Abstract
Fetuin-A is an important inhibitor of extraosseous calcification, but some of the studies that used ELISAs did not identify a significant relationship between serum fetuin-A levels and vascular calcification in patients with chronic kidney disease (CKD). Here, we used centrifugation to separate a fetuin-mineral complex (FMC) composed of fetuin-A, fibrinogen, fibronectin-1, and calcium from the serum of hemodialysis patients. In addition, we analyzed serum fetuin-A levels of 73 patients with diabetes and CKD (predialysis) after centrifugation. Fetuin-A concentrations were significantly lower in supernatants than in serum from patients at any stage of CKD, indicating systemic circulation of FMC in these patients. With greater severity of CKD, the contribution of FMC to total fetuin-A increased. Despite the absence of a correlation between serum fetuin-A and coronary artery calcification scores (CACS), supernatant fetuin-A negatively correlated with CACS and the extent to which centrifugation reduced fetuin-A (reduction ratio [RR]) positively correlated with CACS. In a longitudinal study of 12 hemodialysis patients with secondary hyperparathyroidism, parathyroidectomy and cinacalcet therapy each significantly reduced the RR without changing supernatant fetuin-A levels after 1 month, suggesting a reduction in FMC. Moreover, the magnitude of cinacalcet-induced reduction in parathyroid hormone correlated with the decrease in RR but not with changes in serum or supernatant fetuin-A. These data suggest that a quantitative measure of FMC, not supernatant or serum fetuin-A as measured in previous studies, reflects extraosseous calcification stress.
Medial vascular calcification is a frequent complication of aging, diabetes, and chronic kidney disease (CKD).1–5 It causes blood vessels to stiffen, which leads to increased systolic BP, reduced diastolic BP, increased pulse pressure, and a resultant higher risk for cardiovascular mortality, thus highlighting the importance of accurately evaluating risk status for vascular calcification.6,7
Many promoters and inhibitors participate in the complex pathogenesis of vascular calcification. Among several putative calcification inhibitors, the reverse acute-phase protein fetuin-A is a good candidate extraosseous calcification inhibitor, particularly in patients with CKD.8,9 Abundant serum glycoprotein fetuin-A (also called α-2 Heremans Schmid glycoprotein) is synthesized in the liver and secreted into the bloodstream, from which it potently inhibits ectopic calcification.10 Ketteler et al.11 demonstrated a relationship between all-cause/cardiovascular mortality and low serum fetuin-A levels in patients who were on hemodialysis (HD); since then, fetuin-A has received considerable focus. A significant correlation between low serum fetuin-A and vascular calcification has been verified by several other investigators.1,12–14
Despite such accumulating evidence, we could not identify a significant relationship between serum fetuin-A and coronary artery calcification scores (CACS) in predialysis patients with CKD.15 In addition to our findings, Mehrotra et al.,16 Jung et al.,17 Hermans et al.,18 and Ix et al.19 also reported that low serum fetuin-A is not an independent predictor of vascular calcification in patients with CKD. To determine why serum fetuin-A levels did not correlate with CACS in our previous clinical study,15 we studied rats that had adenine-induced renal failure (AIRF) and exhibited medial vascular calcification with reduced levels of serum fetuin-A.20 Intermittent weekly injection of alendronate eliminated vascular calcification but did not change serum fetuin-A levels, thus showing that these values do not always reflect the extent of vascular calcification; however, the centrifugation of serum from rats with AIRF yielded a small pellet comprising a complex of fetuin-A and calcium (FMC) that was undetectable in serum from normal rats and those that were administered injections of alendronate. Analysis in vitro revealed that the amount of FMC reflects a risk for mineral precipitation. On the basis of these findings, we speculated that a similar phenomenon exists in patients with CKD and explains why a significant relationship between serum fetuin-A levels and CACS could not be identified. Here, we examined whether the serum of patients with CKD actually contains FMC and explored a novel method of analyzing fetuin-A that is helpful for evaluating extraosseous calcification stress.
