Abstract
Arginine-vasopressin (AVP) modulates the water channel aquaporin-2 (AQP2) in the renal collecting duct to maintain homeostasis of body water. AVP binds to vasopressin V2 receptors (V2R), increasing cAMP, which promotes the redistribution of AQP2 from intracellular vesicles into the plasma membrane. cAMP also increases AQP2 transcription, but whether altered degradation also modulates AQP2 protein levels is not well understood. Here, elevation of cAMP increased AQP2 protein levels within 30 minutes in primary inner medullary collecting duct (IMCD) cells, in human embryonic kidney (HEK) 293 cells ectopically expressing AQP2, and in mouse kidneys. Accelerated transcription or translation did not explain this increase in AQP2 abundance. In IMCD cells, cAMP inhibited p38-mitogen-activated protein kinase (p38-MAPK) via activation of protein kinase A (PKA). Inhibition of p38-MAPK associated with decreased phosphorylation (serine 261) and polyubiquitination of AQP2, preventing proteasomal degradation. Our results demonstrate that AVP enhances AQP2 protein abundance by altering its proteasomal degradation through a PKA- and p38-MAPK–dependent pathway.
Aquaporin-2 (AQP2) is the water channel mediating arginine-vasopressin (AVP)–increases in water re-absorption in renal collecting duct principal cells.1–4 AVP binds to plasma membrane–located vasopressin V2 receptors, thereby stimulating adenylyl cyclase and elevating cAMP. cAMP activates protein kinase A (PKA), which phosphorylates AQP2 at serine 256 (S256), inducing its redistribution from intracellular vesicles into the plasma membrane.3,4 This short-term regulation of AQP2 occurs within seconds to minutes. In the case of long-term regulation, cAMP enhances AQP2 mRNA expression, followed by a rise in the AQP2 protein level within hours.5,6
AQP2 can be degraded in proteasomes and lysosomes.7,8 Ubiquitination directs proteins for degradation to both compartments. Monoubiquitination (mUb) is a signal for degradation in lysosomes, whereas polyubiquitination (pUb) is mainly linked to proteasomal degradation.9 mUb of AQP2 is induced by FSK stimulation and occurs at the apical plasma membrane.10 In WT5 cells, a model for AQP2 regulation, increased mUb of AQP2 persists after termination of FSK stimulation, leading to a higher rate of AQP2 retrieval from the plasma membrane into endosomes.10 The extension of monoubiquitin by two or three additional ubiquitin moieties (short-chain ubiquitination) apparently participates in the control of AQP2 degradation.10,11 pUb of AQP2 has not been observed. The signaling processes controlling ubiquitination, and therefore the AQP2 abundance, are largely unknown.
In addition to the phosphorylation of S256, the phosphorylation levels of serines 261 (S261), 264, and 269 within the C-terminus of AQP2 change in response to AVP. S256, S264, and S269 phosphorylations appear to be involved in the regulation of AQP2 trafficking,12–14 whereas the role of S261 phosphorylation in the regulation of AQP2 remains unclear. In suspensions of inner medullary collecting duct cells from rats phosphorylation of S261 decreases upon challenge with the AVP analogue desmopressin (dDAVP).15 A candidate kinase to phosphorylate S261 is p38-mitogen-activated protein kinase (p38-MAPK).15,16 p38-MAPK is downregulated by cAMP in a PKA-dependent manner in HeLa cells and fibroblasts.17 Importantly, phosphorylation by p38-MAPK represents a hallmark for ubiquitination and proteasomal degradation of its targets.18 Here we demonstrate that in renal principal cells AVP controls AQP2 protein abundance through a mechanism involving PKA-dependent p38-MAPK inhibition and a p38-MAPK–dependent regulation of proteasomal degradation of AQP2. Physiologically, this novel regulatory mechanism of AQP2 abundance is likely to play a role in rapidly increasing the osmotic water permeability of the renal collecting duct in response to AVP.
Results
cAMP Elevation Induces a Rapid Increase in AQP2 Protein Abundance in Cultured Inner Medullary Collecting Duct Cells and In Vivo Independently of Accelerated Transcription
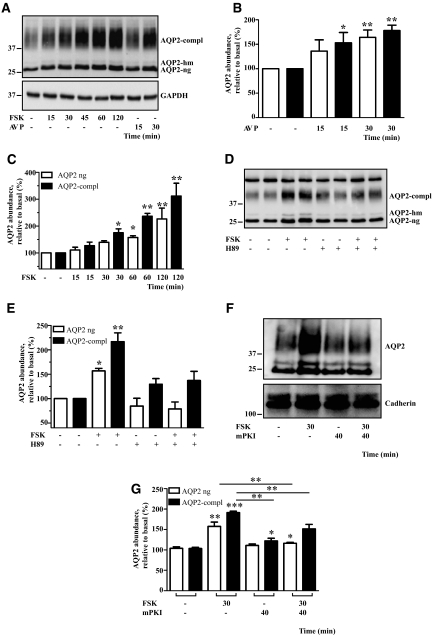
Primary cultured rat inner medullary collecting duct (IMCD) cells represent a model for studies of both short- and long-term regulation of AQP2.4,19–21 We utilized these cells to investigate whether AQP2 protein abundance is also subject to short-term regulation by cAMP. IMCD cells were treated with AVP (100 nM) for 15 or 30 minutes or with forskolin (FSK; 10 μM) for 15, 30, 45, 60, and 120 minutes. AQP2 was immunoprecipitated using antibody H27, directed against the C-terminus of AQP2, and its abundance was analyzed by immunoblotting using another antibody raised against the C-terminus (C-17; Figure 1, A and B). Compared with control cells, AVP significantly increased AQP2 protein abundance after 15 and 30 minutes (Figure 1, A and B). Effects of longer treatments with AVP were not studied because internalization/downregulation of vasopressin V2 receptors under prolonged AVP exposure decreases cAMP production.22,23 FSK also augmented AQP2 abundance within 30 minutes (Figure 1, A and C; Figure 2, C and D). The increase was also detectable with an antibody directed against the N-terminus of AQP2 (N-20) in lysates derived from IMCD cells (Figure 1F; Figure 7, A and E; Figure 8, A and C) and mouse renal inner medullae (Figure 3). In addition, both antibodies, C-17 and N-20, detected the increase in lysates derived from human embryonic kidney 293 (HEK293) cells transiently expressing AQP2 (Supplemental Figure 1). Thus, the increased AQP2 abundance is not due to altered recognition of the protein by the antibodies. A change in AQP2 solubility is unlikely to be responsible for the FSK effect because the ratio of AQP2 between lysates and pellets remained unchanged under FSK treatment (data not shown).
Figure 1.
