Abstract
Objective:
To determine whether microvascular damage, indicated by cerebral microbleeds (CMBs) and retinal microvascular signs, is associated with cognitive function and dementia in older persons.
Methods:
This is a cross-sectional study of 3,906 participants (mean age 76 years; 58% women) in the AGES-Reykjavik Study (2002–2006). We assessed CMBs on MRI and retinal microvascular signs on digital retinal images. Composite Z scores of memory, processing speed, and executive function were derived from a battery of neurocognitive tests. Dementia and subtypes were diagnosed following international criteria. Regression models were used to relate cognitive Z scores and dementia to CMBs and retinal microvascular signs, adjusting for demographics, cardiovascular factors, and brain ischemic lesions.
Results:
People with multiple (≥2) CMBs had lower Z scores on tests of processing speed (β-coefficient −0.16; 95% confidence interval −0.26 to −0.05) and executive function (−0.14; −0.24 to −0.04); results were strongest for having multiple CMBs located in the deep hemispheric or infratentorial areas. The odds ratio of vascular dementia was 2.32 (95% confidence interval 1.02 to 5.25) for multiple CMBs and 1.95 (1.04 to 3.62) for retinopathy. Having both CMBs and retinopathy, compared to having neither, was significantly associated with markedly slower processing speed (−0.25; −0.37 to −0.12), poorer executive function (−0.19; −0.31 to −0.07), and an increased odds ratio of vascular dementia (3.10; 1.11 to 8.62).
Conclusion:
Having multiple CMBs or concomitant CMBs and retinopathy is associated with a profile of vascular cognitive impairment. These findings suggest that microvascular damage, as indicated by CMBs and retinopathy lesions, has functional consequences in older men and women living in the community.
GLOSSARY
- AD
= Alzheimer disease;
- AGES
= Age, Gene/Environment Susceptibility;
- CAA
= cerebral amyloid angiopathy;
- CI
= confidence interval;
- CMB
= cerebral microbleed;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- DSST
= Digit Symbol Substitution Test;
- FA
= flip angle;
- FLAIR
= fluid-attenuated inversion recovery;
- FOV
= field of view;
- FSE
= fast spin-echo;
- MR
= magnetic resonance;
- OR
= odds ratio;
- PD
= proton density;
- TE
= echo time;
- TR
= repetition time;
- VaD
= vascular dementia;
- VCI
= vascular cognitive impairment;
- WMH
= white matter hyperintensity.

CME
The functional impact of microvascular damage has been well-studied in the retina, kidney, and peripheral nervous system, but its effect in the brain is poorly understood.1 However, there has been an increasing interest in studying cerebral microbleeds (CMBs) as a marker of microvascular disease.2–4 CMBs, characterized by foci of signal loss on T2*-weighted gradient-echo MRI, have been histopathologically confirmed as hemorrhagic microvascular lesions or microangiopathy in the brain.5 CMBs are frequently detected among asymptomatic older adults, and the prevalence of CMBs increases with advancing age.6–8 CMBs are more likely to occur in people with hypertension,2–4 cerebral amyloid angiopathy (CAA),2–4,9 cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy,2,10 stroke,2–4 and diabetes and retinopathy,11 all conditions reported to be associated with cognitive impairment.9,10,12,13 Indeed, cognitive consequences of CMBs have been noted in several patient-based studies,2–4,14 but data from population-based studies are lacking.
Extant studies show that retinal microvascular lesions are strongly correlated with cerebral small vessel disease such as white matter lesions and CMBs,11,15–17 suggesting that these retinal microvascular signs are markers for cerebral microvascular disease. Thus, studying retinal microvascular signs is another approach to indirectly measuring cerebral microvascular disease. Indeed, several population-based studies of middle-aged and older people suggest that retinal microvascular signs, in particular retinopathy lesions, are associated with poorer performance in cognitive domains such as processing speed and executive function.18–21
Here, we investigate, in a community-based cohort of older people, whether CMBs and retinal microvascular signs are associated with cognitive function and dementia. Furthermore, we hypothesize that having microvascular lesions in both the brain and retina indicates a heavier burden of cerebral microvascular damage than having lesions in either site alone, and thus, is associated with more severe cognitive consequences.