RESULTS
Serum from Patients Who Were on HD Contained FMC
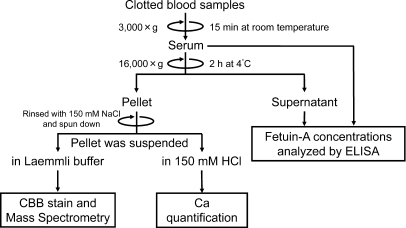
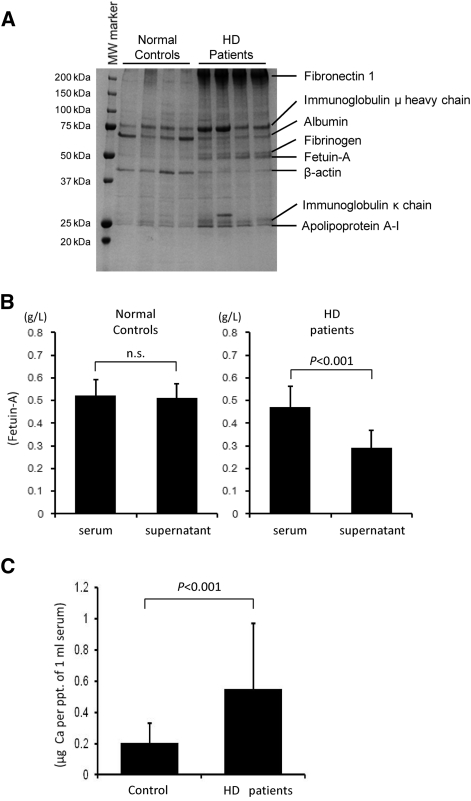
Our previous study of rats with AIRF found that the serum of these rats contained FMC, which could be separated by centrifugation at 16,000 × g.20 The FMC closely correlated with extraosseous calcification stress and was never detected in normal control rats. To determine whether FMC could be similarly separated from patients on dialysis, we compared FMC levels in the serum from normal control subjects and patients who were on maintenance HD. Serum samples that were separated by centrifugation for 15 minutes at room temperature at 3000 × g were centrifuged once again for 2 hours at 16,000 × g at 4°C (Figure 1) to generate a small pellet. Proteins in the pellet were analyzed by Coomassie Brilliant Blue–stained polyacrylamide gel (Figure 2A). The pellets from the patients' serum contained proteins of 250, 78, 68, 53, 50, 40, 26, and 25 kD (Figure 2A). We conducted matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF/MS) and identified the proteins as fibronectin-1, Ig μ heavy chain, albumin, fibrinogen, fetuin-A, β-actin, Ig κ chain, and apolipoprotein A-I, respectively (Figure 2A, Supplemental Table 1). We quantified fetuin-A levels in serum and in the 16,000 × g serum supernatant (Figure 2B) using ELISA kits (Epitope Diagnostic, San Diego, CA). Levels of fetuin-A were decreased by centrifugation in patients who were on HD but not in normal control subjects (Figure 2B), suggesting that the proteins observed both in normal control subjects and in patients who were on HD (Figure 2A, Ig μ heavy chain, albumin, β-actin, Ig κ chain, and apolipoprotein A-I) were less potent components of FMC. In accordance with our previous studies of model rats,20 the pellet in patients' serum contained significantly elevated levels of calcium (Figure 2C). All of these data suggest that the serum of patients were on HD contained FMC and was possibly composed of fetuin-A, fibrinogen, fibronectin-1, and calcium.
Figure 1.
Analytical methods. Serum samples obtained by centrifugation at 3000 × g for 15 minutes at room temperature are centrifuged again for 2 hours at 16,000 × g at 4°C. Levels of serum or supernatant fetuin-A are measured using ELISA. Supernatants are discarded, then centrifuge tubes are rinsed twice with 150 mM NaCl and re-centrifuged. Resultant pellets are dissolved in 150 mM HCl for calcium (Ca) analysis. Pellets are dissolved in Laemmli buffer containing 60 mM EDTA and resolved by Super-Sep PAGE. Proteins of interest are identified by Coomassie Brilliant Blue (CBB) staining and MALDI-TOF/MS.
Figure 2.
Serum of HD patients contains FMC. Physical characteristics of fetuin-A from patients who were on HD considerably differ from those of normal control subjects. Serum is processed as described in Figure 1. (A) Centrifugation at 16,000 × g generates pellets from HD patients' serum (n = 4) but not from normal control subjects (n = 4). Separating constituent proteins by SDS-PAGE followed by CBB staining reveals 250-, 78-, 68-, 53-, 50-, 40-, 26-, and 25-kD proteins in the pellet from the HD patients. Proteins of 78, 68, 40, 26, and 25 kD are also observed in the pellet fraction from normal control subjects. Each protein is identified by MALDI-TOF/MS. (B) Concentrations of fetuin-A in serum and in 16,000 × g supernatant are measured by ELISA (Epitope Diagnostic). Although fetuin-A levels in serum and supernatant are comparable in normal control subjects (n = 11), that in supernatant from HD patients (n = 13) declines (Wilcoxon signed-ranks test). (C) Pellet from HD patients' serum contains more calcium. Normal control subjects, n = 11; HD patients, n = 13 (P < 0.001, nonpaired t test).
Levels of Fetuin-A are Lower in Supernatant than in Serum at All CKD Stages
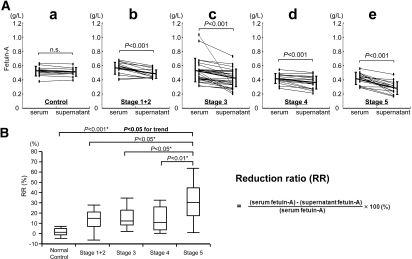
To determine whether the reduction in fetuin-A caused by centrifugation at 16,000 × g is limited to patients who are on HD, we measured fetuin-A levels in the serum and supernatant of predialysis patients who had diabetes and CKD, who presumably have vascular calcification. Fetuin-A levels were comparable in the supernatant and serum of normal individuals (Figure 3Aa) but significantly lower in the supernatant than in serum at all CKD stages (Figure 3A, b through e). We calculated the fetuin-A reduction ratio (RR) by centrifugation to assess the ratio of sedimented fetuin-A (i.e., FMC in total serum fetuin-A; Figure 3B). The Jonckheere-Terpstra test confirmed a stepwise increase in RR with the progression of CKD (P < 0.05 for trend; Figure 3B).
Figure 3.
The ratio of FMC contained in the serum of predialysis patients increases with greater severity of CKD. Fetuin-A levels measured by ELISA (Epitope Diagnostic) are significantly reduced at all stages of CKD after centrifugation at 16,000 × g. Serum is processed as described in Figure 1. (A) Serum and supernatant fetuin-A levels are comparable in normal individuals (a), whereas supernatant levels are significantly lower than those of serum in patients with stages 1 and 2 CKD (b). Results are similar for stages 3 (c), 4 (d), and 5 CKD (e). Normal control subjects, n = 11; stages 1 and 2 CKD, n = 15; stage 3 CKD, n = 28; stage 4 CKD, n = 15; stage 5 CKD, n = 15 (Wilcoxon signed-ranks test). (B) RR of fetuin-A increases with CKD progression (P < 0.05 for trend, Jonckheere-Terpstra test). *Stage 5 serves as reference for Dunnet test.
