Abstract
Carbamylated LDL (cLDL) is a potential atherogenic factor in chronic kidney disease (CKD). However, whether elevated plasma cLDL associates with atherosclerosis in vivo is unknown. Here, we induced CKD surgically in apolipoprotein E–deficient (ApoE−/−) mice fed a high-fat diet to promote the development of atherosclerosis. These mice had two- to threefold higher plasma levels of both oxidized LDL (oxLDL) and cLDL compared with control mice. Oral administration of urea increased cLDL approximately eightfold in ApoE−/− mice subjected to unilateral nephrectomy and a high-fat diet, but oxLDL did not rise. Regardless of the model, the uremic mice with high plasma cLDL had more severe atherosclerosis as measured by intravital ultrasound echography and en face aortic staining of lipid deposits. Furthermore, cLDL accumulated in the aortic wall and colocalized with ICAM-1 and macrophage infiltration. In summary, these data demonstrate that elevated plasma cLDL may represent an independent risk factor for uremia-induced atherosclerosis.
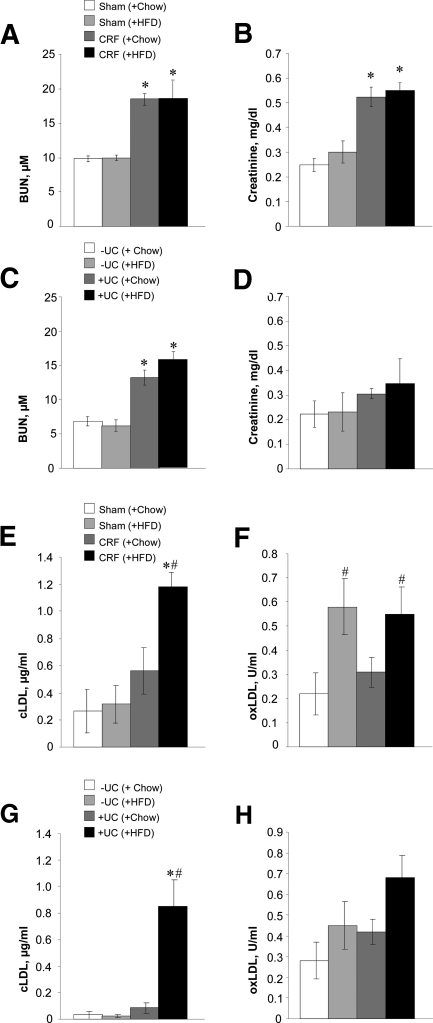
Cardiovascular disease is increased up to 30-fold in patients with chronic renal failure (CRF) compared with the general population, and morbidity and mortality rates rise even in the initial stages of the disease.1,2 In particular, a high incidence of atherosclerosis occurs in CRF patients with uremia, but the pathogenic events that contribute to uremic atherosclerosis are poorly understood.3 Modified LDLs have been suggested to be strong atherogenic factors.4,5 Oxidized LDL (oxLDL), the most recognized modified LDL, has been recently shown to be elevated in uremic patients6,7; however, it was not always associated with cardiovascular disease.8 Recently, it was shown that elevated urea directly induces the formation of potentially atherogenic carbamylated LDL (cLDL).9,10 Using a new ELISA assay, we recently confirmed that cLDL is significantly elevated in the plasma of uremic patients.11 The carbamylation of LDL occurs by spontaneous, nonenzymatic chemical modification of apolipoprotein B, the protein component of LDL, by isocyanic acid derived from urea. An alternative myeloperoxidase-mediated mechanism of LDL carbamylation has been suggested by Wang et al.,12 who also observed a correlation between total plasma protein carbamylation and the incidence of cardiovascular disease in nonuremic patients. cLDL promotes several biologic processes in vitro that are regarded as pro-atherogenic, including vascular smooth muscle cell proliferation, monocyte adhesion to endothelial cells,13,14 and endothelial cell cytotoxicity.9,15,16 However, experimental evidence showing a pro-atherogenic effect of cLDL in vivo is lacking. To obtain this critical evidence, we established two mouse models of elevated plasma urea using (1) a standard model of surgical kidney reduction that mimics CRF in humans17 and (2) a newly developed, urea consumption (UC) model that elevates plasma urea independently of renal failure. To validate both uremic models, basic renal parameters were assessed weekly. Blood urea nitrogen (BUN) was stably elevated in CRF and urea-consuming (+UC) mice compared with control sham-operated (Sham) and non–urea-consuming (−UC) mice for at least 12 weeks (Supplement Figure 1, A and B). CRF and Sham animals fed a high-fat diet (HFD) showed elevated BUN that did not differ from their counterparts fed regular chow (Chow; Figure 1A). Both HFD- and Chow-fed CRF mice showed elevated plasma creatinine (Figure 1B). The HFD resulted in elevated serum cholesterol and triglycerides, although the latter value was not significantly elevated compared with Chow-fed mice (Supplement Figure 1, C and D). In +UC mice, BUN was stably elevated compared with −UC mice (Figure 1C). Notably, creatinine was not elevated in either animal group of the UC model (Figure 1D). Total cholesterol and triglycerides were significantly higher in control or +UC mice supplemented with the HFD (Supplement Figure 1, E and F).
