Abstract
GAMYB was first isolated as a positive transcriptional regulator of gibberellin (GA)-dependent α-amylase expression in barley aleurone cells, and its molecular and biochemical properties have been well characterized. However, the role of GAMYB elsewhere in the plant is not well understood. To investigate the molecular function of GAMYB outside of the aleurone cells, we isolated loss-of-function mutants from a panel of rice mutants produced by the insertion of a retrotransposon, Tos17. Through PCR screening using primers for rice GAMYB (OsGAMYB) and Tos17, we isolated three independent mutant alleles that contained Tos17 inserted in the exon region. No α-amylase expression in the endosperm was induced in these mutants in response to GA treatment, indicating that the Tos17 insertion had knocked out OsGAMYB function. We found no significant defects in the growth and development of the mutants at the vegetative stage. After the phase transition to the reproductive stage, however, shortened internodes and defects in floral organ development, especially a defect in pollen development, were observed. On the other hand, no difference was detected in flowering time. High-level OsGAMYB expression was detected in the aleurone cells, inflorescence shoot apical region, stamen primordia, and tapetum cells of the anther, but only low-level expression occurred in organs at the vegetative stage or in the elongating stem. These results demonstrate that, in addition to its role in the induction of α-amylase in aleurone, OsGAMYB also is important for floral organ development and essential for pollen development.
INTRODUCTION
Gibberellins (GAs) are growth factors with a tetracyclic diterpenoid structure that are essential regulators of diverse growth and developmental processes in plants (Davies, 1995). A series of genes that encode the enzymes involved in the GA biosynthetic pathway has been cloned from various species, and an almost complete picture of GA biosynthesis has been revealed (reviewed by Hedden and Phillips, 2000). In contrast to the rapid progress in the study of GA biosynthesis, much less is known about how plants perceive GA and how the GA signal is transmitted to regulate plant growth.
GAMYB is the first GA signaling protein to have been identified and is a positive transcriptional regulator of α-amylase expression in barley aleurone cells (Gubler et al., 1995). Orthologous proteins have since been identified in other grasses, such as rice, wheat, and Lolium temulentum (Gubler et al., 1997; Gocal et al., 1999; Chen et al., 2001). The GAMYB protein induces the expression of α-amylase and some other GA-inducible genes by interacting directly with the GA-responsive cis-acting elements of these genes in aleurone tissue (Gubler et al., 1999). Interestingly, the gene that encodes GAMYB, GAMYB, also is regulated positively by GA (Gubler et al., 1995).
The biochemical function of GAMYB as a transcription factor in aleurone cells has been studied extensively. For example, the preferential DNA binding sequences of GAMYB have been identified by random PCR and are located in GAMYB-regulated genes in aleurone cells (Gubler et al., 1999). A reporter gene controlled by the promoter region of α-amylase is upregulated in response to GA treatment, but this does not occur after the mutation of the GAMYB binding sequence (Gubler et al., 1995). In addition, the expression of GAMYB is regulated negatively by SLN1, a regulator of GA signaling in barley (Gubler et al., 2002; Zentella et al., 2002). All of these results demonstrate that GAMYB is a positive regulator of GA signaling.
Although the function of GAMYB in the aleurone cells of germinating cereal seeds is relatively well defined, its role elsewhere in the plant is less well understood. Some recent studies have suggested that GAMYB may be involved in other GA-regulated events, such as floral initiation, stem elongation, anther development, and seed development (reviewed by Woodger et al., 2003). For example, GAMYB is expressed at a high level in the floral meristems at the double-ridge stage and in stamen primordia of the grass L. temulentum. Because floral induction of L. temulentum occurs in response to long-day conditions via an increase in bioactive GA, Gocal et al. (1999) proposed that GAMYB might be a positive regulator of floral induction. However, there is no direct evidence for the molecular function of GAMYB outside of the aleurone cells. Phenotypic analyses of loss-of-function mutants of GAMYB are needed to investigate the role of GAMYB. Abnormal phenotypes in other parts of the plant should be observed if GAMYB functions as a positive regulator of GA signaling.
Of the cereal crops, rice is the best model for molecular genetics studies because an almost complete rice genome sequence has been published (Feng et al., 2002; Goff et al., 2002; Sasaki et al., 2002; Yu et al., 2002), reliable transformation techniques are available (Hiei et al., 1994), and a large number of mutants generated by chemical mutagens, γ-rays, and transposons have been produced. In particular, the mutant lines caused by the retrotransposon Tos17 should provide a powerful tool for characterizing the biological functions of genes of interest using a reverse genetics approach (Sato et al., 1999; Hirochika, 2001; Kumar and Hirochika, 2001). Transposons of Tos17 are activated during tissue culture, and several copies of Tos17 are inserted randomly into the rice genome. In this system, it is possible to screen for mutants by inserting Tos17 into genes of interest from a large pool of regenerated plants.
Here, we describe the isolation and characterization of loss-of-function mutants of the rice GAMYB gene (OsGAMYB). We have analyzed the phenotype of plants carrying loss-of-function mutations in OsGAMYB and demonstrate that OsGAMYB is essential for flower development.
RESULTS
Screening for Loss-of-Function Mutations in OsGAMYB
Before starting to screen the loss-of-function mutations of OsGAMYB, we examined the possibility of the presence in the rice genome of GAMYB-like genes homologous with the barley GAMYB in addition to OsGAMYB identified previously by Gubler et al. (1997). We searched all of the available rice DNA databases (Feng et al., 2002; Goff et al., 2002; Sasaki et al., 2002; Yu et al., 2002; Kikuchi et al., 2003) using the barley GAMYB amino acid sequence as a query sequence. We also screened a cDNA library produced from embryonic mRNA and the genomic library under low-stringency conditions using the cDNA sequence of OsGAMYB as a probe. Through these screens, we found some genes encoding the MYB domain homologous with the barley GAMYB protein but no genes that encoded not only the MYB domain but also three other conserved domains shared with AtGAMYB and other grass GAMYB proteins (Gocal et al., 1999, 2001) in addition to OsGAMYB (data not shown). Thus, we concluded that rice has only one GA-related MYB gene.
Rare insertions of a transposable element into a gene can be detected by PCR-based screening. When an insertion of a transposon occurs in a gene of interest, a suitable template will be generated that can be amplified by PCR using gene-specific and transposon-specific primers. We designed transposon-specific primers from the long terminal repeat (LTR) of Tos17 in the sense (LTR4S) and antisense (LTR4A) orientations (Hirochika et al., 1996) and a OsGAMYB-specific primer that was located just behind the translation initiation site (Figure 1A, MYB-5′). We performed PCR-based screening using LTR4S or LTR4R plus the MYB-5′ primer. For efficient screening, we adopted a pool sampling system in which plants were arranged in a three-dimensional matrix as described previously (Kumar and Hirochika, 2001). Because one pool contained genomic DNAs from ∼100 independent plants, we often detected false-positive bands that did not contain the OsGAMYB sequence. To distinguish the real products containing OsGAMYB and Tos17 sequences from false-positive products, we examined which bands were derived from OsGAMYB/Tos17 chimera genes by genomic DNA gel blot hybridization using the OsGAMYB sequence (Figure 1A, Probe). The hybridized PCR products then were sequenced to confirm the insertion of Tos17 into OsGAMYB. In this screening process, we obtained several PCR products hybridized with the probe. All of these products contained the OsGAMYB and Tos17 sequences at different junction sites, indicating that these products were derived from OsGAMYB mutant alleles with independently inserted Tos17. Based on information from the pooled PCR, we identified candidate mutant lines. We then confirmed the insertion of Tos17 in OsGAMYB in these lines. The genomic DNA isolated from 16 progeny of each candidate line was examined by PCR using the same primer combination used in the pooled screening. Three candidate lines gave PCR products of the same size as those obtained in the pooled screening, whereas the other lines gave either no PCR product or PCR products of a different size. The discrepancy between the PCR results obtained from the pooled screening and isolated progeny of candidate lines probably was attributable to the fact that the genomic DNA containing the inserted Tos17 was not always inherited by the next generation.
