Abstract
Dendritic cells (DCs), which maintain tolerance and orchestrate T cell immune responses, comprise a heterogeneous group of cells. For example, in the steady state, murine spleen contains pre-DC-derived CD8+ and CD8− conventional DCs. During inflammation, monocytes become activated and acquire some DC-like features such as expression of CD11c and MHCII. Although each of these cell types can present antigen, the relative efficiency of processing and presentation after antigen capture by different routes has not yet been systematically compared. To this end we administered OVA to various conventional DCs and activated monocytes by receptor-mediated endocytosis, pinocytosis or phagocytosis and measured internalization and presentation to MHCI and MHCII restricted T cells. We find that CD8− DCs are more efficient than any other type of antigen presenting cell tested in terms of presenting antigen to MHCII restricted T cells, irrespective of the route of antigen capture. In contrast both subsets of splenic DCs are highly effective in cross-presenting antigens to CD8+ T cells. DCs and activated monocytes cross-presented antigens delivered by DEC205-mediated endocytosis and pinocytosis. However, DCs differ from activated monocytes in that the latter are several orders of magnitude less efficient in presenting antigens captured by phagocytosis to CD8+ or CD4+ T cells. We conclude that DCs derived from pre-DCs differ from monocyte-derived cells in that DCs process and present antigens efficiently irrespective of the route of antigen capture. Our observations have significant implications for understanding initiation of immune responses and vaccination strategies targeting DCs and activated monocytes.
Introduction
Dendritic cells (DCs) were first recognized by their unique morphology (1) and later shown to be potent stimulators of the mixed leukocyte reaction (2) and effector T cell responses (3, 4). Unlike macrophages or B cells, which are able to present antigen but are specialized for phagocytosis and antibody production respectively, DCs are professional antigen-presenting cells (5-7).
DCs are closely related to monocytes and macrophages; however, they develop from committed progenitors that diverge from monocytes and macrophages in the bone marrow (8) during the transition between macrophage-and-DC progenitor (9, 10) and common-DC progenitor (11, 12). Common-DC progenitors give rise to pre-DCs, which have lost the potential to produce plasmacytoid DCs (8). Pre-DCs circulate in the blood, seed lymphoid (8) and non-lymphoid organs (13-15) where they divide (16, 17) and differentiate into subpopulations of conventional DCs. In the lymphoid organs DCs become incorporated into networks of cells and present antigen to migrating T cells (8, 18).
In humans as well as in mice DCs are a heterogeneous group of cells, composed of several subsets, all of which can express high levels of CD11c and MHC class II (MHCII) (19). In the mouse spleen, resident DCs can be divided into two main subsets based on the expression of CD8α, DEC-205 and DCIR2: CD8+DEC205+, and CD8− DCIR2+ DCs. In the steady state, both spleen DC subsets are derived from the same common progenitor, the pre-DC (8). However, during inflammation or infection the distribution of DCs in lymphoid organs changes, and monocytes can also acquire DC-like features such as expression of CD11c, MHCII and co-stimulatory molecules (20-22). For example, during Listeria infection, monocytes differentiate in the spleen into TNF/inducible nitric oxide synthase producing CD11c+ cells (tip-DCs) (23). Similarly, cells with many features of DCs can be derived from monocytes cultured with cytokines, such as GM-CSF and IL-4 (24, 25). However, the precise function of these activated monocytes in vivo remains to be defined.
Although both subsets of conventional DCs found in the spleen excel in antigen processing and presentation, CD8−DCIR2+ DCs are more efficient than CD8+DEC205+ DCs in processing antigens for MHCII presentation when antigens are captured by endocytic receptors (26). In contrast, CD8+DEC205+ DCs are superior in cross-presenting antigens to MHC class I (MHCI) after ingestion of dead cells or during viral infection (27-29). However, the two subsets have not yet been compared systematically, either to each other or to other cells for presentation of antigens captured by different mechanisms.
Here we compare MHCI and MHCII antigen presentation by resting or activated spleen DCs, activated monocytes, GM-CSF (GM) and FLT3 ligand (FL) bone marrow culture-derived DCs and activated B cells after antigen capture by either receptor-mediated endocytosis, pinocytosis or phagocytosis. We find that all the different cell types tested can present antigens to MHCII or MHCI restricted T cells after antigen capture by DEC205-mediated endocytosis or pinocytosis, albeit with different efficiencies. However, antigens captured by phagocytosis are only presented effectively by conventional DCs. CD8−DCIR2+ DCs are the most efficient antigen presenting cells for the MHCII pathway irrespective of activation status or mode of antigen acquisition. Finally, both major spleen DC subsets (CD8− and CD8+) are similar in cross-presentation, irrespective of the route of antigen uptake.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratory. Transgenic OT-II, OT-I, CD11c-hDEC (26), B1-8hi (30) mice, as well as DEC-205 deficient and GM-CSF receptor-β deficient (31) mice were bred at the Rockefeller University. 6-10-week-old mice were used in experiments. All experiments were performed in accordance with NIH guidelines and approved by the Rockefeller University Animal Care and Use Committee.
Production of chimeric antibodies and other antigens
Chimeric αmDEC-OVA, αDCIR2-OVA and αhDEC-OVA antibodies were expressed by transient transfection of 293T cells and purified with Protein G (GE Healthcare) as described (32). OVA (Sigma, Grade V A5503) was decontaminated from LPS by multiple rounds of extraction with triton-X 114 (Sigma) (33) and dialysed extensively. NP (Biosearch) and Biotin (Invitrogen) conjugation to OVA were performed according to manufacturers’ instructions. All antigens were tested for LPS contamination (Fisher-Cambrex) and decontaminated by triton-X 114 extraction when necessary. OVA was adsorbed to 2 μM red beads (Polysciences) according to the manufacturer’s protocol. Briefly, 0.5 ml of 2.5% suspension of beads was resuspended in 0.1 M borate buffer pH 8.5 and incubated overnight with 500 μg of OVA at 4°C. Alternatively, to obtain 25% OVA-beads, beads were incubated overnight with 125 μg of OVA and 375 μg of keyhole limpet hemocyanin (Sigma). Unbound protein was washed away extensively, and OVA-adsorbed beads were resuspended in 1 ml PBS. Coupling was confirmed by flow-cytometry with rabbit anti-OVA (Cappel) antibodies followed by anti-rabbit-Cy5 staining (Jackson ImmunoResearch).