AVP and FSK rapidly increase the AQP2 protein level in a PKA-dependent manner in IMCD cells. (A) IMCD cells were left untreated or treated with AVP (100 nM) or FSK (10 μM) for the indicated times. AQP2 was immunoprecipitated and detected by Western blotting using antibodies directed against the C-terminus. Shown is a representative blot of five independent experiments. Protein levels of GAPDH were detected by Western blotting in the cell lysates used for immunoprecipitation. Semiquantitative analysis of the effects of AVP (B) and FSK (C) on AQP2 protein levels. Western blots, obtained as indicated in A, were densitometrically analyzed. Shown are means ± SEM of five independent experiments. (D) IMCD cells were left untreated or treated with FSK (10 μM, 30 minutes), the PKA inhibitor H89 (30 μM, 30 minutes), or a combination of FSK (10 μM, 30 minutes) and H89 (30 μM, 30 minutes). AQP2 was immunoprecipitated and detected as in panel A. (F) The cells were left untreated or treated with FSK (10 μM, 30 minutes), the PKA-specific inhibitor mPKI (350 μM, 40 minutes), or a combination of FSK (10 μM, 30 minutes) and mPKI (350 μM, 40 minutes), a peptide derived from the heat-labile PKA inhibitor peptide, rendered membrane-permeable by coupling to myristoylate. The cells were lysed and AQP2 was detected with antibodies directed against the N-terminus of AQP2 (N-20). Cadherin was detected as a loading control. (E and G) The effects of FSK and H89 (E) and of FSK and mPKI (G) on the AQP2 protein abundance were analyzed as indicated in panels B and C. Statistically significant differences from untreated controls are indicated: *P < 0.05, **P < 0.01, and ***P < 0.001. AQP2-compl, complex glycosylated APQ2; AQP2-hm, high mannose form of AQP2; AQP2-ng, nonglycosylated AQP2.
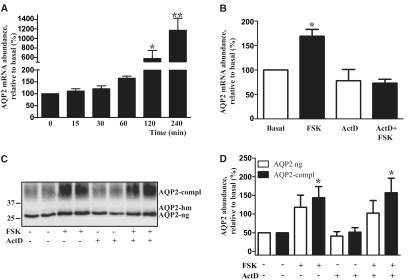
Figure 2.
FSK increases the AQP2 protein abundance in IMCD cells independent from transcriptional regulation. (A) IMCD cells were left untreated or treated with FSK (10 μM, 15 to 240 minutes). The AQP2 mRNA level was quantified by real-time PCR. Shown are means ± SEM of three independent experiments each performed in duplicates. (B) IMCD cells were left untreated or pretreated with the transcription inhibitor, actinomycin D (ActD, 10 μg/ml, 60 minutes), and where indicated, additionally treated with FSK (10 μM) for a further 60 minutes. The AQP2 mRNA was quantified as described in panel A. Shown are means ± SEM of three independent experiments each performed in duplicates. (C) IMCD cells were left untreated or were preincubated with ActD (10 μg/ml) for 60 minutes. FSK (10 μM) was added where indicated and cells were incubated for an additional hour. Cells were lysed, and AQP2 was immunoprecipitated and detected by Western blotting using antibodies directed against the C-terminus. Shown is a representative blot of four independent experiments. (D) Semiquantitative analysis of ActD and FSK effects on the AQP2 protein level. AQP2 was detected as indicated in panel C. Shown are means ± SEM of four independent experiments each performed in duplicates. Statistically significant differences from untreated controls in panels A, B, and D are indicated: *P < 0.05 and **P < 0.01.
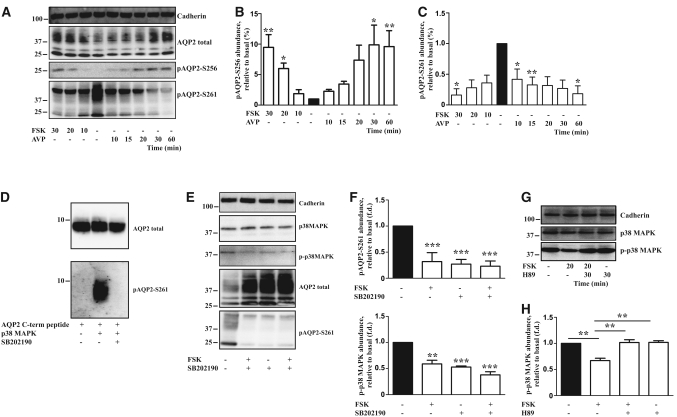
Figure 7.
The phosphorylation of S261 of AQP2 involves control by p38-MAPK in a PKA-dependent manner. (A) IMCD cells were either left untreated or were treated with AVP (100 nM) or FSK (10 μM) for the indicated times. The cells were lysed and the proteins subjected to Western blotting: total AQP2 was detected with an antibody directed against the N-terminus (N-20); AQP2 phosphorylated at S256 or S261 was visualized with two different antibodies, each specifically recognizing one of the phosphorylated serines; detection of cadherin served as a loading control. Shown are representative blots of three independent experiments. Semiquantitative analysis of the levels of AQP2 phosphorylated at serines 256 (B) and 261 (C). Western blots, obtained as indicated in panel A, were analyzed by densitometry. Shown are means ± SEM of three independent experiments. Statistically significant differences versus untreated cells are indicated: *P < 0.05 and **P < 0.01. (D) p38-MAPK phosphorylates AQP2 in vitro. p38-MAPK was preincubated with the inhibitor SB202190 (5 μM, 15 minutes) or DMSO (15 minutes), and a peptide derived from the C-terminus of AQP2 (27 μg) was added (60 minutes). Unphosphorylated peptides and peptides phosphorylated by p38-MAPK were detected by Western blotting using an anti-AQP2 antibody directed against the C-terminus (H27) or antibodies specifically recognizing phospho-S261 of AQP2. (E) IMCD cells were left untreated, treated with FSK (10 μM, 30 minutes), the p38MAPK inhibitor, SB202190 (5 μM, 120 minutes), or a combination of both agents. AQP2 (antibody N-20), phosphorylated at S261, total p38-MAPK, p-p38-MAPK, and as a loading control, cadherin were detected by Western blotting. Shown are representative blots of three independent experiments. (F) Semiquantitative, analysis of the levels of total AQP2, AQP2 phosphorylated at S261, and phosphorylated p38-MAPK. Statistically significant differences versus untreated cells are indicated: ***P < 0.001. (G) IMCD cells were left untreated, treated with FSK (10 μM, 20 minutes), the PKA inhibitor H89 (30 μM, 30 minutes), or a combination of both agents. Total p38-MAPK, phosphorylated p38-MAPK (p-p38-MAPK), and as a loading control, cadherin were detected by Western blotting. Shown are representative blots of three independent experiments. (H) Semiquantitative analysis of the levels of phosphorylated p38-MAPK. Statistically significant differences are indicated: **P < 0.01.
