Abstract
Production of anti-dsDNA antibodies is a hallmark of lupus nephritis, but how these antibodies deposit in organs and elicit inflammatory damage remains unknown. In this study, we sought to identify antigens on the surface of human mesangial cells (HMC) that mediate the binding of human anti-dsDNA antibodies and the subsequent pathogenic processes. We isolated anti-dsDNA antibodies from patients with lupus nephritis by affinity chromatography. We used multiple methods to identify and characterize antigens from the plasma membrane fraction of mesangial cells that crossreacted with the anti-dsDNA antibodies. We found that annexin II mediated the binding of anti-dsDNA antibodies to HMC. After binding to the mesangial cell surface, anti-dsDNA antibodies were internalized into the cytoplasm and nucleus. This also led to induction of IL-6 secretion and annexin II synthesis, mediated through activation of p38 MAPK, JNK, and AKT. Binding of anti-dsDNA antibodies to annexin II correlated with disease activity in human lupus nephritis. Glomerular expression of annexin II correlated with the severity of nephritis, and annexin II colocalized with IgG and C3 deposits in both human and murine lupus nephritis. Gene silencing of annexin II in HMC reduced binding of anti-dsDNA antibody and partially decreased IL-6 secretion. In summary, our data demonstrate that annexin II mediates the binding of anti-dsDNA antibodies to mesangial cells, contributing to the pathogenesis of lupus nephritis. This interaction provides a potential target for therapeutic intervention.
SLE is a prototype autoimmune disease characterized by a loss of immunologic self-tolerance and the production of auto-antibodies against self antigens. Whereas the disease is not organ-specific, kidney involvement is common and is a leading cause of acute or chronic renal failure. Lupus nephritis is characterized by the deposition of auto-antibodies in the mesangial area and along the glomerular basement membrane, complement activation, and the local production of mediators that initiate inflammation and fibrosis.1–4 Histologic features include mesangial cell proliferation, inflammatory cell infiltration, damage and replacement of the normal kidney parenchyma with extracellular matrix, and sclerosis.1,5 Abnormalities in the mesangial area precede lesions in the glomerular capillary loop.5 Whereas the levels of anti-double-stranded (ds) DNA antibodies often correlate with disease activity, their role in pathogenesis remains obscure. Pathogenicity of anti-dsDNA antibodies has been associated with crossreactivity to cell surface antigens or extracellular matrix components,6–10 but the pathogenic relevance of these putative antigens remains unproven in human lupus.
IL-6 is a pleiotropic cytokine produced by T and B lymphocytes, monocytes, mesangial cells, endothelial cells, and fibroblasts in response to trauma, infection, and inflammation.11 It amplifies inflammatory responses through induction of lymphocyte activation and differentiation.12,13 The animal data show that IL-6 stimulates the production of anti-DNA antibodies and exacerbates glomerulonephritis,14,15 whereas interruption of IL-6 signaling could prevent kidney disease.16 Glomerular IL-6 expression is increased in lupus nephritis, and IL-6 levels correlate with nephritic flares.17–19 Recent data on the activation of the IFN-inducible gene Ifi202 by IL-6 also highlight its role in lupus pathogenesis.20 The role of resident kidney cells in IL-6 production in lupus nephritis remains unclear.
We have previously shown that anti-dsDNA antibodies from patients with lupus nephritis could bind to human mesangial cells (HMC)21 and that such binding correlated with disease activity and resulted in the induction of inflammatory cytokines.4,21 In this study we sought to identify the molecule(s) that mediated the binding of human anti-dsDNA antibodies to HMC and to examine the subsequent cellular changes pertaining to pathogenesis, focusing on IL-6 secretion by HMC.
RESULTS
Binding of Human Anti-dsDNA Antibodies to Mesangial Cells
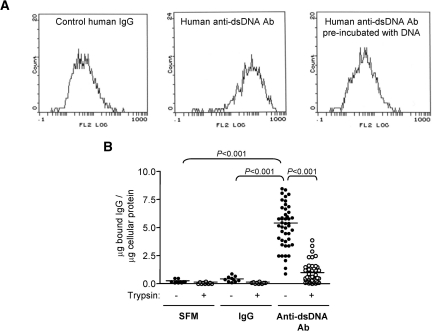
Polyclonal anti-dsDNA antibodies were isolated from 46 serum samples from 32 patients that demonstrated significant IgG binding to HMC as determined by cellular ELISA. The binding of these auto-antibodies to HMC was confirmed by flow cytometry (Figure 1A) and was unaffected by DNase treatment or addition of exogenous DNA.21 Limited trypsinization of HMC significantly reduced anti-dsDNA antibody binding (5.13 ± 1.9 versus 0.96 ± 0.93 μg of bound IgG/μg of cellular protein for anti-dsDNA antibody binding before and after limited trypsin treatment; P < 0.001) (Figure 1B). These data suggest that anti-dsDNA antibodies bind directly to HMC membrane antigen(s) and not through DNA on the cell surface.
Figure 1.
Anti-dsDNA antibodies bind to HMC. (A) Flow cytometric histograms of HMC that have been incubated with control human IgG (left panel) or human polyclonal anti-dsDNA antibodies (middle panel). Preincubation of anti-dsDNA antibodies with exogenous DNA (1 μg/ml) before their addition to HMC inhibited their binding to HMC (right panel). (B) Binding of SFM, control human IgG, or anti-dsDNA antibodies to HMC in cellular ELISA before and after removal of cell surface proteins with limited trypsinization (10 μg/ml). The amount of IgG bound to HMC was expressed as μg of bound IgG/μg of cellular protein.21 The horizontal line represents the mean μg of bound IgG/μg of cellular protein for each group.
Annexin II Mediates Anti-dsDNA Antibody Binding to Mesangial Cells
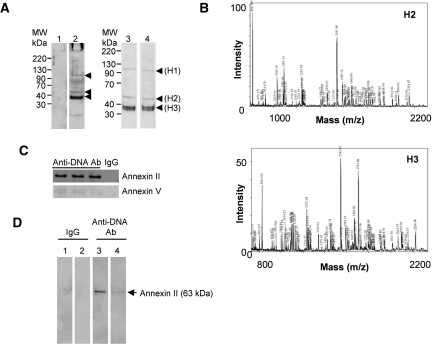
Anti-dsDNA antibodies, but not isotype-matched normal IgG, bound to three proteins bands in the plasma membrane fraction of HMC, including one prominent band with a molecular mass of 36 to 38 kD (denoted as H3) and two minor bands with molecular masses 100 to 110 kD and approximately 55 kD (denoted as H1 and H2 respectively) (Figure 2A). Mass spectrometry identified H2 as annexin II and H3 as annexin II/V (Figure 2B, Tables 1 and 2), whereas the minor band H1 could not be fully identified.
Figure 2.
Anti-dsDNA antibodies bind to annexin II on the surface of HMC. (A) Plasma membrane proteins from HMC were subjected to Western blot and probed with control IgG (lane 1) and three different human anti-dsDNA antibodies (lanes 2 through 4). Three bands (H1, H2, and H3) showed binding with anti-dsDNA antibodies but not control IgG. (B) MALDI-TOF spectra obtained from proteins H2 (upper panel) and H3 (lower panel) after trypsin digestion and tryptic peptide sequencing show that both proteins contain annexin II with a minor quantity of annexin V in H3. (C) Plasma membrane proteins were immunoprecipitated with either monoclonal anti-annexin II (upper panel) or polyclonal goat anti-annexin V antibodies (lower panel), subjected to Western blotting, and then probed with control human IgG or three different anti-dsDNA antibodies. The results demonstrated a high reactivity of anti-dsDNA antibodies to annexin II. (D) DNA-free human recombinant annexin II was subjected to Western blot analysis and probed with control IgG (lane 1), control IgG preincubated with recombinant annexin II (lane 2), anti-dsDNA antibody (lane 3), or anti-dsDNA antibody preincubated with human recombinant annexin II (lane 4). One representative blot is shown.
Table 1.
Tryptic peptide sequences from annexin II-matching peaks of MALDI-TOF spectra obtained from protein H2
| Mass (m/z) | Peptide Sequence | Start | End |
|---|---|---|---|
| 826.39 | (K) VFDRYK (S) | 246 | 251 |
| 879.37 | (R) DLYDAGVK (R) | 215 | 222 |
| 1035.19 | (K) WISIMTER (S) | 231 | 238 |
| 1085.46 | (K) AYTNFDAER (D) | 47 | 55 |
| 1110.53 | (R) QDIAFAYQR (R) | 87 | 95 |
| 1221.55 | (K) TPAQYDASELK (A) | 123 | 133 |
| 1243.58 | (R) TNQELQEINR (V) | 154 | 163 |
| 1266.62 | (R) QDIAFAYQRR (T) | 87 | 96 |
| 1406.72 | (R) IMVSRSEVDMLK (I) | 309 | 320 |
| 1420.68 | (K) SLYYYIQQDTK (G) | 332 | 342 |
| 1459.67 | (K) SYSPYDMLESIR (K) | 252 | 263 |
| 1541.84 | (K) GVDEVTIVNILTNR (S) | 68 | 81 |
| 1587.76 | (K) SYSPYDMLESIRK (E) | 252 | 264 |
| 1633.84 | (K) GTDVPKWISIMTER (S) | 225 | 238 |
| 1639.76 | (K) TPAQYDASELKASMK (G) | 123 | 137 |
| 1649.92 | (K) SALSGHLETVILGLLK (T) | 107 | 122 |
| 1666.83 | (R) SNAQRQDIAFAYQR (R) | 82 | 95 |
| 1719.86 | (K) GLGTDEDDLIEIICSR (T) | 138 | 153 |
| 1770.98 | (K) TKGVDEVTIVNILTNR (S) | 66 | 81 |
| 1810.84 | (K) TDLEKDIISDTSGDFR (K) | 171 | 186 |
| 1843.91 | (K) LSLEGDHSTPPSAYGSVK (A) | 29 | 46 |
| 1907.89 | (R) AEDGSVIDYELIDQDAR (D) | 198 | 214 |
| 2012.00 | (K) SLYYYIQQDTKGDYQK (A) | 332 | 347 |
| 2064.01 | (R) RAEDGSVIDYELIDQDAR (D) | 197 | 214 |
| 2154.07 | (K) AYTNFDAERDALNIETAIK (T) | 47 | 65 |
Table 2.