Isolation and sorting strategy for DCs and activated monocytes
Dendritic cells were isolated from mice that had been injected 9-12 days before euthanasia with 1-2 106 B16-FL melanoma cells (34). All experiments (except those in Fig. 5) were also performed with dendritic cells not expanded by FL isolated from uninjected control mice, with identical results. For in vivo antigen targeting experiments, mice were injected intraperitoneally with 10 μg of chimeric antibodies 8-12 hours before euthanasia. Activated DCs and monocytes were isolated from mice that were primed and boosted with methylated BSA (mBSA) in CFA (Difco) and then injected intraperitoneally 24 hours before analysis with 100 μg mBSA (Sigma) as described (22, 35). For DC and monocyte isolation, spleens were removed, injected with 0.4 U/ml collagenase D type II (Roche) in HBSS with 2% FCS (Gibco), cut into small fragments and incubated at 37°C for 30 min. Digestion was stopped, with 5 mM EDTA for 5 min, before collection of cell suspensions. Red blood cells were removed by ACK lysis (Gibco). All subsequent steps were performed in PBS 2% FCS, and during incubations cells were kept at 4°C. Non-specific binding was blocked with purified Fc block and DCs were enriched with anti-CD11c beads (Miltenyi) according to manufacturer’s instructions. Enriched naive CD11c+ cells were stained and sorted as follow: CD8+ DCs (B220−, NK1.1−, CD11chi, CD8+) and CD8− DCs (B220−, NK1.1−, CD11chi, CD8−, CD4+). Enriched CD11c+ cells from immunized mice were stained and sorted as follow: iMono (CD8−, CD11bhi, CD11cint and Ly6C+), iCD8− DCs (CD8−, Ly6C−, CD11bint and CD11chi) and iCD8+ DCs (CD8+, CD11chi and Ly6C−). In addition, cells were stained with B220, NK1.1, CD3, Ter119, Ly6G to gate out non-DCs/non-monocytes. In experiments with CD11c-hDEC mice, cells were also stained with humanCD205 to sort positive cells. Sorted populations were collected in complete RPMI (RPMI 1640, Gibco, supplemented with 10% FCS, 10 mM HEPES, 2 mM L-Glutamine, Antibiotic/AntiMycotic, 1 mM Sodium Pyruvate and 53 μM 2-ME) and live cells were typically >95% pure.
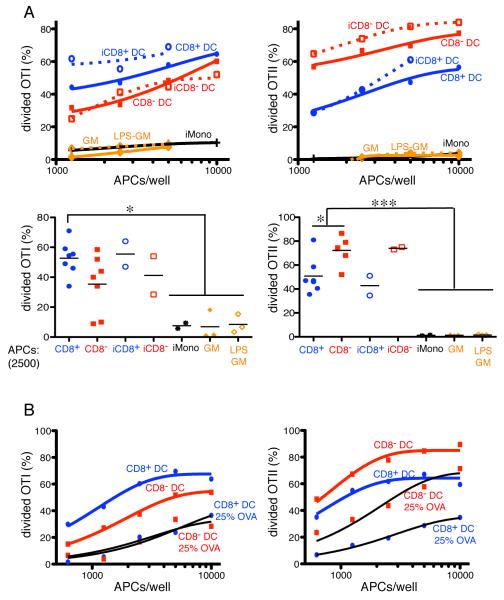
FIGURE 5.
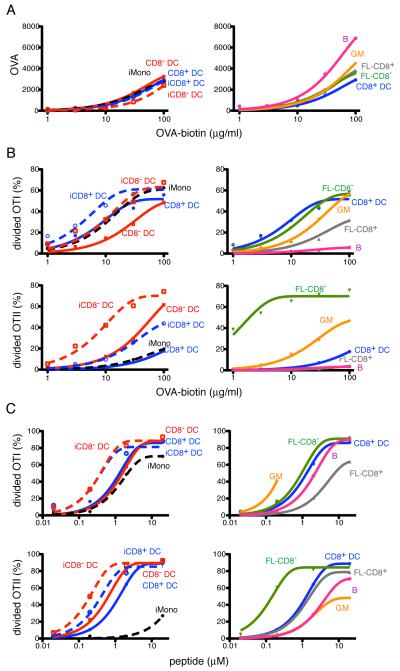
Antigen presentation after phagocytosis. A, APCs were isolated from spleen of naive or mBSA-CFA immunized mice. GM-DCs were derived from bone marrow. Enriched populations were incubated with OVA-beads before sorting. Panels show activation and proliferation of OTI (left panels) and OTII (right panels) T cells after incubation with the indicated number of APCs in the X-axis, that were sorted to contain a single OVA-bead. The Y-axis shows percentage of divided T cells. In lower panels each symbol indicates independent experiments and represents the average of duplicate measurements where 2,500 sorted APCs were incubated with T cells. Data was analyzed by ANOVA and Tukey’s test was used to compare groups: * p < 0.05; ***p<0.001. B, as in A, but DCs from naive mice were incubated with beads adsorbed with 25% OVA and 75% keyhole limpet hemocyanin protein. A and B represent pooled data from 2-7 independent experiments.
Culture and sorting strategy for cultured DCs
Bone marrow cells were obtained by flushing femurs and tibiae with RPMI supplemented with 5% FCS. Red blood cells were removed by ACK lysis (Gibco) and washed with complete RPMI.
For GM-DCs differentiation (24), bone marrow cells were plated at 1 ×106 cells/ml in complete RPMI with 3% vol/vol supernatant of J558L cells transduced with murine GM-CSF (provided by A. Lanzavecchia). Cells were cultured at 37°C and 5% CO2. Media was changed every 2 days, removing loosely adherent and dead cells. GM-DCs were collected at day 7. Non-specific binding was blocked with Fc block and cells were stained with CD11c. CD11chi cells were sorted.
For FL-DC differentiation (36), bone marrow cells were plated at 1.5 ×106 cells/ml in complete RPMI with 100 ng/ml recombinant murine FL. FL was obtained by anti-FLAG purification of supernatant from Chinese hamster ovary (CHO) cells expressing recombinant murine FL-FLAG (kindly provided by C.G. Park, The Rockefeller University). Cells were cultured at 37°C and 5% CO2 and FL-DCs were collected at day 10. Non-specific binding was blocked with purified Fc block and cells were stained and sorted as follow: FL-CD8−DCs (B220−, CD11chi, SIRPαhi and CD24lo) and FL-CD8+DCs (B220−, CD11chi, SIRPαlo and CD24hi) (37).
