Abstract
Receptor-mediated endocytosis is responsible for protein reabsorption in the proximal tubule. This process involves two interacting receptors, megalin and cubilin, which form a complex with amnionless. Whether these proteins function in parallel or as part of an integrated system is not well understood. Here, we report the renal effects of genetic ablation of cubilin, with or without concomitant ablation of megalin, using a conditional Cre-loxP system. We observed that proximal tubule cells did not localize amnionless to the plasma membrane in the absence of cubilin, indicating a mutual dependency of cubilin and amnionless to form a functional membrane receptor complex. The cubilin-amnionless complex mediated internalization of intrinsic factor-vitamin B12 complexes, but megalin considerably increased the uptake. Furthermore, cubilin-deficient mice exhibited markedly decreased uptake of albumin by proximal tubule cells and resultant albuminuria. Inactivation of both megalin and cubilin did not increase albuminuria, indicating that the main role of megalin in albumin reabsorption is to drive the internalization of cubilin-albumin complexes. In contrast, cubulin deficiency did not affect urinary tubular uptake or excretion of vitamin D-binding protein (DBP), which binds cubilin and megalin. In addition, we observed cubilin-independent reabsorption of the “specific” cubilin ligands transferrin, CC16, and apoA-I, suggesting a role for megalin and perhaps other receptors in their reabsorption. In summary, with regard to albumin, cubilin is essential for its reabsorption by proximal tubule cells, and megalin drives internalization of cubilin-albumin complexes. These genetic models will allow further analysis of protein trafficking in the progression of proteinuric renal diseases.
The renal handling of plasma proteins involves ultrafiltration in the glomerulus followed by tubular reabsorption. As a result of the essentially size-selective properties of the glomerular filter, the primary urine contains proteins of low molecular weight (<60 kD) such as vitamin D-binding protein (DBP) or free retinol-binding protein (RBP),1 whereas larger proteins are excluded. Albumin, the single most abundant plasma protein, is partially filtered, and the reported amount present in the glomerular ultrafiltrate varies from 1 to 50 μg/ml.2 Ultrafiltered protein, whatever the total amount in the lumen of the initial proximal tubule may be under physiologic conditions, is reabsorbed because normal urine is virtually protein free. Reabsorption takes place in the proximal tubule via receptor-mediated endocytosis, which, at present, is the only documented process for tubular protein clearance. Two receptors, physically and physiologically associated, have been identified.1 Megalin is a large transmembrane protein (approximately 600 kD) that belongs to the LDL receptor family. Cubilin,3 also known as the intrinsic factor cobalamin receptor,4,5 is a peripheral membrane protein (approximately 460 kD).3 Megalin binds cubilin with high affinity and may contribute to the internalization of cubilin-ligand complexes. Cubilin also binds amnionless (AMN),6,7 a 50-kD transmembrane protein that is required for its membrane expression and may permit internalization. Most proteins potentially present in the glomerular ultrafiltrate, and all of those that have been specifically studied have been identified as ligands of megalin, cubilin, or both. This is in particular the case for the most abundant, albumin, which binds both megalin and cubilin.1
The functional relevance of cubilin for tubular uptake of proteins relies on observations made in patients with Imerslund-Grasbeck syndrome (I-GS; also known as megaloblastic anemia 1, OMIM No. 261100) caused by inheritable cubilin or AMN gene defects.8–11 Functional cubilin deficiency resulting from inappropriate membrane insertion6,12 and/or synthesis of a truncated form of cubilin13 is associated with urine excretion of cubilin ligands such as albumin, transferrin, or apoA-I. Similar observations are made in a model of I-GS in dogs.6,12 On the other hand, the functional relevance of megalin relies on observations made in mice. Megalin-deficient mice14–17 excrete megalin ligands (RBP, DBP, cathepsin B, and albumin) as well as cubilin-specific ligands (transferrin and apoA-I). The latter finding has been tentatively related to the fact that megalin is essential for the internalization of cubilin-ligand complexes. Several questions remain unanswered. For instance can apoA-I (or other cubilin ligands), which does not bind megalin, be reabsorbed in the absence of cubilin? We do not know either whether megalin and cubilin function in parallel or as an integrated system for albumin reabsorption. To evaluate the respective roles of cubilin and megalin, it is necessary to compare the effects of cubilin and megalin deficiency in the same system (mouse or man) as well as to analyze the combined effects of simultaneous cubilin and megalin deficiency. This has not yet been achieved because interference with cubilin function early in utero results in early embryonic lethality18,19 related, at least in part, to dysfunction of extra embryonic tissues. We report here an efficient conditional inactivation system on the basis of loxP and the use of MORE mice that express the Cre recombinase in the whole epiblast but not in extra embryonic tissues.20 The inactivation of cubilin in the kidney results in a dramatic decrease of albumin and intrinsic factor vitamin B12 (IF-B12) complex reabsorption but, unexpectedly, does not affect the reabsorption of several other cubilin ligands.
RESULTS
Cubilin and Megalin Inactivation
Cubilin Inactivation.