Tryptic peptide sequences from annexin II-matching peaks of MALDI-TOF spectra obtained from protein H3
| Mass (m/z) | Peptide Sequence | Start | End |
|---|---|---|---|
| 743.37 | (R) DKVLIR (I) | 285 | 290 |
| 826.43 | (K) VFDRYK (S) | 228 | 233 |
| 1085.46 | (K) AYTNFDAER (D) | 29 | 37 |
| 1091.59 | (R) SEVDMLKIR (S) | 296 | 304 |
| 1110.53 | (R) QDIAFAYQR (R) | 69 | 77 |
| 1221.61 | (K) TPAQYDASELK (A) | 105 | 115 |
| 1243.61 | (R) TNQELQEINR (V) | 136 | 145 |
| 1266.64 | (R) QDIAFAYQRR (T) | 69 | 78 |
| 1406.70 | (R) IMVSRSEVDMLK (I) | 291 | 302 |
| 1420.69 | (K) SLYYYIQQDTK (G) | 314 | 324 |
| 1459.68 | (K) SYSPYDMLESIR (K) | 234 | 245 |
| 1541.85 | (K) GVDEVTIVNILTNR (S) | 50 | 63 |
| 1587.76 | (K) SYSPYDMLESIRK (E) | 234 | 246 |
| 1649.91 | (K) SALSGHLETVILGLLK (T) | 89 | 104 |
| 1666.83 | (R) SNAQRQDIAFAYQR (R) | 64 | 77 |
| 1719.85 | (K) GLGTDEDSLIEIICSR (T) | 120 | 135 |
| 1771.00 | (K) TKGVDEVTIVNILTNR (S) | 48 | 63 |
| 1810.86 | (K) TDLEKDIISDTSGDFR (K) | 153 | 168 |
| 1843.90 | (K) LSLEGDHSTPPSAYGSVK (A) | 11 | 28 |
| 1907.86 | (R) AEDGSVIDYELIDQDAR (D) | 180 | 196 |
| 2011.93 | (K) SLYYYIQQDTKGDYQK (A) | 314 | 329 |
| 2063.97 | (R) RAEDGSVIDYELIDQDAR (D) | 179 | 196 |
| 2154.07 | (K) AYTNFDAERDALNIETAIK (T) | 29 | 47 |
Annexins II and V from the HMC plasma membrane fraction were then immunoprecipitated with commercially available antibodies, and the immunoprecipitants were subjected to SDS-PAGE and immunoblotting with anti-dsDNA antibodies from lupus patients, which showed significant binding to annexin II but minimal binding to annexin V (Figure 2C). To confirm that anti-dsDNA antibodies bound to annexin II, aliquots of full-length DNA-free recombinant annexin II were subjected to Western blot and immunoblotted with normal human IgG control or anti-dsDNA antibodies. The results showed that anti-dsDNA antibodies, but not control IgG, bound to recombinant annexin II (Figure 2D, lanes 3 and 1, respectively). The specificity of binding was confirmed by preincubation of anti-dsDNA antibodies with recombinant annexin II, which reduced their subsequent binding to annexin II on nitrocellulose membrane in immunoblotting experiments (Figure 2D, lane 4).
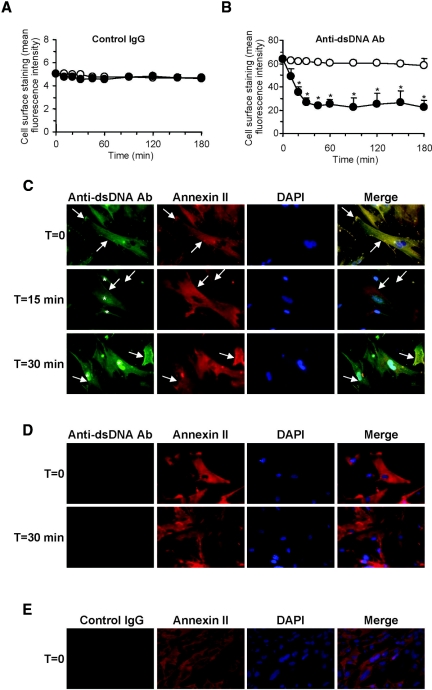
Flow cytometry showed significant cell surface binding of anti-dsDNA antibodies to live HMC (mean fluorescence intensity 68.9 ± 5.8%, compared with 5.01 ± 1.2% for normal IgG) (Figure 3, A and B), and the binding colocalized with cell surface annexin II (Figure 3C). Internalization of cell membrane-bound anti-dsDNA antibodies was evident by the progressive reduction of cell surface fluorescence intensity (maximum reduction after 30 to 45 minutes) and their incorporation into the cytoplasm and nucleus (Figure 3, B and C). Of the 15 polyclonal anti-dsDNA antibodies tested (12 isolated during active disease and three during remission), three localized solely in the cytoplasm, and 12 (80%) showed predominantly intranuclear localization. The internalization process was time- and temperature-dependent and was reduced when the temperature was maintained at 4°C (Figure 3, A and B). Prior incubation of anti-dsDNA antibodies with recombinant human annexin II inhibited the subsequent binding and internalization of the antibodies by HMC (Figure 3D). Control IgG did not show significant binding to HMC (Figure 3, A and E). 4′,6-Diamidino-2-phenylindole (DAPI) counterstaining confirmed the absence of DNA on the HMC surface (Figure 3, C through E); thus these antibodies did not bind via chromatin material.
Figure 3.
Binding of anti-dsDNA antibodies to annexin II on HMC mediates antibody internalization. (A and B) Cell surface staining of control human IgG (A) and anti-dsDNA antibodies (B) to HMC with time at either 4°C (○) or 37°C (●) as detected by flow cytometry. *P < 0.01 for 4°C versus 37°C. Reduced cell surface staining of anti-dsDNA antibodies on HMC was observed in cells incubated at 37°C but not at 4°C (B). (C) Representative images depicting colocalization of anti-dsDNA antibodies with cell surface annexin II expression at T = 0 (arrow, denoted as yellow staining in merged images), followed by internalization of anti-dsDNA antibodies to the nuclei within 30 minutes (depicted by asterisk). (D) Parallel studies were conducted in which anti-dsDNA antibodies were incubated with recombinant human annexin II before incubation with HMC. These representative images show no significant binding of these anti-dsDNA antibodies to cells and no antibody internalization after 30 minutes. (E) Representative images showing HMC incubated with control IgG. The nuclei are stained with DAPI. Magnification, ×400 for all.
Annexin II Binding Activity in Serum and Anti-dsDNA Antibodies from Patients with Lupus Nephritis
The annexin II-binding activity of immunoglobulins in the sera or polyclonal anti-dsDNA antibodies from patients with lupus nephritis was examined.
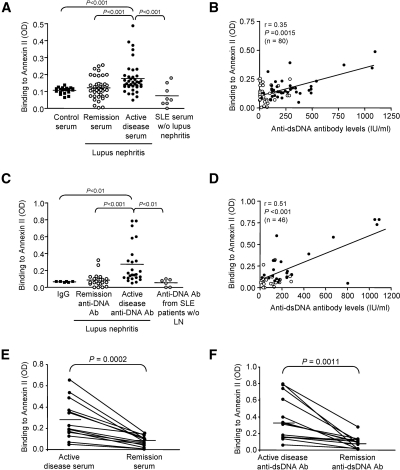
Twenty-six of 80 serum samples (comprising 17 of 39 patients with active lupus nephritis and nine of 41 patients with quiescent disease) showed significant IgG binding to recombinant human annexin II by ELISA. The mean ± SD OD values for annexin II binding by control sera, remission sera, and active disease sera were 0.106 ± 0.018, 0.121 ± 0.062, and 0.176 ± 0.092, respectively (P < 0.001, active disease versus control and remission samples) (Figure 4A), and the binding in lupus sera correlated with the level of anti-dsDNA antibodies (Figure 4B).
Figure 4.
Serum immunoglobulins and anti-dsDNA antibodies from patients with lupus nephritis bind to annexin II. (A) IgG binding activity to annexin II assessed by ELISA in serum samples from patients with lupus nephritis during remission or active disease, patients with nonrenal lupus, or healthy controls. The values represent the mean OD for triplicate wells. The horizontal line in each group represents the mean value. (B) Serum annexin II-binding activity correlated with circulating anti-dsDNA antibody levels in lupus nephritis patients with active disease (●) or remission (○). The P values refer to pooled data. (C) Control IgG or anti-dsDNA antibodies from patients with lupus nephritis during remission or active disease or patients with nonrenal lupus were also tested for their binding to annexin II by ELISA. The values represent the mean OD for triplicate wells. The horizontal line in each group represents mean OD for annexin II binding. (D) Annexin II-binding activity in anti-dsDNA antibody preparations from lupus nephritis patients with active disease (●) or remission (○) correlated with levels of circulating anti-dsDNA antibodies. P values refer to pooled data. (E) Annexin II-binding activity in 14 paired serum samples from patients with lupus nephritis. (F) Annexin II-binding activity in 14 paired anti-dsDNA antibody preparations isolated from patients with lupus nephritis.
Twenty-two (88.0%) of 25 anti-dsDNA antibody preparations obtained during flares and eight (38.1%) of 21 anti-dsDNA antibodies obtained during remission showed significant binding to annexin II. The mean ± SD OD values for annexin II binding by control IgG, remission anti-DNA antibody (Ab), and active nephritis anti-DNA Ab were 0.066 ± 0.005, 0.086 ± 0.078, and 0.273 ± 0.236, respectively (P < 0.01 and P < 0.001, active disease anti-DNA Ab versus control IgG and remission anti-DNA Ab, respectively) (Figure 4C), and the binding correlated with anti-dsDNA antibody levels (Figure 4D). Annexin II binding activity in serum samples and anti-dsDNA antibody preparations from patients with active lupus nephritis was significantly higher than that in nonrenal lupus patients (P < 0.001 and P < 0.01 respectively) (Figure 4, A and C).
Twenty-eight paired samples were obtained from 14 patients with lupus nephritis. Annexin II binding by serum Ig or anti-dsDNA antibodies correlated with disease activity (OD values of 0.28 ± 0.18 and 0.07 ± 0.05 for active disease sera and remission sera, respectively, P = 0.0002; OD values of 0.36 ± 0.26 and 0.08 ± 0.07 for active disease and remission anti-dsDNA antibodies, respectively, P = 0.0011) (Figure 4, E and F).
