Abstract
Overexpression of plant Bax Inhibitor-1 (BI-1) was able to suppress Bax-mediated cell death in yeast and Arabidopsis. Here, we demonstrate that reactive oxygen species production induced by the ectopic expression of Bax was insensitive to the coexpression of AtBI-1. Similarly, H2O2– or salicylic acid–mediated cell death also was suppressed in tobacco BY-2 cells overexpressing AtBI-1. To define the functional domain of AtBI-1 as a cell death suppressor, a truncated series of the AtBI-1 protein was analyzed in yeast possessing a galactose-inducible mammalian Bax. The results showed that ΔC-AtBI-1 (with the C-terminal 14 amino acids deleted) lost the ability to sustain cell growth. Furthermore, a mutant protein in which the C-terminal seven amino acid residues of AtBI-1 were replaced with others lacking a coiled-coil structure failed to inhibit cell death, suggesting that the C-terminal region is essential for the inhibition of cell death. We also noted that the C-terminal hydrophilic region was interchangeable between animal and plant Bax inhibitors.
INTRODUCTION
Like animal cells, plant cells can respond to various stimuli, including fungal toxins and biotic and abiotic stresses, by initiating programmed cell death (PCD). Some morphological and biochemical changes, such as cell shrinkage, chromatin condensation, and DNA fragmentation, seem to be common features of cell death because they occur in both plant and animal cells (Lam et al., 2001). In animals, the key regulators of apoptosis are evolutionarily conserved. For example, the ced-9 protein in Caenorhabditis elegans is homologous with the antiapoptotic members of the Bcl-2 family (Hengartner and Horvitz, 1994), which is a large family of proteins that act as either activators (e.g., Bax and Bak) or suppressors (e.g., Bcl-2 and Bcl-XL) of PCD (reviewed by Reed, 1994; Kroemer, 1997). Similarly, the main executors of PCD, such as caspases, are conserved in the animal kingdom (Yuan et al., 1993). By contrast, in a Basic Local Alignment Search Tool (BLAST) database search of the yeast and plant genomes, no obvious homologs of any crucial regulators of metazoan apoptosis (members of the Bax/Bcl-2 family, caspases, Apaf-a/Ced-4, p53) were detected. However, the introduction of mammalian death regulators in yeast and plant cells induced the proper phenotype with regard to the nature of the gene introduced (Lacomme and Cruz, 1999; Mitsuhara et al., 1999; Kawai-Yamada et al., 2001).
It has been noted that yeast cells, both Saccharomyces cerevisiae and Schizosaccharomyces pombe, can undergo cell death by the expression of the proapoptotic mammalian genes Bax and Bak (Bischoff et al., 1992; Sato et al., 1994; Hanada et al., 1995; Greenhalf et al., 1996). Madeo et al. (2002) demonstrated that Yor197w, a yeast protein with structural homology with mammalian caspases, has caspase-like processing activity and regulates H2O2-induced yeast death. Therefore, elements of the PCD pathway conserved in yeast as well as animals should belong to a basic and ancient, evolutionarily conserved mechanism. In animal cells, the mitochondrion plays a crucial role in PCD. Upon perceiving cell death signals, the proapoptotic protein Bax forms channels in the outer membrane of the mitochondrion and triggers the release of cytochrome c (Green and Reed, 1998). The latter subsequently activates a series of caspases that result in the proteolysis of proteins essential for the maintenance of cell integrity.
Oxidative stress also was found to be involved in plant PCD processes (Lamb and Dixon, 1997; Mittler, 2002). Several investigators have shown that H2O2 induced PCD in suspension cultures of soybean (Levine et al., 1994), Arabidopsis (Desikan et al., 1998), and tobacco (Houot et al., 2001) cells. In addition, Houot et al. (2001) showed that H2O2 induces PCD through a process similar to apoptosis, including cell shrinkage, chromatin condensation, and DNA fragmentation. The process induced by H2O2 depends on active cellular metabolism and can be blocked by protease inhibitors (Solomon et al., 1999). Rao and Davis (1999) demonstrated that treatment with salicylic acid (SA) caused plant cell death by enhanced H2O2 production, lipid peroxidation, and oxidative damage to proteins.
Although no Bax homolog has been identified in plant genomes to date, the overexpression of mammalian Bax in tobacco (Lacomme and Cruz, 1999) and Arabidopsis (Kawai-Yamada et al., 2001) causes cell death. Thus, when the animal Bax gene is expressed in plant cells under a dexamethasone (DEX)-inducible system, such plants exhibit marked cell death at the whole-plant level, with cell shrinkage, membrane destruction, and other apoptotic phenotypes (Kawai-Yamada et al., 2001). Recently, Abramovitch and co-workers (2003) demonstrated that the Pseudomonas type III effecter AvrPtoB suppresses Bax-induced cell death in yeast. They showed that this gene induces plant disease susceptibility by inhibiting host PCD. The morphological and biochemical features resulting from the ectopic expression of human death regulators (Lacomme and Cruz, 1999; Mitsuhara et al., 1999; Kawai-Yamada et al., 2001) strongly suggest that death mechanisms in plants are operational as in animal cells.
Xu and Reed (1998) identified a human cDNA that suppresses Bax-mediated cell death in yeast, and the corresponding protein was named Bax Inhibitor-1 (BI-1). Subsequently, we isolated BI-1 homologs from rice (OsBI-1) and Arabidopsis (AtBI-1) and showed that the overexpression of plant BI-1 also could suppress Bax-mediated cell death in yeast (Kawai et al., 1999). The chlorosis caused by Bax expression was retarded in transgenic Arabidopsis expressing both Bax and AtBI-1, suggesting that the plant antiapoptotic protein AtBI-1 is biologically active in suppressing mammalian Bax action in planta. The BI-1 protein has seven transmembrane domains and is thought to be localized in the endoplasmic reticulum (ER) membrane that includes the nuclear envelope (Xu and Reed, 1998; Kawai-Yamada et al., 2001; Bolduc et al., 2003). Interestingly, the evolutionarily conserved function of both plant and animal BI-1 also was demonstrated in a mammalian cell culture system (Yu et al., 2002; Bolduc et al., 2003).
Direct evidence for the role of AtBI-1 in PCD was presented recently in a study of elicitor-induced hypersensitive response (HR) in rice suspension cells (Matsumura et al., 2003). The elicitors isolated from rice blast pathogen induced cell death through the stimulation of reactive oxygen species (ROS). Such cell death was overcome by the overexpression of AtBI-1. Furthermore, the role of BI-1 in Mlo-mediated resistance to Blumeria graminis also was demonstrated recently by an overexpression analysis of barley BI-1 (Hückelhoven et al., 2003). To determine the mode of action of AtBI-1 in suppressing cell death, AtBI-1 was overexpressed in tobacco suspension-cultured cells. Here, we demonstrate that the C terminus of AtBI-1 is essential for the suppression of H2O2- and SA-induced plant cell death. The precise base substitution analysis of the C-terminal region of AtBI-1 clearly demonstrated the critical role of the C-terminal amino acid composition of the BI-1 protein as ER membrane components, which play a novel role against the detrimental effects associated with oxidative stresses.