METHODS
Study population.
Participants are from the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, which is described elsewhere.22 Briefly, the Reykjavik Study was initiated in 1967 by the Icelandic Heart Association and included men and women born in 1907–1935 and living in Reykjavik, Iceland. During 2002–2006, 5,764 persons from the original cohort of the Reykjavik Study were re-examined as a part of the AGES-Reykjavik Study. The AGES-Reykjavik Study aims to study common mechanisms leading to major clinical and subclinical diseases in neurologic, cardiovascular, musculoskeletal, and metabolic systems. As part of comprehensive assessments at the Reykjavik research center, a questionnaire, clinical examination, and cognitive battery were administered, and images were acquired of the brain and retina.
Standard protocol approvals, registrations, and patient consents.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00–063), the Icelandic Data Protection Authority, Iceland, and the Institutional Review Board for the National Institute on Aging, NIH, United States. Written informed consent was obtained from all participants.
Retinal microvascular measures.
Retinal digital images were taken directly from the fundus of both eyes with a 6.3-megapixel Canon CR6 nonmydriatic digital camera after maximal pupil pharmacologic dilation.11,23 At the Ocular Epidemiology Reading Center, University of Wisconsin, 3 certified graders assessed retinal images for the presence of retinopathy lesions and retinal arteriolar signs (focal arteriolar narrowing and arteriovenous nicking) following a validated protocol.16 Retinopathy lesions included retinal blot hemorrhages, microaneurysms, soft exudates, and other less common lesions such as hard exudates, macular edema, and optic disc swelling.24 The quality control procedure and intergrader and intragrader reliability for assessing retinal microvascular signs have been reported elsewhere.11,16
MRI acquisition and reading protocol.
High-resolution MRI was acquired on a 1.5-T Signa Twinspeed System (General Electric Medical Systems, Waukesha, WI).7,11,16 The image protocol consisted of a proton density (PD)/T2-weighted fast spin-echo (FSE) sequence (time to echo [TE] 1, 22 msec; TE2, 90 msec; repetition time [TR], 3,220 msec; echo train length, 8; flip angle [FA], 90°; field of view [FOV], 220 mm; matrix, 256 × 256) and a fluid-attenuated inversion recovery (FLAIR) sequence (TE, 100 msec; TR, 8,000 msec; inversion time, 2,000 msec; FA, 90°; FOV, 220 mm; matrix, 256 × 256). A T2*-weighted gradient-echo type echoplanar sequence (TE, 50 msec; TR, 3,050 msec; FA, 90°; FOV, 220 mm; matrix, 256 × 256) was used to detect CMBs. The above sequences were acquired in 3-mm-thick interleaved slices. Also acquired were T1-weighted 3-dimensional spoiled gradient-echo sequence (TE, 8 msec; TR, 21 msec; FA, 30; FOV, 240 mm; matrix, 256 × 256; slice thickness, 1.5 mm) images. All images were acquired to give full brain coverage and slices were angled parallel to the anterior commissure–posterior commissure line to give reproducible image views in the oblique-axial plane.
CMBs were first identified and scored by neuroradiologists following the criteria reported elsewhere.7,11 Then, trained and standardized raters accessed the database and recorded the location of each bleed, up to 30; additional bleeds over 30 were not recorded for their size and location due to coalescing of individual microbleeds. Lesion location was recorded as present in the cerebral lobes (frontal, temporal, parietal, and occipital), basal ganglia, external capsule, and infratentorial structure (medulla oblongata, cerebellum, pons, and mesencephalon).7 CMBs were assessed with good interrater reliability (κ = 0.73) and excellent intrarater reliability (κ = 1).7
WMHs were defined as visible hyperintense lesions on T2-weighted FSE/PD and FLAIR images. The load of WMHs was rated separately for subcortical and periventricular regions following a semiquantitative rating scale with known properties.16,25 Cerebral infarct was defined as a defect of the brain parenchyma with a signal intensity that is isointense to that of CSF on all pulse sequences and with a diameter ≥4 mm, with the exception of infarcts in the cerebellum that had no size criteria.26
Cognitive function.
