Abstract
The mechanisms underlying “aldosterone escape,” which refers to the excretion of sodium (Na+) during high Na+ intake despite inappropriately increased levels of mineralocorticoids, are incompletely understood. Because local purinergic tone in the aldosterone-sensitive distal nephron downregulates epithelial Na+ channel (ENaC) activity, we tested whether this mechanism mediates aldosterone escape. Here, urinary ATP concentration increased with dietary Na+ intake in mice. Physiologic concentrations of ATP decreased ENaC activity in a dosage-dependent manner. P2Y2−/− mice, which lack the purinergic receptor, had significantly less increased Na+ excretion than wild-type mice in response to high-Na+ intake. Exogenous deoxycorticosterone acetate and deletion of the P2Y2 receptor each modestly increased the resistance of ENaC to changes in Na+ intake; together, they markedly increased resistance. Under the latter condition, ENaC could not respond to changes in Na+ intake. In contrast, as a result of aldosterone escape, wild-type mice had increased Na+ excretion in response to high-Na+ intake regardless of the presence of high deoxycorticosterone acetate. These data suggest that control of ENaC by purinergic signaling is necessary for aldosterone escape.
Renal sodium (Na+) excretion influences BP by affecting systemic Na+ balance. Consequently, negative feedback in response to changes in Na+ balance, perceived as changes in effective circulating volume (ECV) and BP, control renal Na+ handling. The fine-tuning of Na+ balance occurs in the aldosterone-sensitive distal nephron (ASDN), including the connecting tubule (CNT) and the collecting duct (CD). Here, Na+ reabsorption is highest when ECV and BP are low. Activity of the epithelial Na+ channel (ENaC) is limiting for discretionary Na+ reabsorption across the ASDN.1–7 As ECV declines, activity of the renin-angiotensin-aldosterone system (RAAS) increases with the mineralocorticoid aldosterone, stimulating channel activity to decrease Na+ excretion in correction of falling ECV. Aldosterone increases the number and activity of ENaC in the apical membrane.8–11 Under normal conditions, the opposite is also true; aldosterone and, thus, ENaC activity decline in response to elevations in BP.
The thiazide-sensitive Na-Cl co-transporter (NCC) in the distal convoluted tubule (DCT) is also a target of aldosterone with the mineralocorticoid increasing NCC activity and possibly transporter abundance in the apical membrane of DCT cells.12–16 This increase in NCC activity decreases Na+ excretion. Thus, aldosterone promotes distal nephron Na+ reabsorption by increasing the activity of ENaC and NCC.
The loss of negative-feedback regulation of renal Na+ excretion mediated by ENaC and NCC leads to hypertension. For instance, gain-of-function mutations in ENaC cause the channel to be hyperactive irrespective of mineralocorticoid status and systemic Na+ balance, thus leading to inappropriate Na+ retention and hypertension; conversely, loss-of-function mutations in ENaC can cause decreases in BP and renal salt wasting.2,3,5,7,17–19
The aldosterone-promoted decrease in renal Na+ excretion is overridden in some circumstances.20,21 This disruption of aldosterone action is termed aldosterone escape and results in avid Na+ excretion during high Na+ intake despite elevated mineralocorticoid levels. Aldosterone escape seems to be a protective mechanism to allow appropriate response to positive Na+ balance despite inappropriate mineralocorticoid levels. Such escape is important clinically, for example, in primary aldosteronism, in which it may ameliorate, to some degree, the hypertensive effects of high circulating levels of aldosterone.22–24 The mechanism allowing aldosterone escape is uncertain.
Aldosterone escape is known to be dependent on increases in renal vascular perfusion pressure rather than systemic factors, including changes in the levels of circulating hormones, such as renin and aldosterone, or renal nerve activity.25 This led to the idea that aldosterone escape is a manifestation of a pressure natriuresis response.13,25,26 This is associated with declining Na+ reabsorption in the distal nephron despite elevated levels of aldosterone and occurs independent of changes in GFR.26,27 Indeed, early micropuncture measurements demonstrated increases in urine flow and Na+ delivery to the CD during escape.26,27 Details about the specific site(s) along the distal nephron involved in decreased Na+ reabsorption during aldosterone escape and the exact transport proteins involved in this escape, though, largely remain obscure.
A study by Wang et al.13 revealed that during aldosterone escape, NCC levels in the DCT decrease. This response was selective because the levels of other apical membrane transport proteins, including ENaC, were unaffected. This finding supports the idea that NCC is, at least, one target for regulatory processes that mediate aldosterone escape, whereby decreasing NCC abundance facilitates Na+ excretion despite high levels of mineralocorticoid. The cellular mechanism underpinning declines in NCC levels during aldosterone escape is unknown. Similarly, it is unclear whether other transport proteins are involved in aldosterone escape because Na+ reabsorption is a manifestation not only of the number of transport proteins in the apical membrane but also of their activity.