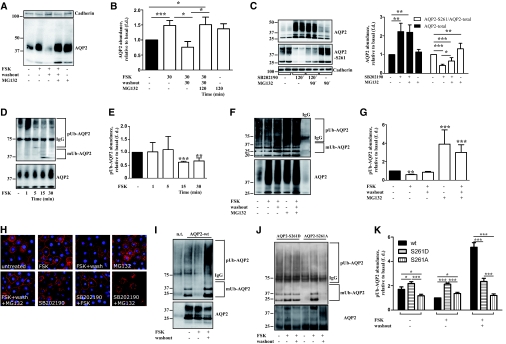
Figure 8.
Elevation of cAMP protects AQP2 from proteasomal degradation by reducing polyubiquitination of AQP2. (A) IMCD cells were left untreated or treated with FSK (10 μM, 30 minutes) or the proteasome inhibitor, MG132 (10 μM), for an additional 90 minutes. Where indicated, FSK was washed out. Subsequently, the cells were left untreated or incubated with MG132 (10 μM, 90 minutes). Cells were lysed, AQP2 (with antibody N-20) and, as a loading control, cadherin were detected by Western blotting. Shown are representative blots of three independent experiments. (B) Semiquantitative analysis of the effects of FSK and MG132 treatments as depicted in panel A on the AQP2 protein abundance. Shown are means ± SEM of three independent experiments. Statistically significant differences are indicated: *P < 0.05 and ***P < 0.001. (C) IMCD cells were treated with SB202190, MG132, or the combination of the two agents and lysed and total AQP2 (with antibody N-20), AQP2 phosphorylated at S261, and cadherin were detected by Western blotting. Shown are representative blots of three independent experiments. Semiquantitative analysis of the levels of total AQP2 and AQP2 phosphorylated at S261. Statistically significant differences are indicated: *P < 0.05, **P < 0.01, and ***P < 0.001. (D) IMCD cells were left untreated or incubated with FSK for the indicated times. AQP2 was immunoprecipitated with antibody N-20 and ubiquitin and AQP2 were detected by Western blotting. Shown are representative blots from three independent experiments. (E) Signals obtained in Western blots shown in panel D were densitometrically evaluated. The amount of polyubiquitinated AQP2 is relative to the abundance of total AQP2 (shown in the lower panel of D). Shown are means ± SEM of three independent experiments. Statistically significant differences from untreated controls are indicated: **P < 0.01 and ***P < 0.001. (F) IMCD cells were left untreated or treated as indicated in panel A. The cells were lysed, AQP2 was immunoprecipitated with antibody N-20, and ubiquitin and AQP2 were detected by Western blotting. Shown are representative blots of four independent experiments. IgG, the immunoprecipitation was carried out with unrelated IgG instead of the specific anti-AQP2 antibody. (G) Semiquantitative analysis of the effects of FSK and MG132 treatments depicted in panel F on the ubiquitination of AQP2. Shown are means ± SEM of three independent experiments. Statistically significant differences from untreated controls are indicated: **P < 0.01 and ***P < 0.001. (H) IMCD cells were treated as indicated in panel A and AQP2 was detected by immunofluorescence microscopy. (I and J) HEK293 cells remained nontransfected (n.t.) or were transiently transfected with plasmids encoding either wild-type AQP2 (AQP2-wt; panel I) or the mutant versions of AQP2, AQP2-S261D or AQP2-S261A (J). The cells were left untreated or treated with FSK (10 μM, 30 minutes). Where indicated, FSK was washed out. The cells were lysed, AQP2 was immunoprecipitated, and ubiquitin and total AQP2 were detected by Western blotting as described in panel D. Shown are representative blots of three independent experiments. (K) Semiquantitative analysis of the effects of the FSK treatments depicted in panels I and J on the ubiquitination of AQP2-wt, AQP2-S261D, and AQP2-S261A. Shown are means ± SEM of three independent experiments. Statistically significant differences are indicated: *P < 0.05 and ***P < 0.001.
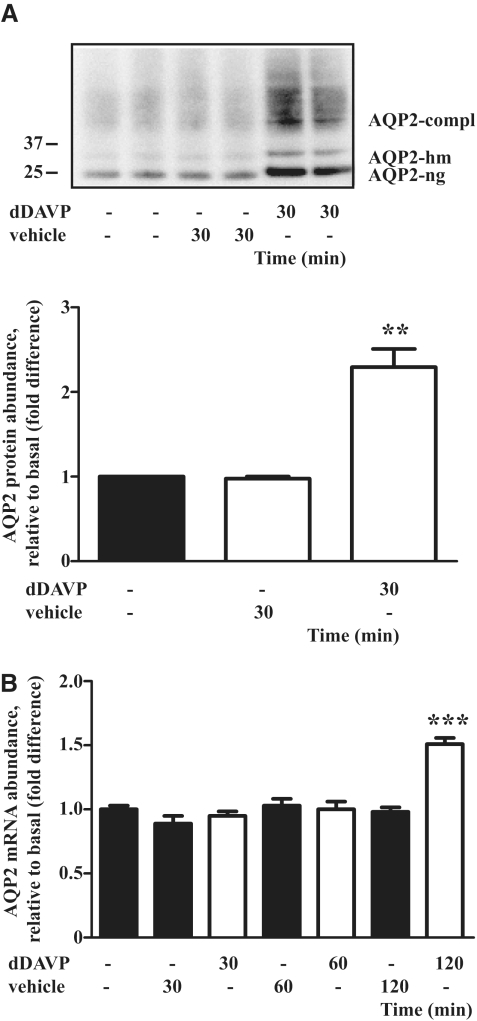
Figure 3.
AVP rapidly upregulates the AQP2 protein level in vivo. (A) Wild-type C57/Bl6 mice received dDAVP (1 ng/g body wt, intraperitoneally) and were sacrificed 30 minutes later and lysates from renal medullae were analyzed for protein levels of AQP2 by Western blotting using anti-AQP2 antibody N-20 directed against the N terminus (upper panel). Shown are the results of two of a total of five untreated animals, five animals treated with vehicle, and five animals treated with dDAVP. Lower panel: The effects of dDAVP on the AQP2 protein abundance were semiquantitatively evaluated by densitometric analysis of the Western blots obtained as indicated in the upper panel. Shown are means ± SEM of five independent experiments. (B) Semiquantitative analysis of the effects of dDAVP on AQP2 mRNA levels in C57/Bl6 mice. Mice were subjected to peritoneal injection of dDAVP (1 ng/g body wt) or vehicle as indicated in panel A and sacrificed 30, 60, or 120 minutes later and relative levels of AQP2 mRNA in renal inner medullae were determined by real-time PCR. Shown are means ± SEM of five animals in each group. Statistically significant differences from vehicle-treated animals are indicated: **P < 0.01 and ***P < 0.001.