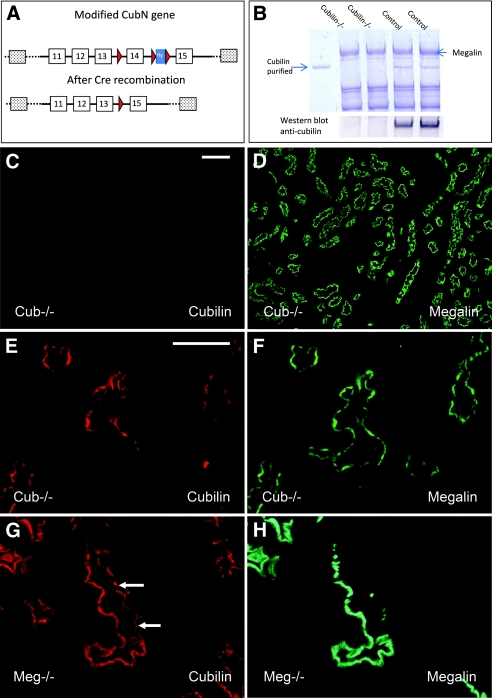
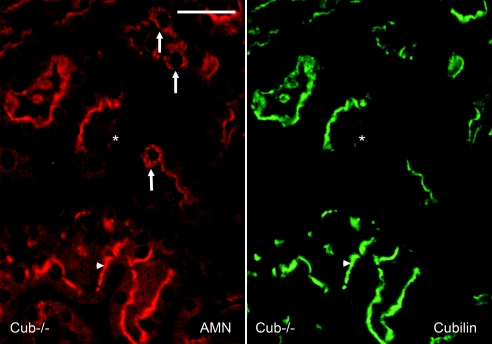
Mice bearing a floxed cubilin allele (Figure 1A) were crossed with MORE mice, which express the Cre recombinase in the epiblast but not in the extraembryonic tissues. Under these conditions embryos carrying all possible genotypes were born. Some degree of lethality was seen in cubilin−/− mice (19% versus 25% expected on a total of 200 mice). Inactivation in the kidney was very efficient as shown by Coomassie Blue staining and Western blot of renal extracts fractionated by SDS-PAGE (Figure 1B). No clear-cut renal developmental defect was found in cubilin-deficient mice. Morphology of the kidney was normal at the light microscopy level. In most mice 90 to 95% of the proximal tubules lacked cubilin (Figure 1C) but expressed megalin normally (Figure 1D). Occasional tubules (1 to 3 cross-sections of proximal tubules/whole kidney cross-section) expressed cubilin, often in a mosaic pattern (Figure 1E), whereas megalin was normal (Figure 1F). Some mice did not have any detectable cubilin. Amnionless was detected on the brush border of the tubular cells expressing cubilin (Figure 2, arrowhead). In contrast, in the absence of cubilin, AMN was either not detectable (Figure 2, asterisk) or present as perinuclear staining (Figure 2, arrows), probably representing cytoplasmic vesicles from the biosynthetic pathway.
Figure 1.
Cubilin and megalin inactivation. (A) The Cubn gene was targeted by homologous recombination using a construct containing three loxP sites (red) inserted into intron 13 and intron 14 flanking exon 14 and a hygromycin resistance cassette (blue) in intron 14. Homologous recombination and subsequent Cre recombinase excision generate a mutant allele that lacks exon 14 and an additional 1 kb of upstream and downstream genomic DNA. (B) Total kidney extracts separated on SDS-PAGE (5 to 16% gradient) were stained with Coomassie Blue or analyzed by Western blotting with anti-cubilin antibodies. Note that there is no detectable cubilin in mutant mice. (C through F) Immunohistochemistry of renal cortex of cubilin-deficient mice tested for cubilin (in red, C and E) or megalin (in green, D and F). (G and H) Immunohistochemistry of renal cortex of megalin-deficient mice tested for cubilin (in red, G) or megalin (in green, H). Bar: 100 μm in C and D; 50 μm in E through H.
Figure 2.
Altered expression of amnionless in cubilin-deficient mice. Immunohistochemical detection of amnionless (in red, left panel) and cubilin (in green, right panel) in mosaic tubules of cubilin-deficient mice. The arrowhead points to coexpression of cubilin and amnionless in cubilin-expressing cells. The arrows point to intracellular, perinuclear expression of amnionless in some cells lacking cubilin. In many cells (*) amnionless is not detectable in the absence of cubilin. Bar, 50 μm.
Megalin Inactivation.
Most of the homozygous megalin-deficient newborns obtained by crossing mice bearing a floxed megalin allele with MORE mice died rapidly after birth, as previously reported for constitutive inactivation.17 A small percentage (approximately 5%) survived in adult life. Sixty to 100% of proximal tubules did not express megalin. Occasional mosaic tubules suggested a mild decrease in cubilin expression in the absence of megalin (Figure 1, G and H, arrows).
Megalin and Cubilin Inactivation.
