Abstract
There is ongoing controversy about the mechanisms that determine the characteristics of the glomerular filter. Here, we tested whether flow across the glomerular filter generates extracellular electrical potential differences, which could be an important determinant of glomerular filtration. In micropuncture experiments in Necturus maculosus, we measured a potential difference across the glomerular filtration barrier that was proportional to filtration pressure (−0.045 mV/10 cm H2O). The filtration-dependent potential was generated without temporal delay and was negative within Bowman's space. Perfusion with the cationic polymer protamine abolished the potential difference. We propose a mathematical model that considers the relative contributions of diffusion, convection, and electrophoretic effects on the total flux of albumin across the filter. According to this model, potential differences of −0.02 to −0.05 mV can induce electrophoretic effects that significantly influence the glomerular sieving coefficient of albumin. This model of glomerular filtration has the potential to provide a mechanistic theory, based on experimental data, about the filtration characteristics of the glomerular filtration barrier. It provides a unique approach to the microanatomy of the glomerulus, renal autoregulation, and the pathogenesis of proteinuria.
In humans, about 180 L of primary urine is produced from plasma containing about 10 kg of protein. Strikingly, only about 1 g of the protein (0.01%) passes the glomerular filtration barrier of the kidney.
The filter is comprised of at least three layers, each of which is essential for its integrity. An empirical mathematic description of the characteristics of the filter has been developed previously (“two-pore” or “heteroporous model”).1,2 However, apart from a few studies, electrical effects have been ruled out thus far in most of the studies.3 The glomerular filtration barrier bears electrostatic charge and shows not only size- but also charge-selective properties.4–7 Thus far, the glomerular filter has been considered to act as a passive filter (similar to a coffee filter). An active filtration process, on the other hand, generates an additional force, which helps to clear the retentate from the filter continuously during the filtration process.
In this work, an effort was undertaken to experimentally test whether filtration-dependent potential differences are generated across the glomerular filtration barrier. Necturus maculosus (common mudpuppy) was chosen as a model organism because its glomeruli and glomerular capillaries are large enough for micropuncture analysis (about 500 and 50 μm, respectively; Figure 1, A–D). To allow direct control of the glomerular perfusion pressure, the Necturi were perfused with a physiologic perfusion solution as described previously.8 A glass microelectrode was placed into a glomerular capillary. A second microelectrode was placed into Bowman's space of the same renal glomerulus, and the anatomic position was verified by lissamine green injection (Figure 1, E and F; Supplemental Movies 1 and 2). When the perfusion pressure was increased, an immediate increase in the electrical potential difference could be measured that was directly proportional to the perfusion pressure in 10 of 10 experimental animals (Figure 2, A and B). The potential difference was generated without temporal delay and was entirely reversible when decreasing the perfusion pressure to baseline levels.
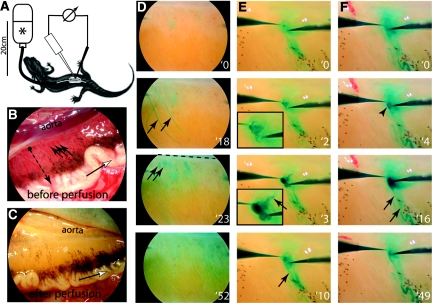
Figure 1.
Micropuncture of a glomerular capillary and Bowman's space within the same glomerulus in Necturus. (A) Schematic of the experimental setup. Anesthetized Necturi were perfused through the cannulated aorta at physiologic pressures (20 cm H2O) with an isoosmolar solution (*). Individual glomeruli were micropunctured by two microelectrodes for potential measurements. (B) Higher magnification of the Necturus kidney. The glomeruli (arrows) are lined up next to the aorta. The tubules (dotted arrow) run from the glomeruli (black dot) laterally to the Wolffian duct, which drains the urine (white arrow). (C) Same view as in B after perfusion of the animal. The glomeruli are no longer visible. (D) View of the kidney after injection of a lissamine bolus into the aorta. After 18 seconds, several superficial arteries are visualized. After 23 seconds, the superficial glomeruli (arrows) lined along the aorta (dashed line) are clearly stained. After 52 seconds, the dye is already washed out. (E) Verification of the anatomical position of the potential microelectrode (left) within a glomerular capillary. At 2 seconds, lissamine green is injected from the tip of the electrode and fills the glomerular capillaries (see magnified inset). At 3 seconds, the lissamine dye exits the glomerular convolute through the efferent arteriole (arrow). At 10 seconds, the staining is already partially washed out. The filtered dye can be seen along the tubule (arrow; Supplemental Movie 1). (F) A lissamine bolus is released from the reference microelectrode at 4 seconds. The dye fills Bowman's space (arrowhead) similar to a shallow pond; the efferent arteriole is not stained. At 16 seconds, the dye stains the tubule (arrows). At 49 seconds, the dye is partially washed out (Supplemental Movie 2).