Annexin II Expression in Kidney Tissue with Human or Murine Lupus Nephritis
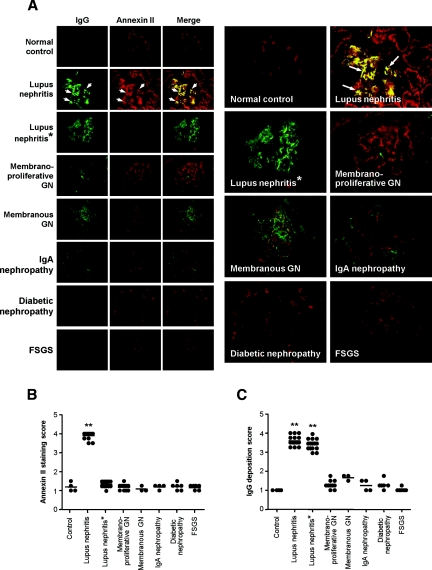
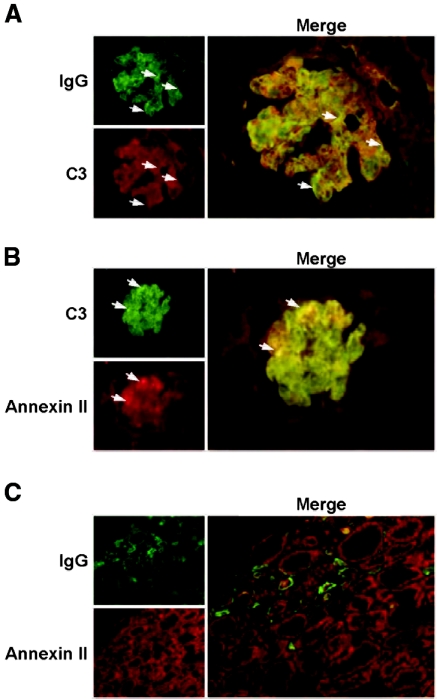
Constitutive expression of annexin II was observed in the glomerulus of normal kidney (Figure 5A). Glomerular and tubulointerstitial annexin II expression was markedly upregulated in lupus nephritis (Figure 5, A and B). Annexin II colocalized with IgG and C3 deposition in the glomeruli of lupus nephritis (Figure 5, A through C, Figure 6, A and B), and the colocalization was not disrupted when subjected to an acidic (HCl) environment, denaturing conditions, or high temperature (55°C). A small number of the lupus nephritis renal biopsies examined also showed tubular IgG deposition, but this did not colocalize with annexin II, which showed a patchy tubular distribution (Figure 6C). Renal biopsies from nonlupus renal diseases did not show increased annexin II expression compared with normal renal specimens (Figure 5, A and B).
Figure 5.
Annexin II expression is increased in renal biopsies from patients with lupus nephritis and colocalizes with IgG deposition. (A) Representative images of IgG deposition and annexin II expression in healthy kidney (top row), lupus nephritis (second and third rows), or nonlupus glomerular diseases (rows 4 through 8). Lupus nephritis* (third row) shows representative images whereby anti-human annexin II antibody was incubated with recombinant human annexin II before use. Enlarged images of the Merge column are shown in the two columns on the right. The arrows depict colocalization of IgG and annexin II, visualized as yellow staining in the Merge column. GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis. (B) Glomerular staining score for annexin II in renal specimens. (C) Glomerular staining score for IgG deposition in renal specimens. The horizontal line in each group represents the mean staining score. **P < 0.001 compared with nonlupus glomerular diseases.
Figure 6.
Annexin II expression colocalizes with glomerular IgG and C3 deposition in renal biopsies from patients with lupus nephritis. (A) Representative images showing IgG and C3 deposition in the glomerulus. The arrows depict colocalization of IgG and C3. (B) Representative images of annexin II expression and C3 deposition in the glomerulus. The arrows depict colocalization of C3 and annexin II. (C) Representative images of annexin II and IgG deposition in the tubulointerstitium. Original magnification, ×400 for all.
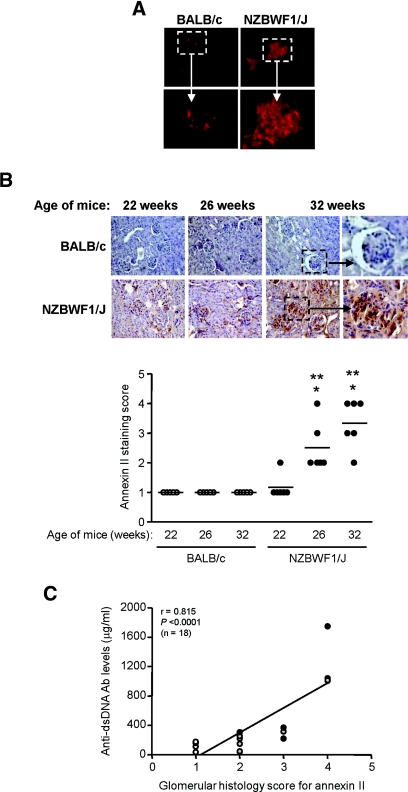
In NZBWF1/J mouse increased glomerular annexin II expression was noted before the development of proteinuria or abnormal kidney function (Figure 7A) and progressively increased as nephritis worsened and the level of anti-dsDNA antibodies increased (Figure 7, B and C). These were not seen in age-matched BALB/c mice controls (annexin II staining scores of 1.00 ± 0.00 and 3.30 ± 0.82 for BALB/c and NZBWF1/J mice at 32 weeks of age, respectively; P < 0.001) (Figure 7B).
Figure 7.
Annexin II expression in the kidneys of NZBWF1/J mice increases with progression of nephritis. (A) Annexin II expression in snap-frozen kidney sections of nonautoimmune BALB/c and NZBWF1/J mice before the onset of nephritis (top panels). Original magnification, ×400. The enlargements of the boxed areas show increased annexin II expression in predisease NZBWF1/J mice compared with BALB/c mice. (B) Expression of annexin II in paraffin renal sections obtained from BALB/c and NZBWF1/J mice at 22, 26, and 32 weeks of age. Annexin II expression increased progressively as nephritis progressed in NZBWF1/J mice, in parallel with the severity of proteinuria and anti-DNA antibody production. No significant change was observed in annexin II expression in the kidneys of the BALB/c mice. The bottom panel shows annexin II staining scores in BALB/c and NZBWF1/J mice. **P < 0.001 for age-matched NZBWF1/J mice versus BALB/c mice; *P < 0.01 for NZBWF1/J mice at 22 weeks versus NZBWF1/J mice at 26 or 32 weeks. (C) Glomerular staining score for annexin II in NZBWF1/J mice at 22 (white circle), 26 (gray circle), and 32 (black circle) weeks correlated with circulating anti-DNA antibody titers.
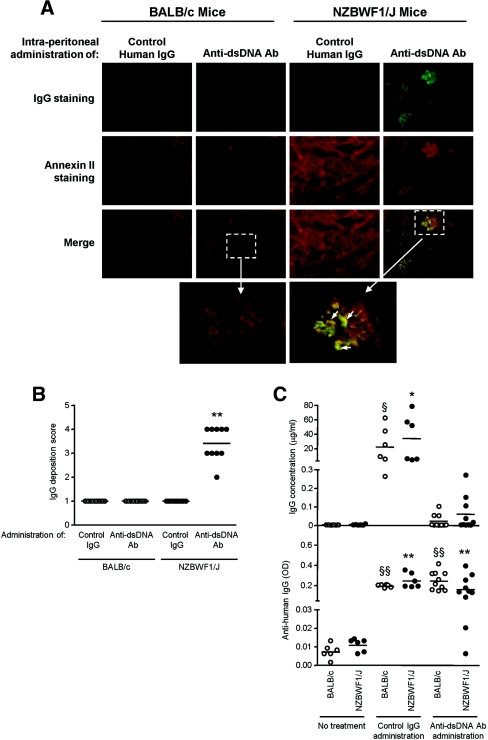
When administered intraperitoneally to predisease NZBWF1/J mice, anti-dsDNA antibodies, but not control IgG, deposited in the glomerulus (semiquantitative scores for IgG staining of 1.00 ± 0.00 and 3.40 ± 0.69 for control IgG and anti-dsDNA antibody, respectively) and colocalized with annexin II (Figure 8, A and B). BALB/c mice administered anti-dsDNA antibodies did not show these changes (Figure 8, A and B), although both the BALB/c mice and the NZBWF1/J mice showed anti-human antibody response after the injection of human immunoglobulins (Figure 8C).
Figure 8.
Human polyclonal anti-dsDNA antibodies deposit in the glomeruli of NZBWF1/J mice and colocalize with annexin II expression. (A) Human polyclonal anti-dsDNA antibodies or normal human IgG (100 μg) were injected intraperitoneally into NZBWF1/J mice or BALB/c mice on 10 consecutive days. Kidney sections were snap frozen, and IgG/annexin colocalization was assessed by immunohistochemical staining. Representative images are presented at magnification, ×400. The enlargements of the boxed area show colocalization of anti-dsDNA antibodies and annexin II in glomeruli from NZBWF1/J mice but not in nonautoimmune mice. The arrows depict areas of colocalization. (B) Glomerular staining score for IgG deposition in renal specimens obtained from BALB/c and NZBWF1/J mice. The horizontal line in each group represents the mean staining score. **P < 0.001 for anti-dsDNA Ab injected into NZBWF1/J mice versus control IgG injected into NZBWF1/J or BALB/c mice or anti-DNA Ab injected into BALB/c mice. (C) Measurement of human IgG concentration (upper panel) and mouse anti-human IgG levels (expressed as OD) (lower panel) in serum samples from BALB/c or predisease NZBWF1/J mice after the last injection of either control human IgG or anti-dsDNA antibodies. Negative controls include sera from aged-matched BALB/c and NZBWF1/J mice without Ig injection (n = 6 for each group). The horizontal line in each group represents the mean level. **P < 0.001 for no treatment versus control IgG or anti-dsDNA Ab administration in NZBWF1/J mice; §§P < 0.001 for no treatment versus control IgG or anti-dsDNA Ab administration in BALB/c mice; *P < 0.01 for no treatment versus control IgG injection in NZBWF1/J mice; §P < 0.01 for no treatment versus control IgG injection in BALB/c mice.
