Blood clots form rapidly in the event of vascular injury, to prevent blood loss. They may also form in undesired places, causing heart attacks, strokes, and other diseases. Blood clots can rupture, and fragments of the clot may lodge in distal blood vessels, causing, for example, ischemic strokes or embolisms. Thus, there has been great interest in understanding the mechanical behavior and failure mechanisms of blood clots and their constituents. To develop a mechanically realistic model of a blood clot, knowledge of the mechanical properties of its constituents is required [1]. The major structural component providing mechanical strength to the clot is a mesh of fibrin fibers. Principally, three pieces of information are needed to develop realistic (fibrin fiber) network models [2]: (i) the architecture of the network; (ii) the properties of the single fibers; and (iii) the properties of the fiber branchpoints.
The architecture of the fibrin fiber network in a blood clot may be obtained from microscopy images. Recently, there has been significant activity in determining the mechanical properties of individual fibrin fibers [3–5]. Here, we investigated the strength and failure of crosslinked and uncrosslinked fibrin fiber branchpoints (Fig. 1). We report two distinct methods of failure: rupture at the joint, and rupture along a fiber. In fully crosslinked fibrin fibers, rupture occurred most often along the fiber. Conversely, in uncrosslinked fibers, failure occurred most often because of the detachment of a leg at the branchpoint. Perhaps the most unexpected finding was that neither crosslinked nor uncrosslinked joints showed failure due to extended unzipping of the joint, indicating that joints are rather resilient to failure. It appears that, in some cases, continuous unzipping may be prevented by a triangular branchpoint architecture, in which fibers (cross-struts) reach across all three legs in a Y-shaped junction. This architecture was revealed in many branchpoints as they were strained. This triangular architecture is consistent with a model in which polymerization occurs in all directions at the branchpoints, as proposed by Ryan et al. [6]. Twisting of protofibrils during aggregation may also prevent unzipping of branchpoints [7]. Finally, we report the strength (stress at failure) of fibrin fiber branchpoints.
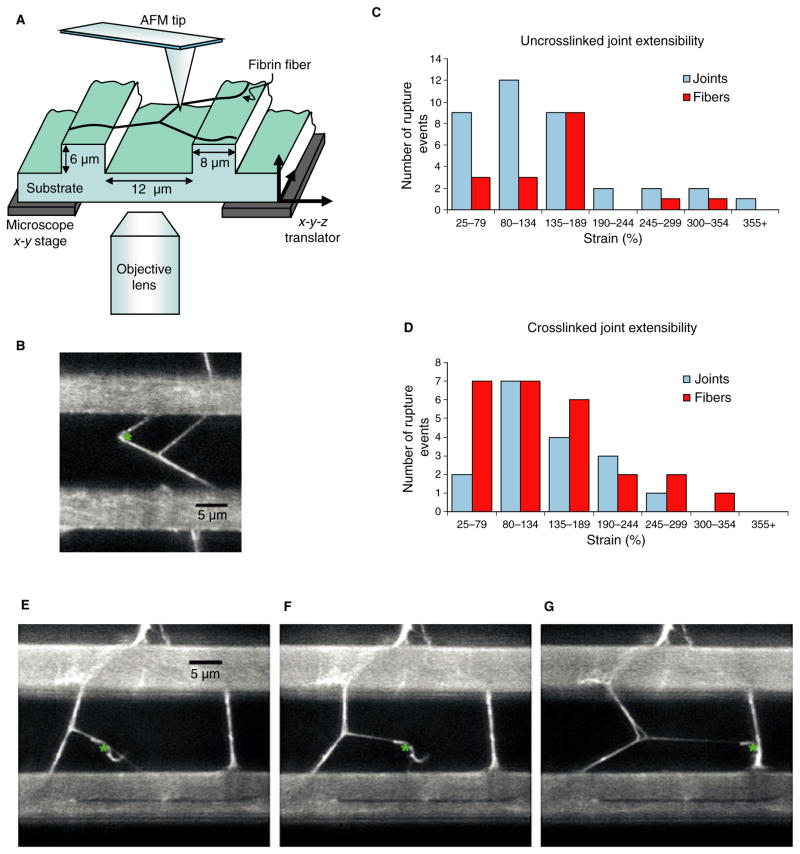
Fig. 1.