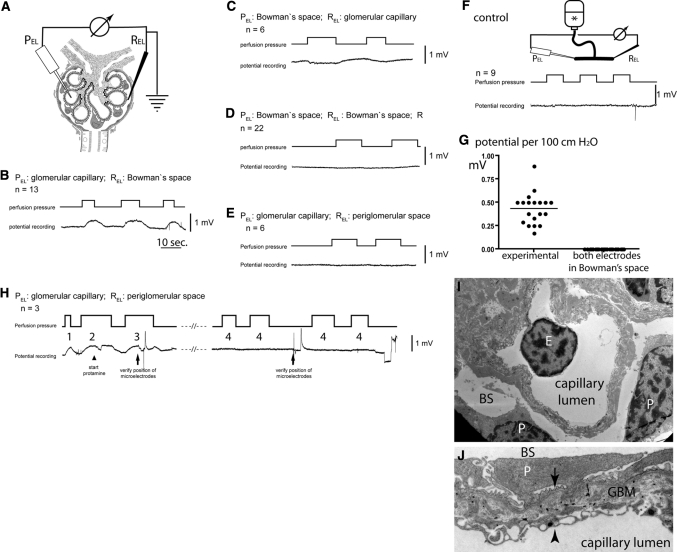
Figure 2.
Direct detection of a filtration-dependent potential difference in vivo. (A) Circuit diagram. The microelectrodes are positioned at defined anatomic locations within the renal glomerulus (i.e., corpuscle). (B–E) Representative potential measurements when perfusion pressures are changed at intervals as indicated. The anatomic location of the respective microelectrodes and the number of independent experiments (n) are indicated (PEL, potential microelectrode; REL, reference electrode). (F) Control experiment. To exclude artifacts from tip or flow potentials, the microelectrodes were placed into a communicating tube system filled with perfusion solution (*), and pressures were increased at intervals as indicated. As shown in a representative recording, no potential changes were observed. (G) Summary of the mean filtration-dependent potential difference in 19 glomeruli of 10 animals (as shown in B and C) and 22 control experiments (as shown in D). (H) Effect of protamine on the filtration-dependent potential difference. After micropuncture of a glomerulus, the correct position of the microelectrodes was verified by lissamine injection (data not shown) and detection of the filtration dependent potential difference (H1 and H2). Subsequently, protamine was added to the perfusate (arrowhead). A filtration dependent potential difference was detectable for up to 100 seconds with decreasing amplitude (H3). After >150 seconds, no filtration-dependent potential difference could be measured in this and at least two additional glomeruli (H4). (I) By transmission electron microscopy, the microanatomy of the Necturus glomerular filtration barrier was unaffected after perfusion with protamine (E, endothelial cell; P, podocyte; BS, Bowman's space). (J) Higher magnification of the Necturus filtration barrier (same animal as in H and I). The endothelial cells form cellular processes and multiple pores to allow filtration to occur (arrowhead). The space (glomerular basement membrane) between the podocyte foot processes (arrow) and endothelial cells is wider than in the mammalian glomerulus and filled with loose extracellular matrix, which also contains type 1 collagen (electron-dense fibers) (P, podocyte primary process; BS, Bowman's space).
The potential difference was consistently positive within the glomerular capillary lumen and negative in Bowman's space. The polarity was confirmed by measurements with reversed anatomical locations of the potential and reference microelectrodes (Figure 2C). No perfusion pressure–dependent potential difference could be measured if both microelectrodes were placed within Bowman's space (Figure 2D). The potential difference almost entirely vanished if the reference microelectrode was placed just outside of Bowman's capsule (Figure 2E). Control experiments excluded artifactual tip potential or pressure- or flow-induced potential differences (Figure 2F). To confirm the polarity of our measurements, classical streaming potentials were generated in an in vitro setup using the identical configuration of our amplifier system. Under these conditions, the predicted polarity was consistently recorded, i.e., positive behind a negatively charged filter (data not shown). Similarly, the predicted polarity was recorded when generating Donnan potentials in an in vitro two-chamber system separated by a semipermeable membrane. On average, the potential difference increased by 0.45 ± 0.16 (SD) mV/100 cm H2O increase in perfusion pressure (Figure 2G): i.e., a potential difference of approximately 0.09 mV at a physiologic perfusion pressure of 20 cm H2O.
To test whether the potential difference could be abolished by interfering with the fixed anionic charges of the glomerular filter, three animals were perfused with the cationic polymer protamine (Figure 2H). During the first 100 seconds, perfusion with protamine reduced the amplitude of the filtration-dependent potential. After about 150 seconds, a potential difference could no longer be detected. