Abstract
Prenatal ethanol exposure is teratogenic, but the effects of ethanol on kidney development and the health of offspring are incompletely understood. Our objective was to investigate the effects of acute ethanol exposure during pregnancy on nephron endowment, mean arterial pressure, and renal function in offspring. We administered ethanol or saline by gavage to pregnant Sprague-Dawley rats on embryonic days 13.5 and 14.5. At 1 month of age, the nephron number was 15% lower and 10% lower in ethanol-exposed males and females, respectively, compared with controls. Mean arterial pressure, measured in conscious animals via indwelling tail-artery catheter, was 10% higher in both ethanol-exposed males and females compared with controls. GFR was 20% higher in ethanol-exposed males but 15% lower in ethanol-exposed females; moreover, males had increased proteinuria compared with controls. Furthermore, embryonic kidneys cultured in the presence of ethanol for 48 hours had 15% fewer ureteric branch points and tips than kidneys cultured in control media. Taken together, these data demonstrate that acute prenatal ethanol exposure reduces the number of nephrons, possibly as a result of inhibited ureteric branching morphogenesis, and that these changes affect adult cardiovascular and renal function.
In Western communities, alcohol (ethanol) consumption by pregnant women is a relatively common occurrence.1 Some women, while reducing their daily alcohol consumption, partake in episodes of acute (binge) drinking while pregnant.2 Whereas in most countries guidelines generally recommend that pregnant women abstain from alcohol consumption, many women still consume alcohol in the early stages of their pregnancy. This is of clinical importance because many pregnancies are unconfirmed until the end of the first trimester.3 Whereas strong evidence suggests chronic consumption of high doses of ethanol are teratogenic, the effects of acute ethanol exposure on the fetus are less well understood. Animal studies have demonstrated that acute ethanol exposure is associated with neuroapoptosis, reduced brain growth, and pulmonary alveolar dysfunction.4–6 However, the effects of ethanol exposure during pregnancy on kidney development and long-term renal and cardiovascular function are yet to be thoroughly explored.
Rats chronically exposed to ethanol during pregnancy have a reduced ability to concentrate urine at 90 days of age, and renal DNA and protein content is reduced at 7 days of age.7,8 Recently, we have shown that repeated maternal ethanol administration during the peak period of nephrogenesis in fetal sheep results in an 11% lower nephron endowment.9 Furthermore, it has been shown that chronic prenatal ethanol exposure can result in an elevated mean arterial pressure (MAP) and altered vascular contractile response.10 However, no previous study has investigated the effects of acute ethanol exposure on nephron endowment or the long-term consequences of such exposure. This is important because a reduced nephron endowment is permanent and has been linked to the development of adult-onset diseases, including hypertension and chronic kidney disease.11–13
In the study presented here, we hypothesized that acute prenatal ethanol exposure in the rat will result in a reduced nephron endowment, elevated BP, and impaired renal function. Our primary aim was to determine whether acute prenatal ethanol exposure results in smaller kidneys with decreased nephron endowment. Because low nephron number has been linked to the development of adult-onset diseases, our second objective was to investigate MAP and renal function in ethanol-exposed offspring at 6 months of age.
The mechanism through which alcohol may act to disrupt the development of the kidney leading to a reduced nephron number is unknown. Mammalian kidney development involves complex molecular reciprocal interactions between the ureteric tree and the surrounding metanephric mesenchyme.14–17 Branching of the ureteric tree is a critical process and plays a significant role in determining nephron number, because new nephrons only form adjacent to ureteric tips. Previous studies have identified a correlation between reduced nephron number and reduced ureteric branching morphogenesis in the developing kidney.18 We hypothesized that any reduction in nephron number would be a consequence of attenuated ureteric branching morphogenesis. This was explored in two ways: (1) by examining the effect of ethanol exposure in vivo on the expression levels of key genes regulating ureteric branching morphogenesis and (2) by exploring the effects of direct ethanol exposure on ureteric branching morphogenesis in metanephric organ culture.
RESULTS
Maternal Weight Gain and Pup Viability
Maternal ethanol administration did not affect gestational weight gain, with dams from all three groups having similar weight gain from E12.5 to E20.5 (ethanol, 52 ± 4 g; sham, 53 ± 6 g; control, 47 ± 3 g). Similarly, ethanol administration did not affect pup viability, with pup numbers being comparable at postnatal day (PN) 2 (males: ethanol, 7 ± 1; sham, 5 ± 1; control, 5 ± 2; and females: ethanol, 6 ± 1; sham, 6 ± 1; control, 7 ± 2).
Blood Alcohol Concentration (BAC)
BAC measured 1 hour after gavage was 0.107 ± 0.001 g/dl in ethanol-treated dams and 0.000 g/dl in sham dams.
Postnatal Growth until PN30
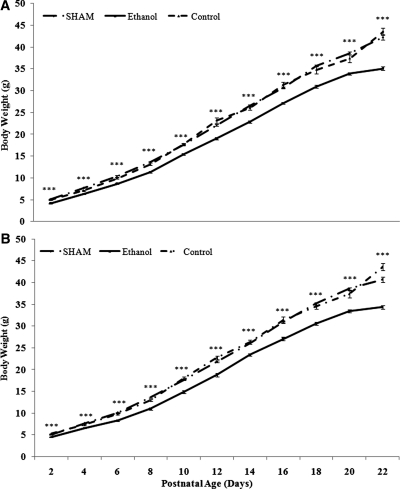
Male and female ethanol-exposed offspring weighed approximately 10 to 18% less than sex-matched sham and control offspring at PN2 (males: ethanol, 4.1 ± 0.1 g; sham, 5.1 ± 0.1 g; control, 4.9 ± 0.1 g, P < 0.001, ethanol versus sham and control; and females: ethanol, 4.5 ± 0.1 g; sham, 5.0 ± 0.1 g; control, 5.3 ± 0.1 g, P < 0.01, ethanol versus sham and control). Similarly, at PN30 ethanol-exposed offspring weighed approximately 12 to 18% less than sex-matched sham and control offspring (Figure 1). Fractional weight gain was similar in all groups (data not shown). The body weights of sham and control offspring were similar at all of the time points studied.
Figure 1.
Offspring exposed to ethanol are growth restricted in postnatal life. Male (A) and female (B) postnatal growth from PN2 to PN22 for the three groups of rats. The data are the means ± SEM. Male and female data were analyzed separately using one-way ANOVA with a Tukey's post hoc test at each time point. n > 20 rats per group, derived from six litters per treatment, Tukey's P value: ***P < 0.001.
