Abstract
High-mobility group box 1 (HMGB1), a nuclear factor released extracellularly as an inflammatory cytokine, is an endogenous ligand for Toll-like receptor 4 (TLR4). TLR4 activation mediates kidney ischemia-reperfusion injury (IRI), but whether HMGB1 contributes to IRI is unknown. Here, treating wild-type mice with neutralizing anti-HMGB1 antibody protected them against kidney IRI, evidenced by lower serum creatinine and less tubular damage than untreated mice. Mice treated with anti-HMGB1 had significantly less tubulointerstitial infiltration by neutrophils (day 1) and macrophages (day 5) and markedly reduced apoptosis of tubular epithelial cells. Furthermore, anti-HMGB1 antibody-treated IRI kidneys had significantly lower levels of IL-6, TNFα, and monocyte chemoattractant protein 1 (MCP1). mRNA, which are downstream of HMGB1. Conversely, administration of rHMGB1 after reperfusion exacerbated kidney IRI in wild-type mice. TLR4 deficient (TLR4−/−) mice were protected against kidney IRI; administration of neither anti-HMGB1 antibody nor rHMGB1 affected this renoprotection. In conclusion, endogenous HMGB1 promotes kidney damage after IRI, possibly through the TLR4 pathway. Administration of a neutralizing antibody to HMGB1 either before or soon after ischemia-reperfusion affords significant protection, suggesting therapeutic potential for acute kidney injury.
Renal ischemia reperfusion injury (IRI) is an inevitable consequence of the procedure of kidney transplantation and impacts negatively on both short- and long-term graft survival.1–3 The initial nonimmune injury leads to the activation of an innate immune response causing variable degrees of tissue damage.4–7 Toll-like receptor (TLR) activation by engagement through TLR endogenous ligands is an important pathway by which IRI triggers innate immunity. IRI causes injured tissues to express or release a variety of endogenous TLR ligands, particularly for TLR2 and TLR4, including heat-shock proteins, high-mobility group box 1 (HMGB1), hyaluronan, fibronectin, heparan sulfate, and biglycan.8–13 Increasing experimental evidence indicates that engagement of TLRs by such endogenous ligands may result in TLR activation, causing initiation and amplification of the local innate immune responses. Expression of TLR2 and TLR4 has been shown to be upregulated in kidney IRI, particularly by tubular epithelial cells.14,15 TLR2 was found to be an important initiator of inflammatory responses after kidney ischemia.16,17 We reported that IRI resulted in upregulation of TLR4 and TLR endogenous ligands including HMGB1, hyaluronan, and biglycan in the IRI kidney and that TLR4−/− mice were protected against kidney dysfunction, tubular damage, neutrophil and macrophage accumulation, and expression of proinflammatory cytokines and chemokines.18 These results have been supported by other groups.19,20 Because TLR4 is likely activated by endogenous ligands, further studies are warranted to elucidate whether blockade of the interaction between endogenous ligands and TLRs can prevent kidney IRI. Recent studies have demonstrated that an endogenous negative regulator of TLRs, single Ig IL-1 receptor-related protein, inhibited kidney ischemia-reperfusion (IR) injury by suppressing the postischemic activation of intrarenal myeloid cells.21,22
HMGB1 is an endogenous molecule known to stimulate TLR4 signaling and has been implicated in the pathogenesis of IRI. HMGB1 is a nuclear factor that is involved in transcriptional activation and DNA folding23,24 but also serves as an extracellular cytokine known to be a critical mediator of innate immune responses to infection and injury.24 HMGB1 has been reported to trigger cellular signaling through TLR2, TLR4, and TLR9,12,25,26 leading to the recruitment of inflammatory cells and the release of proinflammatory cytokines and chemokines that cause organ damage in liver IRI27,28 and acute lung injury.29–31 The role of HMGB1 in kidney IRI is unknown.
We previously reported that TLR4 activation mediated kidney IRI and demonstrated upregulation of the endogenous ligands HMGB1, hyaluronan, and biglycan in the kidney after IRI, providing circumstantial evidence that one or more of these ligands may be the source of TLR4 activation.18 Here we hypothesize that endogenous HMGB1 mediates cell injury and inflammation in kidney IRI via TLR4 signaling. We aimed to determine (1) whether endogenous HMGB1 contributes to kidney IRI; (2) whether this is via direct HMGB1-TLR4 interaction; and (3) whether neutralizing antibody to HMGB1 has therapeutic potential in kidney IRI.
RESULTS
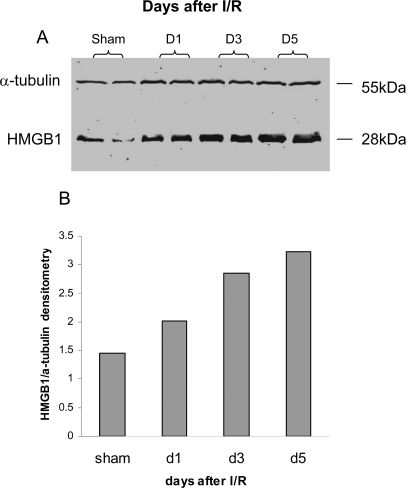
HMGB1 Expression Is Increased in the Kidney after Kidney IRI
We reported that the mRNA level of HMGB1 was significantly increased at day 1 post-IR with further upregulation at day 5 compared with sham-operated controls and that HMGB1 was expressed by tubular epithelial cells by immunofluorescent staining.18 In this study, Western blot analysis of HMGB1 protein expression was performed on kidney homogenates. HMGB1 protein was similarly upregulated at days 1 and 5 after IR compared with sham-operated controls (Figure 1).
Figure 1.
HMGB1 protein is upregulated in IRI kidney from days 1 to 5. Panel A shows the Western blot, and panel B shows quantitation by densitometry. n = 2 per group.