Three domains of cognitive function were measured with a battery of neurocognitive tests.27,28 Memory function was assessed with a modified version of the California Verbal Learning Test that includes subtests of immediate and delayed recall; processing speed was measured with the Digit Symbol Substitution Test (DSST), the Salthouse Figure Comparison Test,29 and the Stroop Test parts 1 and 2; and executive function was tested with the Digits Backward, a shortened version of the CANTAB spatial working memory test, and the Stroop Test part 3. The composite score for each cognitive domain was constructed according to a theoretical grouping of tests as reported elsewhere,30,31 and was computed by converting raw scores to standardized Z scores and averaging them across all tests for the domain. The composite Z scores were based on the distribution of tests in the total AGES-Reykjavik sample.
Diagnosis of dementia and subtypes.
The case finding was based on a 3-step procedure. All participants were screened on the Mini-Mental State Examination and DSST. Screen positives on either of the tests were administered another more complete diagnostic test battery. Those screening positive on the Trails A and B or the Rey Auditory Verbal Learning Test went for a final assessment that included a proxy interview and a neurologic examination. The diagnosis of dementia and subtypes was made during a consensus conference that included a geriatrician, a neurologist, a neuropsychologist, and a neuroradiologist who provided a clinical reading of MRI.28 Dementia was diagnosed according to the guidelines of the DSM-IV.32 Alzheimer disease (AD) was diagnosed according to the criteria of the National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association.33 Vascular dementia (VaD) was diagnosed following the criteria of the State of California AD Diagnostic and Treatment Centers34,35; Clinical medical history and MRI were used in the diagnosis. It was possible to diagnose a subject with possible AD and possible VaD if the 2 pathologies were thought to contribute to dementia.
Covariates.
Demographic, cardiovascular, and brain pathologic factors may confound the association of microvascular lesions to cognitive function. Education and smoking were assessed via questionnaire. Use of blood pressure–lowering drugs, blood glucose–lowering drugs, and anticoagulants was ascertained from medication vials brought to the research center. Body mass index was calculated as measured weight (kg) divided by height (meters) squared. Hypertension was defined as self-reported history of hypertension, use of blood pressure–lowering drugs, or blood pressure ≥140/90 mm Hg. Diabetes was identified through self-reported history of diabetes, use of blood glucose drugs, or fasting blood glucose ≥7.0 mmol/L. Depressive symptomology was defined as a score ≥6 on the 15-item Geriatric Depression Scale. Visual deficit was defined as visual acuity worse than 20/40, and was categorized into having no deficit, deficit in one eye, and deficit in both eyes.
Analytical sample.
Of the 5,764 participants, 762 (13.2%) did not have a magnetic resonance (MR) scan due to contraindications, refusal, or claustrophobia, or only participated in a home visit. An additional 298 (5.2%) did not have the specific image needed for evaluating CMBs. Among those with complete MR scans (n = 4,704), we further excluded 798 (13.8%) subjects who had incomplete data on either retinal images (n = 448) or cognitive function (n = 350), leaving 3,906 (67.8%) persons for current analysis. Compared with individuals who were included, those excluded (n = 1,858) were older (79.1 vs 76.0 years, p < 0.001), less educated (for primary school or less, 27.1% vs 22.5%, p = 0.001), and more likely to report smoking (14.7% vs 11.5%, p = 0.02) and to have diabetes (17.0% vs 11.0%, p < 0.001), but the 2 groups did not differ by sex or hypertension.
Statistical analysis.
We used general linear regression models to estimate the β-coefficient (95% confidence interval [CI]) of the composite cognitive Z score, and logistic regression models to estimate the OR (95% CI) of dementia associated with CMBs and retinal microvascular signs. Retinal vascular lesions were dichotomized, and CMBs were categorized into dichotomous and trichotomous (a single or multiple [≥2] vs no CMBs) variables. Because the distribution of CMBs may reflect different pathologies,2–4 we also examined in secondary analyses CMBs by location36: a strict lobar distribution and separately those in the deep hemispheric or infratentorial regions.