Because ENaC is critical to aldosterone regulation of Na+ excretion, particularly in response to changes in Na+ balance, evidenced by the hypertension associated with gain of ENaC function,3,5,7 we sought to investigate the role of this channel in aldosterone escape. We recently demonstrated that local purinergic tone in the ASDN exerts paracrine downregulation of ENaC activity specifically by affecting channel open probability.28 This paracrine pathway is physiologically relevant because mice lacking the purinergic receptor P2Y2, responsible for the bulk of paracrine regulation in this system, have facilitated Na+ reabsorption in the distal nephron and mild increases in BP.29 Increases in NKCC2 and ENaC activity contribute to this elevated BP.11 It does not seem a coincidence that aldosterone and P2Y2 signaling target the same final effector proteins, possibly allowing one to compensate for the loss of the other. This idea suggests that purinergic regulation of ENaC may contribute to aldosterone escape, a possibility supported by another recent finding from our laboratories: Elevated BP in P2Y2−/− mice is not salt sensitive in the presence of normal-feedback regulation by RAAS but becomes salt sensitive and associated with inappropriately active ENaC when negative-feedback regulation by aldosterone and local purinergic signaling both are disrupted.11,29
To test the role of ENaC and its regulation by mineralocorticoids and purinergic signaling in aldosterone escape and to understand better the mechanism underpinning escape, we quantified the actions of mineralocorticoid on renal Na+ excretion, ENaC activity, and urinary ATP levels in wild-type (WT) and P2Y2−/− mice stressed with different dietary Na+ regimens. We found that urinary [ATP] increases with dietary Na+ intake such that physiologic [ATP] decrease ENaC activity, resulting in increased Na+ excretion. Increased Na+ excretion is greater in WT compared with P2Y2−/− mice and associated with an inability of ENaC, particularly when mineralocorticoid is clamped at high levels, to respond appropriately to changes in dietary Na+ intake in the latter animals. Because ENaC activity normally is sensitive to changes in Na+ balance even when mineralocorticoids are clamped at high levels, these results show that control of ENaC by purinergic signaling is necessary for complete aldosterone escape, consistent with the loss of aldosterone-escape in P2Y2−/− mice and their pronounced hypertension relative to normal mice in the presence of elevated mineralocorticoids and high Na+ intake.
RESULTS
ATP Levels in Urine Reflect Systemic Na+ Levels
To test for a cause-and-effect relation between systemic Na+ balance and urinary [ATP], we measured ATP levels in urine harvested directly from the bladders of WT mice maintained on nominally Na+-free (<0.01%), regular-Na+ (0.32%), and high-Na+ (2%) diets. As summarized in Figure 1A, elevations in dietary Na+ significantly increased urinary ATP concentrations from a minimum of 7.9 ± 1.2 nM (n = 20) on a Na+-free diet to 15.2 ± 3.3 (n = 11) and 50 ± 16 nM (n = 9) for 0.32 and 2% [Na+] diets, respectively (P < 0.05). To exclude the possibility that differences in urinary [ATP] associated with different dietary Na+ regimens primarily reflects variations in GFR and/or urine volume, we normalized urinary [ATP] to urinary creatinine (Ucre). Figure 1B shows Na+-dependent changes in the ratio of urinary [ATP] to [Ucre]. After 1 week on a given dietary Na+ regimen, Ucre levels were similar under each condition with no significant difference (data not shown). Thus, this normalization did not affect the observation that urinary [ATP] increases with higher Na+ intake.
Figure 1.
Urinary ATP concentrations reflect systemic Na+ levels. (A and B) Summary graphs of ATP levels in urine (A) and the urinary ATP (nM)/creatinine (mg/dl) ratio (B) for WT (gray bar), P2Y2−/− (black bar), and Cx30−/− (dark gray bar) mice kept on a nominally Na+-free, regular-Na+, and high-Na+ diet for at least 1 week before experimentation. *Significant (P < 0.05) increase versus WT at <0.01% Na+; **significant (P < 0.05) decrease versus WT.
We tested whether mice with genetic deletion of P2Y2 receptors retain this positive relationship between systemic Na+ balance and urinary [ATP]. Because these animals lack only end-organ response to purinergic stimuli mediated by the P2Y2 receptor, they should produce and release ATP normally. Indeed, we found that ATP levels in urine were 11.6 ± 0.5 (n = 6), 13.2 ± 2.5 (n = 6), and 37.0 ± 13.0 nM (n = 9) for salt-restricted, normal, and salt-loaded P2Y2−/− mice, respectively. These values are not significantly different from those of WT mice.
ATP in urine in the bladder may originate from several distinct nephron segments plus uroepithelium and consists of released nucleotide minus that metabolized.30–32 As a marker for at least one source of urinary ATP, we performed control experiments in mice lacking connexin 30 (Cx30). The expression of this gap-junction protein is mostly restricted to intercalated cells of the CD in the murine ASDN.33 Possibly functioning as an apical hemichannel in intercalated cells, Cx30 serves as a conduit for ATP release into the urine.34 As shown in Figure 1, urinary [ATP] was significantly decreased in Cx30−/− mice at all dietary Na+ concentrations (n = 5 for each condition) compared with that in WT mice. This observation that genetic deletion of Cx30 decreases urinary [ATP] is consistent with evidence that loss of Cx30 decreases ATP release in the murine CD.34 Moreover, the finding that urinary [ATP] is positively related to Na+ feeding is consistent with our previous observation that urinary [ADP], the ATP hydrolytic product, is also positively correlated with dietary Na+ intake.11 Thus, these results support evidence that Cx30 expression contributes to ATP release into the CD and that the likely site for regulation of salt-sensitive ATP release into the urine is in the ASDN.