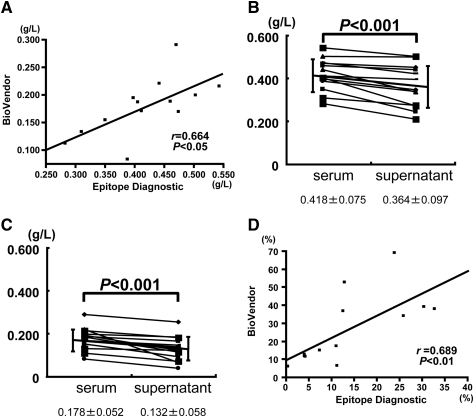
To exclude the possibility that the results shown in Figures 2 and 3 were caused by artifacts that are unique to the Epitope Diagnostic ELISA kits, we analyzed serum and supernatant samples from 13 patients with stage 4 CKD using another fetuin-A ELISA kit purchased from BioVendor (Figure 4). Although the absolute values were lower with this ELISA kit, the serum fetuin-A levels (Figure 4A, vertical axis) closely correlated with those measured using the Epitope Diagnostic ELISA (Figure 4A, horizontal axis). The fetuin-A reduction in the supernatant was similar with both the Epitope Diagnostic (Figure 4B) and BioVendor (Figure 4C) fetuin-A ELISA kits. The RRs derived from the two kits also closely correlated (Figure 4D). All of these data indicate that the fetuin-A reduction after 16,000 × g centrifugation was not specific to either of the ELISA kits. Fetuin-A in the supernatant fraction was indeed reduced after centrifugation at 16,000 × g, and the reduction was not limited to a specific CKD stage. Patients with diabetes at any stage of CKD had FMC.
Figure 4.
Comparison of fetuin-A ELISA kits from two manufacturers. (A) Serum fetuin-A concentrations measured using BioVendor (vertical axis) and Epitope Diagnostic (horizontal axis) ELISA kits closely correlate (r = 0.664, P < 0.05). (B and C) Fetuin-A levels measured using Epitope Diagnostic (B) and BioVendor (C) ELISA kits reveal similar fetuin-A reductions in supernatant from serum of patients (n = 13) with stage 4 CKD (n = 13; P < 0.001, Wilcoxon signed-ranks test). (D) RR also closely correlates between kits (n = 13; r = 0.689, P < 0.01).
Fetuin-A RR but not Serum Fetuin-A Level Correlated with Extraosseous Calcification Stress
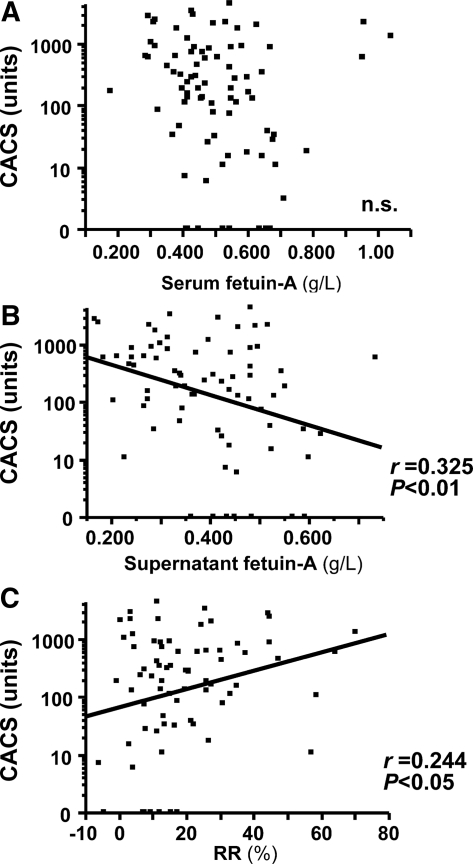
Although many investigators have found that serum fetuin-A levels negatively correlate with extraosseous calcification,1,10,12–14 we did not identify any significant relationship between serum fetuin-A concentrations and CACS in predialysis patients with diabetes and CKD.15 To further our previous clinical findings, we investigated relationships between CACS and serum fetuin-A, supernatant fetuin-A, or RR. Plots in Figure 5 show CACS versus serum fetuin-A, supernatant fetuin-A, or RR. In agreement with our previous findings,15 a significant correlation between CACS and serum fetuin-A (Figure 5A) was not found; however, significant relationships emerged between CACS and fetuin-A after fractionation into supernatant and sediment. Supernatant fetuin-A negatively and RR positively correlated with CACS (Figure 5, B and C, respectively). Moreover, the RR of fetuin-A was significantly associated with serum osteoprotegerin (r = 0.25, P < 0.05), which is an independent determinant of CACS in this population (data not shown).15
Figure 5.
CACS correlates with supernatant fetuin-A and RR but not with serum fetuin-A concentrations. (A) Serum fetuin-A is not significantly associated with CACS. (B) Fetuin-A in supernatant fraction negatively correlates with CACS. (C) RR positively correlates with CACS (n = 73).