Figure 1.
CRF mice develop elevated plasma urea (A), creatinine (B), cLDL (E), and oxLDL (F), whereas +UC mice have increased urea (C) and cLDL (G) without effect on creatinine (D) and oxLDL (H). n = 10 to 16 per group. *P < 0.05 compared with sham-operated or −UC control mice fed with HFD; #P < 0.05 compared with CRF or +UC mice fed with chow diet.
Total plasma protein carbamylation was markedly (2.2 to 3.8 times) increased in the CRF and +UC mice compared with respective controls (Supplement Figure 1, G and H). In contrast, total plasma protein oxidation was not statistically different between experimental mice and respective controls fed Chow or HFD, and there was only a tendency for values to increase in mice with high plasma urea (Supplement Figure 1, I and J).
Next, cLDL and oxLDL were assessed in both uremic models. CRF mice on HFD showed elevated oxLDL and cLDL, but only elevated oxLDL was observed in CRF mice on Chow (Figure 1, E and F). +UC mice on HFD showed a significant difference in plasma cLDL, whereas oxLDL failed to increase significantly despite the high levels of plasma BUN (Figure 1, G and H). The increased cLDL in experimental mice was also confirmed by increased relative electrophoretic mobility and Western blotting using an anti-cLDL antibody (Supplement Figure 2). Therefore, our results indicated that oxLDL was either unchanged or elevated in response to the HFD and regardless of uremia, whereas cLDL was elevated specifically in the uremic mice on HFD.
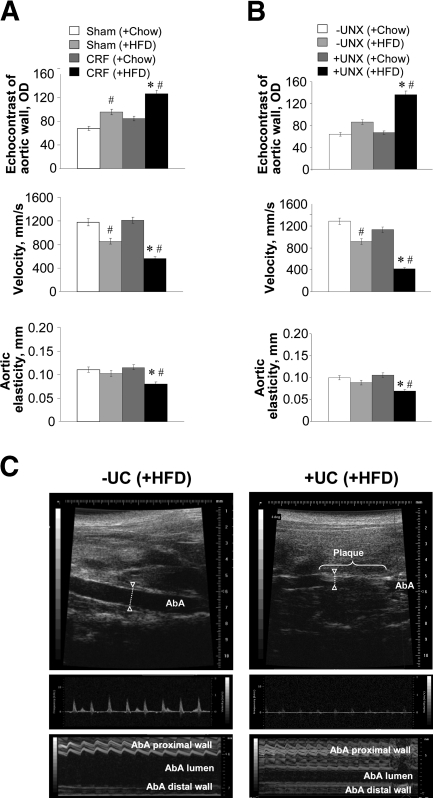
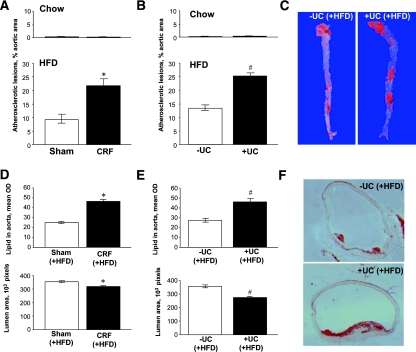
We next determined whether the uremic mice on HFD with elevated plasma cLDL had more atherosclerosis and functional cardiovascular disorders than control animals. Intravital ultrasound echography (IUE) determined significant vascular changes that are specific for atherosclerosis (Figure 2). To verify the intravital findings, the en face Sudan IV staining of aortas (arch and descending part) was performed 12 weeks after the start of diets. Our results suggested that both uremic model mice on HFD had significantly more atherosclerotic lesions than control mice on HFD (Figure 3, A–C). The animals fed with a Chow diet did not have any substantial atherosclerotic lesions, regardless of uremia. The Oil red O–stained cryosections of ascending aortas confirmed the en face staining data by showing a significant increase of lipid deposits in aorta and decrease of aortic lumen area in uremic mice compared with control mice (Figure 3, D–F). Therefore, our data suggest that mice with elevated plasma urea have increased cLDLs and developed the most of the atherosclerosis regardless of whether they had chronic renal failure. Hence, elevated urea seems to be accountable for both cLDL elevation and exacerbation of atherosclerosis in uremia.