Neutralizing Antibody to HMGB1 Protects against Renal IRI
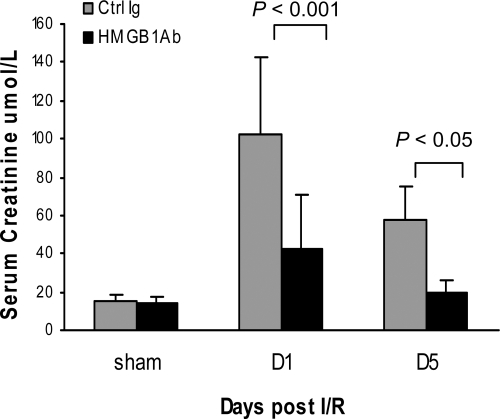
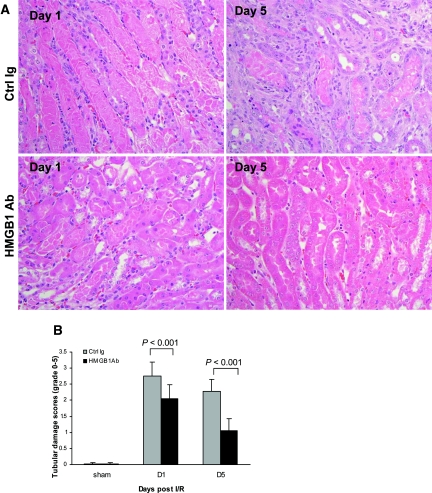
To determine whether endogenous HMGB1 contributes to kidney IRI, wild-type (WT) mice received neutralizing antibody to HMGB1 or isotype Ig as the control 1 hour before ischemia. As shown in Figure 2, IRI caused kidney dysfunction in control Ig-treated mice, reflected by significant elevation of serum creatinine at days 1 and 5 post-IRI. Renal dysfunction was attenuated in anti-HMGB1 antibody (Ab)-treated mice, with serum creatinine lower than the control mice at day 1 (P < 0.001) and day 5 (P < 0.05) post-IR. Pretreatment with anti-HMGB1 Ab also afforded protection as assessed by histology. Control mice incurred severe tubular damage, as evidenced by widespread tubular necrosis, loss of the brush border, cast formation, and tubular dilation at the corticomedullary junction at days 1 and 5 after IRI, which was moderately attenuated in anti-HMGB1 Ab-treated mice (Figure 3, A and B; P < 0.001). Sham-operated mice incurred no tubular injury.
Figure 2.
Anti-HMGB1 Ab-treated mice (black bars) are protected against renal IRI with significantly lower serum creatinine compared with control (Ctrl) Ab-treated mice (gray bars) at days 1 and 5 after reperfusion. Sham-operated mice had normal serum creatinine (10 to 20 μmol/L). The data are the means ± SD. n = 5 per sham group, n = 9 per group for day 1, n = 7 per group for day 5.
Figure 3.
Tubular injury is attenuated in anti-HMGB1 Ab-treated mice. (A) Representative sections of outer medulla from Ctrl Ig-treated mice and anti-HMGB1 Ab-treated mice at days 1 and 5 after reperfusion (hematoxylin- and eosin-stained; magnification, ×400). (B) Semiquantitative analysis of tubular damage in Ctrl Ig-treated (gray bars) and anti-HMGB1 Ab-treated (black bars) kidney at days 1 and 5 after reperfusion. The data shown are the means ± SD. n = 5 to 9 per group.
Interstitial Infiltrates Are Reduced in the Kidney of Anti-HMGB1-treated Mice
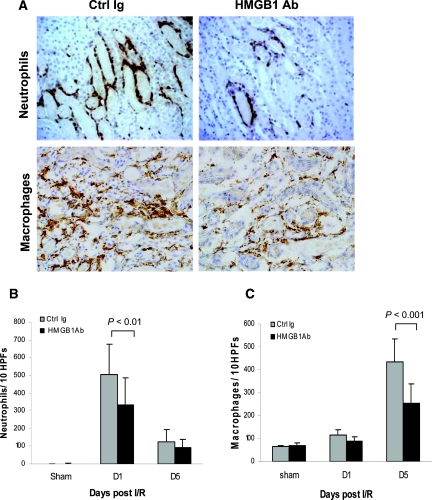
Substantial infiltration of neutrophils was evident in control mice at day 1 after IR versus sham-operated controls (P < 0.001; Figure 4B), and this was attenuated in anti-HMGB1 Ab-treated mice (P < 0.01; Figure 4B). Similarly, significant macrophage accumulation was evident in control mice after IR compared with sham-operated controls but significantly reduced by anti-HMGB1 Ab-treated mice at day 5 post-IR (Figure 4C; P < 0.001).
Figure 4.
Neutrophil and macrophage accumulation within the interstitium of the kidney is significantly less in anti-HMGB1 Ab-treated mice versus Ctrl Ig-treated mice at 24 hours after reperfusion by immunohistochemisty staining. (A) Representative sections of kidney at 24 hours after reperfusion stained for neutrophils in the top panel and at day 5 after reperfusion stained for macrophages in the bottom panel by immunohistochemisty staining (magnification, ×200). (B) Analysis of neutrophil infiltrate in Ctrl Ig-treated (gray bars) and anti-HMGB1 Ab-treated (black bars) kidney (numbers /10 HPFs). (C) Analysis of macrophage infiltrate in Ctrl Ig-treated (gray bars) and anti-HMGB1 Ab-treated (black bars) kidney (numbers per ten HPFs). The data shown are the means ± SD. n = 5 to 9 per group.
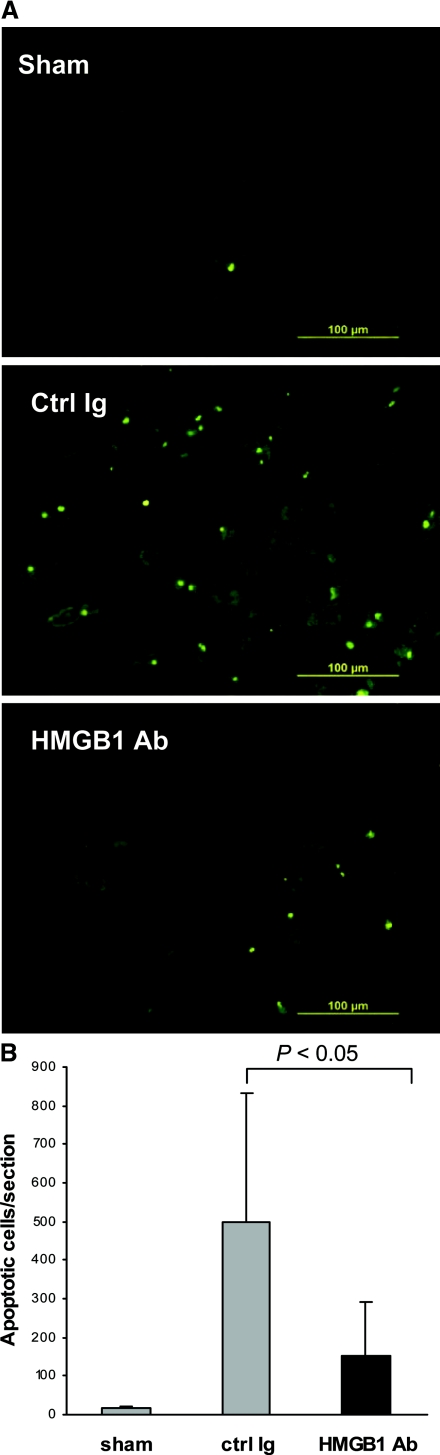
Tubular Epithelial Cell Apoptosis Is Reduced in the IRI Kidney by HMGB1 Blockade
To examine whether the neutralizing HMGB1 prevented apoptosis, we assessed tubular apoptotic cells in the kidney using the terminal deoxynucleotidyl-transferase–mediated dUTP nick end labeling (TUNEL) assay. A substantial increase in tubular epithelial cell apoptosis was evident in control animals at day 1 post-IRI as compared with sham-operated animals (P < 0.05), whereas this increase was significantly abrogated by the administration of anti-HMGB1 Ab (P < 0.05; Figure 5).