Physiologic Concentrations of ATP (and UTP) Rapidly Decrease ENaC Activity in the Native Murine ASDN
We previously demonstrated that high μM concentrations of ATP act via apical membrane P2 receptors to decrease ENaC activity dramatically by reducing Po28,35; however, it is unclear whether physiologic ATP levels in urine (Figure 1) are sufficient to affect ENaC activity. Thus, we defined the concentration dependence of ATP inhibition of murine ENaC in the isolated ASDN by assessing the decrease in Po. Figure 2A shows a representative gap-free current recording and documents ENaC activity in a cell-attached patch on the apical membrane of a principal cell in a freshly isolated, split-open murine ASDN before and after application of 10 μM ATP. ATP rapidly and markedly decreased ENaC Po. The concentration-response curve for this inhibitory effect of ATP on ENaC activity (Figure 2B) yielded an IC50 of 33 ± 2 nM, a concentration in the physiologic range of ATP in urine (Figure 1A), and that is consistent with P2Y2 receptor activation.30–32 Previous work demonstrated that P2Y2 receptor signaling provides the bulk of the inhibitory purinergic regulation of ENaC.28 Unlike ionotropic P2X receptors and other P2Y receptors, the P2Y2 receptor is activated equally well by the purine ATP and the pyrimidine UTP.30 Thus, we also assessed the dosage dependence of UTP actions on ENaC activity in the isolated, split-open murine ASDN (Figure 2B) and found that UTP inhibits ENaC activity as well as ATP, yielding an IC50 of 49 ± 2 nM. These findings demonstrate that activators of P2Y2 receptors at concentrations (in the case of ATP) found in the urine rapidly and markedly decrease ENaC activity in its native environment with the [ATP] associated with a high-Na+ diet inhibiting ENaC activity by at least 50% whereas that associated with a low-Na+ diet has little inhibitory effect on the channel.
Figure 2.
Physiologic concentrations of ATP and UTP rapidly decrease ENaC activity. (A) Representative gap-free current trace from a cell-attached patch on a principal cell in a split-open murine ASDN monitoring ENaC activity before and after addition of 10 μM ATP. Dashed lines show respective current levels with C denoting the closed state. Areas under the bars labeled 1 (control) and 2 (after ATP) are shown below with an expanded time scale. (B) Summary graph of dosage-dependent inhibition of ENaC activity by ATP (black) and UTP (gray). The number of paired experiments, similar to that in A, is shown for each concentration.
Disruption of Local Control of ENaC by Purinergic Tone in P2Y2−/− Mice Impairs Renal Na+ Excretion
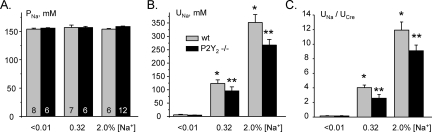
We next assessed the physiologic consequences on renal Na+ excretion of negative regulation of ENaC by the purinergic system intrinsic to the ASDN, which is responsive to changes in Na+ intake as shown in the previous section. For these purposes, we measured and compared plasma Na+ concentrations (PNa), shown in Figure 3A, and urinary Na+ concentrations ([UNa]), shown in Figure 3B, for WT and P2Y2−/− mice maintained on low-, regular-, and high-Na+ regimens. After 1 week on a given Na+ diet, PNa varied little in either WT or P2Y2−/− mice with plasma Na+ concentrations in the latter mice not differing from those in WT mice under any feeding condition tested. In contrast to the findings for PNa, UNa showed a significant dependence on the feeding regimen for both WT and P2Y2−/− mice; UNa was greatest in mice maintained on a high-Na+ diet. With the Na+-deficient diet, UNa was not significantly different between WT and P2Y2−/− mice (6.1 ± 1.7 versus 4.9 ± 1.0 mM). In contrast, UNa was significantly lower for P2Y2−/− compared with WT mice maintained with regular- and high-Na+ diets (96 ± 15 versus 124 ± 13 mM and 265 ± 16 mM versus 352 ± 30 mM, respectively; P < 0.05), although both WT and P2Y2−/− mice had salt-dependent increases in UNa. These findings are consistent with P2Y2−/− mice having a diminished capacity to excrete Na+.
Figure 3.
Na+ excretion is impaired in P2Y2−/− mice. (A and B) Summary data of plasma (A) and urinary (B) Na+ concentrations for WT ( ) and P2Y2−/− (■) mice kept on Na+-free, regular-Na+, and high-Na+ diets for at least 1 week before experimentation. (C) Summary of urine [Na+]/urine [Cre] for WT and P2Y2−/− mice on the various dietary Na+ regimens. UNa in mM/L and UCre in mg/dl. *P < 0.05 versus WT at [Na+] <0.01%; **P < 0.05 versus WT.
