Abstract
Inhibition of p38 mitogen-activated protein kinase and cyclooxygenase-2 reduces albuminuria in models of chronic kidney disease marked by podocyte injury. Previously, we identified a feedback loop in podocytes whereby an in vitro surrogate for glomerular capillary pressure (i.e., mechanical stretch) along with prostaglandin E2 stimulation of its EP4 receptor induced cyclooxygenase-2 in a p38-dependent manner. Here we asked whether stimulation of EP4 receptors would exacerbate glomerulopathies associated with enhanced glomerular capillary pressure. We generated mice with either podocyte-specific overexpression or depletion of the EP4 receptor (EP4pod+ and EP4pod−/−, respectively). Glomerular prostaglandin E2-stimulated cAMP levels were eightfold greater for EP4pod+ mice compared with nontransgenic (non-TG) mice. In contrast, EP4 mRNA levels were >50% lower, and prostaglandin E2-induced cAMP synthesis was absent in podocytes isolated from EP4pod−/− mice. Non-TG and EP4pod+ mice underwent 5/6 nephrectomy and exhibited similar increases in systolic BP (+25 mmHg) by 4 weeks compared with sham-operated controls. Two weeks after nephrectomy, the albumin-creatinine ratio of EP4pod+ mice (3438 μg/mg) was significantly higher than that of non-TG mice (773 μg/mg; P < 0.0001). Consistent with more severe renal injury, the survival rate for nephrectomized EP4pod+ mice was significantly lower than that for non-TG mice (14 versus 67%). In contrast, 6 weeks after nephrectomy, the albumin-creatinine ratio of EP4pod−/− mice (753 μg/mg) was significantly lower than that of non-TG mice (2516 μg/mg; P < 0.05). These findings suggest that prostaglandin E2, acting via EP4 receptors contributes to podocyte injury and compromises the glomerular filtration barrier.
Glomerular filtration barrier (GFB) damage, as indicated by micro/macroalbuminuria, is a renal disease marker.1–3 Interventions that reduce albuminuria slow the progression to ESRD and lower the risk for hypertension and cardiovascular events.4 Although the underlying mechanisms of albuminuria are complex and remain incompletely resolved, a key player in the cause of GFB damage is the podocyte.5 With the increased incidence of kidney disease and a limited number of effective treatments, understanding the role of the podocyte in the progression of glomerular injury is needed to develop novel antiproteinuric therapies.
Damage to the podocyte foot processes is a hallmark of many proteinuric glomerular diseases, including minimal change disease, FSGS, and diabetic kidney disease.1,6–8 A number of maladaptive end points have been identified for the podocyte in glomerular diseases (e.g., foot process effacement, detachment from the glomerular basement membrane, apoptosis, hypertrophy, dedifferentiation).9,10 Although many factors undoubtedly contribute to the initiation and progression of podocyte injury and dysfunction, nonsteroidal anti-inflammatory drugs reduce proteinuria, suggesting that prostanoids derived from cyclooxygenase 1 and 2 (COX-1 and COX-2) activity may compose a portion of the causative mosaic.11,12 Unfortunately, widespread use of COX inhibitors is not feasible, because they diminish vasodilatory prostanoid levels, resulting in reduced GFR and renal blood flow (RBF)13,14; however, inhibition of either COX-1 or COX-2 isoforms attenuates the synthesis of at least five distinct prostanoids (PGE2, PGD2, PGI2, PGF2α, and TxA2) that interact with their respective G-protein–coupled receptors (prostanoid E-type 1 through type 4 [EP-1 through -4], and prostanoid D-, I-, F- and T-type [TP]) to provoke a variety of physiologic actions in the kidney and elsewhere. Targeting those prostanoid receptors that are pro-proteinuric while avoiding those that regulate GFR/RBF may represent a potential therapy for preserving GFB function in renal disease.
Studies have shown that podocyte-specific overexpression of COX-2 in mice renders them more susceptible to glomerular injury in models of minimal change disease.15,16 Furthermore, subtotally nephrectomized (5/6 Nx) rats treated with a selective COX-2 inhibitor reduced glomerular PGE2 levels that correlate with improvements in proteinuria and glomerulosclerosis.17 Glomerular PGE2 synthesis after 5/6 Nx suggests that signaling via one or more of its EP receptor subtypes may contribute to the deleterious effects of COX-2 activity on GFB function.18 Podocytes may be targets of these actions, because they express both EP1 and EP4 receptors.19 We recently uncovered a novel feedback loop in cultured mouse podocytes whereby an in vitro surrogate for glomerular capillary pressure (Pgc; i.e., mechanical stretch) along with PGE2 stimulation of the EP4 receptor induces COX-2 in a p38 mitogen-activated protein kinase–dependent manner.20,21 These findings led us to hypothesize that PGE2-dependent EP4 receptor signaling contributes to podocyte injury in chronic kidney disease (CKD) associated with enhanced Pgc. Here we demonstrate that podocyte-specific overexpression of a desensitization-resistant C-terminal truncated EP4 receptor renders mice more susceptible to the development of albuminuria after 5/6 Nx, whereas conditional deletion of this prostanoid receptor subtype from podocytes confers partial protection from such GFB damage. Our findings therefore support a maladaptive role for the PGE2 EP4 receptor in the context of glomerular injury.
Results
5/6 Nx Induces Renal EP4 Receptor and COX-2 Expression
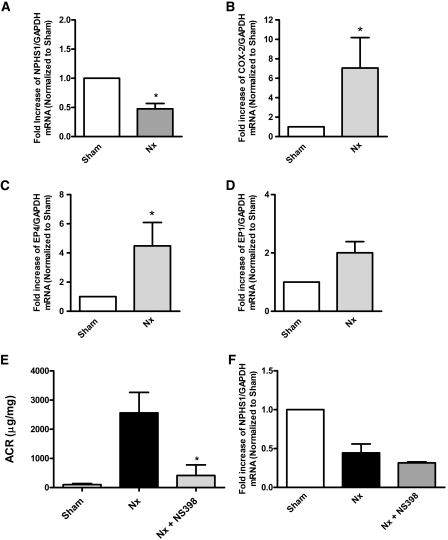
Enhanced renal COX-2 induction was associated with PGE2 synthesis after subtotal renal ablation in rats.18 COX-2 inhibition can blunt albuminuria in such models, suggesting that prostanoid(s) contribute to GFB damage.17 In this study, we investigated whether similar changes are observed in a mouse version of this CKD model. For this purpose, FVB/N mice were subjected to 5/6 Nx as described in the Concise Methods section. GFB injury in FVB/N mice at 12 weeks after 5/6 Nx was evident, because renal mRNA levels for the slit diaphragm component nephrin were significantly reduced as compared with sham-operated controls (Figure 1A). Similar to changes found in rats, renal COX-2 mRNA expression was increased in nephrectomized mice (Figure 1B). In addition, renal EP4 but not EP1 mRNA levels were upregulated (Figure 1, C and D). Administration of NS-398 (1 mg/kg per d) over a 12-week period in the drinking water markedly attenuated 5/6 Nx–induced albumin-creatinine ratios (ACRs; Figure 1E); however, COX-2 inhibition did not restore nephrin mRNA expression at 12 weeks after Nx, indicating that nephrin protein expression may persist despite the reduced mRNA levels (Figure 1F). These data suggest that the intrarenal COX-2/PGE2/EP4 signaling axis is induced and may participate in this model of progressive renal disease.
Figure 1.
COX-2 is upregulated and contributes to 5/6 Nx–induced albuminuria. Eight-week-old FVB/N mice are subjected to 5/6 Nx or sham operation. At 12 weeks after 5/6 Nx, remnant renal mass is removed and total mRNA is isolated. (A) GFB damage is evident as glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-normalized nephrin mRNA levels are significantly decreased in 5/6 Nx mice when compared with sham-operated animals as determined by quantitative RT-PCR. *P < 0.05 versus sham group. (B through D) Renal COX-2 (B) and EP4 receptor (C) but not EP1 (D) mRNA levels are upregulated in 5/6 Nx mice. *P < 0.05 versus sham group. (E) A subset of mice are administered NS-398 (1 mg/kg per d), and ACR is determined. *P < 0.05 versus 5/6 Nx group. (F) At 12 weeks after 5/6 Nx, NS-398 is not able to recover nephrin expression.