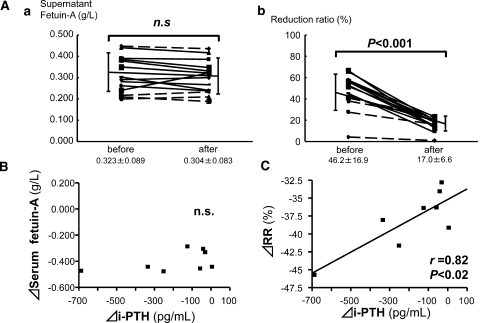
We further analyzed whether potential therapeutic interventions for extraosseous calcification stress alters the fetuin-A fractions. Because secondary hyperparathyroidism is an established extraosseous calcification stressor in patients with CKD and because parathyroidectomy (PTX) often regresses ectopic calcification,21–24 we investigated the effects of PTX on fetuin-A fractions in HD patients. The effects of cinacalcet (25 mg/d) administration, a potential therapeutic intervention for extraosseous calcification stress,25–27 were also investigated. Twelve patients who were on HD underwent PTX (n = 4) or were administered cinacalcet (n = 8), and then serum samples from the two groups were analyzed 1 month later. We confirmed in two of the patients who underwent PTX that CACS decreased 6 months later by the intervention (before 8006 → after 7168, and before 5204 → after 3980). Supernatant fetuin-A levels persisted at essentially the same levels in all patients (Figure 6Aa), whereas serum fetuin-A (data not shown) and RR (Figure 6Ab) were significantly reduced, indicating that the ratio of FMC to total serum fetuin-A was also significantly reduced. We further examined relationships among changes in RR, serum fetuin-A, intact parathyroid hormone (iPTH), serum adjusted calcium, and phosphate and its product in patients who were treated with cinacalcet, in whom none of calcium-based phosphate binders or vitamin D or its analog concomitantly changed. Cinacalcet reduced iPTH from 441.9 ± 226.1 to 249.4 ± 118.7 pg/ml and calcium phosphate product from 47.2 ± 8.1 to 39.1 ± 8.1 mg2/dl2. The reductions in iPTH and in serum fetuin-A levels did not correlate (Figure 6B); only the change in the RR of fetuin-A positively correlated with the reduction in iPTH (Figure 6C). These findings suggested that the RR of fetuin-A—that is, the ratio (%) of FMC to total fetuin-A but not serum or supernatant fetuin-A—accurately reflects extraosseous calcification stress.
Figure 6.
Therapeutic intervention for secondary hyperparathyroidism reduces FMC in HD patients. Differences between pre- and postintervention values are shown as Δ. Postintervention samples are collected 1 month after each intervention. (A) PTX (n = 4) or cinacalcet (25 mg/d; n = 8) to treat secondary hyperparathyroidism in patients with HD reduces RR but does not affect supernatant fetuin-A levels. Solid lines, cinacalcet; dotted lines, PTX. (B) Reduced iPTH levels do not correlate with changes in fetuin-A levels in serum from patients who were treated with cinacalcet. (C) Only RR positively correlates with iPTH reduced by cinacalcet. Pearson correlation coefficient (r) is shown in simple linear regression analysis.
DISCUSSION
We examined the behavior of fetuin-A in patients with CKD and found that the characteristics of fetuin-A also change under extraosseous calcification stress in humans. The following findings support this conclusion: The serum of patients who were on HD contained FMC, which was undetectable in normal serum; the 16,000 × g supernatant that was obtained from serum contained less fetuin-A than serum in patients with diabetes and CKD; the RR of fetuin-A (the ratio of FMC to total fetuin-A) increased with CKD progression; supernatant fetuin-A negatively and RR positively correlated with CACS; and the decrease in iPTH induced by cinacalcet was significantly associated with the decrease in RR but not with that of serum or supernatant fetuin-A. These data suggest that more extraosseous calcification stress in patients with CKD is reflected in a higher ratio of fetuin-A sedimentation. Our results also suggest that previous clinical findings of fetuin-A should be interpreted with care because sample preparation, including blood centrifugation speed, affects the results.
Although Heiss et al.28 described dialysis-associated calcifying peritonitis with calciprotein particle (a synonym for FMC) in ascites, whether FMC really exists in human serum has remained obscure. Here, we discovered that serum from patients who are on HD contains FMC (Figure 2). The FMC described here was quite different from that seen in ascites.28 In contrast to ascites-derived calciprotein particles that contained little fetuin-A but large amounts of albumin,28 FMC in the serum contained at least three proteins: Fetuin-A, fibrinogen, and fibronectin-1. We and others have demonstrated that rat serum contains FMC under several experimental conditions.20,29–31 The FMC in human serum was also different from that in rat serum because FMC in rats with AIRF did not contain fibrinogen and fibronectin-1.20 In addition, matrix Gla protein and secreted phosphoprotein 24 (spp24), components of FMC in etidronate-treated rats, could not be identified in human serum FMC.31,32 Like lipoprotein particles, there may be several types of FMC under different conditions. Further studies are required to address this question. In addition to serum proteins, we detected β-actin in the pellet fraction both in normal control subject and HD patients (Figure 2A). Because of the low centrifugation speed in the first serum separation step, it seems that several cellular components, such as platelets and/or their microparticles, were sedimented with FMC in the second centrifugation step at 16,000 × g.
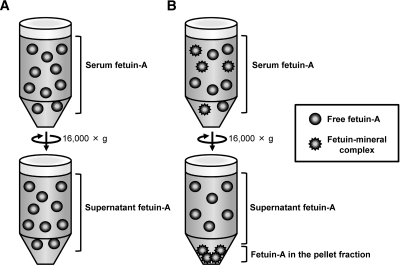
The sedimentation of fetuin-A by centrifugation at 16,000 × g was not restricted to the patients who were on HD. Fetuin-A similarly sedimented in serum samples from patients with diabetes at all stages of CKD but not in that from normal individuals (Figure 3A). Moreover, RR increased with the progression of CKD (Figure 3B). A possible mechanism for these observations on the basis of our findings in animals is schematically summarized in Figure 7.20 Fetuin-A exists in normal serum in the free form; that is, not bound to minerals (Figure 7A, top). Like other serum proteins, free fetuin-A does not sediment at 16,000 × g; therefore, the concentration of fetuin-A in the supernatant (Figure 7A, bottom) is equal to that in serum (Figure 7A, top). In contrast, fetuin-A forms FMC, which could be separated at 16,000 × g, to inhibit mineral precipitation under extraosseous calcification stress (Figure 7B). Under this condition, serum contains both free fetuin-A and fetuin-A as FMC (Figure 7B). Consequently, the amount of fetuin-A in the supernatant (Figure 7B, bottom) becomes lower than that in serum (Figure 7B, top). The difference between serum and supernatant fetuin-A values reflects the amount of fetuin-A as FMC (Figure 7B, bottom, pellet). In addition to these HD patients, patients with CKD at all stages had FMC (Figure 3).
