Summary
Apicomplexa are unicellular eukaryotic pathogens and cause important diseases including malaria and toxoplasmosis. The discovery of an algal endosymbiont, the apicoplast, provides exciting avenues to develop urgently needed new drugs. However, the physiological function of the apicoplast and its integration into the parasite metabolism remain poorly understood and at times controversial. Using a new approach to genetic engineering in Toxoplasma we show here that the apicoplast phosphate translocator (TgAPT) is an essential link between endosymbiont and cytoplasmic metabolism. Our genetic analyses show that TgAPT is required for fatty acid synthesis in the apicoplast, but indicate also that this might not be its most critical function. Detailed biochemical analyses demonstrate that this transporter has unique properties allowing it to supply the apicoplast with carbon, and indirectly with energy and reduction power. Ablation of the transporter results in remarkably strong and fast inhibition of parasite growth underscoring its merit as a target.
Keywords: plastid, Apicomplexa, fatty acid synthesis, transporter, recombineering
Introduction
Plastids are derived from the endosymbiotic integration of a free-living cyanobacterium into a single-celled protist (Gould et al., 2008). This single primary endosymbiosis led to the development of three major autotrophic lineages: the glaucophytes, the red algae and the green algae (and their descendants, the higher plants). Additional lineages are derived from at least three secondary endosymbiotic events, i.e the uptake and integration of a green alga giving rise to the Euglenophyta and the Chlorachniophyta, and the engulfment of a red alga leading to the Alveolata, Heterokontophyta and other groups (Gould et al., 2008).
While primary plastids are enclosed by two envelope membranes, which are derived from the inner and outer membranes of the gram-negative cyanobacterium (Cavalier-Smith, 2000), secondary plastids are surrounded by three or four membranes. Here, the two innermost membranes are thought to represent the two plastid membranes of the green or red algae, the third membrane is a remnant of the red or green algae plasma membrane and the outermost membrane is derived from the host’s endomembrane system. The discovery of a vestigial plastid surrounded by four membranes in Apicomplexa, the apicoplast (Kohler et al., 1997; McFadden et al., 1996), suggests that this group of protozoan parasites likely evolved from a photosynthetic ancestor (Moore et al., 2008). Growing evidence supports the notion that the ancestor of the apicoplast was a red alga, however, some studies have suggested green algal ancestry (Gould et al., 2008). Primary and secondary plastids are the home of photosynthesis, but also perform a range of additional essential biosynthetic functions. The exact physiological functions of apicoplasts, in contrast to plant plastids, are not fully understood, however, it is now well established that the apicoplast is indispensable for parasite survival (Fichera and Roos, 1997; Jomaa et al., 1999; van Dooren et al., 2009). The phylum Apicomplexa contains numerous important pathogens including the causative agents of malaria and toxoplasmosis. As the human host lacks plastids, the apicoplast has become a prime target for the development of new anti-parasitic drugs (Fichera and Roos, 1997; Goodman and McFadden, 2007; Jomaa et al., 1999). Mining the genome sequences of Apicomplexa and subsequent experimental studies have produced three major candidate functions for the apicoplast, namely the synthesis of fatty acids, isoprenoids and heme (Ralph et al., 2004).
Here we focus on how the metabolism of the apicoplast is linked with the metabolism of the parasite cytoplasm. It has been proposed that the integration of transporters into plastid membranes was an early important step in endosymbiosis allowing the host cell to directly profit from its endosymbiont (Cavalier-Smith, 2000; Weber et al., 2006). In higher plants, carbon skeletons from photosynthesis are exported in the form of triose phosphates by the triose phosphate/ phosphate translocator (TPT) (Flügge et al., 2003). The TPTs represent one member of a larger family of plastid phosphate translocators (pPTs) (Knappe et al., 2003). All pPTs function as antiport systems using inorganic phosphate and phosphorylated C3, C5 or C6 compounds as counter substrates. The TPT is involved in the export of carbon, in contrast, the other subfamilies catalyze import of metabolites into plastids, namely phosphoenol pyruvate (PPT; (Fischer et al., 1997)), glucose-6-phosphate (GPT, (Kammerer et al., 1998)) and xylulose-5-phophate (Eicks et al., 2002). In the apicoplast this diversity appears greatly reduced. Species of the genus Plasmodium possess two different pPTs, only a single pPT was identified in T. gondii (TgAPT) (Fleige et al., 2007; Karnataki et al., 2007; Mullin et al., 2006). While the Toxoplasma APT appears to localize to multiple membranes, the Plasmodium transporters are believed to differentially localize to the outer and inner membranes respectively. The physiological functions of the apicoplast PTs and their role in apicoplast metabolism remain unknown.
Here we show in Toxoplasma that loss of the APT results in the rapid death of the parasite. Through biochemical and genetic analyses we demonstrate that APT combines the substrate specificity of plant TPTs and PPTs and is thus able to deliver carbon skeletons for at least two important metabolic pathways of apicoplasts. In addition, this transporter likely plays a major role in delivering redox equivalents and ATP to this organelle.
RESULTS
Construction of targeting cosmids by recombineering
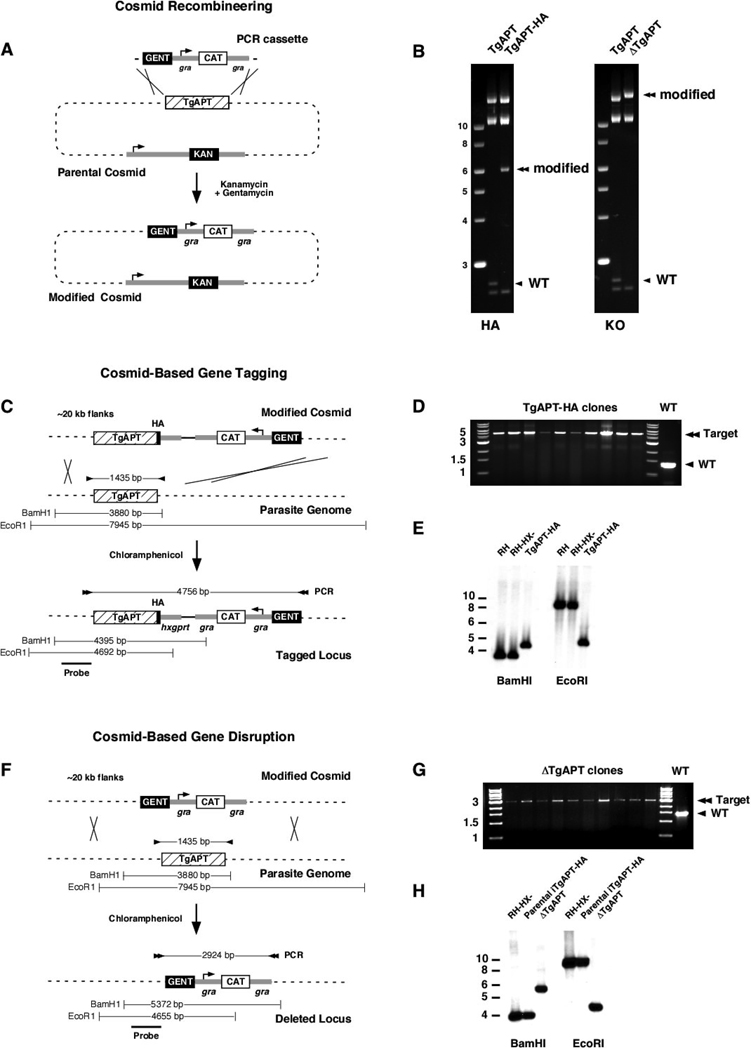
We thought to genetically test the function and importance of the apicoplast phosphate translocator in T. gondii. A number of approaches are available to modify the parasite genome, however the efficiency of these procedures is often low, requiring the screening of large numbers of transgenic clones. The main reason for this is the high level of non-homologous versus homologous recombination observed in T. gondii (see (Striepen and Soldati, 2007) for a detailed discussion). This can be overcome by employing positive/ negative selection using two independent markers (Mazumdar et al., 2006) or by using mutant parasite strains that lack the end -joining repair mechanism (Fox et al., 2009; Huynh and Carruthers, 2009). However, thus far these mutants are not suitable for the construction of conditional gene deletions. We wondered if using large flanking sequences to guide recombination would increase the frequency of homologous replacement and thus negate the need for multiple markers or lack of end-joining repair. An arrayed and end -sequenced genome-wide set of cosmids now provides ready access to large insert clones of T. gondii genomic DNA (Gubbels et al., 2008), but the large size of cosmids (~45,000 bp) precludes standard restriction mediated cloning of targeting constructs. We have therefore adapted recombineering (Datsenko and Wanner, 2000; Lee et al., 2001) to modify cosmids into parasite targeting vectors. This can be accomplished in a single cloning step and the strategy is outlined in Fig. 1. We engineered a series of modification cassettes that allow the construction of epitope tags and gene deletions. These cassettes contain a gentamycin resistance marker derived from a bacterial transposon (Poteete et al., 2006) for selection in bacteria and chloramphenicol or phleomycin markers for the subsequent selection in T. gondii. We selected a suitable cosmid covering the TgAPT gene (PSBYL85), introduced this into the EL250 strain of E. coli and transiently induced the recombination machinery by heat shock. We then PCR amplified a targeting cassette using primers containing 50 bp of gene specific flanking sequence to guide recombination into the desired site, and isolated recombinants by double selection using gentamycin and kanamycin. Fig. 1 B shows restriction mapping of cosmid PSBYL85 before (TgAPT) and after recombination of a c-terminal HA-eptiope tag (TgAPT-HA) or a deletion of the TgAPT gene (ΔTgAPT), respectively. Correct placement of the cassette was also confirmed by sequencing cosmids using primers flanking the insertion sites.