Anti-dsDNA Antibody Binding Induces Annexin II and IL-6 in Mesangial Cells
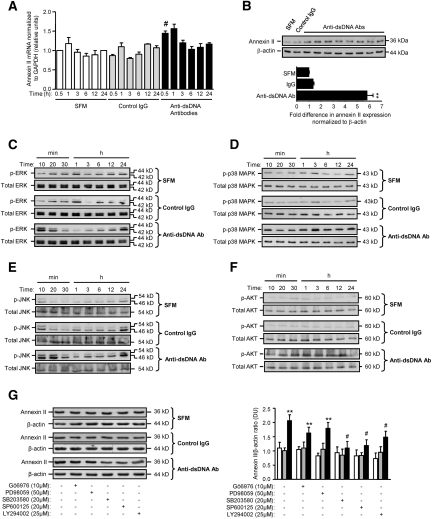
Incubation of HMC with anti-dsDNA antibodies induced 1.46- and 5.8-fold increases in annexin II mRNA and protein expression, respectively (P < 0.05 for anti-dsDNA antibodies versus serum-free medium [SFM] or control IgG with regard to mRNA expression, and P < 0.001 for anti-dsDNA antibodies versus SFM or control IgG with regard to protein expression) (Figure 9, A and B). Whereas anti-dsDNA antibodies induced a moderate increase in annexin II mRNA in HMC, this increase was transient (Figure 9A).
Figure 9.
Anti-dsDNA antibodies induce annexin II and ERK, p38 MAPK, JNK, and AKT activation in HMC. (A) Annexin II transcript in HMC incubated with SFM, control IgG, or six different human polyclonal anti-dsDNA antibodies as determined by real-time PCR. #P < 0.05 for anti-dsDNA Ab versus SFM or control IgG after 30 minutes of incubation. (B) Western blot showing the effect of SFM, control human IgG, or eight different human polyclonal anti-dsDNA antibody preparations on annexin II expression in total mesangial cell lysate (upper panel). The ECL signal was semiquantified by gel documentation, normalized to β-actin, and expressed as the mean ± SD fold difference (lower panel) compared with SFM or control IgG. **P < 0.001 for anti-dsDNA Ab versus SFM or control IgG. (C through F) Representative Western blots showing the effect of SFM, control IgG, and anti-dsDNA antibodies on ERK activation (p-ERK) (C), p38 MAPK activation (p-p38 MAPK) (D), JNK activation (p-JNK) (E), and AKT activation (p-AKT) (F). (G) Representative Western blot (left panel) showing the effects of specific inhibitors to PKC, ERK, p38 MAPK, JNK, and PI3K on annexin II synthesis in HMC incubated with SFM (white bars), control IgG (gray bars), and anti-dsDNA antibodies (black bars). The ECL signal was semiquantified by gel documentation, normalized to β-actin, and expressed as the mean ± SD fold difference compared with SFM (right panel). **P < 0.001 for anti-dsDNA Ab versus SFM or control IgG for the same treatment; #P < 0.05, with versus without inhibitor.
Anti-dsDNA antibodies differentially activated extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and AKT in HMC compared with SFM or control IgG (Figure 9, C through F). Activation of ERK and JNK was biphasic, with maximum induction occurring 10 to 20 minutes and 24 hours after antibody stimulation (P < 0.001 and P < 0.05 for anti-dsDNA antibodies versus SFM or control IgG for 10 to 20 minutes and 24 hours, respectively, for both ERK and JNK). Activation of p38 MAPK occurred within 10 minutes of anti-dsDNA antibody stimulation and was sustained for up to 24 hours (P < 0.01 for anti-dsDNA antibodies versus SFM or control IgG for all time points). In comparison, AKT was activated 30 minutes after stimulation with anti-dsDNA antibodies and was sustained for 12 hours (P < 0.01 for anti-dsDNA antibodies versus SFM or control IgG after 12 hours of incubation) (Figure 9, C through F). Inhibition of p38 MAPK, JNK, and phosphatidylinositol 3-kinase (PI3K) activation significantly reduced anti-dsDNA antibody induction of annexin II (P < 0.05 for all), whereas inhibition of PKC or ERK had no effect on annexin II synthesis (Figure 9G).
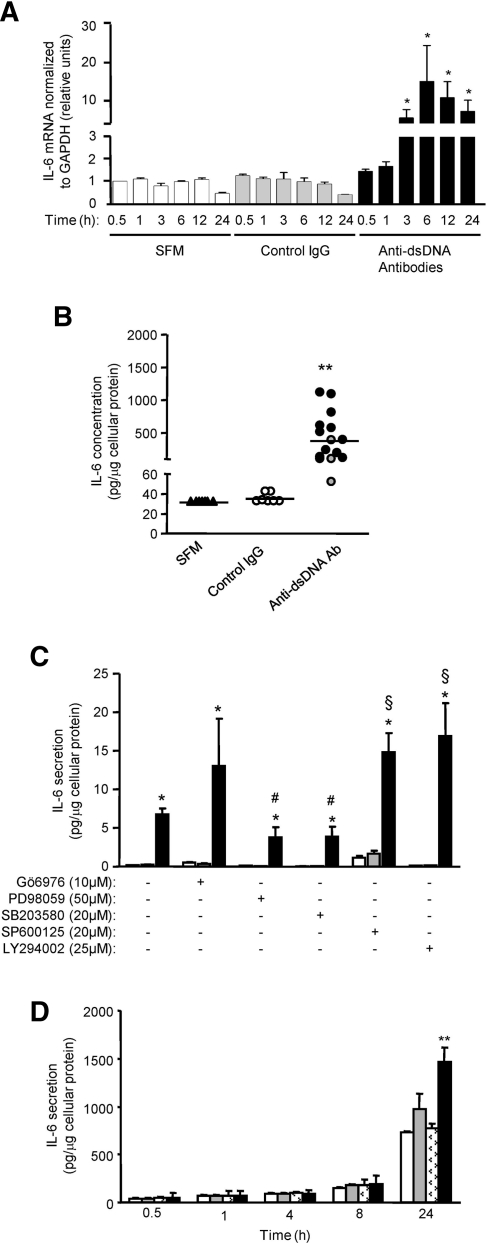
Anti-dsDNA antibodies also induced IL-6 mRNA (Figure 10A) and increased IL-6 secretion (30 ± 3, 36 ± 4, 228 ± 248, and 478 ± 367 pg/μg cellular protein for cells incubated with SFM, control IgG, anti-dsDNA antibodies with cytoplasmic localization, and anti-dsDNA antibodies with nuclear localization, respectively, P < 0.001 for both anti-dsDNA antibodies versus SFM or control IgG) (Figure 10B). Induction of IL-6 by anti-dsDNA antibodies was mediated through ERK and p38 MAPK activation. In contrast, inhibition of JNK and PI3K exacerbated IL-6 secretion by anti-dsDNA antibodies, whereas the inhibition of PKC had no effect on anti-dsDNA antibody induction of IL-6 secretion (Figure 10C). Crosslinking of cell surface annexin II with anti-annexin II antibody before adding anti-dsDNA antibodies resulted in enhanced IL-6 induction after 24 hours of incubation (730 ± 10, 920 ± 150, 770 ± 50, and 1480 ± 150 pg/μg cellular protein for anti-dsDNA antibodies alone, anti-annexin II antibody alone, control IgG before anti-dsDNA antibodies, and anti-annexin II before anti-dsDNA antibodies, respectively) (Figure 10D).
Figure 10.
Anti-dsDNA antibodies induce IL-6 secretion in HMC. (A) IL-6 transcript in HMC incubated with SFM, control IgG, or six different human polyclonal anti-dsDNA antibodies as assessed by real-time PCR. *P < 0.01 for anti-dsDNA Ab versus SFM or control IgG for same time point. (B) IL-6 secretion in HMC incubated with SFM, control IgG, and anti-dsDNA antibodies (encompasses anti-dsDNA antibodies that localize to the cytoplasm [gray circle] and nucleus [black circle]). The horizontal line in each group represents the mean IL-6 secretion. **P < 0.001 for SFM or control IgG versus anti-DNA Ab. (C) IL-6 secretion in HMC incubated with SFM (white bars), control IgG (gray bars), and anti-dsDNA antibodies (black bars) in the presence or absence of specific inhibitors to PKC, ERK, p38 MAPK, JNK, and PI3K. *P < 0.01 for anti-dsDNA antibody versus SFM or control IgG for the same group; #P < 0.05 or §P < 0.01 for with versus without inhibitor. (D) IL-6 secretion from HMC incubated with anti-dsDNA antibodies with (black bars) or without (white bars) prior crosslinking of cell surface annexin II or annexin II antibody (gray bars). Crosslinking of cell surface annexin II with annexin II Ab, but not control Ab (marked white bars), enhanced the subsequent induction of IL-6 by anti-dsDNA antibodies. The data are presented as the means ± SD. **P < 0.001, anti-dsDNA Ab alone, annexin II Ab alone, or control Ab versus cross-linking with annexin II Ab. The horizontal axis denotes the time of incubation with anti-dsDNA antibodies.