Figure 5.
HMGB1 blockade reduces tubular epithelial cell apoptosis in the kidney post-IRI measured by TUNEL assay. (A) Representative sections of kidney at 24 hours after sham operation or reperfusion. (B) The number of apoptotic tubular cells was significantly lower in anti-HMGB1 Ab-treated mice at day 1 post-IRI than in control mice (P < 0.05). The data shown are the means ± SD. n = 5 to 9 per group.
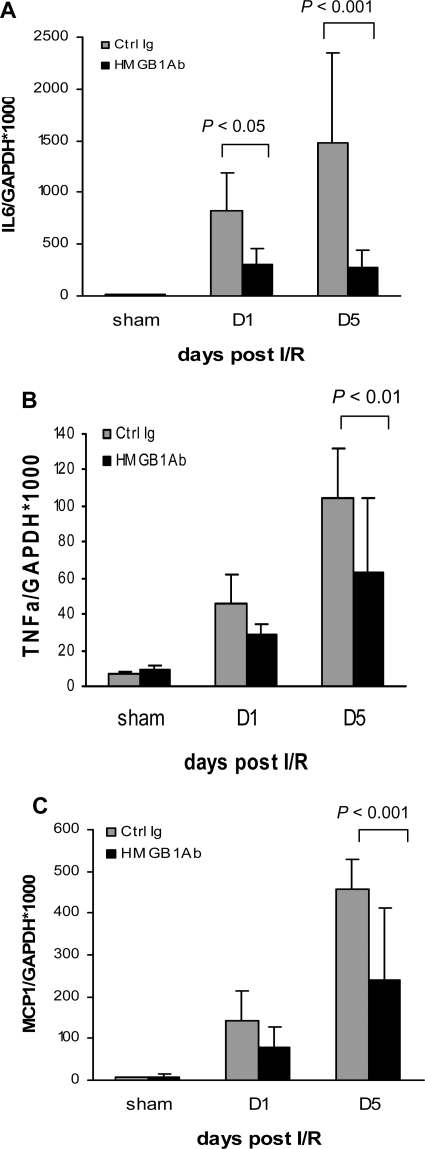
Expression of HMGB1 Downstream Cytokines and Chemokines within the Kidney after IRI Is Attenuated by HMGB1 Blockade
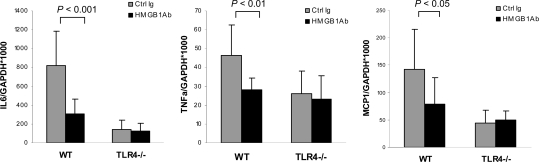
TLR4 engagement initiates a signaling cascade leading to activation of the transcription factor NFκB, which regulates the induction of multiple inflammatory genes. To investigate the effects of anti-HMGB1 Ab pretreatment, we measured mRNA expression of HMGB1 downstream cytokines and chemokines in the kidney by real-time PCR. Sham-operated controls demonstrated a detectable level of TNFα and very low levels of IL-6 and monocyte chemoattractant protein 1 (MCP1). There were no significant differences between control Ab and anti-HMGB1 Ab-treated sham-operated groups. Control mice subjected to IRI demonstrated strong upregulation of IL-6, TNFα, and MCP1 at days 1 and 5 post-IR (Figure 6) compared with sham-operated controls. Mice treated with anti-HMGB1 Ab showed a significant reduction in IL-6, TNFα, and MCP1 expression compared with control mice at days 1 and 5 post-IR (Figure 6).
Figure 6.
HMGB1 blockade inhibits mRNA upregulation of downstream proinflammatory cytokines and chemokines in kidney IRI. Real-time PCR demonstrated that mRNA expression of IL-6 (A), TNFα (B), and MCP-1 (C) in the kidney was significantly reduced in anti-HMGB1 Ab-treated (black bars) mice compared with Ctrl Ig-treated mice (gray bars) on days 1 and 5 after reperfusion. The results have been normalized by expressing the number of transcript copies as a ratio to GAPDH. The data shown are the means ± SD. n = 5 to 9 per group.
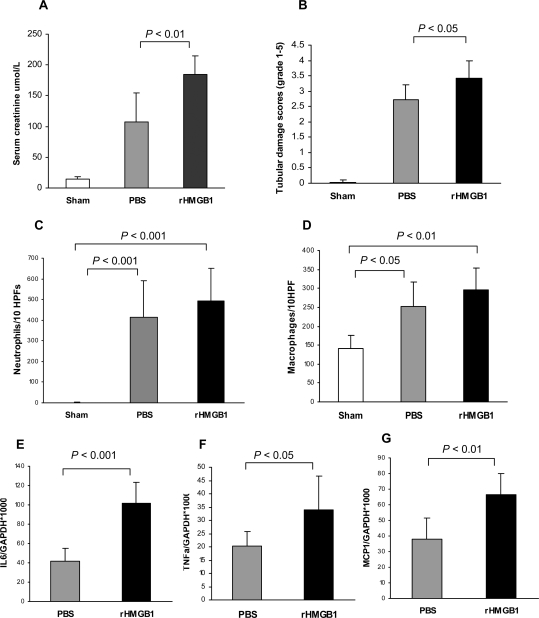
Administration of Recombinant HMGB1 Exacerbates Kidney IRI
To determine whether exogenous HMGB1 would alter kidney IRI, 20 μg of recombinant HMGB1 (rHMGB1) was given to mice immediately after reperfusion. Compared with sham-operated controls, IR caused significant increases in the level of serum creatinine and the score of tubular damage in PBS-treated mice at day 1 post-IR. Kidney injury was exacerbated in mice treated with rHMGB1 with higher serum creatinine and greater tubular damage than PBS-treated mice (Figure 7, A and B). Significant infiltration of neutrophils and macrophages in the kidney was evident in both PBS- and rHMGB1-treated mice at day 1 post-IR compared with sham-operated mice (Figure 7, C and D). A nonsignificant trend toward increased neutrophil and macrophage accumulation in IR kidney was evident in mice treated with rHMGB1 versus PBS. rHMGB1-treated WT mice did show a significant increase in IL-6, TNFα, and MCP1 expression compared with PBS-treated mice at 24 hours post-IR (Figure 7, E through G).