Figure 7.
Under calcification stress, fetuin-A forms FMC, which can be detected by centrifugation. (A) Free fetuin-A circulates in normal serum and does not precipitate at 16,000 × g. Thus, supernatant fetuin-A concentration (bottom) equals that of serum fetuin-A (top). (B) Under extraosseous calcification stress, fetuin-A forms FMC to inhibit mineral precipitation. Serum contains both free and bound fetuin-A (FMC). Because FMC precipitates at 16,000 × g, fetuin-A values in supernatant (bottom) become lower than those in serum (top).
We quantified the ratio of fetuin-A bound as FMC to total fetuin-A using RR, which was calculated using the formula shown in Figure 3B. Although not significantly associated with serum fetuin-A in predialysis patients with CKD (Figure 5A), CACS was positively related to RR (Figure 5C). Moreover, RR positively correlated with serum osteoprotegerin, which is associated with silent coronary artery disease in patients with type 2 diabetes.33 Levels of supernatant fetuin-A negatively correlated in parallel with CACS (Figure 5B). Furthermore, the reduction in iPTH induced by cinacalcet correlated with changes in RR but not with reduced serum or supernatant fetuin-A levels in HD patients with secondary hyperparathyroidism (Figure 6). Consistent with our results from animal experiments,20 these two findings (Figures 5 and 6) show that RR may be a reliable indicator of extraosseous calcification stress. Truly, the correlation of supernatant fetuin-A with CACS seems to be stronger than that with RR (Figure 5). Although there was no significant correlation between RR and serum albumin, we observed a significant positive correlation between supernatant fetuin-A and serum albumin (R = 0.09, P = 0.001). Given that malnutrition is a factor contributing to vascular calcification, it is reasonable that we found a stronger correlation of supernatant fetuin-A with CACS15; however, supernatant fetuin-A levels seem less reliable markers because intervention for secondary hyperparathyroidism did not alter supernatant fetuin-A levels (Figure 6A). These fetuin-A fractions should be considered so that the risk for extraosseous calcification can be evaluated.
Fetuin-A is reported to be associated with intimal calcification as well as medial calcification.34 In fact, it was found in an animal study that overriding a fetuin-A deficiency in apolipoprotein-E–deficient mice that had CKD and were fed a high-phosphate diet promotes the intimal calcification of atheromatous lesions.34 We postulated that the patients with diabetes and CKD in this study had intimal in addition to medial calcification.
The findings of our previous animal experiments showed that the appearance of serum FMC preceded the onset of vascular calcification. Given that FMC prevented the spontaneous precipitation of minerals in vitro,20 the appearance of FMC seems to represent compensation for ongoing calcification stress in CKD; hence, the amount of FMC might serve as a marker of ongoing calcification stress. In this context, early changes in FMC (reflected in RR) by any intervention may predict the progression of ectopic calcification. In fact, in two PTX cases in which we followed CACS 6 months after the interventions, the decrease in RR preceded the decrease in CACS. Future studies are required to confirm the significance of RR as a predictor in clinical practice.
In this study, we did not elucidate the origin of FMC. Because ectopic artery calcification is often accompanied by decreased bone mineral density or disturbed bone turnover, FMC might be generated in the bone. Indeed, Price et al.29–32 reported that exceedingly large, nontherapeutic doses of etidronate generate FMC in rat by inhibiting normal bone mineralization. Because PTX or cinacalcet administration was associated with the decrease in RR in this study, it seems possible that the change in bone health is an important factor for the appearance of FMC in humans, too; however, because we did not directly assess the bone health in these cases, there still remains several other possible origins of FMC, such as calcium and phosphate absorber (i.e., intestine).35 Future studies are required to clarify the origin of FMC in patients with CKD. This would help us to understand the role of FMC in relation to extraosseous calcification stress.
Our results suggested that the results of previous clinical studies should be carefully interpreted because either ELISA or nephelometry has been used to measure fetuin-A (Table 1). We summarized published clinical studies of the relationship between fetuin-A and extraosseous calcification and/or mortality (Table 1). In a previous study, we used an ELISA to analyze fetuin-A levels in clinical serum samples separated by centrifugation at 3000 × g. Although most studies that used fetuin-A ELISA kits did not describe details of sample preparation, serum samples are usually separated at low-speed centrifugation in clinical situations. Thus, the serum fetuin-A determined by ELISA in previous clinical studies corresponds to the serum fetuin-A in this study; that is, the sum of free fetuin-A and fetuin-A in FMC. Several other studies used nephelometry, which determines levels of target molecules on the basis of turbidity. Samples are mixed with anti–fetuin-A antibody, and the turbidity derived from fetuin-A bound to the antibody is determined from light scattering. Because of this analytical principle, samples analyzed by nephelometry must be clarified by centrifugation at 15,000 × g. Although we separated human FMC at a slightly higher speed (16,000 × g) following our animal experiments,20 the fetuin-A levels measured by nephelometry substantially correspond to supernatant fetuin-A in our study; therefore, ELISA and nephelometry measure different fractions of fetuin-A, and this should be considered when interpreting previous clinical findings. This could explain why most studies that used nephelometry yielded positive results whereas most of negative studies of serum fetuin-A used ELISAs (Table 1) and why serum fetuin-A levels are unreliable as a marker of extraosseous calcification stress (Figures 5 and 6).