Effect of HMC Annexin II Knockdown with RNA Interference (RNAi)
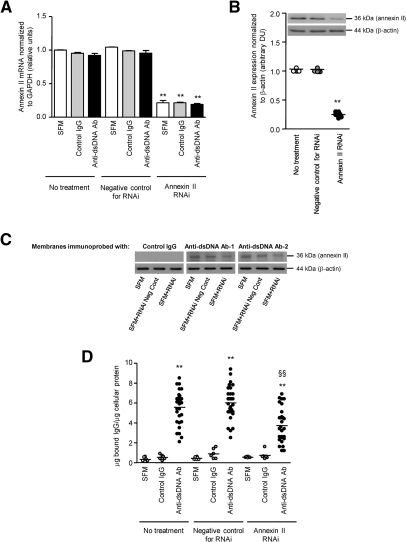
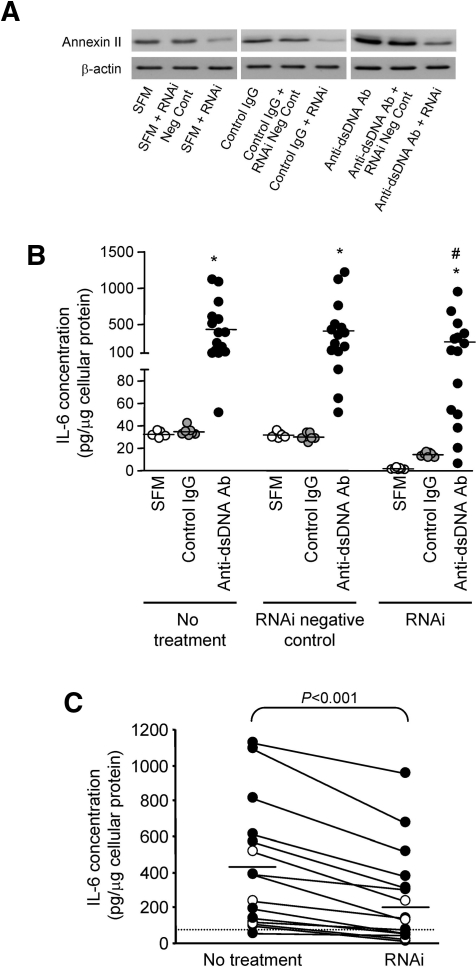
Gene silencing with annexin II-specific RNAi suppressed annexin II mRNA and constitutive protein expression in HMC by 78.5% (Figure 11A) and 74.8% (Figure 11B), respectively (P < 0.001 for anti-dsDNA antibodies versus SFM or control IgG for both mRNA and protein expression). The resultant reduced annexin II expression was associated with decreased binding by anti-dsDNA antibodies as shown in Western blot (Figure 11C), cellular ELISA (5.57 ± 1.67, 6.01 ± 1.63, and 3.73 ± 1.68 μg of bound IgG/μg of cellular protein for anti-dsDNA antibodies alone, anti-dsDNA antibodies with RNAi negative control, and anti-dsDNA antibodies with RNAi, respectively; P < 0.001 comparing RNAi with no treatment or RNAi negative control) (Figure 11D) and immunohistochemistry (Figure 12A). This was accompanied by a 74.8 ± 18.4% reduction in the number of cells showing anti-dsDNA antibody internalization (Figure 12B). Prior suppression of annexin II in HMC resulted in significant reduction of anti-dsDNA antibody induced increase in annexin II (Figure 13A) and IL-6 (428.2 ± 354.2, 406.2 ± 366.9, and 257.7 ± 274.6 pg/μg cellular protein for anti-dsDNA antibodies alone, anti-dsDNA antibodies with RNAi negative control, and anti-dsDNA antibodies with RNAi, respectively; P < 0.05 comparing RNAi versus no treatment or RNAi negative control) (Figure 13, B and C). IL-6 secretion after incubation with anti-dsDNA antibodies was reduced by 42% with prior RNAi treatment of HMC (P < 0.001).
Figure 11.
Suppression of annexin II expression in HMC by RNAi reduces anti-dsDNA binding. (A) Annexin II transcript in HMC incubated with SFM, control IgG, or seven different anti-dsDNA antibodies with or without prior RNAi treatment. **P < 0.001 for RNAi versus RNAi negative control or no treatment for the same stimulus. (B) Annexin II protein expression in HMC incubated with SFM (no treatment) with or without prior RNAi treatment. A representative Western blot is shown. The ECL signal was semiquantified by gel documentation, normalized to β-actin, and expressed as arbitrary densitometric units. **P < 0.001 for RNAi versus RNAi negative control or no treatment. (C) Cell lysates were extracted from transfected and nontransfected HMC, subjected to Western blot, and probed with control IgG and two different human anti-dsDNA antibodies. Annexin II suppression with RNAi resulted in decreased binding by anti-dsDNA antibodies compared with samples isolated from untreated (SFM) cells or those transfected with RNAi negative control. The samples were normalized to β-actin. (D) Binding of anti-dsDNA antibodies to HMC, with or without prior RNAi treatment, was assessed by cellular ELISA and expressed as μg of bound IgG/μg of cellular protein.21 The horizontal line in each group represents the mean level. **P < 0.001 for anti-dsDNA Ab versus SFM or control IgG for same treatment; §§P < 0.001 for anti-dsDNA Ab or anti-dsDNA Ab + RNAi negative control versus anti-DNA Ab + RNAi.
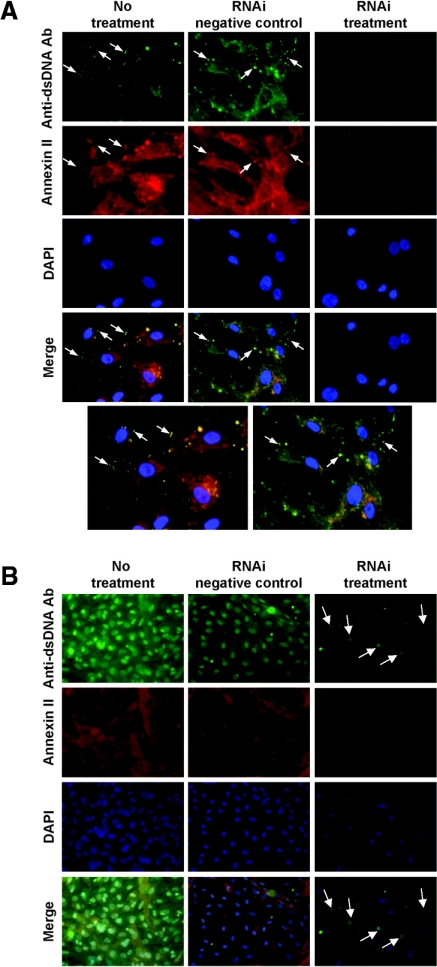
Figure 12.
Immunohistochemical staining showing reduced anti-dsDNA binding and internalization in HMC after suppression of annexin II expression with RNAi. (A) Suppression of annexin II synthesis by RNAi in HMC before their incubation with anti-dsDNA antibodies inhibited anti-dsDNA antibody binding to cells (images taken at T = 0). The arrows depict colocalization of anti-dsDNA antibodies and annexin II at the cell surface. The bottom panel shows enlargement of Merge images for anti-dsDNA Ab alone and anti-dsDNA Ab with RNAi negative control. The nuclei are stained with DAPI. Original magnification, ×400. (B) Suppression of annexin II synthesis by RNAi in HMC before their incubation with anti-dsDNA antibodies resulted in a significant reduction in anti-dsDNA internalization and their localization into the nucleus (arrows) (images taken after 30 minutes of incubation with anti-dsDNA antibodies). Original magnification, ×200.
Figure 13.
Suppression of annexin II expression in HMC by RNAi reduces anti-dsDNA antibody induction of annexin II synthesis and IL-6 secretion. (A) Whole cell lysates extracted from transfected and nontransfected HMC after 24 hours of incubation with SFM, control IgG, or anti-DNA antibodies were subjected to Western blot and probed with monoclonal anti-human annexin II normalized to β-actin. (B) IL-6 secretion was measured in HMC incubated with SFM, control IgG, or a panel of 15 different anti-dsDNA antibodies with or without annexin II gene silencing. Knockdown of annexin II expression was accompanied by reduced IL-6 induction in HMC stimulated with anti-dsDNA antibodies. The reduction in IL-6 stimulation applied to both anti-dsDNA antibodies obtained during remission (○) and those obtained during disease flares (●) as shown in C. The horizontal line in each group in B and C denotes the mean IL-6 level. (C) The dashed line represents the basal level of IL-6 secretion. *P < 0.01 for the comparison of samples within the same group; #P < 0.05 for RNAi versus no treatment or RNAi negative control.
DISCUSSION
Despite much circumstantial evidence that implicates a pathogenic role for anti-dsDNA antibodies, how these antibodies localize to the involved organs and induce damage remains obscure. Data from murine lupus suggested that murine anti-DNA antibodies could bind to nucleosomal or chromatin material entrapped in the glomerular basement membrane.22,23 There is also accumulating evidence that anti-dsDNA antibodies may crossreact with nonchromatin cell surface or extracellular antigens in various cell types.6,8–10,24–26 The putative crossreactive antigens include myosin, α-actinin, heparan sulfate, collagen, and laminin.6,8,9,26,27 However, the clinical pertinence of these data is hampered by the use of nonrenal cells and murine antibodies. In addition, there is no in vitro data on the functional consequence of antibody binding or its correlation with clinical disease.
In this study, we present novel data to show that anti-dsDNA antibodies bind to annexin II of mesangial cells to induce downstream inflammatory processes. Our data show that 65% of anti-dsDNA antibodies isolated from patients with lupus nephritis, and almost all of the antibody samples obtained during disease flares, showed significant binding to annexin II. The pathogenic importance of annexin II in lupus is corroborated by its upregulation in kidney tissue during lupus nephritis but not other renal diseases. Furthermore, annexin II colocalizes with immune deposits in the glomerulus in both human and murine lupus nephritis biopsies, thereby underscoring the pathogenic role of annexin II and its interaction with anti-dsDNA antibodies. Whereas it is not feasible to perform serial renal biopsies in humans, our murine data show that disease progression is accompanied by progressive upregulation of renal annexin II expression, to which anti-dsDNA antibodies localized. The spread of immune deposits from the mesangial area to the glomerular basement membrane in disease progression could result from injury to the normal glomerular architecture or possibly through a direct interaction between autoantibodies and basement membrane components.
Increased annexin II expression was also observed in the tubulointerstitium in both patients and NZBWF1/J mice with active lupus nephritis. Interestingly the tubulointerstitial annexin II expression did not colocalize with IgG deposition. Other investigators have reported that IL-6, IFN-γ, and vascular endothelial growth factor can upregulate annexin II synthesis in epithelial cells.28,29 These peptides play critical roles in the pathogenesis of lupus nephritis30–32 and are synthesized by both infiltrating and resident renal cells. Interstitial inflammation, which is commonly observed in lupus nephritis,33 may thus account for increased tubulointerstitial annexin II expression. Furthermore, annexin II has been implicated in the regulation of macrophage activation and its recognition of apoptotic T lymphocytes,34,35 thereby playing a role in tubulointerstital inflammation.
Data from immunohistochemical studies in cultured HMC and annexin II gene knockdown experiments provide confirmatory evidence that anti-dsDNA antibody binding to annexin II is prerequisite to the internalization of these antibodies and the subsequent cellular changes including IL-6 induction. The observed induction of annexin II synthesis by anti-dsDNA antibodies suggests potential amplification of immune-mediated inflammation, mediated through the activation of p38 MAPK, JNK, and PI3K/AKT. Whereas gene silencing of annexin II resulted in a significant reduction of anti-dsDNA antibody induced IL-6 secretion, our data suggest that IL-6 secretion may also occur through mechanisms independent of annexin II engagement with anti-dsDNA antibodies. Lupus flare is associated with increased serum IL-6 levels and increased glomerular IL-6 expression.18,19 Interruption of IL-6 signaling has been shown to prevent kidney disease in lupus mice.16 Annexin II may thus present a possible point for therapeutic intervention upstream of IL-6.
The time and temperature dependency suggest that the internalization of anti-dsDNA antibodies into mesangial cells is an active process. In this context, it is pertinent to note the role of annexin II in endocytosis and placental Ig transport.36 Annexin II is a 36-kD protein that can exist in the monomeric form or as a tetramer. It may be associated with the annexin II light chain (p11), a member of the S100 family. Annexins may undergo post-translational modifications such as alternative splicing, glycosylation, or phosphorylation.37,38 These modifications may account for the molecular-mass difference between H2 and H3, although this warrants further investigation. Crystal structure analysis has demonstrated 52 and 22% homology between annexins II and V in their core and N-terminal domains, respectively,39 and this may account for the identification of annexin V in H3. Whereas we were unable to characterize the minor cross-reactive antigen with a molecular mass of ∼100 kD, its molecular mass suggests the possibilities of α-actinin, myosin, or a tetramer of annexin II.
Our data show that the induction of IL-6 in HMC was more prominent with anti-dsDNA antibodies that show intranuclear localization. Lung cancer cells have been shown to highly express annexins I and II, and autoantibodies to annexins I and II have been detected in 60% of patients with adenocarcinoma and in 33% of patients with squamous cell lung carcinoma.40 This immune reactivity to tumor antigens was associated with increased serum IL-6 levels, which implicated an enhanced host immune responsiveness. In this context, it is pertinent to note that annexin II-derived peptides could bind to MHC class II molecules and be presented as antigenic stimuli, thereby eliciting an immune response.41 Further investigations along this line might unravel the basis for crossreactivity between anti-DNA antibodies and annexin II. Annexin II has also been implicated in the regulation of cell proliferation and differentiation, and increased annexin II has been associated with a proliferative response in hepatocytes and osteoclast precursors.42,43 Mesangial cell proliferation is common in lupus nephritis. Whether this can be attributed in part to annexin II induction by anti-DNA antibodies remains to be investigated. It is intriguing that in some patients anti-dsDNA antibodies obtained during apparent clinical remission could still bind to annexin II and induce cellular changes. This might suggest a state of ongoing subclinical inflammation that results in progressive organ damage, with important implications on the choice of maintenance immunosuppressive treatment.
In summary, our data show that the binding of human anti-dsDNA antibodies to mesangial cells is mediated via their direct interaction with annexin II and that this initiates the subsequent pathogenic processes of antibody internalization and the induction of inflammatory changes. Annexin II may therefore be a potential novel target for therapeutic intervention in lupus nephritis.
CONCISE METHODS
All chemicals were of the highest purity and were purchased from Sigma (Tin Hang Technology Ltd., Hong Kong, China) unless otherwise stated. Tissue culture flasks were purchased from Falcon (Becton Dickinson, Hong Kong, China). Culture media, supplements, Stealth RNAi specific for annexin II (Stealth Select HSS141224), Stealth RNAi negative control (Stealth Select Negative control Med GC), Lipofectamine 2000, and BLOCK-iT fluorescence Oligo were purchased from Invitrogen Life Technologies (Hong Kong, China). IL-6 ELISA kits were purchased from PharMingen (Bio-Gene, Hong Kong, China). Human anti-dsDNA antibody assay kits (Microplate autoimmune anti-dsDNA quantitative ELISA) were purchased from Bio-Rad (Hong Kong, China). Murine anti-dsDNA antibody ELISA kits were purchased from Alpha Diagnostic International (Onwon Trading Limited, Hong Kong, China). Human recombinant annexin II with a glutathione S-transferase tag (molecular mass, 63 kD) was purchased from Abnova (Onwon Trading Limited, Hong Kong, China). Mouse anti-human annexin II antibody, which reacts against the C-terminal region of the annexin II protein, was purchased from Zymed (Invitrogen Life Technologies, Hong Kong, China). Goat anti-human annexin II antibody, which reacts with the C-terminal region of human annexin II protein, was purchased from Santa Cruz (Gene Company Limited, Hong Kong, China). Inhibitors to PKC (Gö6976) and p38 MAPK (SB203580) were purchased from Merck (Onwon Trading Limited, Hong Kong, China), and JNK inhibitor (SP600125) was purchased from Sigma-Aldrich (Ting Hang Technology Limited, Hong Kong, China). ERK (PD98059) and PI3K (LY294002) inhibitors and antibodies against total and phosphorylated ERK, p38 MAPK, JNK, and AKT were purchased from Cell Signaling Technology (Gene Company Limited, Hong Kong, China). Protein G-Sepharose was purchased from GE Healthcare Life Sciences (Hong Kong, China). All of the clinical samples (renal biopsies and serum samples) from lupus patients were obtained with written informed consent with prior approval from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Patients and Polyclonal Anti-dsDNA Antibody Preparations
Serum samples from 80 patients with biopsy-proven diffuse proliferative lupus nephritis were screened for IgG binding activity to HMC by cellular ELISA. Thirty-nine serum samples were obtained during active nephritis, and 41 were obtained during remission. All nephritic flares (active disease) were confirmed with renal biopsy showing active diffuse proliferative lupus nephritis, significant proteinuria, and active urinary sediment. Remission was defined as quiescent urinary sediment and a SLE disease activity index of ≤4.44 Immune complexes were removed from blood samples by polyethylene glycol precipitation.21 Sera from 32 patients showed high IgG binding activity to HMC (defined as OD above mean ± 3 SD of controls).21 Twenty-five of these samples were obtained during flares, and paired remission sera were further retrieved in 14 patients. Anti-dsDNA antibodies were isolated from a total of 46 serum samples by affinity chromatography as described previously.21 All of the anti-dsDNA antibody preparations were treated with DNase to remove any bound DNA before use. Serum samples from eight patients with active nonrenal lupus were included in this study, and anti-dsDNA antibodies were isolated from six of these patients. The sera from 20 healthy subjects were used as controls.
Measurement of Human and Mouse Anti-dsDNA Antibody Titers and IgG Levels
Anti-dsDNA activity in human or mouse sera or human polyclonal anti-dsDNA antibodies was measured using quantitative commercial ELISAs according to the manufacturers' instructions. IgG concentration was measured by ELISA.21,30
HMC Culture and Cellular ELISA to Measure Ig Binding to HMC
Primary HMC cultures were established from nephrectomized kidneys and maintained in RPMI 1640 medium supplemented with 15% FBS. Cells of the fifth through the seventh passages, growth-arrested for 72 hours, were used in experiments. Ig binding to HMC with or without transfection with annexin II RNAi was assessed as described previously.21 In some experiments HMC were incubated with trypsin (10 μg/ml) for 10 minutes at 37°C and then neutralized with trypsin inhibitor in PBS (1 mg/ml) before the addition of anti-dsDNA antibodies to study the effect of removal of cell surface proteins. For in vitro studies, the cells were incubated with human IgG polyclonal anti-dsDNA antibodies, normal human IgG, or medium alone for selected time periods up to 24 hours. Parallel studies were also conducted in which cells were incubated with inhibitors to PKC, ERK, p38 MAPK, JNK, and PI3K for 1 hour before incubation with control IgG or anti-dsDNA antibodies. The final IgG concentration was 10 μg/ml, an optimal concentration for cell binding experiments on the basis of our previous data.21
Annexin II Knockdown Using Stealth RNAi
HMC were cultured until 50% confluent and transfected with duplex Stealth RNAi specific for annexin II or Stealth RNAi negative control (final concentration, 40 nM) complexed with Lipofectamine 2000. Transfection efficiency was monitored by BLOCK-iT Fluorescence Oligo. After 4 hours of incubation, the RNAi-Lipofectamine complex was removed, and the cells were cultured overnight in RPMI 1640 supplemented with 15% FBS. The cells were growth arrested for 72 hours before incubation with anti-dsDNA antibodies (final IgG concentration, 10 μg/ml), control IgG, or serum-free medium.
Flow Cytometry to Measure Ig Binding to HMC
Confluent, growth-arrested HMC were treated with 0.05% trypsin and 0.02% EDTA for 5 minutes at 37°C, neutralized with trypsin inhibitor (1 mg/ml) in PBS, cultured overnight in suspension, pelleted by centrifugation at 800 × g for 10 minutes, and washed three times with PBS. The cells were incubated with normal IgG (100 μg/ml) to block Fc receptor-mediated binding and incubated with anti-dsDNA antibodies or control IgG (final IgG concentration, 10 μg/ml) for 1 hour at 4°C (T = 0) in Krebs-Ringer bicarbonate buffer containing 1% BSA. Unbound IgG was removed by washing with Krebs-Ringer bicarbonate buffer, and the cells were incubated with fresh medium at either 4 or 37°C for periods up to 3 hours. At selective time points, aliquots of cells were washed with PBS and fixed with 1% paraformaldehyde, incubated with FITC-conjugated anti-human IgG antibody, and resuspended in 0.5 ml of PBS. Cell surface IgG binding was analyzed by flow cytometry, counting 10,000 cells for each sample.
Immunohistochemical Studies of HMC
HMC or HMC transfected with RNAi were incubated with normal IgG (100 μg/ml) for 30 minutes at 4°C to block Fc receptor-mediated binding, incubated with anti-dsDNA antibodies or control IgG (10 μg/ml) for 1 hour at 4°C, washed with PBS, and incubated with serum-free medium at 37°C for periods up to 3 hours. At selective time periods, the cells were fixed with 3.5% paraformaldehyde containing 0.05% Tween 20 for 10 minutes and examined for IgG binding and annexin II expression.21 Epifluorescence was viewed under an Axiovert 135 microscope or a Bio-Rad Radiance 2100 confocal microscope. Cellular internalization of anti-dsDNA antibodies was assessed by calculating the percentage of cells showing cytoplasmic or intranuclear localization of anti-dsDNA antibodies out of the total number of cells per visual field. Six separate images for each antibody preparation were examined, and a mean value ± SD was calculated for each antibody preparation. In some experiments anti-dsDNA antibodies were preincubated with recombinant human annexin II (50 ng) at 37°C for 2 hours before they were added to HMC to confirm the specificity of their binding to annexin II.