) and P2Y2−/− (■) mice kept on Na+-free, regular-Na+, and high-Na+ diets for at least 1 week before experimentation. (C) Summary of urine [Na+]/urine [Cre] for WT and P2Y2−/− mice on the various dietary Na+ regimens. UNa in mM/L and UCre in mg/dl. *P < 0.05 versus WT at [Na+] <0.01%; **P < 0.05 versus WT.
Figure 3C summarizes the effects of varying systemic Na+ levels and disruption of the local purinergic system in the ASDN through genetic deletion of the P2Y2 receptor on relative Na+ excretion (UNa [mM]/UCre [mg/dl]). Ucre did not differ between WT and P2Y2−/− mice and was unaffected by dietary Na+ intake (data not shown). As expected, little Na+ was excreted by either group under salt-restricted conditions. In contrast, relative Na+ excretion rose in mice fed normal- and high-Na+ diets. In these latter conditions, relative Na+ excretion was significantly lower in P2Y2−/− compared with WT mice. Thus, disrupting negative regulation of ENaC by compromising the local purinergic system significantly impairs an appropriate increase in Na+ excretion in response to increased Na+ intake.
Compensation by the Local Purinergic System Allows for Aldosterone Escape
Although impaired in the absence of normal regulation of ENaC by local purinergic signaling, Na+ excretion in P2Y2−/− mice retained a dependence on salt intake. This most likely reflects, in part, the contribution of normal feedback regulation of ENaC in ASDN by the mineralocorticoid system responding to increases in Na+ intake. Other nephron segments also contribute to proper Na+ excretion in response to elevated Na+ intake, other signaling inputs in addition to mineralocorticoids and purinergic signaling may influence ENaC activity in response to changes in systemic Na+ intake, and ATP may target effectors other than ENaC in response to changes in Na+ balance. To predict the involvement of ENaC to Na+ excretion in response to increases in salt intake and to identify the contributions of salt-dependent mineralocorticoid and local purinergic regulation of this channel to salt excretion, we developed an index to gauge the responsiveness of ENaC to changes in Na+ balance. We measured ENaC activity in the isolated split-open murine ASDN with patch-clamp electrophysiology, as in Figure 2A, from mice treated with nominally free- and high-Na+ diets, and then divided the activity observed on a high-Na+ diet by that on a Na+-free diet. As we showed previously,10,11 ENaC activity is normally very responsive to salt intake because ENaC activity decreases and increases in response to high and low salt intake, respectively, through the cumulative actions of feedback regulation by the mineralocorticoid system, local purinergic signaling, and other inputs.10,11,28 As shown in Figure 4, fractional ENaC activity in WT mice, in the absence of any perturbation other than changing salt feeding, is low, indicating a high degree of responsiveness to changes in Na+ balance. Raw ENaC activity data for WT mice maintained with nominally Na+-free and high-Na+ feeding in the absence and presence of deoxycorticosterone acetate (DOCA) are shown and compared in Table 1 (some of these data were presented previously in reference 11). Because ENaC is a primary target for negative-feedback regulation of ECV and BP, as changes in ENaC activity become salt-resistant, BP can increase in a salt-sensitive manner, such as with gain-of-function mutations in ENaC, producing salt-sensitive increases in BP. As shown in Figure 4, clamping DOCA at high levels in WT mice increases salt resistance of ENaC, presumably because the ability of the mineralocorticoid system to govern ENaC activity in response to changes in systemic salt levels has been compromised.
Figure 4.
Disruption of mineralocorticoid and purinergic regulation uncouples ENaC from a normal response to increases in systemic Na+. Summary data comparing the fractional ENaC activity (activity with high dietary Na+ divided by activity with low dietary Na+; n ≥ 30 for high and low Na+ for each condition) in isolated ASDN from WT and P2Y2−/− mice in the absence (control) and presence of exogenous DOCA. Some data used in this figure were presented originally in reference 11.
Table 1.
Na+- and DOCA-sensitive ENaC activity in WT and P2Y2−/− mice
| Genotype | Condition | NPo | f | Activity |
|---|---|---|---|---|
| WT | Low salt | 1.25 ± 0.25 | 0.45 (26/58) | 0.56 ± 0.11 |
| + DOCA | 1.55 ± 0.33 | 0.60 (12/20) | 0.93 ± 0.19 | |
| High salt | 0.29 ± 0.12 | 0.25 (14/57) | 0.07 ± 0.02a | |
| + DOCA | 0.66 ± 0.17 | 0.46 (18/39) | 0.30 ± 0.08a,b | |
| P2Y2 | Low salt | 1.56 ± 0.29 | 0.52 (10/19) | 0.82 ± 0.15 |
| + DOCA | 1.80 ± 0.28 | 0.62 (30/48) | 1.12 ± 0.17 | |
| High salt | 1.03 ± 0.13 | 0.28 (12/43) | 0.29 ± 0.03a,c | |
| + DOCA | 1.88 ± 0.32 | 0.50 (11/22) | 0.94 ± 0.16b,c |
Some data in this table were presented originally in reference 11. f, ENaC frequency (number of seals having at least one active ENaC/total number of gigaohm seals under that condition).
Activity significantly (P < 0.05) different
aversus low salt,
bversus no DOCA, and
cversus WT under identical conditions.