Figure 2.
CRF (A) and UC (B and C) mice develop atherosclerotic lesions in aorta as visualized by intravital ultrasound echography using VisualSonics Vevo 770. Quantification of echo-positive lipid deposits, blood velocity, and aortic wall elasticity measurements in abdominal aortas was performed by B-mode, Doppler mode, and M-mode, respectively. As showed by B-mode and M-mode, both CRF and +UC animals fed with HFD had higher wall densities in the aorta, which may be qualified as lipid deposits. Additionally, these animals had intravascular, echo-positive masses that narrowed the aortic lumen as detected by B-mode and changed blood flow in abdominal aorta as detected by Doppler mode. The elasticity of the abdominal aorta, i.e., the movement of aortic wall between the systole and diastole, was significantly changed as measured by M-mode. Similarly to abdominal aorta, aortic arch and major arteries showed intravital structural and hemodynamic changes that reflect significant vascular system remodeling toward atherosclerosis (Supplement Figure 3). n = 6 to 16 per group. *P < 0.05 compared with sham or −UC control mice fed with HF diet; #P < 0.05 compared with CRF or +UC mice fed with chow diet.
Figure 3.
CRF (A) and UC (B and C) mice develop atherosclerotic plaques and lipid deposits in aortas as detected by Sudan IV en face staining: measurements (A and B) and representative images (C). Staining of atherosclerotic plaques and lipid deposits in ascending aortas by Oil red O (D–F) and their quantification in CRF (D) and UC (E and F) mice fed with HFD. n = 6 to 10 per group. *P < 0.05 compared with sham-operated mice fed with HFD. #P < 0.05 compared with −UC control mice fed with HFD.
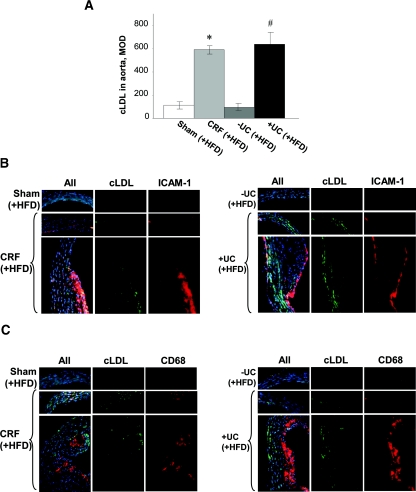
Next, cLDL in aortic cryosections was assayed using a polyclonal cLDL antibody. This immunostaining showed that cLDL in the aortas of CRF and +UC mice fed HFD was substantially increased compared with controls (Figure 4A). Most cLDL was concentrated in atherosclerotic plaques and throughout the aortic wall around subintimal areas and in the adventitial layer. All sham-operated and −UC mice fed HFD, as well as all CRF and +UC mice fed Chow, had little or no cLDL in their aortic walls and plaques. Therefore, our data showed that cLDL-positive atherosclerosis lesions are extensively prevalent in mice with the highest plasma cLDL compared with all other groups.
Figure 4.
Immunohistochemically detectable cLDL is elevated in ascending aortic wall of CRF and +UC mice (A) and colocalized with ICAM-1 and CD68 in aortic walls and atherosclerotic plaques (B and C). n = 6 to 10 per group. *P < 0.05 compared with sham-operated mice; #P < 0.05 compared with −UC mice.