Table 1.
Clinical studies on the relationship between fetuin-A and extraosseous calcification and/or mortality
| Reference | Subjects | Centrifugation | Analytic Methods | Results |
|---|---|---|---|---|
| Ketteler et al. (2003)11 | HD patients | ND | Original indirect ELISA | Serum fetuin-A deficiency enhances mortality. |
| Mehrotra et al. (2005)16 | Patients with type 2 diabetes | ND | Original indirect ELISA | Serum fetuin-A positively correlates with CACS. |
| Moe et al. (2005)1 | Patients with stage 5 CKD | ND | Nephelometry | Low serum fetuin-A correlates with increased CACS. |
| Stenvinkel et al. (2005)12 | Patients with stage 5 CKD | ND | ELISA (ED) | Low serum fetuin-A associates with increased mortality. |
| Wang et al. (2005)13 | PD patients | ND | ELISA (ED) | Low fetuin-A associates with valvular calcification and mortality. |
| Honda et al. (2006)39 | Patients with stage 5 CKD | ND | Nephelometry | Low serum fetuin-A predicts higher mortality. |
| Hermans et al. (2006)18 | HD and PD patients | 15,000 × g | Nephelometry | Low fetuin-A does not predict 60-minute aortic stiffness. |
| Jung et al. (2006)17 | HD patients | 520 × g | ELISA (BV) | Serum fetuin-A levels do not correlate with CACS. |
| Cozzolino et al. (2006)40 | HD patients | ND | ELISA (ED) | Low fetuin-A correlates with increased cardiovascular calcification. |
| Mori et al. (2007)14 | Healthy subjects | ND | ELISA (BV) | Serum fetuin-A levels associate with carotid artery stiffness. |
| Russo et al. (2007)41 | Patients with stages 3 through 5 CKD | ND | ELISA (ED) | Serum fetuin-A is lower in patients with CAC. |
| Hermans et al. (2007)42 | HD and PD patients | 15,000 × g | Nephelometry | Low serum fetuin-A predicts 60-minute high mortality. |
| Ix et al. (2007)43 | Patients with CAD | 15,000 × g | Nephelometry | Low fetuin-A associates with 60-minute increased mitral annular calcification. |
| Ix et al. (2007)19 | Patients with stages 3 and 4 CKD | ND | ELISA (ED) | Serum fetuin-A levels are not related to mortality. |
| Mikami et al. (2008)15 | Patients with stages 1 through 5 CKD | ND | ELISA (ED) | Serum fetuin-A levels do not correlate with CACS. |
| Shroff et al. (2008)44 | HD and PD patients | ND | ELISA (ED) | Aortic PWV negatively correlates with serum fetuin-A. |
| Metry et al. (2008)45 | HD patients | ND | ELISA (ED) | Low serum fetuin-A associates with increased mortality only in the presence of inflammation. |
| Zheng et al. (2009)46 | HD patients | ND | ELISA (ED) | Low serum fetuin-A correlates with increased CACS. |
Published clinical studies on the relationship between fetuin-A and extraosseous calcification and/or mortality in adults are listed. Two analytical methods were used in these studies: ELISA and nephelometry. BV, BioVendor; CAC, coronary artery calcification; CAD, coronary artery disease; ED, Epitope Diagnostic; ND, not described; NRF, normal renal function; PD, peritoneal dialysis; PWV, pulse wave velocity. Bolded references are negative studies about fetuin-A.
In conclusion, the ratio of FMC to total fetuin-A (i.e., RR) potentially reflects extraosseous calcification stress, whereas supernatant fetuin-A is less reliable and serum fetuin-A is not a reliable indicator at all. Our results suggest that the measurement of FMC is required for assessment of ongoing extraosseous calcification stress.
CONCISE METHODS
Patient Recruitment
We analyzed serum samples from 13 patients who were on HD to detect FMC directly (Figure 2). Eleven control subjects did not have diabetes or CKD and did not smoke (Figures 2 and 3). We also recruited 73 predialysis patients with CKD and diabetes from the outpatient services of Osaka University Hospital in Japan between April 2005 and April 2007 (Figures 3 through 5). Individuals who had progressed to ESRD that required dialysis or who had a history of myocardial infarction, coronary angioplasty, or coronary bypass surgery and those who were under or had received glucocorticoid or active vitamin D therapy were excluded from the study. Three patients who were receiving calcium carbonate were included. We established a diagnosis and classification of CKD stages according to the criteria of the Clinical Practice Guidelines for Chronic Kidney Disease from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI).36 The GFR was estimated using the reexpressed Modification of Diet in Renal Disease (MDRD) equation.37
Another 12 patients (5 men; mean age 66 years [range 50 to 75 years]) who were on HD for a median of 14 years (range 5 to 21 years), had severe secondary hyperparathyroidism, and underwent PTX (n = 4) or received cinacalcet (n = 8) were included (Figure 6). While under cinacalcet therapy, doses of other drugs, such as calcium-based phosphate binders and vitamin D or its analogues, were not changed for 1 month, to understand the effect of cinacalcet alone on fetuin-A and FMC; however the dosage of vitamin D or calcium-based phosphate binders had to be increased to treat hypocalcemia soon after PTX. We measured CACS before and 6 months after the interventions in two patients who underwent PTX and provided informed consent. We adhered to the Declaration of Helsinki throughout this study. The ethics committee of Osaka University Hospital approved the study protocol, and all of the patients and control subjects provided written informed consent to participate in all aspects of the study.
