Abstract
We evaluated the characteristics associated with uptake of HPV vaccine by 19-26 year old women seen in primary care university-based clinics. Of the 11,545 women analyzed only 18% had initiated the 3-dose vaccine series. Series completion among the sample overall was only 10% in the 30 month study period. Decreased series initiation was associated with older age, public insurance, white race and non-family medicine specialty. Decreased series completion was associated with public insurance and African American race. Utilization disparities by race and insurance worsened over time suggesting that the highest risk populations of women were not getting vaccinated.
Keywords: Human papillomavirus, vaccine, women
1.0 INTRODUCTION
Human papillomavirus (HPV) is a common sexually transmitted infection that can cause cervical cancer, genital warts and other anogenital cancers. A quadrivalent vaccine against the most clinically relevant HPV types was approved for females in 2006 and recommended for all 11-26 year old females who had not been previously vaccinated, and to girls as young as 9 based on clinician discretion.[1, 2]
In recent years, national and regional data on HPV vaccine utilization among adolescents, the preferred target age group for vaccination, have begun to emerge in the U.S.[3-11] These studies have identified disparities in vaccine uptake by several socio-demographic characteristics, and have deepened our understanding of the barriers to achieving high HPV vaccination rates among adolescents. Much less information has been reported on HPV vaccination of young adults however, even though this age group has the highest rates of HPV infection and related disease.[12, 13] In one national study performed 2-5 months following vaccine licensure, uptake of the first dose of the HPV vaccine by 18-26 year old women was only 10%.[14] Limited sample size precluded statistical analyses of the characteristics associated with vaccination, and because of the time frame, there was no data on utilization of second or third doses. There have been more recent reports using regional samples that have described adult HPV vaccine utilization levels ranging from 9% to 49%, depending on whether vaccine series initiation or completion was analyzed, the time elapsed since vaccine licensure, and population characteristics.[8, 9, 15-17]
Young adult women are a critical component of national HPV vaccination “catch up” strategies in the U.S. However, a better understanding of the factors associated with both beginning and completing the HPV vaccine series are needed in order to appropriately design future interventions to achieve national HPV vaccination targets.[18] The objectives of this study therefore were to determine 1) the uptake of first, second, and third doses of HPV vaccine among a large population of 19-26 year old women seen in primary care clinics within a university-based health system; 2) to examine whether race-, insurance-, age-, or medical specialty-related disparities in HPV vaccine use identified for adolescents[3] were also present among young adults; and 3) to determine how disparities changed over time.
2.0 METHODS
2.1 Study Design and Population
This was an age- and time-based extension of a previous study[3] that used clinical visit data (medical records and billing data) from the University of Michigan Health System to retrospectively assess the factors associated with HPV vaccine utilization. Using methods previously described,[3] we electronically captured all clinical encounters occurring between January 1, 2007 through June 30, 2009 for women ages 19-26 years seen in the outpatient general internal medicine (GM – 10 sites), family medicine (FM – 5 sites), general medicine-pediatrics (MP – 2 sites), gynecology (6 sites) or pediatrics clinics (9 sites). These 32 clinical sites are staffed primarily by faculty physicians with some resident clinics. They include both urban and suburban practice locations and serve a diverse population across four counties in Southeast Michigan. The study start date coincided with the first month when the HPV vaccine was widely available within this health system for both publically and privately insured individuals. For six months during the beginning of the study period, the FM clinics, but not other specialties, had automated computerized prompts to remind providers about HPV vaccination for eligible patients. (The effect of this automated intervention is reported in a separate manuscript). All study activities were approved by the institutional review board at the University of Michigan. Data were analyzed in 2010.
2.2 Outcome Measures
We measured receipt of HPV vaccine doses at the patient, vaccine-dose and visit level. Patient level analyses included the proportion of women who received first, second and/or third doses of HPV vaccine according to the recommended schedule,[2] and the cumulative proportion of eligible women initiating or completing the vaccine series over time. Vaccine-dose-level analyses included the proportion of the HPV vaccine doses administered that were attributable to different patient or clinical characteristics. Visit-level analyses included the proportion of, and factors associated with, visits that were “missed opportunities” for providing HPV vaccine (any eligible dose). Missed opportunity visits were defined as visits where an HPV vaccine dose could have been provided but was not, based on the recommended timing from any previous HPV vaccine doses,[2] if applicable (0, 2 and 6 months, with an extra two weeks allowed to accommodate variability in scheduling vaccination appointments).