Generation of Transgenic Mice with Podocyte-Specific EP4 Expression
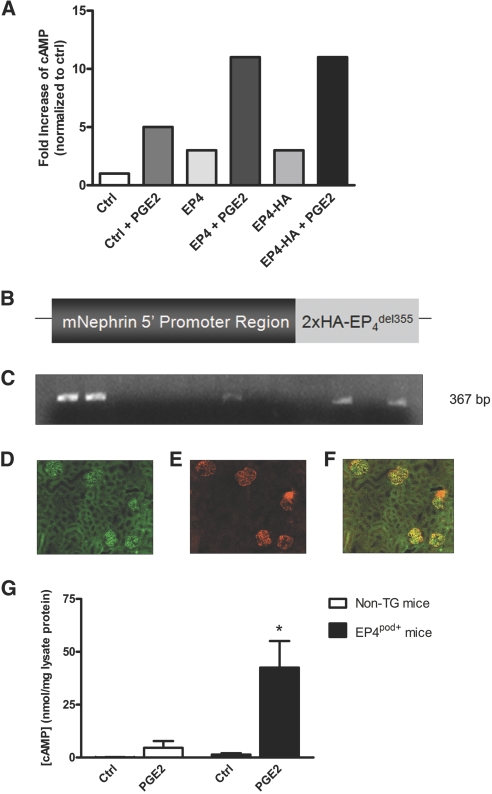
To test the role of podocyte EP4 receptors in GFB injury in a CKD model, we generated mice with podocyte-specific EP4 receptor expression (EP4pod+ mice). To diminish the impact of heterologous desensitization of the overexpressed receptor, we engineered an EP4 construct lacking a significant portion of its C-terminal intracellular tail. This region (from amino acid 355 onward) was shown previously to contain several phosphorylation sites that contribute to its rapid desensitization and sequestration.22 A double hemagglutinin (2×HA) tag was added to the 5′ end of the truncated EP4 receptor open reading frame. Neither the epitope tag nor the loss of the C-terminus had any appreciable impact on acute receptor function (Figure 2A). An 8.3-kb fragment of the mouse nephrin (NPHS1) promoter region was incorporated upstream of the 2×HA-EP4del355 sequence for expression in mice (Figure 2B).23 Transgenic (TG) mice on an FVB/N background were produced, and genotyping of the resulting 36 pups (candidate founders) was performed by PCR using tail-snip–derived DNA (Figure 2C). Seven EP4pod+ founder lines were initiated, and otherwise healthy and fertile offspring were obtained in a Mendelian ratio. For all subsequent studies, two lines exhibiting the highest transgene expression as determined semiquantitatively by immunofluorescence of renal sections with an HA antibody were chosen. Figure 2, D through F, depicts co-localization of the HA-tagged EP4 transgene in one such founder line with the podocyte marker synaptopodin, thereby confirming its podocyte-restricted expression. To verify that transgene expression resulted in a functional GPCR, we isolated glomeruli from age-matched (8 weeks old) non-TG and EP4pod+ littermates and assayed for PGE2-induced cAMP synthesis. As shown in Figure 2G, in response to 1 μM PGE2, glomerular cAMP levels in EP4pod+ mice were severalfold higher than those of their non-TG littermates (43 versus 5 nmol/mg lysate protein respectively; P < 0.05).
Figure 2.
Podocyte-restricted functional expression of an EP4 receptor transgene in mice. (A) COS-7 cells are transfected with full-length or truncated (del355) EP4 constructs. cAMP production is not affected by incorporation of a 2×HA tag. (B) Illustration of the construct used to generate EP4pod+ mice. An 8.3-kb fragment of the mouse NPHS1 immediate promoter region is incorporated upstream of a human EP4 open reading frame that lacks the coding sequence for amino acid 355 onward. A 5′ 2×HA epitope tag is inserted immediately distal to the NPHS1 promoter sequence. (C) TG mice are identified by PCR of tail-snip DNA. The representative image shows agarose gel electrophoresis of the 367-bp PCR product amplified from the transgene (five of 13 mice shown are positive for the transgene). Seven founders with germline transmission of the transgene are obtained from a total of 36 pups. (D) Immunofluorescence of EP4 receptor transgene in glomeruli of EP4pod+ mice using a rabbit HA antibody. (E) Mouse synaptopodin antibody (podocyte marker) immunofluorescence identifies podocytes. (F) Merged images (C/D) reveal co-localization of HA-EP4 and synaptopodin in EP4pod+ mouse kidney sections. (G) Glomeruli isolated from EP4pod+ mice respond to 10 minutes of stimulation with 1 μM PGE2 with cAMP production that significantly exceeds that of non-TG mice. *P < 0.05 versus non-TG group with PGE2. Forskolin (FSK; 10 μM) is used as a positive control. Magnification, ×200.
Exacerbated Renal Phenotype of EP4pod+ Mice after 5/6 Nx
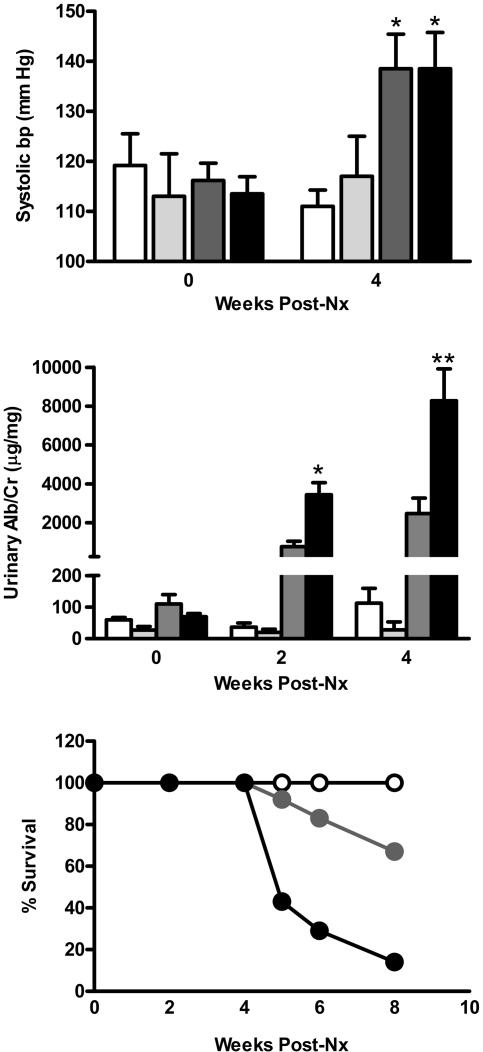
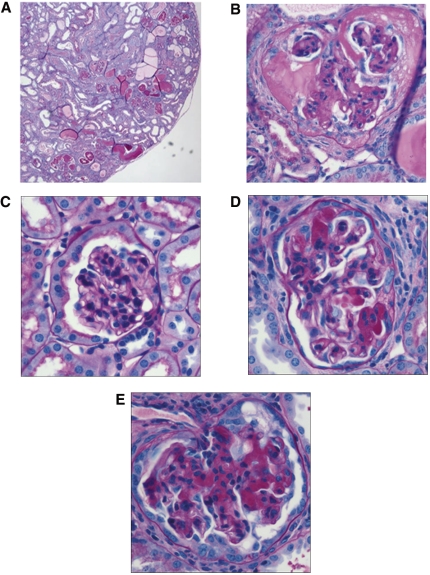
For evaluation of the impact of podocyte-specific overexpression of the EP4 receptor on the GFB, groups of EP4pod+ mice underwent 5/6 Nx. Subtotal renal ablation in mice results in hypertension, albuminuria, and FSGS.24 In these studies, 5/6 Nx non-TG mice developed elevated systolic BP (SBP) compared with sham-operated mice by 4 weeks after Nx (approximately Δ25 mmHg; Figure 3A). Similar increases were observed in 5/6 Nx EP4pod+ mice, suggesting that expression of the transgene in podocytes is without effect on systemic BP. Spot urine ACR analyses revealed that 5/6 Nx non-TG mice became significantly albuminuric as compared with sham-operated mice; however, 5/6 Nx EP4pod+ mice were significantly more albuminuric (3438 μg/mg; n = 7) than 5/6 Nx non-TG mice (773 μg/mg; P < 0.0001; n = 12) as early as 2 weeks after 5/6 Nx, becoming severely affected by 4 weeks (Figure 3B). Furthermore, as compared with their 5/6 Nx non-TG littermates, 5/6 Nx EP4pod+ mice exhibited increased mortality by 8 weeks after 5/6 Nx (67 versus 16%; Figure 3C). Of the mice that did not survive to the end of this 12-week study, renal pathology showed tubulointerstitial fibrosis along with significant tubular abnormalities including dilatations and filling with protein casts (Figure 4A) along with severe glomerular scarring (Figure 4B)—all suggesting that renal deterioration subsequent to exacerbated proteinuria was more likely in EP4pod+ mice than in non-TG mice after 5/6 Nx. Sham-operated controls were devoid of pathologic features (Figure 4C). Conversely, for mice surviving to 12 weeks, renal pathology was much less severe with milder glomerulosclerosis for both 5/6 Nx non-TG and EP4pod+ mice (Figure 4, D and E).