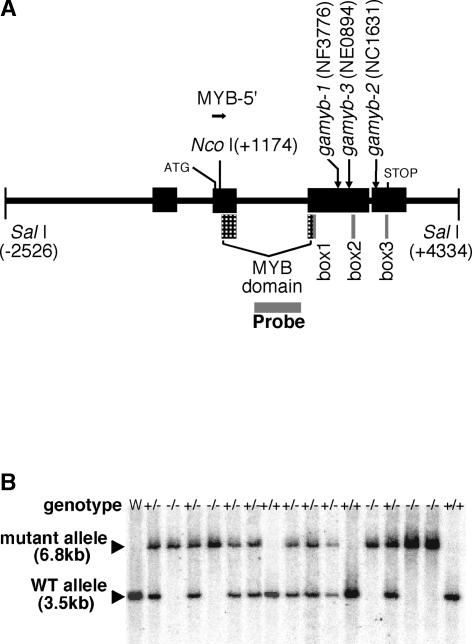
Figure 1.
Sites of Tos17 Insertion in the OsGAMYB Gene and Cosegregation Analysis.
(A) Structure of the OsGAMYB gene. The exons and introns are indicated by boxes and lines, respectively. Insertion sites of Tos17 in the three mutant lines are indicated by vertical arrows showing the names of the mutant lines. ATG and STOP indicate the initiation and stop codons for translation, respectively. The MYB domain and boxes 1 to 3 represent the conserved sequences of GAMYB as reported by Gocal et al. (2001). MYB5′ was used as a primer for the PCR screening of OsGAMYB alleles containing the Tos17 insert. Probe indicates the region used as a probe for cosegregation analysis in (B).
(B) Cosegregation analysis of gamyb-2 by genomic DNA gel blot hybridization. Each lane contained 1 μg of genomic DNA digested with PstI. In the wild type, a single 3.5-kb band was detected (lane W and lanes +/+). A 6.8-kb band was predicted as the mutant allele based on the restriction map of OsGAMYB and Tos17. Thus, individuals with only a single band at 6.8 kb are homozygous (−/−) for the insertion, and those showing bands at 6.8 and 3.5 kb are heterozygous (+/−).
To confirm that Tos17 had been inserted in OsGAMYB and was transmitted germinally in the three candidate lines, we performed genomic DNA gel blot analysis with the progeny of these plants. Genomic DNA of line NC1631 (gamyb-2) was isolated and digested with PstI and hybridized with Probe as a probe (Figure 1B). From 16 individual plants tested, three patterns of hybridizing bands were observed: a single 3.5-kb band; two bands of 3.5 and 6.8 kb; and a single 6.8-kb band. The number of individuals showing each pattern was 3, 8, and 5, respectively, the ratio of which almost fits with the theoretical 1:2:1 predicted for Mendelian inheritance. In the wild-type plant, only the 3.5-kb band was observed, as expected from the restriction map of the OsGAMYB sequence (Figure 1B, lane W). The 6.8-kb band corresponded to the insertion allele of OsGAMYB, based on the restriction maps of OsGAMYB and Tos17. Consequently, individuals showing a single band of 3.5 kb were wild-type (+/+), those with two bands of 3.5 and 6.8 kb were heterozygous (+/−), and those with a single band of 6.8 kb were homozygous for the insertion (−/−). Essentially the same results were obtained using the progeny of the other lines (data not shown). These findings demonstrate that Tos17 had been inserted into OsGAMYB in these lines and transmitted germinally. Tos17 was inserted into exon 3 (gamyb-1 and gamyb-3) or exon 4 (gamyb-2), both of which correspond to the protein-coding region (Figure 1A). As noted previously (Gocal et al., 2001), the GAMYB protein contains four well-conserved regions: the MYB domain and boxes 1 to 3. In gamyb-2, the point of insertion of Tos17 was located at the front of box 3, and in gamyb-1 and gamyb-3, it was at the front of box 2 (Figure 1A). Because the conserved boxes are likely to be essential domains for the expression of GAMYB function, the insertion of Tos17 should affect GAMYB severely. Indeed, all of the gamyb mutant lines failed to induce α-amylase in the aleurone cells, indicating that the C-terminal portion is essential for the expression of GAMYB function (see below).
Induction of α-Amylase in the gamyb Mutant Lines
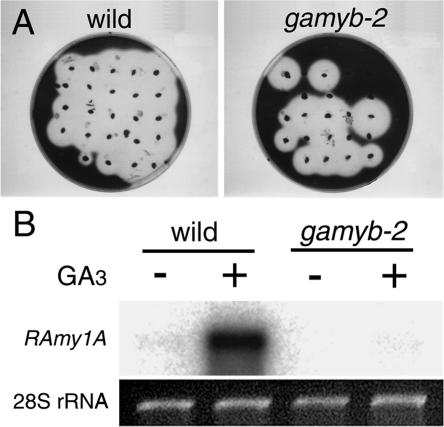
As mentioned previously, it is well established that GAMYB functions as a positive regulator of α-amylase expression in aleurone cells. Consequently, we did not expect α-amylase to be expressed in the gamyb mutants. To verify this, we studied the induction of α-amylase in the mutants. We performed an agar plate assay of amylase activity using the progeny seeds derived from a heterozygous line of gamyb-2. Embryoless half-seeds were placed on a starch plate with 1 μM GA3 for 4 days, and the plate was stained with iodine (Figure 2A). Production and secretion of α-amylase from almost all wild-type embryoless half-seeds was observed as clear plaque-like zones on the plate containing GA3 (Figure 2A, wild). When we used the embryoless half-seeds from progeny of the gamyb line for this assay, only ∼75% of the tested seeds induced α-amylase activity (Figure 2A, gamyb-2). We tested the genotype of the seeds and found that those lacking α-amylase activity contained Tos17-inserted alleles (−/−), whereas seeds expressing α-amylase had the heterozygous (+/−) or wild (+/+) allele of OsGAMYB (data not shown).
Figure 2.
Induction of α-Amylase Expression in Embryoless Half-Seeds of Wild-Type and gamyb Plants.
(A) Embryoless half-seeds of the wild type and gamyb-2 were placed on starch plates containing 1 μM GA3, and starch was detected by staining with iodine.
(B) RNA gel blot analysis of the α-amylase gene (RAmy1A) in aleurone cells. Embryoless half-seeds of the wild type and gamyb-2 were incubated at 30°C for 36 h in culture medium with (+) or without (−) 10−7 M GA3. Two micrograms of total RNA was used for the detection of RAmy1A mRNA. The gel was stained with ethidium bromide as a loading control (28S rRNA).
We also directly studied the induction of α-amylase expression in the gamyb endosperm by RNA gel blot analysis (Figure 2B). In wild-type seeds, α-amylase expression was induced at a concentration of 10−6 M GA3. However, in gamyb seeds, 10−6 M GA3 did not induce any α-amylase expression. These results indicate that the Tos17 insertion causes a severe failure in the induction of α-amylase expression; therefore, the alleles containing the inserted Tos17 have lost GAMYB function.
No Phenotypic Difference between gamyb and Wild-Type Plants Was Observed at the Vegetative Stage
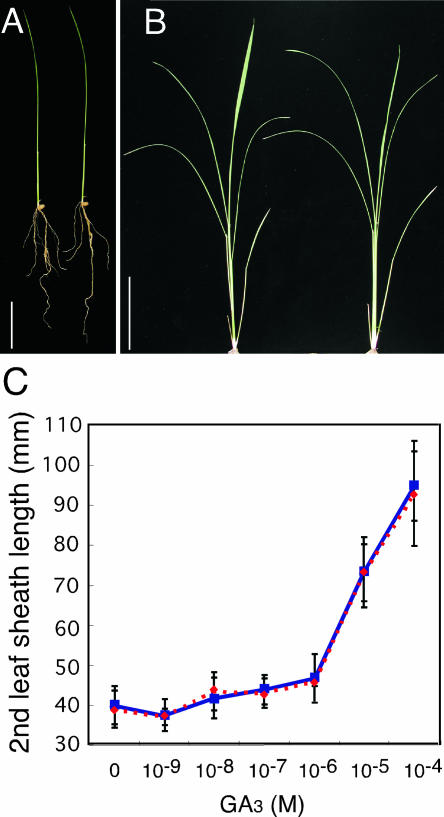
We examined the phenotype of the gamyb lines to investigate the function of OsGAMYB outside of the aleurone cells. Two-week-old mutant seedlings showed no signs of abnormal development or morphology (Figure 3A). At 6 weeks after germination, the mutant plants could not be distinguished from wild-type plants (Figure 3B). There were no differences in root elongation between the wild-type and mutant plants (Figure 3A). We also examined the GA responsiveness of the mutant seedlings. The leaf sheaths of both the wild-type and mutant plants elongated in response to treatment with exogenous GA3, and the elongation ratios were similar for the wild-type and mutant plants (Figure 3C). These results demonstrate that OsGAMYB does not play a role in the growth or development of rice seedlings at the vegetative stage.