Mortality in this context was conditioned by megalin inactivation, and only a few mice lived to adulthood. Renal morphology was identical to that in megalin-deficient mice.
Handling of IF-B12 Complexes
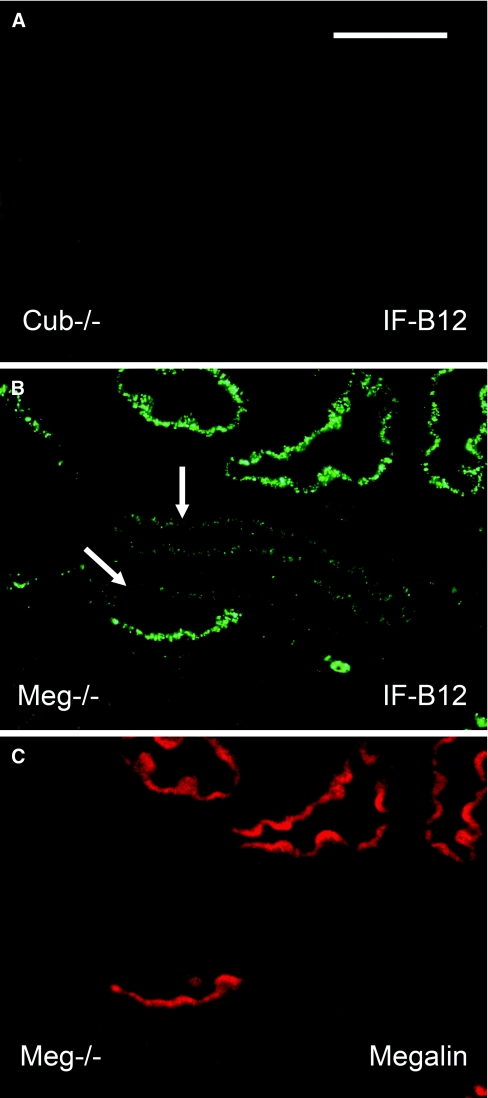
We first analyzed the uptake of IF-B12 complexes, the prototypic ligand of cubilin, by proximal convoluted tubule cells after intravenous injection in cubilin−/− or megalin−/− mice. Uptake could not be detected in cubilin−/− mice (Figure 3A). Definite but limited uptake was detected in megalin mutant cells (Figure 3B, arrows), indicating that, by itself, the cubilin-AMN complex present on the brush border can promote internalization of cubilin-ligand complexes. However, as demonstrated in mosaic tubules (Figure 3, B and C), uptake was massively greater in the presence of megalin, indicating that, under normal conditions, megalin is the main motor for cubilin internalization. Because cubilin is the physiologic receptor of IF-B12 complexes in the ileum, we measured plasma levels of vitamin B12. Values of 1800 and 12,000 pmol/L were measured respectively in cubilin−/− and in control mice (mean values of three mice for each group), demonstrating a dramatic decrease in uptake.
Figure 3.
Uptake of intrinsic factor-cobalamin complexes in cubilin- or megalin-deficient mice. (A) Immunochemical staining for intrinsic factor (in green) in the kidney cortex of cubilin-deficient mice. Note the lack of uptake. (B and C) Double labeling for IF (B in green) or megalin (C in red) in mosaic tubules from the renal cortex of megalin-deficient mice. Uptake of IF is detectable in megalin-deficient cells (arrows) but dramatically increased in cells expressing megalin. The mice were sacrificed 30 minutes after intravenous injection of IF-cobalamin complexes. Bar, 50 μm.
Tubular Handling and Reabsorption of Albumin
Urinary Albumin Excretion.
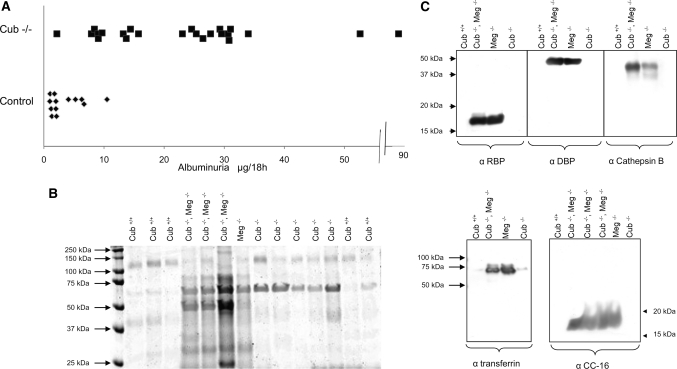
Total protein excretion was not significantly increased in cubilin-deficient mice (data not shown). However, selective daily (18 hour) albumin excretion was increased approximately six-fold in cubilin-deficient mice (P < 0.02) (Figure 4A). Increased albumin excretion was also evident when equivalent amounts of urine were analyzed by SDS-PAGE as shown in Figure 4B. Statistical comparison with albumin excretion in megalin-deficient or megalin- and cubilin-deficient mice could not be carried out because of the small number of available megalin−/− mice. It is, however, apparent that the amount of albumin excreted in megalin-deficient or in cubilin- and megalin-deficient mice is comparable with the amount excreted by the mice deficient in cubilin only.