Figure 1. High frequency targeting of the parasite genome using modified cosmid clones.
(A) Schematic representation of cosmid engineering using a gene replacement cassette as example, homologous recombination is guided by a 50 bp sequence added to the ends by PCR. (B) RsrII Restriction mapping of cosmid PSBYL85 prior (TgAPT, predicted restriction fragments are: 18,280, 11,852, 11,146, 2,635, and 2,490 bp), and after recombineering with cassette pHcG (TgAPT-HA, 18280, 11852,11146, 6028, and 2490 bp) and pICG (ΔTgAPT 22,407, 11,852, 11,146, and 2,490), respectively. Diagnostic restriction fragments for the parental cosmid (arrowhead) and modified cosmid (double arrowhead) are highlighted. Schematic representations of homologous recombination events in the T. gondii genome for cosmid-based genome tagging (C) or gene disruption (F). Diagnostic PCR products and restriction fragments for the native and modified TgAPT locus are highlighted. (D and G) PCR analysis of clones derived from a stable chloramphenicol resistant population after transfection with modified cosmid DNA. RH strain is shown as wild type (WT) control. (E and H) Southern blot analysis of parasite mutant clones along with RH, RH-HXGPRT− and parental iTgAPT-HA strain (TATi strain with TgAPT minigene under control of the tet-regulatable promoter). Blots were probed with a 600 bp fragment of the TgAPT 5’ untranslated region as indicated. Maps shown in this figure are not to scale.
Transfection of T. gondii with modified cosmids results in highly efficient gene targeting
We next tested whether modified cosmids will target the cassette into the appropriate locus of the parasite genome. Fig. 1C shows a schematic representation of the crossover event that will result in the insertion of an HA epitope tag at the 3’ end of the TgAPT gene. Cosmid DNA (TgAPT-HA) was transfected into RH-HX- strain tachyzoites by electroporation, and parasites were cultured in the presence of chloramphenicol. Clonal lines were established and tested for gene targeting by PCR using primers flanking the TgAPT coding region. As shown in Fig. 1D, for all clones tested the 4756 bp product expected for successful replacement was obtained, while wild type controls produced a 1435 bp amplicon. Southern blot analysis using the 5’ untranslated region of the TgAPT gene as probe further supported correct insertion of the cassette. We also tested the knockin mutant at the protein level and performed immunofluorescence and Western blot analyses using an antibody specific to the HA tag (supplementary Fig. S1). We found labeling of the apicoplast and a single protein band of the appropriate size that remains unprocessed as described previously (Fleige et al., 2007; Karnataki et al., 2007) consistent with correct genomic placement of the tag.
TgAPT is required for the activity of the apicoplast fatty acid synthesis (FASII) pathway
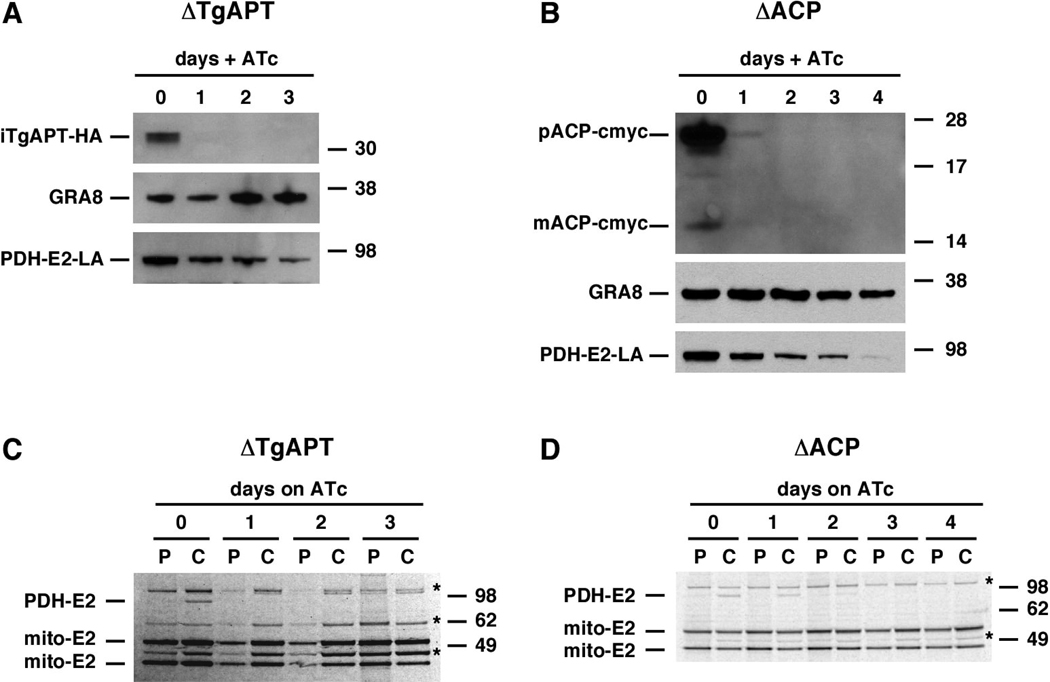
We next engineered a conditional TgAPT mutant. We introduced an HA-tagged minigene version of the TgAPT gene under control of a tetracycline-regulatable promoter into the T. gondii transactivator strain (Meissner et al., 2002). The resulting parasite strain was then transfected with a cosmid in which we had replaced the TgAPT coding sequence with the CAT gene (Fig. 1 F), and stable transformants were selected on chloramphenicol. PCR analysis of the resulting transgenic lines showed that 16 out of 17 clones carried the deletion, and homologous recombination was confirmed by Southern blot (Fig. 1 G, H). We cultured this mutant in 0.5 µg/ ml anhydrotetracycline (ATc) and measured protein level by Western blot using an anti-HA antibody found that TgAPT levels were severely depleted after one day of treatment (Fig. 2A). This swift knockdown is similar to that observed in the previously isolated ACP mutant (Mazumdar et al., 2006), that is shown for comparison (Fig. 2B, note that these are two independent antibody reagents which might have slightly different limits of detection). We hypothesized that TgAPT might import the presumptive precursor molecule for apicoplast fatty acid synthesis, phosphoenol pyruvate (Mazumdar and Striepen, 2007; Ralph et al., 2004), into the apicolast. Currently there is no suitable assay available to directly measure the activity of apicoplast FASII in T. gondii. However, in previous work we have shown that the activity of the FASII pathway can be measured indirectly by following the lipoylation of the apicoplast pyruvate dehydrogenase E2 subunit (PDH-E2) using lipoylation specific antibody reagents (Mazumdar et al., 2006). PDH-E2 lipoylation depends on two apicoplast resident enzymes, LipA and LipB, and uses FASII derived octanyl-ACP as substrate (Crawford et al., 2006; Cronan et al., 2005; Mazumdar et al., 2006; Thomsen-Zieger et al., 2003). Western blot analysis of the TgAPT mutant grown in the presence of ATc shows a marked drop in the steady state level of lipoylated PDH-E2 over the course of the treatment, comparable to that seen in ACP mutant controls (Fig. 2 A and B). Genetic interference with apicoplast metabolism or protein import has been shown to result in biogenesis defects and subsequent loss of the organelle (Agrawal et al., 2009; Mazumdar et al., 2006; van Dooren et al., 2008). To ensure that the loss of PDH lipoylation observed here was not the trivial consequence of organelle loss we quantified apicoplasts using an imaging assay. As shown in supplementary Fig. S3 no significant organelle loss is detectable over the three days of ATc treatment used in all other experiments.