RESULTS
AtBI-1 Does Not Suppress the Bax-Induced Accumulation of O2−
The overexpression of mammalian Bax in Arabidopsis plants triggers cell death (Kawai-Yamada et al., 2001). To test whether ROS production is involved in this process, transgenic Arabidopsis plants were grown in the presence or absence of 5 μM DEX and leaves were stained with nitroblue tetrazolium (NBT) (Figure 1). Blue formazan precipitates, produced by the reaction of NBT with generated O2−, were evident in leaves of Bax transgenic plants within 7 h after Bax treatment (Figures 1B, 1E, and 1H), whereas this effect was not seen in the control plants (Figures 1A, 1D, and 1G). As shown in Figure 1H, magnified images of stained mesophyll cells revealed that the oxidized NBT precipitants were punctuated, indicating the intracellular origin of ROS. When leaves from transgenic plants possessing ΔC-Bax (inactive Bax in which the C-terminal transmembrane domain has been deleted, as described by Kawai-Yamada et al. [2001]) or with an empty vector (pTA7002) were treated with DEX, no ROS generation was observed (data not shown). To determine whether the cell death suppression activity of AtBI-1 was accompanied by the inhibition of ROS generation, transgenic plants expressing both Bax and AtBI-1 also were examined. As shown in Figures 1C, 1F, and 1I, the NBT-stained patterns were similar in plants transformed with Bax (Figures 1B, 1E, and 1H) or with Bax and AtBI-1 (Figures 1C, 1F, and 1I).
Figure 1.
Cytological Observation of ROS Production in Plants Expressing Bax.
To detect superoxide radical (O2−), 3-week-old transgenic Arabidopsis plants treated with DEX were stained with NBT. Each plant was treated with 5 μM DEX for 7 h to induce Bax. Detached leaves of non-DEX-treated ([A], [D], and [G]) or DEX-treated transgenic plants possessing Bax ([B], [E], and [H]) or DEX-treated Bax+AtBI-GFP plants ([C], [F], and [I]) were infiltrated with NBT solution. After the stained leaves were boiled in acetic acid:glycerol:ethanol to remove the chlorophyll, evidence of ROS production was observed with a light microscope. (D) to (I) show magnified images of (A) to (C). Formazan precipitates are indicated with arrowheads in (H) and (I). The presence of the formazan precipitate indicates the location of O2−. The experiment was repeated at least three times, and representative photographs are presented.
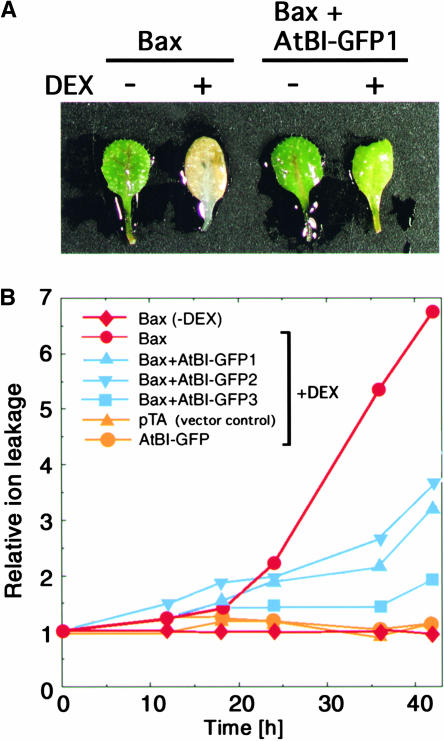
AtBI-1 Suppresses Bax-Induced Ion Leakage
When detached leaves of 3-week-old Bax transgenic plants (Bax) were treated with 5 μM DEX solution, severe chlorosis appeared within 3 to 4 days, whereas leaves of plants transformed with Bax and AtBI-1 (Bax+AtBI-GFP [green fluorescent protein]) remained green (Figure 2A). Ion leakage has been used as an indicator of plant cell death (Mitsuhara et al., 1999; Rizhsky et al., 2002). Likewise, electrolyte leakage was noted in leaves of plants transformed with Bax but not in those transformed with Bax and AtBI (Bax+AtBI-GFP1 to -3; Figure 2B). A Bax transgenic plant without DEX (Bax-DEX) and plants possessing an empty vector (pTA) or AtBI-GFP alone treated with DEX showed no clear ion leakage.
Figure 2.
Suppression of Ion Leakage from Detached Leaves of Transgenic Arabidopsis Expressing Both Bax and AtBI-1.
(A) Three-week-old Bax and Bax+AtBI-GFP1 transgenic plants were used in the experiments. Detached leaves were incubated with (+) or without (−) 5 μM DEX in distilled water at 23°C for 4 days, and photographs were taken.
(B) Detached leaves from transgenic plants containing pTA7002 (vector control; pTA), pTA-Bax (Bax), Bin-AtBI-GFP (AtBI-GFP), or both pTA-Bax and Bin-AtBI-GFP (Bax+AtBI-GFP1 to Bax+AtBI-GFP3) were incubated with 5 μM DEX in distilled water at 23°C. Bax+AtBI-GFP1 to Bax+AtBI-GFP3 are independent lines. The electrical conductivity of the solution was measured with a conductivity meter and is indicated as a relative value. n = 5.
BY2 Cells Overexpressing AtBI-1 Are Tolerant of H2O2-Induced Cell Death
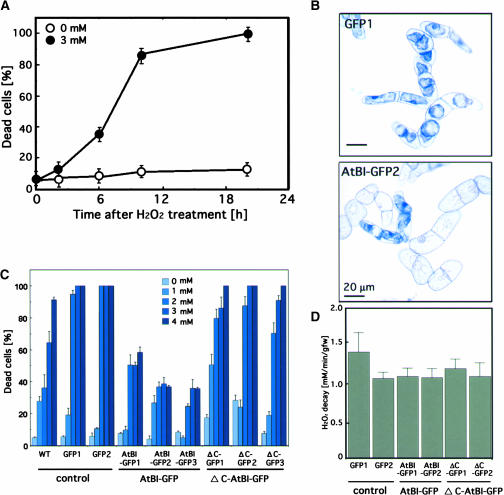
Exposure to H2O2 has been shown to cause death in many kinds of plant cells (Levine et al., 1994; Desikan et al., 1998; Houot et al., 2001). To examine the effect of H2O2 in tobacco BY-2 suspension cells, a 7-day-old cell suspension was transferred to fresh medium (2 mL/50 mL), treated immediately with 3 mM H2O2, and incubated in the dark. After various times, the percentage of dead cells (those stained with Evans blue) was determined by microscopy. As shown in Figure 3A, exposure of cells to H2O2 resulted in a time-dependent increase in the percentage of dead cells within 20 h.
Figure 3.
Inhibition of H2O2-Induced Cell Death by AtBI-1.
(A) Induction of cell death by exogenously supplied H2O2 in tobacco BY-2 suspension cells. Evans blue treatment was performed at 0, 2, 6, 10, and 20 h after the addition of 3 mM H2O2, and dead cells showing shrunken blue cytoplasm were scored with a light microscope. Data shown are means ± SE of three experiments.
(B) Comparison of AtBI-1 (AtBI-GFP2) and control (GFP1) cells stained with Evans blue at 18 h after treatment with 3 mM H2O2.