To examine the joint effects of CMBs and retinal vascular lesions, we divided subjects into 4 groups: those with no CMBs or retinal lesion (reference), with only CMBs, only retinal lesions, or both. We tested statistical interaction by simultaneously including CMBs, retinopathy, and the cross-product of these variables. We reported results from 2 models: model 1 adjusted for age, sex, and education; and model 2, which was further adjusted for depressive symptomology, visual acuity, smoking, hypertension, diabetes, body mass index, use of anticoagulants, brain infarcts (present vs absent), and load of subcortical and periventricular WMHs (entered as continuous variables). The SPSS Statistics 17.0 for Windows (SPSS Inc., Chicago, IL) was used for all analyses.
RESULTS
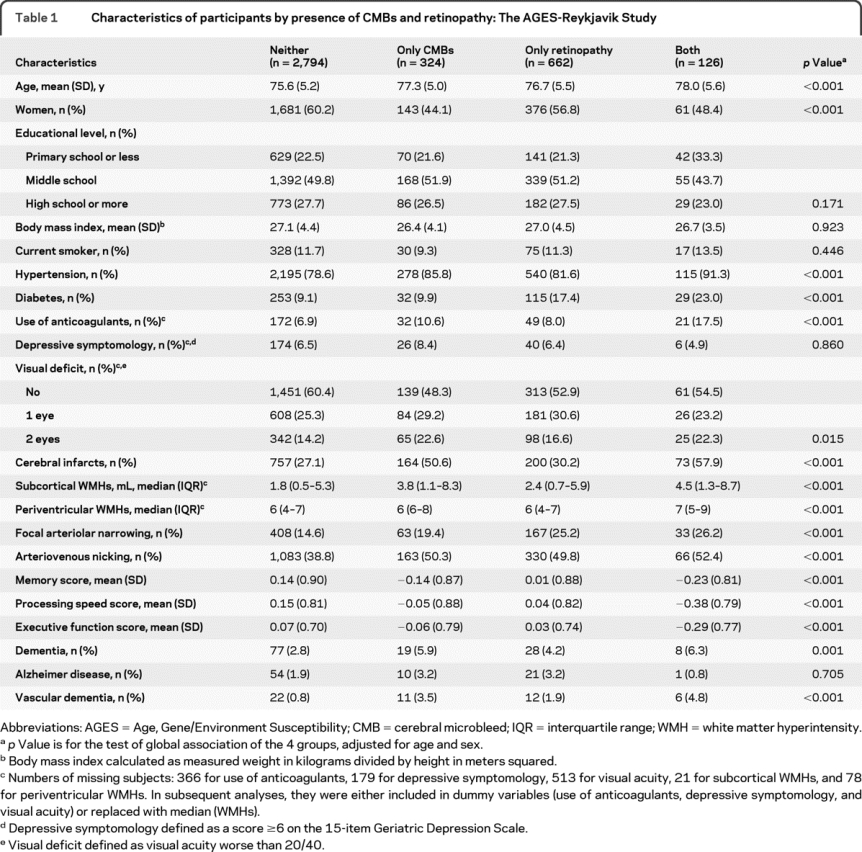
Participants had a mean age of 76 years (SD 5.2; range 66–96) and 58% were women. The mean composite Z score for memory was 0.09 (SD 0.90), for processing speed 0.09 (0.82), and for executive function 0.04 (0.72). Of the 3,906 participants, 132 (3.4%) were diagnosed with dementia, including 66 with AD, 31 with VaD, and 20 with both possible AD and possible VaD. Evidence of CMBs was found in 450 (11.5%) subjects, 176 (39.1%) of whom had multiple CMBs. Retinopathy lesions were detected in 788 (20.2%) subjects, of which 94.4% were retinal microaneurysms or hemorrhages. Subjects with CMBs or with both retinopathy and CMBs are older and more likely to have an elevated risk of cardiovascular disorders, brain infarcts, and a heavier WMH load (table 1).
Table 1 Characteristics of participants by presence of CMBs and retinopathy: The AGES-Reykjavik Study
Having CMBs was significantly associated with lower scores on tests of all 3 cognitive domains (table 2, model 1). The association with lower test scores on processing speed and executive function remained significant after further adjusting for potential confounders, including brain infarcts and WMHs (table 2, model 2). The associations were strongest in subjects with multiple CMBs (table 2), and particularly in those having multiple CMBs in the deep hemispheric or infratentorial regions (for processing speed: multiadjusted β-coefficient −0.20; −0.34 to −0.06; for executive function: −0.19; −0.33 to −0.05). Finally, having multiple CMBs was significantly associated with an increased OR for VaD. There was no location-specific association of CMBs with dementia and subtypes (table 3).