Figure 2. Genetic ablation of TgAPT results in loss of apicoplast FASII dependent PDH(E2) lipoylation.
(A and B) Western blot analyses of TgAPT and ACP mutant after 0 to 4 days of ATc treatment (TgAPT mutant could only be analyzed for 3 days due to parasite death). The product of the regulated minigene is detectable in the absence of ATc (using an antibody against the HA epitope (A) or ACP (B)) and shows a severe reduction within 24 hours. Extracts of equal numbers of parasites (5 × 106) were loaded and GRA8 serves as loading control. We also probed steady state levels of lipoylated PDH(E2) with a lipoylation specific antibody and found that levels are reduced in both mutants in response to ATc treatment. (C and D) Pulse-chase analysis of PDH(E2) lipoylation. Levels of newly synthesized lipoylated PDH(E2) are severely reduced in the TgAPT and ACP mutants after one or two days, respectively. Lipoylation of mitochondrial E2 enzyme subunits (mito-E2) is not affected in either mutant. Human PDH and mitochondrial E2 subunits (a contamination from the human host cell (Crawford et al., 2006; Mazumdar et al., 2006; van Dooren et al., 2008)) are marked by an asterisks. This experiment uses an antibody that recognizes all lipoylated proteins (we demonstrate that the 92 kDa lipoylated protein represents the apicoplast PDH-E2 through immunoprecipitation experiments using a specific antibody, see supplementary figure S2). P, pulse, C, chase.
To measure the rate of synthesis of lipoylated PDH-E2 in response to ATc we used a highly sensitive pulse-chase assay (van Dooren et al., 2008). Parasites were labeled with 35Samino acids for one hour (pulse) followed by washout and incubation in label-free media for three hours (chase). Lipoylated proteins were isolated from parasite lysates by immunoprecipitation and analyzed by gel electrophoresis and autoradiography (Fig. 2 C and D). In the absence of ATc PDH-E2 lipoylated during the chase period can be clearly detected in the TgAPT and ACP mutants. ATc knockdown results in loss of de novo PDH lipoylation in the TgAPT mutant after one day, and in the ACP mutant after two days, respectively. Note that ATc treatment of wild type parasites has no effect on lipoylation (Agrawal et al., 2009; Mazumdar et al., 2006; van Dooren et al., 2008). We conclude that metabolites transported by TgAPT are essential for PDH-E2 lipoylation and hypothesize that this is due to the lack of the required precursor a product of the apicoplast FASII pathway.
Loss of TgAPT results in rapid death of parasites
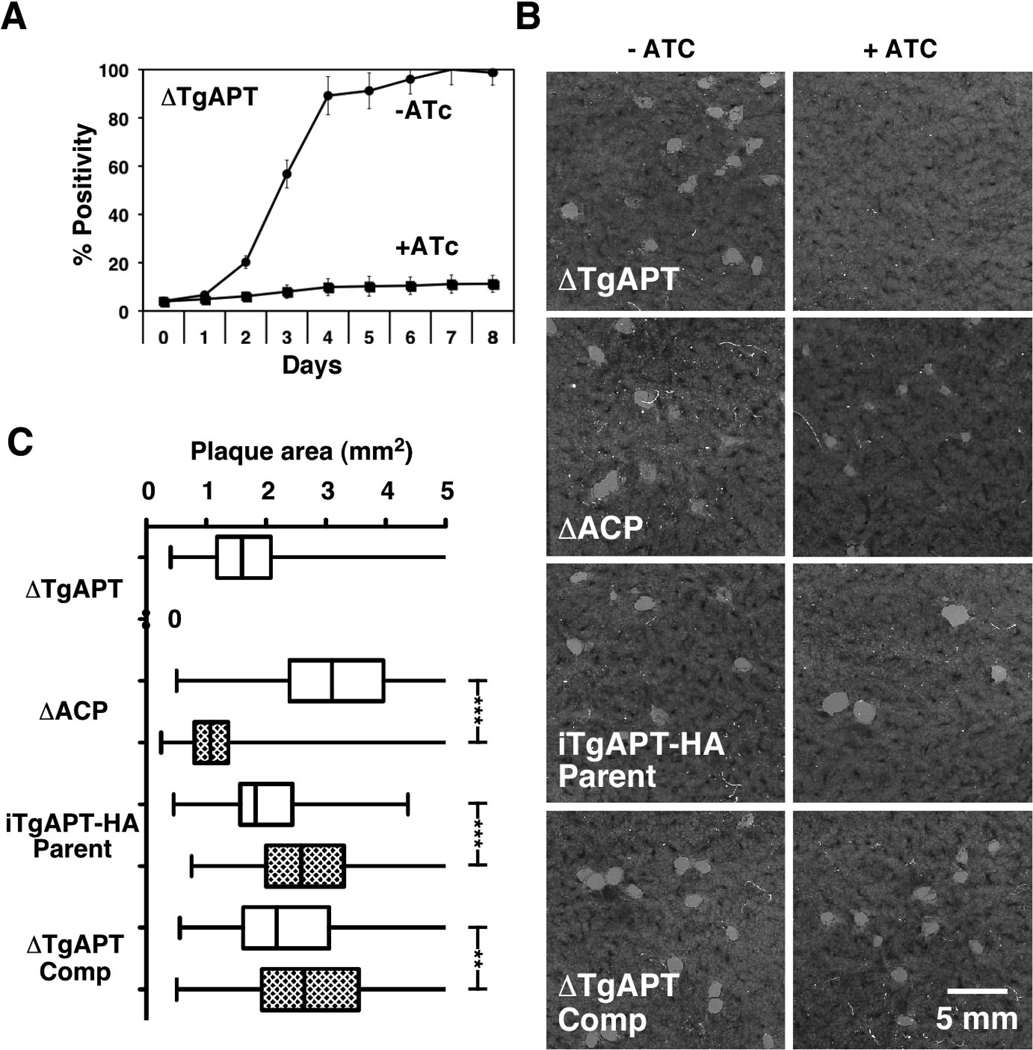
Apicoplast FASII is essential for the growth of T. gondii (Mazumdar et al., 2006). One would therefore expect the transporter supplying FASII with substrate to be equally essential. To test this we introduced a red fluorescent protein gene into the mutant strain and recorded fluorescence intensity as a measure of growth. As shown in Fig. 3A, ATc treatment results in negligible growth of the mutant strain when compared to untreated control, consistent with a block of the essential FASII system. Furthermore, this growth impact appeared remarkably fast when compared to the previously engineered apicoplast FASII mutant (Mazumdar et al., 2006). We directly compared the TgAPT with the ACP mutant in parallel plaque assays (Fig. 3 B and C). As previously reported, knockdown of ACP results in marked reduction of plaque size. In contrast, in the TgAPT mutant we did not observe any plaque formation. We noted that the TgAPT mutant shows a reduction in growth already in the absence of ATc. We wondered if this might be due to secondary mutations or due to lower activity of the ectopic transporter (e.g due to the C-terminal epitope tag, see below, or inappropriate expression level or timing from the tet-regulated promoter). To test this we introduced a TgAPT minigene carrying an N -terminal Ty-1 tag under control of a constitutive promoter into the mutant background using a BLE marker and phleomycin selection. This strain shows restored growth arguing against unspecific secondary mutations (Fig. 3 B and C). In fact, in both the parental and the complemented strain ATc treatment results in improved growth suggesting that the C but not the N -terminally tagged transporter exerts a slight dominant negative effect (pPTs usually are homo-dimers; additional fluorescence based growth data produce similar results and are provided in the supplementary figure S4). To exclude that the fast death of the conditional APT mutant was due to crippling resulting from a C-terminal regulated copy of APT we reengineered this mutant starting with an N -terminal regulated ectopic APT gene. As shown in supplementary figure S5 this mutant dies as fast as the previous mutant suggesting that death is a direct consequence of loss of APT.
Figure 3. Parasites lacking TgAPT show a more pronounced growth defect than parasites lacking apicoplast FASII.