Data Collection
Blood specimens were obtained from predialysis patients with diabetes on the day of consultation. Blood specimens were collected from HD patients before starting HD at 2 days after the last HD session. Clotted blood samples were separated by centrifugation for 15 minutes at room temperature at 3000 × g, and then the decanted serum was frozen at −80°C. iPTH was assayed using Elecsys PTH immunoradiometric assays kit (Roche Diagnostics, Meylan, France). Fetuin-A was measured using Human Fetuin-A ELISA kits (Epitope Diagnostic) according to the manufacturer's instructions. Another Human Fetuin-A ELISA kit (BioVendor Laboratory Medicine, Brno, Czech Republic) was also used where indicated. A schema of the analytical procedure for fetuin-A is shown in Figure 1.
Serum samples (50 μl) were centrifuged for 2 hours at 4°C at 16,000 × g, and then fetuin-A levels in the supernatant fraction were measured using the Human Fetuin-A ELISA kits. For analysis of FMC (Figure 2), serum samples (1 ml) in 1.5-ml tubes were centrifuged for 2 hours at 4°C at 16,000 × g. The supernatant was discarded, and the tubes were rinsed twice with 1 ml of 150 mM NaCl and separated again by centrifugation for 5 minutes at 16,000 × g. Portions of pellets were dissolved in 15 μl of 150 mM HCl for calcium analysis (Figure 2C) using clinical diagnostic reagents (Wako, Osaka, Japan). Other portions of pellets were dissolved in 80 μl of Laemmli buffer containing 60 mM EDTA, and 15-μl portions were resolved by 10 to 20% Super-Sep polyacrylamide gel (Wako, Osaka, Japan) electrophoresis and then stained with Coomassie Brilliant Blue.
Protein Identification by MALDI-TOF/MS
Gel pieces containing proteins of interest were excised and subjected to in-gel digestion with sequencing-grade modified trypsin (Promega, Madison, WI). ZipTip C18–concentrated (Millipore, Schwalbach, Germany) tryptic digested samples were prepared by co-crystallization of a saturated solution of the matrix (α-cyano-4-hydroxycinnamic acid in 50/50 vol/wt acetonitrile/0.1% trifluoroacetic acid in water). Mass spectra were recorded on a Bruker Ultraflex TOF/TOF (Bruker Daltonics, Bremen, Germany) in positive ion reflectron mode. Tryptic monoisotopic peptide masses were searched against National Center for Biotechnology Information and SwissProt databases using the Mascot (Matrix Science, London, UK) program.
Multidetector Computed Tomography
We evaluated CACS using multidetector computed tomography (16-slice technique; Light Speed Ultra 16; GE Yokokawa Medical Systems, Tokyo, Japan). Image acquisition was timed to coincide with the diastolic phase of the cardiac cycle at approximately 70% (total three phases) of the R wave-to-R wave interval as determined through electrocardiographic monitoring with a 2.5-mm gap between slices. Scanning time was approximately 30 seconds for the entire zone of interest that encompassed the whole heart. This equipment was capable of detecting lesions of a density of at least 130 Hounsfield units and a minimum area of 0.4882 mm2. Total CACS was calculated using Smartscore software (GE Yokokawa Medical Systems) in modified Agatston units.38 Scanning proceeded at 120 kV and 300 mA. The mean radiation dose defined as volume computed tomography dose index was 11.85 mGy, the dose product length was 142.15 mGy, and the effective dose was 97.4%.
Statistical Analyses
Serum fetuin-A and supernatant fetuin-A were compared by the Wilcoxon signed ranks test. Differences in RR of fetuin-A among patients at various stages CKD and control patients were compared using Dunnet analysis with stage 5 CKD as a reference. Linear trends between CKD stages and RR were confirmed using the Jonckheere-Terpstra trend test. Linear regression analyses of two continuous parameters were performed using the least-squares method. All statistical tests were two-sided, and P < 0.05 was considered significant. All data were statistically analyzed using JMP 5.1.2J for Windows (SAS Institute, Cary, NC).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 18790564).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M: Medial artery calcification: A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16: 978–983, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Moe SM, O'Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA: Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19: 2387–2393, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM: Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107: 363–369, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K: Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 61: 638–647, 2002 [DOI] [PubMed] [Google Scholar]
- 6. London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 7. London GM, Marchais SJ, Guerin AP, Metivier F, Adda H: Arterial structure and function in end-stage renal disease. Nephrol Dial Transplant 17: 1713–1724, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Vattikuti R, Towler DA: Osteogenic regulation of vascular calcification: An early perspective. Am J Physiol Endocrinol Metab 286: E686–E696, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Abedin M, Tintut Y, Demer LL: Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24: 1161–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W: The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112: 357–366, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J: Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 361: 827–833, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, Barany P, Lindholm B, Jogestrand T, Heimburger O, Holmes C, Schalling M, Nordfors L: Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int 67: 2383–2392, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE: Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 20: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Mori K, Emoto M, Araki T, Yokoyama H, Teramura M, Lee E, Motoyama K, Koyama H, Shoji T, Inaba M, Nishizawa Y: Association of serum fetuin-A with carotid arterial stiffness. Clin Endocrinol (Oxf) 66: 246–250, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Mikami S, Hamano T, Fujii N, Nagasawa Y, Isaka Y, Moriyama T, Matsuhisa M, Ito T, Imai E, Hori M: Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens Res 31: 1163–1170, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Mehrotra R, Westenfeld R, Christenson P, Budoff M, Ipp E, Takasu J, Gupta A, Norris K, Ketteler M, Adler S: Serum fetuin-A in nondialyzed patients with