcet-treated 1α(OH)ase−/− mice on a rescue diet, mineralization of the epiphyses also improved compared with the vehicle-treated 1α(OH)ase−/− mice on a high-calcium intake, but there were reduced trabeculae in cinacalcet-treated animals on a rescue diet, reflecting the increased bone resorption (Figure 6).
Discussion
In the 1α(OH)ase null mice on a high-calcium intake, both 1,25(OH)2D3 and doxercalciferol normalized serum calcium and dramatically reduced circulating PTH levels and parathyroid gland size. Both compounds increased serum phosphorus levels on this normal phosphorus, 0% lactose diet, but at the dose used only 1,25(OH)2D3 induced hyperphosphatemia. Whether this observation represents a systematic difference between the two active vitamin D compounds is unclear and whether there are differential effects on phosphate absorption and/or differential potency of these two sterols in their capacity to increase the phosphaturic hormone FGF-23 will require further study.
The effects of 1,25(OH)2D3 and of doxercalciferol on the parathyroid glands likely stemmed from both direct inhibitory actions of these compounds on parathyroid growth9,10 and PTH production,4,5 as well as from indirect effects due to increased serum calcium activating the CaSR. In contrast, although cinacalcet significantly reduced circulating PTH concentrations, the results were biphasic20 and less pronounced than the reductions produced by 1,25(OH)2D3 or doxercalciferol. The lesser effectiveness of cinacalcet in reducing PTH in this model likely reflects both the potency and the pharmacokinetics of cinacalcet as well as the absence of any direct inhibitory action of active vitamin D. A recent communication of the reduced effectiveness of cinacalcet relative to active vitamin D in other rodent models supports the current observations.21 Calcimimetics have been reported to reduce parathyroid hyperplasia,22 but parathyroid gland size was not significantly decreased by cinacalcet in the 1α(OH)ase null mice on a high-calcium intake, in contrast to the effects produced by the active vitamin D compounds. Furthermore, the significant hypocalcemia observed in the vehicle-treated 1α(OH)ase null mice was not improved by the use of cinacalcet, suggesting that the CaSR, although expressed in the gastrointestinal tract,23 is not capable of substituting for vitamin D-mediated intestinal calcium transport.
When 1α(OH)ase null mice were maintained on a rescue diet, animals became normocalcemic and normophosphatemic and had normal PTH levels. The normalization of these blood analytes likely reflects the capacity of the 20% lactose in the rescue diet to facilitate vitamin D–independent calcium and phosphorus absorption in the gut.18 Treatment of these animals with cinacalcet induced modest hypocalcemia and hypercalciuria, reflecting the reduced renal calcium re-absorption consequent to stimulation of the renal CaSR. Although serum PTH was decreased at 2 hours, at 24 hours PTH was not significantly different than vehicle-treated levels. The elevated serum phosphorus in these animals at 2 hours but not at 24 hours likely reflects the induction by the calcimimetic of a mild hypoparathyroid state over a portion of the 24-hour period. In recent studies we have reported an inverse relationship between bone formation and serum FGF-23 levels in the 1α(OH)ase null mice.24 It will be of importance in future studies to measure FGF-23 levels in response to cinacalcet to determine if this hormone may be modified directly by the calcimimetic.
Osteoblast numbers and activity were increased in bone of vehicle-treated null animals on a high-calcium intake, consistent with the skeletal anabolic effect of the high circulating concentrations of PTH.18 Osteoblast activity was reduced by 1,25(OH)2D3 and doxercalciferol as PTH fell, and this likely reflected reduction of excess osteoblast stimulation by the supraphysiologic serum PTH levels. Although cinacalcet cyclically inhibited PTH in this model, the transient peaks in circulating PTH produced by cinacalcet were insufficient to maintain osteoblast numbers at the elevated levels seen in vehicle-treated null animals on a high-calcium intake. Furthermore, reductions in osteoblast numbers were greater with cinacalcet than with the vitamin D sterols, possibly because of intrinsic bone anabolic activity of active forms of vitamin D.18,19,25 On a rescue diet, the cinacalcet-treated null mice also maintained osteoblast numbers and activity at a level significantly lower than in the severely hyperparathyroid vehicle-treated mutant mice on a high-calcium intake. CaSR has been reported to function in vitro in a variety of skeletal cells including cells of the osteoblast lineage.26–28 In our studies in these adult mice, however, any putative positive effects of cinacalcet on osteoblasts due to stimulation of the CaSR27 or other potential cation-sensing receptors29 were not clearly evident in the absence of the skeletal anabolic effect of high circulating PTH.