Table 2 Association [β-coefficient (95% confidence interval)] of composite cognitive Z scores to CMBs and retinopathy: The AGES-Reykjavik Study
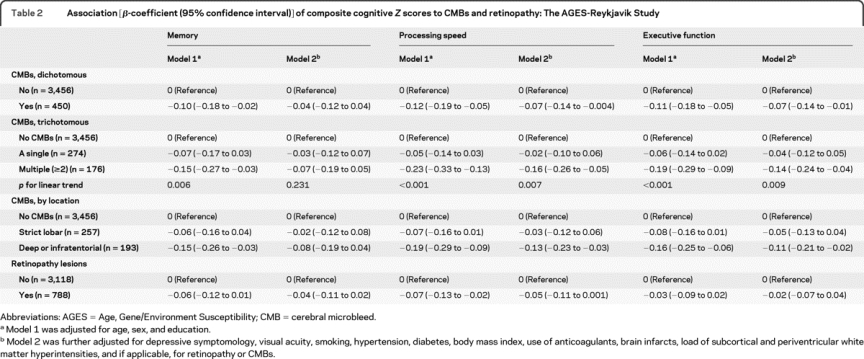
Table 3 Association [odds ratio (95% confidence interval)] of dementia and subtypes to CMBs and retinopathy: The AGES-Reykjavik Study
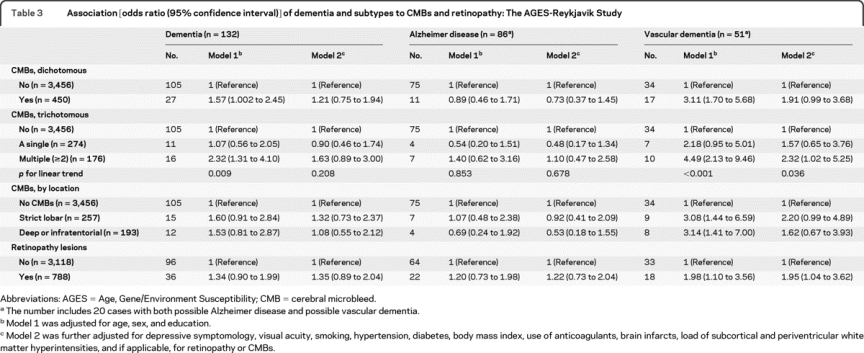
Retinopathy was significantly associated with a lower score on tests of processing speed in model 1, but this association was no longer significant when additional covariates were included in model 2 (table 2). Having retinopathy lesions was significantly associated with an increased OR for VaD, independent of cardiovascular risk factors and comorbid ischemic brain lesions (table 3). We found no significant association of retinal focal arteriolar narrowing and arteriovenous nicking to cognitive performance or dementia (data not shown).
Compared to those with no CMBs or retinopathy, individuals with only CMBs or only retinopathy had similar test scores on all 3 cognitive domains, but people having both CMBs and retinopathy had markedly lower scores on processing speed (multiadjusted β-coefficient −0.25; 95% CI −0.37 to −0.12; pinteraction = 0.008) and executive function (−0.19; −0.31 to −0.07; pinteraction = 0.020) (figure 1). Results were strongest for processing speed (−0.23; −0.41 to −0.04; pinteraction = 0.006) and executive function (−0.26; −0.45 to −0.08; pinteraction = 0.006) in those with strict lobar CMBs and retinopathy (table e-1 on the Neurology® Web site at www.neurology.org). Finally, compared to people having no CMBs or retinopathy, the multiadjusted OR for VaD was 2.31 (95% CI 1.05 to 5.06) in those having only CMBs, 2.31 (1.10 to 4.85) in those with only retinopathy, and 3.10 (1.11 to 8.62) in those having both lesions.
