Abstract
Objective:
Motor recovery after stroke depends on the integrity of ipsilesional motor circuits and interactions between the ipsilesional and contralesional hemispheres. In this sham-controlled randomized trial, we investigated whether noninvasive modulation of regional excitability of bilateral motor cortices in combination with physical and occupational therapy improves motor outcome after stroke.
Methods:
Twenty chronic stroke patients were randomly assigned to receive 5 consecutive sessions of either 1) bihemispheric transcranial direct current stimulation (tDCS) (anodal tDCS to upregulate excitability of ipsilesional motor cortex and cathodal tDCS to downregulate excitability of contralesional motor cortex) with simultaneous physical/occupational therapy or 2) sham stimulation with simultaneous physical/occupational therapy. Changes in motor impairment (Upper Extremity Fugl-Meyer) and motor activity (Wolf Motor Function Test) assessments were outcome measures while functional imaging parameters were used to identify neural correlates of motor improvement.
Results:
The improvement of motor function was significantly greater in the real stimulation group (20.7% in Fugl-Meyer and 19.1% in Wolf Motor Function Test scores) when compared to the sham group (3.2% in Fugl-Meyer and 6.0% in Wolf Motor Function Test scores). The effects outlasted the stimulation by at least 1 week. In the real-stimulation group, stronger activation of intact ipsilesional motor regions during paced movements of the affected limb were found postintervention whereas no significant activation changes were seen in the control group.
Conclusions:
The combination of bihemispheric tDCS and peripheral sensorimotor activities improved motor functions in chronic stroke patients that outlasted the intervention period. This novel approach may potentiate cerebral adaptive processes that facilitate motor recovery after stroke.
Classification of evidence:
This study provides Class I evidence that for adult patients with ischemic stroke treated at least 5 months after their first and only stroke, bihemispheric tDCS and simultaneous physical/occupational therapy given over 5 consecutive sessions significantly improves motor function as measured by the Upper Extremity Fugl-Meyer assessment (raw change treated 6.1 ± 3.4, sham 1.2 ± 1.0).
GLOSSARY
- CST
= corticospinal tract;
- FLAIR
= fluid-attenuated inversion recovery;
- LI
= laterality index;
- MRC
= Medical Research Council;
- PT/OT
= physical/occupational therapy;
- rTMS
= repetitive transcranial magnetic stimulation;
- tDCS
= transcranial direct current stimulation;
- UE-FM
= Upper Extremity Fugl-Meyer assessment;
- WMFT
= Wolf Motor Function Test.
Motor impairment due to ischemic stroke is one of the leading disabilities in adults in Western countries.1 In addition to established means of facilitating motor recovery after stroke such as physical and occupational therapy, a variety of experimental rehabilitation approaches have been tested.2 Recent developments include noninvasive brain stimulation techniques such as repetitive transcranial magnetic stimulation (rTMS)3 and transcranial direct current stimulation (tDCS).4 The use of these tools is based on neurophysiologic studies demonstrating an imbalance of interhemispheric interactions which appears to interfere with the recovery process.5,6
The model of interhemispheric imbalance provides a framework for developing hypotheses based on its 2 facets: 1) upregulating excitability of intact portions of the ipsilesional motor cortex and 2) downregulating excitability of the contralesional motor cortex to modulate its unrestrained inhibitory influence on ipsilesional regions.2,4 Pilot and proof-of-principle studies, using either rTMS7–10 or tDCS,11–14 have shown both approaches to have beneficial effects on motor skills and motor learning. Furthermore, the combination of tDCS and peripheral stimulation (e.g., peripheral nerve stimulation or peripheral sensorimotor activities) seems to enhance the effects of each intervention by itself.13,14
To date, however, no study has tested the efficacy of bihemispheric stimulation, i.e., upregulation of ipsilesional and simultaneous downregulation of contralesional motor regions, in combination with peripheral sensorimotor activities in chronic stroke patients. Bihemispheric tDCS may potentiate the effects of anodal stimulation to the lesional hemisphere11,14 through additional modulation of interhemispheric interactions15 via cathodal stimulation to the contralesional motor cortex.12
METHODS
Subjects.