2.3 Predictor Variables
Patient-level variables included insurance type, age and self-reported race. Based on the data distribution, race designations were condensed into three categories (African American, White, and Other/Not Specified). Data on Latino ethnicity is not systematically collected in this health system and therefore could not be assessed in our study. Age was stratified into four 2-year blocks (19-20, 21-22, 23-24, 25-26 years). Included in the 25-26 year category were patients who began the HPV vaccination series at age 26, but received subsequent vaccinations at age 27. Insurance categorization was based on billing data. “Public” insurance was defined as patients with Medicaid and/or Medicare billing, “private/other” as patients were those with private and/or military insurance, and “no insurance” patients were those were billed as “self pay” and/or “collection agency” without another identified billing source. For patients with >1 visit during the study period, race, age and insurance were defined using data from the first visit where an HPV vaccine dose was provided, or the first visit during the study period when if no vaccine doses were administered.
Visit-level variables included the medical specialty of the provider and the visit type. Because of the low numbers of medicine-pediatrics (MP) visits, these were combined with general internal medicine (GM) visits for most analyses. Visit types were categorized as either “preventive”, “problem-focused” or “immunization-only”, based on HEDIS criteria and our previous work.[3, 19]
2.4 Statistical Analyses
The proportion of women vaccinated was determined by dividing the number of individuals who received first, second or third HPV vaccine doses by the number of individuals who were eligible for those doses. The proportion of vaccine doses attributable to different patient or clinical factors was determined by dividing the number doses with a given characteristic by the total number doses, with separate analyses for the first, second and third doses in the series. The proportion of missed opportunity visits was determined by dividing the number of missed opportunity visits by the total number of visits where a vaccine dose could have been/was administered. Kaplan-Meier methods were used to determine the cumulative proportion of the eligible population receiving first, second or third doses over time. Individuals were censored if they reached the end of the study period or received a vaccine dose. Analyses were stratified by patient and/or clinic characteristics and chi-square tests assessed for associations. Logistic regression models assessed the factors independently associated with either HPV vaccine series initiation or series completion. Based on our a priori hypotheses, each of these models controlled for patient age, insurance type, race, visit type and medical specialty. Because of small cell sizes, pediatrics visits were dropped from both models, and immunization-only visits were dropped from the model assessing vaccine series initiation. Analyses were performed using a combination of SAS®, version 9.1 and STATA®, version 10 (STATA Corporation, College Station, TX 2008). A p-value of ≤ 0.05 was considered significant.
3.0 RESULTS
3.1 Sample Characteristics
During the 30 month study period, there were 11,535 young adult women who participated in 41, 672 outpatient visits in the selected clinics. Only 3% of the sample was uninsured. Very few visits occurred in pediatrics clinics. Sample characteristics are described in detail in Table 1.
Table 1.
Characteristics of sample by patient- and visit-level factors
| Characteristics | % |
|---|---|
|
Characteristics of patients (n=11,535) at time of first qualifying visit
| |
| Age | |
| 19-20 years | 22% |
| 21-22 years | 24% |
| 23-24 years | 26% |
| 25-26 years | 28% |
|
| |
| Race | |
| White | 76% |
| African American | 11% |
| Other/Not specified | 13% |
|
| |
| Insurance | |
| Private/other | 76% |
| Public | 21% |
| No Insurance | 3% |
|
| |
|
Characteristics of qualifying visits (n=41,672)
| |
| Medical Specialty | |
| Pediatrics | 1% |
| FM | 34% |
| Gynecology | 20% |
| GM/MP | 45% |
|
| |
| Visit Type | |
| Preventive Care | 20% |
| Problem-focused | 72% |
| Immunization-only | 7% |
3.2 Patient-level Disparities in the Uptake of First, Second and Third Doses of HPV Vaccine
As shown in Table 2, only 18% of women eligible to receive the HPV vaccine actually started the series. However, once the series had begun, a large proportion of women eligible for 2nd and/or 3rd doses received them. As age increased, the likelihood of initiating the vaccine series decreased significantly (Chi-square statistic for linear trend = 220.441, p<0.0001), but there was no association between age and receipt of 2nd or 3rd doses. Compared to other race groups, African Americans were slightly more likely to initiate the vaccine series, but significantly less likely to receive subsequent doses. Both series initiation and completion was lower among those with public insurance when compared to those with private insurance. However, those without insurance were the least likely to initiate or to complete the series (though the small sample size warrants caution when making statistical conclusions). When the analysis was repeated with individuals in the “no insurance” category eliminated, a similar pattern of results was found (data not shown).