Both AVP and FSK significantly increased the abundance of glycosylated before nonglycosylated AQP2, presumably because glycosylated AQP2 is the mature protein that is rather accessible for posttranslational modifications in the cytotsol (ubiquitination, see below). Neither AVP or FSK treatments for up to 2 hours affected the protein level of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Figure 1A).
dbcAMP increased AQP2 abundance threefold (Supplemental Figure 2, A and B). To test whether the cAMP-dependent elevation of the AQP2 protein abundance is mediated by PKA, the kinase was inhibited with H89, and a membrane-permeable peptide derived from the heat-labile inhibitor of PKA (mPKI). Both prevented the effect of FSK on AQP2 abundance (Figure 1, D through G), strongly suggesting that AVP and FSK increase AQP2 abundance in a PKA-dependent manner.
Long-term AVP stimulation increases AQP2 protein abundance in renal principal cells by inducing gene transcription.6,7,24 FSK did not affect AQP2 mRNA levels within 30 minutes but increased it thereafter (Figure 2A). To determine whether increased transcription contributes to the initial rise in AQP2 protein levels, we measured AQP2 mRNA and protein levels in the presence of a selective transcription inhibitor, Actinomycin D (ActD; 10 μg/ml). ActD completely prevented the FSK-induced increase in AQP2 mRNA levels (Figure 2B). The FSK-induced increase in AQP2 protein abundance (60 minutes) was not affected by ActD (Figure 2, C and D). Therefore, the rapid increase in AQP2 protein abundance solely depends on posttranscriptional regulation.
To evaluate whether the rise in cAMP increases AQP2 protein abundance in vivo, wild-type C57/Bl6 mice were treated with the AVP analog dDAVP. As in cultured cells, the AQP2 protein abundance increased after 30 minutes of treatment (Figure 3A), whereas AQP2 mRNA levels were significantly enhanced only 120 minutes after dDAVP application (Figure 3B).
cAMP Increases AQP2 Abundance through Control of the AQP2 Protein Itself
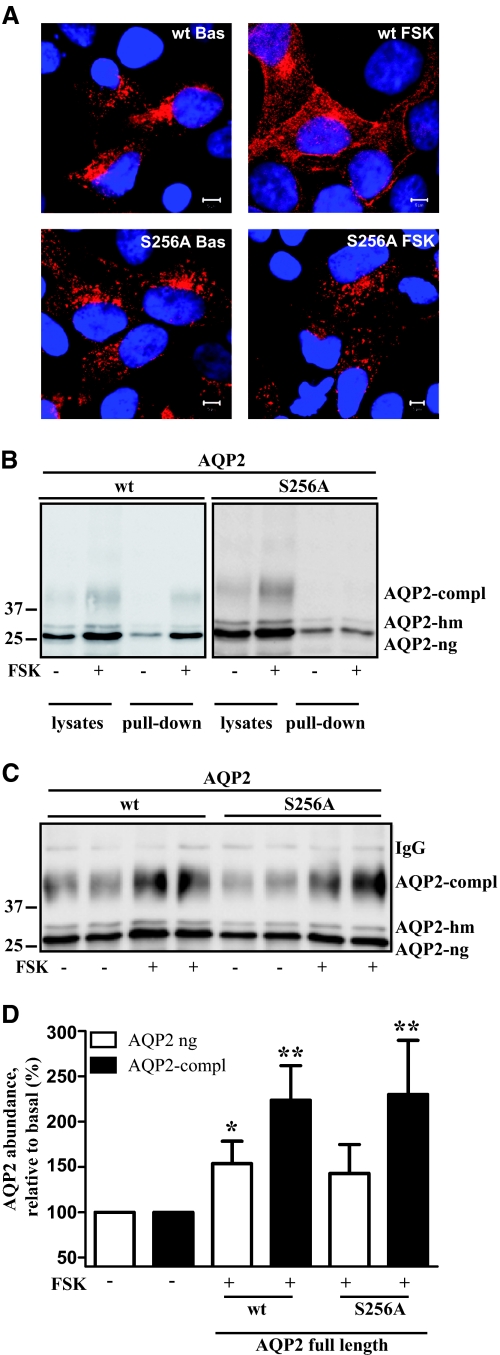
Next, we investigated whether the ability of cAMP to elevate the AQP2 protein level depends on the AQP2 protein itself. We used HEK293 cells ectopically expressing AQP2 under the control of the cytomegalovirus (CMV) immediate-early promoter. Under basal conditions, AQP2 was localized in the perinuclear region (Figure 4A), and co-localized with Rab11 (data not shown) as already demonstrated for IMCD, CD8, and MDCK cells.21,25 FSK induced a redistribution of AQP2 to the plasma membrane (Figure 4A). As in other models AQP2-S256A, an AQP2 form lacking the PKA consensus motif and which thus mimics the constitutively unphosphorylated protein, does not translocate to the plasma membrane in response to FSK (Figure 4A).26,27 Cell surface biotinylation experiments confirmed that increased amounts of wild-type AQP2 but not of AQP2-S256A were inserted into the plasma membrane in response to cAMP elevation (Figure 4B). Thus, in HEK293 cells ectopically expressed AQP2 is subject to regulation as it occurs in primary IMCD cells and in vivo. Therefore, HEK293 cells may be used to study AQP2 regulation. Although the cells are not polarized, this system has the advantage that it is of human origin and efficiently transfectable.
Figure 4.
AQP2 is subject to short-term regulation in HEK293 cells. (A) HEK293 cells transiently expressing wild-type AQP2 (upper panel; wt) or AQP2-S256A (lower panel; S256A) were either left untreated (left side; Bas) or were treated with FSK (right side; FSK; 10 μM, 30 minutes). Cells were fixed, permeabilized, and stained for AQP2 (red). Nuclei were stained with DAPI (blue). Scale bars, 5 μm. (B) Cells were transfected and treated as indicated in panel A. To detect AQP2 on the cell surface, surface proteins were biotinylated with Sulfo-NHS-SS-Biotin and then precipitated with streptavidin-Sepharose. Proteins were separated by NuPAGE and AQP2 was detected by Western blotting with antibody directed against the C-terminus (C-17). Shown are representative blots of four independent experiments. (C) HEK293 cells transiently expressing full-length, untagged wild-type AQP2 or AQP2-S256A remained untreated or after 24 hours were treated with FSK (10 μM, 60 minutes). AQP2 was immunoprecipitated and detected by Western blotting as described in the legend to Figure 1A. Shown is a representative blot of five independent experiments. (D) Semiquantitative analysis of the effect of FSK on the AQP2 protein level. AQP2 was detected as indicated in panel C. Shown are means ± SEM of five independent experiments. Statistically significant differences from untreated controls are indicated: *P < 0.05 and **P < 0.01.
FSK (for 30 or 60 minutes) increased the protein content of ectopically expressed wild-type AQP2 or AQP2-S256A in HEK293 cells as compared with untreated controls (Figure 4, B through D). Selective inhibition of transcription with ActD did not prevent this effect (data not shown).