Twenty chronic stroke patients participated in the study (see table 1 for demographic, clinical, and imaging data; figure 1 for individual lesion maps). Inclusion criteria consisted of occurrence of ischemic stroke in the territory of the medial cerebral artery at least 5 months prior to enrollment; no previous or subsequent strokes; Medical Research Council (MRC) strength grade of ≤3/5 in extensor muscles of the affected upper extremity in the acute phase with at least 15 degrees of active wrist dorsiflexion at enrollment; no additional neurologic or psychiatric disorders; and no concurrent use of CNS-affecting drugs.
Table 1 Demographic, imaging, and behavioral motor data
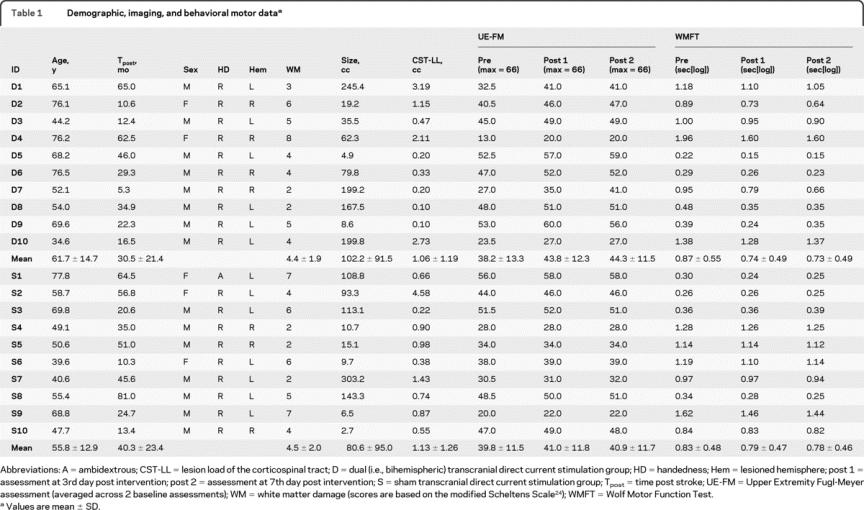
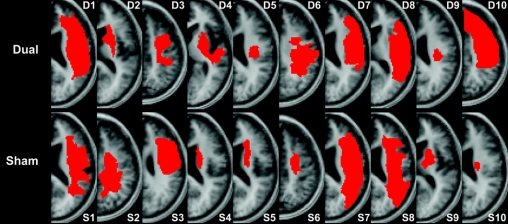
Figure 1 Representative individual lesion maps
After spatial normalization to the Montreal Neurological Institute space using SPM5, individual lesion maps of patients in the real stimulation group (dual) and control group (sham) were drawn and superimposed onto a canonical T1-weighted image. The slice closest to the internal capsule level with the greatest lesion expansion is shown to illustrate each patient's lesion.
Sample size calculations were based on a previous unihemispheric tDCS study (cathodal stimulation of the contralesional motor cortex), in which patients improved by 4 points on the Upper Extremity Fugl-Meyer assessment (UE-FM) after 5 days of treatment.4,16 Since bihemispheric tDCS was shown to be about 50% more effective than unihemispheric tDCS in healthy subjects,17 we assumed an improvement of about 6 ± 3 points in our real stimulation group and, according to this previous study, 1 ± 2 points in our control group. A power analysis revealed the necessary sample size to be n = 8 per group to achieve a statistical power of at least 95% (2-tailed α = 0.05).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local Institutional Review Board, and all patients gave written informed consent. This trial is registered at the public trials registry (NCT00792428).
Study design.
Patients were randomly assigned to one of two groups—real tDCS with physical/occupational therapy (PT/OT) or sham tDCS with PT/OT—using a block randomization with 3 strata of impairment based on UE-FM score (13–28, 29–41, and 42–56 points) to achieve similar distributions of impairment between the 2 groups.
Each patient underwent motor impairment assessments and MRI at baseline and after the intervention, conducted by trained individuals who were blinded to the type of intervention the patients received.
For both conditions, tDCS (30 minutes) and PT/OT (60 minutes) commenced at the same time. An experienced occupational therapist used a combination of PT/OT techniques including functional motor tasks to promote sensory-motor integration, coordination of movement, and goal-directed activities of practical relevance (e.g., reaching, grasping, or object manipulation). All patients received similar exercises within their own capabilities. The patients and the therapist were blinded as to whether the patients received real or sham tDCS.