Figure 3.
EP4pod+ mice are significantly more proteinuric following 5/6Nx. (Top) SBP increases in 5/6 Nx mice. SBP is assessed via tail-cuff plethysmography. At 4 weeks after 5/6 Nx, both EP4pod+ and non-TG mice display similar BP elevation compared with sham-operated animals (grouped as non-TG and EP4pod+ mice). (Middle) At 2 weeks after 5/6 Nx, ACR of EP4pod+ mice (3438 μg/mg; n = 7) is significantly higher than that of non-TG mice (773 μg/mg; P < 0.0001; n = 12).This effect persists at 4 weeks after 5/6 Nx, when EP4pod+ mice (8280 μg/mg; n = 7) are significantly more albuminuric that non-TG mice (2471 μg/mg; n = 12). **P < 0.0001, *P < 0.05. (Bottom) Severe albuminuria observed in 5/6 Nx EP4pod+ mice is associated with a significant drop in mouse survival over the course of the experiment. Percentage survival of nephrectomized mice over 8 weeks for EP4pod+ mice (16%) is much lower than that for non-TG Nx mice (67%). No sham-operated animals are lost at 8 weeks following 5/6 Nx.
Figure 4.
Mice exhibit severe renal pathology following 5/6Nx. A subpopulation of 5/6 Nx EP4pod+ mice (n = 6) die suddenly between 5 to 8 weeks after Nx. Kidneys are recovered and disease pathology is visualized by periodic acid–Schiff staining of sections. (A and B) Of the mice that do not survive for 12 weeks, renal pathology shows severe glomerular scarring, tubulointerstitial fibrosis, and widespread accumulation of tubular protein casts. For mice that survive to 12 weeks, renal pathology is milder with less glomerulosclerosis and no grossly apparent differences between 5/6 Nx non-TG and EP4pod+ mice. (C) Sham (12 weeks after 5/6 Nx). (D) 5/6 Nx non-TG (12 weeks after 5/6 Nx). (E) 5/6 Nx EP4pod+ (12 weeks after 5/6 Nx). Magnifications: ×40 in A; ×400 in B through E.
Generation of EP4pod−/− Mice
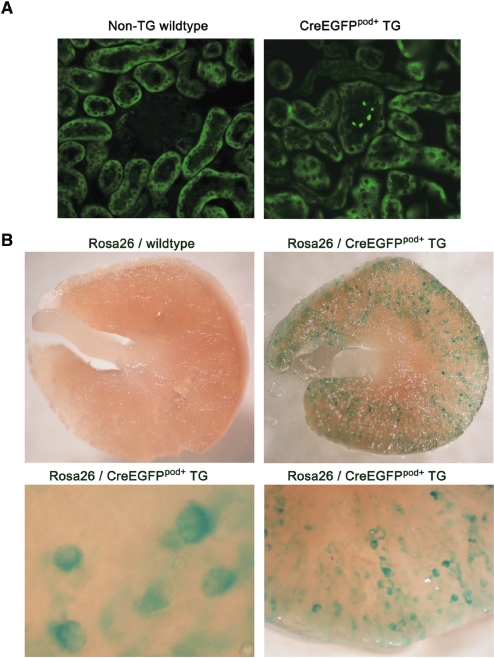
To test further the role of podocyte EP4 receptors in the regulation of the GFB, we generated a line of mice with conditional deletion of this PGE2 receptor subtype. Briefly, TG mice with podocyte-specific expression of a Cre-recombinase/enhanced green fluorescence protein (GFP) fusion protein were engineered using an 8.3-kb fragment of the mouse NPHS1 promoter (CreEGFPpod+ mice). Three founders of mixed C57Bl/6J × C3H/HeJ background, carrying the NPHS1-CreEGFP transgene, were identified by PCR-based genotypic analysis and confirmed by Southern analysis (data not shown). Confirmation of podocyte-specific CreEGFP expression and activity was carried out by immunofluorescence detection of EGFP and by intercrossing with the ROSA26 reporter strain (Figure 5). Expression of the fusion protein was restricted to the podocytes (Figure 5A). Expression was absent from any other tissues examined, including liver, lung, heart, and muscle (data not shown). Organs from 6-week-old ROSA26/CreEGFPpod+ mice were dissected and stained with X-gal. As shown in Figure 5B, extensive and widespread glomerular staining was achieved in ROSA26/CreEGFPpod+ mice. Staining intensities were similar between littermates, as well as across the three founder lines (data not shown). Weaker X-gal staining was observed in brain, but no signal could be found in any of the other tissues examined, a finding generally consistent with the expression pattern observed by others using this promoter fragment.25 CreEGFPpod+ mice were subsequently backcrossed 10 generations onto the FVB/N strain, and the retention of Cre-recombinase activity was confirmed using the ROSA26 mice (data not shown).
Figure 5.
In vivo expression and catalytic activity of CreEGFP is confirmed in TG mice. (A) Expression of the CreEGFP fusion protein in TG mice. Kidneys from 6-week-old non-TG wild-type and CreEGFPpod+ mice are dissected and processed for immunofluorescence as described in the Concise Methods section using an FITC-conjugated mouse monoclonal anti-GFP antibody. Shown are representative images of kidney sections from non-TG wild-type and CreEGFPpod+ mice. Only glomerular cells of the CreEGFPpod+ mice display immunofluorescence. (B) In vivo catalytic activity of the CreEGFP fusion protein in TG mice. CreEGFPpod+ mice are intercrossed with ROSA26 mice, and kidneys from double-positive 6-week-old compound TG mice are dissected and processed for X-gal staining as described in the Concise Methods section. Whole kidneys from ROSA26 and CreEGFP pod+ mice are grossly sectioned by razor blade and visualized under low-power light microscopy. X-gal staining is restricted to glomerular cells of the CreEGFP pod+ mice. Magnification, ×200 in A; ×20 and ×50 in upper and lower panels in B, respectively.
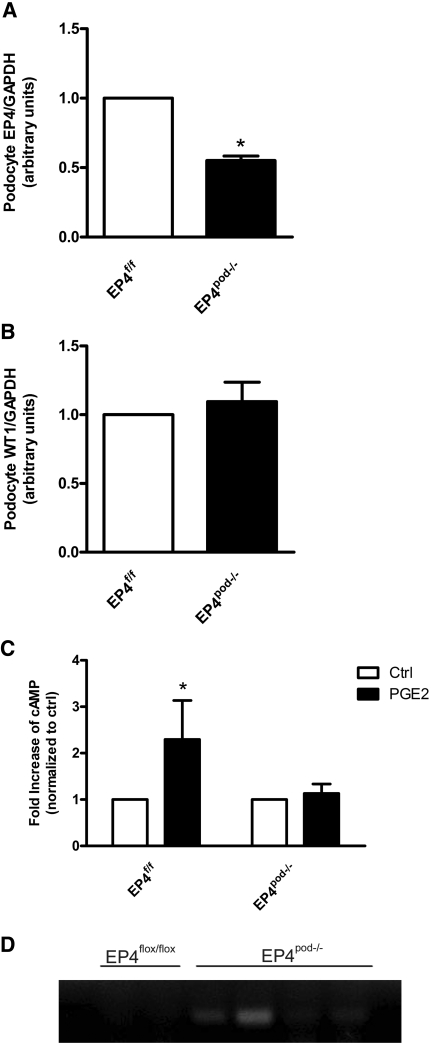
CreEGFPpod+ mice were then bred to floxed EP4 receptor mice (EP4flox/flox) obtained from Dr. Matthew Breyer (Vanderbilt University) to generate podocyte-specific EP4-deleted mice (EP4pod−/−). EP4flox/flox mice have loxP sites flanking exon 2 of the EP4 gene, making it a conditional knockout.26 EP4flox/flox mice are healthy and fertile, exhibiting no obvious renal or other phenotypes, and were backcrossed for 10 generations to obtain congenic mice on the FVB/N background. For EP4pod−/− studies, EP4flox/flox mice were used as controls. EP4pod−/− mice seemed healthy and normoalbuminuric and were obtained in a Mendelian ratio. Initial attempts to verify the efficacy of the conditional deletion of the EP4 gene using immunohistochemical approaches that use EP4 receptor antibodies were unsuccessful. Immunoblotting was negative for EP4 protein in glomeruli isolated from EP4flox/flox, EP4pod−/−, and even EP4pod+ TG mice; therefore, we isolated and cultured primary podocytes from EP4flox/flox and EP4pod−/− mice. As shown in Figure 6A, quantitative reverse transcriptase–PCR (RT-PCR) of mRNA obtained from primary cultured podocytes of EP4pod−/− mice showed a statistically significant 50% reduction in EP4 receptor expression as compared with EP4flox/flox mice. In contrast, Wilms' tumor expression remained similar between groups (Figure 6B). Furthermore, in contrast to EP4flox/flox podocytes, PGE2-stimulated cAMP production was absent in EP4pod−/− podocytes, suggesting functional EP4 receptor deletion in podocytes of EP4pod−/− mice (Figure 6C). Cre-mediated recombination of the floxed EP4 alleles was verified by PCR of genomic DNA obtained from EP4pod−/− primary podocyte cultures (Figure 6D).