(C) Effects of AtBI-1 or ΔC-AtBI-1 on H2O2-induced cell death. Tobacco BY-2 cells expressing GFP, AtBI-GFP, or ΔC-AtBI-GFP were incubated with H2O2 (0 to 4 mM) for 18 h. Dead cells were scored, and the results are expressed as means ± sd of at least three experiments. WT, wild type.
(D) Turnover of H2O2 after its addition to transgenic cell lines expressing GFP, AtBI-GFP, or ΔC-AtBI-GFP. One millimolar H2O2 was added to each cell line, and the H2O2 concentration of the medium was measured after 5 min. Data shown are means ± SE of three experiments. gfw, grams fresh weight.
To investigate whether AtBI-1 could suppress H2O2-induced cell death, we used BY-2 cells expressing either AtBI-1 or ΔC-AtBI-1. Three independent cell lines expressing GFP-tagged AtBI-1 were selected. Compared with the control (wild type, GFP1, and GFP2), repression of cell death in the AtBI-1 transgenic lines (AtBI-GFP1 to AtBI-GFP3) was apparent even at H2O2 concentrations up to 4 mM (Figures 3B and 3C). By contrast, ΔC-AtBI lines showed marked cell death. As seen in Figure 3B, H2O2-induced cell death was denoted by extensive cell shrinkage. It has been demonstrated that exogenously supplied H2O2 is metabolized rapidly (Varner and Lin, 1989; Levine et al., 1994). We examined the turnover of H2O2 in each line, and no apparent changes were observed in the decay of exogenously added 1 mM H2O2 in cells overexpressing AtBI-1 or ΔC-AtBI-1 (Figure 3D).
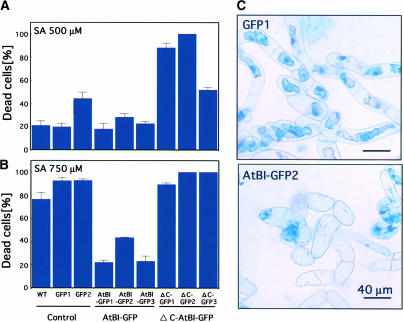
Enhanced Resistance of AtBI-1 Transgenic Cells to SA
In our previous work, rice suspension cells expressing AtBI-1 were confirmed to be resistant to SA-induced cell death (Matsumura et al., 2003). To examine whether the C-terminal region of AtBI-1 is required for the inhibition of SA-induced cell death, 500 or 750 μM SA was added to cell cultures expressing GFP, AtBI-GFP, or ΔC-AtBI-GFP. After 18 h of treatment, dead cells were stained with Evans blue. As shown in Figure 4A, only ΔC-AtBI (ΔC-GFP1 to ΔC-GFP3) showed higher sensitivity to 500 μM SA. Control lines (wild type, GFP1, and GFP2) or cells expressing full-length AtBI-1 (AtBI-GFP1 to AtBI-GFP3) showed low levels of staining with Evans blue. On the other hand, 750 μM SA resulted in the death of almost all control cells and ΔC-AtBI lines (Figure 4B), whereas cell lines overexpressing AtBI-1 (AtBI-GFP1 to AtBI-GFP3) demonstrated enhanced tolerance to 750 μM SA. The results of control experiments without SA treatment were identical to those shown in Figure 3C (0 mM). Microscopic observation confirmed that SA also induced cell shrinkage in control lines (Figure 4C).
Figure 4.
Inhibition of SA-Induced Cell Death by AtBI-1.
Tobacco BY-2 cells expressing GFP, AtBI-GFP, or ΔC-AtBI-GFP were treated with 500 μM (A) or 750 μM (B) SA and then cultured for 18 h before examination with Evans blue staining. Dead cells stained with Evans blue were scored, and values are expressed as percentages. Data shown are means ± SE of more than three replications. (C) shows microscopic images of cells at 18 h after 750 μM SA treatment. WT, wild type.
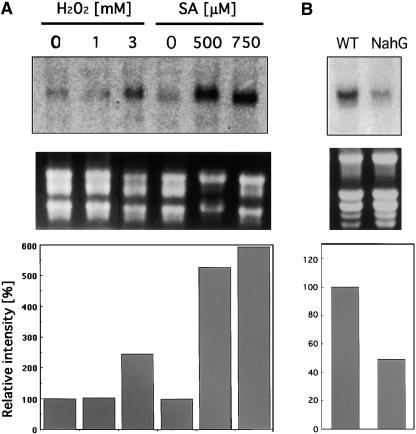
H2O2 or SA Results in the Accumulation of AtBI-1 mRNA
We then examined the possible involvement of AtBI-1 in the oxidative stress response in Arabidopsis suspension cells. Seven-day-old suspension-cultured cells were transferred to fresh medium and treated with either H2O2 or SA followed by incubation for 24 h. Total RNA then was isolated from samples. As shown in Figure 5A, AtBI-1 mRNA level was increased in cells treated with 3 mM H2O2 and 500 or 750 μM SA. We also analyzed AtBI-1 mRNA accumulation in NahG transgenic plants, in which the SA signaling pathway is downregulated (Delaney et al., 1994). RNA gel blot analysis indicated that AtBI-1 mRNA was reduced by 50% in NahG transgenic plants compared with wild-type cells (Figure 5B).
Figure 5.
Accumulation of AtBI-1 mRNA in Arabidopsis Cells under Oxidative Stress or in NahG Transgenic Plants.
(A) Arabidopsis suspension cells (3 days old) were treated with H2O2 (1 and 3 mM) or SA (500 and 750 μM) for 24 h under continuous shaking. Equal amounts of total RNAs (10 μg) were electrophoresed on a 1.2% formaldehyde agarose gel and transferred to a membrane. An α-32P-dCTP–labeled 3′ untranslated region of AtBI-1 cDNA was used as a probe.
(B) Analysis of AtBI-1 mRNA accumulation in NahG transgenic plants. Total RNAs (20 μg) from 2-week-old wild-type (WT) and NahG transgenic plants were electrophoresed and hybridized.
The middle panels in (A) and (B) show gels stained with ethidium bromide for rRNA. The bottom panels show relative levels of AtBI-1 expression in Arabidopsis suspension cells treated with H2O2 and SA (A) and NahG transgenic plants (B). Quantitative measurements of RNA gel blots were performed using the BAS1500 imaging plate scanner.
The C-Terminal 14 Amino Acids of AtBI-1 Are Essential for the Suppression of Bax-Induced Death
The AtBI-1 protein is estimated to have seven transmembrane domains (Xu and Reed, 1998; Kawai et al., 1999) and is associated with ER membranes (Kawai-Yamada et al., 2001). Bolduc et al. (2003) demonstrated that the C-terminal region of Brassica napus BI-1 (BnBI-1) is located on the cytosolic side of the ER membrane.