Figure 3.
Phenotypes of gamyb Mutants at the Vegetative Stage and GA Responsiveness of Elongation in the Second Leaf Sheath.
(A) Wild-type (left) and gamyb-1 (right) seedlings at 2 weeks after germination. Bar = 5 cm.
(B) Wild-type (left) and gamyb-1 (right) plants at 6 weeks after germination. Bar = 10 cm.
(C) Elongation of the second leaf sheath in response to GA3 treatment in gamyb-1 (red) and wild-type (blue) plants. Data presented are means of results from eight plants. Bars indicate standard deviations.
gamyb Impaired Floral Organ Development but Not Flower Initiation
The phase transition of the rice shoot from the vegetative to the reproductive stage is morphologically observed by the development of the first bract primordium on the shoot apical meristem (SAM) at the site opposite the flag leaf primordium (Figure 4A). We grew wild-type and gamyb plants in a paddy field under natural summer conditions and collected the shoot apical regions from 85-day-old plants, when the floral transition can be predicted approximately, and observed the SAM structure. We examined >20 SAMs for each plant and found no difference in the timing of the phase transition between these plants (Figures 4A and 4B). We also compared the morphological transition of the SAM from the bract primordium to the primary rachis branch stage (88-day-old plants) (Figures 4C and 4D) and to the secondary rachis branch stage (data not shown), but we observed no differences in the stage progression between these plants. We further examined heading dates in photoperiod-controlled growth chambers under long-day (14.5 h of light/9.5 h of dark) and short-day (10 h of light/14 h of dark) conditions and obtained results consistent with the paddy field results (data not shown). These observations indicate that OsGAMYB is not essential for the phase transition or for inflorescence meristem development in rice plants.
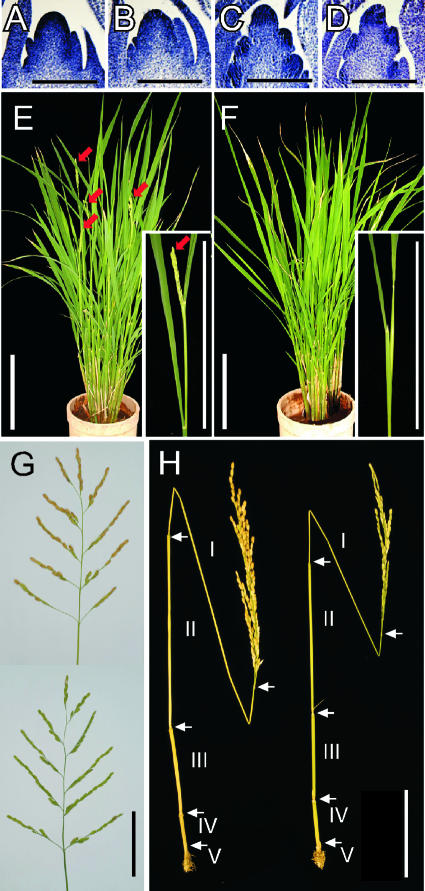
Figure 4.
Phenotypes of gamyb Mutants at the Reproductive Stage.
(A) to (D) Longitudinal sections of the shoot apical region at the bract primordium stage of an 85-day-old plant ([A] and [B]) or at the primary rachis branch stage of an 88-day-old plant ([C] and [D]) from wild-type ([A] and [C]) and gamyb-1 ([B] and [D]) plants. Bars = 0.1 mm.
(E) and (F) Wild-type (E) and gamyb-1 (F) plants at heading time. Red arrows indicate panicles of the wild-type plants. At the same time, the mutant plants showed no panicles. Close-up views of the panicles are shown in the insets. Bars = 15 cm.
(G) Panicle structure of wild-type (top) and gamyb-1 (bottom) plants. Bar = 5 cm.
(H) Elongation of the upper five internodes of wild-type (left) and gamyb-1 (right) plants. Bar = 10 cm.
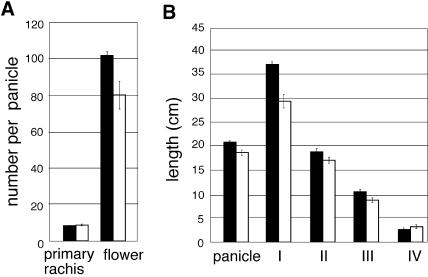
Although floral induction was not delayed in the mutants, the heading time was delayed slightly by several days compared with that of wild-type plants (Figures 4E and 4F). After heading, the mutant plants developed the primary and secondary rachis branches in a normal manner (Figure 4G). The numbers of rachis branches of the mutant were almost the same as in the wild type (Figures 4G and 5A). The development of spikelets also occurred normally in the mutant (Figure 4G), whereas the number of spikelets per panicle was reduced slightly relative to that of the wild type (Figure 5A). There was not a large difference in spikelet number between these plants, but it was significant according to t2 analysis. Thus, OsGAMYB contributes partially to the formation of the floral meristem.
Figure 5.
Comparison of the Number of Primary Branches and Glumous Flowers per Panicle and the Length of the Panicle and Upper Four Internodes of Wild-Type and gamyb-1 Plants.
Number data are shown in (A) and length data are shown in (B). Black bars, wild-type; white bars, gamyb-1. Data presented are means of results from eight plants. Error bars indicate standard deviations.
We also compared internode elongation between the wild-type and mutant plants. The internodes of gamyb plants elongated almost normally (Figure 4H, right), but the length of each internode seemed to be less than that of the wild-type plants (Figure 4H, left). Thus, we precisely compared the length of each internode in the mutant and wild-type plants. The upper four or five internodes of almost all japonica rice cultivars elongate under natural conditions. There were five elongated internodes in the mutant and wild-type plants (Figure 4H). However, the first (top) internode of the mutant was shorter than that in the wild type, whereas the lower elongated internodes (third and fourth) were similar in length in both mutant and wild-type plants (Figure 5B). The defect in the elongation of the first internode may cause the delay of heading time in the mutant.
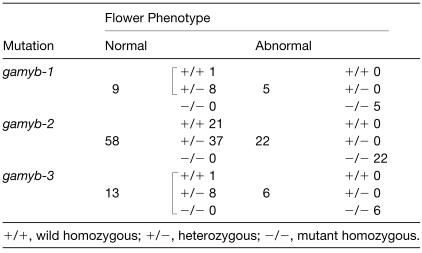
We also examined the development of the floral organs of the mutant. The rice flower is composed of one pair of glumes, one lemma, one palea, two lodicles, six stamens, and two carpels arranged from the peripheral to the central direction (Figure 6A, far left). The flowers of the gamyb mutants showed varying levels of abnormalities (Figure 6A, three right flowers). Flowers with the mild phenotype, which was the dominant phenotype in all alleles, developed shrunken and whitened anthers but had no defects in any other organs (Figures 6A, left center, and 6B, center). The shrunken anthers did not contain pollen, and consequently, the mutant was male sterile (see below). On the other hand, the pistil in this mutant flower had developed normally (Figure 6C, center), and its fertility was almost the same as that of the wild-type plant. When we crossed the pollen of an indica rice, Kasarath, with the pistil of the mutant, we obtained normal seeds with the heterozygous genotype of japonica and indica, and the seeds grew normally after germination (data not shown). Flowers with the intermediate phenotype developed whitened lemma and palea with a slightly malformed structure (Figure 6A, center right) and also developed severely malformed stamens with degraded anthers (Figure 6B, right) and a malformed pistil (Figure 6C, right). In contrast to the flowers with the mild phenotype, the fertility of this type of pistil was lost completely; consequently, we obtained no seeds when we crossed it with the pollen of Kasarath (data not shown). Flowers with the severe phenotype developed whitened lemma and palea with a severely malformed structure (Figure 6A, far right) and also developed malformed pistils and stamens similar to those of the intermediate phenotype (Figures 6B and 6C, right). The three different flower phenotypes were observed on the one mutant plant. Therefore, the severity of the flower abnormalities does not depend on different alleles but might depend on physical (environmental) conditions. The abnormal phenotypes in flowers were cosegregated with the loss-of-function alleles of OsGAMYB (Table 1), confirming that the aberrant flower development is caused by the defect in OsGAMYB function.