Table 2.
Overall HPV Vaccination and Factors Associated with Receipt of First, Second and Third Doses of HPV Vaccine
|
Vaccination Among Cohort Overall | ||||||
|---|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 3 | ||||
|
# women eligible to receive dose |
11,535 | 2,047 | 1,413 | |||
|
| ||||||
|
% eligible women vaccinated |
18% | 74% | 69% | |||
|
| ||||||
|
Vaccination by Patient Characteristics
| ||||||
| Characteristic |
% eligible women vaccinated (n) |
p-value |
% eligible women vaccinated (n) |
p-value |
% eligible women vaccinated (n) |
p-
value |
|
| ||||||
| Age | ||||||
| 19-20 years | 25% (647) | 75% (472) | 72% (320) | |||
| 21-22 years | 21% (558) | <.0001 | 73% (401) | 0.47 | 67% (250) | .44 |
| 23-24 years | 18% (540) | 72% (384) | 69% (238) | |||
| 25-26 years | 11% (345) | 77% (261) | 70% (172) | |||
|
| ||||||
| Race | ||||||
| White | 18% (1564) | 76% (1163) | 72% (781) | |||
| African American | 21% (275) | <.0106 | 64% (171) | <.0003 | 54% (85) | <.0001 |
| Other/Not Specified | 18% (251) | 75% (184) | 68% (114) | |||
|
| ||||||
| Insurance † | ||||||
| Private | 20% (1751) | <.0001 | 77% (1318) | <.0001 | 72% (888) | <.0001 |
| Public | 13% (326) | 60% (190) | 52% (90) | |||
| No Insurance | 4% (13) | 77% (10) | 33% (2) | |||
Similar patterns of statistical significance were obtained when individuals with no insurance were removed from the analysis.
In the sample there were 10,465 women (91% of the sample) who had ≥6.5 months of eligibility in the study period from the date of their first qualifying visit. Theoretically, all of these women should have been able to initiate and complete the 3-dose HPV vaccination series according to the recommended schedule (doses at 0, 2 and 6 months plus an extra 2 weeks for scheduling variations). However, only 1% (n=60) got all 3 doses of the HPV vaccine within this time frame. When eligibility was further narrowed to those with ≥12 months of eligibility (79% of the sample) the series was completed by only 8% within a year from the date of their first qualifying visit, and only 10% when the entire time study period was included in the analysis.
3.3 Clinic-level Disparities in Vaccine Utilization
As shown in Table 3, patients utilized all three visit types to initiate the vaccine series, but the majority of 2nd and 3rd doses were provided at immunization-only appointments. There were statistically significant differences in visit types used when comparing 1st to 2nd and 1st to 3rd doses, but not when comparing 2nd to 3rd doses (data not shown). FM providers were more likely than other medical specialties to both initiate and provide subsequent doses at problem-focused visits. However, immunization-only appointments were still the most common type of visit used for 2nd and 3rd doses across all medical specialties. A similar pattern of results was found when the small proportion of pediatrics visits was eliminated from the analysis (data not shown).
Table 3.
Proportion of first, second and third doses of HPV vaccine administered at each visit type, overall and subdivided by medical specialty.
| Vaccine Dose |
1* | 2* | 3* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit Type | Preventive | Problem | Imm. Only |
Preventive | Problem | Imm. Only |
Preventive | Problem | Imm. Only |
| FM | 35% | 52% | 13% | 12% | 32% | 55% | 10% | 28% | 62% |
| GM/MP | 51% | 27% | 22% | 7% | 14% | 79% | 7% | 14% | 79% |
| Gynecology | 47% | 25% | 28% | 7% | 4% | 89% | 6% | 12% | 82% |
| Pediatrics | 36% | 28% | 36% | 2% | 21% | 77% | 10% | 13% | 77% |
|
| |||||||||
|
Overall Average Across Medical Specialties |
44% | 36% | 20% | 9% | 19% | 72% | 8% | 18% | 74% |
P-value for within-dose comparisons is <0.0001. A similar result is found with Pediatrics visits are eliminated.