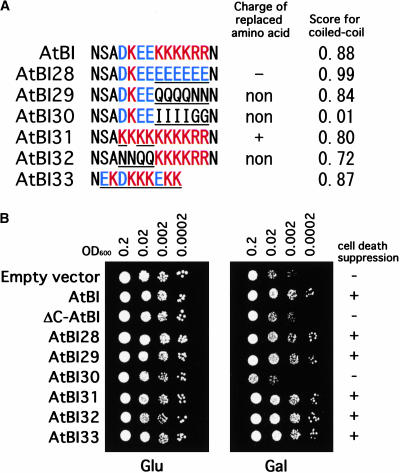
To determine the functional domains of AtBI-1 essential for the death-suppressor activity, ΔN (amino acids 1 to 31 deleted), ΔC (amino acids 234 to 247 deleted), and ΔNC (amino acids 1 to 31 and 234 to 247 deleted) AtBI-1 proteins were constructed and expressed in yeast possessing galactose-inducible mammalian Bax (Figures 6A and 6B). The AtBI-1 protein lacking the N-terminal domain (ΔN) inhibited Bax-induced cell death in yeast, whereas mutants with the C-terminal region deleted (ΔC) or with both the N and C termini deleted (ΔNC) did not inhibit Bax effects. In this regard, expression of the C-terminal domain alone could not inhibit Bax-induced cell death (data not shown). As shown in Figure 6C, the C-terminal region of BI-1 proteins from various organisms consists of clusters of charged amino acids (D and E, which are negatively charged, and K and R, which are positively charged), and this feature is evolutionarily conserved in plants and animals. Furthermore, such a region was estimated to form a coiled-coil structure (Figure 6D).
Figure 6.
Evaluation of Cell Death Inhibition Activity in a Truncated Series of AtBI-1 Proteins to Bax.
(A) Schemes of truncated AtBI-1 proteins. ΔN-, ΔC-, and ΔNC-AtBI-1 were constructed using PCR as described in Methods. The red bar shown in the C-terminal region of AtBI-1 indicates the conserved domain exhibited in (C). The results of cell death suppression from (B) are shown at right. ++, suppressed; + weakly suppressed; −, not suppressed. TM1 to TM7, transmembrane domains 1 to 7.
(B) The full-length AtBI-1 (AtBI), constructed AtBI-1 mutants (ΔN, ΔC, and ΔNC), and the empty vector pYX112 (pYX) were transformed to a yeast strain possessing galactose-inducible mammalian Bax and streaked on glucose (Glu)- or galactose (Gal)-containing medium. The photograph was taken after 3 days of incubation at 30°C.
(C) Amino acid comparisons of the C-terminal region of BI-1 proteins from various organisms. AtBI-1, Arabidopsis thaliana (Kawai et al., 1999); OsBI-1, Oryza sativa (Kawai et al., 1999); BnBI-1, Brassica napus; NtBI-1, Nicotiana tabacum; HvBI-1, Hordeum vulgare; HsBI-1, Homo sapiens; RnBI-1, Rattus norvegicus; MmBI-1, Mus musculus; PoBI-1, Paralichthys olivaceus. Red letters (K and R) indicate positively charged amino acids. Blue letters (D and E) indicate negatively charged amino acids.
(D) The probability of forming the coiled-coil structure calculated using the COILs program (available at http://www.ch.embnet.org/software/COILS_form.html). A putative coiled-coil region is predicted in the C-terminal region of the AtBI-1 protein. aa, amino acids.
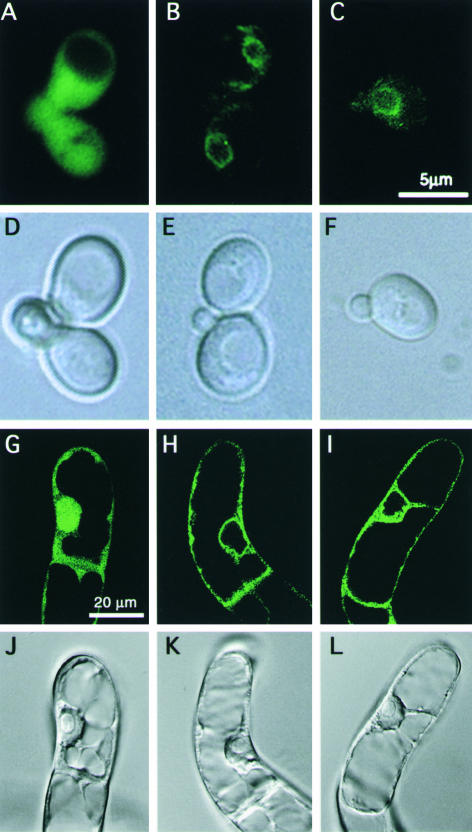
As mentioned above, plant and animal BI-1 proteins are located mostly in the ER and the perinuclear region (Xu and Reed, 1998; Kawai-Yamada et al., 2001; Bolduc et al., 2003). To determine the significance of the C-terminal region of AtBI-1 in terms of cellular localization, a plasmid containing GFP-tagged ΔC-AtBI-1 (ΔC-AtBI-GFP) was prepared and expressed in yeast and tobacco suspension cells. As shown in Figure 7, the fluorescent images of AtBI-GFP and ΔC-AtBI-GFP in yeast (Figures 7B, 7C, 7E, and 7F) and in BY-2 cells (Figures 7H, 7I, 7K, and 7L) revealed a perinuclear pattern consistent with ER localization. The control expressing only GFP (Figures 7A, 7D, 7G, and 7J) showed cytosolic localization. These findings indicate that the absence of the C-terminal domain did not affect the cellular localization of AtBI-1.
Figure 7.
Localization of a C-Terminal Truncated AtBI-GFP (ΔC-AtBI-GFP) Protein in Yeast and Plant Cells.
Distribution of GFP ([A], [D], [G], and [J]), AtBI-GFP ([B], [E], [H], and [K]), and ΔC-AtBI-GFP ([C], [F], [I], and [L]) in yeast ([A] to [F]) or tobacco BY-2 ([G] to [L]) was observed with a fluorescence microscope (DMRD; Leica) ([A] to [F]) or a confocal laser scanning microscope (BX50; Olympus) ([G] to [L]). Fluorescence ([A] to [C] and [G] to [I]) and bright-field ([D] to [F] and [J] to [L]) images are displayed.
To further investigate the role of the C-terminal region in cell death–suppression activity, site-directed mutagenesis was performed (Figures 8A and 8B). As mentioned above, the C-terminal part of AtBI-1 consists of charged amino acids and exhibits a coiled-coil structure. To dissect the importance of such characteristics, amino acids located in this domain were replaced with negatively charged (E in AtBI28) or noncharged (Q and N in AtBI29 and AtBI32) amino acids. Furthermore, to reduce the possibility of a coiled-coil structure, K and R were replaced with I and G, respectively, in AtBI30. When such plasmids were transformed into a yeast strain harboring galactose-inducible Bax, only AtBI30 lost the activity. These results suggest that the coiled-coil structure located in the C-terminal region of AtBI-1 is essential for the suppression of Bax-induced death in yeast. On the other hand, a chimeric gene in which the 14–amino acid C terminus of AtBI-1 was replaced with the 9–amino acid C terminus of human BI-1 (AtBI33 in Figure 8) was as effective as the full-length AtBI-1 in inhibiting Bax-induced death in yeast.
Figure 8.
Comparison of the Cell Death–Suppression Activity of C-Terminal Mutants of AtBI-1.
(A) Various mutations of the C terminus of AtBI-1 were constructed, and their ability to suppress Bax-induced cell death was analyzed. Mutated series of AtBI-1 (AtBI28 to AtBI33) were constructed using PCR as described in Methods. The amino acids replaced are underlined. The estimated charges (+, −, or none) of the replaced regions and calculated scores for the coiled-coil structure also are indicated. In AtBI33, the C-terminal 14 amino acids were replaced with the 9 amino acids of human BI-1.