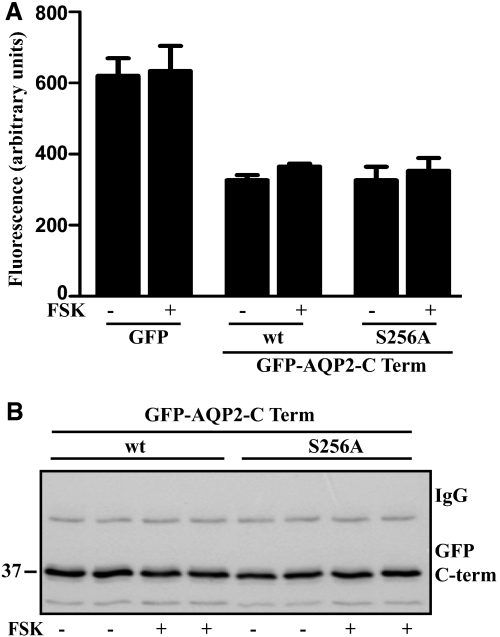
The C-terminus of AQP2 harbors several phosphorylation and protein interaction motifs. We wondered whether it alone is sufficient to endow cAMP-dependent protein upregulation. To test this, the wild-type and S256A mutant versions of the AQP2 C-terminus (amino acids 225 to 271) were fused with green fluorescent protein (GFP) and transiently expressed in HEK293 cells. Fluorimetric measurements revealed no overt differences in GFP fluorescence between control cells and cells treated for 60 minutes with FSK (Figure 5A). Even after prolonged treatment for 4 hours, FSK did not alter fluorescence signals (data not shown). Western blotting (Figure 5B) confirmed that FSK did not affect the abundance of the fusions. Therefore, the FSK-induced upregulation of the AQP2 protein level requires full-length AQP2 protein and does not involve phosphorylation of S256 (Figures 4 and 5).
Figure 5.
The C-terminus of AQP2 is not sufficient to mediate the FSK-induced rapid increase in the protein level of AQP2. HEK293 cells transiently expressing GFP alone or GFP-fusions with the C-terminal cytosolic part (amino acid residues 225 to 271) of wild-type AQP2 or of AQP2-S256A under control of the CMV promoter remained untreated or after 24 hours were treated with FSK (10 μM, 60 minutes). (A) GFP fluorescence was measured in living cells or (B) GFP-fusion proteins were immunoprecipitated with anti-AQP2 antibodies and detected by Western blotting with the same antibodies (antibody H27). Shown is a representative blot of three independent experiments.
FSK-Induced Increases in AQP2 Protein Abundance Are Independent of De Novo Protein Synthesis
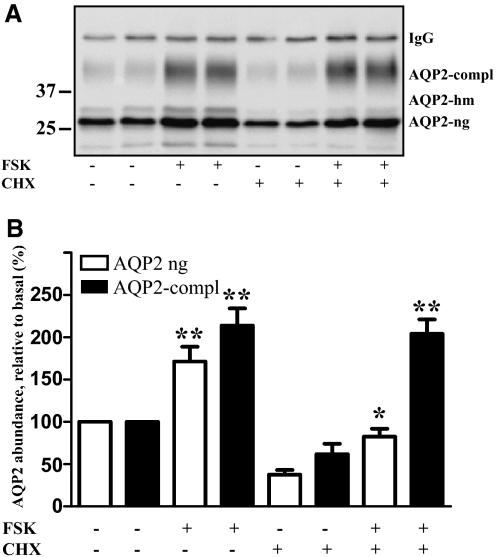
De novo protein synthesis is apparently not responsible for the FSK-induced increase in the AQP2 level. To confirm this, we inhibited protein synthesis in HEK293 cells transiently expressing AQP2 with cycloheximide (CHX; 10 μg/ml) before treatment with FSK. Protein synthesis was fully blocked, as measured by incorporation of 35S-cysteine and 35S-methionine into total protein (data not shown). CHX lowered AQP2 protein levels in resting cells. FSK prevented this decrease (Figure 6, A and B). As in IMCD cells, the complex glycosylated AQP2 apparently increases in a more pronounced manner than the nonglycosylated form. Because, in HEK293 cells, glycosylated AQP2 represented only a small fraction of AQP2 compared with the nonglycosylated form, it is feasible that glycosylation of a small portion of AQP2 explains the large percentage increase of glycosylated AQP2 in the presence of CHX. Taken together, de novo protein synthesis is not required for the FSK-induced increases in AQP2 protein levels.
Figure 6.
Inhibition of protein synthesis does not prevent the FSK-induced increase in the AQP2 protein level in HEK293 cells. (A) HEK293 cells transiently expressing wild-type AQP2 were left untreated or after 24 hours were treated with an inhibitor of protein synthesis, cycloheximide (CHX; 10 μg/ml, 60 minutes). Where indicated, FSK (10 μM) was added and cells were incubated for an additional hour. Cells were lysed and AQP2 was immunoprecipitated and detected by Western blotting as described in the legend to Figure 1A. Shown is a representative blot of five independent experiments. (B) Semiquantitative analysis of the effects of CHX and FSK on the AQP2 protein level. AQP2 was detected as indicated in panel A. Shown are means ± SEM of five independent experiments each performed in duplicates. Statistically significant differences from untreated cells are indicated: *P < 0.05 and **P < 0.01.
A Rise in cAMP Leads to Decreases in the Phosphorylation of p38-MAPK and of AQP2 at S261 in IMCD Cells
cAMP elevation increases AQP2 phosphorylation at S256 and decreases the phosphorylation of S261 in rat IMCD6,28 and in IMCD cells (Figure 7, A through C). p38-MAPK is a putative kinase phosphorylating AQP2 at S261.15,16 Indeed, recombinant p38-MAPK phosphorylates a peptide derived from the C-terminus of AQP2 (amino acids 243 to 271) at S261 in vitro (Figure 7D).16 In IMCD cells, phosphorylated p38-MAPK (p-p38MAPK; the activated form) decreased upon FSK treatment, whereas total levels of p38-MAPK remained constant (Figure 7, E and F). The effect of FSK on p38-MAPK phosphorylation was ablated by H89 (Figure 7, G and H). The selective p38-MAPK inhibitor SB202190 decreased phosphorylations of p38-MAPK, AQP2 at S261 (Figure 7, E and F), and cyclin D3, a known p38-MAPK substrate29 (Supplemental Figure 3). Moreover, SB202190 increased the AQP2 protein level (Figure 7E). Thus, SB202190 had effects similar to FSK. The results suggest that phosphorylation of AQP2 at S261 by p38-MAPK contributes to controlling the protein level of AQP2 in a PKA-dependent manner in IMCD cells.
cAMP Elevation Causes De-polyubiquitination of AQP2 and Prevents Its Proteasomal Degradation
p38-MAPK activation results in proteasomal degradation of different proteins.18,30 To elucidate p38-MAPK's role in regulating AQP2 abundance, AQP2 protein abundance and p38-MAPK activity were evaluated in the presence of MG132, a specific proteasomal degradation inhibitor. FSK increased while its washout decreased AQP2 protein levels (Figure 8, A and B). The decrease was prevented by MG132, suggesting that FSK increases AQP2 abundance by preventing its proteasomal degradation (Figure 8, A and B). MG132 alone reproducibly but statistically did not significantly increase AQP2 abundance. Inhibition of p38-MAPK with SB202190 led to a decrease in the phosphorylation of AQP2 at S261 (see above) and incubation with MG132 partially reversed this SB202190 effect (Figure 8C). Therefore, inhibition of proteasomal degradation overcomes the FSK-dependent block of p38-MAPK.
Polyubiquitination targets proteins to proteasomes for degradation.31 To investigate pUb's role in the FSK-induced short-term upregulation of the AQP2 protein level, AQP2 was immunoprecipitated from IMCD cells. Immunoblotting for ubiquitin revealed that FSK stimulation is associated with a time-dependent decrease to the extent of AQP2 pUb detectable as a reduction of the smeary higher molecular weight bands (higher than 50 kD; Figure 8, D and E). Conversely, mUb of AQP2 increased within 15 to 30 minutes in response to FSK (Figure 8F). Detection of ubiquitination was specific as it was not detectable in immunoprecipitations with control IgG (Figure 8F).
Next, we tested whether the same treatments as shown in Figure 8A lead to changes in pUb of AQP2. Incubation of IMCD cells with FSK for 30 minutes decreases pUb of AQP2 (Figure 8, F and G), whereas 30 minutes after FSK washout levels of polyubiquitinated AQP2 are at the level similar to that from untreated controls. MG132 co-incubation with FSK and additional MG132 incubation during and after FSK washout increased the levels of pUb-AQP2. These various treatments did not prevent the FSK-induced redistribution of AQP2 to the plasma membrane of IMCD cells (Figure 8H). Also MG132 or SB202190 alone apparently did not alter the cellular distribution of AQP2 (Figure 8H). To confirm the role of S261 in the control of pUb, we utilized S261A and S261D mutants of AQP2 (Figure 8, I through K). In HEK293 cells, FSK decreased the level of polyubiqutinated wild-type AQP2 similarly to the effect observed in IMCD cells, but the pUb of S261D did not change. A slight but reproducible increase in the pUb S261A was observed. Although we cannot explain this small increase, the data indicate that the loss of regulated phosphorylation of S261 through FSK interferes with the cAMP-dependent control of pUb of AQP2 (Figure 8, I through K). Collectively, the data show that FSK causes a decrease in pUb of AQP2 and thus lowers its proteasomal degradation. S261 of AQP2 appears to be crucial in the control of pUb.
Discussion
Here we report that AVP- and FSK-induced elevations of cAMP in cultured renal principal cells (Figure 1) and in vivo (Figure 3) rapidly upregulate AQP2 protein levels (Figures 1 through 4). Several lines of evidence indicate that this increase is independent of enhanced transcription and translation. Although the effect of cAMP is PKA-dependent, phosphorylation of AQP2 at S256 by PKA is apparently not involved because the effects of FSK on levels of wild-type AQP2 and AQP2-S256A were similar (Figure 4). In IMCD cells, FSK decreased p38-MAPK activity in a PKA-dependent manner (Figure 7). In addition, FSK or a p38-MAPK inhibitor reduced levels of AQP2 phosphorylated at S261, indicating that p38-MAPK activity is involved in maintaining AQP2 phosphorylated at S261 under resting conditions (Figure 7). The phosphorylation of S261 is not fully blocked by the p38-MAPK inhibitor SB201290, FSK, or the combination of the two agents. Therefore, other kinases such as extracellular signal-regulated kinases (ERK) 1 and 2, c-Jun-N-terminal kinases (JNK), and cyclin-dependent kinases (CDK) 1 and 5, which have been shown to phosphorylate AQP2 at S261, should phosphorylate AQP2 at this residue and contribute to its regulation in these cells.16 In HEK293 cells, FSK increased AQP2 protein levels without decreasing phosphorylation of p38 (data not shown), indicating that other kinases are involved.
The dephosphorylation of AQP2 at S261 is associated with a reduction in pUb. It remains to be established whether pUb occurs at the cell surface or intracellularly, but the reduction in pUb will lower AQP2's proteasomal degradation, most likely explaining the observed increase in AQP2 abundance. Polyubiquitination has functions beyond directing proteins to proteasomal degradation. Because it controls intracellular trafficking of proteins,32 it may, under basal condition, play a role in retaining AQP2 intracellularly. The AVP-induced reduction of AQP2 pUb might promote accumulation of AQP2 at the plasma membrane.
AQP2 targeting to the plasma membrane in response to cAMP elevation is elicited by phosphorylation of AQP2 at S256 by PKA.4 Consistently, AQP2-S256D, mimicking the PKA-phosphorylated state, is constitutively present in the plasma membrane in various cell models,4 whereas AQP2-S256A, which cannot be phosphorylated by PKA, does not translocate to the plasma membrane upon FSK challenge in HEK293 cells (Figure 4) or other cell models.4 Similarly to AQP2-S256D, the double mutants AQP2-S256D-S261A and AQP2-S256D-S261D are located at the plasma membrane,33 indicating that S256 phosphorylation directs AQP2 to the plasma membrane independently of S261 phosphorylation. In line with these observations, inhibition of p38-MAPK and the associated dephosphorylation of S261 do not influence the localization of AQP2 (Figure 8H). p38-MAPK has no effect on S256 phosphorylation.13 Inhibition of the proteasome with MG132 increases S261 phosphorylation, but consistent with the observations described above, this does not influence the localization of AQP2 (Figure 8H).
Water deprivation enhances AVP release, increasing water permeability of the collecting duct.6,7,34,35 In extreme situations of water deprivation (e.g., hypovolaemia) short- and long-term regulation may not sufficiently prevent dehydration. An AVP-dependent reduction of AQP2 pUb, increasing the AQP2 abundance in the short term (Figure 3), substantiates short- and long-term regulations by further enhancing water re-absorption.
Taken together, our data show that cAMP/PKA controls the phosphorylation of S261 of AQP2 via p38-MAPK and thereby its pUb and abundance. These observations might have clinical implications; for example, inhibition of the S261 phosphorylation may prevent excessive water loss in patients suffering from X-linked nephrogenic diabetes insipidus by increasing the abundance of AQP2.