Figure 6.
EP4 mRNA expression is reduced in primary EP4pod−/− podocytes. The efficacy of Cre recombinase–mediated EP4 receptor deletion is assessed indirectly. (A) Podocytes from EP4pod−/− mice (n = 5) are isolated and cultured as described in the Concise Methods section. Subsequently, total mRNA is isolated from these cultures, and EP4 mRNA levels are determined by quantitative RT-PCR. EP4pod−/− podocytes express significantly lower EP4 receptor mRNA compared with EP4flox/flox mice (normalized to GAPDH mRNA). *P < 0.05. (B) No differences are observed between groups with respect to Wilms' tumor expression. (C) PGE2-dependent cAMP production is blunted in podocytes isolated from EP4pod−/− (versus EP4flox/flox podocyte cultures). (D) PCR confirmation from genomic DNA isolated from either EP4pod−/− or EP4flox/flox podocytes showing Cre recombination in EP4pod−/− podocytes only (representative image shows agarose gel electrophoresis of the 200-bp PCR product amplified where recombination has occurred; each lane represents podocytes isolated from either EP4flox/flox [n = 2] or EP4pod−/− [n = 4 mice]).
Milder Renal Phenotype of EP4pod−/− Mice after 5/6 Nx
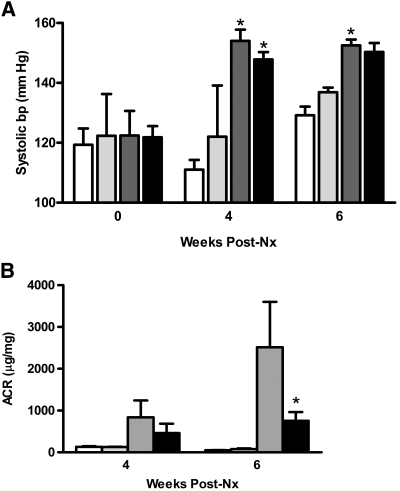
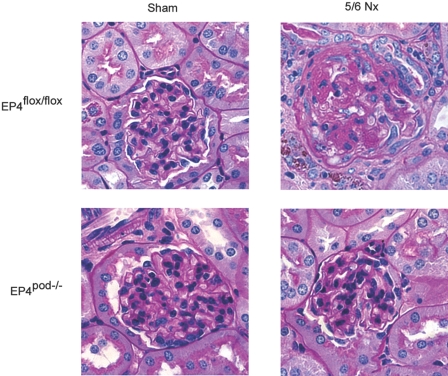
We next investigated the impact of EP4 receptor deletion from the podocytes on the GFB in progressive kidney disease. For this purpose, groups of EP4pod−/− mice underwent 5/6 Nx as described for the EP4pod+ mice. Similar to our findings with EP4pod+ mice (Figure 3A), SBP increased equivalently for EP4flox/flox and EP4pod−/− mice by 4 weeks after 5/6 Nx (Δ+25 mmHg) compared with sham-operated controls (Figure 7A); however, partial attenuation of GFB damage was evident as early as 4 weeks after 5/6 Nx and became statistically significant by 6 weeks after 5/6 Nx, because the ACR of EP4pod−/− mice (753 μg/mg) was significantly lower than that of EP4flox/flox mice (2516 μg/mg; P < 0.05; Figure 7B). Consistent with the partial preservation of GFB function in mice lacking podocyte EP4 receptors, glomerular pathology of 5/6 Nx EP4pod−/− mice exhibited qualitatively milder glomerular lesions, with less matrix deposition than their 5/6 Nx EP4flox/flox littermate controls (Figure 8).
Figure 7.
EP4pod−/− mice are less proteinuric following 5/6Nx. (A) SBP increases in 5/6 Nx mice. SBP is assessed via tail-cuff plethysmography. Beginning at 2 weeks after Nx, both EP4pod−/− (black bars) and EP4flox/flox (dark gray bars) mice displayed similar elevated SBP as compared with sham-operated animals (white and light gray bars, respectively). *P < 0.05 versus sham. (B) Proteinuria increases after 5/6 Nx. At 6 weeks after Nx, ACR is significantly lower for EP4pod−/− mice (753 μg/mg; n = 12) than for EP4flox/flox mice (2516 μg/mg; n = 6). *P < 0.05.
Figure 8.
Milder renal pathology is observed in 5/6Nx EP4pod−/− mice. Kidneys are removed and disease pathology is visualized by periodic acid–Schiff staining of paraffin-embedded sections at 8 weeks after 5/6 Nx. Milder glomerular pathology is observed in 5/6 Nx EP4pod−/− mice with less matrix deposition than 5/6 Nx EP4podflox/flox mice. Magnification, ×400.
EP4 Receptor Overexpression Exacerbates Stretch-Induced Detachment from the Extracellular Matrix
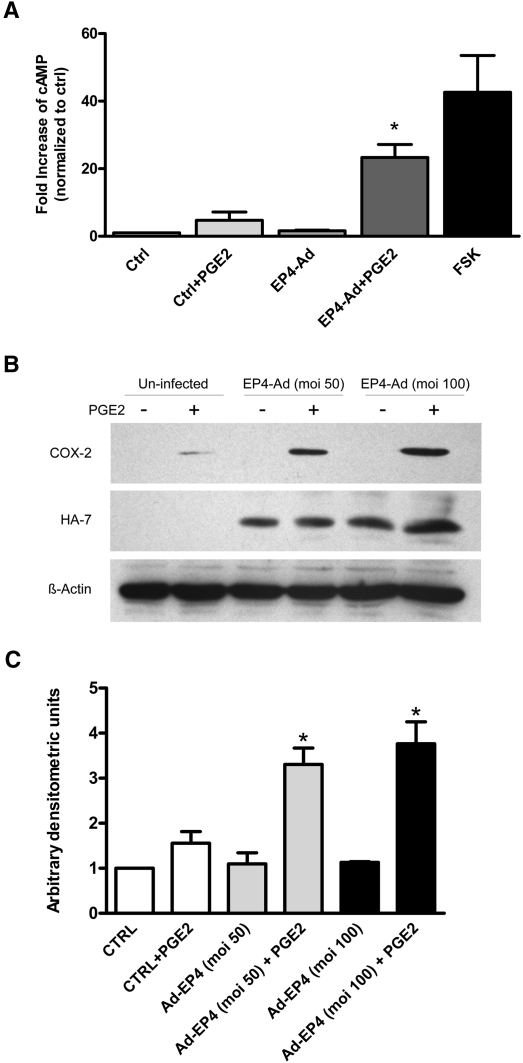
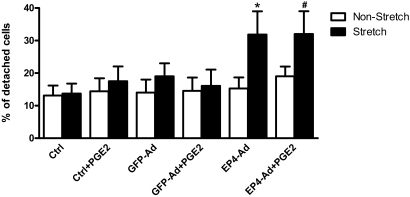
Podocyte loss through detachment from the glomerular basement membrane is associated with proteinuria and subsequent sclerosis in glomerular disease.27 Excessive mechanical forces as a result of enhanced Pgc could facilitate podocyte detachment in vivo. We previously demonstrated that in vitro mechanical stretch of conditionally immortalized mouse podocytes induces both EP4 receptor and COX-2 expression.21 Moreover, we showed that PGE2 acting via the EP4 receptor can likewise induce COX-2 in cultured podocytes.20 In this study, EP4 overexpression by adenoviral infection and subsequent stimulation with PGE2 resulted in a significantly greater level of cAMP production (Figure 9A) and COX-2 expression (Figure 9, B and C) compared with noninfected controls. Thus, both mechanical stretch and PGE2 trigger an autocrine feedback loop in podocytes; however, whether PGE2 acting via EP4 receptors along with mechanical stretch could promote podocyte detachment is not known. We therefore exposed conditionally immortalized mouse podocytes to mechanical stretch as described previously by our laboratory.21 In cell detachment assays, PGE2 alone was without effect on the number of podocytes adhering to the surface of the culture dish (Figure 10). Furthermore, the application of mechanical stretch either alone or in the presence of PGE2 was likewise without effect on podocyte adhesion. In contrast, when EP4 expression was enhanced via adenoviral-mediated transduction, a significant increase in cell detachment after mechanical stretch was observed. Curiously, the addition of PGE2 to EP4-transduced cells did not contribute further to stretch-induced podocyte detachment from the substratum, suggesting possible agonist-independent receptor activation in response to mechanical stretch. GFP overexpression by adenoviral transduction was without effect on podocyte adhesion.