In contrast to osteoblast levels, osteoclast numbers and activity were increased by cinacalcet in mutant mice on both a high-calcium intake and a rescue diet. The induction of osteoclast resorption by cinacalcet in our studies is consistent with in vitro evidence demonstrating the presence of CaSR in monocyte-macrophages,30 and osteoclasts,31,32 and with in situ hybridization showing the presence of CaSR transcripts in bone marrow cells and osteoclasts.28 In addition, in vivo studies have shown that transgenic mice with a constitutively active mutant CaSR targeted to mature osteoblasts demonstrated osteopenia because of enhanced bone resorption, apparently through the stimulation of RANK ligand production,33 and a recent communication has reported that rats with CKD treated with cinacalcet also demonstrate increased bone resorption and reduced bone volume.21 Finally, when the CaSR is genetically disrupted in the same 1α(OH)ase null mouse model, osteoclastic bone resorption, even in the face of normocalcemia, is reduced.34 Consequently, it would appear that the increased osteoclastic bone resorption stimulated by cinacalcet is occurring via activation of the skeletal CaSR. Further studies will be required to determine whether this increased bone resorption occurs via an indirect action in osteoblasts or a direct effect on osteoclast precursors or mature osteoclasts, or both. Interestingly, this increase in osteoclastic activity occurred in the presence of hypocalcemia, suggesting that the effect of cinacalcet on the osseous calcium receptor superseded the effect of low calcium, or that cinacalcet rendered the osseous CaSR more sensitive to activation by [Ca2+]e.
A previous study described the effects of therapy with calcimimetics on bone histology in patients treated with hemodialysis35 and noted that a few patients developed adynamic bone, although the sample size was small and the patients all received concurrent vitamin D and/or phosphate binder therapy. Overall, our studies in the current model are not consistent with suppression of bone turnover by cinacalcet.
We and others have previously noted that in models expressing the null mutation of either the 1α(OH)ase gene18,36 or the VDR gene,37 osteoclast activity is either only modestly increased or not increased despite markedly elevated circulating PTH. This observation has been interpreted as evidence that active vitamin D and the VDR are required for optimal PTH stimulated bone resorption, and active vitamin D forms may in fact be more potent osteoclast activators than PTH. The present studies suggest that the mechanism for this effect of vitamin D may lie, in part, in correcting the hypocalcemia and thereby enhancing the stimulation of osteoclastic bone resorption via CaSR.
Calcium, phosphorus and 1,25(OH)2D have all been implicated in the normal development and mineralization of the growth plate. However, the role of the calcium receptor in growth plate development remains unclear. In the present study, treatment of 1α(OH)ase null mice on a high-calcium intake with active vitamin D compounds improved both the architecture and mineralization of the growth plate and normalized long bone growth. However, our studies in 1α(OH)ase null mice failed to show any positive effect of cinacalcet alone on growth plate architecture, or longitudinal bone growth. There are several potential explanations for this observation. It is possible that calcimimetic-induced modulation of calcium signaling at the CaSR differs in the chondrocyte, as compared with the parathyroid and osseous cells, or that calcium functions independently of the CaSR in the growth plate. Finally, in view of the fact that the growth of vehicle-treated normocalcemic and normophosphatemic mutant mice on the rescue diet was not completely normalized, direct vitamin D action on the growth plate may be required and this requirement was not met in the 1α(OH)ase null mice treated with cinacalcet alone.
In this study, treatment of null mice on a high-calcium intake with doxercalciferol or 1,25(OH)2D3 not only reversed rickets but also, by normalizing ambient calcium and phosphorus,18 repaired osseous mineralization. In contrast, cinacalcet only modestly reversed osteomalacia in null mice on a high-calcium intake. Recently, it was reported that mice with osteoblast-specific deletion of CaSR exhibited severely undermineralized skeletons,38 suggesting an important role for the CaSR in osteoblast-mediated bone mineralization, yet in our studies cinacalcet only partially improved bone mineralization. The persistent hypocalcemia occurring with cinacalcet treatment may therefore have limited matrix mineralization despite any putative activation of the osseous CaSR. On a rescue diet, mineralization was normalized with cinacalcet treatment despite the fact that transient mild hypocalcemia was present. The marked improvement in serum calcium in the null mice on the rescue diet even after cinacalcet treatment, coupled with the transient increase in serum phosphorus in null mice on the rescue diet after cinacalcet treatment, may therefore have improved osseous mineralization.