Our previous studies showed various effects of cLDL on endothelial cells in vitro, suggesting its pro-atherosclerosis properties.9,13–16 Among those, the role of the intercellular adhesion molecule 1 (ICAM-1) and monocyte adhesion by endothelium were most prominent and, in some conditions, exceeded those induced by oxLDL.13 To test whether ICAM-1 overexpression and macrophage housing occurred in vivo as a result of uremic atherosclerosis associated with LDL carbamylation, the aortic samples were simultaneously stained for cLDL and for ICAM-1 or CD68, respectively. Our data showed that ICAM-1 protein expression was significantly higher in CRF and +UC mice fed HFD compared with all controls (Figure 4B). In addition, both CRF and +UC mice exhibited higher quantities of housed macrophages (Figure 4C). Interestingly, although both ICAM-1 and macrophages were located in close proximity to cLDL deposits, the latter were not always colocalized with macrophages and often were found in vascular intima around plaques. These data suggested that ICAM-1 overexpression and extensive macrophage housing are associated with cLDL-mediated atherosclerosis in vivo.
Our study showed for the first time that elevated plasma cLDL induced by chronic elevation of plasma urea in apolipoprotein E−/− mice is associated with the accelerated progression of atherosclerosis in the absence of elevated plasma concentrations of oxLDL. The CRF model results were in strong agreement with the data on uremic atherosclerosis development in CRF models reported by others.18,19 However, because CRF is a complex model, the atherogenic role of other “uremic toxins” cannot be excluded. Our new UC model allowed us to narrow down the existent chronic uremia model to the effects of elevated urea, a major source of cyanate and protein carbamylation.10 Our data suggest that cLDL, but not oxLDL, is directly produced in presence of elevated plasma urea, which accelerates atherogenesis in the absence of other factors and toxins produced by CRF. Because the UC model data were similar to the CRF model for atherosclerosis development, this may indicate that elevated urea or its metabolites are direct atherogenic factors. We can speculate that cLDL could be the factor that contributes to uremia-induced atherogenesis because high cholesterol-controlling and high urea–controlling groups did not show similar atherosclerotic lesions.
Finally, for the first time, cLDL was detected and quantified in the aortic wall and atherosclerotic lesions using a highly specific cLDL antibody.11 Wang et al.12 reported carbamylated proteins in atherosclerotic plaques that mainly colocalized with macrophages. Our findings suggest that cLDL may compose one of these carbamylated proteins, and the majority of it is likely to derive from urea, not thiocyanate, as suggested by Wang et al. We also observed colocalization of cLDL and macrophages and detected cLDL accumulation in macrophage-free zones of intima and plaques. The latter finding may relate to our previous in vitro and in vivo findings showing the high capacity of cLDL for internalization and accumulation by endothelium, followed by endothelial transcytosis.14 This high affinity of vascular endothelium to cLDL may explain the abnormal cLDL metabolism reported by Gonen et al. and Horkko et al.20,21 We also showed a significant increase of ICAM-1 on the aorta's endothelial surface in experimental mice with the highest plasma cLDL. Our previous data established strong links between cLDL, expression of ICAM-1, and monocyte adhesion and showed that ICAM-1 is more relevant to cLDL than to other LDLs.13 Therefore, our current in vivo data confirm our previous results obtained in vitro and verify that ICAM-1 is involved in cLDL atherogenesis. In conclusion, our findings provide initial in vivo evidence to implicate cLDL resulted from elevated urea as an independent pathogenic factor for uremia-induced atherosclerosis.
CONCISE METHODS
Animals and Surgical Procedures
All animal experiments were approved by the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System. Mice were fed ad libitum either a regular chow diet (5.7% fat, 18.9% protein, 72.3% carbohydrates; Harlan Teklad diet) or a HFD (21% total fat, 0.15% cholesterol; Harlan Teklad diet).
The CRF model was established in 8-week-old male B6.129P2-apolipoprotein Etm1Unc/J mice (background C57BL/6, Jackson Lab 002052) using a two-step surgery described by Gagnon et al.17 First, approximately 80% of the right kidney surface was electrocoagulated, avoiding areas adjacent to the ureter, blood vessels, and adrenal gland. Two weeks later, the left kidney was removed using the same anesthesia. Control mice received sham operations. The UC model was initiated in 10-week-old male mice. The animals were subjected to left-sided unilateral nephrectomy (UNX) under anesthesia.19,22 One week later, the experimental mice were provided drinking water containing 20 mg/ml urea for 12 weeks, whereas the control mice were supplied with urea-free water. Although UNX without urea (−UC) did not affect renal function, the supplementation of UNX mice with 2% urea (+UC) in drinking water yielded plasma urea concentrations similar to the CRF model. These results allowed for comparison between the two models.