WT mice showed only a slight increase in salt resistance of ENaC in the presence of DOCA. This presumably occurs because control of ENaC by systems other than the RAAS also able to respond to Na+ balance, such as the local purinergic system intrinsic to the ASDN, are retained and responding appropriately. To test this idea, we performed similar experiments in P2Y2−/− mice; we assessed ENaC activity in the ASDN from P2Y2−/− mice maintained with high- and nominally Na+-free diets in the absence and presence of DOCA. Raw data are shown and compared in Table 1. As shown in Figure 4, ENaC activity in mice lacking purinergic signaling but retaining the ability to respond to a Na+ load via the mineralocorticoid system also showed only slight increases in salt resistance in the absence of DOCA. This is consistent with P2Y2−/− mice having only modest increases in BP when maintained on a normal-salt diet with increases in BP having little salt sensitivity; in contrast, clamping DOCA at high levels in combination with disruption of purinergic regulation in P2Y2−/− mice produces markedly increased salt-resistance of ENaC.29 Such results predict and we have found that BP would be greatest in P2Y2−/− mice treated with high salt plus DOCA and that under these conditions, BP in these mice should show a pronounced salt sensitivity.11 These experiments demonstrate that ENaC activity is regulated in response to changes in systemic salt in parallel by negative-feedback regulation via the mineralocorticoid pathway and local purinergic signaling, such that one may compensate for the loss of the other.
The understanding provided by the data and analysis just described suggested the possibility that decreases in ENaC Po dependent on a local purinergic signaling system in response to increases in systemic salt could possibly compensate, at least in part, for inappropriate elevations in mineralocorticoid levels during positive Na+ balance. Thus, proper regulation of ENaC by the local purinergic system intrinsic to the ASDN might account for the renal Na+ excretion associated with aldosterone escape. To test this, we compared relative Na+ excretion in WT and P2Y2−/− mice maintained on a high-Na+ diet in the absence and presence of DOCA. As shown in Figure 5, we found that Na+ excretion was appropriately elevated in WT mice on a high-Na+ diet. After 3 days of DOCA treatment on the high-Na+ diet, which permits aldosterone escape,13 WT mice maintained elevated UNa/UCre not significantly different from that in the absence of DOCA. This suggests that a protective mechanism enables appropriate Na+ excretion even in the presence of a continuous mineralocorticoid signal. In contrast, relative Na+ excretion in P2Y2−/− mice treated with a high-Na+ diet plus DOCA is significantly reduced. This is an inappropriate response considering that Na+ balance likely leads to the elevation in BP observed in these mice when treated with a high-Na+ diet plus DOCA, an effect on BP not noted in WT mice on a similar regimen.11
Figure 5.
Compensation for loss of normal mineralocorticoid feedback regulation of ENaC by the local purinergic system allows for aldosterone escape. Summary data of UNa/UCre for WT and P2Y2−/− mice kept on a high-Na+ diet (for 1 week) in the absence (■) and presence ( ) of supplementing (for 3 days) with exogenous DOCA. UNa in mM/L and UCre mg/dl. *P < 0.05 versus WT at 2% [Na+]; **P < 0.05 versus WT at 2% [Na+] + DOCA and P2Y2−/− at 2% [Na+].
) of supplementing (for 3 days) with exogenous DOCA. UNa in mM/L and UCre mg/dl. *P < 0.05 versus WT at 2% [Na+]; **P < 0.05 versus WT at 2% [Na+] + DOCA and P2Y2−/− at 2% [Na+].
DISCUSSION
Our results are consistent with the presence of local regulation of ENaC by a purinergic system intrinsic to the ASDN that enables, at least in part, aldosterone escape: Elevated Na+ excretion in the presence of high levels of mineralocorticoid. This purinergic regulation is mediated by P2Y2 receptors and presumably by the increase in the urinary levels of purinergic agonists (ATP and/or UTP) that accompanies increased dietary Na+ intake and results in decreases in ENaC activity and increases in Na+ excretion. Loss of proper feedback control, by disrupting both mineralocorticoid and purinergic regulation, uncouples ENaC from responding normally to changes in systemic Na+. This affects regulation of BP in P2Y2−/− mice with these mice, in contrast to WT mice, having pronounced elevations in BP in the presence of high salt intake and high levels of DOCA because ENaC is no longer responsive to changes in Na+ balance.
We did not investigate the interplay between regulation of ENaC by local purinergic signaling during aldosterone escape along with other factors and targets, such as ANP, nitric oxide signaling, and NCC, that may be involved in such escape.13,36 Rather, our goal was to assess the role played by local purinergic regulation of ENaC in aldosterone escape and to understand the consequences of loss of purinergic regulation during the escape process.