Figure 4.
Urinary proteins in cubilin mutants. (A) Comparison of albumin excretion (μg/18 hours) in control and cubilin-deficient mice. (B) Profile of urinary proteins in control mice and cubilin-deficient, megalin-deficient, or cubilin- and megalin-deficient mice. Equivalent amounts of urine (normalized as in A) were subjected to SDS-PAGE and stained with Coomassie Blue. (C) Identification by Western blotting of RBP, DBP, cathepsin B, transferrin, and CC16 in the urine of cubilin, megalin, or cubilin- and megalin-deficient mice.
Internalization by Proximal Tubule Cells.
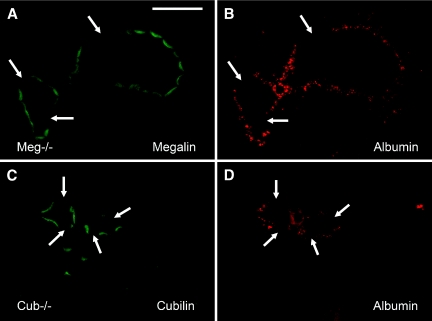
Proximal tubule sections of cubilin-deficient, megalin-deficient, or cubilin- and megalin-deficient mice displayed no or very few vesicles containing albumin that are found in control kidneys. The importance of the two receptors was confirmed by an extensive analysis of mosaic tubules. Figure 5 shows that selective inactivation of megalin (upper panels) or cubilin (lower panels) impedes formation of albumin vesicles, indicating that the simultaneous expression of the two receptors is required for the endocytic uptake of albumin.
Figure 5.
Decreased tubular reabsorption of albumin in cubilin- or megalin-deficient mice. Immunohistochemical detection of mouse albumin (in red, B and D) and megalin (in green, A) or cubilin (in green, C) in mosaic tubules of the kidney cortex from megalin-deficient (A and B) or cubilin-deficient (C and D) mice. Albumin-containing vesicles cannot be identified in cells lacking either megalin or cubilin. Bar, 50 μm.
Tubular Handling of Other Cubilin Ligands
Urine Excretion.
As shown in Figure 4C, plasma proteins known to be ligands of cubilin (transferrin and CC16), or megalin (RBP and cathepsin B) or shared ligands (DBP) were easily detectable in the unconcentrated urine of megalin-deficient or megalin- and cubilin-deficient mice. Mice deficient in cubilin only did not excrete “specific” megalin ligands, a “shared” ligand such as DBP, or cubilin-specific ligands. Modest amounts of transferrin were detected in some cubilin-deficient mice, but traces were also found in control mice. ApoA-I was not found in any group (not shown in Figure 4).
Internalization by Proximal Tubule Cells
Sections of renal cortex from cubilin-deficient mice were examined for cubilin and/or megalin ligands. Endocytic vesicles containing megalin ligands RBP and cathepsin B but also the shared ligand DBP were detected in cubilin-deficient mice as well as in control mice. We could not detect any decrease in the uptake of three ligands of cubilin: CC16, transferrin, and apoA-I (data not shown).
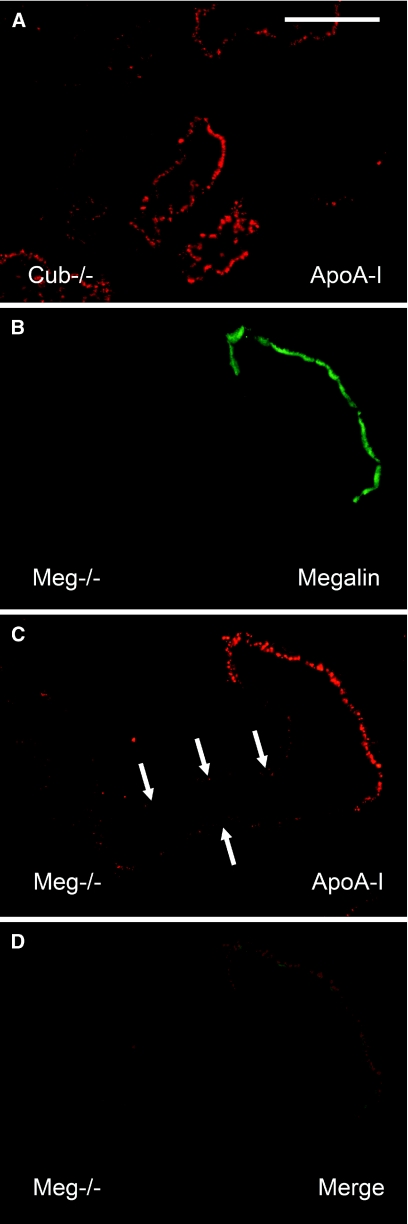
To analyze further the uptake of apoA-I, we injected fluorescently labeled apoA-I intravenously in cubilin and/or megalin-deficient mice, which were sacrificed after 30 minutes. As shown in Figure 6A, injected apoA-I was internalized efficiently in cubilin mutants. Megalin mutants internalized apoA-I, but uptake was limited as compared with cells expressing megalin (Figure 6, B and C), illustrating the importance of cubilin for uptake as well. There was no detectable uptake in mice lacking both cubilin and megalin (data not shown).