Transcranial direct current stimulation.
Direct current was delivered using a Phoresor® II autostimulator (IOMED, Salt Lake City, UT) through 2 saline-soaked surface gel-sponge electrodes (16.3 cm2 active area).4 Real stimulation consisted of 30 minutes of 1.5 mA direct current with the anode placed over the ipsilesional and the cathode over the contralesional motor cortex (C3 and C4 of the international 10–20 EEG electrode system). For sham stimulation, the same electrode positions were used. The current was ramped up to 1.5 mA and slowly decreased over 30 seconds to ensure the typical initial tingling sensation.18
Motor impairment and motor activity assessments.
Each patient underwent the UE-FM,19 a standardized motor impairment scale (primary outcome measure), and the Wolf Motor Function Test (WMFT),20 a battery of proximal and distal motor activity tasks (secondary outcome measure), on 2 different days prior to the intervention to assure measurement stability at baseline.21 Patients were assessed again 3 days (post 1) and 7 days (post 2) after the last intervention session. A 2-tailed paired t test revealed no difference between baseline assessments (p > 0.62) so the 2 preintervention tests were averaged for the overall analysis.
A generalized linear mixed-effects regression model with robust variance estimation was used to evaluate the association between treatment assignment (dual vs sham) and outcome measures over time (pre, post 1, post 2) while controlling for age, time poststroke, and lesion size. All statistical analyses were performed using Stata 10.0 (StataCorp, College Station, TX).
Functional MRI tasks.
Prior to and following the experimental intervention, all patients were scanned while performing repetitive elbow and wrist extension/flexion movements, which were paced auditorily by a metronome, delivered through MRI-compatible headphones. Subjects were asked to close their eyes, listen to the metronome, and alternate between flexion and extension up to the maximal excursion they could perform while still synchronizing with the auditory pacer at a rate of 1 Hz. The fMRI session consisted of alternating 35-second epochs of elbow or wrist movements.
Each active task was followed by a nonmovement rest condition (elbow and wrist in neutral position), resulting in 20 volumes each for affected limb movements, unaffected limb movements, and rest conditions in each run. To minimize training effects and their influence on the activation pattern,22 patients practiced until they were able to perform the movements at the required pace and at maximal excursion without mirror movements. An investigator remained in the scanner room to observe whether the tasks were performed as instructed. No mirror movements were detected.
MRI acquisition, preprocessing, and analysis.
All patients underwent MRI using a 3-T GE scanner, which included a set of highly T1-weighted images (0.93 × 0.93 × 1.5 mm3), a fluid-attenuated inversion recovery (FLAIR) sequence (0.5 × 0.5 × 5 mm3), and a gradient echo T2*-weighted EPI sequence. Head motion was minimized using foam pads and forehead restraining straps. The T1-weighted images were spatially normalized (2-mm isotropic voxels). Each patient's stroke lesion was mapped using the coregistered FLAIR images as an additional guide to confirm the location and extent of the chronic lesion (figure 1). We calculated an overlap of each lesion with a canonical corticospinal tract (CST) derived from a group of 10 age-matched healthy control subjects (58.2 ± 12.2 years) using diffusion tensor imaging.23 We used lesion volume as well as CST lesion load, a combined measure of lesion site and size that has been shown to be an excellent predictor of motor impairment in chronic stroke patients, to test for group differences at baseline. Furthermore, we rated white matter hyperintensities on the FLAIR images using the modified Scheltens scale24 in order to test for differences in the presence and severity of small vessel disease between the 2 groups.
Functional imaging data were acquired with the following parameters: field of view = 64 × 64, 32 contiguous axial slices covering the whole brain (voxel size 3.75 × 3.75 × 4 mm3). We used a sparse temporal design with clustered volume acquisition (acquisition time of 2.6 seconds and effective repetition time of 7 seconds). A long repetition time was chosen to ensure that patients heard the metronome during the interscan intervals and to exclude the possibility that the scanner noise would serve as a guide for movements.