Our observation that urinary [ATP] and [UTP] increase as a function of dietary Na+ intake suggests a tight link between systemic Na+ balance and release of ATP and UTP into the urine, although the specific mechanisms for this relationship remain obscure (see also reference 11). Moreover, which nephron segments serve as the primary source of salt-sensitive ATP release are not known. Whether ATP versus UTP or some other purinergic receptor agonist is the primary physiologic stimulus of P2Y2 receptors during ENaC regulation also is obscure. Here, we followed ATP as a representative P2Y2 agonists found in urine. Some released ATP must arise from the ASDN because we see autocrine/paracrine (tonic) regulation of ENaC via apical P2Y2 receptors in this nephron segment when it is isolated from the rest of the kidney.11,28 Elevation of systemic Na+ increases plasma and urine volume and Na+ content, as well as urine flow, and can influence tubular cell volume. It is not clear which of these is the proximate trigger for ATP release into the urine: Both changes in tubular flow rate and cell volume have been reported to promote tubular ATP release.34,37–39
Our findings with Cx30−/− mice and data for ATP release in these animals performed previously33,34 provide evidence that Cx30−/− in the distal nephron, particularly in intercalated cells, is important for ATP release into the urine. Our finding that the bulk of salt-sensitive ATP release is lost in Cx30−/− mice suggests, in addition, that this route of release may be particularly important during responses to changes in Na+ balance. This idea is consistent with the findings that renal Na+ excretion and pressure natriuresis are impaired in Cx30−/− mice and that these mice develop ENaC-dependent, salt-sensitive elevations in BP.34 Principal cells in the ASDN are another possible source of ATP release with release from these cells seeming to depend on the presence of the apical primary cilia.37,40 Interestingly, this monocilia may be involved in sensing urine flow, which rises upon increases in systemic Na+ levels. Other possible sources of urinary ATP potentially capable of affecting transport proteins in the distal nephron are upstream nephron segments, including the thick ascending limb.38,39 For instance, ATP released from the thick ascending limb in response to flow acts in a paracrine manner to mobilize Ca2+ in tubules from WT but not P2Y2−/− mice.39
Although the precise mechanisms that trigger ATP release into the urine in response to elevations in Na+ intake and the intrarenal source of this paracrine-acting ATP remain to be definitively established, the observation that increases in Na+ balance increase urinary [ATP] is consistent with previous findings.11,30,31 Micropuncture studies have shown that ATP levels in the ultrafiltrate of the proximal tubule climb as high as 300 nM with distal tubule levels being approximately 3.5-fold lower.41 In agreement with this, we found urinary ATP levels in the nanomolar range. As a result of the actions of ecto-ATPase and ecto-5′-nucleotidase and other extracellular enzymes capable of metabolizing ATP, quantification of [ATP] from urine in the bladder may underestimate, somewhat, actual values. Alternatively, water reabsorption distal to the site of ATP release may concentrate yielding ATP values modestly higher in the final urine compared with urine in the ASDN. Nevertheless, [ATP] concentrations consistent with those measured in urine have a marked effect on ENaC activity in its native environment in isolation from possible secondary effects, such as changes in perfusion pressure, urine flow, and other factors that may complicate interpretation. Thus, the [ATP] associated with high Na+ levels causes marked inhibition of ENaC, whereas the channel is uninhibited with [ATP] at lower levels of dietary Na+, results consistent with the regulation of ENaC by ATP signaling in the ASDN playing an important role in the renal Na+ excretory response to changes in systemic Na+ levels. Our findings showing that genetic deletion of the P2Y2 receptor, which is the primary target for paracrine ATP regulation of ENaC in the ASDN,11,28 impairs excretion of Na+ particularly during elevations of Na+ intake are in agreement with this idea. Our findings also agree with previous results showing that renal Na+ excretion is impaired in P2Y2−/− mice.29
Together, our results demonstrate that increasing systemic Na+ increases urinary [ATP] to levels capable of suppressing ENaC activity and facilitating Na+ excretion. RAAS has a similar role in mediating an ENaC response to salt: Increases in total body Na+, sensed as pressure and/or volume changes, suppress plasma renin activity and lower aldosterone levels, leading to less active ENaC and thereby facilitating Na+ excretion. ENaC activity thus changes inversely with respect to changes in systemic Na+ levels.
P2Y2−/− mice have facilitated renal Na+ reabsorption associated with suppressed plasma renin and aldosterone levels and lower-than-normal plasma K+.29 The latter is because K+ is secreted in the distal nephron to maintain charge neutrality as Na+ is reabsorbed via ENaC. This profile along with salt-sensitive changes in BP is the clinical manifestation of Liddle's syndrome: Gain of ENaC function.3,5,7,17 BP in P2Y2−/− animals, in the absence of any other perturbation, however, is salt resistant.29 This suggests little involvement of ENaC; however, on the basis of our previous work11,28 and results of this study, we conclude that regulation of ENaC by ATP functions in parallel and complements regulation by aldosterone. This means that ENaC activity can change, to some degree, in response to changes in Na+ intake when one but not both salt-sensing control systems is lost. In other words, ENaC activity, as shown in Figure 4, retains its ability to respond to changes in Na+ intake in the absence of mineralocorticoid or purinergic regulation but not when both are absent. Likewise, renal Na+ handling and BP are mostly normal in the absence of either but not of both salt-sensing regulatory mechanisms that govern ENaC activity. Consistent with this idea are our findings showing that relative Na+ excretion, although modestly lower in P2Y2−/− mice compared with WT mice, retains most of its dependence on Na+ balance and that Na+ excretion remains elevated during aldosterone escape despite elevated mineralocorticoid levels. In contrast, simultaneously disrupting regulation by both purinergic and mineralocorticoid signaling uncouples ENaC from being able to respond appropriately to Na+, giving it a high salt resistance and driving Na+ excretion to inappropriately low levels in the presence of high salt intake (see Figure 5).