Figure 6.
Tubular reabsorption of apoA-I in cubilin- or megalin-deficient mice. (A) Immunohistochemical detection of apoA-I in cubilin-deficient mice. Cubilin was not detectable in the tissue. (B through D) Detection of apoA-I (in red, C) and megalin (in green, B) in mosaic tubules from the renal cortex of megalin-deficient mice. (D) Merge. Uptake of apoA-I is detectable in megalin-deficient cells (arrows) but dramatically increased in cells expressing megalin. Mice were sacrificed 30 minutes after intravenous injection of labeled apoA-I. Bar, 50 μm.
DISCUSSION
From our initial studies using monoclonal antibodies to cubilin,18 we predicted that a lack of cubilin would not be compatible with full embryonic development. This assumption was indeed confirmed by Smith et al.19 We therefore turned to conditional invalidation using a “Cre-loxP” approach. In these studies we used MORE mice, which express the recombinase in the epiblast, albeit in a mosaic pattern, but not in the extraembryonic tissues, therefore preserving cubilin function in this structure. Embryonic lethality is not abolished, but it is possible to obtain viable mice lacking cubilin in the kidney. Inactivation of cubilin in the kidney was remarkably efficient, approximately 90% in most mice, on the basis of immunohistological analysis, Western blotting of whole kidney extracts, and reverse transcription-PCR analysis. The level of inactivation achieved compares favorably with other models of the literature using the Cre-loxP approach and in particular with the megalin model.15 In our system, inactivation of megalin reproduced faithfully the renal phenotype observed with constitutive inactivation,17 confirming the efficiency of the inactivation in the kidney. It is not within the scope of this study to analyze the role of cubilin during development. Similarly, although we measured very low levels of vitamin B12 in cubilin-deficient mice, this aspect will not be discussed further because the role of cubilin for ileal uptake of vitamin B12 has been extensively demonstrated in clinical studies.13,21 We will focus here on the renal consequences of cubilin deficiency. Three conclusions emerge from our studies. First, cubilin and AMN are mutually dependent for brush border expression; the cubilin-AMN complex can mediate uptake of cubilin ligands by PCT cells in the absence of megalin albeit at a very limited rate. Second, cubilin plays an essential role in the physiologic reabsorption of albumin in the proximal tubule, and megalin, rather than AMN, is the main motor for internalization of cubilin-albumin complexes. Third, uptake of high-affinity cubilin ligands such as apoA-I, CC16, or transferrin can take place in mice in the absence of cubilin.
Our initial report of cubilin as a peripheral protein3 devoid of a transmembrane domain left unanswered its mechanisms of endocytosis and membrane association. The observation that AMN, a 50-kD transmembrane protein, was mutated in I-GS patients in whom cubilin was normal22 led to the conclusion that expression of AMN was indispensable for membrane targeting of cubilin6,7,23,24 and possibly sufficient in vitro to mediate internalization of IF-B12 complexes.6 Analysis of mosaic tubules in this study demonstrates not only that AMN is required for the membrane expression of cubilin but also that cubilin in vivo is required for membrane expression of AMN as it is in cell culture studies.25 Whereas in cubilin-expressing cells AMN is co-localized with cubilin to the apical membrane, in cubilin-deficient cells it is either not detectable or restricted to intracellular vesicles. The results also show in vivo that the sole cubilin-AMN complex (in the absence of megalin) is only sufficient to mediate minimal uptake of IF-B12 complexes in PCT cells. The amount of IF-B12 complexes taken up when megalin is expressed is considerably greater, demonstrating that megalin (rather than AMN) is the main promoter of the uptake of cubilin-ligand complexes.
A second conclusion of this study concerns the uptake of albumin, a shared ligand of cubilin and megalin, by proximal tubule cells. Our data show that urinary albumin excretion in cubilin-deficient mice is increased approximately six-fold over background, a level comparable with that measured in megalin-deficient mice.26 Because both megalin and cubilin bind albumin with similar affinity, one could propose that the effects of megalin and cubilin inactivation on albumin excretion would be additive. This is not the case, however, because the inactivation of megalin in addition to cubilin does not result in a substantial increase in albumin excretion. Furthermore, albumin-containing vesicles are virtually absent from either cubilin- or megalin-deficient tubular cells. Taken together, these findings indicate that, under physiologic conditions, albumin is reabsorbed by a mechanism involving both cubilin and megalin, the latter promoting internalization of cubilin-albumin complexes. The amount of albumin reabsorbed by megalin independently of cubilin is small. Recent controversial studies suggest that the amount of albumin filtered by the glomerulus is considerably higher than previously suspected or measured. It has been proposed that megalin and cubilin only reabsorbed a fraction of the filtered albumin, the bulk being reabsorbed by yet unidentified mechanisms of vesicular transport.27 Such a possibility is in sharp disagreement with our studies indicating that albumin-containing vesicles are not detectable in proximal tubule cells lacking megalin or cubilin or both.