Body and Organ Weights at PN30
Both male and female ethanol-exposed offspring weighed significantly less than their sex-matched sham and control offspring at PN30 (Table 1). Male ethanol-exposed offspring had significantly lighter kidneys than sham and control offspring, whereas control offspring had smaller kidneys than sham offspring. The brains of male ethanol-exposed offspring were significantly lighter than those of sham and control offspring. However, once corrected for body weight, the brain was significantly heavier in male ethanol-exposed offspring than in sham and control offspring, whereas the relative weights of all other organs were comparable (Table 1).
Table 1.
Absolute and corrected organ weights for male and female offspring at PN30
| Absolute Weight (g) |
||||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| Control | Sham | Ethanol | Control | Sham | Ethanol | |
| Body weight | 75 ± 2a | 80 ± 2a | 66 ± 1c | 71 ± 2d | 72 ± 2d | 60 ± 1e |
| Left kidney | 0.431 ± 0.01a | 0.473 ± 0.01b | 0.401 ± 0.01c | 0.401 ± 0.01d | 0.404 ± 0.01d | 0.395 ± 0.01d |
| Right kidney | 0.434 ± 0.01a | 0.485 ± 0.01b | 0.418 ± 0.01c | 0.407 ± 0.01d | 0.417 ± 0.01d | 0.397 ± 0.01d |
| Brain | 1.557 ± 0.01a | 1.616 ± 0.02a | 1.531 ± 0.01c | 1.493 ± 0.02d | 1.566 ± 0.02d | 1.495 ± 0.02d |
| Liver | 3.680 ± 0.11a | 4.075 ± 0.25a | 3.630 ± 0.12a | 3.547 ± 0.11d | 3.371 ± 0.21d | 3.359 ± 0.08d |
| Lung | 0.818 ± 0.09a | 0.804 ± 0.03a | 0.748 ± 0.05a | 0.812 ± 0.10d | 0.807 ± 0.06d | 0.643 ± 0.01d |
| Heart | 0.442 ± 0.02a | 0.464 ± 0.01a | 0.448 ± 0.01a | 0.418 ± 0.02d | 0.425 ± 0.01d | 0.382 ± 0.01e |
| Organ Weight:Body Weight Ratio (mg/g) |
||||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| Control | Sham | Ethanol | Control | Sham | Ethanol | |
| Left kidney | 5.79 ± 0.22a | 5.94 ± 0.20a | 6.06 ± 0.20a | 5.94 ± 0.21d | 5.61 ± 0.21d | 6.53 ± 0.22e |
| Right kidney | 5.83 ± 0.18a | 6.09 ± 0.25a | 6.32 ± 0.24a | 5.79 ± 0.25d | 5.80 ± 0.22d | 6.55 ± 0.22e |
| Brain | 20.91 ± 0.60a | 20.29 ± 0.56a | 23.01 ± 0.40c | 21.14 ± 0.62d | 21.75 ± 0.62d | 24.68 ± 0.68e |
| Liver | 49.18 ± 1.34a | 51.28 ± 3.54a | 54.61 ± 2.05a | 49.85 ± 1.08d | 46.67 ± 3.03d | 55.49 ± 2.01d |
| Lung | 10.90 ± 1.14a | 10.08 ± 0.44a | 11.20 ± 0.77a | 11.51 ± 1.47d | 11.20 ± 1.0d | 10.61 ± 0.30d |
| Heart | 6.04 ± 0.55a | 5.83 ± 0.21a | 6.74 ± 0.27a | 5.74 ± 0.37d | 5.89 ± 0.22d | 6.32 ± 0.22d |
The male and female data were analyzed separately using one-way ANOVA with a Tukey's post hoc test. The data are the means ± SEM, n = 8 rats per group, derived from six litters per treatment. The values that do not share a common letter are significantly different from each other (P < 0.05).
Female ethanol-exposed offspring had significantly lighter hearts than sham and control offspring. When organ weight was corrected for body weight, female ethanol-exposed offspring had significantly larger brains than sham and control offspring. The kidneys of ethanol-exposed females were significantly larger in proportion to body weight than sham and control female offspring, whereas the relative weights of all other organs were comparable (Table 1).
Nephron Number at PN30
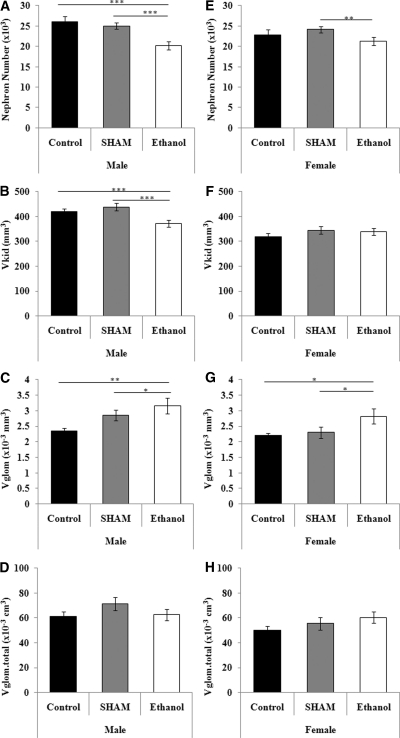
Male ethanol-exposed offspring had significantly lower glomerular number than male sham and control offspring, whereas female ethanol-exposed offspring had a significantly lower nephron number than sham offspring (Figure 2) (males: ethanol, 20,210 ± 1006; sham, 25,000 ± 744; control, 26,061 ± 1288, P < 0.001, ethanol versus sham and controls; females: ethanol, 21,239 ± 926; sham, 24,144 ± 350; control, 22,751 ± 273, P < 0.01, ethanol versus sham). In both male and female ethanol-exposed offspring, mean glomerular volume was significantly larger than in sex-matched sham and control offspring (males: P < 0.01; females: P < 0.05). The mean renal corpuscle volume and total glomerular and total renal corpuscle volumes were similar in the three groups.
Figure 2.
Prenatal ethanol exposure results in a reduction in nephron number. Male and female nephron number (A and E), kidney volume (B and F), mean glomerular volume (C and G), and total glomerular volume (D and H) at PN30 in the three groups of rats. The data are the means ± SEM. The male and female data were analyzed separately using one-way ANOVA with a Tukey's post hoc test. n = 8 rats per group, derived from six litters per treatment, Tukey's P values: *P < 0.05, **P < 0.01, ***P < 0.001.
Body and Organ Weights at 6 Months of Age
Both male and female ethanol-exposed offspring were approximately 5 to 10% smaller in body weight than their sex-matched sham and control counterparts; however, this difference was not statistically significant. Similarly, organ weights were not significantly different from their sex-matched sham and control offspring at 6 months of age (Table 2).
Table 2.