Western Blot Analysis
HMC cultured under control or experiment conditions were solubilized in 20 mM sodium acetate, pH 6.0, containing 4 M urea and 1% Triton X-100 (200 μl) to obtain whole cell lysates. Plasma membrane preparations were purified from DNase I-treated HMC.45 Protein content was measured with a modified Lowry assay. Whole cell lysates or plasma membrane preparations (10 μg) were separated by SDS-PAGE followed by Western blotting. The membranes were probed with anti-dsDNA antibody preparations or monoclonal antibody to human annexin II and then incubated with relevant secondary antibodies. Visualization was by enhanced chemiluminescence (ECL), semiquantitated with ChemiGenius analysis, and expressed as arbitrary densitometric units. Annexin II expression was normalized to β-actin.
In separate studies, aliquots of DNA-free human recombinant annexin II (5 μg/ml) were electrophoresed on 10% polyacrylamide gels and probed with either human control IgG or anti-dsDNA antibodies. In parallel studies, anti-dsDNA antibodies were preincubated with human recombinant annexin II before use.
To determine whether anti-dsDNA antibodies induced cell signaling pathways in HMC, cells cultured under control or experiment conditions were lysed with 50 mM Tris, pH 8.0, containing 150 mM NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, 0.5% SDS, and a cocktail of protease inhibitors (200 μl). Equal quantities of proteins (20 μg) were separated by SDS-PAGE followed by Western blotting. The membranes were probed with antibodies against total and phosphorylated ERK, p38 MAPK, JNK, and AKT, and the bands were visualized as described above. Expression of phosphorylated ERK, p38 MAPK, JNK, and AKT was normalized to the total ERK, p38 MAPK, JNK, and AKT, respectively.
Matrix-assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry (MS)
HMC plasma membrane proteins immunoprecipitated with anti-dsDNA antibodies were separated by SDS-PAGE, stained with Coomassie Blue, and destained in 40% methanol/10% acetic acid. Detectable bands were excised for in-gel tryptic digestion and mass spectrometric analysis.46 Peptide mass fingerprinting analysis softwares Prowl ProFound (http://prowl.rockefeller.edu/), Prospector (http://prospector.ucsf.edu), and Mascot (http://www.matrixscience.com) were used for protein identification. Searches were conducted using the NCBI nonredundant protein and SwissProt databases, utilizing a maximum of one missed cleavage site by trypsin and a mass tolerance of 1 Da.
Immunoprecipitation of Annexins II and V
Annexins II and V from preadsorbed HMC plasma membrane preparations were immunoprecipitated overnight with 10 μl of anti-human annexin II or V bound to 100 μl of protein A-Sepharose. Bound proteins were then eluted with SDS-PAGE sample buffer, electrophoresed on 10% polyacrylamide gels, and transferred to nitrocellulose. The membranes were probed with anti-dsDNA antibodies, and the bands were visualized by ECL.
ELISA to Measure Ig Binding to Annexin II
Ninety-six-well microtitre plates were coated overnight with DNA-free human recombinant annexin II (1 ng/ml) in 0.1 M carbonate buffer, pH 9.6. The plates were washed three times with PBS containing 0.05% Tween 20 between incubations, and all of the incubations were for 1 hour at 37°C. The plates were blocked with 3% BSA and incubated with serum samples from healthy individuals or lupus nephritis patients (starting dilution, 1:100), control IgG, or isolated human polyclonal anti-dsDNA antibody preparations (final IgG concentration, 10 μg/ml) in triplicate in serial dilutions, before incubation with horseradish peroxidase-conjugated goat anti-human IgG (5 μg/ml). Color development was with o-phenylenediamine for 30 minutes, and absorbance was measured at 450 nm. OD above the mean ± 2 SD of control sera or IgG was considered significant.
Measurement of Anti-human IgG Response in Mice Injected with Human Immunoglobulins
Mouse sera (5 μl) diluted in PBS (80 μl) were incubated with protein G-Sepharose beads (10 μl) for 15 minutes at room temperature with constant agitation and centrifuged to remove the protein G-Sepharose-bound immune complex.47 The immune complex was dissociated from protein G-Sepharose by the addition of Tris-HCl, pH 6.0, containing 2 M NaCl. Human IgG concentration in immune complex fractions and the resultant supernatants after incubation with protein G-Sepharose, i.e. serum samples free of immune complexes, were determined as described previously.21 To determine the presence of anti-human IgG antibodies in these samples, 96-well microtitre plates were coated overnight with human control IgG (100 μg/ml) in 0.1 M carbonate buffer, pH 9.6. The plates were washed three times with PBS containing 0.05% Tween 20 between incubations, and all of the incubations were for 1 hour at 37°C. The plates were blocked with 3% BSA, and the samples were incubated in serial dilution (starting dilution, 1:100) in triplicate before incubation with horseradish peroxidase-conjugated rabbit anti-mouse IgG (1 μg/ml). Color development was with o-phenylenediamine for 30 minutes, and the absorbance was measured at 450 nm. Sera from age-matched predisease NZBWF1/J and BALB/c mice not administered human immunoglobulins were used as controls.
Quantitative Real Time PCR for Annexin II and IL-6
HMC were stimulated with SFM, control IgG, or anti-dsDNA antibodies (final concentration, 10 μg/ml of IgG) for selected time periods up to 24 hours. Total RNA from HMC was extracted using RNeasy mini kits according to the manufacturer's instructions (Qiagen, Hong Kong, China). One microgram of total RNA was reverse-transcribed to cDNA with Moloney murine leukemia virus reverse transcriptase (Invitrogen Life Technologies, Hong Kong, China) using the random hexamer method. SYBR Green-based quantitative real-time PCR were performed in triplicate in a LightCycler 480 II Real-time PCR Detection System (Roche Diagnostics, DKSH Hong Kong Limited, Hong Kong, China) using specific paired primer sequences for annexin II (5′-TCTGCTCCAGAACCAACCAG-3′ and 5′-TTGCGGAAGTCACCAGATG-3′), IL-6 (5′-GGCACTGGCAGAAAACAACC-3′ and 5′-GCAAGTCTCCTCATTGAATCC-3′), and glyceraldehyde-3-phosphate dehydrogenase (5′-CAAATTCCATGGCACCGTCA-3′ and 5′-CTCGCTCCTGGAAGATGGTGA-3′). Results of comparative real-time PCR were analyzed using LightCycler 480 Software v1.5.0.SP3 (Roche Diagnostics, DKSH Hong Kong Limited, Hong Kong, China).
Studies in Murine Lupus Nephritis
All of the animal procedures were approved by the Institutional Committee on the Use of Live Animals in Teaching and Research. Ten female NZBWF1/J mice (The Jackson Laboratory, Bar Harbor, ME) were followed weekly from 15 weeks. Once fixed proteinuria >300 mg/dl was noted on two separate occasions 2 days apart (at around 20 weeks), the mice were sacrificed at 2, 6, and 12 weeks (n = 6 for each group) thereafter, and the kidneys were harvested for assessment of annexin II expression. In separate experiments, female BALB/c and predisease NZBWF1/J mice (15 weeks of age, n = 10 for both groups) were given 100 μg of human polyclonal anti-dsDNA antibodies or control human IgG in 100 μl of saline by intraperitoneal injection for 10 consecutive days48 before sacrifice and renal immunohistochemical studies.
Histologic Studies of Human and Murine Lupus Nephritis
Fifteen renal biopsies showing active diffuse proliferative lupus nephritis (ISN/RPS class IV G or S, A, or A/C) were studied. Normal renal tissue from five nephrectomy samples was included as controls. The samples were washed three times with PBS in between all steps, and unless otherwise stated, all incubations were for 1 hour at 37°C. To study colocalization of annexin II and IgG, cryosections (8 μm) were incubated with mouse anti-human annexin II, followed by Alexa Fluor 594 nm conjugated anti-mouse secondary antibody and FITC-conjugated goat anti-human IgG. To confirm the specificity of the anti-annexin II antibody, aliquots of this antibody were incubated with recombinant human annexin II (50 ng) before use in parallel experiments. Annexin II colocalization with C3 was assessed with FITC-conjugated anti-human C3. Epifluorescence was viewed using a Bio-Rad Radiance 2100 confocal microscope or an Axiovert 135 microscope. Nonlupus glomerular disease biopsy controls included membranoproliferative glomerulonephritis (n = 10), idiopathic membranous glomerulonephritis (n = 3), IgA nephropathy (n = 4), diabetic nephropathy (n = 6), and focal segmental glomerulosclerosis (n = 6). Four separate images were examined in a blinded manner for each sample. Deposition of human anti-dsDNA antibodies in kidneys of BALB/c and NZBWF1/J mice was assessed with goat anti-human IgG. Annexin II expression was detected using rabbit polyclonal antibody to annexin II followed by a peroxidase-anti-peroxidase method and hematoxylin counterstaining. Staining was scored semiquantitatively (1, 0 to <5%; 2, 5 to 25%; 3, 25 to 75%; and 4, >75%), and a mean score was calculated for each sample.
Effect of Anti-dsDNA Antibodies on HMC IL-6
HMC in 25-cm2 flasks were incubated with anti-dsDNA antibodies or control IgG for 24 hours, before assessment of IL-6 secretion. The supernatant was decanted and centrifuged at 1000 × g for 10 minutes to remove cell debris, and the level of IL-6 was measured using a commercial ELISA. The results were expressed as pg/μg cellular protein. In some experiments HMC were preincubated with medium alone, mouse anti-human annexin II antibody (10 μg/ml), or isotype-matched control (10 μg/ml) for 1 hour at 4°C before anti-dsDNA antibody stimulation to study the effect of cross-linking of cell surface annexin II.49
Statistical Analyses
All of the experiments were repeated three times. The results are expressed as the means ± SD. Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California). The differences were assessed by paired t test, Mann-Whitney test, or ANOVA with or without repeated measurements followed by Bonferroni's multiple comparison post test as appropriate. Correlation was examined using the Spearman's method. Two-tailed P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This study was supported by the Hong Kong Research Grants Council General Research Fund (HKU 7374/03M and HKU 7603/09M), Committee on Research and Conference Grants (10203476 and 10400647), an Outstanding Researcher Award (10207175), and a University Grants Committee Matching Grant Scheme (Phase III, 20730358). Dr. S. Yung is supported by the Endowment Fund established for the Yu Professorship in Nephrology awarded to Tak Mao Chan and the Wai Hung Charitable Foundation Limited.