Such an important role for cubilin does not seem to apply to the reabsorption of DBP, another shared ligand of cubilin and megalin. DBP is not found in the urine of cubilin-deficient mice, whereas it is easily detectable in the urine of megalin-deficient mice. Also, DBP-containing vesicles are detected equally well in the cortex of normal and cubilin-deficient mice, whereas they are not found in the cortex of megalin-deficient mice. This result was unexpected because patients with I-GS carrying the FM2 mutation or AMN mutations, which result in truncated or lack of membrane expression of cubilin, excrete DBP. Also dogs with abnormal membrane expression of cubilin caused by AMN mutations excrete DBP, and DBP-containing vesicles cannot be found in their proximal tubules. The small amounts of cubilin remaining after genetic inactivation of cubilin cannot account for the lack of DBP excretion because DBP-containing vesicles are found equally in cubilin-negative cells as well as in the few remaining cubilin-expressing cells. In addition, all mice undergoing inactivation of megalin using the same process with the same efficiency display the characteristic excretion of DBP reported in full megalin KOs. This might suggest that mice handle DBP differently from dogs and humans.
The third and most unexpected conclusion concerns the cubilin-independent uptake in our mice of transferrin, CC16, and apoA-I, identified as specific cubilin ligands. We previously reported that this set of proteins was detected in the urine of dogs or I-GS patients lacking expression of functional cubilin. We also identified transferrin and CC16 in the urine of megalin-deficient mice and related the latter finding to defective internalization of cubilin-ligand complexes. At variance these results in cubilin−/− mice show that, at least in this species, cubilin is not strictly required for uptake of transferrin, apoA-I, or CC16 and suggest that the role of megalin may not be limited to cubilin internalization. One cannot conclude, however, that cubilin plays no role in the uptake of these proteins. For instance, our results using intravenously administered fluorescence apoA-I reveal that the cubilin-AMN complex can take up apoA-I. Unfortunately we could not analyze further the traffic of transferrin and CC16 because of a combination of technical difficulties including the lack of antibodies for immunochemistry and/or the unavailability of purified proteins and/or size preventing tubular access for injection of labeled proteins.
Three potential categories of explanations, not mutually exclusive, can account for the discrepancies between these results and previously published observations. One could first propose that factors in addition to the lack of cubilin on the cell surface might contribute to the uptake defect in dogs and I-GS patients. Abnormal intracellular traffic is observed in dogs, but it affects the biosynthethic pathway, and there is no evidence that it has any consequence on the endocytic pathway or on the membrane expression of other receptors such as megalin. Second, it is also conceivable that, in mice, mechanisms other than cubilin contribute to the binding and uptake of apoA-I, transferrin, and CC16. Because of its low efficiency, in particular for compounds present in low concentration, it is unlikely that fluid phase endocytosis can account for the results. It appears more likely that, in mice, transferrin, CC16, and apoA-I bind to some yet undefined membrane “receptor” with (very) low affinity, accounting for the fact that it has escaped biochemical identification. AMN can be excluded because it is not expressed on the membrane in cubilin-deficient mice. Megalin, which has a very large number of ligands with a wide range of affinities, could play this role. Another yet unknown receptor may come into play that, like cubilin, would require the assistance of megalin for optimal uptake. Finally, it is also possible that the proteins under consideration might complex with other compound(s) themselves bound by megalin. For example an apoA-I–binding protein, synthesized in tubule cells, has been identified and may be a megalin ligand.28
In conclusion, using a set of genetic models allowing inactivation of cubilin, megalin, or both simultaneously, we describe the essential role of cubilin in albumin reabsorption by proximal tubule cells and provide evidence that the main role of megalin in albumin reabsorption is to drive internalization of cubilin-albumin complexes. In addition, the models developed, and in particular the simultaneous inactivation of cubilin and megalin, provide new and powerful means to analyze the role of protein trafficking in the progression of proteinuric diseases toward terminal renal failure.
CONCISE METHODS
Production of Mice
Cubilin KO.
Standard techniques were used to generate a conditional cubilin targeting vector. As shown in Figure 1A, exon 14 was flanked with loxP sites for the Cre recombinase, and a hygromycin cassette was inserted in intron 14. The linearized vector was transfected in embryonic stem cells by electroporation, and hygromycin-resistant clones were selected. Screening for homologous recombination was carried out by long-range PCR using an internal primer and primers situated upstream of the 5′ end and downstream of the 3′ end of the targeting vector. Correctly targeted embryonic stem cell clones were confirmed by Southern blot analysis, injected into C57BL/6J blastocysts, and implanted into recipient females. Germ line transmission of the recombinant Cubn allele was obtained for several chimeric mice. For the generation of Cubn-deficient mice, homozygous Cubnloxlox were crossed with MORE mice,20 a deleter strain that selectively expresses the recombinase in the epiblast, sparing the extraembryonic tissue. The mice were maintained on a mixed C57BL/6-129/Svj background. Genotypic analysis of offspring was performed by PCR using allele-specific primers (Supplemental Table 1). The deletion of the targeted exon was verified by Southern blotting and reverse transcription-PCR. Details concerning screening of embryonic stem cell clones and genotyping are given as Supplemental Data. The lack of cubilin protein was verified by Western blotting (Figure 1). Mouse breeding and handling were carried out in a certified animal facility according to procedures that were approved by the local animal care and experimentation authorities.