Concise Methods
Reagents and Plasmids
Dibutyryl cyclic adenosine monophosphate (dbcAMP) was purchased from Biolog (Bremen, Germany). AVP and peptides derived from the C-terminus of AQP2 (AQP2 C-term WT, DTDWEEREVRRRQSVELHSPQSLPRGTKA-NH2; AQP2 p-S261, DTDWEEREVRRRQSVELH(pS)PQSLPRGTKA-NH2) were synthesized by Dr. M. Beyermann (Leibniz-Institut für Molekulare Pharmakologie, Berlin, Germany). Actinomycin D and cycloheximide were obtained from Calbiochem (Darmstadt, Germany) and SB202190 from SuperArray. All other chemicals, if not indicated otherwise, were obtained from Sigma (Deisenhofen, Germany). TaqMan gene expression assays for AQP2 and GAPDH were obtained from Applied Biosystems (Darmstadt, Germany). AQP2 was amplified from rat kidney cDNA and cloned into the vector pEGFP-C2 via EcoRI and BamHI restriction sites, resulting in GFP-AQP2. To obtain an AQP2 construct without tag under the control of the CMV promoter, a restriction fragment from GFP-AQP2 containing the CMV promoter and the GFP sequence was replaced by a fragment from the vector pEGFP-N1 containing the CMV promoter without GFP (via EcoRI and ApaLI restriction sites). AQP2-S256A was generated by site directed mutagenesis using the Quickchange II kit (Stratagene, Heidelberg, Germany) using wild-type AQP2 as a template. To obtain GFP-AQP2-C-Term constructs, cDNA fragments coding for amino acid 225 to 271 of wild-type AQP2 or AQP2-S256A mutant were amplified by PCR and inserted in the pEGFP-C2 vector via EcoRI and BamHI restriction sites.
Cell Cultures
Primary cultured rat IMCD cells were cultured as described previously.21 HEK293 cells (ATCC; catalog no. CRL-1573) were grown in DMEM (with 5% FBS, 100 IU of penicillin per milliliter, and 100 μg of streptomycin per milliliter). HEK293 cells were transfected 48 hours after seeding with 1 μg of plasmid DNA per 60-mm dish using Transfectin (Biorad, Munich, Germany) and were subjected to treatment and immunoprecipitation 24 hours after transfection.
Immunoprecipitation and Western Blotting
IMCD cells and HEK293 cells transiently expressing AQP2 (both grown on 60-mm dishes) were treated as described in the legends to the figures. For immunoprecipitation of total AQP2, cells were lysed with lysis buffer (10 mM K2HPO4, 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 0.5% Triton X-100, 1 mM benzamidine, 0.5 mM phenylmethanesulfonyl fluoride, 3.2 μg/ml trypsin inhibitor I-S, 1.4 μg/ml aprotinin; total volume 200 μl) for 15 minutes on ice, and lysates were collected and sonicated. Cell debris was removed by centrifugation (12,000g, 15 minutes, 4°C). Supernatant fractions (500 μl) were incubated with protein-A Sepharose, preincubated with rabbit polyclonal anti-AQP2 antiserum H2736 (1 μl per 350 μg of Sepharose beads in 50 μl of lysis buffer), and rotated overnight at 4°C. Beads were washed twice with 500 μl of lysis buffer. NuPAGE sample buffer (120 μl) was added and samples were boiled (10 minutes). Proteins in lysates and immunoprecipitates were separated by NuPAGE and AQP2 was detected by Western blotting using goat polyclonal anti-AQP2 antibodies directed against the C-terminus (C-17, Santa Cruz Biotechnology, Inc.) and N-terminus (N-20; Santa Cruz) of AQP2. AQP2, phosphorylated at S261, was detected with phospho-specific antibody (phospho-S261; rabbit polyclonal antibody; Santa Cruz). AQP2 protein levels were quantified by densitometric analysis of Western blots (LumiAnalyst 3.0 software; Roche Diagnostics GmbH, Mannheim, Germany).
Detection of Ubiquitinated AQP2
IMCD cells were washed with ice-cold PBS and lysed with lysis buffer (see above) containing 20 mM N-ethylmaleimide; Sigma). The supernatant was incubated overnight at 4°C with protein-A Sepharose beads (Invitrogen) and anti-AQP2 antibody H27. Proteins bound to the beads were washed three times with ice-cold lysis buffer. NuPAGE sample buffer containing N-ethylmaleimide was added and samples were heated to 70°C (10 minutes). Total AQP2 and ubiquitinated AQP2 were analyzed by Western blotting using an anti-AQP2 antibody (Santa Cruz, N-20) and a monoclonal mouse antibody against ubiquitin (Cell Signaling). The amounts of total and ubiquitinated AQP2 were analyzed densitometrically.
p38-MAPK Phosphorylation of Peptides Derived from the C-Terminus of AQP2
p38-MAPK (0.4 μg; N-terminal glutathione S-transferase-tagged, recombinant human full-length p38α/SAPK2α; Millipore) in kinase buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 2 mM dithiothreitol) supplemented with 100 μM ATP was preincubated with 5 μM of the p38-MAPK inhibitor SB202190 or DMSO for 15 minutes on ice. AQP2-derived peptide (27 μg; for sequences see above) was added and incubated at 30°C for 1 hour. Samples were denatured by adding Laemmli sample buffer (4* Rotiload; Carl Roth) and heating to 60°C for 10 minutes. Peptides (10 μg per lane) were subjected to NuPAGE and transferred to polyvinylidene difluoride membranes. Western blotting was performed as described.20 In brief, blocking was carried out using 1% BSA in Tris-buffered saline-Tween-20 with antibodies directed against AQP2 (C-17, Santa Cruz; goat polyclonal IgG; dilution 1:1000) and AQP2 (phospho-S261; rabbit polyclonal antibody; Santa Cruz; dilution 1:750). The specificity of the AQP2 C-17 and phospho-S261 antibodies was confirmed by loading 3.75 μg per lane of the indicated peptides (WT, pS256, pS261, pS264; data not shown).
Real-Time Reverse Transcription-PCR
Total RNA from mice kidney inner medullae was isolated using TRIzol (Invitrogen), further purified on RNeasy columns (Qiagen) and subjected (100 ng) to reverse transcription using Superscript reverse transcriptase III kit (Invitrogen). cDNA encoding AQP2 and GAPDH were amplified in an ABI real-time PCR device as described previously37 and in accordance with manufacturer's instructions (Applied Biosystems). Samples contained 1 μl of universal Taqman PCR master mix (Applied Biosystems), 1 μl of AQP2 or GAPDH primers (Taqman gene expression assays, Applied Biosystems), 7 μl of RNAse free water (Sigma), and 1 μl of cDNA.