(A) Schematic view of the experimental setup. The atomic force microscope (AFM) tip is located above the fibrin fiber joint, which is formed on a striated substrate. The inverted fluorescence microscope allows visualization and movie collection of the manipulation from below. (B) Movie frame of a fibrin fiber joint manipulation. The ridges of the striated surface are the bright horizontal bars. The location of the AFM tip is represented by a green asterisk. (C, D) Histograms of the maximum extensibility of the uncrosslinked and crosslinked joints. The data are color-coded by failure mechanism. The maximum extensibility before detachment at the node is depicted in blue, and the maximum extensibility before fiber rupture is depicted in red. The error in the strain is due to error in the measurement of the length of the fiber, and is approximately 8%. All manipulations were performed in fibrin buffer (140 mM NaCl, 10 mM Hepes, 5 mM CaCl2, pH 7.4). (E–G) Movie frames of a fibrin fiber joint manipulation in buffer, showing triangular joint architecture. The triangular structure becomes visible as the joint is stretched. (A small piece of fiber from a previous manipulation can be seen stuck to the AFM tip.)
Fibrin is formed by thrombin-mediated cleavage of fibrino-peptides A and B from soluble fibrinogen. Fibrin monomers self-assemble into half-staggered protofibrils, which associate to form fibers. During the formation of fibrin fibers, factor XIIIa covalently rigidifies molecule–molecule interactions through three types of crosslink: (i) γ–γ crosslinks, which form between the γ-nodules of abutting molecules; (ii) α–α crosslinks, which form multiple bonds between the αC regions of adjacent molecules; and (iii) the less frequent α–γ crosslinks. Crosslinking of fibrin films and fibers increases the modulus (stiffness) while making fibrin fibers less extensible [8–10].
During clot formation, the fibrin fibers branch and grow, forming a network with interspersed platelets and red blood cells. The fiber joints, or branchpoints, in this network are mostly formed by the intersection of three fibers at a node [6,11]. At a molecular level, there are two models of how these joints form: the trimolecular joint model, and the tetramolecular joint model [12,13].
To examine fibrin joints, a striated substrate was prepared by a micromolding technique, as previously reported [3]. Fibrin fibers were polymerized directly on the substrate, with a human fibrinogen concentration between 0.5 and 1 mg mL−1 (American Diagnostica, Stamford, CT, USA; FXIII-depleted), and a thrombin concentration between 0.05 and 0.1 units/mL (Enzyme Research Laboratories, South Bend, IN, USA). To form crosslinked fiber joints, 25 Loewy units/mL of FXIII were added during polymerization (Enzyme Research Laboratories). The fibrin fibers were labeled with 24-nm carboxyl yellow–green fluospheres for visualization (Invitrogen, Carlsbad, CA, USA). To test the extent of crosslinking, samples were prepared in parallel and subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis. To within the sensitivity limits of the gel, crosslinking was complete (≥90%).
Mechanical manipulations of joints were performed with a combined atomic force microscope/fluorescence microscope (ThermoMicroscope Explorer atomic force microscope, Zeiss Axiovert 200, Hamamatsu EM-CCD C9100 camera, 3rd Tech NanoManipulator), as described in [3,8]. The atomic force microscope tip applied a force to one leg of the joint. Fluorescence microscopy images were recorded and used to determine failure mechanism and measure initial length and strain at rupture of each leg of the joint (Fig. 1A,B).
Straining fibrin fiber joints resulted in two methods of joint failure: rupture along a fiber, and rupture at the node (for movies of failure mechanisms, see supplement). Additionally, we observed detachment of a fiber at the ridge; however, these data were excluded, as they result from the experimental setup and have no physiologic relevance.
Uncrosslinked and crosslinked joints showed different rupture behaviors. Uncrosslinked joints ruptured 68.5% of the time at the node and 31.5% of the time along a fiber. Conversely, crosslinked joints ruptured 39.5% of the time at the node and 60.5% of the time along a fiber (Fig. 1C,D). This suggests that individual crosslinked fibrin fibers are weaker than crosslinked joints, whereas the opposite is true for uncrosslinked fibrin fibers. Failure of crosslinked fibers before failure of joints may also be due to the lower extensibility of crosslinked fibers (147–217%) [4,8] than of uncrosslinked fibers (226%) [3]. Also, the difference between crosslinked and uncrosslinked fibrin joints suggests that FXIIIa crosslinking provides a mechanism that fortifies and strengthens fiber branchpoints. Thus, natural, fully crosslinked clots may mainly fail by fiber rupture rather than by rupture of branchpoints.