IUE
The development of atherosclerosis in mice was monitored by an independent person blinded to the treatment. Animals were anesthetized by isoflurane inhalation (2.0 to 2.5%, 1.5 L/min oxygen) and similarly maintained (1.5 to 1.75% isoflurane, 1 L/min oxygen). After fur removal, animals were immobilized on a warming table and connected to electrodes to monitor cardiac ECG and respiration. IUE was performed using VisualSonics Vevo 77023 using the probes RMV707B-231 (30 MHz) and RMV704–215 (40 MHz) to visualize the aortic arch and abdominal aorta, respectively. The ascending aorta, aortic arch, and its major branches were visualized from a right parasternal long-axis view with the ultrasound probe offset 120 to 140° from animal's frontal surface. Blood flow and velocity were measured in the left common carotid artery 0.8 to 10 mm from the aortic arch using Doppler ultrasound. The abdominal aorta was visualized by longitudinal access using B-mode and M-mode. For quantification of blood flow and velocity, the Doppler mode was applied to the lower abdominal aorta (2 mm below renal arteries) using longitudinal access with the same ultrasound probe oriented at a 45 to 55° angle, sagittally.
Measurements of aortic wall echocontrast were performed at three locations: (1) the aortic arch, (2) the major aortic arch branches, and (3) the abdominal aorta (cross-sectional view between the celiac artery and iliac bifurcation). Three consecutive, 5-second cines were captured and saved for the anatomic and hemodynamic quantification at each site using a cardiovascular algorithm provided by VisualSonics. Measurements were obtained during at least three diastolic periods free of breathing movement. Aortic atherosclerotic lesions, including fatty streaks and plaques, were visualized by B-mode (plain, two-dimensional analysis of a single scan) as focal wall thickening. Aortic wall elasticity was measured as movement of the aortic wall during five cardiac cycles.
Blood Collection and Measurements
At the end of the experiment, mice were euthanized, and blood (approximately 1 ml) was collected by left ventricular puncture using a syringe containing 0.2 μmol EDTA. Creatinine, urea, total cholesterol, and triglycerides were measured by commercial kinetic assays (International Bioanalytical Industries, Boca Raton, FL) using a Synergy-HTI plate reader (BioTek, Winooski, VT). Plasma cLDL measurements, LDL electrophoresis, and Western blotting were performed as described earlier.11 oxLDL measurements were performed using a commercial kit (Mercodia, Uppsala, Sweden). Assays were validated for mouse plasma before use. Total plasma protein carbamylation was assayed using diacetyl monoxime,24 and total plasma protein oxidation was measured by the thiobarbituric acid assay.25
Evaluation of Aortic Atherosclerosis En Face
The cardiovascular system of the euthanized mouse was flushed with PBS and perfused with 10% buffered formalin (3 to 4 ml). The ascending aorta, aortic arch, and descending aorta, as well as innominate and common carotid arteries, were removed to the bifurcation of the abdominal aorta. Macroscopic morphometric analysis of aortic fatty deposits (index of atherosclerotic lesion formation) were made with Sudan IV as described previously.26
Histology and Quantitative Immunohistochemistry
A 3-mm length of ascending aorta (2 to 3 mm proximal to the brachiocephalic artery) was removed, cryosected into 5-μm sections, and assayed for fatty deposits by Oil red O staining and for cLDL, ICAM-1, or CD68 by immunohistological staining.16
Statistical Analysis
Continuous measures were evaluated for normality, and if marked deviations existed, transformations were done. Parametrical results were expressed as the mean ± SE. Significant differences in mean values between groups were examined with t test and an ANOVA, or, if the ANOVA assumptions were not fulfilled, the Mann-Whitney nonparametric test was used (SPSS 13.0 for Windows; SPSS, Chicago, IL). A value of P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Nancy Rusch, PhD, University of Arkansas for Medical Sciences (UAMS), for critical review of the manuscript; Sue Theus, PhD, and Kimberly Henning, BS, at the Little Rock Veterans Administration Veterinarian Medical Unit, for assistance with mouse intravital ultrasound, and the DNA Damage and Toxicology Core Center at UAMS for help with the histologic part of study. This research was supported by American Heart Association South Central Affiliate Grant 0865046F (to E.O.A.), National Institutes of Health/NHLBI Grant 1R21HL087405 (to A.G.B.), and Veterans Administration Merit Review grants (to A.G.B. and S.V.