In a multivariable model assessing correlates of series initiation (Table 4), increasing age, public insurance on non-FM specialty were all associated with a decreased odds of this outcome and African American race was associated with an increased odds of this outcome. In a second model that assessed correlates of series completion among those who had received the first dose in the series and had at least one year of time available in the study to receive subsequent doses, African American race was associated with a decreased odds of completing the series when compared to other groups, as was public insurance. However, series completion was not associated with age or medical specialty.
Table 4.
Independent predictors of HPV vaccine series initiation and completion*.
| Characteristic | HPV Vaccine Series Initiation | HPV Vaccine Series Completion | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | ||||||
| 19-20 | Ref. | - | - | Ref. | - | - |
| 21-22 | 0.81 | 0.70-0.94 | 0.006 | 0.93 | 0.71-1.23 | 0.63 |
| 23-24 | 0.62 | 0.54-0.72 | <0.0001 | 0.96 | 0.72-1.26 | 0.74 |
| 25-26 | 0.35 | 0.30-0.41 | <0.0001 | 1.17 | 0.86-1.59 | 0.32 |
|
| ||||||
| Race | ||||||
| White | Ref. | - | - | Ref. | - | - |
| African Am. | 1.43 | 1.21-1.71 | <0.0001 | 0.48 | 0.33-0.69 | <0.0001 |
| Other | 1.04 | .087-1.23 | 0.70 | 0.99 | 0.72-1.39 | 0.99 |
|
| ||||||
| Insurance | ||||||
| Private | Ref. | - | - | Ref. | - | - |
| Public | 0.67 | 0.57-0.78 | <0.0001 | 0.48 | 0.34-0.68 | 0.0004 |
|
| ||||||
|
Medical Specialty |
||||||
| FM | Ref. | - | - | Ref. | - | - |
| GM/MP | 0.48 | 0.42-0.54 | <0.0001 | 1.10 | 0.86-1.40 | 0.45 |
| Gyn | 0.37 | 0.31-0.43 | <0.0001 | 0.95 | 0.72-1.26 | 0.72 |
|
| ||||||
| Visit Type | ||||||
| Preventive | Ref. | - | - | Ref. | - | - |
| Problem | 0.36 | 0.32-0.40 | <0.0001 | 1.06 | 0.83-1.34 | 0.66 |
| Imm Only | Not included | N.A. | N.A. | 1.24 | 0.95-1.63 | 0.12 |
Each model is controlled for the variables listed in the first column. In both models, individuals in the “No insurance” and “Pediatrics” categories were removed because of small cell sizes and to improve model fit. In addition, in the series initiation model the “Immunization-only” category was also removed for this reason.
Note: Series completion was assessed among the subset of women who had gotten the first dose of vaccine and had at least 12 months in the study period in which to receive the subsequent two doses in the series.
3.4 Patterns of Utilization Over Time
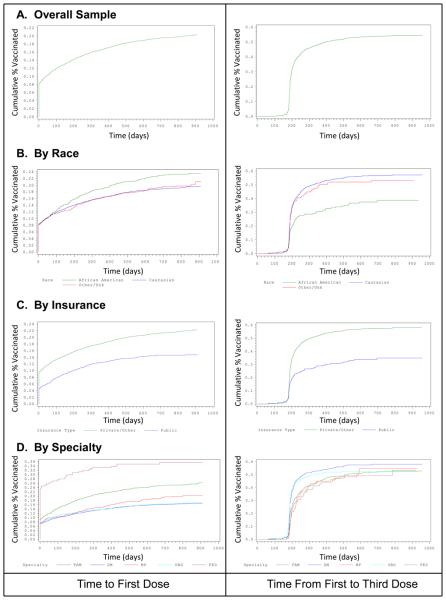
Using Kaplan-Meier techniques, we examined the patterns of vaccine utilization over time among the sample overall and disaggregated by various characteristics. As shown in Figure 1A, the first visit captured in our analysis included initiating the HPV vaccine series for approximately 8% of women. As shown in Figure 1B, most women who completed the vaccine series did so within 180-300 days after the first dose. After >400 days from the first dose the proportion of eligible women who completed the series was negligible.
Figure 1.