(A) Fluorescence growth assay of the TgAPT mutant in the presence and absence of ATc (a dTomato-RFP reporter was introduced by transfection and stable transformats were isolated by flow cytometry as previously described (van Dooren et al., 2008). Each data point represents the mean of six wells and the error bar gives the standard deviation). (B) Confluent host cell cultures were infected with 400 tachyzoites of various parasite strains (as indicated) and incubated for 10 days in the absence and presence of ATc. Cells were fixed and stained as described (Striepen and Soldati, 2007). Assays were performed in duplicate flasks and plaque areas were measured by image analysis after scanning. Areas are shown as whisker plots (C) providing the mean and 1.5 times the interquartile distance as the box. Statistical significance was evaluated using the unpaired t-test. No ATc white boxes, plus ATc, hatched boxes. No plaques were observed in the ATc treated APT mutant.
We next asked if the TgAPT mutants simply grow extremely slow or if they suffer more pronounced defects that result in a swift death of the parasite. A frequently used measure of cellular viability is the energetic state of the mitochondrion, and more specifically its membrane potential. We measured parasite mitochondrial membrane potential using 5,5’,6,6’-tetrachloro-1,1’,3,3’ tetraethylbenzimidazolylcarbocyanine iodide (JC-1). JC-1 is a green fluorescent delocalized lipophilic cation that accumulates in the mitochondrion to concentrations that result in the formation of red fluorescent J-aggregates (Cossarizza et al., 1993). Mitochondrial accumulation depends on an intact membrane potential. JC-1 labeled parasites showed green fluorescence in the cytoplasm and red fluorescence in the mitochondrion that was dissipated by CCCP and valinomycin treatment (Fig. 4). We measured the effect of ACP and TgAPT knockdown on the mitochondrial accumulation of JC-1 by flow cytometry after 0 to 3 days of ATc treatment. We did not observe a significant change in the ACP mutant. However, we note a dramatic drop of mitochondrial fluorescence in TgAPT knockdown parasite (56.6%, compared to 73.2 and 79.9% with the strong uncouplers valinomycin and CCCP). We conclude that loss of TgAPT leads to a significantly more severe phenotype than loss of ACP suggesting that TgAPT likely supports essential apicoplast functions beyond the FASII pathway.
Figure 4. TgAPT but not ACP mutant shows dramatic loss of mitochondrial membrane potential.
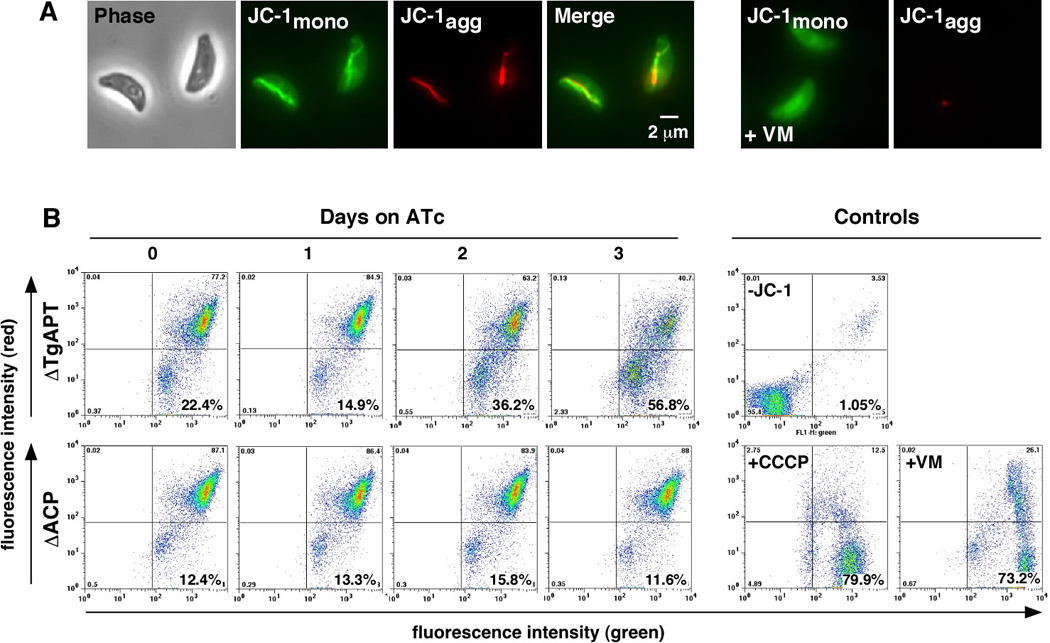
(A) Fluorescence microscopy of JC-1 labeled parasites shows accumulation of green fluorescent dye in the cytoplasm and the formation of red fluorescent J aggregates in the mitochondrion. This red fluorescence is abolished when parasites are treated with ionophores (valinomycin (+VM) is shown here). (B) TgAPT and ACP mutants were treated for 0 to 3 days with ATc, labeled with JC-1 and analyzed by flow cytometry. Untreated parasites show bright green and red fluorescence. Sustained ATc treatment leads to a pronounced decline in red fluorescence in the TgAPT mutant, but not the ACP mutant over the observation period.
Transport characteristics of heterologously expressed TgAPT
To identify potential pathways supplied by TgAPT we next sought to establish the molecules that are imported by this transporter into the apicoplast. As the apicoplast cannot be purified in sufficient quantity and purity, we expressed TgAPT heterologously in yeast and reconstituted the transport activities in liposomes (Hanke et al., 1999). The entire TgAPT coding sequence was introduced into the yeast expression vectors pYES-N and pYES-C thereby adding a 6× His-tag to the N- and C-terminus of TgAPT, respectively. The TgAPT constructs were used to transform yeast cells and several clones were tested for TgAPT expression by Western blot analysis using an antibody directed against the His-tag (data not shown).
In a first experiment, the phosphate transport activities of both the N- and C-terminal fusion proteins were determined. We noted that the specific activity of the TgAPT-C protein was less than 30% of the activity of TgAPT-N (data not shown). We conclude that C-terminal tagging impedes optimal transporter function, a finding that is consistent with our in vivo data in parasites. Therefore, only the TgAPT-N protein was used for further experiments.
Substrate specificities of TgAPT were determined by measuring the transport of radiolabelled Pi into proteo-liposomes preloaded with different counter -exchange substrates. These data are shown in table 1 in comparison with the substrate specificities of pPTs from higher plants and a red alga. Evidently, the TgAPT protein equally accepts inorganic phosphate and phosphoenol pyruvate (PEP) as substrates and, to an even higher extent, triose phosphate and 3 phosphoglycerate (trioseP and 3-PGA). In contrast, glucose-6-phosphate (Glc6P, table 1) and other sugar phosphates like Glc1P and fructose-6-phosphate (data not shown) are not transported by the TgAPT. These data are corroborated by measurements of the apparent kinetic constants of TgAPT for the above-mentioned substrates (table 1). The KM (app) for inorganic phosphate is 1.39 mM similar to the KM values of the plant transporters, which are in a range between 0.8 mM and 1.1 mM (Fischer et al., 1997; Kammerer et al., 1998). The Ki values, i.e. the apparent constants for competitive inhibition, for the other substrates transported by TgAPT are in the same range as the KM for phosphate, with Ki values of 1.63 mM, 1.33 mM and 1.65 mM for triose phosphate, 3-PGA and PEP, respectively. In contrast, Glc6P and other hexose phosphates are only poorly transported by TgAPT under the experimental conditions. Under physiological conditions in which the hexose phosphates have to compete with the other phosphorylated substrates these metabolites are likely not transported at all. Thus, the TgAPT represents a plastid phosphate translocator with a remarkable substrate specificity not found in pPTs from plants and algae, transporting triose phosphates and 3-PGA, i.e metabolites phosphorylated at carbon 3, and PEP which is phosphorylated at position 2.
Table 1.