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Reis SE, Olson MB, Fried L, Reeser V, Mankad S, Pepine CJ, Kerensky R, Merz CN, Sharaf BL, Sopko G, Rogers WJ, Holubkov R: Mild renal insufficiency is associated with angiographic coronary artery disease in women. Circulation 105: 2826–2829, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Chade AR, Lerman A, Lerman LO: Kidney in early atherosclerosis. Hypertension 45: 1042–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Steinbrecher UP, Zhang HF, Lougheed M: Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med 9: 155–168, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Jialal I, Fuller CJ: Oxidatively modified LDL and atherosclerosis: An evolving plausible scenario. Crit Rev Food Sci Nutr 36: 341–355, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Van Tits L, De Graaf J, Hak-Lemmers H, Bredie S, Demacker P, Holvoet P, Stalenhoef A: Increased levels of low-density lipoprotein oxidation in patients with familial hypercholesterolemia and in end-stage renal disease patients on hemodialysis. Lab Invest 83: 13–21, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Takenaka T, Takahashi K, Kobayashi T, Oshima E, Iwasaki S, Suzuki H: Oxidized low density lipoprotein (Ox-LDL) as a marker of atherosclerosis in hemodialysis (HD) patients. Clin Nephrol 58: 33–37, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Tertov VV, Kaplun VV, Orekhov AN: Lack of correlation between degree of human plasma low density lipoprotein oxidation and its atherogenic potential. Biofactors 6: 139–143, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV: Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int 68: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Kraus LM, Kraus AP, Jr: Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl 78: S102–S107, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Apostolov EO, Shah SV, Ok E, Basnakian AG: Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin Chem 51: 719–728, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Apostolov EO, Shah SV, Ok E, Basnakian AG: Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 27: 826–832, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Apostolov EO, Shah SV, Ray D, Basnakian AG: Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler Thromb Vasc Biol 29: 1622–1630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asci G, Basci A, Shah SV, Basnakian A, Toz H, Ozkahya M, Duman S, Ok E: Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 13: 480–486, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Apostolov EO, Basnakian AG, Yin X, Ok E, Shah SV: Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am J Physiol Heart Circ Physiol 292: H1836–H1846, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Gagnon RF, Duguid WP: A reproducible model for chronic renal failure in the mouse. Urol Res 11: 11–14, 1983 [DOI] [PubMed] [Google Scholar]
- 18. Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drueke TB, Massy ZA: Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 112: 2875–2882, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB: Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol 14: 2466–2474, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gonen B, Cole T, Hahm KS: The interaction of carbamylated low-density lipoprotein with cultured cells. Studies with human fibroblasts, rat peritoneal macrophages and human monocyte-derived macrophages. Biochim Biophys Acta 754: 201–207, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Horkko S, Huttunen K, Kervinen K, Kesaniemi YA: Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur J Clin Invest 24: 105–113, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Buzello M, Tornig J, Faulhaber J, Ehmke H, Ritz E, Amann K: The apolipoprotein e knockout mouse: A model documenting accelerated atherogenesis in uremia. J Am Soc Nephrol 14: 311–316, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Gan LM, Gronros J, Hagg U, Wikstrom J, Theodoropoulos C, Friberg P, Fritsche-Danielson R: Non-invasive real-time imaging of atherosclerosis in mice using ultrasound biomicroscopy. Atherosclerosis 190: 313–320, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Trepanier DJ, Thibert RJ, Draisey TF, Caines PS: Carbamylation of erythrocyte membrane proteins: An in vitro and in vivo study. Clin Biochem 29: 347–355, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Fernando RL, Varghese Z, Moorhead JF: Differential ability of cells to promote oxidation of low density lipoproteins in vitro. Clin Chim Acta 269: 159–173, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T: Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100: 1634–1642, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.