Cumulative proportion of women vaccinated overall, and subdivided by race, insurance type and medical specialty of appointment.
Disparities in vaccine series initiation by race and insurance type (Figure 1, Panels B and C, respectively) appeared to worsen over time (demonstrated by an increasing distance between lines over time). However, the “level” of disparities by race and insurance appeared relatively stable over time for series completion (lines run generally parallel over time). Disparities in series initiation by medical specialty appeared to be sustained, but relatively fixed, over time (Figure 1, panel D). Specialty-based disparities were minimized by the time of series completion.
3.5 Missed Opportunities for Vaccination
The proportion of visits that were “missed opportunities” for vaccination (any eligible dose) was high (88% for all visit types and medical specialties combined). There were significant differences in the proportion of missed opportunity visits by medical specialty (FM and gynecology −88% each, pediatrics − 73%, GM/MP − 91%; p<0.0001). The highest proportion of missed opportunities occurred at problem-focused visits (91-98%, depending on medical specialty). However, even among preventive care visits, where vaccines are known to be preferentially provided,[20-22] missed opportunities were still common (61-92% of visits, depending on medical specialty).
4.0 DISCUSSION
Two to five months following recommendations for HPV vaccination of women, the CDC reported that only 10% of 18-26 year olds had initiated the vaccine series.[14] Results of our study, which assessed HPV vaccination among 19-26 year old women seen in a university-based primary care clinic system 2 ½ years after the vaccine was available, suggest that HPV vaccine uptake among young adult women continues to be low. Only 18% of these women had initiated the 3-dose series and only 10% of the cohort with ≥1 year of study eligibility had received all three vaccine doses within the 30 month period. HPV vaccination use among adult women is notably lower than adolescents seen in the same clinical system. In a previous analysis that assessed within the same clinical setting HPV vaccination among adolescent female 15 months after the vaccine was available we found that 28% of 11-17 year olds had initiated the vaccine series and 15% of the cohort with ≥1 year of study eligibility had received all three vaccine doses.[3]
Among adolescents, national and regional assessments have demonstrated disparities in HPV vaccine utilization by race, age, insurance and poverty level.[3, 11] Similar findings have not been confirmed nationally for adult women, but have been supported by regional analyses [4, 5, 14, 15] and are also supported by our results. Our study is one of the first to demonstrate disparities in adult HPV vaccine uptake by medical specialty, and to describe differences in these disparities by different doses in the series. Our longitudinal analyses also present evidence to suggest that the race- and insurance-related disparities in HPV vaccine use are worsening over time.
In our study African American race was associated with increased vaccine series initiation but decreased receipt of second and third doses when compared to whites and those of other/unreported race. These results are similar to our previous study of adolescent HPV vaccine utilization within the same university-based clinical system and to other studies.[3, 4, 15] The consistency across the age spectrum of lower series completion among African Americans is concerning given that minority women are at increased risk of HPV-related morbidity and mortality.[23, 24] Lower utilization of both Pap testing and HPV vaccination among minority women suggests that cultural mediators may create barriers to participation in these important preventive activities. It also raises the broader question of whether HPV vaccination efforts as they currently stand will have any impact on population-level rates of cervical cancer in the U.S. It appears that additional work is needed to identify culturally relevant educational messages and interventions that can improve compliance with both HPV vaccination and Pap smear screening among this population.
Disparities in vaccine uptake among young adult women with regard to age and insurance type were also identified in this study. Younger age was associated with an increased odds of starting the vaccine series but age had no association with completing the series once it had begun. One explanation for this age effect is women may believe it is “too late” for them to benefit from HPV vaccination as they age and become more sexually experienced.[25] In our previous study of adolescents[3] a reverse association with age was found – younger adolescents were less likely to initiate the series when compared to older adolescents (with no associations between age and second/third doses). Taken together these results suggest that age primarily affects the decision of whether or not to vaccinate, not the ability or willingness to comply with subsequent doses once the vaccine series is begun. Interventions that target series initiation may therefore be an effective “starting point” to improve HPV vaccine uptake. However, it is important to note that the women completing the vaccination series in this study deviated significantly from the ideal schedule used in HPV vaccine efficacy trials.[26] Theoretically, this deviation could reduce the efficacy of the vaccine, though this hypothesis remains to be proven.