Substrate specificities of recombinant plastid phosphate translocators from T. gondii, the red alga Galdieria sulphuraria and land plants.
| Recombinant translocator proteins | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TgAPT | Translocators from other species | Kinetic constantd | ||||||||
| Substratea | WT | K67A | K145A | K310A | GsTPTb | GsPPTb | SoTPTc | BoPPTc | PsGPTc | TgAPT |
| Phosphate | 100 | 21 ± 2 | 0 ± 2 | 0 ± 1 | 100 | 100 | 100 | 100 | 100 | Km 1.39 ± 0.28 mM |
| Triose-P | 192 ± 50 | 19 ± 3 | 0 ± 3 | 0 ± 2 | 87 | 25 | 92 | 22 | 112 | Ki 1.63 ± 0.26 mM |
| 3-PGA | 167 ± 34 | 23 ± 3 | 1 ± 1 | 0 ± 2 | 25 | 22 | 90 | 16 | 50 | Ki 1.33 ± 0.49 mM |
| PEP | 99 ± 16 | 17 ± 2 | 0 ± 2 | 0 ± 1 | 20 | 90 | 5 | 72 | 20 | Ki 1.65 ± 0.52 mM |
| Glucose-6-P | 25 ± 5 | 1 ± 3 | 0 ± 1 | 0 ± 1 | 25 | 15 | 5 | 2 | 90 | |
Liposomes were preloaded with 20 mM substrates as indicated. Transport activities are given as percentage of the Pi/Pi counter-exchange set to 100%. Mean values are from at least three different experiments.
Data are from (Linka et al., 2008)
Data are from (Kammerer et al., 1998); Spinacea oleracea, So; Brassica olerace, Bo; Pisum sativum, Ps.
Mean values (± SE) are from 4–6 different experiments.
Lysine residues conserved across phosphate translocators are critical to TgAPT activity in vivo and in vitro
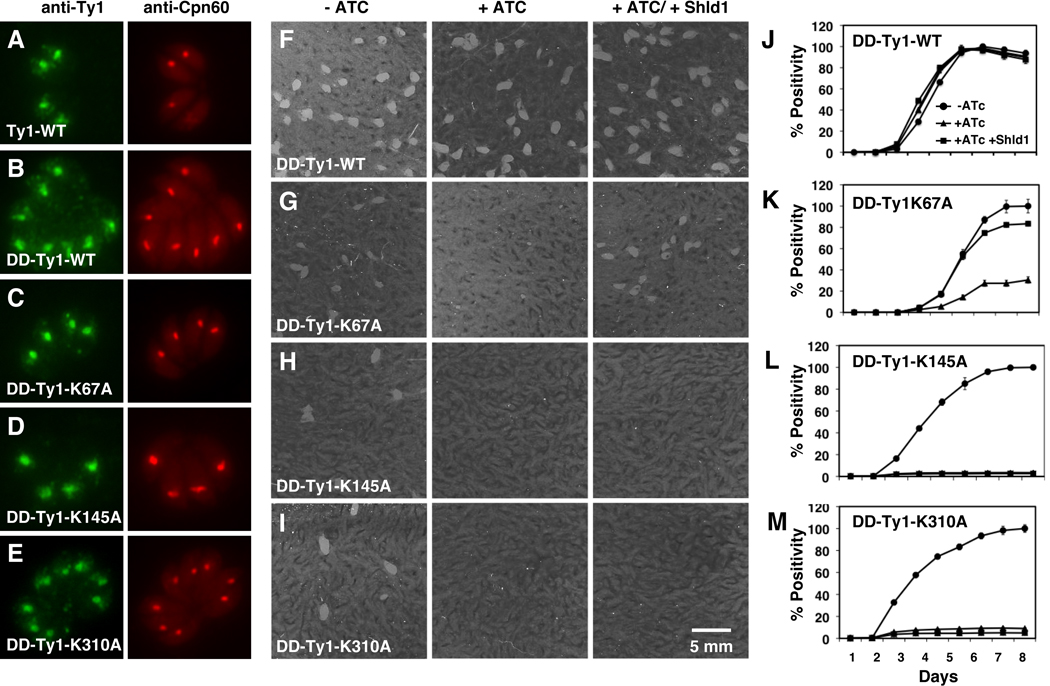
To begin to dissect the molecular basis of the unique substrate specificity of TgAPT we established a complementation assay to test mutant versions of the transporter in vivo. We subcloned a wild type version of the TgAPT coding sequence into a construct that results in N -terminal fusion to a Ty-1 epitope tag and a FKB12 destablization domain (DD). We introduced this domain to allow for conditional modulation of protein levels using Shield -1 -Götz et al., 2007) in the case point mutants should exert dominant negative effects. We introduced this plasmid stably into the mutant background using phleomycin selection and demonstrated by immunofluorescence that the protein is expressed and correctly targeted to the apicoplast (Fig. 5B). Measuring protein levels by Western blot we noted that despite the presence of a DD domain, the protein was stable in the absence of Shield1 (supplementary Fig. S6). This might be due to poor destruction of multi-pass membrane proteins, or due the fact that TgAPT localizing to the inner membranes of the apicoplast might be protected from proteosomal degradation. Importantly, WT-TgAPT fully restores growth of the mutant under ATc in the presence or absence of Shield -1 when measured by plaque or fluorescence assay (Fig. 5F, J).
Figure 5. Identification of lysine residues critical for TgAPT activity by mutational analysis and in vivo complementation assay.
A ΔTgAPT/iAPT-HA mutant clone carrying the dTomato-RFP marker was transfected with the wild type APT coding sequence expressed from a constitutive promoter carrying an N-terminal Ty1 epitope tag (Ty-WT), the same transgene carrying in addition a FKB12 destabilization domain (DD-Ty1-WT), and three point mutations of the latter (DD-Ty1-K 67, 145 and 310 A respectively). (A–E) Immunofluorescence assays showing parasites cultured in the presence of 0.1 µM Shield1 for 36 hours and subsequently labeled with antibodies to the Ty-1 epitope tag (green) and the apicoplast resident protein Cpn60 (red, note that cytoplasmic red background fluorescence is due to residual dTomato-RFP autofluorescence after fixation). Transgenic parasite strains are as indicated. (F–I) Plaque assays of indicated strains grown in the absence (−ATc) or presence of 0.5 µg/ml ATc (+ATc), or the presence of ATc and Shield1 (+ATc +Shld1). (J–M) Fluorescene growth assays circles, −ATc; triangles, +ATc; squares, +ATc +Shld1 (each data point represents the mean of six wells and the error bar gives the standard deviation).
Several lysine residues are highly conserved in a wide range of members of the drug/ metabolite transporter superfamily including TgAPT (Flügge et al., 2003; Karnataki et al., 2007; Knappe et al., 2003; Martin and Kirk, 2004). These positively charged residues are believed to interact with the negatively charged substrates, and are therefore thought to be critical for substrate recognition and transport activity (Knappe et al., 2003). To directly test this hypothesis we engineered three lysine to alanine point mutations (K67A, K145A and K310A) and introduced them into the TgAPT mutant. All three mutants are expressed at levels comparable to the wild type transgene and the proteins are correctly targeted to the apicoplast (Fig. 5 and S6). We next tested the growth of these mutants by fluorescence and plaque assays in the absence and presence of ATc and Shield -1. We find that K145A and K310A fail to complement under ATc regardless of the presence of Shield -1. Interestingly, mutant K67A displayed a Shield1 dependent phenotype. In the presence, but not the absence, of Shield -1 growth of the mutant is restored. We next wanted to correlate these in vivo observations with the transport capabilities of the mutant proteins. Mutant TgAPT proteins were heterologously expressed and tested in reconstituted liposomes (table 1). Mutations K145A and K310A abolished transport activity while mutation K67 reduced the activity to about 10–20% for all transport substrates tested. It appears that this partial attenuation of transport efficiency is responsible for Shield1 dependency of complementation in vivo. The exact molecular mechanism underlying this phenomenon (subtle differences in expression level or additive effects of point mutants and DD destabilization) remains to be defined. We conclude that residues implicated in substrate binding are key in mediating transporter activity of TgAPT both in vivo and in vitro, suggesting that substrate selection is vital for the correct functioning of this apicoplast membrane transporter. We note that transport activity in vitro and biological complementation in vivo are highly consistent, making this an ideal system for further mechanistic dissection of this transporter and its function in apicoplast metabolism.
Discussion
T. gondii possesses a single protein that shows significant similarity to plant plastid phosphate translocators and that is located in the apicoplast envelope membranes ((Fleige et al., 2007; Karnataki et al., 2007) and figure S1). By heterologous expression of the TgAPT gene in yeast and reconstitution of the encoded protein we showed that the TgAPT is able to transport triose phosphates, 3-PGA and PEP, but does not possess Glc6P transport activity. With the substrate specificities of the single Toxoplasma phosphate translocator presented here several conclusions can be drawn with respect to its evolution and physiological functions.