Figure 9.
EP4 overexpression and stimulation with PGE2 results in significantly greater cAMP production and COX-2 expression. (A) After 14 days of differentiation, conditionally immortalized mouse podocytes are transduced with adenovirus for the human full-length HA-tagged EP4 receptor (MOI = 50). Three days after infection, cells are stimulated with 1 μM PGE2 for 10 minutes in the presence of 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) and 5 μM indomethacin. Forskolin (FSK; 10 μM) is used as a positive control. Cells infected with EP4 adenovirus and stimulated with PGE2 display a significant increase in cAMP production compared with uninfected controls (n = 3). *P < 0.05. (B) Podocytes infected with HA-tagged EP4 adenovirus are stimulated with 1 μM PGE2 for 6 hours, inducing a significant increase in COX-2 expression over noninfected controls. Western blots are probed with COX-2, HA, and β-actin antibodies (n = 3). MOI = 50 or 100 as shown. (C) Quantification of COX-2 expression is achieved using densitometric values normalized to β-actin levels. Cells infected with EP4 adenovirus had significantly higher COX-2 expression than uninfected controls after PGE2 stimulation. *P < 0.05.
Figure 10.
EP4 signaling and mechanical stretch promote podocyte detachment. After 14 days of differentiation, conditionally immortalized mouse podocytes are plated onto collagen I–coated silicone stretch plates and transduced with adenovirus for either the EP4 receptor or GFP control (MOI = 50). Three days after infection, cells are stimulated with 1 μM PGE2 and exposed to 12% equibiaxial mechanical stretch for 24 hours in the presence of 5 μM indomethacin. After mechanical stretch, medium is collected and nonadherent cells are counted using a hemocytometer. For determination of the number of adherent cells, trypsin is added to wells, the dislodged cells are centrifuged, the pellets are resuspended, and cells are counted (n = 5). *P < 0.05, #P < 0.01.
Discussion
COX inhibition reduces albuminuria in clinical and experimental renal disease. Work by Harris's group17 showed that COX-2 inhibition with SC58236 blunted PGE2 synthesis and proteinuria while improving glomerulosclerosis in nephrectomized rats. Our data are consistent with these findings. We showed that in a mouse version of the 5/6 Nx CKD model, renal COX-2 was upregulated whereas its inhibition with NS-398 significantly attenuated albuminuria. A maladaptive role for COX-2 in podocytes is supported by studies using TG mice with podocyte-specific COX-2 overexpression. The elevated COX-2 levels render the mice susceptible to GFB injury after either adriamycin or puromycin aminonucleoside–induced nephrosis, whereas non-TG mice remain resistant.15,16
The factors that initiate COX-2 upregulation in podocytes have only recently been investigated. Spurney et al.28 showed that Gq-dependent signaling induces COX-2 expression in cultured podocytes, which was associated with increased PGE2 synthesis. Our previous work showed that both mechanical stretch and PGE2 induce COX-2 expression in cultured mouse podocytes.20,21 Specifically, it is the Gs-coupled EP4 receptor, not the Gq-coupled EP1 subtype, that mediates PGE2-stimulated COX-2 induction in conditionally immortalized mouse podocytes. We therefore asked whether the EP4 receptor as expressed in podocytes mediates the injurious effects of COX-2 activity and therefore contributes to GFB damage encountered in a model of CKD. Two approaches were chosen to test this hypothesis. First, overexpression of a desensitization-resistant EP4 receptor in podocytes of mice allowed us to maximize PGE2 signaling. This strategy rendered the mice more susceptible to injury during disease progression. In contrast, sham-operated EP4pod+ mice were normoalbuminuric, indicating that overexpression of this GPCR was without effect on GFB function. These findings suggest that in addition to overexpression of the EP4 receptor, glomerular COX-2 induction coupled with enhanced PGE2 synthesis is likely required to activate the EP4 signaling pathway. Although other prostanoids could potentially interact with the EP4 receptor expressed at such high levels, this possibility seems remote because the EP4 receptor is selective for PGE2 over most other prostanoids (Ki for PGE2, 1.1 nM; PGE1, 0.66 nM; PGD2, 1240 nM; PGF2α, 570 nM; iloprost [a prostanoid I-type receptor selective agonist], 277 nM; and U46619 [a TP receptor ligand], 2330 nM).29 Although overexpression of the wild-type EP4 receptor may have yielded a similar phenotype, because of the potential for heterologous desensitization we were not convinced that we would have observed enhanced expression/signaling. Our second approach was to ablate podocyte EP4 expression genetically. Accordingly, podocyte-specific conditional EP4 deletion from mice conferred significant GFB protection after 5/6 Nx with overexpression of the receptor enhancing significantly the glomerular/tubulointerstitial damage after 5/6 Nx. Renal damage was so severe that nearly 80% of EP4pod+ mice died earlier than 8 weeks after 5/6 Nx. Loss of the EP4 receptor did not prevent the 20% mortality observed with 5/6 Nx wild-type mice. In contrast, others showed that renal pathology and GFR after subtotal renal ablation in rats are ameliorated with an EP4 agonist (CP-044,519-02).30 These findings suggest that vascular EP4 receptor activation may be beneficial in preserving RBF in CKD; however, in contrast to our work, renal ablation in those studies failed to induce EP4 expression. Furthermore, the effects on the podocyte were not explored. The EP4 receptor may therefore exert pleiotropic effects on the kidney during the onset of injury. Our findings contrast with those of Cheng et al.,31 who showed that thromboxane receptor deletion but not podocyte-specific EP4 receptor knockout in COX-2–overexpressing mice protected against adriamycin-induced albuminuria and renal pathology. We speculate that in addition to the obvious differences in the severity and etiology of the models used (adriamycin versus 5/6 Nx, the latter being significantly more injurious than the former), another factor contributing to the observed GFB phenotypes could be the background strain of mice (C57Bl/6J × B6/D2 versus FVB/N). Whereas C57Bl/6J mice are relatively resistant to a variety of kidney disease models,24 the FVB/N strain of mice used in this study are susceptible to various forms of renal injury.32–34
In this study, attenuation of albuminuria in 5/6 Nx EP4pod−/− mice was incomplete, which may reflect the inefficient nature of the Cre-mediated EP4 gene deletion. Despite that our CreEGFPpod+/ROSA26 mice exhibited qualitatively robust glomerular Cre-recombinase activity, we were unable to obtain a reduction in EP4 mRNA beyond 50% in podocytes isolated from EP4pod−/− mice. Although this could be due to incomplete excision of the floxed allele, basal podocyte EP4 expression may be relatively weak (and therefore difficult to detect reliably) in healthy glomeruli, being induced to significant levels only under pathophysiologic conditions. Consistent with this possibility, glomeruli isolated from healthy non-TG mice responded to PGE2 with a modest cAMP synthesis, whereas those obtained from EP4pod+ TG mice produced cAMP levels in excess of 40-fold above nonstimulated controls. Although we cannot discount the possibility of off-target activity of Cre-recombinase in podocytes of EP4pod−/− mice, functional deletion of EP4 receptor expression was sufficient to abrogate the modest cAMP synthesis induced by PGE2 in EP4pod−/− primary podocytes. Conversely, we cannot discount the contribution to podocyte damage of additional factors not directly affected by the loss of EP4 expression in this model (e.g., angiotensin II, TGF-β); however, the interplay between these and the COX-2/PGE2/EP4 expression and activity is not yet known. The mechanism by which the EP4 receptor might alter podocyte structure/function will require additional studies. An impact on cell survival is unclear; however, Takano and colleagues35 demonstrated that PGE2/EP4 receptor coupling protects rat glomerular epithelial cells from apoptosis. We were unable to detect changes in apoptosis for conditionally immortalized mouse podocytes in response to PGE2. Similarly, Harris and co-workers31 showed that puromycin aminonucleoside–induced apoptosis was attenuated in response to antagonism of the Gq-coupled TP receptor but not antagonism of the Gs-coupled EP4 receptor. The work of Spurney and colleagues36 is