In vitro activation of DCs was by addition of 1 μg/ml LPS (Sigma) for the last 18 hours of culture. In experiments with CD11c-hDEC mice, cells were also stained with humanCD205 to sort positive cells. Sorted populations were collected in complete RPMI and live cells were typically >95% pure.
B cell culture
Naive B cells were isolated from WT C57BL/6 mice or B1-8hi mice, as indicated. For WT mice, single cell suspensions from spleen were incubated with anti-CD43 beads (Miltenyi) and enriched in LS columns for CD43− naive B cells. For B1-8hi mice, single cell suspensions from spleen and skin-draining lymph nodes were incubated with anti-Igκ-PE (187.1, BD) followed by anti-PE (Miltenyi) beads and anti-CD43 beads (38). Cells were enriched in LS columns (Miltenyi) for CD43−Igκ− naive λ+ B cells (NP-specific). Enriched B cells were plated at 0.7 ×106 cells/ml in complete RPMI supplemented with 25 μg/ml LPS (Sigma) and 5 ng/ml IL-4 (Sigma) and cultured 37°C and 5% CO2 for 2-3 days.
In vitro phagocytosis
Enriched CD11c+ cells from spleen (naive control or immunized mice) or GM-DCs were incubated with OVA-adsorbed beads at 108 cells/ml in complete RPMI, for 30 min at 37°C and 5% CO2. Cells were washed 3 times with cold PBS (900 rcf, 5 min) and stained for cell sorting, as described above.
In vitro antigen delivery
Sorted APCs were counted, and 15,000 live cells/well were plated in complete RPMI in 96-well round bottom plates. For B cell blasts, 30,000 live cells/well were plated. Antigen was added at the indicated concentrations. For receptor-mediated endocytosis (DEC-205 targeting and BCR targeting), all media was kept cold and antigen was pulsed for 20 min at 4°C. For pinocytosis, warm media with antigen (OVA-biotin) was added and plates were incubated at 37°C with 5% CO2 for 2 hours. As controls in all experiments, APCs were also incubated with peptides for 20 min at 4°C: for OTI co-culture we used OVA peptide EQLESIINFEKLTEW and for OTII co-culture we used OVA peptide LSQAVHAAHAEINEAGR, synthesized by the Proteomics Resource Center, The Rockefeller University. After antigen or peptide incubation, cells were washed 3 times with complete RPMI to remove excess free antigen, before assessment of antigen uptake or before incubation at 37°C and 5% CO2, with OVA-specific T cells. All incubations were done in duplicate wells.
Antigen presentation assay
CD8+ and CD4+ OVA-specific T cells were isolated from OTI and OTII mice respectively. CD8+ and CD4+ T cells were enriched with a CD8+ T cell isolation kit or a CD4+ T cells isolation kit, according to the manufacturer’s instructions (Miltenyi) with the addition of anti-CD11c-biotin to the antibody cocktail. Enriched T cells were labeled with 1 μM CFSE (Molecular Probes) in PBS 0.1% BSA for 10 min at 37°C. The reaction was quenched with FCS and cells were washed 2-3 times with complete RPMI. 100,000 to 125,000 T cells were added to each well containing APCs. Activation and division of OVA-specific T cells was determined by flow cytometry after culture at 37°C and 5% CO2 for 60-65 hours (OTI cells) or 80-85 hours (OTII cells). For analysis, cells were stained with Va2 PE, CD69 APC, CD8 or CD4 PerCP and 0.5 μg/ml DAPI before acquisition on LSR II (BD).
Quantification of antigen uptake
After removal of free antigen, APCs were resuspended in cytofix/cytoperm (BD) for 15 min at room temperature and washed in perm-wash (BD) and then in PBS 1% BSA 0.1% saponin. αDEC-OVA and NP-OVA captured by receptor-mediated endocytosis were detected with rabbit polyclonal anti-OVA (Cappel) followed by F(ab)’2 donkey anti-Rabbit IgG-HRP (Jackson ImmunoResearch). OVA-biotin was detected with streptavidin-HRP (Jackson ImmunoResearch). HRP content was assessed by a fluorometric assay with amplex red (Invitrogen). Plates were excited at 530 nm and emission was collected at 590 nm on Cytofluor II (Perseptive Biosystems).
Flow cytometry analysis
The following antibodies, purchased from either BD or eBioscience, were used: CD3 (145-2C11), CD4 (L3T4), CD8 (53-6.7), CD11b (M1/70), CD11c (N418, HL3), CD16 and CD32/Fc block (2.4G2), CD19 (MB19-1 or eBio1D3), CD24/HSA (M1/69), CD45R/B220 (RA3-6B2), CD45.1 (A20), CD45.2 (104), CD69 (H1.2F3), CD86 (GL1), CD115/CSF-1R (AFS98), CD135/Flk-2/Flt3 (A2F10), CD172α/SIRPα (P84), mouseCD205/mDEC-205 (205yefta), humanCD205/hDEC-205 (Mg38), DCIR2 (33D1), Fas/CD95 (Jo2), GL7, H2Kb (AF6-88.5), Ly6C (AL-21 or HK1.4), Ly6G (1A8), MHCII (AF6-120.1 or M5/114.15.2), NK1.1 (PK136), Ter-119 and Vα2 (B20.1). Streptavidin-PE and –PE-Cy5.5 were from eBioscience. Streptavidin APC was from BD. Streptavidin Pacific Blue and DAPI were from Invitrogen. Data was acquired on LSR-II (BD). Analysis was performed using Diva (BD) or FlowJo (TreeStar).
Data analysis
Graphs were compiled on Prism software (GraphPad Software, Inc). Antigen pulse curves representing antigen uptake or T cell proliferation were adjusted to exponential one-phase association curves. Statistical analysis was performed using Prism software as described in each figure.
Results
Antigen acquisition by DEC-205 or B cell receptor-mediated endocytosis
CD8+ DCs excel in cross-presentation during infection with Herpes Simplex Virus and West Nile Virus or after ingestion of apoptotic cells (27-29, 39, 40). However, both CD8+ and CD8− DCs can cross-present when they are incubated with high concentrations of soluble OVA (41), OVA immune-complexes (42) or bacteria expressing OVA (40, 43). Whether these differences are related to the amount of antigen acquired, the mechanism of uptake or some other cell intrinsic difference between the two DC subsets is not known.