Preprocessing (including realignment, spatial normalization, and smoothing using a kernel size of 8 mm) and statistical analysis were done with SPM5 (Wellcome Department of Neurology, London, UK) implemented in Matlab (The Mathworks Inc., Sherborn, MA). In the elbow task, 3 patients' imaging studies had to be excluded due to head motion that could not be adequately corrected, resulting in 9 sets of preintervention and postintervention data for the dual group and 8 for the sham group. Only 7 patients in each group could perform the wrist task at the required pace and were included in this analysis.
Voxels with task-related activity were identified using a General Linear Model approach.25 For the first level group analysis data were high-pass filtered (128 seconds) and modeled with the standard canonical hemodynamic response function. The analyses were conducted using a threshold of p < 0.05 (corrected for family-wise error).
The images of patients with right hemispheric lesions were mirrored so that all lesions were displayed on the left side. In addition to group analyses for the dual and sham groups, we extracted individual regional β parameters using WFU Pick Atlas templates of the precentral gyri in order to compare changes in laterality indices (LI) from preintervention to postintervention:
LI = (contralesional − ipsilesional)/(|contralesional| + |ipsilesional|)26
Correlation analyses were used to examine changes in LI with respect to changes in motor impairment/motor activity scores.
RESULTS
No adverse effects were observed by the investigators or reported by the patients except a mild tingling sensation at the site of the electrodes at the beginning of the stimulation, which is a common finding across different studies.18
Motor assessments and demographic data.
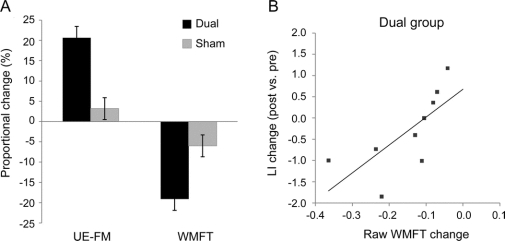
The groups did not differ with respect to age, time poststroke, gender distribution, presence and severity of small vessel disease,24 lesion volume, CST lesion load,23 or baseline motor scores (all p > 0.35; table 1). In the dual group, we observed an increase in UE-FM from 38.2 ± 13.3 to 43.8 ± 12.3 (post 1) and 44.3 ± 11.5 (post 2) and a decrease in WMFT scores from 0.87 ± 0.55 to 0.74 ± 0.48 (post 1) and 0.73 ± 0.49 seconds[log] (post 2). This corresponded to raw changes of 6.1 ± 3.4 in UE-FM (20.7 ± 18.9% proportional change) and −0.14 ± 0.11 in WMFT (−19.1 ± 9.0%; figure 2A).
Figure 2 Change in motor impairment scores and fMRI laterality index
(A) Proportional change of motor impairment scores from baseline to postintervention assessments ([post − pre]/pre × 100). Note that an increase in Upper Extremity Fugl-Meyer assessment (UE-FM) scores reflects an improvement in impairment of the affected limb and that a decrease in logarithmized Wolf Motor Function Test (WMFT) scores indicates a better function of the affected limb (i.e., shorter completion times). (B) Linear regression of change in the precentral gyrus activation laterality index (LI) and WMFT change (sec[log]) in the real stimulation group (Pearson coefficient r = 0.72, p = 0.029). No significant correlation between functional imaging measures and behavioral motor measures were found in the sham group.
In the sham group, UE-FM score increased from 39.8 ± 11.5 to 41.0 ± 11.8 (post 1) and 40.9 ± 11.7 (post 2), and WMFT score decreased from 0.83 ± 0.49 to 0.79 ± 0.47 (post 1) and 0.78 ± 0.46 seconds[log] (post 2). This corresponded to raw changes of 1.2 ± 1.0 in UE-FM (3.2 ± 3.2%) and −0.05 ± 0.06 in WMFT (−6.0 ± 10.5%).
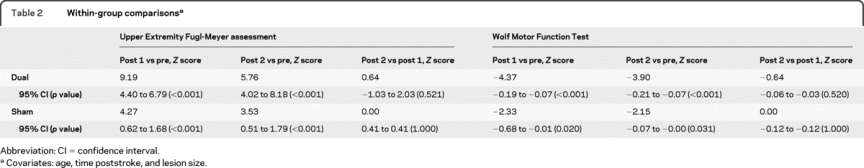
The linear mixed-effects regression analysis (controlling for age, time poststroke, and lesion size) revealed a main effect of time for both groups for UE-FM (Z = 9.44 for post 1, p < 0.001; Z = 5.92 for post 2, p < 0.001) and WMFT (Z = −2.40 for post 1, p = 0.016; Z = −2.21 for post 2, p = 0.027). The interaction between group and time revealed that the effect of time was different between the dual and sham groups for UE-FM (Z = 6.86 for post 1, p < 0.001; Z = 4.59 for post 2, p < 0.001) and WMFT (Z = −2.82 for post 1, p = 0.005; Z = −2.70 for post 2, p = 0.007). Linear regression analyses were also conducted for each group separately to evaluate the effects of time on outcome measures (table 2).