Figure 7.
Administration of recombinant HMGB1 worsens kidney IRI. (A and B) Compared with sham-operated control, IR caused significant increases in the level of serum creatinine (A) and the tubular damage score (B) in PBS-treated mice at day 1 post-IR. Kidney injury was exacerbated in mice treated with rHMGB1 (n = 5) with higher serum creatinine (A) and more severe tubular damage (B) than PBS-treated mice (n = 7). (C and D) Significant infiltration of neutrophils and macrophages in the kidney was evident in both PBS- and rHMGB1-treated mice at day 1 post-IR compared with sham-operated mice with a trend toward greater numbers of both cell types in rHMGB1-treated mice. (E through G) rHMGB1-treated WT mice showed a significant increase in IL-6, TNFα, and MCP1 expression compared with PBS-treated mice at 24 hours post-IR.
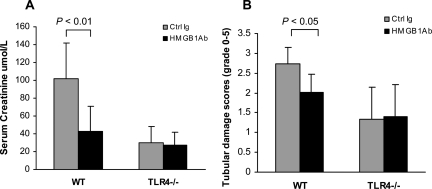
HMGB1-mediated IRI Involves TLR4
We previously reported that TLR4−/− mice were protected against kidney IRI and that several endogenous ligands were upregulated in IR kidney. TLR4 is likely activated by endogenous ligands released or expressed by ischemic cells within the kidney. Recent studies suggest that HMGB1 serves as an endogenous ligand for TLR4. To determine whether HMGB1-mediated kidney IRI is via HMGB1-TLR4 interaction, TLR4−/− animals were pretreated with anti-HMGB1 or control Ab and underwent kidney IR. IRI caused a significant increase in serum creatinine and extensive tubular damage in WT mice treated with control Ab at day 1, and these effects were attenuated in WT mice treated with anti-HMGB1 Ab (Figure 8). TLR4−/− mice treated with control Ab were protected against renal dysfunction and tubular damage with no further protection provided by anti-HMGB1 Ab (Figure 8). TLR4−/− mice were not significantly different in terms of renal function and tubular injury compared with WT animals treated with HMGB1 Ab.
Figure 8.
HMGB1-mediated IRI involves TLR4 signaling. TLR4−/− mice treated with control Ab were protected against renal dysfunction (A) and tubular damage (B) with no further protection afforded by anti-HMGB1 Ab administration. The data shown are the means ± SD. n = 9 per group.
We further examined mRNA expression of IL-6, TNFα, and MCP1 in the kidney in these mice. As stated above, anti-HMGB1 Ab-treated WT mice showed a significant reduction in IL-6, TNFα, and MCP1 expression compared with control Ab-treated mice at 24 hours post-IR. No significant difference was seen in mRNA levels of IL-6, TNFα, and MCP1 in TLR4−/− mice treated with either control or anti-HMGB1 Ab (Figure 9).
Figure 9.
Inhibition of IL-6, TNF, and MCP-1 by anti-HMGB1 Ab requires TLR4. mRNA levels of IL-6, TNFα, and MCP-1 in TLR4−/− mice treated with either control antibody (gray bars) or neutralizing anti-HMGB1 Ab (black bars) by real-time PCR analysis. The data shown are the means ± SD. n = 7 to 9 per group.
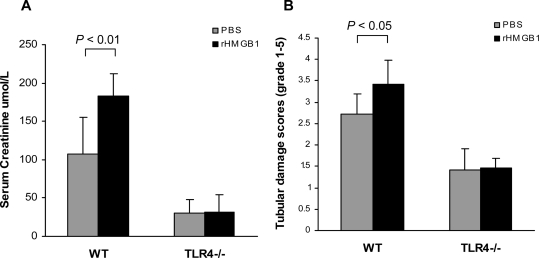
To confirm whether HMGB1-mediated kidney IRI requires TLR4 signaling, WT and TLR4−/− mice were administered rHMGB1 or PBS immediately after reperfusion. rHMGB1 exacerbated kidney injury in WT mice but had no effect on serum creatinine or tubular damage in TLR4−/− mice (Figure 10).
Figure 10.
HMGB1-mediated kidney IRI involves TLR4 signaling. rHMGB1 exacerbated kidney injury in WT mice; however, there was no significant difference in serum creatinine (A) and tubular damage (B) between TLR4−/− mice treated with either rHMGB1 or PBS.
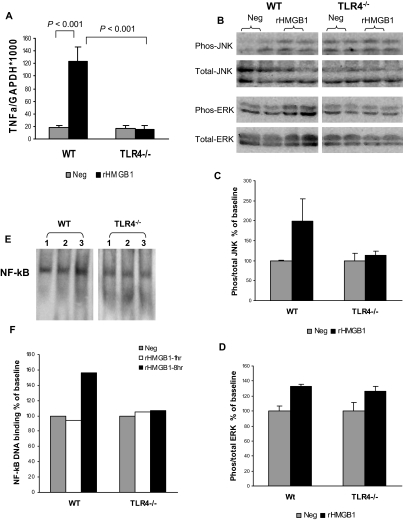
We and others have reported that tubular epithelial cells (TECs) express TLR4. To test whether HMGB1 can directly mediate renal inflammation, primary TEC cultures were stimulated in vitro with rHMGB1, LPS (TLR4-specific ligand, positive control), or medium only (negative control). rHMGB1 induced marked upregulation of TNFα expression in WT TECs compared with negative control; however, this was not observed in TLR4−/− TECs (Figure 11A). LPS stimulation produced similar results (data not shown). To examine whether HMGB1 upregulated TNFα via the mitogen-activated protein kinase (MAPK) signaling pathway, we further examined phosphorylation of MAPK-1(c-Jun N-terminal kinase [JNK]), MAPK-8(extracellular signal-regulated kinase [ERK]), and MAPK-14(P38) by Western blot. Phosphorylation of MAPK-1(JNK) was upregulated by rHMGB1 stimulation in WT TECs compared with negative control, but upregulation was not observed in TLR4−/− TECs (Figure 11, B and C). Phosphorylation of MAPK-8(ERK) expression was increased by rHMGB1 stimulation in WT and TLR4−/− TECs compared with their negative controls (Figure 11, B and D). Phosphorylation of MAPK-14(P38) was not detectable by Western blot in WT or TLR4−/− TECs, with or without rHMGB1. NF-κB activation was measured by electrophoretic mobility shift assay (EMSA). NF-κB DNA binding activity was increased in WT TECs by rHMGB1 stimulation for 8 hours compared with negative control but not in TLR4−/− TECs (Figure 11, E and F).