TgAPT is the source of carbon for essential apicoplast anabolic pathways
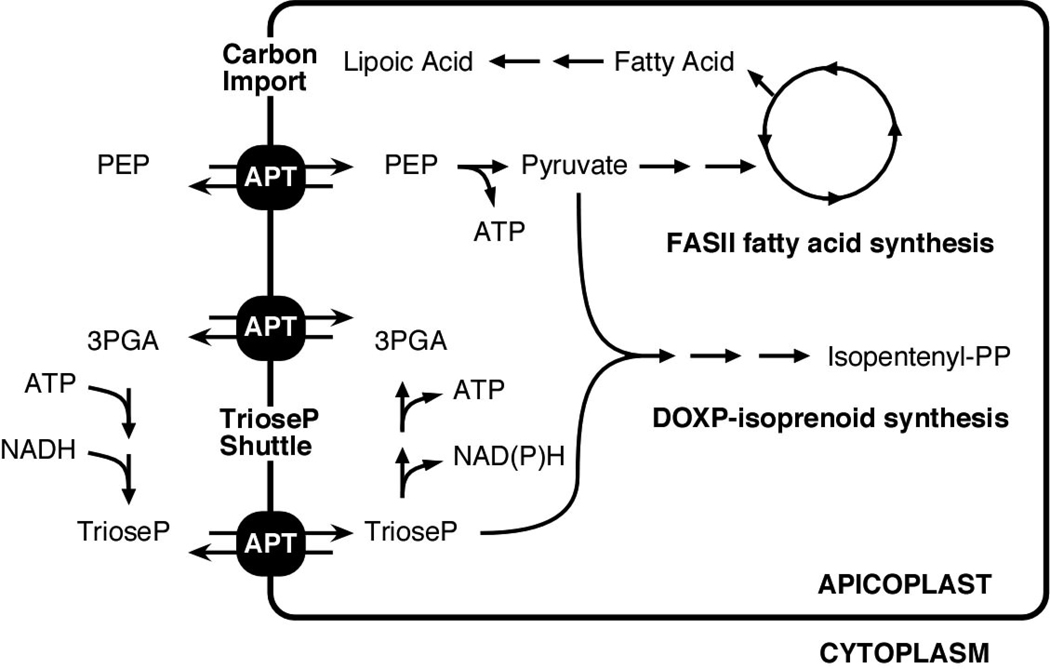
Our biochemical analyses indicate that TgAPT is capable of exchanging triose phosphate and PEP for inorganic phosphate. This leads to a net import of carbon into the apicoplast to be used for anabolic pathways (Fig. 6). Through genetic ablation, we demonstrate that TgAPT is required for the activity of the apicoplast FASII pathway thus providing a direct link between cytoplasmic and apicoplast metabolism. However, as we show here, loss of TgAPT results in a significantly more severe phenotype than loss of FASII alone. Taken together with recent genetic studies on FASII in Toxoplasma and Plasmodium, this suggests that the production of fatty acids is likely not the primary metabolic role of the apicoplast. In Plasmodium FASII was shown to be dispensable in the blood and insect stages, but essential in the liver stage, and in T. gondii, while clearly essential, ablation of the pathway results in delayed effects on growth (Mazumdar and Striepen, 2007; Mazumdar et al., 2006; Vaughan et al., 2008; Yu et al., 2008). A second pathway likely depending on TgAPT imported substrates is the DOXP isoprenoid synthesis pathway (see Fig. 6). Comparative genomic analyses across the Apicomplexa find this pathway to be conserved in all apicoplast-bearing species while FASII and heme synthesis have been lost in Theileria and Babesia (Brayton et al., 2007; Gardner et al., 2005). The recent discovery of an important role for the plant hormone absisic acid in Toxoplasma has produced an intriguing candidate for a parasite molecule that might depend on apicoplast derived isoprenoids (Nagamune et al., 2008). Inhibition of the rate-limiting step of the DOXP pathway with the antibiotic fosmidomycin results in inhibition of Plasmodium in culture and cure of the infection in animal models and human clinical trials (Jomaa et al., 1999; Oyakhirome et al., 2007). Surprisingly however, Toxoplasma and Theileria appear to be highly resistant to fosmidomycin (Clastre et al., 2007; Lizundia et al., 2009). While this might suggest that this pathway is not essential in these parasites, our data raises alternative explanations. Based on the TgAPT mutant phenotype and the comparative genomic data we hypothesize that fosmidomycin resistance in T. gondii is more likely due to differences in the target enzyme DOXP reductoisomerase, or differences in drug import into the parasite or the apicoplast. Clearly, more work is needed to discern these models. The genetic and biochemical approaches developed in the current study should provide the necessary tools to address this important question.
Figure 6. TgAPT links apicoplast metabolism to the parasite cytoplasm.
Schematic representation of apicoplast key anabolic pathways (note that this is a highly simplified outline and that the number of arrows does not reflect the number of enzymatic steps needed). Note that TgAPT serves as a hub to supply carbon (through PEP and triose phosphate) as well as energy and reduction power through the triose shuttle. TgAPT is a phosphate antiporter and Pi is not shown for simplicity.
Triose phosphate/3-PGA exchange can provide energy and reducing equivalents for the Toxoplasma apicoplast
The apicoplast has lost its ability to photosynthesize during the adaptation of its host to parasitism. This required adaptations of the flow of energy and carbon between endosymbiont and host reflected in the transporter. With the import of triose phosphates into the apicoplast, their conversion to 3-PGA and subsequent export of 3-PGA, a triose phosphate/ 3-PGA shuttle is constituted which now likely runs in the opposite direction when compared to plants (see Fig. 6). All necessary components required for such a shuttle are expressed in T. gondii, namely cytosolic and plastid isoforms of phosphoglycerate kinase, GAPDH, triose phosphate isomerase, and the TgAPT linking them (Fleige et al., 2007). This shuttle results in a net transfer of ATP and reduction power from the cytosol to the apicoplast. GAPDH and PDH are the only biochemical processes identified in the apicoplast producing reducing equivalents in the form of NADH or NADPH while the phosphoglycerate kinase and pyruvate kinases are the only enzymes producing ATP. The importance of the triose phosphate/ 3-PGA shuttle is reflected in the transport characteristics of the TgAPT. When compared to plant transporters TgAPT shows an unusually high relative transport rate of triose phosphates and 3-PGA which are 190% and 172% of the phosphate transport rate. Strikingly, while the T. gondii genome encodes two GAPDH genes, of which one is of red algal origin and likely apicoplast targeted (Fast et al., 2001; Fleige et al., 2007), in Plasmodium only a single GAPDH is apparent from the genome sequence and the protein appears to be localized to the cytosol (Daubenberger et al., 2003). This suggests important differences in the apicoplast metabolism between the two parasite species.
Evolution of the Toxoplasma phosphate translocator
Toxoplasma has a phosphate translocator that uniquely combines the substrate specificites of a TPT and a PPT. None of the phosphate translocators from algae and higher plants have the ability to transport C3 compounds phosphorylated at both carbon 2 and 3. In higher plants four specialized subfamilies of transporters have evolved to enable exchange of carbon with the chloroplast (Flügge et al., 2003) and a survey of green algal genomes indicates that they share three of these pPT subfamilies (K.F., unpublished). In contrast, the red alga Galdieria sulphuraria possesses only a TPT and a PPT (Linka et al., 2008). Equivalent transport activities have also been detected in the plastid of the cryptophyte Guillardia theta which is also derived from red algae by secondary endosymbiosis, however, these activities have not yet been assigned to a particular protein (Haferkamp et al., 2006). Given the presence of both a PPT and TPT in red algae, it is likely that the ancestor of the apicoplast inherited multiple, specific pPTs. Over time, likely driven by the biochemical and genomic consequences of losing photosynthesis, some pPTs were lost and others experienced a broadening of substrate specificity. Analysis of pPTs in apicomplexan relatives such as dinoflagellates and the recently discovered, early-diverging photosynthetic apicomplexan Chromera promises to illuminate the evolution of pPTs in the Apicomplexa. Both red algae and Apicomplexa lack transporter for Gl6P and other hexose phosphates.