Figure 6.
Phenotypes of Floral Organs of the gamyb Mutants.
(A) Flowers of a wild-type plant (far left) and mild (center left), intermediate (center right), and severe (far right) gamyb-1 plants. Cp, carpel; Le, lemma; Lo, lodicle; Pl, palea; St, stamen.
(B) Close-up view of the stamens of a wild-type plant (left), a gamyb plant with the mild phenotype (center), and a gamyb plant with the intermediate phenotype (right). An, anther; Fl, filament.
(C) Close-up view of the pistils of a wild-type plant (left), a gamyb plant with the mild phenotype (center), and a gamyb plant with the intermediate phenotype (right).
Bars = 2 mm.
Table 1.
Cosegregation between the Abnormal Phenotype and gamyb Mutations in the Progeny from Self-Pollinated Heterozygous Plants
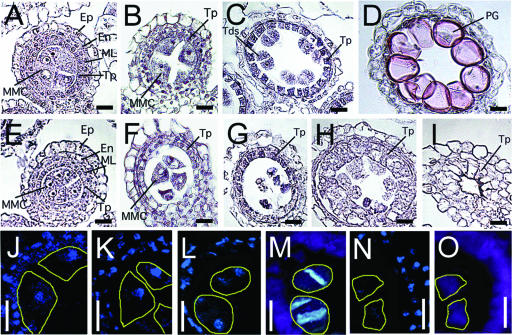
We also studied the development of the anther and pollen in mutant flowers with the mild phenotype. We crossed the developing loculi of the anther and observed these with a microscope. Before microspore mother cells started meiotic cell division, there was no difference between the wild-type and mutant plants, and morphologically normal microspore mother cells containing the 4′,6-diamidino-2-phenylindole dihydrochloride n-hydrate (DAPI)–stainable nuclei were surrounded by the anther wall, consisting of epidermal, endothelial, middle layer, and tapetal cells from outside to inside (Figures 7A, 7E, 7J, and 7K). In the wild type, the microspore mother cells were constricted, adhered to the tapetal layer, and then started meiotic cell division (Figures 7B and 7C). The dividing nuclei and the newly synthesized callose walls that accompany the meiotic divisions were observed clearly by DAPI and aniline blue staining (Figures 7L and 7M). A group of four haploid cells adhering closely to each other (pollen tetrad) was produced by two meiotic division cycles. Pollen tetrads then dispersed, and each developed a mature pollen cell (Figure 7D). In this process, the tapetum cells were a source of nutrition for pollen development; consequently, the cells were almost degraded in the mature anther. In the mutants, the microspore mother cells also were constricted but not arranged to adhere to the tapetal layer (Figure 7F). At the stage in the mutants equivalent to the meiotic division in wild-type plants, the microspore mother cells became shrunken (Figure 7G). We observed no clear DAPI-stainable nuclei or any newly synthesized callose walls in the mutants (Figures 7N and 7O). The tapetum cells then expanded (Figure 7H) and spread to occupy almost the whole space of the anther (Figure 7I). At this stage, we detected no microspore mother cells in the anther. These observations demonstrate that OsGAMYB is essential for the adherence of microspore mother cells to the tapetal layer and the subsequent meiotic division of the microspore mother cells.
Figure 7.
Anther Development in Wild-Type and gamyb-1 Plants.
(A) to (D), (J), (L), and (M) Wild type.
(E) to (I), (K), (N), and (O) gamyb-1.
(A) to (I) Samples from plants stained with hematoxylin.
(J) to (L) and (N) Samples from plants stained with DAPI to detect nuclei.
(M) and (O) Samples from plants stained with aniline blue to detect callose walls.
(A) and (E) Cross-sections of anthers before meiotic cell division.
(B), (F), (J), and (K) Cross-sections of anthers at the premeiotic cell division stage.
(C), (G), and (L) to (O) Cross-sections of anthers at the meiotic stage.
(D) Cross-section of the developed wild-type anther.
(H) and (I) Cross-sections of shrunken anthers of gamyb-1. The tapetum cells expanded as the anther aged.
The yellow lines in (J) to (O) indicate the section of the cells observed under the bright field. En, endothecium; Ep, epidermis; ML, middle layer; MMC, microspore mother cell; PG, pollen grain; Tds, tetrads; Tp, tapetum. Bars = 25 μm.
Expression Pattern of the OsGAMYB Gene
Because the loss-of-function mutation of OsGAMYB affected the induction of α-amylase activity in aleurone cells as well as flower development but had relatively little impact on other GA-dependent growth and developmental processes, such as leaf elongation and flowering time, we suspected that the expression of OsGAMYB was restricted to the specific tissues or cells that showed abnormal phenotypes in the mutants. To investigate this possibility, we analyzed the expression pattern of OsGAMYB by introducing a chimeric gene consisting of its promoter region (−2562 to +1174 bp) and a reporter gene, β-glucuronidase (GUS), into wild-type rice plants (Figure 1A). First, we studied GUS expression in germinating seeds of the transformants (Figure 8A). GUS activity was observed in the epithelial layer of the embryo, and moderate activity was seen in the aleurone layer of the endosperm. The expression of OsGAMYB in the epithelium and aleurone layer of germinating seeds overlaps with the spatial pattern of α-amylase expression (Sugimoto et al., 1998).
Figure 8.