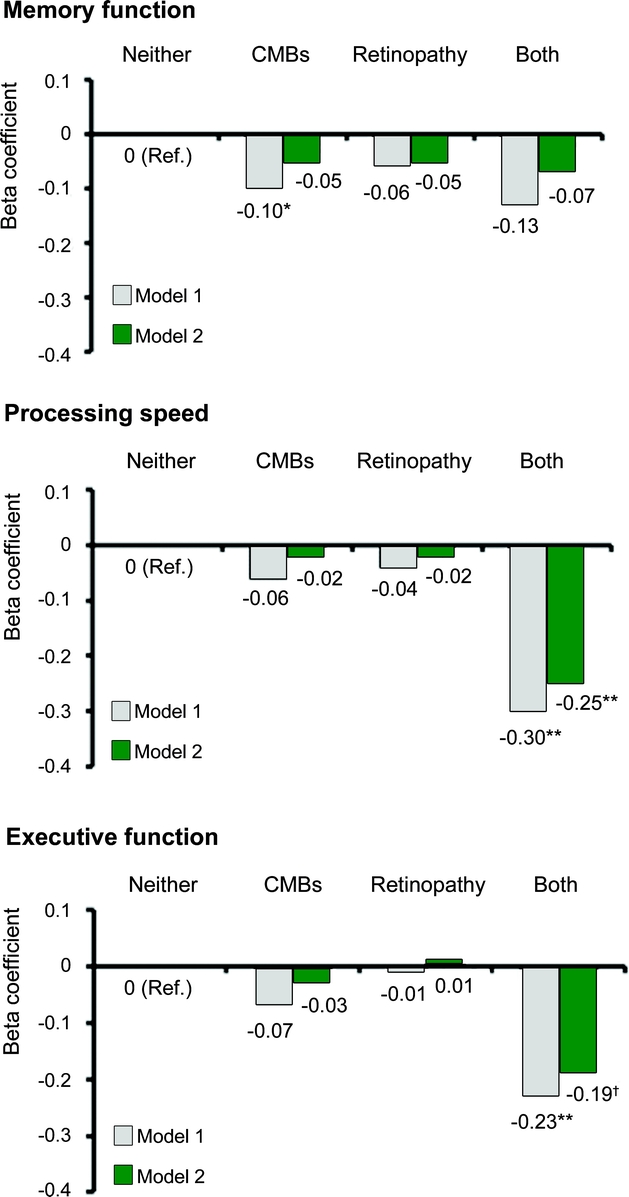
Figure 1 Joint effect of CMBs and retinopathy on cognitive function: Age, Gene/Environment Susceptibility–Reykjavik Study
Model 1 was adjusted for age, sex, and education; model 2 was further adjusted for depressive symptomology, visual acuity, smoking, hypertension, diabetes, body mass index, use of anticoagulants, brain infarcts, and load of subcortical and periventricular WMHs. *p < 0.05; **p < 0.001; †p < 0.01. CMBs = cerebral microbleeds; WMHs = white matter hyperintensities.
We repeated the main analyses of CMBs, retinopathy, and cognitive function among subjects without dementia (table e-2 and figure e-1); results were generally comparable to those reported in table 2 and figure 1. Finally, we obtained results for AD and VaD that were similar to those reported in table 3 when 20 cases with both possible AD and possible VaD were excluded from the analyses (data not shown).
DISCUSSION
This community-based study of older adults suggests that having multiple CMBs is associated with a range of indicators for vascular cognitive impairment (VCI), including slower processing speed, poorer executive function, and VaD. The association of CMBs to poorer cognitive performance is strongest for having multiple CMBs and is strengthened in the presence of retinopathy lesions. All these associations are independent of major cardiovascular factors, WMHs, and cerebral infarcts. The magnitude of the associations with multiple CMBs or concomitant CMBs and retinopathy is approximately equal to that of per 3- to 5-year increment in age (multiadjusted β-coefficients range −0.12 to −0.30). These findings suggest that loss of microvascular integrity, as indicated by CMBs and retinopathy lesions, has functional consequences in older men and women living in the community.
This study has several strengths. It is based on a large cohort of older people living in the community that is well-characterized with regard to health factors, retinal microvasculature, cerebral small vessel lesions, and cognitive function. Moreover, we are able to control for a broad range of factors that may potentially confound the association of cognitive function to vascular lesions in the brain and retina. Finally, the 3 cognitive domains were measured with multiple tests, which gave more robust assessments compared to those based on any single cognitive test.