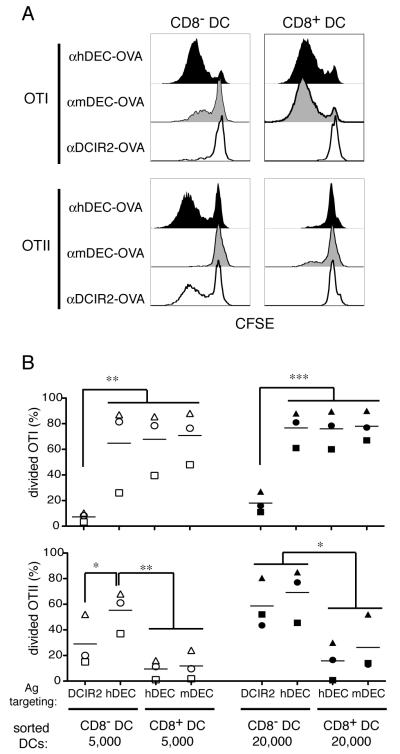
In order to compare the antigen presenting activity of the different DC subsets after receptor-mediated endocytosis we made use of mice that express human DEC-205 under the control of the CD11c promoter (CD11c-hDEC) because all DC subsets in these mice express hDEC-205 (26). OVA was targeted to DCs in vivo by injecting chimeric anti-human-DEC-205 monoclonal antibodies that carry intact OVA (αhDEC-OVA). As controls we used anti-mouse-DEC-OVA (αmDEC-OVA) and anti-DCIR2-OVA (αDCIR2-OVA) antibodies which target either the CD8+ or CD8− DCs in the spleen respectively (26, 32). Following antibody injection, hDEC expressing CD8− and CD8+ splenic DCs were purified by cell sorting and evaluated for MHCI or MHCII presentation by co-culture with CFSE labeled OVA specific OTI and OTII transgenic T cells. As previously documented, CD8− DCs targeted in vivo with αDCIR2-OVA efficiently induced OTII proliferation but little OTI proliferation; whereas CD8+ DCs targeted in vivo with αmDEC-OVA induced robust OTI and more modest OTII proliferation (Fig. 1) (26). Consistent with the low but detectable levels of mDEC-205 expressed by CD8− DCs (Supplementary Fig. 1A)(44), we found small amounts of cross-presentation by CD8− DCs upon αmDEC-OVA targeting (Fig. 1A). When both DCs were targeted with αhDEC-OVA, CD8− DCs were more effective at stimulating OTII proliferation than CD8+ DCs from the same mice (Fig. 1) (26). In contrast, both CD8− and CD8+ DCs targeted in vivo with αhDEC-OVA elicited similarly robust levels of OTI proliferation (Fig. 1). These results indicate that both DC subsets have the same intrinsic potential to cross-present antigens when the antigen is delivered by DEC-205.
FIGURE 1.
hDEC205-mediated endocytosis in vivo promotes cross-presentation by both CD8− and CD8+ DC subsets. A, Representative histograms show proliferation as measured by CFSE dye dilution of OTI (upper panels) and OTII (lower panels) T cells upon culture with 5×103 CD8− or CD8+ DCs isolated from CD11c-hDEC transgenic mice injected with αhDEC-OVA, αmDEC-OVA or αDCIR2-OVA, as indicated. B, Summary of 3 independent experiments as in A. Panels show the percentage of divided, CFSE low OTI (upper panel) and OTII (lower panel) after incubation with 5 × 103 (open symbols) or 20 × 103 (solid symbols) CD8− or CD8+ DCs that were targeted with OVA, as indicated. Each symbol indicates independent experiments and represents the average of duplicate measurements. Spleens of 3-10 injected mice were pooled in each experiment. Data was analyzed by repeated measures ANOVA and Tukey’s test was used to compare groups: * p < 0.05; **p < 0.01; ***p<0.001.
Although both subsets of DCs express similar levels of hDEC-205 in the CD11c-hDEC transgenic mice (26), differences in antigen presentation may nevertheless be the result of differences in the amounts of antigen captured. To explore this possibility we isolated spleen CD8− and CD8+ DCs from CD11c-hDEC mice, pulsed them at 4°C with varying amounts of αhDEC-OVA and measured the amount of cell-associated OVA (Fig. 2A, left). Replica plates of the pulsed DCs were then co-cultured with CFSE labeled OTI or OTII transgenic T cells at 37°C for 3-4 days to measure their ability to present OVA (Fig. 2B, left). As a further control, DCs were pulsed with peptides to measure their antigen presenting activity independent of antigen capture and processing (Fig. 2C, left).
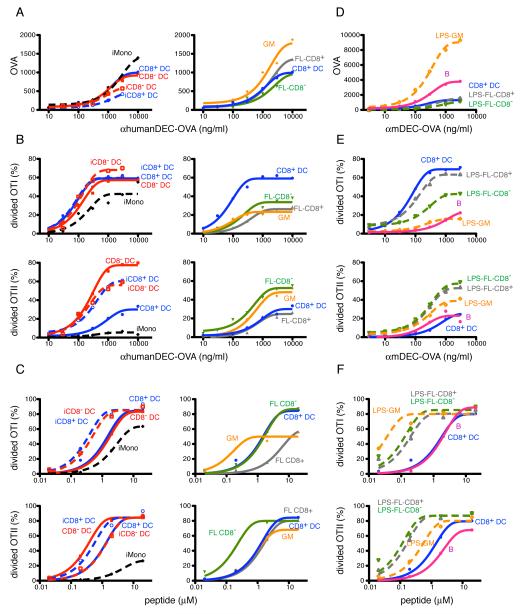
FIGURE 2.
Antigen presentation after DEC205-mediated endocytosis in vitro. A-C, APCs from CD11c-hDEC mice were isolated from naive (solid lines) or mBSA-CFA immunized (dashed lines) mice (left panels), or cultured from bone marrow (right panels). A, The Y-axis shows relative cell-associated OVA, as measured by rabbit anti-OVA and developed with anti-rabbit-HRP, after targeting with the indicated concentrations of αhDEC-OVA on the X-axis. B, Activation and proliferation of OTI (upper panels) and OTII (lower panels) T cells in response to OVA containing APCs. The Y-axis shows the percentage of divided T cells. C, Graphs show OTI (upper panels) and OTII (lower panels) T cell proliferation in response to peptide pulsed APCs. D-F, APCs were isolated or cultured from spleen or bone marrow of WT mice. D and E, as in A and B, but targeting was with αmDEC-OVA. F, As in C. A-F, graphs represent pooled data from 3-7 independent experiments.