consistent with these findings; they demonstrated that overexpression of a constitutively active Gq α subunit stimulated calcineurin activity and was associated with podocyte apoptosis in vitro. The notion that Gq- but not Gs-coupled signaling promotes podocyte apoptosis is supported by a number of studies examining effects of the angiotensin II type 1 (AT1) receptor. Mechanical stretch of conditionally immortalized podocytes contributed to AT1-dependent apoptosis.37 TG overexpression of the AT1 receptor in rats produced glomerulosclerosis and podocyte loss.38 In light of these findings, how, then, might activation of the Gs-coupled EP4 receptor be detrimental to podocyte health? Our findings demonstrate that exposure of immortalized podocytes, transduced to overexpress the EP4 receptor, to both mechanical stretch and PGE2 resulted in detachment from the extracellular matrix. Furthermore, we showed previously that mechanical stretch of podocytes in vitro followed by acute PGE2 stimulation dramatically reduced actin stress fiber content21; therefore, excessive EP4 activation might promote cell survival pathway activation yet nevertheless contribute to injury through its detrimental effects on podocyte attachment and cytoskeletal architecture. The loss of stress fibers along with the detachment defect observed in this study may be mechanistically related. Stress fiber formation is dependent on the small GTPase RhoA. Protein kinase A can phosphorylate RhoA on Ser188 to inhibit its activity by sequestering it from active sites associated with the membrane.39 For example, cAMP-dependent Protein kinase A induction in cultured human trabecular meshwork cells directly phosphorylated and inactivated RhoA, resulting in stress fiber dissociation.40 Whether PGE2 modulates its effects on the podocyte cytoskeleton and adhesion through regulation of Rho GTPase activity or other pathways such as those involving integrin receptor expression or focal adhesion kinase activity remains to be determined.
In summary, our findings point to a maladaptive role for PGE2 acting through its Gs-coupled EP4 receptor subtype in podocytes. Activation of this receptor contributes to the early deterioration of the GFB in a mouse model of chronic progressive renal injury and highlights the pleiotropic nature of the intrarenal actions of the prostanoid family.
Concise Methods
Cell Culture
Conditionally immortalized mouse podocytes (a gift from Dr. Karlhans Endlich, University of Greifswald, Greifswald, Germany) were cultured using previously described methods.41,42 Briefly, cells were grown on collagen I–coated culture plates (0.1 mg/ml; Sigma-Aldrich, St. Louis, MO) in RPMI-1640 medium supplemented with 10% FBS (Invitrogen, Carlsbad, CA), 100 μg/ml normocin (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada), and penicillin-streptomycin solution (1:100; Invitrogen). Podocytes were propagated at 33°C in the presence of 10 U/ml recombinant mouse γ-IFN (Invitrogen). For induction of podocyte differentiation, cells were maintained at 38°C for 14 days in the absence of γ-IFN.
Adenoviral Constructs and Infection of Cultured Podocytes
An adenoviral construct containing full-length mouse 2×HA-tagged EP4 receptor sequence was generated by the University of Ottawa Viral Vector Core Facility. Briefly, a human cDNA coding sequence clone was obtained from Invitrogen via the National Institutes of Health Mammalian Gene Collection (IMAGE Id 8069040; accession no. BC101534). The EP4 coding sequence was subcloned out of its host plasmid (pCMV-Sport6) into pcDNA3 using EcoRI/XhoI restriction enzyme sites. An N-terminal 2×HA tag was introduced by PCR-based cloning using the following primers: forward gatccatgtatccatatgacgtcccagactctgcctatccatatgacgtcccagactctgccg; reverse aattcggcagagtctgggacgtcatatggataggcagagtctgggacgtcatatggatacatg.
For adenovirus production, the HA-tagged EP4 construct was subcloned into pShuttle-CMV (Stratagene, La Jolla, CA). This shuttle vector undergoes homologous recombination in bacteria with pAdEasy-1 to constitute the recombinant adenoviral plasmid. Linearized recombinant adenoviral plasmid was transfected into AD-293 cells to obtain a primary viral stock, which was amplified, purified, and titered. For optimization of infection conditions, differentiated mouse podocytes were infected with EP4 adenovirus at a 0 to 100 multiplicity of infection (MOI) for 72 hours. Cell lysates were then prepared by washing podocytes twice with ice-cold PBS followed immediately by lysis with RIPA buffer. Samples were centrifuged at 16,000 × g for 15 minutes, and supernatants were collected. Equal quantities of protein (10 μg) lysate were loaded onto 10% SDS-PAGE resolving gel and transferred to nitrocellulose membrane. Immunoblots were incubated with antibodies against COX-2 (1:1000; Caymen Chemical, Ann Arbor, MI), HA-7 (1:1000; Sigma-Aldrich), or β-actin (1:1000; Sigma-Aldrich) in conjunction with an anti-rabbit IgG horseradish peroxidase as secondary antibody (1:1000; Cell Signaling Technologies, Danvers, MA). Proteins were detected using SuperSignal West Pico Substrate (Biolynx, Brockville, Ontario, Canada) and exposed to Kodak X-Omat Blue film. For all subsequent experiments, podocytes were infected at an MOI of 50 pfu/cell.
cAMP Assay
Cells were grown for 14 days on 12-well cluster dishes at 38°C. After 10 minutes of preincubation with 5 μM indomethacin (Sigma) and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma), podocytes were stimulated with 1 μM PGE2 (Caymen Chemical) for 10 minutes. cAMP levels were determined by ELISA (Cayman Chemical).
Adhesion Studies
Differentiated podocytes (14 days of differentiation) were seeded onto six-well collagen I–coated stretch plates (Flexcell Int., Hillsborough, NC) for 3 days. Plates were mounted onto vacuum-based loading docks of the Flexcell FX-4000T apparatus (Flexcell Int.). Podocytes were incubated with 1 μM PGE2 in RPMI containing 0.5% FBS and subjected to 12% elongation at a frequency of 0.5 Hz for 24 hours in the presence of 5 μM indomethacin (to block endogenous prostanoid production). Nonstretched cells (control) were exposed to identical experimental conditions but without mechanical stretch. After mechanical stretch, medium was collected and nonadhering cells were counted using a hemocytometer. Adherent cells were similarly quantified after trypsinization.
Generation and Genotypic Analysis of EP4pod+ TG Mice
A construct containing the EP4 receptor (EP4del355) but lacking the nucleotides that encode the 133 C-terminal amino acids was engineered and cloned into pcDNA 3 identically to previously described methods.22 An N-terminal 2×HA tag was cloned in frame with the truncated receptor open reading frame using overlapping and complementary oligonucleotides (forward gatccatgtatccatatgacgtcccagactctgcctatccatatgacgtcccagactctgccg; reverse aattcggcagagtctgggacgtcatatggataggcagagtctgggacgtcatatggatacatg). An 8.3-kb fragment of the mouse NPHS1 promoter was cloned immediately upstream of the 2×HA-EP4del355 sequence and included the 5′ untranslated and 5′ flanking regions of the mouse NPHS1 gene. Briefly, the plasmid was cut by restriction enzyme digestion with Acc65I; its 5′ nucleotide overhang was subsequently filled in and blunted with T4 DNA polymerase, and the plasmid was then digested by HindIII. The 8.3-kb NPHS1 promoter fragment was likewise excised in a stepwise procedure from its pcDNA3 host vector using XhoI and blunted as already described with T4 DNA polymerase followed by HindIII restriction enzyme digestion. The HindIII/blunt end fragment was cloned into pcDNA3–2×HA-EP4del355 to generate pNPHS1-2×HA-EP4del355. This NPHS1-2×HA-EP4del355 transgene was linearized with HindIII/RsrII, isolated by agarose gel electrophoresis/QIAEX II gel extraction and then microinjected into FVB/N mouse embryos. Embryos were then surgically transferred to the oviduct of pseudopregnant CD1 recipient female mice. Both the embryo donor mice and the recipient mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Genotyping of resulting pups was performed by PCR of genomic DNA isolated from tail snips of 3-week-old mice using the following primer pair: Forward gagaaagaactgttaacggg and reverse accaccccgaagat. A total of seven candidate founders were identified. After determination of the highest expressing founders, two lines were initiated and maintained through intercrossing with non-TG FVB/N mice. Both EP4pod+ lines yield healthy, fertile, hemizygous TG offspring at the predicted Mendelian ratio (1:1).