For women >18 years old, it is important to consider any age effects on vaccine uptake in the context of insurance status. Individuals >18 years old are not eligible for the Vaccines For Children (VFC) program, a federally funded program that provides free vaccines to millions of under or un-insured individuals ≤18 years of age.[27] In addition, between the ages of 19-26 years coverage under parents' insurance plans also declines, often without replacement by employer-sponsored individual health coverage.[28, 29] These age-based differences in insurance status could explain, at least in part, the finding of lower vaccine series initiation with advancing age among young adult females in our study. However, only a small proportion of women in our study were without insurance, and the majority of private and public payers were covering the HPV vaccine for adult women at the time of the study.
This study also demonstrated disparities in HPV vaccine use by medical specialty. FM providers were substantially more likely to initiate the vaccine series, and to use “problem-focused” visits to administer vaccine doses than the other medical specialties. In addition, there were specialty-based differences in series initiation but not series completion – a finding that has also been described for HPV vaccination among adolescents.[3] One explanation for this finding is that, because they care for children (who require many vaccines), FM providers may be more adept or comfortable than gynecologists or GM providers at convincing adult patients to receive the HPV vaccine. Anecdotal reports from our institution suggest that gynecologists might have a particularly difficult time in initiating the series because some patients believe their insurance company will deny coverage of the vaccine since the gynecologist is not designated as their “primary care provider.” The lack of specialty-based differences in administration of 2nd and 3rd doses again highlights the importance of vaccine series initiation. Importantly, “missed opportunities” for providing vaccines were commonplace across all medical specialties.
4.1 Limitations
Thus study's results should be interpreted in light of several important limitations. First, the study population was limited to one university-based health system located in Michigan. Though this population is both economically and racially diverse, results may not be generalizable to other geographic locales, to patients seen in private practice or public health settings, or to non-medical-based populations. Furthermore, Latino ethnicity, a variable that has been shown to be associated with differences in vaccination levels for childhood vaccines[30], could not be assessed in our analysis. In other studies of HPV vaccination, Latina ethnicity has been associated with increased series initiation, but decreased series completion for HPV vaccines.[11] Second, the sample included very few individuals without health insurance. Young adults have higher rates of un-insurance when compared to other ages[28, 29] and the high out-of-pocket costs of HPV vaccines likely prevent many without insurance from being vaccinated. Thus at a population level, HPV vaccination uptake among 19-26 year old females is expected to be lower than that found in this study. Third, the analysis did not capture doses of vaccine provided outside of the university setting, though our results appear generally consistent with other studies on HPV vaccine uptake among adults.[14] Fourth, we were unable to discern reasons why vaccination did not occur among eligible patients. Some patients may have been offered the vaccine and refused. In addition, inconsistency in provider recommendation could have caused variability in uptake between different clinical settings or among different patient populations.
4.2 Conclusions
Clinicians caring for women and men eligible for the HPV vaccine need to explore options to promote the initiation of the vaccine at every encounter with patients. Once the vaccination series is started, then systems need to be tested which achieve completion of the series in the ideal time intervals. In the era of electronic medical records, systematic prompts to clinicians, support staff, and patients could significantly improve this process.
ACKNOWLEDGEMENTS
This work was funded by the University of Michigan Bridging Interdisciplinary Research Careers in Women's Health (BIRCWH) award (5 K12 HD001438-07).
Funding Statement:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.U.S. Food and Drug Administration Product Approval Information - Licensing Action, Gardasil. 2006. 2006 August 1; Available from: http://www.fda.gov/cber/products/hpvmer060806qa.htm.