The impaired ability of ENaC to respond to changes in Na+ intake leads to the salt-sensitive hypertension of P2Y2−/− mice treated with DOCA. As a result of aldosterone escape, WT mice treated with DOCA have BP that is not salt sensitive. We conclude that inhibition of ENaC by local purinergic signaling provides a mechanism for escaping the actions of aldosterone.
CONCISE METHODS
All chemicals and materials were from Sigma (St. Louis, MO) unless noted otherwise and were of reagent grade. Animal use and welfare adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. For experiments, WT (C57BL/6), P2Y2−/− (backcrossed and inbred into the C57BL/6 background; described in detail previously11,28,29) and Cx30−/− (also inbred on the C57BL/6 background and described previously33,34) mice were maintained on a nominally Na+-free diet (<0.01% Na+; Harlan TEKLAD TD.90228), regular-Na+ diet containing 0.32% Na+ (Harlan TEKLAD TD.7912), or a high-Na+ diet (2% Na+; Harlan TEKLAD TD.92034) 1 week before experimentation. For some experiments, mice were administered a subcutaneous injection of 2.4 mg of DOCA dissolved in 150 μl of olive oil for 3 consecutive days before being killed.
Isolation of the ASDN containing CNT and CD suitable for electrophysiology has been described previously.10,11,28 Briefly, mice were killed by CO2 administration followed by cervical dislocation. Kidneys were immediately removed and were cut into thin slices (<1 mm), which were placed into ice-cold physiologic saline solution buffered with HEPES (pH 7.4). The ASDN was identified as merging of CNT into CD and was mechanically isolated from cortical sections of kidney slices by microdissection using watchmaker forceps under a stereomicroscope. Isolated ASDN was allowed to settle onto 5 × 5-mm coverglass coated with poly-l-lysine. Coverglass containing ASDN was placed in a perfusion chamber mounted on an inverted Nikon Eclipse TE2000 microscope and superfused with room temperature HEPES-buffered (pH 7.4) saline solution. ASDN were split open with two sharpened micropipettes controlled with different micromanipulators to gain access to the apical membrane and were used within 1 to 2 hours of isolation.
ENaC activity in principal cells of murine ASDN was determined in cell-attached patches on the apical membrane made under voltage-clamp conditions (−Vp = −60 mV) using standard procedures.10,28 Current recordings were made in a still bath with experimental reagents added directly to the recording chamber. Recording pipettes had resistances of 10 to 15 MΩ. Typical bath and pipette solutions were as follows: 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 5 mM glucose, and 10 mM HEPES (pH 7.4) and 140 mM LiCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.4), respectively. For each experimental condition, ASDN from at least three mice were assayed. Gap-free single-channel current data from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B (Axon Instruments) or EPC-9 (HEKA Instruments) patch-clamp amplifier interfaced via a Digidata 1322A (Axon Instruments) to a PC running pClamp 9.2 software (Axon Instruments). Currents were low-pass-filtered at 100 Hz with an eight-pole Bessel filter (Warner Instruments). Unitary current (i) was determined, as normal, from all-point amplitude histograms fitted with single- or multi-Gaussian curves using the standard 50% threshold criterion to differentiate between events. Events were inspected visually before acceptance. Channel activity, defined as NPo, was calculated using the equation NPo = (t1 + 2t2 + … + ntn), where N and Po are the number of ENaC in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. For calculating Po in paired experiments, N was fixed as the greatest number of active channels observed in control or experimental conditions. In such paired patch-clamp experiments, N cannot change in response to the experimental maneuver (e.g., ATP), so any detected effect must be an effect on Po. The error associated with calculating Po increases as this variable moves away from 0.5 and approaches 0 or unity. To ensure reliable calculation of Po, we measured Po with standard and accepted tools using long recording times (>1 minute) and patches containing five or fewer channels. This approach, which provides the most confidence other than using seals with only one channel, is routinely used to determine Po.42,43 The frequency (f) of observing ENaC in a patched membrane for a given condition was calculated by dividing the number of seals containing at least one active channel for that condition by the total number of gigaohm seals formed under that condition.
Urine and blood (on 8 U heparin/500 μl serum) samples were collected directly from the bladder and heart, respectively, immediately after mice were killed. Heparinized blood samples were centrifuged at 1000 rpm for 40 minutes to separate plasma from blood cells. Urinary and plasma Na+ concentrations were quantified in fresh samples using a PFP7 flame photometer (Techne, Burlington, NJ). Relative Na+ excretion (UNa/UCre) was calculated by dividing [UNa] (in mM) by [Ucre] (in mg/dl). Creatinine values were assessed with an improved Jaffe method using the QuantiCrom DICT-500 Kit (BioAssay Systems, Hayward, CA) following the manufacturer's protocol. In addition, urinary ATP levels were quantified using a luciferase bioluminescence assay (ATP Determination Kit A22066; Molecular Probes, Eugene, OR) following the manufacturer's instructions.