CD8− and CD8+ splenic DCs captured similar amounts of OVA when pulsed with αhDEC-OVA (Fig. 2A, left) and showed similar intrinsic antigen presenting activity when pulsed with peptides (Fig. 2C, left). When antigen was titrated under conditions when DCs were present in excess, the results of in vitro targeting mirrored those obtained in vivo in that the two subsets were equivalent for MHCI cross-presentation and also that CD8− DCs were intrinsically more efficient than CD8+ DCs in processing and presenting antigens in MHCII (Fig. 2B, left and supplemental Fig. 2A). Only small differences in presentation were found when the number of antigen presenting cells were titrated: CD8+ DCs were about 3-fold more effective than CD8− DCs for cross-presentation when less than 10,000 DCs were present (Supplemental Fig. 2B, upper panels). In contrast, CD8− DCs were several orders of magnitude more efficient than CD8+ DCs for MHCII presentation, irrespective on the number of DCs assayed (Supplemental Fig. 2B, lower panels). We conclude that CD8− and CD8+ splenic DCs are similar in terms of their ability to cross-present antigens on MHCI when the antigen is captured by DEC-205.
During some infections or inflammation, monocytes become activated and can differentiate into GM-CSF dependent tip-DCs (22, 23, 35, 45) (Supplemental Fig. 3). Conversely, conventional DCs are FL-dependent, GM-CSF-independent and derived from pre-DCs (16). In order to compare activated monocytes to conventional DCs we immunized hDEC-205 transgenic mice with CFA (35) to induce monocyte activation. Activated monocytes and conventional DCs were purified by cell sorting and compared after targeting with αhDEC-OVA. Activated monocytes had similar levels of CD86, 2-fold higher levels of MHCI but lower levels of MHCII when compared to conventional DCs from naive control mice (Supplemental Fig. 3C and 4). Conventional DCs isolated from the immunized mice (iCD8+ and iCD8− DCs) had higher levels of CD86, MHCI and MHCII when compared to control DCs (Supplemental Fig. 3C and 4).
All cell types isolated from the immunized mice (iCD8+ and iCD8− DCs, and iMono) were similar to control DCs in antigen uptake after αhDEC-OVA targeting (Fig. 2A, left) (46). However, DCs from immunized mice were more efficient than their naive counterparts with regard to the presentation of pulsed peptides. On the other hand, activated monocytes were less efficient than DCs, especially for MHCII presentation, when pulsed with peptides (Fig. 2C, left). Consistent with the peptide presentation experiments, activated monocytes performed poorly in MHCII presentation, but showed levels of cross-presentation that approached those of conventional DCs (Fig. 2B, left). We conclude that activated monocytes resemble conventional DCs in their ability to cross-present antigens captured by DEC205-mediated endocytosis. However, under the same conditions, activated monocytes are far inferior to naive or activated conventional spleen DCs with regard to MHCII presentation.
Cultured DCs and B cells
DCs obtained by culturing murine bone marrow cells with GM-CSF (GM-DCs) are widely used as antigen-presenting cells (24). A second method for producing DCs in vitro involves murine bone marrow culture in the presence of FL (FL-DCs)(36). FL-DCs are heterogeneous, and contain cells that resemble CD8+ and CD8− splenic DCs (FL-CD8+ and FL-CD8− DCs respectively) as well as plasmacytoid DCs in terms of cell surface marker and transcription factor expression (37, 47). Both FL- and GM-DCs can be activated with TLR agonists such as LPS to increase the expression of MHC and co-stimulatory molecules (36, 48, 49), as well as antigen processing (50, 51) and the stability of MHC-peptide complexes on the cell surface (6, 52). Moreover, endogenous mouse DEC-205 expression is induced on cultured DCs upon TLR ligation (36, 53).
In order to compare the ability of tissue culture-derived DCs and conventional DCs to present antigens captured by receptor-mediated endocytosis, we targeted GM- and FL-DCs obtained from hDEC transgenic mice with αhDEC-OVA. CD86, MHCI and MHCII expression by GM- and FL-DCs was equal to or higher than spleen DCs (Supplemental Fig. 4). But GM-DCs accumulated more OVA than spleen DCs or FL-DCs after αhDEC-OVA targeting (Fig. 2A, right). Nevertheless, GM-DCs were less effective than spleen DCs in cross-presentation (Fig. 2B, right and supplemental Fig. 2A). Consistent with their high levels of MHCII expression, GM-DCs were efficient in presenting pulsed peptides to MHCII restricted T cells (Fig. 2C, right). However, the same cells were intermediate, between CD8+ and CD8− DCs, for MHCII presentation after antigen capture by DEC205-mediated endocytosis (Fig. 2B, right and supplemental Fig. 2A).
FL-DCs were separated into CD8− (Sirpαhi) and CD8+ (CD24hi) subsets. Both FL-DC subsets induced less OTI proliferation than splenic DCs (Fig. 2B, right). However, FL-CD8+ DCs were almost 10-fold less efficient than their splenic counterparts for MHCI presentation after exogenous peptide loading (Fig. 2C, right). For MHCII presentation FL-CD8− DCs were more efficient that FL-CD8+ DCs, the latter showing similar efficiency as their splenic counterpart CD8+ DCs (Fig. 2B, right). We conclude that both GM- and FL-DCs are capable of MHCI and MHCII presentation when antigen is captured by DEC205-mediated endocytosis; however, these cells are less active than conventional spleen DCs.
In order to determine how activation by TLR ligation alters DCs ability to present antigens acquired through receptor-mediated endocytosis, we stimulated GM- or FL-DCs with LPS and measured antigen presentation after targeting with αmDEC-OVA. Naive CD8+ DCs from spleen were used as controls in all experiments. As expected, stimulation with LPS enhanced expression of MHCI and II and co-stimulatory molecules in all culture-derived DCs (Supplemental Fig. 4)(6). GM-DCs activated with LPS express high levels of DEC-205 and thus captured more antigen than any of the other DCs tested (Fig. 2D). In addition, when loaded with exogenous peptide, LPS-GM-DCs performed better than control CD8+ DCs in activating MHCI and MHCII dependent T cell responses (Fig. 2F). However, LPS-GM-DCs were far less effective than CD8+ DCs and no better than resting GM-DCs in cross-presentation of antigens acquired by DEC205-mediated endocytosis (Fig. 2E and supplemental Fig. 2C). Finally, LPS stimulation also decreased the relative efficiency of GM-DCs with regards to processing antigen to be presented on MHCII (Fig. 2E).