In summary, our studies show that when 1α(OH)ase null mice are exposed to an environment in which they exhibit persistent hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, rickets, and osteomalacia, doxercalciferol or 1,25(OH)2D3 treatment can normalize serum calcium and PTH, parathyroid gland size, and bone parameters, although 1,25(OH)2D3 may cause significant hyperphosphatemia. In contrast, cinacalcet treatment can improve hyperparathyroidism in this model, but does not normalize the accompanying mineral and skeletal abnormalities. When 1α(OH)ase null mice are exposed to an environment in which they become normocalcemic and normophosphatemic, cinacalcet treatment may perturb mineral homeostasis by causing transient hypocalcemia and hyperphosphatemia. Furthermore, our data suggest that cinacalcet may exert direct effects on the skeletal calcium receptor that may independently increase bone resorption and modify skeletal homeostasis. Therefore, in the setting of reduced active vitamin D, calcimimetics do not appear optimal for sole treatment of secondary hyperparathyroidism and skeletal abnormalities, especially in the presence of rickets and osteomalacia.
Concise Methods
Mice and Materials
The derivation of the 1α(OH)ase homozygous null mice [1α(OH)ase−/−], by homologous recombination in embryonic stem cells, was previously described by Panda et al.17 Briefly, a neomycin resistance gene replaced exons VI, VII, and VIII of the mouse 1α(OH)ase gene, removing both the ligand-binding and the heme-binding domains.17 RT-PCR of renal RNA from homozygous 1α(OH)ase−/− mice confirmed the lack of 1α(OH)ase expression. Mice were generated through breeding of heterozygous mice on a C57BL/6J genetic background and identified by Southern blots and/or PCR with tail genomic DNA as the template as described previously;17,18 wild-type littermates were used as controls in all of the experiments. To enhance fertility of females, all breeders were maintained on a high-calcium diet containing 1.5% calcium in the drinking water and autoclaved chow containing 1% calcium, 0.85% phosphorus, 0% lactose, and 2.2 units per gram of vitamin D3 (Ralston Purina Co., St. Louis, MO). At approximately 21 days of age, mice were weaned and maintained on drinking water containing 1.5% calcium gluconate and either a normal diet of autoclaved chow containing 1% calcium, 0.85% phosphorus, 0% lactose, and 2.2 units per gram of vitamin D3 (Ralston Purina Co.) or a rescue diet18 of γ-irradiated chow containing 20% lactose, 2% calcium, 1.25% phosphorus, and 2.2 units per gram of vitamin D2 (TD96348; Harlan Teklad, Madison, WI). No significant differences in any parameter determined were observed in wild-type mice on a high-calcium intake or a rescue diet.18 Consequently, control data are shown for the wild-type mice on the high-calcium intake. Cinacalcet (Amgen, Thousand Oaks, CA) was obtained via the local pharmacy. Doxercalciferol and 1,25(OH)2D3 were provided by Genzyme Corporation, Middleton, WI.
In Vivo Experiments
All animal experiments were carried out in compliance with and approval by the Institutional Animal Care and Use Committee. Mice were maintained in a virus- and parasite-free barrier facility and exposed to a 12-hour/12-hour light/dark cycle. From weaning, animals received, every 2 days by subcutaneous injection, either of the following: vehicle (50% propylene glycol, 40% saline, 10% ethanol); doxercalciferol, 150 pg/g; 1,25(OH)2D3, 50 pg/g; or cinacalcet, 30 μg/g daily by oral gavage. The doses of doxercalciferol and 1,25(OH)2D3 used were based on preliminary studies in the null mice on a high-calcium intake; the doses selected for each compound were those that reduced serum PTH to the normal range without causing hypercalcemia. The dose of cinacalcet used was based on previous studies in mice and rats19,21,39 and on preliminary studies in the null mice on a high-calcium intake in which doses of 10, 20, 30, and 50 μg/g were employed; no further PTH suppression was seen at 50 μg/g than at 30 μg/g, so the latter dose was selected. At about 3 and one-half months of age, the animals were killed, and blood and urine samples were collected for analysis. Serum was obtained 2 and 24 hours after the final cinacalcet administration and 24 hours after the final doxercalciferol and 1,25(OH)2D3 injections. Tibias, femurs, and thyroparathyoidal tissues were also obtained, at the time of death, for histology and for histomorphometric analysis.