Table 2 Within-group comparisons
Functional MRI.
In the dual group, the post > pre contrast of affected elbow movements vs rest yielded a distinct cluster of significant change in the primary motor/premotor cortex of the lesional hemisphere. The post > pre contrast of affected wrist movements vs rest showed significantly more activation after therapy than before therapy in the ipsilesional primary motor/premotor cortex and in the contralesional inferior frontal gyrus (figure 3). The post > pre contrast of affected elbow and wrist movements vs rest in the sham group did not show any significant cortical activation changes. No significant changes were seen in the reverse contrast (pre > post) in either group.
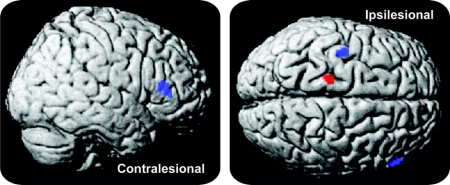
Figure 3 Contrast of affected limb movements postintervention vs preintervention for the dual group
Contrasting postintervention vs preintervention movements of the affected upper limb overlaid onto a standard anatomic template (p < 0.05, familywise error corrected; cluster extent threshold: 20 voxels). Both wrist (blue) and elbow (red) movements yielded stronger activations of ipsilesional primary motor/premotor cortex after the 5-day intervention. For the elbow movement task, the cluster was located in primary motor cortex (Talairach coordinates: x = −18, y = −23, z = 68; T = 5.39). For the wrist movement task, one cluster was located at the border between primary motor and premotor cortex (x = −40, y = −8, z = 60; T = 5.28); an additional positive activation change was found in the contralesional inferior frontal gyrus (x = 56, y = 29, z = 14; T = 5.66).
In the dual group, we found a significant correlation between changes in the precentral gyrus LI (using the elbow movement task) and changes in the WMFT scores (Pearson r = 0.72, p = 0.029; figure 2B). No correlations were seen between LI (using the elbow movement task) and UE-FM changes. No correlations were found with the wrist movement task. No correlations were seen in the sham group between LI (elbow and wrist movement tasks) and changes in WMFT or UE-FM scores.
DISCUSSION
In this sham-controlled noninvasive brain stimulation trial of chronic stroke patients with residual moderate to severe hemiparesis, we found significantly greater improvements in motor function in the group receiving bihemispheric tDCS with simultaneous PT/OT, compared with a control group receiving only PT/OT. The observed effects persisted beyond the intervention by at least 1 week and were accompanied by functional changes in motor cortex activation.