Megalin KO.
Mice of mixed genetic background carrying a floxed megalin allele have been previously reported.15 They were bred with MORE mice to obtain megalin invalidation.
Cubilin and Megalin KO.
Mice carrying floxed megalin gene were crossed for several generations with mice carrying a floxed cubilin gene. The offspring were selected for homologous recombination resulting from meiotic crossing over to obtain mice bearing both modified genes. These mice were also crossed with MORE mice to obtain invalidation of both cubilin and megalin.
Characterization of Mutants.
Kidney extracts were initially tested by Western blotting for cubilin and megalin using previously described rabbit29 and sheep26 antibodies raised against purified rat proteins. Tissue sections were also analyzed for the presence or absence of cubilin and megalin using the above antisera and anti-AMN23 antibodies.
Handling of IF-B12 Complexes
The plasma levels of vitamin B12 were determined using a commercially available competitive protein binding assay with IF as the binding protein (Centaur™ Automated Chemiluminescence System) (Bayer A/S; Diamond Diagnostics, Holliston, MA). In addition, control, cubilin, or megalin knockout (KO) mice were injected via the saphenous vein with recombinant human intrinsic factor-cobalamin complexes (IF-B12) (330 μg) (Cobento, Aarhus, Denmark). Thirty minutes after injection, the kidneys were fixed as described below.
Analysis of Urine Proteins
Urine was collected in the presence of a mix of protease inhibitors (Complete; Roche, Hvidovre, Denmark) from 23 cubilin-deficient and 13 control mice placed in metabolic cages for 18 hours. Total protein and albumin excretion were measured on an Olympus auto-analyzer using the chemical molybdate-pyrogallol and immunonephelometry, respectively. The protein profile in urine from knockout and control mice was examined by SDS-PAGE and Western blot using rabbit antibodies against rat albumin, human DBP, and human RBP (Dako, Glostrup, Denmark), goat anti-mouse transferrin (Dunn Labortechnik GmbH, Asbach, Germany), goat anti-mouse apoA-I (Rockland Immunochemicals, Gilbertsville, PA), CC16,30 and goat anti-mouse cathepsin B (R&D Systems Inc., Minneapolis, MN). Identical blots were made with urine from megalin as well as cubilin- and megalin-deficient mice.
Morphology and Immunohistology
For immunohistological studies, the kidneys were fixed by retrograde perfusion through the abdominal aorta with 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4. The tissue was dehydrated and embedded in paraffin by standard methods. Sections from control and cubilin-deficient mice were analyzed using the above mentioned antisera for the presence of endocytic vesicles containing the following cubilin and/or megalin ligands: albumin, DBP, RBP, transferrin, apoA-I, and cathepsin B. For comparison, mice deficient in megalin only or in cubilin and megalin were studied for apoA-I and albumin. In all instances simultaneous labeling was performed on identical or consecutive sections with anti-megalin and anti-cubilin antibodies. In separate experiments, apoA-I (100 μg) was injected intravenously. Thirty minutes after injection, the kidneys were fixed and processed as above.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported in part by INSERM, Université Pierre et Marie Curie, Paris 6, the University of Aarhus, the Danish Medical Research Council, the NOVO-Nordisk Foundation, the programs of the European Community, EuReGene (FP6, GA#5085) and EUNEFRON (FP7, GA#201590), the Belgian agencies Fonds National de la Recherche Scientifique and Fonds de la Recherche Scientifique Médicale, the “Fondation Alphonse & Jean Forton, ” an Interuniversity Attraction Pole (IUAP P6/05), and the DIANE project (Communauté Française de Belgique). J. Gburek was the recipient of a Marie Curie Intra-European fellowship.