LPS-FL-DCs captured similar amounts of antigen as did control splenic CD8+ DCs (Fig. 2D) but showed increased activation of MHCI and MHCII restricted T cells after peptide loading (Fig. 2F). Surprisingly, LPS stimulation had a positive impact on antigen presentation on FL-CD8+ but not on FL-CD8− DCs (Fig. 2E, and supplemental Fig. 2C). In conclusion, LPS failed to enhance MHCI and MHCII presentation by all cultured DCs, with the exception of FL-CD8+ DCs.
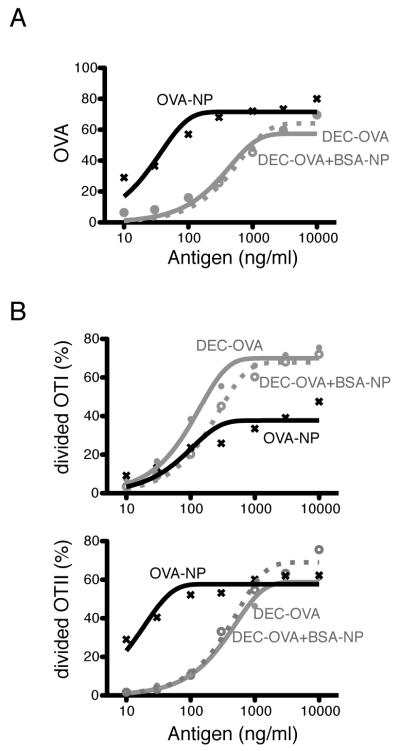
B cells express constitutive low levels of DEC-205 (53) but increase DEC-205 expression upon activation: germinal center B cells have higher DEC-205 expression than naive B cells (Supplemental Fig. 1A) and B cells express high levels of DEC-205 after culture with LPS and IL-4 or anti-CD40 and IL-4 (Supplemental Fig. 1B). LPS/IL-4 activated B cells, which also expressed higher levels of CD86, MHCI and II than splenic DCs (Supplemental Fig. 4), were compared to DCs for antigen processing and presentation after targeting with αmDEC-OVA. Activated B cells captured more antigen than CD8+ DCs (Fig. 2D), and were equivalent to CD8+ DCs in presenting exogenously loaded peptide to MHCI and MHCII restricted T cells (Fig. 2F). Consistent with these observations, activated B cells were also similar to CD8+ DCs in antigen presentation to MHCII restricted T cells after antigen targeting to DEC-205 (Fig. 2E). However, B cell blasts induced far less OTI proliferation and were much less efficient than DCs in cross-presentation (Fig. 2E and supplemental Fig. 2C). When the relative amount of antigen uptake is taken into account, similar results were obtained for MHCII presentation for antigens targeted to the B cell antigen receptor instead of DEC-205 (Fig. 3A and 3B) on B cells from B1-8hi mice, specific for NP (4-hydroxy-3-nitrophenil) (30). However, antigen captured by DEC-205 was approximately three times more efficient than the B cell antigen receptor for cross-presentation (Fig. 3A and 3B). This difference is not dependent on B cell receptor cross-linking, since αDEC-OVA together with BSA-NP was equivalent to αDEC-OVA alone.
FIGURE 3.
DEC205-mediated endocytosis promotes cross-presentation in B cells. NP-specific B cells were isolated from B1-8hi transgenic mice and stimulated with LPS and IL-4 for 50-60 hours. A, The Y-axis shows relative cell-associated OVA, after targeting with the indicated concentrations of αmDEC-OVA, or αmDEC-OVA and BSA-NP, or OVA-NP on the X-axis. Maximum cell-associated OVA was normalized to 100 in each experiment. B, Activation and proliferation of OTI (upper panel) and OTII (lower panel) T cells in response to OVA containing B cells. The Y-axis shows percentage of divided T cells. A and B, represent pooled data from 3 independent experiments.
We conclude that, when antigen is captured by DEC205-mediated endocytosis, FL-DCs (both CD8+ and CD8−) resemble conventional spleen DCs with regards to efficiency of antigen presentation. Furthermore, antigen delivery by endocytosis through DEC-205 is not sufficient to ensure high efficient cross-presentation, since B cells and LPS-GM-DCs were almost 10-fold less effective in cross-presentation than splenic DCs.
Antigen presentation after pinocytosis
To determine whether the observed differences in antigen presentation were cell intrinsic or dependent on the route of antigen capture, we loaded cells with antigen by pinocytosis, using high concentrations of soluble OVA (up to 100 μg/ml). Although some preparations of OVA display mannose residues that serve as ligands for mannose receptors, we found no detectable binding at 4°C of our OVA preparation to any of the cell types tested (Supplemental Fig. 5 A and B).
Both spleen DC subsets obtained from naive and immunized mice showed similar levels of OVA endocytosis after incubation at 37°C (Fig. 4A, left). Despite their similarities in antigen uptake and presentation of exogenous peptides, OVA captured by bulk pinocytosis was presented more effectively by CD8− than CD8+ DCs to MHCII restricted T cells (Fig. 4B and 4C, left). In addition, DCs obtained from immunized mice were more efficient in MHCII antigen presentation than naive DCs, but the differences between the subsets did not change qualitatively (Fig. 4B, left). Therefore, CD8− DCs are more efficient than CD8+ DCs at presentation to MHCII restricted T cells, irrespective of the route of antigen uptake or activation status.
FIGURE 4.
Antigen presentation after pinocytosis. APCs were isolated from spleen or cultured from bone marrow or spleen of WT mice. A, The Y-axis shows relative cell-associated OVA, developed with streptavidin-HRP, after incubation of APCs with the indicated concentrations of OVA-biotin on the X-axis. B, Activation and proliferation of OTI (upper panels) and OTII (lower panels) T cells in response to OVA containing APCs. The Y-axis shows percentage of divided T cells. C, Graphs show OTI (upper panels) and OTII (lower panels) T cell proliferation in response to peptide pulsed APCs. A-C represent pooled data from 3-5 independent experiments.
Despite antigen uptake at levels similar to conventional DCs, activated monocytes showed low levels of MHCII presentation of pinocytosed antigen, which correlated with low levels of presentation with pulsed peptide (Fig. 4, left). However, activated monocytes were similar to spleen DCs for cross-presentation (Fig. 4B, left). We conclude that activated monocytes can cross-present pinocytosed antigens as efficiently as spleen DCs; however, these cells are far less effective in MHCII presentation when antigen is captured by either DEC205-mediated or bulk phase endocytosis.