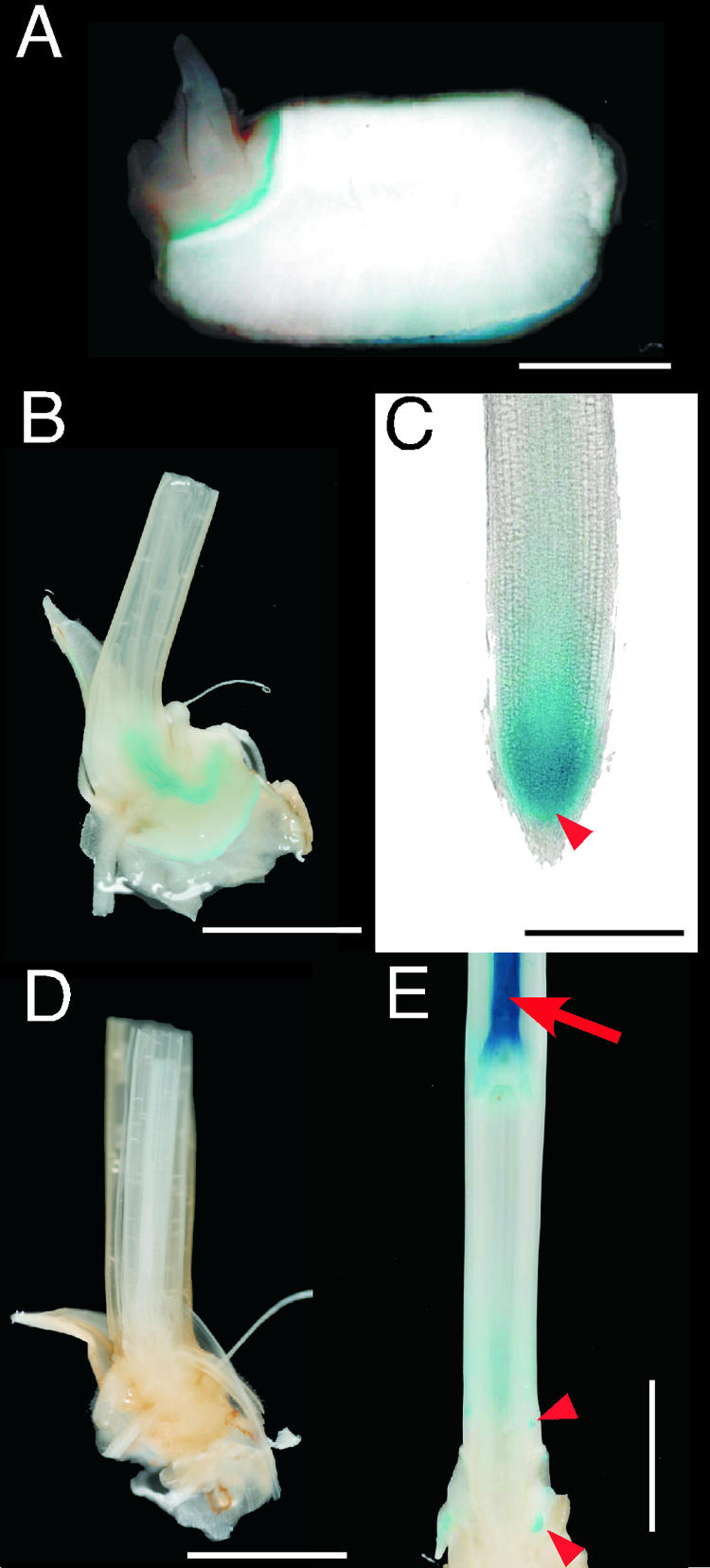
Localization of GUS Activity in Transgenic Rice Carrying OsGAMYB:GUS.
(A) Longitudinal section of a rice seed after 24 h of imbibition. Blue staining by GUS activity was localized in the epithelium of the embryo and the aleurone layer of the endosperm. Bar = 2 mm.
(B) Longitudinal section of a 2-week-old seedling. GUS activity was observed only in the vascular tissue and epithelium of the embryo but not in any organs of the seedling. Bar = 2 mm.
(C) GUS staining of a root. GUS activity was localized in the root tip (arrowhead). Bar = 1 mm.
(D) Longitudinal section of a 2-month-old plant. GUS activity was not observed in any organs. Bar = 2 mm.
(E) Longitudinal section of a stem at the elongating stage. Strong staining was observed around the shoot apical region, whereas moderate staining was seen in the elongation zone of the internode and in the tips of adventitious roots. Arrow and arrowheads indicate the SAM and root tips, respectively. Bar = 1 cm.
Very low GUS activity was detected in the young seedlings of transformants, whereas moderate activity remained in the vascular bundle and the epithelial layer of the embryo (Figure 8B). GUS activity was observed at the root tip but not in other tissues of the root (Figure 8C). No, or very low, GUS activity was observed in any aboveground tissues or organs at the vegetative stage (Figure 8D). However, when internode elongation had commenced after the phase change, strong activity was seen around the shoot apical region, including the SAM and young leaf primordia, whereas moderate GUS activity was observed in the basal region of the elongating internode, corresponding to its divisional and elongating zones (Figure 8E).
Strong GUS activity also was observed in the primordia of floral organs (data not shown), but we could not determine precisely from this analysis which organs expressed such a high level of GUS activity. Therefore, we conducted in situ hybridization analysis using the antisense strand of the 3′-teminal sequence of OsGAMYB cDNA as a probe. Before we studied the expression of OsGAMYB in the flower, we confirmed that its expression was very low around the SAM at the vegetative stage (Figure 9A). At the phase transition stage, a low level of OsGAMYB expression was observed in the SAM itself and also in young primordia (Figure 9B).
Figure 9.
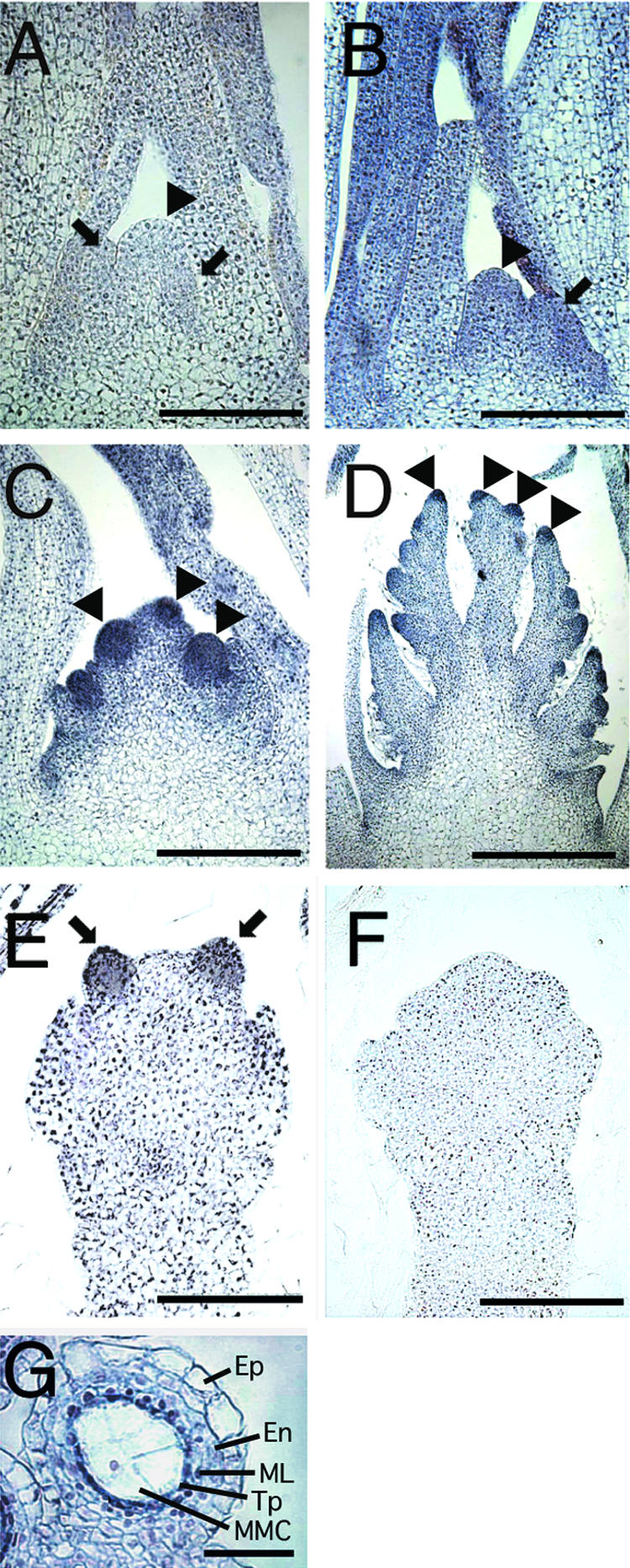
In Situ Hybridization of OsGAMYB in the Shoot Apex Regions and Anthers.
Sections were hybridized with the OsGAMYB antisense RNA probe ([A] to [E] and [G]) or the sense probe (F) labeled with digoxigenin-UTP. Bars = 0.1 mm in (A) to (F) and 25 μm in (G).
(A) Vegetative shoot meristem of a 2-month-old plant. No signal was seen in any tissues or organs. Arrowhead and left and right arrows indicate the SAM, P1, and P0 leaf primordia, respectively.
(B) Shoot meristem at the phase exchange. A low-level hybridization signal was observed in the SAM and young leaf primordia. The arrowhead and arrow indicate the SAM and bract primordium, respectively.
(C) Inflorescence shoot meristem at the primary rachis differentiation stage. Strong signal was observed in the inflorescence meristems of primary rachis branches. Arrowheads indicate the primary rachis meristems.