We wish to thank M. Pontoglio and P. Soriano for providing MORE mice and E. Lacy for providing the anti-amnionless antibody. Also, the skillful technical assistance of Hanne Sidelmann, Inger Blenker Kristoffersen, Anne Merete Hass, and Nicolas Sorhaindo is gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tubular Reabsorption of Albumin: It's All About Cubilin,” on pages 1810–1812.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Christensen EI, Verroust PJ, Nielsen R: Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch 458: 1039–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Maack T, Park CH, Camargo MJF: Renal filtration, transport and metabolism of proteins. In: The Kidney, 2nd Ed., edited by Seldin DW, Giebisch G. New York, Raven Press, 1992, pp 3005–3038 [Google Scholar]
- 3. Moestrup SK, Kozyraki R, Kristiansen M, Kaysen JH, Rasmussen HH, Brault D, Pontillon F, Goda FO, Christensen EI, Hammond TG, Verroust PJ: The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J Biol Chem 273: 5235–5242, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Birn H, Verroust PJ, Nexø E, Hager H, Jacobsen C, Christensen EI, Moestrup SK: Characterization of an epithelial approximately 460-kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and binds receptor-associated protein. J Biol Chem 272: 26497–26504, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Seetharam B, Christensen EI, Moestrup SK, Hammond TG, Verroust PJ: Identification of rat yolk sac target protein of teratogenic antibodies, gp280, as intrinsic factor-cobalamin receptor. J Clin Invest 99: 2317–2322, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fyfe JC, Madsen M, Hojrup P, Christensen EI, Tanner SM, de la Chapelle A, He Q, Moestrup SK: The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 103: 1573–1579, 2004 [DOI] [PubMed] [Google Scholar]
- 7. He Q, Madsen M, Kilkenney A, Gregory B, Christensen EI, Vorum H, Hojrup P, Schaffer AA, Kirkness EF, Tanner SM, de la Chapelle A, Giger U, Moestrup SK, Fyfe JC: Amnionless function is required for cubilin brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo. Blood 106: 1447–1453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burmeister R, Boe IM, Nykjaer A, Jacobsen C, Moestrup SK, Verroust P, Christensen EI, Lund J, Willnow TE: A two-receptor pathway for catabolism of Clara cell secretory protein in the kidney. J Biol Chem 276: 13295–13301, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui S, Christensen EI, Aminoff M, de la Chapelle A, Krahe R, Verroust PJ, Moestrup SK: The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med 5: 656–661, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK: Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A 98: 12491–12496, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI: Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A 98: 13895–13900, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fyfe JC, Ramanujam KS, Ramaswamy K, Patterson DF, Seetharam B: Defective brush-border expression of intrinsic factor-cobalamin receptor in canine inherited intestinal cobalamin malabsorption. J Biol Chem 266: 4489–4494, 1991 [PubMed] [Google Scholar]
- 13. Aminoff M, Carter JE, Chadwick RB, Johnson C, Grasbeck R, Abdelaal MA, Broch H, Jenner LB, Verroust PJ, Moestrup SK, de la Chapelle A, Krahe R: Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet 21: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjær A, Blomhoff R, Willnow TE, Moestrup SK: Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 10: 685–695, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE: Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J 17: 247–249, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI: Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci U S A 104: 5407–5412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J: Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A 93: 8460–8464, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sahali D, Mulliez N, Chatelet F, Dupuis R, Ronco P, Verroust P: Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med 167: 213–218, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith BT, Mussell JC, Fleming PA, Barth JL, Spyropoulos DD, Cooley MA, Drake CJ, Argraves WS: Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation. BMC Dev Biol 6: 30, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tallquist MD, Soriano P: Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26: 113–115, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kozyraki R, Kristiansen M, Silahtaroglu A, Hansen C, Jacobsen C, Tommerup N, Verroust PJ, Moestrup SK: The human intrinsic factor-vitamin B12 receptor, cubilin: Molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. Blood 91: 3593–3600, 1998 [PubMed] [Google Scholar]
- 22. Tanner SM, Aminoff M, Wright FA, Liyanarachchi S, Kuronen M, Saarinen A, Massika O, Mandel H, Broch H, de la Chapelle A: Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nat Genet 33: 426–429, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Strope S, Rivi R, Metzger T, Manova K, Lacy E: Mouse amnionless, which is required for primitive streak assembly, mediates cell-surface localization and endocytic function of cubilin on visceral endoderm and kidney proximal tubules. Development 131: 4787–4795, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Xu D, Kozyraki R, Newman TC, Fyfe JC: Genetic evidence of an accessory activity required specifically for cubilin brush-border expression and intrinsic factor-cobalamin absorption. Blood 94: 3604–3606, 1999 [PubMed] [Google Scholar]
- 25. Coudroy G, Gburek J, Kozyraki R, Madsen M, Trugnan G, Moestrup SK, Verroust PJ, Maurice M: Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J Am Soc Nephrol 16: 2330–2337, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI: Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM: Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 295: F1589–F1600, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Ritter M, Buechler C, Boettcher A, Barlage S, Schmitz-Madry A, Orso E, Bared SM, Schmiedeknecht G, Baehr CH, Fricker G, Schmitz G: Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I. Genomics 79: 693–702, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Sahali D, Mulliez N, Chatelet F, Laurent Winter C, Citadelle D, Sabourin JC, Roux C, Ronco P, Verroust P: Comparative immunochemistry and ontogeny of two closely related coated pit proteins: The 280-kd target of teratogenic antibodies and the 330-kd target of nephritogenic antibodies. Am J Pathol 142: 1654–1667, 1993 [PMC free article] [PubMed] [Google Scholar]
- 30. Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB: Mice lacking renal chloride channel, CLC-5, are a model for Dent's disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9: 2937–2945, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.