- 2.Markowitz LE, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 3.Dempsey A, Cohn L, Dalton VA, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a unveristy-based health system. Vaccine. 2010;28(4):989–995. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao C, et al. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin Proc. 2009;84(10):864–70. doi: 10.4065/84.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubrand TP, et al. Factors associated with completion of the human papillomavirus vaccine series. Clin Pediatr (Phila) 2009;48(9):966–969. doi: 10.1177/0009922809337534. [DOI] [PubMed] [Google Scholar]
- 6.Kahn JA, et al. Rates of Human Papillomavirus Vaccination, Attitudes About Vaccination, and Human Papillomavirus Prevalence in Young Women. Obstet Gynecol. 2008;111(5):1103–1110. doi: 10.1097/AOG.0b013e31817051fa. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal SL, et al. Uptake of HPV vaccine: demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008;43(3):239–45. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health. 2009;45(5):453–62. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Conroy K, et al. Human Papillomavirus Vaccine Uptake, Predictors of Vaccination, and Self-Reported Barriers to Vaccination. J Womens Health (Larchmt) 2009;18(10):1679–1686. doi: 10.1089/jwh.2008.1329. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525–33. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National, state, and local area vaccination coverage among adolescents aged 13-17 years --- United States, 2009 MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–23. [PubMed] [Google Scholar]
- 12.Dunne EF, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 13.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med, 1997. 102(5A):3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, et al. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, National Immunization Survey-Adult 2007. Prev Med. 2009;48(5):426–31. doi: 10.1016/j.ypmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal SL, et al. Predictors of HPV vaccine uptake among women aged 19-26: Importance of a physician's recommendation. Vaccine. 2010 doi: 10.1016/j.vaccine.2009.12.063. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Licht AS, et al. Is use of the human papillomavirus vaccine among female college students related to human papillomavirus knowledge and risk perception? Sex Transm Infect. 2010;86(1):74–8. doi: 10.1136/sti.2009.037705. [DOI] [PubMed] [Google Scholar]
- 17.Roberts ME, et al. Mother-daughter communication and human papillomavirus vaccine uptake by college students. Pediatrics. 2010;125(5):982–9. doi: 10.1542/peds.2009-2888. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services . Healthy People 2010. 2nd ed U.S. Government Printing Office; Washington, D.C.: 2000. [Google Scholar]
- 19.HEDIS: Healthcare Effectiveness Data and Information Set . Technical Specifications for Physician Measurement. Vol. 2. National Committee for Quality Assurance; Washington, DC 2005: 2009. [Google Scholar]
- 20.Halpern-Felsher BL, et al. Preventive services in a health maintenance organization: how well do pediatricians screen and educate adolescent patients? Arch Pediatr Adolesc Med. 2000;154(2):173–9. doi: 10.1001/archpedi.154.2.173. [DOI] [PubMed] [Google Scholar]
- 21.Kottke TE, et al. Delivery rates for preventive services in 44 midwestern clinics. Mayo Clin Proc. 1997;72(6):515–23. doi: 10.4065/72.6.515. [DOI] [PubMed] [Google Scholar]
- 22.Schaffer SJ, et al. Adolescent immunization practices: a national survey of US physicians. Arch Pediatr Adolesc Med. 2001;155(5):566–71. doi: 10.1001/archpedi.155.5.566. [DOI] [PubMed] [Google Scholar]
- 23.McDougall JA, et al. Racial and ethnic disparities in cervical cancer incidence rates in the United States, 1992-2003. Cancer Causes Control. 2007;18(10):1175–86. doi: 10.1007/s10552-007-9056-y. [DOI] [PubMed] [Google Scholar]
- 24.Watson M, Saraiya M, Wu X. Update of HPV-Associated Female Genital Cancers in the United States, 1999-2004. J Womens Health (Larchmt) 2009;18(11):1731–8. doi: 10.1089/jwh.2009.1570. [DOI] [PubMed] [Google Scholar]
- 25.Ferris DG, et al. HPV Vaccine Acceptance Among Mid-Adult Women. J Am Board Fam Med. 2008;21(1):31–7. doi: 10.3122/jabfm.2008.01.070103. [DOI] [PubMed] [Google Scholar]
- 26.Villa LL, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee GM, et al. Gaps in vaccine financing for underinsured children in the United States. JAMA. 2007;298(6):638–43. doi: 10.1001/jama.298.6.638. [DOI] [PubMed] [Google Scholar]
- 28.Adams SH, et al. Health insurance across vulnerable ages: patterns and disparities from adolescence to the early 30s. Pediatrics. 2007;119(5):e1033–9. doi: 10.1542/peds.2006-1730. [DOI] [PubMed] [Google Scholar]
- 29.Park MJ, et al. The health status of young adults in the United States. J Adolesc Health. 2006;39(3):305–17. doi: 10.1016/j.jadohealth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Wooten KG, Luman ET, Barker LE. Socioeconomic factors and persistent racial disparities in childhood vaccination. Am J Health Behav. 2007;31(4):434–45. doi: 10.5555/ajhb.2007.31.4.434. [DOI] [PubMed] [Google Scholar]