Isolation of mRNA from IMCD cells was performed with the Magnetic mRNA Isolation Kit according to the manufacturer's instructions (New England Biolabs, Frankfurt am Main, Germany). cDNA was synthesized by reverse transcription from 100 ng of total RNA (SuperScript First-Strand Synthesis System for RT-PCR; Invitrogen, Karlsruhe, Germany). cDNA synthesis and real-time PCR were performed as described previously.38
Fluorimetric Determination of the Amount of GFP Fusion Proteins in Living Cells
GFP and GFP-fusion proteins were transiently expressed in HEK293 cells (see above). Cells were treated as indicated in the legends to the figures. Changes in fluorescence were measured after 30, 60, 120, and 240 minutes by use of a Speedscan device (Analytic Jena, Jena, Germany).
Immunofluorescence Microscopy
Immunofluorescence microscopic detection of AQP2 in IMCD cells with affinity-purified primary rabbit anti-AQP2 (H-27) and Cy3-coupled anti-rabbit secondary antibodies (Dianova, Hamburg, Germany) was performed as described.20,21 Fluorescence signals were detected by confocal laser scanning microscopy (LSM 510 META; Carl Zeiss, Jena, Germany) with a Plan Neofluar 100×/1.3 oil objective.
Biotinylation of Cell Surface Proteins
Biotinylation of proteins on the surface of HEK293 cells was carried out as described.39
Animal Treatment
The local council on animal care approved the protocols (Landesamt für Gesundheit und Soziales, Berlin); the standards correspond to the local and international requirements. Adult mice (C57/Bl6) were obtained from Charles River (Bad Soden, Germany) and kept on standard diet and tap water. Mice were randomly divided into two groups: (1) control, vehicle-treated animals for 30, 60, and 120 minutes (n = 5 each time point) and (2) animals treated with dDAVP (1 ng/g body wt intraperitoneally) for 30, 60, and 120 minutes (n = 5 each time point). At the end of the experiments, the animals were sacrificed by an overdose of sodium pentobarbital (Nembutal), the kidneys were removed, and medullae were dissected, homogenized, and subjected to Western blotting or real-time PCR for the detection of AQP2 (see above).
Statistic Analysis
Data were analyzed with GraphPad Prism software with the One-Way ANOVA test with the Bonferonni post hoc test.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Andrea Geelhaar, Beate Eisermann, Janin Junker, and Frauke Serowka for excellent technical assistance and Prof. Dr. Roger A. Johnson for critical reading of the manuscript. Prof. Dr. Peter Deen, Radboud University Nijmegen, The Netherlands, kindly provided plasmids encoding AQP2-S261A and AQP2-S261D. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Kl1415/3-2 and 4-2) and the GoBio program of the Bundesministerium für Bildung und Forschung (FKZ 0315097 and FKZ 0315516).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S: Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Fushimi K, Sasaki S, Marumo F: Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 3. King LS, Kozono D, Agre P: From structure to disease: The evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E: Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol (190): 133–157, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Yasui M, Zelenin SM, Celsi G, Aperia A: Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol 272: F443–F450, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F: Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY: Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Puliyanda DP, Ward DT, Baum MA, Hammond TG, Harris HW, Jr: Calpain-mediated AQP2 proteolysis in inner medullary collecting duct. Biochem Biophys Res Commun 303: 52–58, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Woelk T, Oldrini B, Maspero E, Confalonieri S, Cavallaro E, Di Fiore PP, Polo S: Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol 8: 1246–1254, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM: Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A 103: 18344–18349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Balkom BW, Boone M, Hendriks G, Kamsteeg EJ, Robben JH, Stronks HC, van der Voorde A, van Herp F, van der Sluijs P, Deen PM: LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J Am Soc Nephrol 20: 990–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA: Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci U S A 105: 3134–3139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA: Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moeller HB, Knepper MA, Fenton RA: Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA: Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA: Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci U S A 107: 3882–3887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang B, Bui TN, Xiang J, Lin A: Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol Cell Biol 4: 1223–1224, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gianni M, Parrella E, Raska I, Jr, Gaillard E, Nigro EA, Gaudon C, Garattini E, Rochette-Egly C: P38MAPK-dependent phosphorylation and degradation of SRC-3/AIB1 and RARalpha-mediated transcription. EMBO J 25: 739–751, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maric K, Oksche A, Rosenthal W: Aquaporin-2 expression in primary cultured rat inner medullary collecting duct cells. Am J Physiol 275: F796–F801, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E: Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18: 199–212, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA, 3rd, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E: A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8: 110–123, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Bouley R, Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D: Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in ‘endocytosis-resistant’ membrane domains after vasopressin treatment. Biol Cell 98: 215–232, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Katsura T, Ausiello DA, Brown D: Direct demonstration of aquaporin-2 water channel recycling in stably transfected LLC-PK1 epithelial cells. Am J Physiol 270: F548–F553, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Terris J, Ecelbarger CA, Nielsen S, Knepper MA: Long-term regulation of four renal aquaporins in rats. Am J Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Tajika Y, Matsuzaki T, Suzuki T, Ablimit A, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K: Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol 124: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Katsura T, Gustafson CE, Ausiello DA, Brown D: Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 27. van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM: The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA: Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Casanovas O, Jaumot M, Paules AB, Agell N, Bachs O: P38SAPK2 phosphorylates cyclin D3 at Thr-283 and targets it for proteasomal degradation. Oncogene 23: 7537–7544, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Uchida S, Yoshioka K, Kizu R, Nakagama H, Matsunaga T, Ishizaka Y, Poon RY, Yamashita K: Stress-activated mitogen-activated protein kinases c-Jun NH2-terminal kinase and p38 target Cdc25B for degradation. Cancer Res 69: 6438–6444, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Li W, Ye Y: Polyubiquitin chains: Functions, structures, and mechanisms. Cell Mol Life Sci 65: 2397–2406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Welchman RL, Gordon C, Mayer RJ: Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6: 599–609, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D: The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lorenz D, Krylov A, Hahm D, Hagen V, Rosenthal W, Pohl P, Maric K: Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells. EMBO Rep 4: 88–93, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hasler U, Nielsen S, Feraille E, Martin PY: Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol 290: F177–F187, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Liebenhoff U, Rosenthal W: Identification of Rab3-, Rab5a- and synaptobrevin II-like proteins in a preparation of rat kidney vesicles containing the vasopressin-regulated water channel. FEBS Lett 365: 209–213, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Bieche I, Olivi M, Champeme MH, Vidaud D, Lidereau R, Vidaud M: Novel approach to quantitative polymerase chain reaction using real-time detection: application to the detection of gene amplification in breast cancer. Int J Cancer 78: 661–666, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Mutig K, Paliege A, Kahl T, Jons T, Muller-Esterl W, Bachmann S: Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Alken M, Rutz C, Kochl R, Donalies U, Oueslati M, Furkert J, Wietfeld D, Hermosilla R, Scholz A, Beyermann M, Rosenthal W, Schulein R: The signal peptide of the rat corticotropin-releasing factor receptor 1 promotes receptor expression but is not essential for establishing a functional receptor. Biochem J 390: 455–464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.