(D) Inflorescence shoot meristem at the secondary rachis differentiation stage. Moderate signal was observed in the inflorescence meristems of secondary rachis branches. Arrowheads indicate the secondary rachis meristems.
(E) Developing floral shoot meristem. Strong signal was localized in the stamen primordia, and moderate signal was seen in other organ primordia. Arrows indicate the stamen primordia.
(F) Developing floral shoot meristem hybridized with the sense RNA probe. No signal was seen in any tissues or organs.
(G) Cross-section of a developing anther. Strong signal was localized in the tapetum cell layer (arrowhead). En, endothecium; Ep, epidermis; ML, middle layer; MMC, microspore mother cell; Tp, tapetum.
When longitudinal sections of the shoot apex region at the primary rachis branch differentiation stage were stained with the probe, a strong signal was localized in the inflorescence meristems of the primary branches (Figure 9C). This expression pattern was essentially the same at the secondary rachis branch differentiation stage, and OsGAMYB was expressed in the inflorescence meristems of the branches, but the level of expression was reduced compared with that observed at the primary rachis branch differentiation stage (Figure 9D). We also studied OsGAMYB expression in the floral meristem itself and in floral organ primordia. A strong signal was detected in the stamen primordia at the floral organ development stage, and a moderate signal was seen in other floral organ primordia (Figure 9E). These signals were never detected when we used the sense strand of OsGAMYB cDNA as a probe (Figure 9F). After the differentiation of the anther and the filament, OsGAMYB expression was localized in the stamen primordia, and its strong expression was localized finally to the tapetum cells of the anther (Figure 9G). Moderate expression also was localized to the endothecium and the middle layer, whereas no expression was seen in microspore mother cells (Figure 9G). This localized expression of OsGAMYB to the outer cell layers of anthers is in agreement with the previous observation of the barley GAMYB expression in anthers (Murray et al., 2003).
Molecular Complementation of gamyb by the Wild-Type OsGAMYB Gene
To confirm that the abnormal phenotype of the mutant was caused by the inserted Tos17 transposon, we performed a complementation analysis of gamyb by introducing the wild-type OsGAMYB gene. Transformation of the mutant with a control vector that carried no rice genomic DNA had no effect on pollen development. However, when a 6.86-kb DNA fragment digested with SalI (Figure 1A) and containing the entire wild-type OsGAMYB gene was introduced, the normal phenotype was recovered by linkage with hygromycin resistance (data not shown). This result confirms that the mutant phenotype that we characterized is caused by the loss-of-function mutation in the OsGAMYB gene.
DISCUSSION
Target Inactivation of OsGAMYB by Transposon Insertion
To gain an understanding of the biological function of OsGAMYB, we screened for loss-of-function mutants in ∼40,000 plants that had been mutagenized by the random insertions of the rice transposon Tos17. RNA gel blot analysis demonstrated that the mutant alleles containing the inserted Tos17 did not produce the stable mRNA for OsGAMYB (data not shown). We also examined GA-dependent α-amylase expression in aleurone cells of the mutant lines to determine whether the mutants maintained OsGAMYB activity, because OsGAMYB is considered to play an essential role in GA-dependent α-amylase expression (Zentella et al., 2002). α-Amylase mRNA or activity was not induced in the GA-treated endosperm of any of the three mutant lines (Figures 2A and 2B). These results indicate that the mutant alleles containing the inserted Tos17 produce an inactive form of the OsGAMYB protein.
OsGAMYB Does Not Appear to Play an Important Role at the Vegetative Stage or Flower Initiation
None of the three mutant lines showed any abnormal phenotypes during the vegetative stage. The growth and development of the leaves and roots of the mutants were indistinguishable from those of wild-type plants (Figures 3A and 3B). Moreover, the GA responsiveness of the mutants also was the same as that of the wild type (Figure 3C). Other rice GA-insensitive mutants, such as d1 (a loss-of-function mutant of the Gα protein) and GA-insensitive dwarf2, show defects in leaf and root elongation and GA insensitivity of leaf sheath elongation (Ueguchi-Tanaka et al., 2000; Sasaki et al., 2003), demonstrating that GA signaling is essential for normal leaf and root development. Thus, our results suggest that OsGAMYB is not an important factor in these biological events at the vegetative stage. The lack of an abnormal phenotype in the gamyb mutants is consistent with no or very low-level expression of the OsGAMYB gene in the vegetative tissues or organs.
We found no significant difference in the timing of the floral transition between wild-type and mutant plants (Figures 4A to 4D). A few articles have reported the relationship between GAs, GAMYB-like genes, and petiole elongation and flowering in Arabidopsis. Gocal et al. (2001) observed the accumulation of GAs and the induction of GAMYB-like gene expression during the transition to flowering. Blázquez et al. (1998) found that GA promotes the flowering time of Arabidopsis by activating a floral meristem identity gene, LEAFY, and the LEAFY gene contains a GAMYB binding motif that is required for normal LEAFY expression (Blázquez and Weigel, 2000). Based on these observations, Gocal et al. (2001) proposed that Arabidopsis GAMYB-like genes might mediate GA signaling in flowering responses by inducing the expression of LEAFY by interacting with its cis-acting element.
The same group also investigated the relationship between GAs, GAMYB, and floral induction in the grass L. temulentum (Evens et al., 1990; King et al., 1993). The content of bioactive GAs in L. temulentum leaves and the SAM increases with exposure to flower-inductive (long-day) conditions (Gocal et al., 1999; King et al., 2001). Similarly, the expression of genes encoding late GA biosynthesis enzymes (GA20 oxidase and GA3 oxidase) and GA signal–related proteins (Gα protein and SLR1) was upregulated in the SAM and young leaf primordia just before the phase transition from the vegetative to the reproductive stage (Kaneko et al., 2003). These results indicate that GA biosynthesis and signaling are activated around the SAM before flowering time; therefore, GA and GA signaling may play important roles in mediating the flowering response. By contrast, the upregulation of LtGAMYB does not occur at the same time as floral initiation but is delayed by the double-ridge stage of flowering (Gocal et al., 1999). Similarly, high-level expression of OsGAMYB was seen only after the phase transition—that is, at the primary rachis branch primordia differentiation stage (Figure 9C)—but low-level expression was seen before and just after the phase change (Figures 9A and 9B). Low-level expression of GAMYB around the SAM before and just after the phase transition supports a significant role of GAMYB not in the phase transition itself but in inflorescence meristem development after the transition.
OsGAMYB Is Important for Pollen Development, and Its Knockout Mutant Displays Altered Floral Development
The defect in GAMYB function impaired the development of floral organs such as the lemma, palea, lodicle, and carpel (Figure 6A) but had the greatest impact on the stamen (Figure 6B). In fact, mutant flowers with the mild phenotype, which was the dominant phenotype of the mutants, developed shrunken anthers without pollen grains, whereas the other floral organs had no abnormalities. The carpels of this type of mutant flower retained their fertility and could develop normal seeds when crossed with wild-type plants. These results indicate that GAMYB function is important, but not essential, for the normal development of floral organs. The reason why opportunistic abnormalities in the floral organs were observed has not been determined, but it is possible that environmental conditions such as temperature and/or humidity may be important for the induction of these events. In fact, the mutant plants developed abnormal flowers at a higher frequency under high-temperature conditions than at lower temperatures.
The most consistent abnormal phenotype in the mutant flowers was a defect in pollen development (Figures 6 and 7). The flowers with the mild phenotype developed morphologically normal microspore mother cells, but the meiotic cell division of these cells did not occur and the cells degenerated at a later stage (Figure 7). However, this defect in cell division may not be caused directly by the loss of function of OsGAMYB, because clear expression of OsGAMYB was not observed in the microspore mother cells but rather in the tapetum cells of the anther (Figure 9G). According to a precise microscopic observation of the development of pollen in the rice anthers, the meiotic division of microspore mother cells depends on an interaction between the tapetum layer and the microspore mother cell itself (Wada et al., 1990). In fact, before meiotic division commences, the microspore mother cells adhere to the tapetum layer and then the contraction of the cytoplasm occurs. The first meiotic division occurs in a direction parallel to the tapetum layer. It is possible that a defect in the tapetum layer caused by the loss of function of OsGAMYB affects the interaction between the tapetum layer and the microspore mother cells.