We thank Ms. Shuk Kei Lau, Mr. Ryan C.W. Tsang, and Mr. Jack Leung for technical assistance; Dr. Kwok Wah Chan (Department of Pathology, Queen Mary Hospital, Hong Kong, China) for the retrieval of renal biopsy specimens; and Dr. Joseph Fernandez and colleagues (The Rockefeller University Proteomics Resource Centre) for performing mass spectrometry analyses. We thank the Core Imaging Facility at the Li Ka Shing Faculty of Medicine, University of Hong Kong for the use of the Bio-Rad Radiance 2100 confocal microscope.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Cameron JS: Lupus nephritis. J Am Soc Nephrol 10: 413–424, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Foster MH, Cizman B, Madaio MP: Nephritogenic autoantibodies in systemic lupus erythematosus: Immunochemical properties, mechanisms of immune deposition, and genetic origins. Lab Invest 69: 494–507, 1993 [PubMed] [Google Scholar]
- 3. Peterson KS, Huang JF, Zhu J, D'Agati V, Liu X, Miller N, Erlander MG, Jackson MR, Winchester RJ: Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest 113: 1722–1733, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yung S, Tsang RC, Leung JK, Chan TM: Increased mesangial cell hyaluronan expression in lupus nephritis is mediated by anti-DNA antibody-induced IL-1beta. Kidney Int 69: 272–280, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz MM: The pathological classification of lupus nephritis. In: Lupus Nephritis, edited by Lewis EJ, Schwartz MM, Korbet SM. New York, Oxford University Press, 1999, pp 126–158 [Google Scholar]
- 6. Berden JH, Termaat RM, Brinkman K, Smeenk RJ, Swaak AJ, Faaber P, Assmann K, van de Heuvel LP, Veerkamp JH: Binding of anti-DNA antibodies to glomerular heparan sulfate: A new clue for the pathogenesis of SLE nephritis? Nephrologie 10: 127–132, 1989 [PubMed] [Google Scholar]
- 7. Sun KH, Liu WT, Tsai CY, Tang SJ, Han SH, Yu CL: Anti-dsDNA antibodies cross-react with ribosomal P proteins expressed on the surface of glomerular mesangial cells to exert a cytostatic effect. Immunology 85: 262–269, 1995 [PMC free article] [PubMed] [Google Scholar]
- 8. Mostoslavsky G, Fischel R, Yachimovich N, Yarkoni Y, Rosenmann E, Monestier M, Baniyash M, Eilat D: Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: A case for tissue injury by molecular mimicry. Eur J Immunol 31: 1221–1227, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Deocharan B, Qing X, Lichauco J, Putterman C: Alpha-actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J Immunol 168: 3072–3078, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Du H, Chen M, Zhang Y, Zhao MH, Wang HY: Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritis. Clin Exp Immunol 145: 21–27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishimoto N, Kishimoto T: Interleukin 6: From bench to bedside. Nat Clin Pract Rheumatol 2: 619–626, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Nagafuchi H, Suzuki N, Mizushima Y, Sakane T: Constitutive expression of IL-6 receptors and their role in the excessive B cell function in patients with systemic lupus erythematosus. J Immunol 151: 6525–6534, 1993 [PubMed] [Google Scholar]
- 13. Takeno M, Nagafuchi H, Kaneko S, Wakisaka S, Oneda K, Takeba Y, Yamashita N, Suzuki N, Kaneoka H, Sakane T: Autoreactive T cell clones from patients with systemic lupus erythematosus support polyclonal autoantibody production. J Immunol 158: 3529–3538, 1997 [PubMed] [Google Scholar]
- 14. Alarcon-Riquelme ME, Moller G, Fernandez C: Age-dependent responsiveness to interleukin-6 in B lymphocytes from a systemic lupus erythematosus-prone (NZB x NZW)F1 hybrid. Clin Immunol Immunopathol 62: 264–269, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ: Interleukin-6 exacerbates glomerulonephritis in (NZB x NZW)F1 mice. Am J Pathol 144: 927–937, 1994 [PMC free article] [PubMed] [Google Scholar]
- 16. Kiberd BA: Interleukin-6 receptor blockage ameliorates murine lupus nephritis. J Am Soc Nephrol 4: 58–61, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Fukatsu A, Matsuo S, Tamai H, Sakamoto N, Matsuda T, Hirano T: Distribution of interleukin-6 in normal and diseased human kidney. Lab Invest 65: 61–66, 1991 [PubMed] [Google Scholar]
- 18. Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I: Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 18: 565–570, 2000 [PubMed] [Google Scholar]
- 19. Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, Avalos-Diaz E: Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus 7: 154–158, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Pramanik R, Jorgensen TN, Xin H, Kotzin BL, Choubey D: Interleukin-6 induces expression of Ifi202, an interferon-inducible candidate gene for lupus susceptibility. J Biol Chem 279: 16121–16127, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Chan TM, Leung JK, Ho SK, Yung S: Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol 13: 1219–1229, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Berden JH, Licht R, van Bruggen MC, Tax WJ: Role of nucleosomes for induction and glomerular binding of autoantibodies in lupus nephritis. Curr Opin Nephrol Hypertens 8: 299–306, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP: Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol 168: 1779–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Andrea DM, Coupaye-Gerard B, Kleyman TR, Foster MH, Madaio MP: Lupus autoantibodies interact directly with distinct glomerular and vascular cell surface antigens. Kidney Int 49: 1214–1221, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Raz E, Brezis M, Rosenmann E, Eilat D: Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol 142: 3076–3082, 1989 [PubMed] [Google Scholar]
- 26. Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP: Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest 100: 25–31, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kootstra JC, Veninga A, Baelde JJ, van Eendenburg J, de Heer E, Bruijn JA: Characterization of reactivity of monoclonal autoantibodies with renal antigens in experimental lupus nephritis. J Clin Lab Immunol 48: 201–218, 1996 [PubMed] [Google Scholar]
- 28. Fang YT, Lin CF, Liao PC, Kuo YM, Wang S, Yeh TM, Shieh CC, Su IJ, Lei HY, Lin YS: Annexin A2 on lung epithelial cell surface is recognized by severe acute respiratory syndrome-associated coronavirus spike domain 2 antibodies. Mol Immunol 47: 1000–1009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao S, Huang L, Wu J, Zhang Y, Pan D, Liu X: Vascular endothelial growth factor upregulates expression of annexin A2 in vitro and in a mouse model of ischemic retinopathy. Mol Vis 15: 1231–1242, 2009 [PMC free article] [PubMed] [Google Scholar]
- 30. Yung S, Tsang RC, Sun Y, Leung JK, Chan TM: Effect of human anti-DNA antibodies on proximal renal tubular epithelial cell cytokine expression: Implications on tubulointerstitial inflammation in lupus nephritis. J Am Soc Nephrol 16: 3281–3294, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Carvalho-Pinto CE, Garcia MI, Mellado M, Rodriguez-Frade JM, Martin-Caballero J, Flores J, Martinez AC, Balomenos D: Autocrine production of IFN-gamma by macrophages controls their recruitment to kidney and the development of glomerulonephritis in MRL/lpr mice. J Immunol 169: 1058–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Navarro C, Candia-Zuniga L, Silveira LH, Ruiz V, Gaxiola M, Avila MC, Amigo MC: Vascular endothelial growth factor plasma levels in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Lupus 11: 21–24, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Jeruc J, Jurcic V, Vizjak A, Hvala A, Babic N, Kveder R, Praprotnik S, Ferluga D: Tubulo-interstitial involvement in lupus nephritis with emphasis on pathogenesis. Wien Klin Wochenschr 112: 702–706, 2000 [PubMed] [Google Scholar]
- 34. Swisher JF, Khatri U, Feldman GM: Annexin A2 is a soluble mediator of macrophage activation. J Leukocyte Biol 82: 1174–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Fan X, Krahling S, Smith D, Williamson P, Schlegel RA: Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell 15: 2863–2872, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kristoffersen EK, Matre R: Surface annexin II on placental membranes of the fetomaternal interface. Am J Reprod Immunol 36: 141–149, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Goulet F, Moore KG, Sartorelli AC: Glycosylation of annexins I and annexin II. Biochem Biophys Res Commun 188: 554–558, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Fey MF, Moffat GJ, Vik DP, Meisenhelder J, Saris CJ, Hunter T, Tack BF: Complete structure of the murine p36 (annexin II) gene: Identification of mRNAs for both the murine and the human gene with alternatively spliced 5′ noncoding exons. Biochim Biophys Acta 1306: 160–170, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Kim J, Hajjar KA: Annexin II: a plasminogen-plasminogen activator co-receptor. Front Biosci 7: d341–d348, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, Hanash SM: An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A 98: 9824–9829, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li K, Adibzadeh M, Halder T, Kalbacher H, Heinzel S, Muller C, Zeuthen J, Pawelec G: Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and annexin II eluted from melanoma cells. Cancer Immunol Immunother 47: 32–38, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masaki T, Tokuda M, Fujimura T, Ohnishi M, Tai Y, Miyamoto K, Itano T, Matsui H, Watanabe S, Sogawa K, Yamada T, Konishi R, Nishioka M, Hatase O: Involvement of annexin I and annexin II in hepatocyte proliferation: Can annexins I and II be markers for proliferative hepatocytes? Hepatology 20: 425–435, 1994 [PubMed] [Google Scholar]
- 43. Menaa C, Devlin RD, Reddy SV, Gazitt Y, Choi SJ, Roodman GD: Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J Clin Invest 103: 1605–1613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH: Derivation of the SLEDAI: A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35: 630–640, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Lin PH, Selinfreund R, Wakshull E, Wharton W: Rapid and efficient purification of plasma membrane from cultured cells: Characterization of epidermal growth factor binding. Biochemistry 26: 731–736, 1987 [DOI] [PubMed] [Google Scholar]
- 46. Fernandez J, Gharahdaghi F, Mische SM: Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using MALDI-TOF-MS. Electrophoresis 19: 1036–1045, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Nezlin R: A quantitative approach to the determination of antigen in immune complexes. J Immunol Methods 237: 1–17, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Bynoe MS, Spatz L, Diamond B: Characterization of anti-DNA B cells that escape negative selection. Eur J Immunol 29: 1304–1313, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, McCrae KR: Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 105: 1964–1969, 2005 [DOI] [PubMed] [Google Scholar]