Figure 11.
HMGB1 induces inflammation in TECs via TLR4. Primary TECs from WT mice and TLR4−/− mice were cultured with medium alone (Neg, gray bars) or with rHMGB1 (5 μg/ml, black bars) for 8 hours. (A) Real-time PCR analysis demonstrated that rHMGB1 induced marked upregulation of TNFα expression in WT TECs compared with negative control; however, this was not seen in TLR4−/− TECs. The data shown are the means ± SD. n = 4 per group. (B) Phosphorylated and total JNK and ERK expression was assessed by Western Blot and measured by densitometry. (C and D) n = 2 per group. (E) NF-κB activation was measured by using EMSA. (F) NF-κB DNA binding activity was increased in WT TECs by rHMGB1 stimulation for 8 hours compared with negative control, but not in TLR4−/− TECs. The assay is representative of three experiments.
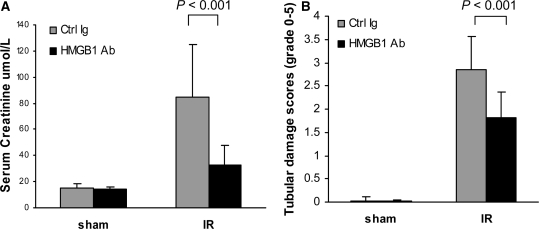
Treatment with Neutralizing Antibody to HMGB1 after Reperfusion Is Also Protective in Renal IRI
Given the protection from IRI afforded by pretreatment with neutralizing anti-HMGB1 Ab, we also assessed the efficacy of anti-HMGB1 Ab given after the onset of IRI as a potentially more clinically relevant strategy. Treatment of WT mice with anti-HMGB1 Ab after reperfusion was also renoprotective. Serum creatinine levels after anti-HMGB1 Ab treatment were significantly less than that of control mice at 24 hours post-IR (P < 0.001; Figure 12A). HMGB1 Ab treatment also significantly reduced tubular damage compared with control mice at 24 hours post-IR (P < 0.001; Figure 12B).
Figure 12.
Anti-HMGB1 Ab treatment after the onset of IRI is protective in kidney IRI. Mice treated with α-HMGB1 Ab demonstrated significantly lower serum creatinine (A) and less tubular damage, assessed by semiquantitative scoring of hematoxylin- and eosin-stained tissue sections (B) compared with Ctrl Ig-treated mice at 24 hours after reperfusion. n = 5 to 7 per group.
DISCUSSION
In this study, we confirmed upregulation of HMGB1 within the kidney after IRI and, using two complementary strategies to block (anti-HMGB1 Ab) or enhance (rHMGB1) HMGB1 activity, were able to demonstrate a pathogenic role for HMGB1 in kidney IRI. The mechanisms by which HMGB1 promotes kidney damage in this setting appear to involve activation of the innate immune system, resulting in the generation of inflammatory molecules IL-6, TNFα, and MCP1. Activation of the innate immune system by endogenous HMGB1 required TLR4 signaling, consistent with our previous observations that TLR4−/− mice were protected against kidney IRI. These results, and specifically the capacity of anti-HMGB1 Ab to attenuate kidney damage when given either before or after ischemia, indicate significant therapeutic potential.
HMGB1 is an evolutionarily conserved protein present in the nucleus of almost all eukaryotic cells, where it functions to stabilize nucleosomes and acts as a transcription factor.23,24,32 In addition to its nuclear roles, HMGB1 was identified as a potent proinflammatory cytokine in experiments showing that HMGB1 is actively secreted by activated macrophages and functions as a late mediator of lethal endotoxemia in sepsis models.24,33,34 HMGB1 is also released from necrotic or damaged cells and serves as a signal to trigger inflammation.35 When released into the extracellular milieu, HMGB1 also acts as an early inflammatory mediator and causes local and systemic inflammatory responses in liver IRI,27,28 acute lung injury,29–31 and hemorrhagic shock.31 Ischemic hepatocytes upregulate HMGB1, and administration of neutralizing anti-HMGB1 antibody protects against hepatic IRI, whereas administration of recombinant HMGB1 immediately after reperfusion worsens liver injury.27 In contrast to the proinflammatory role of HMGB1, preconditioning with HMGB1 provides protection against liver ischemia-reperfusion injury.36 HMGB1 is a distal mediator of acute inflammatory lung injury. HMBG1 given intratracheally caused acute inflammatory injury to the lungs with neutrophil accumulation, the development of lung edema, and increased pulmonary production of IL-1β, TNFα, and MIP-2, whereas administration of anti-HMGB1 Abs reduced the lung injury in endotoxin-induced acute lung inflammation.29 In this study, we have demonstrated upregulation of HMGB1 and HMGB1-triggered downstream cytokines and chemokines in IRI kidney, consistent with a proposed role for HMGB1 in promoting kidney injury. HMGB1 blockade afforded protection from kidney IRI and consequently reduced production of HMGB1 downstream inflammatory molecules, whereas administration of recombinant HMGB1 aggravated kidney IRI, suggesting that endogenous HMGB1 plays a pathogenic role in kidney IRI. In this study, neutralization of HMGB1 after reperfusion also provided renoprotection against kidney IRI, thus strategies targeting HMGB1 using neutralizing Ab may have therapeutic possibilities in a clinical setting.