Generation of CreEGFPpod+ Mice
The CreEGFP plasmid was obtained from Dr. David Threadgill (University of North Carolina) and was constructed as follows: pBluescript containing the coding sequence for Cre-recombinase was enzymatically digested with XhoI and BamHI to isolate a 5′ fragment of the Cre coding sequence (1068 bp). A corresponding 3′ fragment of Cre, lacking the stop codon, was isolated from pnCre-noStop after BamHI and ApaI restriction enzyme digestion. These two pieces were inserted into pEGFP-N2 (Clontech, Mountain View, CA) at the XhoI and ApaI sites to generate pCreEGFP. The CreEGFP sequence was subcloned into a pCDNA3.1 plasmid containing an 8.3-kb fragment of the 5′ murine nephrin promoter (pCDNA-NPHS1) as follows: Briefly, the entire CreEGFP sequence was excised from the plasmid after BglII and NotI digestion. This fragment was blunted with T4 DNA polymerase and cloned into pCDNA-NPHS1 to generate pNPHS1-CreEGFP. Orientation was verified by automated sequencing. The transgene was resolved as an 11.3-kb fragment after restriction digestion with HindIII and FspI and includes the BGH polyadenylation signal sequence and was purified using a QiaEX II kit according to the manufacturer's instructions (Qiagen, Mississauga, Ontario, Canada). After pronuclear injection of the NPHS1-CreEGFP transgene into oocytes and subsequent implantation into pseudopregnant females, three founder mice were obtained as determined by PCR-based genotypic analysis of tail-snip genomic DNA (isolated with a Qiagen tissue DNA purification kit) using Cre-specific primers (forward aggtgtagagaaggcacttag and reverse ctaatcggcatcttccagcagg). Subsequent backcrossing to wild-type C57Bl/6J mice yielded healthy, fertile, hemizygous TG offspring at the predicted Mendelian ratio (1:1). Mice were subsequently backcrossed for 10 generations to obtain a congenic FVB/N CreEGFPpod+ line.
In Vivo Expression and Catalytic Activity of CreEGFP
Kidneys from 6-week-old non-TG wild-type and CreEGFPpod+ mice were dissected, fixed with 4% paraformaldehyde (PFA), and embedded in paraffin wax. Seven-micrometer sections were processed for immunofluorescence using an FITC-conjugated mouse monoclonal anti-GFP antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution). Immunofluorescence was detected with a Zeiss Axioskop 2 fluorescence microscope (Zeiss Axioskop 2 MOT, Zeiss Germany), and images were captured with a Zeiss AxioCam. For determination of in vivo catalytic activity of the CreEGFP fusion protein in TG mice, CreEGFPpod+ mice were intercrossed with ROSA26 mice. We next determined whether the CreEGFP fusion protein could efficiently catalyze the excision of loxP-containing DNA sequences in vivo. For analysis, CreEGFPpod+ founder mice were bred with homozygous ROSA26 mice (R26R), a reporter line that expresses LacZ upon Cre-mediated recombination.43 More specific, this mouse line contains two loxP sites inserted into the ROSA26 locus so that no functional β-galactosidase is expressed. Excision of the loxP-flanked DNA sequence by Cre restores β-galactosidase expression.43,44 After appropriate intercrosses, the genotype of CreEGFPpod+/ROSA26 compound mice were determined by PCR using the Cre-specific and ROSA26-specific primers (R26Fo primer gcgaagagtttgtcctcaacc; R26Rev1 primer ggagcgggagaaatggatatg; R26Rev2 primer aaagtcgctctgagttgttat). Kidneys from double-positive 6-week-old compound TG mice were dissected and processed for X-gal staining as follows: Decapsulated kidneys were immersed in 0.1 M NaH2PO4/4% PFA solution (pH 7.3) for 1 hour at 4°C and were subsequently washed in phosphate buffer containing 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% NP-40. Samples were then placed in X-gal staining solution [1 mg/ml X-gal, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6] for 6 hour at 37°C, followed by three brief washes in PBS. Kidneys were postfixed for 24 hours in 0.1 M NaH2PO4/4% PFA solution (pH 7.3) at 4°C. Whole kidneys from ROSA26 and CreEGFPpod+ mice were grossly sectioned by razor blade and visualized under low-power light microscopy.
Primary Podocyte Experiments
Primary podocyte cultures were generated following previously described methods.45 Briefly, glomeruli were isolated from either EP4flox/flox or EP4pod−/− mice and seeded onto collagen I–coated plates in 1:1 K-1 Media:media harvested from cultured NIH-3T3 cells. K-1 Media (Fisher Scientific) contained Ham's F-12, DME-low glucose (Fisher), 10% FBS, 0.1% ITS Premix (Fisher Scientific), 1% sodium bicarbonate (Fisher Scientific), and 1% penicillin-streptomycin. Experiments assaying for cAMP or for genomic DNA isolation were performed 7 to 10 days after isolation.
Genomic DNA was isolated from cultured podocytes using the QIAamp DNA Mini Kit (Qiagen). For determination of whether cre recombination had occurred in the podocytes, primers were designed to flank exon 2 of the EP4 receptor (forward gaagggtcttgctcatctcg; reverse ctaacccgcactctctctc). Generation of a 200-bp PCR product was indicative of successful recombination.
5/6 Nx
All procedures were approved by the University of Ottawa Animal Care Committee. Under isoflurane-induced anesthesia, age-matched male and female mice (8 to 10 weeks) underwent renal ablation via removal of five sixths of total renal mass. The surgical procedure was carried out by resecting the left kidney and cauterizing the upper and lower poles of the right kidney. Briefly, surgeries were carried out under standard sterile conditions with the mouse lying ventrally with shaved area of back exposed. A 1-in incision was made along the skin of the back followed by dissection down the right lateral side to expose the muscle. A small incision was made through the muscle, and the right kidney was exposed and tied off at its blood supply at three different locations before its removal. The site was then stitched closed before proceeding with the left kidney. The left kidney was located and exposed, and the poles were excised to achieve 75% reduction in total renal mass. The ends of the kidney were cauterized, and the organ was placed back into the mouse. Once the left side was stitched, the dorsal incision was closed using stainless steel wound clips before the mouse was placed in recovery. Control mice underwent sham operations without the removal of any renal mass.
Urinalysis
Morning spot urine samples were collected from mice at specified time points after the 5/6 Nx procedure and analyzed for albumin and creatinine content using the Albuwell M Test Kit (Exocell, Philadelphia, PA) and Creatinine Companion Kit (Exocell), respectively, consistent with the Animal Models of Diabetic Complications Consortium guidelines. Results are reported as μg albumin:mg creatinine.
BP Measurements
SBP of mice was measured by tail-cuff plethysmography (BP- 2000; Visitech Systems, Apex, NC). Briefly, mice were placed on a heated platform (30°C) in an isolated chamber, and SBP measurements were obtained. Before nephrectomy, mice were trained for 5 days followed by an additional 3 days of measurements. Beginning at 2 weeks after 5/6 Nx, measurements were collected biweekly. Each session included five preliminary measurements followed by 10 measurements of data acquisition.
Renal Pathology
Mice were anesthetized with isoflurane and perfused with 20 ml of ice-cold PBS through the left ventricle. A small cut was made in the right atrium to allow the escape of return circulation. Kidneys were harvested from mice and decapsulated, and the poles were removed. Kidneys were either placed in 4% PFA/PBS for paraffin embedding or embedded in OCT Compound (Sakura Finetek USA, Torrance, CA) for frozen sectioning. Kidneys to be used for light microscopic analysis were sectioned at 4 μm and stained with periodic acid–Schiff and visualized with a Zeiss AxioCam HRc (Zeiss Axio Imager.A1, Zeiss Germany).