Among cultured DCs, FL-DCs acquired similar amounts of OVA by pinocytosis as their spleen DC counterparts (Fig. 4A, right). Whereas FL-CD8− DCs were equivalent to spleen DCs for cross-presentation and far more efficient with regards to MHCII presentation of pinocytized antigen, FL-CD8+ DCs were less effective in both tasks (Fig. 4B, right and supplemental Fig. 5C). GM-DCs accumulated similar amounts of OVA by bulk phase pinocytosis and performed similarly to CD8+ DCs for cross-presentation and intermediate between the two DC subsets for MHCII presentation (Fig. 4A and 4B, right).
In contrast, activated B cells, which were active in endocytosis and presentation of peptides, were nearly inactive in processing antigen acquired by bulk phase pinocytosis for presentation on MHCII or MHCI (Fig. 4 right and supplemental Fig. 5C). Therefore, we conclude that both the cell type and the route of endocytosis influence the efficiency of antigen presentation.
Antigen presentation after phagocytosis
To further analyze the effects of the route of antigen uptake on antigen presentation we delivered OVA to antigen-presenting cells by phagocytosis of OVA-adsorbed polystyrene beads. Flow cytometry was used to purify cells that had captured a single fluorescent bead to normalize the amount of antigen captured. Internalization was confirmed by analysis on Image Stream cytometry (Supplemental Fig. 6). Naive and activated splenic DCs presented antigen acquired by phagocytosis in a manner that was similar to antigen captured by DEC205-mediated or bulk phase pinocytosis (Fig. 5A). CD8− DCs remained more efficient than CD8+ in MHCII presentation, and cross-presentation was similar in both types of DCs (Fig. 5A). Decreasing the amount of OVA on the beads did not alter the results (Fig. 5B). However, neither activated monocytes nor GM-DCs, nor LPS activated GM-DCs presented phagocytized antigens to MHCI or MHCII restricted T cells to any appreciable degree (Fig. 5A). Similar results were obtained when we assayed splenic monocytes from Listeria infected mice (tip-DCs) (Supplemental Fig. 7). We conclude that activated monocytes and GM-DCs are far less effective in antigen presentation than conventional DCs when the antigen is acquired by phagocytosis.
Discussion
We have systematically compared conventional spleen DCs to tissue culture-derived DCs and to activated monocytes for presentation of antigen acquired by DEC205-mediated endocytosis, pinocytosis or phagocytosis. We find that conventional spleen DCs differ from all other cells tested in that they excel in antigen presentation irrespective of the route of antigen uptake and activation status. In contrast, the mechanism of antigen capture has a profound influence on the efficiency of antigen presentation by other antigen presenting cells. For example, activated monocytes and GM-DCs (which are monocyte derived) are nearly as effective as conventional DCs in cross-presentation of antigens captured by DEC205-mediated and bulk phase endocytosis, but they are nearly inactive when the same antigens are captured by phagocytosis. Similarly, activated B cells are as effective as CD8+ DCs for MHCII antigen presentation when the antigen is delivered by receptor-mediated endocytosis (DEC-205 or the B cell antigen receptor), but the same cells are ineffective when the antigen enters the cell by bulk phase endocytosis.
As has been previously documented, antigen uptake was at least 100-fold more efficient when delivered through specific receptors as compared to bulk phase pinocytosis (54). However, not all receptors deliver antigen to intracellular compartments with equal efficiency. For example, DEC-205 is more efficient in antigen internalization than DCIR2 (26). But when equivalent amounts of captured antigen are compared, the efficiency of presentation by DCs was similar for bulk phase and DEC205-mediated endocytosis. B cells differed from DCs in that antigen taken up by bulk phase pinocytosis were presented inefficiently when compared to receptor-mediated uptake. Unlike DCs, whose primary function is to initiate immunity, B cells present antigens to recruit specific cognate T cell help. The relative inefficiency at processing and presentation of antigens acquired by bulk phase endocytosis guarantees that T cell help remains focused during an immune response. Only B cells that capture antigen through specific antigen receptors will recruit T cell help. In contrast, the DCs’ ability to present a range of antigens acquired by pinocytosis serves to broaden the scope of T cell immunity.
Several groups have suggested that cross-presentation is a specialized function of CD8+ DCs (27-29, 39), while others have come to the opposite conclusion (43, 55-57). However, many of these experiments failed to consider the amount or route of antigen capture. For instance, CD8+ DCs are far better at taking up dead cells than CD8− DCs and therefore are favored in terms of presenting antigen contained in dead cells (41). Similarly, CD8+ DCs are the preferred antigen cross-presenting cells in viral (29, 58) or Listeria monocytogenes (27) infections; however, this too may be due to differential access to the antigen. We have shown that CD8+ DCs are enriched in components of the MHCI processing pathway but we did not measure the intrinsic MHCI antigen presenting capacity of CD8+ vs CD8− cells directly (26). The data presented here show that when the amount of antigen captured and the route of internalization are taken into consideration, the two subsets of conventional spleen DCs have similar abilities with regards to cross-presentation. In agreement with others, we found that there were small differences in MHCI cross-presentation by the two DC subsets: CD8+ DCs were slightly more efficient than CD8− DCs after phagocytosis of OVA-adsorbed beads (40) and also after DEC205-mediated endocytosis when limiting number of DCs are compared. However, these differences were far less pronounced than the difference between either subset of DCs and antigen presenting cells derived from monocytes.
In contrast to cross-presentation, CD8− DCs are more efficient than CD8+ DCs and most other APCs tested for processing and presenting antigens in MHCII, irrespective of the route of antigen acquisition or maturation status (26). Thus in the steady state this subset may be key to maintain CD4+ T cell tolerance to self-antigens (32, 59-61). Nevertheless, activation improves MHCII presentation by CD8+ DCs and may enhance their ability to activate CD4+ T cells during infection.
Activated monocytes, including GM-DCs and tip-DCs share many of the features of conventional spleen DCs, but arise from distinct progenitors (8, 10, 22, 62). For example, whereas conventional DCs are normal in CCR2−/− mice, tip-DCs do not develop in the spleen of these mice after Listeria infection because monocytes fail to emigrate from the bone marrow (63). In addition, activated monocytes do not express characteristic DC lectins (DCIR2 or DEC-205), or high levels of Flk2 (FL receptor), but instead express the M-CSF receptor, like their monocyte progenitors (Supplemental Fig. 3D and 7B). Finally, CD8+ DCs and activated monocytes rely on different mechanisms to enhance cross-presentation (64). Although activated monocytes contribute to immunity against influenza (65), Lesihmania (66) and Listeria (23), they do not appear to be responsible for the initiation of adaptive immune responses. This is consistent with these cells’ lower levels of MHCII surface expression and poor performance in MHCII presentation, irrespective of the mechanism of antigen capture, including peptide pulsing. Notably, monocyte-derived DCs do not contribute significantly to the steady state pool of DCs in lymphoid (8) and may only contribute to CD103− populations of CD11c+ cells in non-lymphoid organs (13, 14), which have poor T-cell stimulation capacity (14, 67).