Recent experimental data suggest that IRI rapidly activates innate immune responses through TLR signaling systems. TLRs are germ line-encoded innate immune receptors that are utilized by the host immune system to detect the presence of invading pathogens.37 TLRs recognize specific molecular patterns that are present on invading microorganisms38–40 but also recognize endogenous ligands released by damaged cells8,41 and are involved in “sterile inflammation” where they contribute to inflammation in the absence of infection. IRI causes injured tissues to express or release a variety of endogenous TLR ligands, particularly for TLR4 and TLR2, including heat-shock proteins, HMGB1, hyaluronan, fibronectin, heparan sulfate, and biglycan.8–13 TLR9 recognizes bacterial CpG as well as self-DNA that may be released from ischemic tissues. HMGB1 has been reported to trigger cellular signaling by interacting with three receptors: RAGE, TLR2, and TLR4.12,32 HMGB1 binds to TLR2 and TLR4, which leads to NF-κB activation through a MyD88-dependent pathway to promote inflammatory responses including the release of proinflammatory cytokines and chemokines.32 Related to its capacity to bind DNA, HMGB1 was found to be a component of DNA-containing immune complexes that stimulated cytokine production through a TLR9 and MyD88 pathway.25 Furthermore, binding of HMGB1 to bacterial DNA (CpG-oligodeoxynucleotides) in vitro augments the inflammatory responses of dentritic cells and macrophages through TLR9 and RAGE.25,26
HMGB1 has been implicated in the pathogenesis of IRI because HMGB1 produced by ischemic hepatocytes binds to TLR4, and this interaction is critical for the development of lethal hepatic IRI.27 A recent study investigated the role of TLR9 in liver IRI and showed that TLR9−/− mice were protected from liver IRI.42 In vitro, liver nonparenchymal cells were activated by endogenous hepatocyte DNA through a TLR9-dependent mechanism. TLR9−/− mice that received anti-HMGB1 antibody enjoyed greater protection from liver IRI than either TLR9−/− mice or WT mice treated with an anti-HMGB1 antibody,42 supporting the previous finding that HMGB1 mediates liver IRI via TLR4. We and other groups reported that TLR4−/− mice were protected against kidney IRI.18–20 To further investigate the mechanism of HMGB1-mediated kidney IRI, we explored whether HMGB1-mediated kidney IRI required TLR4 signaling. Here we found that TLR4−/− mice were protected against kidney IRI and that additional blockade or augmentation of HMGB1 activity, by administration of anti-HMGB1 Ab or rHMGB1, respectively, had no effect on that level of protection. These results suggest that endogenous HMGB1 released from damaged tissue may promote kidney IRI through engagement of TLR4.
HMGB1 also binds to TLR2, another receptor involved in the pathogenesis of kidney IRI. Genetic absence of TLR2 in a mouse kidney IRI model resulted in reduced cytokine and chemokine production, reduced leukocyte infiltration, and protection from kidney dysfunction and tubular damage.16 Given that both TLR2 and 4 have similar downstream effects after binding by HMGB1, redundancy between their effects may be expected, and indeed both TLR2-deficient16 and TLR4-deficient18 mice are significantly protected against kidney IRI, with MyD88-deficient mice also protected to a similar degree.17,18
TLRs are expressed by a variety of immune cell types, such as macrophages, dendritic cells, T and B cells, and NK cells, and also by a number of nonimmune cells, including kidney tubular epithelial cells, endothelial, podocytes, and mesangial cells.9 We have reported that tubular epithelial cells express TLR418 and IRI upregulates TLR4 expression. In this study, we also showed that in vitro, rHMGB1 induced marked upregulation of TNFα expression by TECs from WT mice, but this was not observed in TLR4−/− TECs, suggesting that HMGB1 directly interacts with TLR4 in this setting. This result is consistent with a recent study in humans where stimulation of human tubular cells (HK-2) with rHMGB1 in vitro caused upregulation of proinflammatory cytokines and chemokines. This effect was completely blocked by prior transfection of HK-2 cells with TLR4 siRNA, thus demonstrating that HMGB1 can stimulate proinflammatory responses in kidney in a TLR4-dependent manner.43 All TLRs, except TLR3, can signal via MyD88, which leads to translocation of NF-κB and/or the activation of MAPKs with consequent upregulation of proinflammatory cytokines and chemokines, which in turn initiate inflammation. Furthermore, we found that marked upregulation of TNFα expression in response to rHMGB1 stimulation of TECs from WT mice was associated with the upregulation of MAPK-1(JNK) activation and NF-κB DNA binding activity.
In this study, neutralization of HMGB1 with anti-HMGB1 antibody, when given either before or after ischemia, provided renoprotection against kidney IRI. Thus strategies targeting HMGB1 by using anti-HMGB1 antibodies may have therapeutic potential in a clinical setting. Kidney transplantation may provide the ideal opportunity, given the potential for pretreatment of the donor kidney, before implantation, to attenuate the currently inevitable IRI that occurs and contributes to problems of delayed graft function and increased risks of acute rejection and graft loss.
In conclusion, endogenous HMGB1 is a mediator of kidney damage after IRI that may operate through the TLR4 pathway. Administration of a neutralizing antibody to HMGB1 either before or soon after IR affords significant kidney protection, indicating therapeutic potential.
CONCISE METHODS
Animals
Male WT (C57BL/6) mice were obtained from the Animal Resource Centre (Perth, Australia). TLR4−/− mice on C57BL/6 background have been described previously.18 The mice were housed in filter-top cages with free access to sterile acidified water and irradiated food in a specific pathogen-free facility in the University of Sydney. Male mice weighing 25 to 30 g were used in all of the experiments. The experiments were conducted by following established guidelines for animal care and were approved by the animal ethics committee of the University of Sydney.
Induction of Kidney IRI
Induction of kidney IRI was as described previously.18 Briefly, using a midline abdominal incision, both renal pedicles were clamped for 22 minutes with microaneurysm clamps. During the period of ischemia, body temperature was maintained by placing the mice on a 37°C heat pad. After removal of the clamps, the kidneys were inspected for 1 minute for restoration of blood flow, returning to their original color. The abdomen was closed. Sham-operated mice received identical surgical procedures except that microaneurysm clamps were not applied and were sacrificed at day 1 after surgery. To maintain fluid balance, all of the mice were supplemented with 1 ml of saline administered subcutaneously. The mice were sacrificed 1, 3, and 5 days after reperfusion (n = 5 to 9 per group).
Experimental Design
Anti-HMGB1 and isotype control Abs were sourced from the Shino-Test Corporation (Sagamihara-shi, Kanagawa, Japan). This anti-HMGB1 antibody was characterized in vitro and used at 200 μg per mouse for in vivo studies.44–48 Mice received polyclonal chicken IgY chicken anti-HMGB1 antibody (300 μg per mouse) or isotype control IgY by intraperitoneal injection 1 hour before the induction of ischemia or immediately after reperfusion. The mice received recombinant HMGB (20 μg per mouse) (Sigma-Aldrich) or vehicle PBS by intraperitoneal injection immediately after reperfusion. rHMGB1 reagent was endotoxin-tested and contained 0.003 endotoxin units/μg. Thus this product is considered to be endotoxin-free.
Blood and Tissue Samples
Blood and kidney tissues were harvested at sacrifice. The tissue slices were either fixed with 10% neutral-buffered formalin for paraffin embedding, fixed with periodate-lysine-paraformaldehyde fixative to be frozen in optimal cutting temperature compound (Sakura Finetek Inc., Torrance, CA), or snap frozen in liquid nitrogen for subsequent mRNA extraction.
Assessment of Renal Function
Serum creatinine was measured using the modified Jaffe rate reaction by the Biochemistry Department of the Royal Prince Alfred Hospital (Sydney, Australia).
Histology Examination
Formalin-fixed kidney sections were stained with hematoxylin and eosin. The percentage of tubules in the corticomedullary junction that displayed cellular necrosis and a loss of brush border were counted and scored in a blinded fashion as follows: 0 = none, 1 = <10%, 2 = 11 to 25%, 3 = 26 to 45%, 4 = 46 to 75%, and 5 = >76%. At least ten high-power fields (magnification, ×200) per section for each sample were examined.
Immunohistochemisty Staining
Formalin-fixed sections of 5-μm thickness were deparaffinized and boiled for 10 minutes in 10 mM sodium citrate buffer (pH 6.0) for neutrophil detection. Periodate-lysine-paraformaldehyde-fixed frozen sections (7 μm) for macrophage detection were blocked with a biotin blocker system (DAKO, Carpinteria, CA). The sections were then blocked with 10% normal horse serum. Primary antibody, either rat anti-mouse neutrophil antibody clone 7/4 (ABD Serotec Inc., Oxford, UK) or rat anti-mouse F4/80 antibody (ABD Serotec), was applied to the sections for 60 minutes. Concentration-matched rat IgG was used as an isotype-negative control. The sections were exposed to 3% H2O2 in methanol for 5 minutes and then incubated with the biotinylated secondary antibody anti-rat IgG (BD Biosciences, Pharmingen). A Vector stain ABC kit (Vector Laboratories Inc.) was applied to the tissue followed by 3,3′-diaminobenzidine substrate-chromogen solution (DAKO). The slides were counterstained with Harris' hematoxylin.
Analysis of the cellular infiltrate was performed in a blinded manner by assessing ten consecutive high-power fields (HPFs; magnification, ×400) of the outer medulla and corticomedullary junction on each section. Using an ocular grid, the number of cells staining positively for each antibody was counted and expressed as cells per ten HPFs.
TUNEL Assay
Apoptotic cells in IRI kidney were detected by TUNEL assay following the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany). Briefly, formalin-fixed sections of 5-μm thickness were deparaffinized, microwave-heated for 1 minute in 200 ml of 0.1 M citrate buffer (pH 6.0), and rapidly cooled with the addition of distilled H2O. The sections were then blocked with 0.1 M Tris-HCl (pH 7.5) containing 20% normal goat serum and 3% bovine serum albumin for 45 minutes. DNA fragments in apoptotic cells were then labeled and identified by terminal transferase dUTP conjugated with fluorescein (Roche Diagnostics) for 30 minutes at 37°C. The number of apoptotic cells was counted across the whole section for each sample in a blinded manner.
Stimulation of Primary Renal Tubular Epithelial Cells with rHMGB1 In Vitro
Primary mouse renal TECs were generated following the method as described previously.18 Briefly, the kidneys were flushed with saline in vivo to remove blood cells and then removed. The kidney cortices from WT mice were cut into pieces of approximately 1 mm3 and then digested in HBSS containing 3 mg/ml of collagenase at 37°C for 25 minutes. The kidney digest was washed through a series of sieves (mesh diameters of 250, 150, 75, and 40 μm). The cortical tubular cells were spun down and further washed. The cell pellet was resuspended in defined K1 medium.49 The cell suspension was then placed on cell culture Petri dishes and incubated at 37°C for 2 to 3 hours to facilitate adherence of contaminating glomeruli. The nonadherent tubules were then collected and cultured on collagen-coated Petri dishes (BD Biosciences, Bedford, MA) in K1 medium until epithelial colonies were established. The experiments were commenced after the cells had reached 70 to 90% confluence. Expression of the epithelial cell marker cytokeratin was verified by immunofluorescent staining with an anti-cytokeratin antibody (Sigma-Aldrich) as described previously.18 The cells were 96 to 100% cytokeratin positive.
TECs were placed in serum-free K1 medium for 24 hours and then stimulated at 37°C for 1 or 8 hours in fresh serum-free K1 medium in the presence of rHMGB1 (5 μg/ml; Sigma-Aldrich), purified LPS (1 μg/ml; Sigma-Aldrich) as a positive control, or medium alone as a negative control. After 8 hours of stimulation, the cells were washed with PBS and harvested by adding 1 ml of TRIzol (Invitrogen) for mRNA expression or by adding prechilled CelLytic MT reagent (Sigma-Aldrich) with a 1% protease inhibitor cocktail (Sigma-Aldrich) for use with mammalian cell extracts for protein expression.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from kidney tissue and cells using TRIzol (Invitrogen). cDNA was synthesized using oligo(dT)16 (Applied Biosystems, Foster City, CA) and the SuperScript III reverse transcriptase kit (Invitrogen).
Real-time PCR
cDNA was amplified in 1× Universal Master Mix (Applied Biosystems) with gene-specific primers and probe on Rotor-Gene 6000 (Corbett Life Science). Specific TaqMan primers and probes for IL-6, TNFα, MCP1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were previously described.18 All of the results are expressed as ratios to GAPDH.
Kidney Tissue and TEC Protein Extraction
Snap-frozen kidney tissue was added into prechilled CelLytic MT reagent (Sigma-Aldrich) with a 1% protease inhibitor cocktail (Sigma-Aldrich) for use with mammalian tissue extracts and then homogenized. The samples were incubated for 30 minutes at 4°C and centrifuged at 16,000 × g at 4°C for 15 minutes to pellet the tissue debris. The supernatants were stored at −70°C. Protein concentrations were determined by a colorimetric protein assay (Bio-Rad, Oakland, CA) using protein standards from Sigma-Aldrich.