Biochemical and Hormone Analyses
Serum calcium and phosphorus and urine calcium were determined by autoanalyzer (Beckman Synchron 67, Beckman Instruments). Serum intact PTH was measured by a two-site immunoradiometric assay (Immutopics, San Clemente, CA). A mouse osteocalcin two-site immunoradiometric assay (IRMA; Immutopics) was used for the measurement of serum osteocalcin levels according to the manufacturer's specifications. A mouse TRAP5b assay (IDS, Fountain Hills, AZ) was used according to the manufacturer's specifications to determine osteoclast-derived TRAP5b in mouse serum samples.
Histology
Femurs, tibias, and thyroparathyroidal tissue were removed and fixed in PLP fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate) overnight at 4°C and processed histologically as described previously.40 The bones were decalcified in EDTA glycerol solution for 14 days at 4°C. Decalcified bones and thyroparathyroidal tissue were dehydrated and embedded in paraffin, after which 5-μm sections were cut on a rotary microtome. The sections were stained with hematoxylin and eosin (H&E) or histochemically for TRAP activity41 and alkaline phosphatase (ALP) activity as described below. Alternatively, undecalcified bones were embedded in LR White acrylic resin (London Resin Co., London), and 1-μm sections were cut on an ultramicrotome. These sections were stained for mineral with the von Kossa staining procedure and counterstained with toluidine blue. Histomorphometric indices were determined as suggested by the ASBMR Histomorphometry Nomenclature Committee.42
Histochemical Staining for TRAP
Enzyme histochemistry for TRAP staining was performed as described previously.41 De-waxed sections were preincubated for 20 minutes in buffer containing 50 mM sodium acetate and 40 mM sodium tartrate at pH 5.0. Sections were then incubated for 15 minutes at room temperature in the same buffer containing 2.5 mg/ml naphthol AS-MX phosphate (Sigma-Aldrich, St. Louis) in dimethylformamide as substrate and 0.5 mg/ml fast garnet GBC (Sigma-Aldrich) as a color indicator for the reaction product. After the product was washed with distilled water, the sections were counterstained with methyl green and mounted in Kaiser's glycerol. ALP staining was performed on methyl methacrylate (MMA)-embedded undecalcified bone samples using a vector red alkaline phosphatase substrate kit (Vector Laboratories43).
Double Calcein Labeling
Double calcein labeling was performed by intraperitoneal injection of mice with 10 μg of calcein per gram of body weight (C-0875; Sigma-Aldrich) at 10 and 3 days before the mice were killed. Bones were harvested and embedded in LR White acrylic resin as described above. Serial sections were cut, and the freshly cut surface of each section was viewed and imaged using fluorescence microscopy. The double calcein interlabel width was measured using Northern Eclipse image analysis software version 6.0 (Empix Imaging Inc., Mississauga, ON, Canada), and the mineral apposition rate (MAR; MAR = interlabel width/labeling period) was calculated. The bone formation rate was calculated according to the following formula: BFR = MAR × (MS/BS), where MS is the mineralizing surface and BS is the bone surface.41 Calculations were made on a minimum of duplicate specimens from replicate mice of each group.