There is some evidence from the phenotypic analyses of GA-deficient mutants to suggest that bioactive GAs are essential for anther development. For example, Arabidopsis ga1 (loss of function of the copalyl diphosphate synthetase gene) has poorly developed anthers (Koornneef and van der Veen, 1980; Sun and Kamiya, 1994). In the anthers of this mutant, microsporogenesis occurs but pollen grains are not viable (Koornneef and van der Veen, 1980). Pollen development also is defective in the tomato GA-deficient mutants gib-1 (loss of function of the copalyl diphosphate synthetase gene) and gib-2 (loss of function of the kaurenoic acid oxidase gene) (Nester and Zeevaart, 1988; Jacobsen and Olszewski, 1991). In contrast to Arabidopsis ga1, the development of the anthers of these gib mutants is arrested at an earlier developmental stage. In gib-2, cells of the sporogenous layer are initiated but degenerate without meiosis at a later stage (Nester and Zeevaart, 1988). Similarly, in gib-1, pollen development is arrested at the G1 phase of premeiotic interphase (Jacobsen and Olszewski, 1991). These observations demonstrate that defects in GA biosynthesis block pollen development before or after meiosis of microspore mother cells (Izhaki et al., 2002). Our study of the rice gamyb mutants has revealed that the loss of function of GAMYB also causes a defect in pollen development before meiosis, as in the GA-deficient tomato mutants gib-1 and gib-2. Recently, Murray et al. (2003) reported that the overexpression of barley GAMYB (HvGAMYB) in transgenic barley causes abnormal anther development (i.e., decrease in anther length, lighter anther color, failure in anther dehiscence, and male sterility). However, the male sterility of the overproducers was attributable to the unbroken septum in the anthers, whereas microspore mother cells underwent normal meiosis and developed. This observation indicates that the overproduction of GAMYB does not influence the meiotic division of microspore mother cells, for which GAMYB is essential, but has an important influence on anther dehiscence. Consequently, it is likely that the loss-of-function phenotype does not correlate directly with the overproduction phenotype.
METHODS
Plant Materials
The wild-type rice (Oryza sativa) cv Nipponbare and three mutants derived from Nipponbare and containing Tos17 inserted at the OsGAMYB gene were used in this study. Rice seeds were immersed in water for 2 days, grown for 1 month in a greenhouse, and then transplanted to the field.
PCR Screening for Loss-of-Function Mutations of Rice OsGAMYB
To screen for loss-of-function mutations, DNA fragments in which Tos17 had been inserted into the rice OsGAMYB gene were amplified by PCR using transposon-specific primers (LTR4S and LTR4A) and OsGAMYB-specific primers (MYB-5′) from DNA pools constructed using the three-dimensional sampling method from ∼40,000 Tos17-inserted plants. The PCR products were hybridized with the partial OsGAMYB sequence as a probe (Figure 1A, Probe), and the positive products were gel purified and sequenced to identify those containing the real OSGAMYB sequence. Mutagenesis with Tos17 and the pool sampling system were performed as described previously (Hirochika, 2001; Kumar and Hirochika, 2001).
Sequence Analysis
Nucleotide sequences were determined by the dideoxynucleotide chain-termination method using an automated sequencing system (model ABA3100; Applied Biosystems, Foster City, CA). Sequences were analyzed with GENETYX computer software (Software Development, Tokyo, Japan).
DNA and RNA Gel Blot Analyses
Rice genomic DNA was isolated from leaf tissue using the ISOPLANT DNA isolation kit (Nippon Gene Co., Tokyo, Japan), and 1 μg was digested with suitable restriction enzymes, transferred onto Hybond N+ membranes (Amersham) under alkaline conditions, and analyzed. Hybridization was performed at 65°C in 0.25 M Na2HPO4, 1 mM EDTA, and 7% SDS. Filters were washed twice in 2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) and 0.1% SDS at 65°C for 15 min and once in 0.2× SSC and 0.1% SDS at 65°C for 15 min. Isotope probes were prepared according to the manufacturer's instructions (Ready-to-Go DNA Labeling Beads; Amersham Pharmacia).
For RNA gel blot analysis, total RNA from various organs was prepared as described by Sambrook et al. (1989). RNA (10 μg per sample) was electrophoresed and transferred to Hybond N+ nylon membranes. Hybridization was performed in 5× SSC, 10% (w/v) dextran sulfate, 0.5% (w/v) SDS, and 0.1 mg/mL denatured salmon sperm DNA at 65°C. Filters were washed using the procedure described above.
Assay for α-Amylase
The preparation of embryoless half-seeds and the induction of α-amylase activity were performed as described previously (Yamaguchi, 1998). Twenty-four embryoless half-seeds per plate were sterilized, washed, and positioned perpendicularly on a starch plate (0.2% starch and 2% agar) without GA3. The plates were incubated in the dark for 4 days at 30°C and then placed in a box saturated with iodine vapor. After a few minutes, the reaction between starch and iodine turned the agar plates blue-purple. The agar around half-seeds with α-amylase activity remained colorless because of the hydrolysis of starch by α-amylase.
For RNA gel blot analysis, 96 embryoless half-seeds were placed in the wells of a 96-microwell titer plate. The seeds were sterilized, washed, and incubated in 2 mL of culture medium supplemented with 10−7 M GA3 for 36 h at 30°C. After incubation, the embryoless half-seeds were stored at −80°C until RNA extraction. The corresponding half-seeds with embryo were grown, and genomic DNA was isolated from the harvested seedlings to examine the genotype of each seed. Seeds with the homozygous Tos17-inserted allele, and seeds with the heterozygous or wild homozygous alleles, were collected independently to isolate total RNA.
Construction of OsGAMYB:GUS and Rice Transformation
The 5′ flanking sequence of rice OsGAMYB (from −2526 to +1174 numbered from the transcription initiation site) was prepared by digesting the OsGAMYB genomic clone with SalI (5′ end) and NcoI (3′ end), treated with the Klenow fragment after digestion to fill in (Figure 1A), and cloned into the SalI-SmaI site of the hygromycin-resistant binary vector pBI-Hm (Sato et al., 1999) to produce a fusion with the GUS reporter gene (OsGAMYB:GUS). The chimeric construct was introduced into Agrobacterium tumefaciens strain EHA101 and used to infect rice callus according to Hiei et al. (1994). Transformed cells and plants were screened by hygromycin selection and maintained in sterile culture; regenerated plants then were grown to maturity in pots in a greenhouse. Primary transformants were self-pollinated, and the resulting seeds (T1) were collected.
Histological Analysis
Plant materials fixed in formalin:acetic acid:70% ethanol (1:1:18) were dehydrated through a graded ethanol series and embedded in Paraplast Plus (Sherwood Medical, St. Louis, MO). Microtome sections (10 μm thick) were stained with 0.2% hematoxylin to stain the cells, 0.2% aniline blue to stain the callose walls, or 0.2% 4′,6-diamidino-2-phenylindole dihydrochloride n-hydrate to stain the nuclei.
In Situ Hybridization
Plant materials were fixed in 4% (w/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4, overnight at 4°C, dehydrated through a graded ethanol series followed by a t-butanol series, and finally embedded in Paraplast Plus. Microtome sections (8 to 10 μm thick) were mounted on glass slides treated with silane. Digoxigenin-labeled RNA probe was prepared from the 3′-terminal half of a cDNA clone of OsGAMYB. Hybridization and immunological detection of the hybridized probes were performed according to the method of Kouchi and Hata (1993).
Complementation Analysis
To construct pBI-OsGAMYB, a restriction fragment covering the entire region of the OsGAMYB sequence (from −2526 to +4334 in Figure 1A) was cloned into the SalI site of pBI101-Hm3 (Sato et al., 1999). The plasmid was introduced into A. tumefaciens strain EHA101.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact M. Matsuoka, makoto@nuagr1.agr.nagoya-u.ac.jp.