(B) Spot assay of cell death–suppression activity in yeast. Yeast transformed with plasmids containing mutagenized AtBI-1 genes were cultured in SD medium containing glucose. After 1 day of shaking, the OD600 of each culture was adjusted to 0.2 and diluted to 0.0002. Each dilution was spotted on SD medium containing glucose (Glu) or galactose (Gal). The results show the growth of each line after 2 days (Glu) and 4 days (Gal) of incubation at 30°C. The results of cell death suppression are shown at right: +, suppressed; −, weak suppression or not suppressed.
DISCUSSION
AtBI-1 Suppresses Bax-Induced Cell Death without Downregulating ROS Production
It has been reported that mammalian Bax might activate an endogenous cell death program in plants that mimics the HR induced by Tobacco mosaic virus in tobacco plants carrying the N gene (Lacomme and Cruz, 1999). The ability of Bax to induce cell death in plants suggests the existence of common features in animal and plant cell death processes. One of the earliest events in the HR is a burst of oxidative metabolism leading to the generation of O2− and the subsequent accumulation of H2O2 (Lamb and Dixon, 1997). These are mediators for the signal network for defense gene induction and consequent cell death.
In the animal system, the mitochondria are involved directly in the generation of ROS and the activation of Bax-induced cell death processes via the release of cytochrome c (Green and Reed, 1998). Similarly, Bax targets mitochondria in yeast (Zha et al., 1996) and induces the release of cytochrome c (Manon et al., 1997) and the generation of ROS (Madeo et al., 1999), suggesting that a toxic effect related to oxidative metabolism is involved. The C-terminal transmembrane domain is known to be required for Bax targeting of the mitochondria in yeast. Similarly, it was demonstrated that the C-terminal transmembrane domain of Bax is needed to induce cell death in tobacco (Lacomme and Cruz, 1999) and Arabidopsis (Kawai-Yamada et al., 2001).
In this study, a NBT assay was performed to determine whether Bax-induced plant death is accompanied by oxidative stress. Our results showed that the expression of mammalian Bax could lead to enhanced ROS generation, which appeared <7 h after Bax expression. Ion leakage indicative of the loss of membrane permeability was observed at 20 to 30 h after Bax expression. Thus, ROS accumulation preceded the destruction of the plasma membrane in Bax-induced plant cell death.
We reported previously that the expression of Bax in Arabidopsis plants resulted in apoptotic cell death, which could be suppressed by the coexpression of AtBI-1 (Kawai-Yamada et al., 2001). Thus, we used a similar genetic system to evaluate the functional significance of AtBI-1 with respect to oxidative cell death. Our results indicated that AtBI-1 did not affect the O2− production caused by Bax. Furthermore, when tobacco BY-2 cells were treated with 1 mM H2O2, its decomposition did not change in control and AtBI-1–overexpressing lines, indicating that AtBI-1 was not responsible for scavenging the oxidant. Thus, it is reasonable to assume that AtBI-1 suppresses Bax-induced cell death downstream of ROS generation.
AtBI-1 Downregulates the Cellular Defect Caused by Oxidative Stress in Plants
Plants continuously produce ROS such as O2−, H2O2, and single oxygen as products of normal cellular metabolism. Consequently, plants rapidly metabolize these ROS with the help of antioxidant enzymes or metabolites. However, once plants are subjected to stresses such as high light, pathogens, ozone (O3), and UV irradiation, excess ROS is generated, which upon reaching a threshold level triggers PCD (Hernandez et al., 1993; Prasad et al., 1994; Chamnongpol et al., 1996; Lamb and Dixon, 1997). H2O2 plays an important role in signal transduction, cell wall reinforcement, HR, and phytoalexin production (Brisson et al., 1994; Dixon et al., 1994), and it has been demonstrated that exogenous H2O2 can induce a typical PCD in tobacco BY-2 cells (Houot et al., 2001).
Treatment of plant cells with SA is believed to enhance H2O2 levels (Rao and Davis, 1999). SA plays an important role in influencing the oxidative burst, and the defense responses and subsequent cell death ultimately lead to the development of the systemic acquired resistance and HR (Draper, 1997; Shirasu et al., 1997). Arabidopsis expressing the bacterial enzyme salicylate hydroxylase (NahG) does not accumulate SA where R gene–mediated HR is not observed (Delaney et al., 1994). Furthermore, NahG plants do not exhibit a HR-like response to O3 (Rao and Davis, 1999), and virus-induced HR is replaced by spreading necrotic lesions in NahG tobacco (Chivasa and Carr, 1998). On the other hand, increased production of H2O2 enhances the activities of enzymes involved in the biosynthesis of SA (Leon et al., 1995; Summermatter et al., 1995). Subsequently, increased SA levels promote H2O2 production, and high levels of free radicals are generated (Kauss et al., 1994; Fauth et al., 1996).
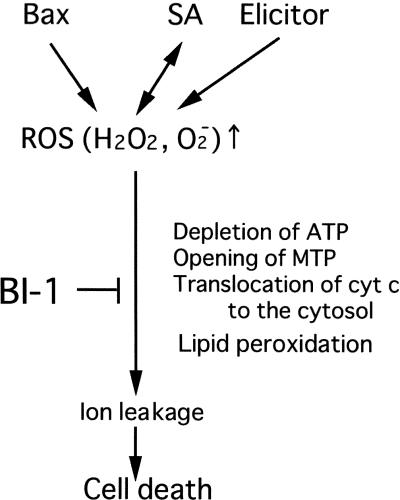
Concerning the effects of fungal elicitors on cell death, rice cells expressing AtBI-1 were found to downregulate the HR induced by the Magnaporthe grisea elicitor (Matsumura et al., 2003). Interestingly, AtBI-1 accumulation was delayed in the Arabidopsis coi1 mutant compared with wild-type plants (Sanchez et al., 2000). Reduced AtBI-1 levels might contribute to the enhanced susceptibility shown by coi1 plants to infections by various fungal pathogens. The relevance of BI-1 gene expression to the defense response has been addressed in Arabidopsis (Sanchez et al., 2000) and barley (Hückelhoven et al., 2001). Bolduc and Brisson (2003) observed that BY-2 cells expressing antisense NtBI-1 were more susceptible to autophagy, internucleosomal DNA fragmentation, and death when subjected to sucrose starvation or hypoosmotic shock. These data strongly indicate that BI-1 may play a ubiquitous role in responses to biotic and abiotic stresses and thus a general protective role against oxidative cell death. A scheme illustrating the position of AtBI-1 in the cell death pathway is shown in Figure 9. As described in this work, AtBI-1 confers the resistance of plant cells to H2O2 or SA stress. A marked increase in the proportion of dead cells was seen in ΔC-AtBI-1 transformants treated with H2O2 or SA. Because AtBI-1 is not responsible for scavenging ROS, it may interfere with ROS-specific execution steps.
Figure 9.
Scheme of the Role of the BI-1 Protein in the ROS-Induced Cell Death Pathway.