Previous unihemispheric brain stimulation studies revealed transient beneficial effects after modulation of either ipsilesional7,10,11,14 or contralesional motor cortex excitability.9,12 The novel bihemispheric stimulation design used in the present study allowed us to test the efficacy of simultaneously affecting both components of the hypothesized imbalance of interhemispheric interactions after stroke.5,6 The rationale for targeting ipsilesional motor regions was based on functional neuroimaging studies showing that reactivation of intact portions of the ipsilesional motor cortex is associated with better outcome after stroke.27,28 In the present study, the electrodes were centered on the precentral gyrus to target the primary motor cortex and indirectly affect its interhemispheric connections. Due to the relatively large electrode size, however, tDCS most likely exerted its effects on adjacent premotor and primary sensory regions as well.4 Modulating excitability of ipsilesional perimotor regions, which has previously been shown to be important for motor recovery,29,30 possibly contributed to the overall effects observed in our patient group. Similarly, cathodal stimulation of the contralesional primary motor cortex might have also affected the adjacent premotor cortex. Although the exact role of these contralesional motor regions in recovery remains elusive,31,32 downregulating excitability of contralesional motor regions consistently showed transient improvements of motor function in chronic stroke patients.9
Taken together, our study demonstrates the feasibility and efficacy of a bihemispheric stimulation approach in chronic stroke patients that affects bilateral motor regions differentially. We suggest that the cathodal component augments the direct effects of the anodal component on intact portions of the ipsilesional motor cortex by modulating a potentially unbalanced inhibitory effect of the contralesional hemisphere.6,15 In healthy individuals, it has been demonstrated that cathodal and anodal stimulation of the motor cortex result in differential effects on motor skill acquisition15,33,34 and that bihemispheric tDCS produces greater behavioral effects than unihemispheric stimulation.17 However, it is difficult to directly compare our results with those of others due to methodologic differences. Furthermore, the multisession design of the present study cannot be easily related to previous single session experiments of chronic stroke patients.11,12,14 To adequately compare the efficacy of different tDCS montages in combination with peripheral sensorimotor stimulation and to assess differences in short-term and longer-term functional improvements, larger patient samples are needed. Furthermore, although we chose to combine tDCS with PT/OT, which is commonly applied in routine poststroke rehabilitation, more standardized peripheral stimulation programs such as constraint-induced movement therapy may be an alternative in future studies. Constraint-induced movement therapy has been shown to have efficacy35 and can lead to functional reorganization of the motor system.36
The combination of central and peripheral stimulation has been successfully used to enhance motor recovery in animal models of stroke.37 In humans, proof-of-principle studies have combined tDCS and peripheral sensorimotor activities to improve motor function in stroke patients. In a previous investigation, anodal tDCS was applied to the ipsilesional motor cortex while patients received robot-assisted arm training.13 Only 3 out of 10 subacute stroke patients showed significant improvements after the multisession intervention. However, it should be noted that 2 of these 3 patients had subcortical strokes while the remaining 8 had large hemispheric strokes with involvement of the motor cortex (mean UE-FM of 7.2). In contrast, the mean UE-FM was 38.9 in the present study and the lesions spared (at least) parts of the ipsilesional motor cortex. A recent rTMS study demonstrated similar results: therapeutic responses were only observed when at least parts of the motor cortex were spared by the stroke.7
The stronger effects of peripheral sensorimotor activities with concurrent brain stimulation observed in our present study are in agreement with an investigation of unihemispheric tDCS and peripheral nerve stimulation.14 The combination of tDCS and peripheral training may enhance skill acquisition/consolidation through long-term potentiation-like mechanisms38 by increased afferent inputs to the cortex while its intrinsic excitability is being modulated by tDCS. Notably, a previous study in which constraint-induced movement therapy was administered (consecutively, but not simultaneously) with rTMS revealed no significant differences between real and sham stimulation on motor function of chronic stroke patients.8 Thus, the concurrent modulation of cortical excitability and peripheral sensorimotor stimulation has the potential to enhance motor improvement and corresponding plastic changes more significantly than any one intervention by itself.
With regard to neural correlates underlying the functional improvement, a stronger activation of ipsilesional motor regions after the intervention occurred in the real stimulation group, but not in the control group. This activation change was located more dorsally in the motor cortex than one would expect, given the typical limb representation. A possible explanation can be found in natural stroke recovery studies, which report increased activation of intact ipsilesional primary and adjacent nonprimary motor regions in association with a more favorable outcome.27,28 Similarly, functional imaging and electrophysiologic studies of reorganization processes in the chronic stroke phase demonstrated shifts and enlargement in cortical output centers associated with motor recovery.36 In our current study, additional activation postintervention was found in the contralesional inferior frontal gyrus, a component of the putative human mirror neuron system which has been suggested to play a role in motor recovery after stroke.39 The ipsilesional inferior frontal gyrus was part of the stroke lesion in 4 of 10 patients in the dual group.
Changes in the hemispheric asymmetry index of motor cortex activation in our study were related to changes in the WMFT completion times. This significant behavior-imaging correlation strengthens our assumption that modulating brain activity in ipsilesional and contralesional motor cortices with simultaneous peripheral sensorimotor activities leads to functional reorganization of the ipsilesional motor cortex. A possible explanation for the correlation between WMFT but not UE-FM scores and fMRI measures is that the WMFT incorporates measures of task accomplishment and is thought to more accurately distinguish between motor recovery and compensation.40
ACKNOWLEDGMENT
The authors thank Dr. Hsiao-Ting Wu for help with the statistical analysis.