This cross-sectional study also has limitations. First, a temporal relationship of retinal and cerebrovascular lesions to cognitive dysfunction cannot be established, although a reverse temporal relationship is unlikely. Furthermore, a cross-sectional study is subject to bias due to selective survival or selective participation, but only if CMBs, retinal microvascular lesions, and cognitive dysfunction are differentially related to survival and participation. There is no evidence to suggest this is the case here. Finally, we cannot completely rule out the residual confounding due to imperfect measurement of confounders or unmeasured confounders.
Our findings of CMBs in association with lower scores in processing speed and executive function are similar to those reported for cerebral ischemic small vessel disease.27,28,30 The additional finding that VaD, but not AD, is associated with CMBs and retinopathy lesions is consistent with the hypothesis that microvascular damage is associated with a profile of VCI.37,38 The interaction of CMBs with retinopathy suggests that more extensive microvascular lesions, which could manifest as increasingly lower cognitive function, underlie vascular disease not necessarily captured in the brain MR scans. There was no interaction of CMBs with retinopathy on VaD, although people with both lesions had a slightly higher odds for VaD compared to those having lesions only in one site. However, the number of VaD cases is small, which results in relatively wide CIs.
In secondary analyses, we found that the associations of poorer cognitive function to CMBs vary by their location and presence of retinopathy. Specifically, the association of poorer cognitive function to multiple CMBs was strongest for lesions located in the deep hemispheric or infratentorial regions, thought to reflect hypertensive vasculopathy.2–4 This finding, together with others,2–4,14 suggests that CMBs may be part of the pathologic spectrum linking hypertension to VCI and dementia. We also found that the interaction of retinopathy with CMBs on poorer cognitive function was strongest for CMBs with a strictly lobar distribution, which are thought to reflect CAA.2–4 Although CAA and CMBs commonly occur in AD,2–4,39,40 our findings suggest that more pervasive microstructural damage is associated with cognitive disturbances that are common in vascular origin. Additional investigations are needed to clarify why memory is not associated with these microvascular lesions.
CMBs may reflect tiny focal destructive lesions in strategic and nonstrategic subcortical structures and connection fibers relevant to cognitive function.2–4,14 Retinopathy lesions mainly include microaneurysms and retinal hemorrhages, which indicate a breakdown of retinal blood barrier.15,17,18 Previous studies have shown that people with both WMHs and retinopathy had a much higher incidence of stroke than those without cerebral or retinal vascular lesions.24 We also have reported that retinopathy lesions are correlated specifically with CMBs.16 Thus, compared to having lesions in one site, having comorbid CMBs and retinopathy lesions may indicate more extensive and more severe subclinical microvascular pathologies, which can result in substantially worse cognitive performance.
Our findings and those of other studies suggest that multiple CMBs or concomitant CMBs and retinopathy are markers for clinically relevant cerebrovascular disease in patients with mild cognitive impairment or dementia. Recognition and treatment of modifiable vascular risk factors should be pursued in patients with these lesions. Furthermore, the presence of CMBs and retinopathy lesions might define a clinical phenotype that would benefit from therapeutic interventions aimed at microvascular pathology.
DISCLOSURE
Dr. Qiu serves as an Associate Editor for the Journal of Alzheimer's Disease and receives research support from the Swedish Council for Working Life and Social Research for the Future Leaders on Ageing Research in Europe (FLARE) Program (2007-1728 and 2009-1934) and the Swedish Research Council (K2008-69X-20821-01-3 [PI]). Dr. Cotch receives research support from the Intramural Research Program of the NIH (ZIAEY000401). S. Sigurdsson, Dr. Jonsson, Dr. Jonsdottir, Dr. Sveinbjörnsdottir, and G. Eiriksdottir report no disclosures. Dr. Klein serves on a scientific advisory board for AstraZeneca; serves on the editorial board of Ophthalmology; has served/serves as a consultant for Pfizer Inc., Eli Lilly and Company, Novartis, Genentech Inc., CoMentis, Inc., Allergan, Inc., and Merck Serono; and has received/receives research support from the NIH (NEI 2 U10 EY006594-21A1 [PI], NEI 1 R01 HL6997 [PI], NEI 1 R01 EY016379 [PI], 1 R01 DK073217-01 [PI], and N01-AG-12100 [PI]). Dr. Harris serves on the editorial boards of the Journal of Gerontology: Medical Sciences, Age and Nutrition, and Ageing: Clinical and Geriatric Science. Dr. van Buchem reports no disclosures. Dr. Gudnason receives research support from the NIH (N01-AG-12100 [PI]) and the Icelandic Heart Association. Dr. Launer reports no disclosures.