ROS cause mitochondrial permeability transition, ATP depletion, release of cytochrome c from the mitochondrial membranes, and lipid peroxidation, which cause ion leakage and cell death (Rao and Davis, 1999; Houot et al., 2001; Maxwell et al., 2002; Tiwari et al., 2002). BI-1 proteins, as components of the ER membrane, are involved in counteracting “oxidative stress.”
The C-Terminal Region of AtBI-1 Is Essential for the Inhibition of Bax-Induced Cell Death
In the present study, molecular dissection of the AtBI-1 gene demonstrated that the C-terminal region is necessary for the inhibition of Bax-induced cell death in yeast. ΔC-AtBI-1 (lacking the C-terminal 14 amino acid residues of AtBI-1) completely abolished the cell death–suppression ability. On the other hand, ΔN-AtBI-1, in which the N-terminal 31 amino acids of AtBI-1 were deleted, maintained its activity. When the amino acids purported to form the coiled-coil structure in the C-terminal region were replaced with other amino acids, such mutated constructs failed to inhibit Bax-induced cell death.
Both mammalian and plant BI-1s are localized to the ER (Xu and Reed, 1998; Kawai-Yamada et al., 2001; Bolduc et al., 2003). Analysis of the cellular localization of plant BI-1 indicated that the C-terminal region of BnBI-1 is located on the cytosolic face of the ER (Bolduc et al., 2003). We demonstrated here that the deletion of the C-terminal 14 amino acid residues did not affect the localization of ΔC-AtBI-1 to the ER in yeast or in BY-2 cells. Thus, the presence of a putative coiled-coil domain at the C-terminal region of AtBI-1 is crucial to prevent the detrimental effects of ROS stress. The protection against Bax-induced cell death by the AtBI-1/human BI-1 chimeric gene shows that the conserved C-terminal domains of BI-1s are interchangeable between the animal and plant kingdoms. This evidence suggests the existence of a conserved death mechanism in diverse organisms involving the regulation of oxidative metabolism. Further studies are necessary to define the precise mechanism of suppression by BI-1, particularly at the level of ER membrane homeostasis in relation to oxidative stress.
METHODS
Plant Materials
The Columbia ecotype of Arabidopsis thaliana was used in all experiments. Transgenic plants possessing mouse Bax and AtBI-1 were described previously (Kawai-Yamada et al., 2001). Arabidopsis plants were cultivated in growth chambers at 23°C under continuous light.
Suspension-cultured cells of tobacco (Nicotiana tabacum) Bright Yellow 2 (BY-2) were cultured weekly in liquid Murashige and Skoog (1962) medium supplemented with sucrose (3% [w/w]), KH2PO4 (200 mg/L), thiamine (1 mg/L), and 2,4-D (0.2 mg/L), as described previously (Nagata et al., 1992). For the induction of cell death, refreshed cells (diluted 1:25 from 7-day-old cells at stationary phase) were treated immediately with H2O2 (0 to 4 mM) or salicylic acid (0 to 750 μM) and cultured with continuous shaking at 27°C.
Detection of Ion Leakage
Three leaves from 3-week-old plants were floated on autoclaved distilled water with or without 5 μM dexamethasone. Electrolyte leakage was monitored using an electrical conductivity meter (B-173; Horiba, Kyoto, Japan) and expressed as a relative value.
In Situ Detection of Reactive Oxygen Species
In situ detection of O2− was performed by treating leaves with nitroblue tetrazolium as described by Rao and Davis (1999). Seven hours after the addition of 5 μM dexamethasone, leaves were detached from seedlings, vacuum-infiltrated with 10 mM NaN3 in 10 mM potassium phosphate buffer, pH 7.8, for 1 min, and incubated in 0.1% nitroblue tetrazolium (in 10 mM potassium phosphate buffer, pH 7.8) for 30 min at room temperature. Stained leaves were cleared by boiling in acetic acid:glycerol:ethanol (1:1:3 [v/v/v]) solution before photographs were taken.
Transformation of BY-2 Cells with GFP Constructs
To express GFP-tagged AtBI-1 and ΔC-AtBI-1 in BY-2 cells, PCR-amplified fragments of full-length AtBI-1 and ΔC-AtBI-1 were cloned into the SalI site of a GFP cassette plasmid containing the 35S promoter of Cauliflower mosaic virus and the nopaline synthase terminator (Niwa et al., 1999). Subsequently, the EcoRI-HindIII fragment was ligated into the EcoRI-HindIII–digested binary vector Bin19, as described by Kawai-Yamada et al. (2001). The resulting plasmids, Bin19-GFP (vector control), Bin19-AtBI-GFP, and Bin19-ΔC-AtBI-GFP, were transferred to Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993) using the freeze-thaw method (An et al., 1998). Transformation of tobacco BY2 cells was performed as described by Shaul et al. (1996). Finally, two to three different lines were established for cells expressing GFP, AtBI-GFP, and ΔC-AtBI-GFP. These cell lines were maintained in Linsmaier and Skoog medium (1965) by transferring 2 mL of culture into 50 mL of fresh medium every week and culturing on a rotating shaker at 100 rpm at 27°C in the dark.
Antioxidant Activity
The antioxidant activity of BY-2 suspension cells was assayed by measuring the decay of 1 mM H2O2 with time as described by Tiwari et al. (2002). The media of cells treated for various time intervals (0, 3, 5, and 10 min) with 1 mM H2O2 were mixed with 0.1 mL of titanium sulfate and incubated for 15 min at room temperature. The oxidation of titanium sulfate was recorded by reading OD410. These values were converted to corresponding concentrations using a standard calibration plot of known concentrations of H2O2.
RNA Gel Blot Analysis
Total RNAs were isolated from H2O2– or salicylic acid–treated Arabidopsis suspension cells using guanidinium thiocyanate as described previously (Kawai et al., 1999). Total RNAs, electrophoresed on a denaturing 1.2% agarose gel, were transferred to a nylon membrane (Biodyne B; Pall, Port Washington, NY), which then was hybridized to the α-32P-labeled 3′ untranslated region fragment of AtBI-1 (Kawai et al., 1999) in 10% dextran sulfate, 1 M NaCl, 1% SDS, and 100 μg/mL heat-denatured salmon sperm DNA. Washing was performed at high stringency (0.1× SSC [1× SSC is 0.15 M NaCl and 0.015 M sodium citrate] and 0.1% SDS at 65°C) . The membrane was analyzed with a BAS1500 imaging plate scanner (Fuji Film, Tokyo, Japan).
Yeast Strains and Expression
Yeast strains and plasmids used in this study have been described previously (Kawai et al., 1999; Kawai-Yamada et al., 2001). Truncated AtBI-1 genes (ΔN, amino acids 1 to 31 deleted; ΔC, amino acids 234 to 247 deleted; and ΔNC, amino acids 1 to 31 and 234 to 247 deleted) tagged with EcoRI sites by PCR were ligated to the EcoRI site of the expression vector pYX112, possessing a 2-μm replicon. The Saccharomyces cerevisiae strain QX95001, which is BF264-15au (MATα ade1 his2-3, 112 trp1-1a ura3) (Xu and Reed, 1998) containing the LEU2-marked mouse Bax-encoding plasmid Yep51-Bax, was transformed with the plasmids pYX-AtBI, pYX-ΔN, pYX-ΔC, and pYX-ΔNC using the lithium acetate method. Ura+Leu+ transformants were streaked on either a synthetic dropout (SD)–glucose (2%) plate or a SD-galactose (5%) plate and incubated at 30°C.