diabetic nephropathy: Relationship with coronary artery calcification. Kidney Int 67: 1070–1077, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Jung HH, Kim SW, Han H: Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 21: 1915–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hermans MM, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Gladziwa U, Rensma PL, Bartelet K, Konings CJ, Hoeks AP, Floege J, Leunissen KM: Study on the relationship of serum fetuin-A concentration with aortic stiffness in patients on dialysis. Nephrol Dial Transplant 21: 1293–1299, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Ix JH, Shlipak MG, Sarnak MJ, Beck GJ, Greene T, Wang X, Kusek JW, Collins AJ, Levey AS, Menon V: Fetuin-A is not associated with mortality in chronic kidney disease. Kidney Int 72: 1394–1399, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Matsui I, Hamano T, Mikami S, Fujii N, Takabatake Y, Nagasawa Y, Kawada N, Ito T, Rakugi H, Imai E, Isaka Y: Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int 75: 915–928, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Derici U, El Nahas AM: Vascular calcifications in uremia: Old concepts and new insights. Semin Dial 19: 60–68, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Drueke TB, Massy ZA: Advanced oxidation protein products, parathyroid hormone and vascular calcification in uremia. Blood Purif 20: 494–497, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Di Leo C, Gallieni M, Bestetti A, Tagliabue L, Cozzolino M, Carpani P, Pozzato C, Tarolo GL, Brancaccio D: Cardiac and pulmonary calcification in a hemodialysis patient: Partial regression 4 years after parathyroidectomy. Clin Nephrol 59: 59–63, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Bleyer AJ, Burkart J, Piazza M, Russell G, Rohr M, Carr JJ: Changes in cardiovascular calcification after parathyroidectomy in patients with ESRD. Am J Kidney Dis 46: 464–469, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Aladren Regidor MJ: Cinacalcet reduces vascular and soft tissue calcification in secondary hyperparathyroidism (SHPT) in hemodialysis patients. Clin Nephrol 71: 207–213, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Wang HY, Yu CC, Huang CC: Successful treatment of severe calciphylaxis in a hemodialysis patient using low-calcium dialysate and medical parathyroidectomy: Case report and literature review. Ren Fail 26: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Mohammed IA, Sekar V, Bubtana AJ, Mitra S, Hutchison AJ: Proximal calciphylaxis treated with calcimimetic ‘Cinacalcet.’ Nephrol Dial Transplant 23: 387–389, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schafer C, Jahnen-Dechent W: Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem 283: 14815–14825, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Price PA, Lim JE: The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem 278: 22144–22152, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Price PA, Caputo JM, Williamson MK: Bone origin of the serum complex of calcium, phosphate, fetuin, and matrix Gla protein: Biochemical evidence for the cancellous bone-remodeling compartment. J Bone Miner Res 17: 1171–1179, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Price PA, Thomas GR, Pardini AW, Figueira WF, Caputo JM, Williamson MK: Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem 277: 3926–3934, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Price PA, Nguyen TM, Williamson MK: Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem 278: 22153–22160, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Avignon A, Sultan A, Piot C, Elaerts S, Cristol JP, Dupuy AM: Osteoprotegerin is associated with silent coronary artery disease in high-risk but asymptomatic type 2 diabetic patients. Diabetes Care 28: 2176–2180, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Westenfeld R, Schafer C, Kruger T, Haarmann C, Schurgers LJ, Reutelingsperger C, Ivanovski O, Drueke T, Massy ZA, Ketteler M, Floege J, Jahnen-Dechent W: Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol 20: 1264–1274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jahnen-Dechent W, Schafer C, Ketteler M, McKee MD: Mineral chaperones: A role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med 86: 379–389, 2008 [DOI] [PubMed] [Google Scholar]
- 36. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 37. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Shemesh J, Apter S, Rozenman J, Lusky A, Rath S, Itzchak Y, Motro M: Calcification of coronary arteries: Detection and quantification with double-helix CT. Radiology 197: 779–783, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Honda H, Qureshi AR, Heimburger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Cozzolino M, Galassi A, Biondi ML, Turri O, Papagni S, Mongelli N, Civita L, Gallieni M, Brancaccio D: Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol 26: 423–429, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, Brancaccio D, Cozzolino M, Biondi ML, Andreucci VE: Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27: 152–158, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Hermans MM, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW, Leunissen KM, Krediet RT, Dekker FW: Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 72: 202–207, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA: Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: Data from the Heart and Soul Study. Circulation 115: 2533–2539, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shroff RC, Shah V, Hiorns MP, Schoppet M, Hofbauer LC, Hawa G, Schurgers LJ, Singhal A, Merryweather I, Brogan P, Shanahan C, Deanfield J, Rees L: The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not Matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant 23: 3263–3271, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Metry G, Stenvinkel P, Qureshi AR, Carrero JJ, Yilmaz MI, Barany P, Snaedal S, Heimburger O, Lindholm B, Suliman ME: Low serum fetuin-A concentration predicts poor outcome only in the presence of inflammation in prevalent haemodialysis patients. Eur J Clin Invest 38: 804–811, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Zheng S, de Las Fuentes L, Bierhals A, Ash-Bernal R, Spence K, Slatopolsky E, Davila-Roman VG, Delmez J: Relation of serum fetuin-A levels to coronary artery calcium in African-American patients on chronic hemodialysis. Am J Cardiol 103: 46–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.