Supplementary Material
Address correspondence and reprint requests to Dr. Chengxuan Qiu, Aging Research Center, Karolinska Institutet, Gävlegatan 16, S-113 30 Stockholm, Sweden chengxuan.qiu@ki.se; or Dr. Lenore J. Launer, LEDB, National Institute on Aging, NIH, Gateway Building, Suite 3C-309, 7201 Wisconsin Avenue, Bethesda, MD 20892 launerl@nia.nih.gov
Supplemental data at www.neurology.org
Study funding: The AGES-Reykjavik Study was funded by NIH contract N01-AG-12100, the Intramural Research Program of the National Institute on Aging, the National Eye Institute (ZIAEY000401), NIH, USA, and the Icelandic Heart Association and the Icelandic Parliament, Iceland.
Disclosure: Author disclosures are provided at the end of the article.
Received May 4, 2010. Accepted in final form September 2, 2010.
REFERENCES
- 1.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology 2006;66:165–171. [DOI] [PubMed] [Google Scholar]
- 4.Werring DJ. Cerebral microbleeds: clinical and pathophysiological significance. J Neuroimaging 2007;17:193–203. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 6.Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 2004;35:1831–1835. [DOI] [PubMed] [Google Scholar]
- 7.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology 2002;58:1629–1634. [DOI] [PubMed] [Google Scholar]
- 10.Liem MK, Lesnik Oberstein SA, Haan J, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology 2009;72:143–148. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Cotch MF, Sigurdsson S, et al. Retinal and cerebral microvascular signs and diabetes: the Age, Gene/Environment Susceptibility-Reykjavik Study. Diabetes 2008;57:1645–1650. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski JS, Jónsdóttir MK, Garcia ME, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: the Age, Gene/Environment Susceptibility-Reykjavik study. Am J Epidemiol 2008;168:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 2004;127:2265–2275. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth W Jr., Larsen EK, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol 2007;165:78–84. [DOI] [PubMed] [Google Scholar]
- 16.Qiu C, Cotch MF, Sigurdsson S, et al. Microvascular lesions in the brain and retina: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol 2009;65:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindley RI, Wang JJ, Wong MC, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol 2009;8:628–634. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492. [DOI] [PubMed] [Google Scholar]
- 19.Baker ML, Larsen EKM, Kuller LH, et al. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke 2007;38:2041–2047. [DOI] [PubMed] [Google Scholar]
- 20.Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology 2009;73:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liew G, Mitchell P, Wong TY, et al. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc 2009;57:1892–1896. [DOI] [PubMed] [Google Scholar]
- 22.Harris T, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein R, Meuer SM, Moss SE, Klein BE, Neider MW, Reinke J. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol 2004;122:1642–1646. [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67–74. [DOI] [PubMed] [Google Scholar]
- 25.Achten E, Breteler M, de Leeuw FE, et al. Rating scale for age related brain changes. Imaging Decisions MRI 2001;4:10–19. [Google Scholar]
- 26.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci 2008;63:848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke 2009;40:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salthouse T, Babcock R. Decomposing adult age differences in executive function. Dev Psychol 1991;27:763–776. [Google Scholar]
- 30.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 34.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42:473–480. [DOI] [PubMed] [Google Scholar]
- 35.Lopez OL, Kuller LH, Becker JT, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology 2005;64:1539–1547. [DOI] [PubMed] [Google Scholar]
- 36.Vernooij MW, Haag MD, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol 2009;66:714–720. [DOI] [PubMed] [Google Scholar]
- 37.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 38.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 39.Jellinger KA, Attems J. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J Neurol Sci 2005;229–230:37–41. [DOI] [PubMed]
- 40.Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol 2010;119:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