For site-directed mutagenesis of the C-terminal region of AtBI-1, the following oligonucleotide primers were used for the amplification of mutated fragments by PCR: 27, 5′-AAGCTTGTTTCTCCTTCTCCTCCTCCTCTCTTC-3′; 28, 5′-AAGCTTGTTTTCCTCTTCCTCCTCCTCCTCTTC-3′; 29, 5′-AAGCTTGTTCTTCTTTTGCTGCTGCTGCTCTTC-3′; 30, 5′-AAGCTTGTTTCCCCCTATAATGATAATCTCTTC-3′; 31, 5′-AAGCTTGTTTCTCCTTTTCTTCTTCTTCTTCTTTTTCTT-3′; 32, 5′-AAGCTTGTTTCTCCTTTTCTTCTTCTTCTGTTGGTTATT-3′; and 33, 5′-AAGCTTTTTCTTCTCTTTCTTCTTATCCTTTTCATTCTTCAACATTATGATGAGAATCCG-3′. Mutagenized fragments amplified by PCR were cloned into a pGEM T-Easy vector (Promega, Madison, WI) for sequencing. After digestion with EcoRI and HindIII, each fragment was cloned into the EcoRI-HindIII site of the pYX112 vector.
In the spot assay, yeast culture was diluted to various concentrations (OD600 = 0.2, 0.02, 0.002, and 0.0002), and an aliquot (5 μL) from each dilution was spotted onto SD-glucose and SD-galactose medium and incubated for 2 days (glucose medium) or 4 days (galactose medium) at 30°C.
For the analysis of the cellular localization of ΔC-AtBI protein in yeast, a chimera gene of AtBI-1 and GFP was constructed in the cassette vector pTS910 (provided by Y. Kikuchi, University of Tokyo, Japan). The DNA fragment tagged with the EcoRI-HindIII site by PCR was ligated into the EcoRI-HindIII–digested yeast expression vector pYX112. The plasmids pYX112-GFP and pYX112-AtBI-GFP were described in the previous report (Kawai-Yamada et al., 2001).
Cytological Methods
GFP fluorescence was examined at a 488-nm excitation wavelength with a fluorescence microscope (DMRD; Leica, Wetzlar, Germany) or with a confocal laser scanning microscope (BX50; Olympus, Tokyo, Japan). The viability of cells was measured with the addition of Evans blue (0.05%; Nakalai, Kyoto, Japan), which only penetrates dead cells and results in blue staining of the cellular contents (Kawai et al., 1998; Kawai and Uchimiya, 2000). The percentage of dead cells in each treatment was determined by scoring several hundred cells with a microscope.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Hirofumi Uchimiya, uchimiya@iam.u-tokyo.ac.jp.
Accession Numbers
The accession numbers for the sequences shown in Figure 6 are as follows: AtBI-1, AB025927; OsBI-1, AB025926; BnBI-1, AF390555; NtBI-1, AF390556; HvBI-1, AJ290421; HsBI-1, AF033095; RnBI-1, X75855; MmBI-1, BC005588; and PoBI-1, AF220548.
Acknowledgments
We thank J. Reed, N.H. Chua, Y. Kikuchi, Y. Niwa, and A. Hasezawa for their help and gifts of materials. We thank H. Ogawa-Suzuki for her help in all experiments. We thank Louise Brisson, Patrick Gallois, and Chris Hawes for editing the manuscript. This research was supported by a Research for the Future grant from the Japan Society for the Promotion of Science.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.014613.
References
- Abramovitch, R.B., Kim, Y.J., Chen, S., Dickman, M.B., and Martin, G.B. (2003). Pseudomonas type III effecter AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 22, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, G., Ebert, R.R., Mitra, A., and Ha, S.B. (1998). Binary vectors. In Plant Molecular Biology Manual, S.B. Gelvin and R.A. Schilperoort, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 1–19.
- Bischoff, J.R., Casso, D., and Beach, D. (1992). Human p53 inhibits growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 12, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson, L.F., Tenhanken, R., and Kamb, C. (1994). Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6, 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc, N., and Brisson, L.F. (2003). Antisense down regulation of NtBI-1 in tobacco BY-2 cells induces accelerated cell death upon carbon starvation. FEBS Lett. 532, 111–114. [DOI] [PubMed] [Google Scholar]
- Bolduc, N., Ouellet, M., Pitre, F., and Brisson, L.F. (2003). Molecular characterization of two plant BI-1 homologues which suppress Bax-induced apoptosis in human 293 cells. Planta 216, 377–386. [DOI] [PubMed] [Google Scholar]
- Chamnongpol, S., Willekens, H., Langebartels, C., van Montagu, M., Inze, D., and van Camp, W. (1996). Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J., 10, 491–503. [Google Scholar]
- Chivasa, S., and Carr, J.P. (1998). Cyanide restores N gene–mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic hydroxylase. Plant Cell 10, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, T.P., Uknes, S., Vernooji, B., Friedrich, L., Weymann, K., Negrotto, D., Gaffne, T., Gu-Rella, M., Kessmann, H., Ward, E., and Ryals, J. (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Reynolds, A., Hancock, J.T., and Neill, S.J. (1998). Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defense gene expression in Arabidopsis suspension cultures. Biochem. J. 330, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R.A., Harrison, M.J., and Lamb, C.J. (1994). Early events in the activation of plant defense responses. Annu. Rev. Phytopathol. 32, 479–501. [Google Scholar]
- Draper, J. (1997). Salicylate, superoxide synthesis and cell suicide in plant defense. Trends Plant Sci. 2, 162–165. [Google Scholar]
- Fauth, M., Merten, A., Hahn, M.G., Jeblick, E., and Kauss, H. (1996). Competence for elicitation of hydrogen peroxide in hypocotyls of cucumber is induced by breaching the cuticle and is enhanced by salicylic acid. Plant Physiol. 110, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D.R., and Reed, J.C. (1998). Mitochondria and apoptosis. Science 281, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Greenhalf, W., Steohan, C., and Chaudhuri, B. (1996). Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 380, 169–175. [DOI] [PubMed] [Google Scholar]
- Hanada, M., Aimé-Sempé, C., Sato, T., and Reed, J.C. (1995). Structure-function analysis of Bcl-2 protein: Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270, 11962–11968. [DOI] [PubMed] [Google Scholar]
- Hengartner, M., and Horvitz, H.R. (1994). C. elegans cell survival gene ced-9 encodes a functional homologue of the mammalian proto-oncogene bcl-2. Cell 76, 665–676. [DOI] [PubMed] [Google Scholar]
- Hernandez, J.A., Corpas, F.J., Gomez, M., Del Rio, L.A., and Sevilla, F. (1993). Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant. 89, 103–110. [Google Scholar]
- Hood, E.E., Gelvin, S.B., Melchers, L.S., and Hoekema, A. (1993). New Agrobacterium vector for plant transformation. Transgenic Res. 2, 208–218. [Google Scholar]
- Houot, V., Etienne, P., Petitot, A.S., Barbier, S., Blein, J.P., and Suty, L. (2001). Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 52, 1721–1730. [PubMed] [Google Scholar]
- Hückelhoven, R., Dechert, C., and Kogel, K.H. (2003). Overexpression of barley BAX inhibitor 1 induces breakdown of Mlo-mediated penetration resistance to Blumeria graminis. Proc. Natl. Acad. Sci. USA 100, 5550–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven, R., Dechert, C., Trujillo, M., and Kogel, K.H. (2001). Differential expression of putative cell death regulator genes in near-isogenic, resistant and susceptible barley lines during interaction with the powdery mildew fungus. Plant Mol. Biol. 47, 739–748. [DOI] [PubMed] [Google Scholar]