TgPT is an essential part of the Toxoplasma metabolism
The TgAPT delivers carbon units for at least two different anabolic processes in the apicoplast, fatty acid synthesis and likely the DOXP pathway. In addition, it has an essential role in indirectly supplying the apicoplasts with ATP and redox equivalents. Thus, TgAPT is a metabolic hub that links cytosolic metabolism with essential processes in the apicoplast. In support of this central role we show here that genetic ablation of TgAPT results in the rapid death of the parasite. The unique substrate specificity of this transporter combined with the dramatic consequences of loss of its activity suggest that targeting TgAPT could be a viable strategy to identify anti-parasitic compounds. Apicoplast pathways, in particular the FASII and DOXP pathways, which both are of cyanobacterial origin, already are intensely pursued for drug development (Goodman and McFadden, 2007; Wiesner et al., 2008). However, several apicoplast-directed pharmacological and genetic manipulations have yielded slow inhibition kinetics, a phenomenon that has been dubbed delayed death (Fichera and Roos, 1997). Such slow kinetics might limit potency, and potentially could favor the development of drug resistance, which is a particular concern with respect to the malaria parasite. We note that the rapid death of the TgAPT mutant strongly suggests that delayed death is not an inevitable outcome of interference with the apicoplast. Careful genetic and biochemical analyses should yield a more detailed understanding of the truly essential functions of this unique organelle, and produce a list of targets that are unique to the parasite and for which inhibition results in the rapid demise of the pathogen.
Experimental Procedures
Cosmid recombineering
We constructed plasmids as templates from which to PCR amplify inserts that we could then recombineer into cosmids. We designed vectors containing T. gondii knockout cassettes using both chloramphenicol and phleomycin as selectable markers. We also designed vectors to enable 3’-HA epitope tagging of genes-of-interest. In our initial experiments we noted that employing the frequently used untranslated regions from the SAG1 and DHFR-TS genes, which are also found in the backbone of the two cosmid libraries interferes with the efficiency of recombineering. We therefore developed constructs using the flanking regions of the GRA1 and HXGPT genes (see supplementary material for detail on cloning procedures).
Cosmid PSBYL85 was introduced into EL250 cells by electoroporation (Genetronics ECM 630, 1 mm cuvette, 1.75kV, 250 Ω and 25 µF) and bacteria were grown on LB plates containing 50 µg/ ml kanamycin (note that EL250 cells should not be grown at temperatures above 30°C). Clones were picked, confirmed, and grown in liquid culture to an OD600 of 0.4, heat shocked for 20 min at 43°C, and rendered electro-competent. Modification cassettes were amplified from plasmids piCG and pHcG, respectively flanked with 50 bp of gene specific sequence in the primer. The PCR product was introduced by electroporation and cells were selected on agar using 50 µg/ml kanamycin and 10 µg/ ml gentamycin. A detailed protocol for cosmid recombineering and schematic maps of the available tags and libraries (Fig. S7) can be found in the supplementary materials.
Targeting the TgAPT locus
Parasites were maintained and genetically manipulated as described (Striepen and Soldati, 2007). We subcloned the coding sequence for TgAPT from a vector kindly provided by Manami Nishi and David Roos (U. Pennsylvania) into pDt7s4H3 (S. Ramakrishnan and GvD, unpublished) to generate a vector that expresses APT from a tetracycline-inducible promoter with a 3xHA tag at the 3’ end of the gene. We transfected this into TATi strain parasites (Meissner et al., 2002) and selected with pyrimethamine. A clonal line was isolated and transfected with 25–75 µg TgAPT-KO cosmid. Note that we found that linearizing cosmids by restriction did not increase transformation efficiency or the frequency of successful gene targeting. We therefore use unrestricted cosmids for transfection.
JC-1 labelling and flow cytometry
Parasites were harvested from infected host cells by needle passage and filteration, washed in phenol-red free medium and adjusted to 2.5 × 107 cells per ml. Where appropriate, we added valinomycin and carbonyl cyanide 3-chlorophenylhydrazone (CCCP) to final concentrations of 10 µM. Parasites were incubated at 37°C for 30 minutes, JC-1 was added to a final concentration of 1.5 µM, followed by incubation for 15 minutes at 37°C. Cells were washed and analyzed by flow cytometry or microscopy. Flow cytometry was performed on a FACSCalibur cytometer (Becton Dickinson, San Jose, CA).
Microscopy
Immunofluorescence assays were performed as previously described (van Dooren et al., 2008). We used anti-HA antibodies (Roche) at a dilution of 1:100, anti-Cpn60 (Agrawal et al., 2009) at a dilution of 1:2000 and anti-ATrx1 (a kind gift from Peter Bradley, UCLA) at a dilution of 1:1000. Fluorescence images were acquired using a Delta Vision or a Leica DM IRBE microscope.
Western Blotting and pulse-chase analyses
Western blotting was performed as previously described (van Dooren et al., 2008). We used anti-HA antibodies at a dilution of 1:100, anti-ACP (a kind gift from Geoff McFadden, U. Melbourne (Waller et al., 1998)) at 1:500, anti-GRA8 (a kind gift from Gary Ward, U. Vermont (Carey et al., 2000)) at 1:2000 and anti-lipoylated-PDH-E2 (clone3H-2H4; a kind gift from Eric Gershwin, UC Davis; (Migliaccio et al., 1998)) at 1:1000. Pulse-chase analyses were performed as described previously (van Dooren et al., 2008) with the modification that experiments were performed in T75 flasks and the chase time was extended to three hours.
Heterologous expression of the TgAPT in yeast and isolation of membrane proteins
The coding sequence of TgAPT was amplified from RH strain cDNA and subcloned into yeast pYES2/ NTC vector (Invitrogen), resulting in N and C-terminal fusions, respectively. These plasmids were transformed into the yeast strain InvSc1. For membrane preparation, cells were grown in liquid media to an OD600 of 0.8–1.0. Expression was induced by resuspension in liquid media containing 2% galactose. Cells were harvested after 6–8 hours and stored at −2°C. After thawing, cells were disrupted in a buffer containing 10 mM TRIS-HCl pH 7.5, 1 mM EDTA and 1 mM phenylmethylsulfonyl-fluoride (PMSF). Cell debris was removed by centrifugation for 1 min at 500 × g and total membranes were collected by centrifugation for 20 min at 100 000 × g.
Reconstitution and measurement of transport activities
Liposomes were prepared from acetone-washed phosphatidylcholine (120–130 mg/ ml) by sonication for 4 min on ice in 100 mM Tricine-KOH pH 7.6 and 60 mM potassium gluconate (buffer A) in absence or presence of 20 mM of substrate as indicated. Yeast membranes were solubilized using 100 µl 1.25% (w/ v, final concentration) Triton X-100 as detergent and directly added to 750 µl liposomes and proteins were integrated into liposomes by a freeze-thaw step. After sonication, the external medium was removed by passage over Sephadex G-25 columns and proteoliposomes were eluted with buffer A. The transport experiments were started by addition of [32P]-Pi (0.2–10 mM) as the external counter-exchange substrate and terminated at different time points (15–120 s) by passing the liposomes over a Dowex AG1-X8 anion exchange column. The radioactivity of the eluate was determined by liquid scintillation counting. Background activity for membranes purified from yeast transfected with empty plasmid was measured (typically ~1% of TgAPT activity) and subtracted from all measurements.