All summarized data are reported as mean ± SEM. Data from before and after treatment within the same experiment were compared with the paired t test. Data from different experiments were compared with a (two-tailed) t test or an one-way ANOVA using the Dunnett posttest comparing treatment groups with a single control group (Na+-free diet). P ≤ 0.05 considered significant. For presentation, current data from some cell-attached patches were subsequently software filtered at 50 Hz and slow baseline drifts were corrected.
DISCLOSURES
None.
Acknowledgments
This research was supported by National Institutes of Health grants R01DK59594 (to J.D.S.), R01DK56248, R01DK28602, and R01HL94728 (to V.V.), and R01GM66232 (to P.A.I.); American Heart Association (AHA) Establish Investigator Awards 0640054N (to J.D.S.) and 0640056N (to J.P.-P.); AHA Grant in Aid 10GRNT3440038 (to V.V.); AHA SDG2230391 (to O.P.); German Research Foundation (RI1535/3-1 and 3-2 to T.R.); a National Kidney Foundation Fellowship (to T.R.); and the Research Service of the Department of Veterans Affairs (to V.V.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Hummler E: Implication of ENaC in salt-sensitive hypertension. J Steroid Biochem Mol Biol 69: 385–390, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bonny O, Hummler E: Dysfunction of epithelial sodium transport: From human to mouse. Kidney Int 57: 1313–1318, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Hummler E, Horisberger JD: Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol 276: G567–G571, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Rossier BC, Pradervand S, Schild L, Hummler E: Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Garty H, Palmer LG: Epithelial sodium channels: Function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Lifton RP, Gharavi AG, Geller DS: Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Kemendy AE, Kleyman TR, Eaton DC: Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol 263: C825–C837, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG: Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J: Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity 6. J Am Soc Nephrol 20: 721–729, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V: Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH: Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA: The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215–222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH: Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(−)-cotransporter abundance: Role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez-Nunez D, Morales-Ruiz M, Leivas A, Hebert SC, Poch E: In vitro characterization of aldosterone and cAMP effects in mouse distal convoluted tubule cells. Am J Physiol Renal Physiol 286: F936–F944, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Tiwari S, Packer RK, Hu X, Sugimura Y, Verbalis JG, Ecelbarger CA: Increased renal alpha-ENaC and NCC abundance and elevated blood pressure are independent of hyperaldosteronism in vasopressin escape. Am J Physiol Renal Physiol 291: F49–F57, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Carey RM, Douglas JG, Schweikert JR, Liddle GW: The syndrome of essential hypertension and suppressed plasma renin activity: Normalization of blood pressure with spironolactone. Arch Intern Med 130: 849–854, 1972 [PubMed] [Google Scholar]
- 18. Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ: Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83: 969–978, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC: A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci U S A 92: 5699–5703, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Relman AS, Schwartz WB: The effect of DOCA on electrolyte balance in normal man and its relation to sodium chloride intake. Yale J Biol Med 24: 540–558, 1952 [PMC free article] [PubMed] [Google Scholar]
- 21. Schrier RW: Aldosterone ‘escape’ vs ‘breakthrough.’ Nat Rev Nephrol 6: 61, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Endocrine Society: Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 93: 3266–3281, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Young WF: Primary aldosteronism: Renaissance of a syndrome. Clin Endocrinol 66: 607–618, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Young WF, Jr: Primary aldosteronism: A common and curable form of hypertension. Cardiol Rev 7: 207–214, 1999 [PubMed] [Google Scholar]
- 25. Hall JE, Granger JP, Smith MJ, Jr, Premen AJ: Role of renal hemodynamics and arterial pressure in aldosterone “escape.” Hypertension 6: I183–I192, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Knox FG, Burnett JC, Jr, Kohan DE, Spielman WS, Strand JC: Escape from the sodium-retaining effects of mineralocorticoids. Kidney Int 17: 263–276, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Haas JA, Berndt TJ, Youngberg SP, Knox FG: Collecting duct sodium reabsorption in deoxycorticosterone-treated rats. J Clin Invest 63: 211–214, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD: Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V: Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Vallon V: P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Rieg T, Vallon V: ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Unwin RJ, Bailey MA, Burnstock G: Purinergic signaling along the renal tubule: The current state of play. News Physiol Sci 18: 237–241, 2003 [DOI] [PubMed] [Google Scholar]
- 33. McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J: Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J: Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD: Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Bae EH, Cho HJ, Kim IJ, Joo SY, Shin JH, Ma SK, Lee J, Kim SW: Altered regulation of renal nitric oxide, atrial natriuretic peptide and cyclooxygenase systems in aldosterone escape in rats. Regul Pept 159: 117–122, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Burnstock G: Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA: Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch 455: 1105–1117, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J: Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Praetorius HA, Spring KR: A physiological view of the primary cilium. Annu Rev Physiol 67: 515–529, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Vekaria RM, Unwin RJ, Shirley DG: Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ: Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 43. Sakmann B, Neher E: Single-Channel Recording, New York, Plenum Press, 1983 [Google Scholar]