Absolute and corrected organ weights for male and female offspring at 6 months of age
| Absolute Weight (g) |
||||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| Control | Sham | Ethanol | Control | Sham | Ethanol | |
| Body weight | 503 ± 11 | 549 ± 20 | 507 ± 13 | 275 ± 13 | 320 ± 16 | 284 ± 12 |
| Left kidney | 1.68 ± 0.04 | 1.84 ± 0.08 | 1.74 ± 0.07 | 0.99 ± 0.03 | 1.08 ± 0.04 | 0.96 ± 0.04 |
| Right kidney | 1.71 ± 0.04 | 1.85 ± 0.08 | 1.72 ± 0.06 | 1.03 ± 0.04 | 1.11 ± 0.04 | 1.01 ± 0.06 |
| Liver | 17.35 ± 0.69 | 17.82 ± 0.64 | 16.14 ± 0.78 | 9.66 ± 0.39 | 10.85 ± 0.52 | 9.51 ± 0.33 |
| Lung | 2.29 ± 0.05 | 2.35 ± 0.11 | 2.46 ± 0.25 | 1.87 ± 0.11 | 1.95 ± 0.10 | 1.73 ± 0.09 |
| Heart | 1.53 ± 0.05 | 1.55 ± 0.082 | 1.51 ± 0.04 | 0.98 ± 0.05 | 1.00 ± 0.03 | 0.92 ± 0.04 |
| Corrected Organ:Body Weight Ratio (g/kg) |
||||||
|---|---|---|---|---|---|---|
| Male |
Female |
|||||
| Control | Sham | Ethanol | Control | Sham | Ethanol | |
| Left kidney | 3.67 ± 0.11 | 3.38 ± 0.18 | 3.44 ± 0.13 | 3.80 ± 0.15 | 3.14 ± 0.18 | 3.43 ± 0.14 |
| Right kidney | 3.42 ± 0.13 | 3.39 ± 0.20 | 3.39 ± 0.10 | 3.80 ± 0.15 | 3.51 ± 0.19 | 3.57 ± 0.17 |
| Liver | 34.50 ± 1.18 | 32.54 ± 0.96 | 31.68 ± 1.03 | 35.46 ± 1.39 | 33.93 ± 0.76 | 33.68 ± 1.00 |
| Lung | 4.57 ± 0.13 | 4.31 ± 0.19 | 4.84 ± 0.44 | 6.98 ± 0.64 | 6.19 ± 34 | 6.15 ± 0.35 |
| Heart | 3.06 ± 0.08 | 2.82 ± 0.11 | 3.01 ± 0.15 | 3.34 ± 0.06 | 3.18 ± 0.18 | 3.28 ± 0.15 |
The male and female data were analyzed separately using one-way ANOVA with a Tukey's post hoc test. The data are the means ± SEM, n = 8 rats per group, derived from six litters per treatment. There were no statistical differences for any parameter.
MAP and Heart Rate (HR) at 6 Months of Age
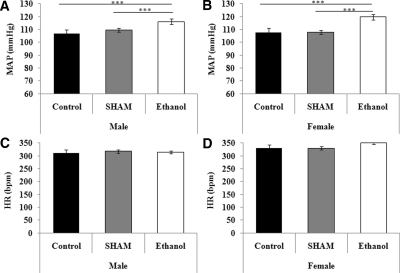
Both male and female ethanol-exposed offspring had significantly higher MAP than sex-matched sham and control offspring at 6 months of age, with no changes in heart rate identified (Figure 3).
Figure 3.
Mean arterial pressure increases as a result of prenatal ethanol exposure. Male and female mean arterial pressure (A and C) and heart rate (B and D) at 6 months of age in the three groups of rats. The data are the means ± SEM. The male and female data were analyzed separately using a one-way ANOVA with a Tukey's post hoc test. n = 8 rats per group, derived from six litters per treatment, Tukey's P values: ***P < 0.001.
Renal Function at 6 Months of Age
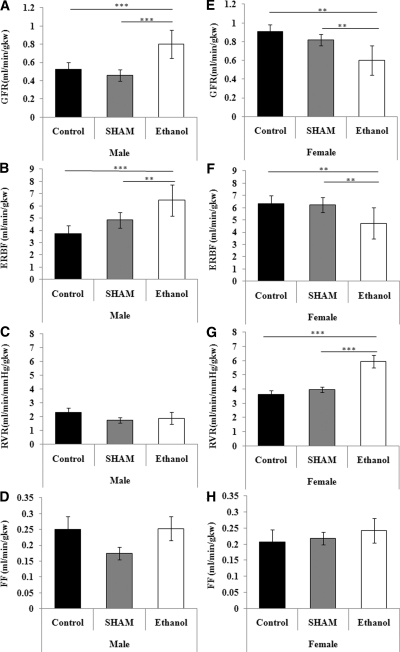
At 6 months of age, male ethanol-exposed offspring had a significantly higher GFR and effective renal blood flow (ERBF), whereas female ethanol-exposed offspring had significantly lower GFR and ERBF than their sex-matched sham and control offspring (Figure 4). Renal vascular resistance (RVR) was significantly elevated in ethanol-exposed female offspring compared with female sham and control offspring. However, male ethanol-exposed offspring had a similar RVR to sham and control offspring. Both male and female ethanol-exposed offspring had a similar filtration fraction as their sex-matched controls. Male ethanol-exposed offspring had higher concentrations of urinary protein in comparison with sex-matched controls, whereas female ethanol-exposed offspring had similar urinary protein. Urine flow was not different between the treatment groups, although urine flow was greater in females than males (P < 0.01). Urine osmolality and excretion rates of sodium, potassium, and chloride in ethanol-exposed male and female offspring were similar to that in sex-matched controls (Table 3). Plasma osmolality; plasma concentrations of sodium, potassium, and chloride; and hematocrit in ethanol-exposed male and female offspring were similar to their sex-matched controls (data not shown).
Figure 4.
Prenatal ethanol exposure results in sex-specific changes in renal function. Male and female GFR (A and E), renal blood flow (B and F), renal vascular resistance (C and G), and filtration fraction (D and H) at 6 months of age in the three groups of rats. The data are the means ± SEM. The male and female data were analyzed separately using a one-way ANOVA with a Tukey's post hoc test. n = 8 rats per group, derived from six litters per treatment, Tukey's P values: **P < 0.01, ***P < 0.001.
Table 3.
Urine excretion analyses performed at 6 months of age
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Control | Sham | Ethanol | Control | Sham | Ethanol | |
| Protein (g/min) | 313.04 ± 48.70a | 202.65 ± 63.13a | 517.66 ± 44.94b | 86.95 ± 20.98 | 108.77 ± 43.26 | 75.07 ± 25.42 |
| Osmolality excretion (mOsmol/min) | 21.33 ± 3.80 | 17.08 ± 3.23 | 13.19 ± 6.17 | 19.20 ± 1.74 | 18.83 ± 7.40 | 19.44 ± 4.60 |
| Na+ excretion (mmol/min) | 1.67 ± 0.94 | 1.55 ± 0.36 | 1.24 ± 0.69 | 2.46 ± 0.10 | 3.87 ± 2.27 | 2.46 ± 0.90 |
| K+ excretion (mmol/min) | 1.13 ± 0.26 | 0.45 ± 0.20 | 0.58 ± 0.21 | 1.54 ± 0.13 | 1.03 ± 0.35 | 1.11 ± 0.24 |
| Cl− excretion (mmol/min) | 3.15 ± 0.82 | 2.98 ± 0.45 | 2.13 ± 0.91 | 2.21 ± 0.19 | 4.73 ± 2.30 | 3.87 ± 1.06 |
The male and female data were analyzed separately using one-way ANOVA with a Tukey's post hoc test. The data are the means ± SEM, n = 8 rats per group, derived from six litters per treatment. The values that do not share a common letter are significantly different from each other (P < 0.05).
Renal Morphology at 6 Months of Age
There was no evidence of renal pathology at 6 months of age in any of the treatment groups. Glomeruli showed no signs of hypercellularity or sclerosis. There was no evidence of renal interstitial fibrosis or cellular infiltration, and vessels appeared normal (data not shown).
Metanephric Gene Expression
The relative mRNA levels of GDNF, FGF7, Wnt11, TGFβ2, and TGFβ3 in E15.5 kidneys of ethanol-exposed fetuses were significantly reduced compared with saline-treated fetuses. mRNA levels for all of the other genes analyzed were similar in control and ethanol-treated fetuses (Table 4).
Table 4.
Real-time PCR analysis performed on E15.5 fetal kidneys after maternal exposure to saline or ethanol (1 g/kg) at E13.5 and E14.5
| Saline | Ethanol | |
|---|---|---|
| GFRα1 | 1.19 ± 0.32 | 1.37 ± 0.55 |
| GDNF | 1.00 ± 0.04 | 0.66 ± 0.13a |
| TGFβ1 | 1.06 ± 0.21 | 0.63 ± 0.07 |
| TGFβ2 | 1.03 ± 0.14 | 0.44 ± 0.20a |
| TGFβ3 | 1.01 ± 0.08 | 0.62 ± 0.09a |
| FGF7 | 1.00 ± 0.04 | 0.50 ± 0.16a |
| Wnt4 | 1.00 ± 0.06 | 1.23 ± 0.51 |
| Wnt11 | 1.06 ± 0.17 | 0.53 ± 0.14a |
| BMP4 | 1.10 ± 0.22 | 0.97 ± 0.12 |
| Bcl-2 | 1.03 ± 0.14 | 0.95 ± 0.33 |
| BAX | 1.00 ± 0.07 | 1.17 ± 0.12 |
The data are the means ± SEM and were analyzed using a two-tailed unpaired t test, n = 5 kidney pairs per group.
aP < 0.05.
Ureteric Branching Morphogenesis In Vitro
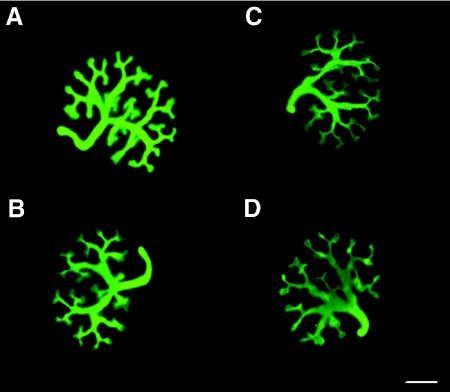
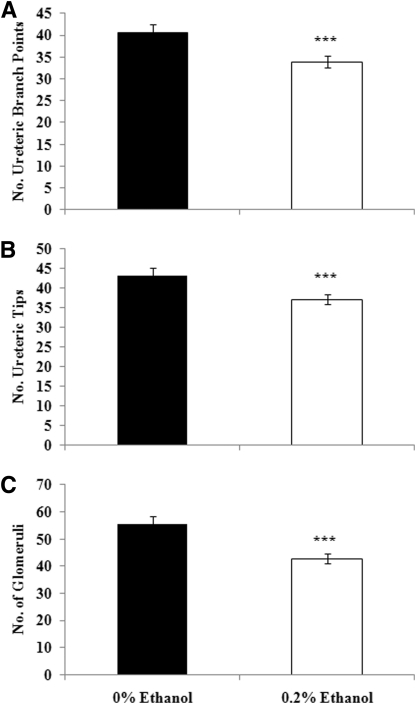
Culture of whole rat metanephroi in the presence of ethanol did not influence the qualitative appearance of the ureteric tree (Figure 5). However, quantitative analysis of ureteric trees after 2 days of culture showed that kidneys cultured in the presence of ethanol contained 20% fewer ureteric branch points and tips than metanephroi cultured in control media (Figure 6).
Figure 5.
Culture of embryonic metanephroi in the presence of ethanol reduces growth and branching morphogenesis. Immunofluorescence images showing the ureteric trees in whole rat metanephroi after 48 hours of culture in 0% ethanol (A and B) and 0.2% ethanol (C and D). Scale bar, 500 μm.
Figure 6.
Culture of embryonic metanephroi in ethanol reduces ureteric branch points, ureteric tips, and glomeruli number. The number of ureteric branch points (A) and tips (B) after 48 hours culture and glomerular number (C) after 5 days of culture in 0% ethanol or 0.2% ethanol. The data are the means ± SEM and were analyzed using a two-tailed unpaired t test. ***P < 0.001. n = 10 to 15 metanephroi per group.
Glomerular Number In Vitro
Culture in the presence of ethanol also adversely affected glomerular number. Metanephroi cultured for 5 days in the presence of 0.2% ethanol contained 25% fewer glomeruli than metanephroi cultured in control media (Figure 6).
DISCUSSION
This study has shown for the first time that acute ethanol exposure at a time when the kidney is in its early stages of development causes postnatal growth restriction and a permanent 10 to 20% reduction in nephron number. The findings support our hypothesis that acute ethanol exposure has long-term consequences for adult health, causing a 10-mmHg elevation in MAP in ethanol-exposed offspring and sex-specific changes in renal function at 6 months of age. Utilizing metanephric organ culture, we have also shown that exposure to ethanol reduces by approximately 20% the number of ureteric branch points, tips, and glomeruli that are formed. Furthermore, we found that levels of gene expression of critical regulators of ureteric branching morphogenesis were downregulated in the kidneys of ethanol-exposed fetuses. Together, these findings demonstrate that prenatal ethanol exposure at an early stage of renal development results in long-term renal and cardiovascular deficits and that the observed reduction in nephron number is due to partial attenuation of ureteric branching morphogenesis.
Our rat model of acute prenatal ethanol exposure has significant relevance to humans in terms of the timing of exposure and the BAC obtained. Epidemiologic studies report that pregnant women are more likely to consume alcohol in acute doses rather than in a chronic capacity.19,20 Approximately 28.5% of pregnant women in the USA report engaging in binge drinking episodes at some point in their pregnancy, defined as consuming more than four standard drinks in the one sitting.2 In this study, maternal BAC measured 1 hour after ethanol administration was approximately 0.1 g/dl. Similar models of maternal ethanol exposure find that maximal BAC ranges from 0.13 to 0.2 g/dl when alcohol is presented to animals in their drinking water or administered by gavage.21,22 The timing of ethanol exposure in this study relates to the earliest stages of kidney development, when the ureteric bud has just started to invade the metanephric mesenchyme. This corresponds to the fifth through seventh weeks of human pregnancy.23 This is a critical time point in kidney development, because previous studies using maternal glucocorticoids have identified that perturbations at this time point contribute to the programming of reduced nephron endowment.18,24 Our data further add to the concept that early kidney development is a “critical window” in which in utero perturbations can elicit long-term cardiovascular effects.
Reductions in birth weight are common in animal models of fetal programming, including maternal low-protein diet25 and placental insufficiency models.26,27 Birth weight was not recorded in this study because we did not want to disturb pups in the first day; therefore it is possible that the growth restriction identified could be a product of postnatal growth restriction and not in utero growth restriction due to ethanol exposure. However, our early measures of body weight suggest that growth restriction in ethanol-exposed offspring probably occurred in utero. Furthermore, because we did not identify any differences in maternal weight gain throughout pregnancy in response to the ethanol administration, we believe that the observed growth restriction is a result of the ethanol exposure and not undernutrition during development. Importantly, during lactation, offspring exposed to ethanol did not become further growth restricted, nor did they undergo significant “catch-up” growth. Catch-up in body weight occurred postweaning in these animals as evidenced by the fact that ethanol-exposed animals had similar body weights to control animals at 6 months of age. Fetal growth restriction has previously been demonstrated in animal models of prenatal ethanol exposure and in human patients with fetal alcohol syndrome.21,28–30
In many models of prenatal programming, a reduction in nephron number is coupled with a reduction in kidney weight at birth. Previous work investigating the effects of prenatal low-protein exposure found that animals had smaller kidneys and a 13% reduction in nephron number.26 However, this is not always the case. For example, short-term prenatal glucocorticoid exposure can result in a reduced nephron endowment without changes in kidney weight at birth.30 Recently, we found no change in kidney weight but an 11% reduction in nephron number in response to ethanol exposure during the latter half of gestation in an ovine model.9 In the study presented here, we identified a reduction in kidney weight in male ethanol-exposed offspring at PN30 but no reduction in kidney weight in ethanol-exposed female offspring, even although there was a reduction in nephron number. The degree of nephron deficit was less in female ethanol-exposed offspring compared with male ethanol-exposed offspring, being approximately 10% in females and 20% in males. The exact cause of this difference between the sexes is unknown; however, it has been established that sex hormones play a role in kidney development.31 This suggests that the kidneys of male fetuses may be more susceptible than female kidneys to ethanol in causing a reduction in nephron number. The observed reduction in nephron number is permanent, because at the time of analysis (PN30), nephrogenesis is complete. Interestingly, both male and female ethanol-exposed offspring displayed an increase in mean glomerular volume, which is not a common finding in studies of a reduced nephron number, particularly at such an early age,9,18,27 suggesting a potential ethanol-specific effect on the glomerulus.
The precise mechanism leading to the reduced nephron number is unclear; it is possible that the deficit in nephron number identified within this study is a product of reduced kidney development in the postnatal period, because kidney development in the rodent continues postnatally.15 However, these results suggest that the mechanism leading to reduced nephron number likely involves reduced ureteric branching morphogenesis. Such an association has previously been reported after exposure to dexamethasone. In vivo exposure to dexamethasone for 2 days in rats (E15 to E16) results in a significant nephron deficit,32,33 whereas metanephric culture in the presence of dexamethasone resulted in a reduction in ureteric branching morphogenesis and the number of glomeruli formed.18
It has previously been identified that altered ureteric branching morphogenesis correlates with changes in the levels of expression of genes critically involved in kidney development.18 Here we showed that expression of TGFβ2, TGFβ3, GDNF, FGF7, and Wnt11, which are all involved in ureteric branching morphogenesis and nephrogenesis, was significantly downregulated in ethanol-exposed embryonic kidneys. GDNF has a direct role in kidney development by promoting ureteric branching through its receptor c-ret.15 Within our laboratory we have identified that GDNF heterozygous mice have reduced nephron endowment compared with wild-type mice,34 and thus reduced GDNF expression identified in ethanol-exposed in vivo kidneys is consistent with a reduced number of nephrons. Members of the TGFβ superfamily are known to inhibit ureteric branching and promote ureteric branch lengthening.35 The lengthening of ureteric branches ensures that branch tips are kept within the nephrogenic zone, the site of reciprocal signaling between the mesenchyme, and the ureteric tips, which promote branching. The identified downregulation of both TGFβ2 and TGFβ3 expression in this study suggests that the reduced branching morphogenesis in kidneys cultured in ethanol may be due, at least in part, to inadequate branch lengthening. Maintenance of nephron induction and survival of the mesenchyme are essential in establishing normal nephron complement, because defects in these processes have been shown to result in reduced nephron number.36–38 Members of the FGF and Wnt signaling pathways play crucial roles in nephron induction.37 The study presented here identified a reduction in both Wnt11 and FGF7 expression. Kidneys of knockout FGF7 embryos are smaller than wild-type embryos, and there are 30% fewer nephrons in adulthood.38 The identified reduction in both Wnt11 and FGF7 expression suggests that the reduced nephron number identified in vivo and in vitro could be in part a product of failure of nephron induction. Future studies of the underlying mechanisms involved in ethanol teratogenesis on kidney development should involve separate analyses in males and females. In addition, comparative studies are warranted to identify whether the mechanism of a reduction in ureteric branching morphogenesis identified in vitro is occurring in vivo.
Many studies have demonstrated a relationship between reduced nephron number and elevated MAP in both animal models and human studies.11–13,18,24,25,27,39 In the study presented here, we demonstrated that as a result of acute ethanol exposure, there was an elevation in MAP of approximately 10 mmHg at 6 months of age in offspring of both sexes. Similar elevations have been observed after short-term prenatal glucocorticoid exposure.18,24 Singh et al.18 reported a 20% deficit in nephron number and a 12-mmHg increase in MAP in rats prenatally exposed to an acute elevation in the glucocorticoid corticosterone. Similarly, dexamethasone exposure in the early stages of kidney development in fetal sheep resulted in a nephron deficit and a 10-mmHg increase in MAP in offspring at 7 years of age.24 What is interesting is that both of these studies administered exogenous glucocorticoids at a similar stage of kidney development (in the earliest stages of ureteric branching) as the ethanol in this study and for a similar duration (2 days). This strongly suggests that the early stages of metanephric development are particularly sensitive to perturbation and that the effects of ethanol on the kidney may be influenced by the actions of maternal glucocorticoids. Prenatal ethanol exposure is known to influence the activity of the hypothalamic-pituitary-adrenal axis and to result in elevated maternal glucocorticoid levels.40 Thus it is plausible to suggest that the findings observed within this study may be mediated at least in part by the actions of glucocorticoids.
Investigations to identify whether the reduction in nephron number had any effect on renal function identified sex-specific changes in GFR, ERBF, and RVR, with no changes in the excretion rates of sodium, potassium, or chloride. The elevations in GFR and ERBF in male ethanol-exposed offspring at 6 months of age suggest that these animals are hyperfiltering above and beyond that expected with reduced nephron number, wherein whole-kidney GFR is restored to or toward normal; thus renal autoregulation may be impaired. In support of this contention, recently our studies in another model of reduced nephron endowment, fetal uninephrectomy, have suggested changes in autoregulatory control of renal function.41 It has been proposed that perturbations in tubuloglomerular feedback are a primary factor in the development of renal dysfunction and hypertension.42 Furthermore, ethanol-exposed male offspring had an elevation in urinary protein concentration, a known independent risk factor for the future development of kidney disease.
Surprisingly, in female ethanol-exposed offspring there was an elevation in RVR coupled with a reduction in GFR and ERBF in association with a modest reduction in nephron number. These changes in renal hemodynamics occurred in the absence of any evidence of histologic damage. Additional studies are required to determine whether these perturbations in renal function progress in the long-term or if an increase in preglomerular resistance could potentially protect the glomerular capillaries from the damaging consequences of hypertension in females. The reasons for these sex differences are not immediately apparent. One possibility is that the expression levels and/or function of the organic anion transporters responsible for p-aminohippuric acid (PAH) secretion may have been altered in ethanol-exposed offspring in a sex-specific manner. Sex differences in expression levels of organic anion transporters have been widely reported,43 and so it is possible that PAH secretion, and thus ERBF, may be effected in females but not males. However, this seems an unlikely possibility given that it is hard to imagine similar problems with inulin clearance, which was also decreased. Another possibility is that the increases in RVR in the ethanol-exposed females were due to volume depletion; however, we discount this explanation because fluid replacement was administered on a per body weight basis, and no differences in hematocrit were observed between groups. Therefore, accepting that the increases in RVR and the decreases in GFR and ERBF in ethanol-exposed female rats were real, how might this sexual dimorphism be induced? One possibility is that vascular function was differentially affected by ethanol exposure in the sexes. It is well recognized that there are sex differences in the role that NO plays in the renal vasculature with NO being enhanced in females as compared with males,44 and thus alterations in renal vascular function might be sexually dimorphic. Previous studies investigating NO blockade have reported changes in ERBF and GFR similar to those identified in this study.45–47 In addition, there is evidence to suggest that prenatal ethanol exposure does alter vascular function.48 Further studies to examine renal and vascular function in ethanol-exposed offspring are warranted.
In conclusion, acute prenatal ethanol exposure at a time when the kidney is in its earliest stages of development results in postnatal growth restriction and a permanent reduction in nephron number in rat offspring. Male and female ethanol-exposed offspring had significant elevations in MAP at 6 months of age and sex-specific changes in renal function, with male offspring having an elevation in GFR and ERBF, whereas female offspring had an elevation in RVR with a decrease in GFR and ERBF. Further studies are warranted to fully evaluate these sex-specific changes in renal function. This study has demonstrated a potential developmental pathway through which alcohol acts on the developing kidney. Embryonic kidneys cultured in the presence of ethanol had a significant reduction in ureteric branching morphogenesis and glomerular number. Gene expression studies identified changes in the expression of ureteric branching regulators from ethanol-exposed kidneys. These data suggest that the identified reduction in nephron number in vivo could be in part a consequence of a reduction in ureteric branching morphogenesis in the developing kidney. The findings from this study have important implications for pregnant women who drink alcohol in binge episodes early in gestation and those who are considering becoming pregnant.
CONCISE METHODS
Animal Groups
All of the experiments were approved by an Animal Ethics Committee of Monash University and conducted according to the National Health and Medical Research Council of Australia guidelines. At 8 weeks of age, 18 virgin Sprague-Dawley female rats were time-mated overnight, and the presence of a seminal plug the following morning was termed E0.5. Dams were randomly allocated to one of three experimental groups (ethanol, sham, and control) and then housed individually. Food and water were provided ad libitum with a cycle of 12 hours of light/12 hours of dark. At E13.5 the ethanol group was given 1 g of ethanol per kg of maternal body weight diluted in saline by oral gavage at 10 a.m. The animals were then returned to their home cages. The ethanol administration was repeated on E14.5. The sham group received an equivalent volume of saline on E13.5 and E14.5 by gavage, with the control group not receiving any treatment (to control for any possible effects of gavage-associated stress on fetal and/or kidney development). Pregnant dams were weighed at E12.5, E13.5, E14.5, E15.5, E16.5, E18.5, and E20.5 and were allowed to litter down naturally. Pup viability was assessed with each pup being individually weighed every second day from PN2 until PN30. The pups were weaned on PN21.
BAC
To determine the BAC achieved in the dam, a separate cohort of animals were used (n = 4 sham and n = 4 ethanol). Blood was collected from the tail vein (500 μl) of pregnant dams 1 hour after gavage on E13.5. BAC was measured using QuantiChromTM Ethanol Assay Kit (BioAssay Systems).
Postmortem Examination at PN30
Two males and two females from each litter in all three groups (control, sham, and ethanol) were culled at PN30 by an overdose of pentobarbital sodium (100 mg/kg, intraperitoneally). The brain, liver, heart, lungs, and kidneys were removed and weighed. Left kidneys were fixed in 10% formalin and prepared for stereological analysis.
Stereological Analysis
Total glomerular number and mean glomerular volume were determined using unbiased stereological methods (physical disector/fractionator combination) as described previously.34,49
MAP and HR
At 6 months of age conscious MAP and HR were recorded via the tail artery catheter in rats that were kept in home cages over a 30-minute time frame, as described previously.18,50 At completion of all recordings, the animals were anesthetized (150 mg/kg inactin intravenously; thiobutabarbital sodium; Sigma-Aldrich) for the renal function experiments.
Renal Function
Renal function (GFR, ERBF, filtration fraction, and RVR) was analyzed via PAH (0.5 μCi/h [14C]PAH; PerkinElmer) and inulin (1 μCi/h [3H]inulin; PerkinElmer) clearance as described previously.51 Bovine serum albumin was continuously infused to replace fluid losses (2% BSA, 0.5 ml/min per100 g of body weight). Urine and plasma samples were analyzed for Na+, K+, and Cl− concentrations. Excretion rates of these ions were calculated as the product of urine flow rate × ion concentration (expressed in mmol/min). Urinary protein concentration was assessed using a Synchron CX-5 clinical system (Beckman). Osmolality was measured using freezing point depression (Advanced Osmometers).
Postmortem Examination at 6 Months of Age
After measuring arterial pressure and renal function, the animals were culled with an overdose of pentobarbital sodium (100 mg/kg intravenously). The heart, lung, liver, and kidneys were removed and weighed. The left kidneys were fixed in 10% formalin for histologic analysis.
Renal Morphology
Samples of left kidneys removed from 6-month-old animals were embedded in paraffin and sectioned at 5-μm thickness. The sections were stained with hematoxylin and eosin, periodic acid-Schiff, or Masson's trichrome.
Gene Expression Studies
To investigate whether maternal ethanol consumption alters fetal metanephric gene expression, pregnant Sprague-Dawley rats were administered 1 g/kg of ethanol via gavage on E13.5 and E14.5 of gestation, with a control group receiving a similar volume of saline. Fetuses were removed at E15.5, cleaned, and weighed, and paired kidneys were collected and frozen for later extraction of RNA. Total RNA was extracted using RNeasy extraction kits (Qiagen), and 1 μg of each RNA sample was reverse transcribed as described previously.18 The levels were determined via real-time PCR using an Applied Biosystems One Step Machine. Sequences and optimal concentrations for Wnt4, AT1bR, AT2R, BMP-4, and TGFβ1 have previously been reported,18,52 whereas primer and probe sequences for GFRα1, TGFβ2, TGFβ3, FGF7, and Wnt11 are presented in Table 5. GDNF, BAX, Bcl-2, and ADH were analyzed using an Assay-on-Demand from Applied Biosystems. A comparative cycle of threshold fluorescence (CT) method was used with 18 S as an internal control; all of the genes were multiplexed with 18 S, except for AT2R, because optimization experiments revealed that the CT was altered if run in a multiplex reaction.
Table 5.
Primer and probe sequences for genes analyzed using real-time PCR
| Gene | Forward (5′-3′) Reverse (5′-3′) | Probe (5′-3′) |
|---|---|---|
| GFRα1 | CGTCTTAACTGCAATACAC | AAATGTCACTGACTTGGGTTTGGGC |
| AACCGCTACAATATCGAAAG | ||
| TGFβ2 | CGACATGCCGTCCCACTTC | CCCTCCGAAACTGTCTGCCCAG |
| CTGCCCACTGAGCCAGAG | ||
| TGFβ3 | TTCAGCCCAATGGAGACATAC | TGGAAAATGTTCACGAGGTGATGGAA |
| CGGCCATGGTCATCTTCGT | ||
| FGF7 | GACAAACGAGGCAAAGTGAAAGG | ACCCAGGAGATGAGGAACAGCTACA |
| TGCCACAGTCCTGATTTCCA | ||
| Wnt11 | TGCGTCTACACAACAGTGAAGTG | AGACAGGCTCTACGTGCCTCCC |
| GGAGCAGGAGCCAGATACC |
Calculation of Relative Gene Expression
The CT values for 18 S were subtracted from the CT value for the gene of interest to give a ΔCT for each sample. The ΔCT of the calibrator (the mean ΔCT for the saline group) was then subtracted from each sample to give a ΔΔCT value. This was then inserted into the equation 2−ΔΔCT to give a final relative expression relative to the calibrator.
Metanephric Organ Culture
Sprague-Dawley female rats were time-mated for 4 hours where the presence of a vaginal plug indicated the time of mating, designated as E0.5. Whole metanephroi were isolated from E14 embryos within a weight range of 130 to 150 mg and placed on 3.0-μm pore polycarbonate transfilter membranes (Osmonics, Poretics) in 24-well tissue culture plates with wells containing 350 μl of serum-free culture medium at 37°C and 5% CO2. The culture medium consisted of DME:Ham's F-12 liquid medium (Sigma-Aldrich) supplemented with 5 μg/ml transferrin (Sigma-Aldrich), 12.9 μl/ml l-glutamine (Sigma-Aldrich), penicillin (100 units/ml), and streptomycin (100 μg/ml). Metanephroi were randomly allocated into either the 0% ethanol (control) group or the 0.2% ethanol group. To investigate the effects of ethanol on ureteric branching morphogenesis, metanephroi were cultured for 48 hours, with the medium being replaced after 24 hours. To determine the effects of ethanol on glomerular number, metanephroi were cultured for 5 days, with the medium being replaced after 24 hours and then left for the following 4 days. At the conclusion of the culture period, metanephroi were fixed in methanol at −20°C for 15 minutes.
Whole Mount Immunofluorescence Staining and Quantitation of Ureteric Branching
After fixation, for visualization of the ureteric tree, whole metanephroi were stained using monoclonal mouse anti-pan cytokeratin (Sigma-Aldrich) and Alexa 488 goat anti-mouse IgG (Molecular Probes, Eugene, OR) as described previously.18 The metanephroi were visualized using an epifluorescence microscope (Olympus), and the ureteric branches were manually skeletonized. The number of ureteric branch points and tips was determined. The branch points were defined as the intersection of three or more branches (lines on the skeletonized image).
Whole Mount Lectin Histochemistry and Quantitation of Glomerular Number
After fixation, those metanephroi to be prepared for glomerular number estimation were briefly washed in PBS and stained using peanut agglutinin (50 μg/ml; Sigma-Aldrich). The metanephroi were then viewed under an epifluorescence microscope for direct counting of glomeruli as described previously.18
Data Analysis
The data are expressed as the means ± SEM, except where otherwise indicated. For in vivo studies, the males and females were analyzed separately, with offspring weight gain being analyzed using a one-way ANOVA weighted for litter testing for the effect of treatment at each time point. All other data were analyzed using a one-way ANOVA weighted for litter testing for the effect of treatment, followed by Tukey's post hoc test. For in vitro studies, a two-tailed unpaired t test was utilized for analysis of ureteric branching, glomerular number, and real-time PCR data. A P < 0.05 was classed as being statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by a National Health & Medical Research Council grant (Grant 511162).
The authors acknowledge the expert technical assistance of Prof. John Dowling, Associate Prof. Mary Wlodek, Ms. Rebecca Flower, Ms. Rebecca Douglas-Denton, Ms. Debbie Arena, Ms. Sue Connell, Ms. Julie Hickey, and Mr. Chaminda Premaratne.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. WHO Department of Mental Health and Substance Abuse: Global Status Report on Alcohol: 2004, Geneva, Switzerland, 2004 [Google Scholar]
- 2. Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C: The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res 30: 1023–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Nayak MB, Kaskutas LA: Risky drinking and alcohol use patterns in a national sample of women of childbearing age. Addiction 99: 1393–1402, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Maier SE, West JR: Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol 23: 49–57, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Young C, Olney JW: Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis 22: 548–554, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Gomutputra P, Wolgemuth DJ, Baxi L: Effects of acute alcohol intoxication in the second trimester of pregnancy on development of the murine fetal lung. Am J Obstet Gynecol 197: 269.e1–269.e4, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Gallo PV, Weinberg J: Organ growth and cellular development in ethanol-exposed rats. Alcohol 3: 261–267, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Assadi FK, Manaligod JR, Fleischmann LE, Zajac CS: Effects of prenatal ethanol exposure on postnatal renal function and structure in the rat. Alcohol 8: 259–263, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Gray SP, Kenna K, Bertram JF, Hoy WE, Yan EB, Bocking AD, Brien JF, Walker DW, Harding R, Moritz KM: Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am J Physiol Regul Integr Comp Physiol 295: R568–R574, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Turcotte LA, Aberle Ii NS, Norby FL, Wang GJ, Ren J: Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol 26: 75–81, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure: Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Clark AT, Bertram JF: Molecular regulation of nephron endowment. Am J Physiol Renal Physiol 276: F485–F497, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Burrow CR: Regulatory molecules in kidney development. Pediat Nephrol 14: 240–253, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Moritz KM, Wintour-Coghlan M, Black MJ, Bertram JF, Caruana G: Morphological development of the mammalian kidney. Adv Anat Embryol Cell Biol 196: 1–78, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA: Effects of dexamethasone exposure on rat metanephric development: In vitro and in vivo studies. Am J Physiol Renal Physiol 293: F548–F554, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Tsai J, Floyd LR, Green PP, Boyle CA: Patterns and average volume of alcohol use among women of childbearing age. Matern Child Health J 11: 437–445, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA: Alcohol consumption by women before and during pregnancy. Matern Child Health J 13: 274–285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vavrousek-Jakuba EM, Baker RA, Shoemaker WJ: Effect of Ethanol on Maternal and Offspring Characteristics: Comparison of Three Liquid Diet Formulations Fed During Gestation. Alcohol Clin Exp Res 15: 129–135, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Knee DS, Sato AK, Uyehara CFT, Claybaugh JR: Prenatal exposure to ethanol causes partial diabetes insipidus in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R277–R283, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Moritz KM, Wintour EM: Functional development of the meso- and metanephros. Pediat Nephrol 13: 171–178, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M: Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langley-Evans SC, Welham SJM, Jackson AA: Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Woods LL, Weeks DA, Rasch R: Programming of adult blood pressure by maternal protein restriction: Role of nephrogenesis. Kidney Int 65: 1339–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM: Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Spohr HL, Willms J, Steinhausen HC: Prenatal alcohol exposure and long-term developmental consequences. Lancet 341: 907–910, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Lan N, Yamashita A, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J: Prenatal ethnaol exposure alters the effects of gonadectomy on hypothalamic-pituitary-alcohol activity in male rats. J Neuroendocrinol 18: 672–684, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Moritz KM, Singh RR, Probyn ME, Denton KM: Developmental programming of a reduced nephron endowment: More than just a baby's birth weight. Am J Physiol Renal Physiol 296: F1–F9, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Walker KA, Caruana G, Bertram JF, McInnes KJ: Sexual dimorphism in mouse metanephroi exposed to 17β-estradiol in vitro. Nephron Exp Nephrol 111: e42–e50, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Ortiz LA, Quan A, Weinberg A, Baum M: Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M: Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cullen-McEwen LA, Drago J, Bertram JF: Nephron endowment in glial cell line-derived neurotrophic factor (gdnf) heterozygous mice. Kidney Int 60: 31–36, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Oxburgh L, Chu GC, Michael SK, Robertson EJ: TGFβ2 superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131: 4593–4605, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP: Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D: FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126: 547–554, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE: Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Weinberg J, Sliwowska JH, Lan N, Hellemans KGC: Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol 20: 470–488, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh RR, Denton KM, Bertram JF, Jefferies AJ, Moritz KM: Reduced nephron endowment due to fetal uninephrectomy impairs renal sodium handling in male sheep. Clin Sci 118: 669–680, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Vallon V: Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 18: 169–174, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I: Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287: F124–F138, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Baylis C: Sexual dimorphism in the aging kidney: Differences in the nitric oxide system. Nat Rev Nephrol 5: 384–396, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Baylis C, Harton P, Engels K: Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol 1: 875–881, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Johnson RA, Freeman RH: Sustained hypertension in the rat induced by chronic blockade of nitric oxide production. Am J Hypertens 5: 919–922, 1992 [DOI] [PubMed] [Google Scholar]
- 47. Denton KM, Anderson WP: Intrarenal haemodynamic and glomerular responses to inhibition of nitric oxide formation in rabbits. J Physiol 475: 159–167, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parkington HC, Coleman HA, Wintour EM, Tare M: Prenatal alcohol exposure: implications for cardiovascular function in the fetus and beyond. Clin Exp Pharmacol Physiol 37: E91–E98, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Bertram JF: Counting in the kidney. Kidney Int 59: 792–796, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Zimanyi MA, Denton KM, Forbes JM, Thallas-Bonke V, Thomas MC, Poon F, Black MJ: A developmental nephron deficit in rats is associated with increased susceptibility to a secondary renal injury due to advanced glycation end-products. Diabetologia 49: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Denton KM, Li M, Anderson WP, Whitworth JA: Glomerular hypertension and hyperfiltration in adrenocorticotrophin-induced hypertension in rats: The role of nitric oxide. J Hypertens 19: 327–334, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Dickinson H, Walker DW, Wintour EM, Moritz K: Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol 292: R453–R461, 2007 [DOI] [PubMed] [Google Scholar]