- Kauss, H., Jeblick, W., Zielger, J., and Kraber, W. (1994). Pretreatment of parsley (Petroselinum crispum L.) suspension culture with methyl jasmonate enhances the elicitation of active oxygen species. Plant Physiol. 105, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, M., Pan, L., Reed, J.C., and Uchimiya, H. (1999). Evolutionally conserved plant homologue of the Bax inhibitor-1 (BI-1) gene capable of suppressing Bax-induced cell death in yeast. FEBS Lett. 464, 143–147. [DOI] [PubMed] [Google Scholar]
- Kawai, M., Samarajeewa, P.K., Barrero, R.A., Nishiguchi, M., and Uchimiya, H. (1998). Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta 204, 277–287. [Google Scholar]
- Kawai, M., and Uchimiya, H. (2000). Coleoptile senescence in rice (Oryza sativa L.). Ann. Bot. 86, 405–414. [Google Scholar]
- Kawai-Yamada, M., Jin, L., Yoshinaga, K., Hirata, A., and Uchimiya, H. (2001). Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBI-1). Proc. Natl. Acad. Sci. USA 98, 12295–12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer, G. (1997). The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat. Med. 3, 614–620. [DOI] [PubMed] [Google Scholar]
- Lacomme, C., and Cruz, S.S. (1999). Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc. Natl. Acad. Sci. USA 96, 7956–7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, E., Kato, N., and Lawton, M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 848–853. [DOI] [PubMed] [Google Scholar]
- Lamb, C., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Leon, J., Lawton, M.A., and Raskin, I. (1995). H2O2 stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108, 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Linsmaier, E.M., and Skoog, F. (1965). Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 18, 100–127. [Google Scholar]
- Madeo, F., Fröhlich, E., Ligr, M., Grey, M., Sigrist, S.J., Wolf, D.H., and Fröhlich, K.U. (1999). Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., Herker, E., Maldener, C., Wissing, S., Lächelt, S., Herlan, M., Fehr, M., Lauber, K., Sigrist, S.J., Wesselborg, S., and Fröhlich, K.U. (2002). A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9, 911–917. [DOI] [PubMed] [Google Scholar]
- Manon, S., Chaudhuri, B., and Bueerin, M. (1997). Release of cytochrome c and decrease of cytochrome c oxidase in Bax expressing yeast cells, and prevention of these effects by coexpression of Bcl-XL. FEBS Lett. 415, 29–32. [DOI] [PubMed] [Google Scholar]
- Matsumura, H., Nirasawa, S., Kiba, A., Urasaki, N., Saitoh, H., Ito, M., Kawai-Yamada, M., Uchimiya, H., and Terauchi, R. (2003). Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L.) cells. Plant J. 33, 425–434. [DOI] [PubMed] [Google Scholar]
- Maxwell, D.P., Nickels, R., and McIntosh, L. (2002). Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J. 29, 269–279. [DOI] [PubMed] [Google Scholar]
- Mitsuhara, I., Malik, K.A., Miura, M., and Ohashi, Y. (1999). Animal cell-death suppressors Bcl-x(L) and Ced-9 inhibit cell death in tobacco plants. Curr. Biol. 9, 775–778. [DOI] [PubMed] [Google Scholar]
- Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 15, 473.–497. [Google Scholar]
- Nagata, T., Nemoto, Y., and Hasezawa, S. (1992). Tobacco BY2 cell line as the “HeLa” cell line in the cell biology of higher plants. Int. Rev. Cytol. 132, 1–30. [Google Scholar]
- Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M., and Kobayashi, H. (1999). Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 18, 455–463. [DOI] [PubMed] [Google Scholar]
- Prasad, T.K., Anderson, M.D., Martin, B.A., and Stewart, C.R. (1994). Evidence for chilling-induced oxidative stress in maize and a regulatory role for hydrogen peroxide. Plant Cell 6, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.Y., and Davis, K.R. (1999). Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 17, 603–614. [DOI] [PubMed] [Google Scholar]
- Reed, J.C. (1994). Bcl2 and the regulation of programmed cell death. J. Cell Biol. 124, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky, L., Hallak-Herr, E., Van Breusegem, F., Rachmilevitch, S., Barr, J.E., Rodermel, S., Inze, D., and Mittler, R. (2002). Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 32, 329–342. [DOI] [PubMed] [Google Scholar]
- Sanchez, P., de Torres-Zabala, M., and Grant, M. (2000). AtBI-1, a plant homologue of Bax inhibitor-1, suppresses Bax-induced cell death in yeast and is rapidly up regulated during wounding and pathogen challenge. Plant J. 21, 393–399. [DOI] [PubMed] [Google Scholar]
- Sato, R., Hanada, M., Bodrug, S., Irie, S., Iwama, N., Boise, L.H., Thompson, C.B., Golernis, E., Fong, L., Wang, H.G., and Reed, J.C. (1994). Interactions among members of the bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 91, 9238–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul, O., Nironov, V., Burssens, S., Montague, M.V., and Inze, D. (1996). Two Arabidopsis cyclin promoters mediate distinctive transcriptional oscillation in synchronized tobacco BY-2 cells. Proc. Natl. Acad. Sci. USA 93, 4868–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu, K., Nakajima, H., Rajashekar, K., Dixon, R.A., and Lamb, C. (1997). Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, M., Belenghi, B., Delledonne, M., and Levine, A. (1999). The involvement of cysteine proteases and protease inhibitor genes in programmed cell death in plants. Plant Cell 11, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summermatter, K., Sticher, L., and Metraux, J.P. (1995). Systemic response in Arabidopsis thaliana infected and challenged with Pseudomonas syringae pv. syringae. Plant Physiol. 108, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, B.S., Belenghi, B., and Levine, A. (2002). Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 128, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner, J.E., and Lin, L.S. (1989). Plant cell wall architecture. Cell 56, 231–239. [DOI] [PubMed] [Google Scholar]
- Yu, L.H., Kawai-Yamada, M., Naito, M., Watanabe, K., Reed, J.C., and Uchimiya, H. (2002). Induction of mammalian cell death by a plant Bax inhibitor. FEBS Lett. 512, 308–312. [DOI] [PubMed] [Google Scholar]
- Yuan, J.Y., Shaham, S., Ledoux, S., Ellis, H.M., and Horvitz, R. (1993). The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β converting enzyme. Cell 75, 641–652. [DOI] [PubMed] [Google Scholar]
- Xu, Q., and Reed, J.C. (1998). Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1, 337–346. [DOI] [PubMed] [Google Scholar]
- Zha, H., Fisk, H.A., Yaffe, M.P., Mahajan, N., Herman, B., and Reed, J.C. (1996). Structure-function comparison of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell. Biol. 16, 6494–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]