DISCLOSURE
Dr. Lindenberg, Dr. Renga, L.L. Zhu, and Dr. Nair report no disclosures. Dr. Schlaug receives research support from the NIH (NINDS 1R01NS045049 [PI], NIDCD 1RO1 DC008796 [PI], and NIDCD R01 DC009823-01 [PI]) and the National Science Foundation.
Address correspondence and reprint requests to Dr. Gottfried Schlaug, Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Ave., Boston, MA 02215 gschlaug@bidmc.harvard.edu
Editorial, page 2146
e-Pub ahead of print on November 10, 2010, at www.neurology.org.
Study funding: Supported by the NIH/NINDS (NS045049).
Disclosure: Author disclosures are provided at the end of the article.
Received April 7, 2010. Accepted in final form August 13, 2010.
REFERENCES
- 1.Clarke PJ, Black SE, Badley EM, Lawrence JM, Williams JI. Handicap in stroke survivors. Disabil Rehabil 1999;21:116–123. [DOI] [PubMed] [Google Scholar]
- 2.Cramer SC. Repairing the human brain after stroke: II: restorative therapies. Ann Neurol 2008;63:549–560. [DOI] [PubMed] [Google Scholar]
- 3.Ziemann U. Improving disability in stroke with RTMS. Lancet Neurol 2005;4:454–455. [DOI] [PubMed] [Google Scholar]
- 4.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol 2008;65:1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55:400–409. [DOI] [PubMed] [Google Scholar]
- 6.Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res 1998;803:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 2009;66:298–309. [DOI] [PubMed] [Google Scholar]
- 8.Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil 2007;86:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 2005;64:1802–1804. [DOI] [PubMed] [Google Scholar]
- 10.Yozbatiran N, Alonso-Alonso M, See J, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke 2009;40:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490–499. [DOI] [PubMed] [Google Scholar]
- 12.Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005;16:1551–1555. [DOI] [PubMed] [Google Scholar]
- 13.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci 2007;25:9–15. [PubMed] [Google Scholar]
- 14.Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke 2009;40:1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res 2004;156:439–443. [DOI] [PubMed] [Google Scholar]
- 16.Nair D, Renga V, Lindenberg R, Zhu LL, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci (in press 2011). [DOI] [PMC free article] [PubMed]
- 17.Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci 2008;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006;117:845–850. [DOI] [PubMed] [Google Scholar]
- 19.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: 1: a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 20.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001;32:1635–1639. [DOI] [PubMed] [Google Scholar]
- 21.Whitall J, Savin DN Jr, Harris-Love M, Waller SM. Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper-extremity hemiparesis. Arch Phys Med Rehabil 2006;87:656–660. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci 1994;14:3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–811. [DOI] [PubMed] [Google Scholar]
- 25.Friston KJ, Holmes AP, Worsley KJ, Poline J, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995;2:189–210. [Google Scholar]
- 26.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 2008;26:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage 2007;34:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cramer SC, Mark A, Barquist K, et al. Motor cortex activation is preserved in patients with chronic hemiplegic stroke. Ann Neurol 2002;52:607–616. [DOI] [PubMed] [Google Scholar]
- 29.Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol 1998;55:1081–1088. [DOI] [PubMed] [Google Scholar]
- 30.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003;126:2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 2006;129:791–808. [DOI] [PubMed] [Google Scholar]
- 32.Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol 1992;31:463–472. [DOI] [PubMed] [Google Scholar]
- 33.Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 2009;106:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci 2008;28:1667–1673. [DOI] [PubMed] [Google Scholar]
- 35.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296:2095–2104. [DOI] [PubMed] [Google Scholar]
- 36.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke 2000;31:1210–1216. [DOI] [PubMed] [Google Scholar]
- 37.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res 2003;25:780–788. [DOI] [PubMed] [Google Scholar]
- 38.Stefan K, Kunesch E, Benecke R, Cohen LG, Claassen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 2002;543:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol 2006;19:55–63. [DOI] [PubMed] [Google Scholar]
- 40.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair 2009;23:313–319. [DOI] [PubMed] [Google Scholar]