Acknowledgments
We are grateful to Masako Hattori (Nagoya University) for technical assistance. This work was supported in part by a Grant-in-Aid for Center of Excellence to Y.I. and M.M. and by a Grant-in-Aid from the Program for the Promotion of Basic Research Activities for Innovation Biosciences to M.U.-T. and M.M.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017327.
References
- Blázquez, M.A., Green, R., Nilsson, O., Sussmen, M.R., and Weigel, D. (1998). Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892. [DOI] [PubMed] [Google Scholar]
- Chen, L., Nishizawa, T., Higashitani, A., Suge, H., Wakeui, H., Takeda, K., and Takahashi, H. (2001). A variety of wheat tolerant to deep-seedling conditions: Elongation of the first internode depends on the response to gibberellin and potassium. Plant Cell Environ. 24, 469–476. [Google Scholar]
- Davies, P.J. (1995). Plant Hormones: Physiology, Biochemistry and Molecular Biology. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Evens, L.T., King, R.W., Chu, A., Mander, L.N., and Pharis, R.P. (1990). Gibberellin structure and florigenic activity in Lolium temulentum, a long-day plant. Planta 182, 97–106. [DOI] [PubMed] [Google Scholar]
- Feng, Q., et al. (2002). Sequence and analysis of rice chromosome 4. Nature 420, 316–320. [DOI] [PubMed] [Google Scholar]
- Gocal, G.F., Poole, A.T., Gubler, F., Watts, R.J., Blundell, C., and King, R.W. (1999). Long-day up-regulation of a GAMYB gene during Lolium temulentum inflorescence formation. Plant Physiol. 119, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal, G.F., Sheldon, C.C., Gubler, F., Moritz, T., Bagnall, D.J., MacMillan, C.P., Li, S.F., Parish, R.W., Dennis, E.S., Weigel, D., and King, R.W. (2001). GAMYB-like genes, flowering, and gibberellin signaling in Arabidopsis. Plant Physiol. 127, 1682–1693. [PMC free article] [PubMed] [Google Scholar]
- Goff, S.A., et al. (2002). A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 5, 92–100. [DOI] [PubMed] [Google Scholar]
- Gubler, F., Chandler, P.M., White, R.G., Llewellyn, D.J., and Jacobsen, J.V. (2002). Gibberellin signaling in barley aleurone cells: Control of SLN1 and GAMYB expression. Plant Physiol. 129, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler, F., Kalla, R., Roberts, J.K., and Jacobsen, J.V. (1995). Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI α-amylase gene promoter. Plant Cell 7, 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler, F., Raventos, D., Keys, M., Watts, R., Mundy, J., and Jacobsen, J.V. (1999). Target genes and regulatory domains of the GAMYB transcriptional activator in cereal aleurone. Plant J. 17, 1–9. [DOI] [PubMed] [Google Scholar]
- Gubler, F., Watts, R.J., Kalla, R., Matthews, P., Keys, M., and Jacobsen, J.V. (1997). Cloning of a rice cDNA encoding a transcription factor homologous to barley GAMYB. Plant Cell Physiol. 38, 362–365. [DOI] [PubMed] [Google Scholar]
- Hedden, P., and Phillips, A.L. (2000). Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 12, 523–530. [DOI] [PubMed] [Google Scholar]
- Hiei, Y., Ohta, S., Komari, T., and Kumashiro, T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Hirochika, H. (2001). Contribution of the Tos17 retrotransposon to rice functional genomics. Curr. Opin. Plant Biol. 4, 118–122. [DOI] [PubMed] [Google Scholar]
- Hirochika, H., Sugimoto, K., Otsuki, Y., Tsugawa, H., and Kanda, M. (1996). Retrotransposons of rice involved in mutations induced by tissue culture. Proc. Natl. Acad. Sci. USA 93, 7783–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhaki, A., Borochov, A., Zamski, E., and Weiss, D. (2002). Gibberellin regulates post-microsporogenesis processes in petunia anthers. Physiol. Plant. 115, 442–447. [DOI] [PubMed] [Google Scholar]
- Jacobsen, S.E., and Olszewski, N.E. (1991). Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiol. 97, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, M., Itoh, H., Inukai, Y., Sakamoto, T., Ueguchi-Tanaka, M., Ashikari, M., and Matsuoka, M. (2003). Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J. 35, 104–115. [DOI] [PubMed] [Google Scholar]
- Kikuchi, S., et al. (2003). Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301, 376–379. [DOI] [PubMed] [Google Scholar]
- King, R.W., Blundell, C., and Evans, L.T. (1993). The behavior of shoot apices of Lolium temulentum in vitro as the basis of an assay system for florigenic extracts. Aust. J. Plant Physiol. 20, 337–348. [Google Scholar]
- King, R.W., Moritz, T., Junttila, O., and Evens, L.T. (2001). Long-day induction of flowering in Lolium temulentum involves sequential increases in specific gibberellins at the shoot apex. Plant Physiol. 127, 624–632. [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., and van der Veen, J.H. (1980). Induction and analysis of gibberellin-sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 58, 257–263. [DOI] [PubMed] [Google Scholar]
- Kouchi, H., and Hata, S. (1993). Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol. Gen. Genet. 238, 106–119. [DOI] [PubMed] [Google Scholar]
- Kumar, A., and Hirochika, H. (2001). Applications of retrotransposons as genetic tools in plant biology. Trends Plant Sci. 6, 127–134. [DOI] [PubMed] [Google Scholar]
- Murray, F., Kalla, R., Jacobsen, J., and Gubler, F. (2003). A role of HvGAMYB in anther development. Plant J. 33, 481–491. [DOI] [PubMed] [Google Scholar]
- Nester, J.E., and Zeevaart, J.A.D. (1988). Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am. J. Bot. 75, 45–55. [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sasaki, A., Itoh, H., Gomi, K., Ueguchi-Tanaka, M., Ishiyama, K., Kobayashi, M., Jeong, D.-H., An, G., Kitano, H., Ashikari, M., and Matsuoka, M. (2003). A defect in an F-box gene causes accumulation of the phosphorylated repressor protein for GA signaling. Science 299, 1896–1898. [DOI] [PubMed] [Google Scholar]
- Sasaki, T., et al. (2002). The genome sequence and structure of rice chromosome 1. Nature 420, 312–316. [DOI] [PubMed] [Google Scholar]
- Sato, Y., Sentoku, N., Miura, Y., Hirochika, H., Kitano, H., and Matsuoka, M. (1999). Loss-of-function mutations in the rice homeobox gene OSH15 affect the architecture of internodes resulting in dwarf plants. EMBO J. 18, 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, N., Takeda, G., Nagato, Y., and Yamaguchi, J. (1998). Temporal and spatial expression of the α-amylase gene during seed germination in rice and barley. Plant Cell Physiol. 39, 323–333. [Google Scholar]
- Sun, T., and Kamiya, Y. (1994). The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell 6, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka, M., Fujisawa, Y., Kobayashi, M., Ashikari, M., Iwasaki, Y., Kitano, H., and Matsuoka, M. (2000). Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 97, 11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, T., Ogawa, K., Ito, T., Suzuki, H., and Takeoka, Y. (1990). Light microscopic observations on pollen and anther development in rice (Oryza sativa L.). I. Stages from pollen mother cells to tetrads. Jpn. J. Crop Sci. 59, 769–777. [Google Scholar]
- Woodger, F.J., Miller, A., Murray, F., Jacobsen, J.V., and Gubler, F. (2003). The role of GAMYB transcription factors in GA-regulated gene expression. J. Plant Growth Regul. 22, 176–184. [Google Scholar]
- Yamaguchi, J. (1998). Analysis of embryo-specific α-amylase using isolated mature rice embryos. Breeding Sci. 48, 365–370. [Google Scholar]
- Yu, J., et al. (2002). A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296, 79–92. [DOI] [PubMed] [Google Scholar]
- Zentella, R., Yamauchi, D., and Ho, T.-h.D. (2002). Molecular dissection of the gibberellin/abscisic acid signaling pathways by transiently expressed RNA interference in barley aleurone cells. Plant Cell 14, 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]