Immunohistochemistry and Immunofluorescence
Embedded kidney sections were deparaffinized in mixed xylenes (Fisher Scientific) and rehydrated, and antigen unmasking was carried by immersing the slides in citrate buffer, micowaving for 2 minutes, and steaming for 25 minutes. Sections were blocked in 5% goat serum, incubated with antibody against Wilms' tumor (added neat; Spring Bioscience, Fremont, CA) followed by anti-rabbit IgG, horseradish peroxidase secondary (dilution 1:100; Cell Signaling Technologies). Sections were then washed in PBS before applying the Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA). Slides were stained using DAB substrate solution according to manufacturer's instructions (DAB Substrate Kit for Peroxidase; Vector Laboratories). Images were then captured using a Zeiss AxioCam HRc.
Frozen tissues embedded in OCT were cut in serial 6-μm sections. Sections were allowed to thaw, were washed three times with PBS, and then were blocked with 5% goat serum (Vector Laboratories) in PBS. After blocking, sections were incubated with primary antibodies (rabbit anti-HA [1:1000; Zymed, San Francisco, CA] and mouse anti-synaptopodin [1:100; Fitzgerald Industries International, Concord, MA]). Sections were then incubated with either Alexa 488– or Alexa 584–conjugated secondary antibodies (1:500; Molecular Probes, Eugene, OR). Images were then captured using a Zeiss AxioCam.
RNA Extraction and Quantitative RT-PCR
Snap-frozen kidneys were retrieved from −80°C storage, and RNA was extracted using an RNeasy Kit (Qiagen). After RNA isolation, mRNA levels of EP4 receptor, EP1 receptor, COX-2, and nephrin were determined by quantitative RT-PCR using TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems Research, Foster City, CA) and an ABI Prism 7000 Sequence Detection System. The primers and TaqMan probe used for the EP4 receptor were forward atggtcatcttactcatcgccac, reverse ccttcaccacgtttggctgat, and probe 6FAM-catctgctccattccgctcgtggt-TAMRA; for the EP1 receptor were forward agtgccaagggtggtccaa, reverse ccgggaactacgcagtgaac, and probe 6FAM- tgggcctaaccaagagtgcctgta-TAMRA; for COX-2 were forward ggggtgcccttcacttctttca, reverse tgggaggcacttgcattga, and probe 6FAM-tgtgcaagatccacagcctaccaaaaca-TAMRA; and for nephrin were forward aagctggacgtgcattatgct, reverse cggtgcagactatatccacagaac, and probe 6FAM-tgccctgaaccctactgaggtgaa-TAMRA. All values were normalized to rodent glyceraldehyde-3-phosphate dehydrogenase (TaqMan Rodent GAPDH Control Kit, Applied Biosystems).
Statistical Analysis
All experimental data were analyzed using GraphPad PRISM software. Data are means ± SEM. Where appropriate either t test or ANOVA followed by Newman-Keuls multiple comparison test was used.
Disclosures
None.
Acknowledgments
W.H.F carried out experiments with the Cre-EGFPpod+ and EP4pod−/− mice. All other experiments were conducted by E.M.S.-C.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The EP4 Receptor for Prostaglandin E2 in Glomerular Disease: A Good Receptor Turned Bad?” on pages 1597–1599.
References
- 1. Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A: In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. Am J Pathol 166: 1309–1320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tryggvason K, Pettersson E: Causes and consequences of proteinuria: The kidney filtration barrier and progressive renal failure. J Intern Med 254: 216–224, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y: Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension 45: 198–202, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: Podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Barisoni L, Mundel P: Podocyte biology and the emerging understanding of podocyte diseases. Am J Nephrol 23: 353–360, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM: Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 119: 943–953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziyadeh FN, Wolf G: Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Stitt-Cavanagh E, MacLeod L, Kennedy C: The podocyte in diabetic kidney disease. ScientificWorldJournal 9: 1127–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vriesendorp R, Donker AJ, de Zeeuw D, de Jong PE, van der Hem GK, Brentjens JR: Effects of nonsteroidal anti-inflammatory drugs on proteinuria. Am J Med 81: 84–94, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Velosa JA, Torres VE: Benefits and risks of nonsteroidal antiinflammatory drugs in steroid-resistant nephrotic syndrome. Am J Kidney Dis 8: 345–350, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD: Prostanoid receptors: Subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Murray MD, Brater DC: Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol 33: 435–465, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X, Kennedy C, Breyer MD, Moeckel GW, Harris RC: Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 18: 551–559, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Jo YI, Cheng H, Wang S, Moeckel GW, Harris RC: Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp Nephrol 107: e87–e94, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Wang JL, Cheng HF, Shappell S, Harris RC: A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int 57: 2334–2342, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Wang JL, Cheng HF, Zhang MZ, McKanna JA, Harris RC: Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol 275: F613–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Bek M, Nüsing R, Kowark P, Henger A, Mundel P, Pavenstädt H: Characterization of prostanoid receptors in podocytes. J Am Soc Nephrol 10: 2084–2093, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Faour WH, Gomi K, Kennedy CR: PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell Signal 20: 2156–2164, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Martineau LC, McVeigh LI, Jasmin BJ, Kennedy CR: p38 MAP kinase mediates mechanically induced COX-2 and PG EP4 receptor expression in podocytes: Implications for the actin cytoskeleton. Am J Physiol Renal Physiol 286: F693–F701, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Slipetz D, Buchanan S, Mackereth C, Brewer N, Pellow V, Hao C, Adam M, Abramovitz M, Metters KM: Sequestration and phosphorylation of the prostaglandin E2 EP4 receptor: Dependence on the C-terminal tail. Biochem Pharmacol 62: 997–1012, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Michaud JL, Lemieux LI, Dubé M, Vanderhyden BC, Robertson SJ, Kennedy CR: Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol 14: 1200–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ma LJ, Fogo AB: Model of robust induction of glomerulosclerosis in mice: Importance of genetic background. Kidney Int 64: 350–355, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Moeller MJ, Kovari IA, Holzman LB: Evaluation of a new tool for exploring podocyte biology: Mouse Nphs1 5′ flanking region drives LacZ expression in podocytes. J Am Soc Nephrol 11: 2306–2314, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD: Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis 40: 7–14, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kriz W, Lehir M: Pathways to nephron loss starting from glomerular diseases: Insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Fields TA, Pazmino K, Dai Q, Burchette JL, Howell DN, Coffman TM, Spurney RF: Activation of Galpha q-coupled signaling pathways in glomerular podocytes promotes renal injury. J Am Soc Nephrol 16: 3611–3622, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Boie Y, Stocco R, Sawyer N, Slipetz DM, Ungrin MD, Neuschäfer-Rube F, Püschel GP, Metters KM, Abramovitz M: Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol 340: 227–241, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Vukicevic S, Simic P, Borovecki F, Grgurevic L, Rogic D, Orlic I, Grasser WA, Thompson DD, Paralkar VM: Role of EP2 and EP4 receptor-selective agonists of prostaglandin E(2) in acute and chronic kidney failure. Kidney Int 70: 1099–1106, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC: Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol 20: 1953–1962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD: Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Teiken JM, Audettey JL, Laturnus DI, Zheng S, Epstein PN, Carlson EC: Podocyte loss in aging OVE26 diabetic mice. Anat Rec (Hoboken) 291: 114–121, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M: Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54: 2328–2335, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Aoudjit L, Potapov A, Takano T: Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol 290: F1534–F1542, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Flannery PJ, Rosenberg PB, Fields TA, Spurney RF: Gq-dependent signaling upregulates COX2 in glomerular podocytes. J Am Soc Nephrol 19: 2108–2118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG, Shankland SJ: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65: 30–39, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ellerbroek SM, Wennerberg K, Burridge K: Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Shen X, Koga T, Park B-C, SundarRaj N, Yue BY: Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J Biol Chem 283: 603–612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P: Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A 94: 3789–3794, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shankland SJ, Pippin JW, Couser WG: Complement (C5b-9) induces glomerular epithelial cell DNA synthesis but not proliferation in vitro. Kidney Int 56: 538–548, 1999 [DOI] [PubMed] [Google Scholar]