Although they appear to be most closely related to activated monocytes and tip-DCs (62, 68), the physiologic counterparts of bone marrow-derived GM-DCs remain to be defined. GM-DCs can present antigens captured by DEC205-mediated or bulk phase endocytosis; these cells also transport MHCII to the cell surface after activation by TLR ligation (6, 48, 50, 51). However, prior activation did not have a positive impact on their ability to present antigens to T cells. In contrast, FL-DCs resembled conventional spleen DCs in this important respect; i.e., antigen presentation was enhanced by activation through TLR ligation. Also, mirroring their splenic counterparts, FL-CD8− DCs were better than FL-CD8+ DCs in activating MHCII restricted T cells. FL-DCs originate from the same progenitor (pre-DCs), rely on the same cytokine (FL) for differentiation and expansion, and are more similar to conventional DCs with regard to antigen processing and presentation. Thus, GM-DCs may not be the best cell type to investigate the role of conventional DCs in immune responses.
Keeping with the idea that the primary function of activated monocytes and GM-DCs might be as innate immune effector cells that destroy phagocytized antigen (23, 62), these cells were far less efficient in presenting phagocytized antigen than conventional DCs. This observation is consistent with the finding that monocyte-derived mononuclear phagocytes tend to have more developed lysosomes that may hamper the escape of processed peptides into MHC loading compartments (7, 69).
We have shown that cell intrinsic differences have a profound impact on antigen handling for MHCII and cross-presentation. Although most APCs studied have the ability to process antigen, in non-DCs the route of antigen capture has a profound impact on its subsequent processing and presentation. Our observations have significant implications for understanding the development of adaptive immune responses and targeted vaccination strategies.
Supplementary Material
Acknowledgments
The authors thank C.G. Park for cells producing rFL; K. Yao for production and purification of rFL; D. Bosque and T. Eisenreich for genotyping and caring for mice; E. Pamer for L. monocytogenes; T. Schwickert for B1-8hi mice; C.G. Park and C. Chong for CD11c-hDEC mice; and G. Begley (Amgen) for GMCSF-Rβ deficient mice. We thank K.Velizon and O. Uche for the multiple long sorts and we also thank C. Bare from The Rockefeller University Flow Cytometry facility for operating the Image Stream100 flow cytometer. We are grateful to members of the lab and R.M. Steinman for helpful discussions and critical reading of the manuscript.
This work was supported in part by NIH grant number AI051573. M.C. Nussenzweig is an HHMI investigator. D. Dudziak is supported by the German Research Foundation (DU548/1-1 and DU548/2-1). A.O. Kamphorst was supported by a CRI fellowship.
Abbreviations used in this paper
- DC
dendritic cell
- MHCI
MHC class I
- MHCII
MHC class II
- tip-DCs
TNF/inducible nitric oxide synthase producing DCs
- FL
Fms-like tryrosine kinase 3 ligand
- GM
GM-CSF
- NP
4-hydroxy-3-nitrophenil
- αmDEC-OVA
anti-mouse-DEC205-OVA mAb
- αDCIR2-OVA
anti-DCIR2-OVA mAb
- αhDEC-OVA
anti-human-DEC205-OVA mAb
- CD11c-hDEC mice
mice carrying a transgene with the human-DEC205 receptor under the control of the CD11c promoter
- mBSA
methylated BSA
Footnotes
Diana Dudziak present address: Laboratory of Dendritic Cell Biology, Department of Dermatology, University Hospital of Erlangen, Glückstr. 6, 91054 Erlangen
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig MC, Steinman RM, Gutchinov B, Cohn ZA. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980;152:1070–1084. doi: 10.1084/jem.152.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 7.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 10.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 12.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 13.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 19.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 20.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 23.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 27.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jirmo AC, Nagel CH, Bohnen C, Sodeik B, Behrens GM. Contribution of direct and cross-presentation to CTL immunity against herpes simplex virus 1. J Immunol. 2009;182:283–292. doi: 10.4049/jimmunol.182.1.283. [DOI] [PubMed] [Google Scholar]
- 29.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 31.Robb L, Drinkwater CC, Metcalf D, Li R, Kontgen F, Nicola NA, Begley CG. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci U S A. 1995;92:9565–9569. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 34.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 35.Cook AD, Braine EL, Hamilton JA. Stimulus-dependent requirement for granulocyte-macrophage colony-stimulating factor in inflammation. J Immunol. 2004;173:4643–4651. doi: 10.4049/jimmunol.173.7.4643. [DOI] [PubMed] [Google Scholar]
- 36.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 37.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8-dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 38.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 39.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C. Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse. Blood. 1999;93:590–598. [PubMed] [Google Scholar]
- 45.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weigel BJ, Nath N, Taylor PA, Panoskaltsis-Mortari A, Chen W, Krieg AM, Brasel K, Blazar BR. Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 2002;100:4169–4176. doi: 10.1182/blood-2002-04-1063. [DOI] [PubMed] [Google Scholar]
- 48.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 49.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 50.Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–936. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 52.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 53.Inaba K, Swiggard WJ, Inaba M, Meltzer J, Mirza A, Sasagawa T, Nussenzweig MC, Steinman RM. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. 1995;163:148–156. doi: 10.1006/cimm.1995.1109. [DOI] [PubMed] [Google Scholar]
- 54.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 55.Yrlid U, Wick MJ. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J Immunol. 2002;169:108–116. doi: 10.4049/jimmunol.169.1.108. [DOI] [PubMed] [Google Scholar]
- 56.Robson NC, Donachie AM, Mowat AM. Simultaneous presentation and cross-presentation of immune-stimulating complex-associated cognate antigen by antigen-specific B cells. Eur J Immunol. 2008;38:1238–1246. doi: 10.1002/eji.200737758. [DOI] [PubMed] [Google Scholar]
- 57.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci U S A. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 63.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 64.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrenreich BA, Cohn ZA. The uptake and digestion of iodinated human serum albumin by macrophages in vitro. J Exp Med. 1967;126:941–958. doi: 10.1084/jem.126.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.