Histomorphometry
After H&E staining or immunohistochemical staining, histomorphometric measurements were performed as described previously42 in the secondary spongiosa in the metaphyseal area (0.5 mm below the growth plate) at the distal end of femur. The parameters obtained for bone formation were the osteoblast surface per bone surface (Ob.S/BS; percentage), and the MAR (micrometers per day) and the BFR/BS(μm3/μm2 per day). The parameter measured for bone resorption was the number of osteoclasts per bone perimeter [N.Oc./bone perimeter (mm)]. After H&E staining or histochemical staining of sections from replicate mice of each group, images of fields were photographed with a digital camera. Images of micrographs from single sections were digitally recorded using a rectangular template, and recordings were processed and analyzed using Northern Eclipse image analysis software as described.44–46
BMD Analysis
Densitometry was performed by PIXImus on the right femur under anesthesia as described previously.46 In brief, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (0.1 mg/kg) in PBS and placed prone on the platform of a PIXImus densitometer (software version 1.46.007; Lunar Corp., Madison, WI) for BMD measurements according to the manufacturer's instruction. In some experiments, the variability in measurements was examined by repeating scans after repositioning the animals. Percent coefficient of variation of BMD for the repeated scans was 1 to 3%.
Skeletal Radiography
After removal of soft tissue, radiographs were taken using a Faxitron model 805 radiographic inspection system (Faxitron Contact, Faxitron, 22-kV voltage, and 4-minute exposure time). X-Omat TL film (Eastman Kodak, Rochester, NY) was used and processed routinely.
Microcomputed Tomography
Femurs obtained from mice were dissected free of soft tissue, fixed overnight in 70% ethanol, and analyzed by microcomputed tomography with a SkyScan 1072 scanner and associated analysis software (SkyScan, Antwerp, Belgium) as described.46 Scans were performed on the distal part of the femur. Image acquisition was performed at 100 kV and 98 μA with a 0.9° rotation between frames. Thresholding was applied to the images to segment the bone from the background. Three-dimensional renderings of the distal 3.5 mm of the femora was reconstructed using two-dimensional data from scanned slices with the 3D Creator software supplied with the instrument. The trabecular bone region of interest was drawn to include all cancellous bone in the metaphysis, and three-dimensional analysis was performed to calculate bone volume (BV)/tissue volume (TV). The resolution of the microcomputed tomography images was 18.2 μm.
Computer-Assisted Image Analysis
After H&E staining or histochemical staining of sections from replicate mice of each group, images of fields were photographed with a Sony digital camera. Images of micrographs from single sections were digitally recorded using a rectangular template, and recordings were processed and analyzed using Northern Eclipse image analysis software.46 To measure the size of the parathyroid glands, the border of the glands were traced on micrographs of H&E-stained sections and traced areas of parathyroid glands were recorded automatically by Northern Eclipse image.
Statistical Analysis
Statistical comparisons, using Graph-Pad Prism (GraphPad Software Inc., San Diego) analysis software were made using t test or ANOVA, followed by a Bonferroni adjustment or a Neuman-Keuls multiple comparison test. P < 0.05 was considered significant.
Disclosures
D.G. has served as a consultant for Amgen, Genzyme, Eli Lilly, Merck, Novartis, Proctor and Gamble/Aventis, and Servier. S.S. is an employee of Genzyme.
Acknowledgments
This work was supported by an investigator-initiated grant to D.G. from Genzyme Corporation and by a grant to D.G. from the Canadian Institutes of Health Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. Brown EM: Calcium receptor and regulation of parathyroid hormone secretion. Rev Endocr Metab Disord 1: 307–315, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, Hsieh JC, Kaczmarska M, Haussler CA, Haussler MR: Vitamin D receptor: Key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res 22 Suppl 2: V2–V10, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Andress DL, Coyne DW, Kalantar-Zadeh K, Molitch ME, Zangeneh F, Sprague SM: Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract 14: 18–27, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Russell J, Lettieri D, Sherwood LM: Suppression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinology 119: 2864–2866, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Silver J, Naveh-Many T, Mayer H, Schmelzer JH, Popovtzer MM: Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest 78: 1296–1301, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto M, Igarashi T, Muramatsu M, Fukagawa M, Motokura T, Ogata E: Hypocalcemia increases and hypercalcemia decreases the steady-state level of parathyroid hormone messenger RNA in the rat. J Clin Invest 83: 1053–1056, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moallem E, Silver J, Kilav R, Naveh-Many T: RNA protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem 273: 5253–5259, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N: The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest 100: 2977–2983, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kremer R, Bolivar I, Goltzman D, Hendy GN: Influence of calcium and 1,25-dihydroxycholecalciferol on proliferation and proto-oncogene expression in primary cultures of bovine parathyroid cells. Endocrinology 125: 935–941, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Cozzolino M, Lu Y, Finch J, Slatopolsky E, Dusso AS: p21WAF1 and TGF-alpha mediate parathyroid growth arrest by vitamin D and high calcium. Kidney Int 60: 2109–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ: Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxycholecalciferol in uremic patients. J Clin Invest 74: 2136–2143, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW: Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis 43: 877–890, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Van Wagenen BC, Delmar EG, Balandrin MF: Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Block GA, Martin KJ, de Francisco ALM, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, Schwanauer LE, Olson KA, Klassen P, Bilezikian JP: Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92: 3803–3808, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD: Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol 3: 658–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D: Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 98: 7498–7503, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D: Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279: 16754–16766, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D: Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum Mol Genet 14: 1515–1528, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D: Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308: 627–635, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Finch JL, Tokumoto M, Nakamura H, Yao W, Dr, Shahnazari M, Lane N Md, Slatopolsky E: Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298: F1315–F1322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colloton M, Shatzen E, Miller G, Stehman-Breen C, Wada M, Lacey D, Martin D: Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int 67: 467–476, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Herbert SC, Cheng S, Geibel J: Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium 35: 239–247, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D: Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology 150: 4835–4845, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D: Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improves mineral ion homeostasis in mice that are homozygous for both the 1alpha-hydroxylase and parathyroid hormone null alleles. Endocrinology 147: 4801–4810, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, Bandyopadhyay S, Ren X, Terwilliger E, Brown EM: Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145: 3451–3462, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D: Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A 101: 5140–5145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA, Miller S, Shoback D: Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140: 5883–5893, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD: Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280: 40201–40209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, Glowacki J, Brown EM: Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 12: 1959–1970, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Kanatani M, Sugimoto T, Kanzawa M, Yano S, Chihara K: High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun 261: 144–148, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, Izumi N, Kawashima H, Ozawa H, Ikeda K, Kameda A, Hakeda Y, Kumegawa M: Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 245: 419–422, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Dvorak MM, Chen TH, Orwoll B, Garvey C, Chang W, Bikle DD, Shoback DM: Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology 148: 3156–3163, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Richard C, Huo R, Samadfam R, Bolivar I, Miao D, Brown EM, Hendy GN, Goltzman D: The calcium sensing receptor and 25-hydroxyvitamin D-1alpha-hydroxylase interact to modulate skeletal growth and bone turnover. J Bone Miner Res 25: 1627–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Malluche HH, Monier-Faugere MC, Wang G, Frazã O JM, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG: An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol 69: 269–278, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Dardenne O, Prud'homme J, Hacking SA, Glorieux FH, St-Arnaud R: Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficientfor the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone 32: 332–340, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB: Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140: 4982–4987, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Chang W, Tu C, Chen TH, Bikle D, Shoback D: The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1: ra1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawata T, Imanishi Y, Kobayashi K, Kenko T, Wada M, Ishimura E, Miki T, Nagano N, Inaba M, Arnold A, Nishizawa Y: Relationship between parathyroid calcium-sensing receptor expression and potency of the calcimimetic, cinacalcet, in suppressing parathyroid hormone secretion in an in vivo murine model of primary hyperparathyroidism. Eur J Endocrinol 153: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Miao D, Bai X, Panda D, McKee MD, Karaplis AC, Goltzman D: Osteomalacia in Hyp mice is associated with abnormal Phex expression and with altered bone matrix protein expression and deposition. Endocrinology 142: 926–939, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Miao D, Scutt A: Recruitment, augmentation and apoptosis of rat osteoclasts in 1,25-(OH)2D3 response to short-term treatment with 1,25-dihydroxyvitamin D3 in vivo. BMC Musculoskelet Disord 3: 16–26, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR: Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Samadfam R, Xia Q, Goltzman D: Co-treatment of PTH with osteoprotegerin or alendronate increases its anabolic effect on the skeleton of oophorectomized mice. J Bone Miner Res 22: 55–63, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC: Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J Clin Invest 115: 2402–2411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miao D, Li J, Xue Y, Su H, Karaplis AC, Goltzman D: Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 145: 3554–3562, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Samadfam R, Xia Q, Goltzman D: Pretreatment with anticatabolic agents blunts but does not eliminate the skeletal anabolic response to parathyroid hormone in oophorectomized mice. Endocrinology 148: 2778–2787, 2007 [DOI] [PubMed] [Google Scholar]