Supplementary Material
Acknowledgments
We thank Silvia Moreno (U. Georgia) and Rowena Martin (Australian National University) for helpful discussions, Peter Bradley, Gary Ward, Geoff McFadden and Eric Gershwin for antibodies, Donald Court (NCI Fredrick) for EL250 cells, Anthony Poteete (U. Massachusetts) for TP997 cells, David Roos, and David Sibley for plasmids, and especially Julie Nelson for assistance with flow cytometry. This work was supported by National Institutes of Health grants AI64671 and AI084415 to B.S. and a C.J. Martin Overseas Fellowship (400489) from the Australian National Health and Medical Research Council to G.vD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal S, van Dooren GG, Beatty WL, Striepen B. Genetic evidence that an endosymbiont-derived ERAD system functions in import of apicoplast proteins. J Biol Chem. 2009 doi: 10.1074/jbc.M109.044024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, Bidwell SL, Brown WC, Crabtree J, Fadrosh D, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–1413. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KL, Donahue CG, Ward GE. Identification and molecular characterization of GRA8, a novel, proline- rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol. 2000;105:25–37. doi: 10.1016/s0166-6851(99)00160-7. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Membrane heredity and early chloroplast evolution. Trends Plant Sci. 2000;5:174–182. doi: 10.1016/s1360-1385(00)01598-3. [DOI] [PubMed] [Google Scholar]
- Clastre M, Goubard A, Prel A, Mincheva Z, Viaud-Massuart MC, Bout D, Rideau M, Velge-Roussel F, Laurent F. The methylerythritol phosphate pathway for isoprenoid biosynthesis in coccidia: presence and sensitivity to fosmidomycin. Exp Parasitol. 2007;116:375–384. doi: 10.1016/j.exppara.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, Seeber F. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. Embo J. 2006 doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE, Zhao X, Jiang Y. Function, attachment and synthesis of lipoic acid in Escherichia coli. Advances in microbial physiology. 2005;50:103–146. doi: 10.1016/S0065-2911(05)50003-1. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenberger CA, Tisdale EJ, Curcic M, Diaz D, Silvie O, Mazier D, Eling W, Bohrmann B, Matile H, Pluschke G. The N'-terminal domain of glyceraldehyde-3-phosphate dehydrogenase of the apicomplexan Plasmodium falciparum mediates GTPase Rab2-dependent recruitment to membranes. Biol Chem. 2003;384:1227–1237. doi: 10.1515/BC.2003.135. [DOI] [PubMed] [Google Scholar]
- Eicks M, Maurino V, Knappe S, Flugge UI, Fischer K. The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol. 2002;128:512–522. doi: 10.1104/pp.010576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast NM, Kissinger JC, Roos DS, Keeling PJ. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Mol Biol Evol. 2001;18:418–426. doi: 10.1093/oxfordjournals.molbev.a003818. [DOI] [PubMed] [Google Scholar]
- Fichera ME, Roos DS. A plastid organelle as a drug target in apicomplexan parasites. Nature. 1997;390:407–409. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- Fischer K, Kammerer B, Gutensohn M, Arbinger B, Weber A, Hausler RE, Flugge UI. A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. Plant Cell. 1997;9:453–462. doi: 10.1105/tpc.9.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. Carbohydrate Metabolism in the Toxoplasma gondii Apicoplast: Localization of Three Glycolytic Isoenzymes, the Single Pyruvate Dehydrogenase Complex, and a Plastid Phosphate Translocator. Eukaryot Cell. 2007;6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U, Häusler RE, Ludewig F, Fischer K. Functional genomics of phosphate antiport systems. Physiol Plant. 2003;118:475–482. [Google Scholar]
- Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end-joining. Eukaryot Cell. 2009 doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Bishop R, Shah T, de Villiers EP, Carlton JM, Hall N, Ren Q, Paulsen IT, Pain A, Berriman M, et al. Genome sequence of Theileria parva, a bovine pathogen that transforms lymphocytes. Science. 2005;309:134–137. doi: 10.1126/science.1110439. [DOI] [PubMed] [Google Scholar]
- Goodman CD, McFadden GI. Fatty acid biosynthesis as a drug target in apicomplexan parasites. Current drug targets. 2007;8:15–30. doi: 10.2174/138945007779315579. [DOI] [PubMed] [Google Scholar]
- Gould SB, Waller RF, McFadden GI. Plastid evolution. Annu Rev Plant Biol. 2008;59:491–517. doi: 10.1146/annurev.arplant.59.032607.092915. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, Flynn J, Parrot B, Radke J, Striepen B, et al. Forward Genetic Analysis of the Apicomplexan Cell Division Cycle in Toxoplasma gondii. PLoS Pathog. 2008;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferkamp I, Deschamps P, Ast M, Jeblick W, Maier U, Ball S, Neuhaus HE. Molecular and biochemical analysis of periplastidial starch metabolism in the cryptophyte Guillardia theta. Eukaryot Cell. 2006;5:964–971. doi: 10.1128/EC.00381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke G, Bowsher CG, Jones MN, Tetlow I, Emes MJ. Proteoliposomes and plant transport proteins. J Exp Bot. 1999;50:1715–1726. [Google Scholar]
- Herm-Götz A, Agop-Nersesian C, Münter S, Grimley JS, Wandless TJ, Frischknecht F, Meissner M. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat Methods. 2007;4:1003–1005. doi: 10.1038/nmeth1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009 doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flugge UI. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell. 1998;10:105–117. doi: 10.1105/tpc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnataki A, Derocher A, Coppens I, Nash C, Feagin JE, Parsons M. Cell cycle-regulated vesicular trafficking of Toxoplasma APT1, a protein localized to multiple apicoplast membranes. Mol Microbiol. 2007;63:1653–1668. doi: 10.1111/j.1365-2958.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Knappe S, Flugge UI, Fischer K. Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol. 2003;131:1178–1190. doi: 10.1104/pp.016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS. A plastid of probable green algal origin in Apicomplexan parasites. Science. 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Linka M, Jamai A, Weber AP. Functional characterization of the plastidic phosphate translocator gene family from the thermo-acidophilic red alga Galdieria sulphuraria reveals specific adaptations of primary carbon partitioning in green plants and red algae. Plant Physiol. 2008;148:1487–1496. doi: 10.1104/pp.108.129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizundia R, Werling D, Langsley G, Ralph SA. Theileria apicoplast as a target for chemotherapy. Antimicrob Agents Chemother. 2009;53:1213–1217. doi: 10.1128/AAC.00126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, Kirk K. The malaria parasite's chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol Biol Evol. 2004;21:1938–1949. doi: 10.1093/molbev/msh205. [DOI] [PubMed] [Google Scholar]
- Mazumdar J, Striepen B. Make it or Take it Fatty Acid Metabolism of Apicomplexan Parasites. Eukaryot Cell. 2007 doi: 10.1128/EC.00255-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Wilson E, Masarek K, Hunter C, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci U S A. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden GI, Reith ME, Munholland J, Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, Coppel RL, Gershwin ME. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161:5157–5163. [PubMed] [Google Scholar]
- Moore RB, Obornik M, Janouskovec J, Chrudimsky T, Vancova M, Green DH, Wright SW, Davies NW, Bolch CJ, Heimann K, et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature. 2008;451:959–963. doi: 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- Mullin KA, Lim L, Ralph SA, Spurck TP, Handman E, McFadden GI. Membrane transporters in the relict plastid of malaria parasites. Proc Natl Acad Sci U S A. 2006;103:9572–9577. doi: 10.1073/pnas.0602293103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyakhirome S, Issifou S, Pongratz P, Barondi F, Ramharter M, Kun JF, Missinou MA, Lell B, Kremsner PG. Randomized controlled trial of fosmidomycin-clindamycin versus sulfadoxine-pyrimethamine in the treatment of Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2007;51:1869–1871. doi: 10.1128/AAC.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteete AR, Rosadini C, St Pierre C. Gentamicin and other cassettes for chromosomal gene replacement in Escherichia coli. Biotechniques. 2006;41:261–262. doi: 10.2144/000112242. 264. [DOI] [PubMed] [Google Scholar]
- Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. Tropical infectious diseases: Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- Striepen B, Soldati D. Genetic manipulation of Toxoplasma gondii. In: Weiss LM, Kim K, editors. Toxoplasma gondii: The Model Apicomplexan - Perspective and Methods. Elsevier; 2007. pp. 391–415. [Google Scholar]
- Thomsen-Zieger N, Schachtner J, Seeber F. Apicomplexan parasites contain a single lipoic acid synthase located in the plastid. FEBS Lett. 2003;547:80–86. doi: 10.1016/s0014-5793(03)00673-2. [DOI] [PubMed] [Google Scholar]
- van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B. A novel dynamin-related protein has been recruited for apicoplast fission in Toxoplasma gondii. Curr Biol. 2009;19:267–276. doi: 10.1016/j.cub.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc Natl Acad Sci U S A. 2008;105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, Lang-Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci U S A. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AP, Linka M, Bhattacharya D. Single, ancient origin of a plastid metabolite translocator family in Plantae from an endomembrane-derived ancestor. Eukaryot Cell. 2006;5:609–612. doi: 10.1128/EC.5.3.609-612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner J, Reichenberg A, Heinrich S, Schlitzer M, Jomaa H. The plastid-like organelle of apicomplexan parasites as drug target. Curr Pharm Des. 2008;14:855–871. doi: 10.2174/138161208784041105. [DOI] [PubMed] [Google Scholar]
- Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, et al. The Fatty Acid Biosynthesis Enzyme FabI Plays a Key Role in the Development of Liver-Stage Malarial Parasites. Cell Host Microbe. 2008;4:567–578. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.