Perhaps most surprising is the finding that joints are so resistant to failure. We expected that uncrosslinked fiber joints, in particular, would easily unzip, as bonds implicated in lateral aggregation are weak [14]. However, we did not observe extended unzipping events. A possible explanation might be that fibrin protofibrils aggregate in a helical manner around the fiber, limiting fiber size [7] and preventing two-dimensional unzipping. Unzipping may be further prevented by a triangular architecture, in which three cross-struts prevent continuous, extended unzipping of the joint (Fig. 1E–G). In this architecture, observed in 27% of both crosslinked and uncrosslinked joints, each fiber is connected via cross-struts to the two additional fibers involved in the joint, resulting in a triangle at the branchpoint. The formation of this architecture suggests that fiber polymerization at joints occurs in all directions, as all three fibers of the joint participate in the formation of the triangle. We believe that this interpretation is consistent with the model of fibrin branching proposed by Ryan et al., in which branchpoints originate at the point at which protofibrils or filaments diverge. Additional protofibrils are then added to form a mature branchpoint [6]. In about half of the joints showing triangular architecture, the shape is visible prior to manipulation. In the other half, it is not visible until the joint is stretched. The triangular architecture may only be visible in the early stages of joint formation, and as the joint matures, the triangle closes. In cases of triangular architecture, one cross-strut would often rupture without complete failure of the joint.
The results suggest that crosslinking, twisting of protofibrils during aggregation and aggregation in all directions at the joints, which may result in triangular architecture, all help to strengthen fibrin fiber joints. Crosslinked and uncrosslinked joints display remarkable resilience to rupture. On average, the legs of crosslinked and uncrosslinked joints can be stretched to strains of 132% and 146%, respectively, before rupture. Using moduli of 8 MPa and 4 MPa for crosslinked and uncrosslinked fibers, respectively, this corresponds to rupture stresses (strengths) of 11 MPa and 7 MPa, respectively [8]. As well as decreasing extensibility and increasing stiffness, crosslinking also decreases the likelihood of joint failure before fiber failure. The properties of the joints and the single fibers [8] can now be used to construct a realistic model of a blood clot.
Supplementary Material
Joint rupture at the node.
Joint rupture along a fiber.
Fig. S1. Joint rupture at the node.
Fig. S2. Joint rupture along a fiber.
Acknowledgments
We thank the NIH research resource P41 EB002025 for general support. This research was supported with funds from the NSF (CMMI-0646627) (M. Guthold) and the American Heart Association (081503E) (C. R. Carlisle).
Addendum
C. R. Carlisle, E. A. Sparks, C. Der Loughian and M. Guthold designed, performed, analyzed and interpreted the research; C. R. Carlisle and M. Guthold wrote the article.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–76. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–4. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, Lord ST, Guthold M. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falvo MR, Millard D, O’Brien ETI, Superfine R, Lord ST. Length of tandem repeats in fibrin’s alphaC region correlates with fiber extensibility. J Thromb Haemost. 2008;6:1991–3. doi: 10.1111/j.1538-7836.2008.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collet JP, Shuman H, Ledger RE, Lee ST, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci USA. 2005;102:9133–7. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisel J, Nagaswami C, Makowski L. Twisting of fibrin fibers limits their radial growth. Proc Natl Acad Sci USA. 1987;84:8991–5. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8:1030–6. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelman RA, Gladner JA, Nossal R. Rigidity of fibrin gels as measured by quasielastic light scattering. Biopolymers. 1980;19:1259–70. doi: 10.1002/bip.1980.360190702. [DOI] [PubMed] [Google Scholar]
- 10.Shen LL, Hermans J, McDonagh J, McDonagh RP, Carr M. Effects of calcium ion and covalent crosslinking on formation and elasticity of fibrin gels. Thromb Res. 1975;6:255–65. doi: 10.1016/0049-3848(75)90073-0. [DOI] [PubMed] [Google Scholar]
- 11.Baradet TC, Haselgrove JC, Weisel JW. Three-dimensional reconstruction of fibrin clot networks from stereoscopic intermediate voltage electron microscope images and analysis of branching. Biophys J. 1995;68:1551–60. doi: 10.1016/S0006-3495(95)80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosesson MW, DiOrio JP, Siebenlist KR, Wall JS, Hainfeld JF. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood. 1993;82:1517–21. [PubMed] [Google Scholar]
- 13.Mosesson MW, Siebenlist KR, Amrani DL, DiOrio JP. Identification of covalently linked trimeric and tetrameric domains in crosslinked fibrin. Proc Natl Acad Sci USA. 1989;86:1113–17. doi: 10.1073/pnas.86.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litvinov RI, Yakovlev S, Tsurupa G, Gorkun OV, Medved L, Weisel JW. Direct evidence for specific interactions of the fibrinogen alpha C-domains with the central E region and with each other. Biochemistry. 2007;46:9133–42. doi: 10.1021/bi700944j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Joint rupture at the node.
Joint rupture along a fiber.
Fig. S1. Joint rupture at the node.
Fig. S2. Joint rupture along a fiber.