Western Blot
Aliquots (50 μg) of kidney homogenates or TECs were separated on 12% polyacrylamide gel (Sigma-Aldrich) and transferred to a polyvinylidene difluoride membrane (NEN Life Science Products, Boston, MA). The membrane was blocked overnight in skim milk, incubated with rabbit polyclonal antibodies to HMGB1 (Abcam, Cambridge, MA) or phosphorylated or total ERK, JNK, and P38 (Cell Signaling Technology) for 1 hour, washed, incubated with anti-rabbit IgG conjugated with horseradish peroxidase (Sigma-Aldrich), and then washed. Positive bands were detected by chemiluminescence technology (Sigma-Aldrich) using the G-Box gel documentation (SYNGENE, Frederick, MD) and analyzed using Image J software. The HMGB1 membrane was also probed with anti-α-tubulin antibody (Sigma-Aldrich) for α-tubulin expression as a control to normalize the protein content of each sample.
Nuclear Extraction and Electrophoretic Mobility Shift Assay (EMSA)
NF-κB DNA binding activity was measured by EMSA using nuclear extracts from TECs. Nuclear extracts were prepared using a NucBuster™ Protein Extraction Kit (Novagen, Darmstadt, Germany) as per the manufacturer's instructions. The DIG Gel Shift Kit (Roche Applied Science, Indianapolis, IN) was used in the EMSA. In brief, 20 μg of nuclear extract were incubated with 1 μg of poly[d(I-C)] as the nonspecific competitor, 1 μg of poly l-lysine in a binding buffer (100 mM Hepes, pH 7.6, 5 mM EDTA, 50 mM (NH4)2SO4, 5 mM dithiothreitol, Tween 20, 1% w/v, 150 mM KCl), and digoxigenin-labeled NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) consensus oligonucleotide (Sigma) for 30 minutes at room temperature. The reaction mixture was electrophoresed through 6% polyacrylamide gels, transferred onto a positively charged nylon membrane (Roche Applied Science), and then crosslinked using a UV-transilluminator for 3 minutes. The membrane was subjected to immunological detection using anti-digoxigenin-AP conjugate and chemiluminescence. The results were analyzed using Image J software, and the shift bands were measured.
Statistical Analysis
All of the data are expressed as the means ± SD. The statistical differences between two groups were analyzed by unpaired, two-tailed t tests, and multiple groups were compared using one- or two-way ANOVA with post-hoc Bonferroni's correction (Graph Pad Prism 4.0 software). P values less than 0.05 were considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Project Grants #402539 and #512489).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Tilney NL, Guttmann RD: Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 64: 945–947, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Nankivell BJ, Chapman JR: Chronic allograft nephropathy: Current concepts and future directions. Transplantation 81: 643–654, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Herrero-Fresneda I, Torras J, Cruzado JM, Condom E, Vidal A, Riera M, Lloberas N, Alsina J, Grinyo JM: Do alloreactivity and prolonged cold ischemia cause different elementary lesions in chronic allograft nephropathy? Am J Pathol 162: 127–137, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonventre JV, Zuk A: Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH: Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA: Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol 170: 3883–3889, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Marshak-Rothstein A: Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6: 823–835, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anders HJ, Banas B, Schlondorff D: Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H: HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H: Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 276: 31332–31339, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H: HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26: 174–179, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ: The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C: In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW: Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S: Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB: TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC: Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoSONE 3: e3596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mkaddem SB, Werts C, Goujon JM, Bens M, Pedruzzi E, Ogier-Denis E, Vandewalle A: Heat shock protein gp96 interacts with protein phosphatase 5 and controls toll-like receptor 2 (TLR2)-mediated activation of extracellular signal-regulated kinase (ERK) 1/2 in post-hypoxic kidney cells. J Biol Chem 284: 12541–12549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F, Garlanda C, Mantovani A, Anders HJ: Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol 183: 4109–4118, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Noris M, Cassis P, Azzollini N, Cavinato R, Cugini D, Casiraghi F, Aiello S, Solini S, Cassis L, Mister M, Todeschini M, Abbate M, Benigni A, Trionfini P, Tomasoni S, Mele C, Garlanda C, Polentarutti N, Mantovani A, Remuzzi G: The Toll-IL-1R member Tir8/SIGIRR negatively regulates adaptive immunity against kidney grafts. J Immunol 183: 4249–4260, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Javaherian K, Liu JF, Wang JC: Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 199: 1345–1346, 1978 [DOI] [PubMed] [Google Scholar]
- 24. Yang H, Wang H, Czura CJ, Tracey KJ: The cytokine activity of HMGB1. J Leukoc Biol 78: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ: Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8: 487–496, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM: A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110: 1970–1981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR: The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201: 1135–1143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR: HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204: 2913–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ: HMG-1 as a mediator of acute lung inflammation. J Immunol 165: 2950–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, McIntyre RC: Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol 287: R592–R599, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP: Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med 12: 105–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lotze MT, Tracey KJ: High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ: HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ: Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 101: 296–301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR: Cutting edge: High-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol 176: 7154–7158, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Medzhitov R, Preston-Hurlburt P, Janeway CA, Jr.: A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Matzinger P: The danger model: A renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Gordon S: Pattern recognition receptors: Doubling up for the innate immune response. Cell 111: 927–930, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Medzhitov R, Janeway CA, Jr.: Decoding the patterns of self and nonself by the innate immune system. Science 296: 298–300, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Beg AA: Endogenous ligands of Toll-like receptors: Implications for regulating inflammatory and immune responses. Trends Immunol 23: 509–512, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, Dematteo RP: Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology 51: 621–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B: Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A 106: 3390–3395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JY, Park JS, Strassheim D, Douglas I, Diaz d V, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E: HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 288: L958–L965, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Yamada S, Inoue K, Yakabe K, Imaizumi H, Maruyama I: High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem 49: 1535–1537, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, Maruyama I: The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115: 1267–1274, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y: Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J. Gastroenterol 12: 7666–7670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M, Yamada S, Hashimoto S, Fukata S, Abraham E, Maruyama I, Kitajima M, Ishizaka A: Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg 30: 1755–1762, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE: MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int 37: 783–792, 1990 [DOI] [PubMed] [Google Scholar]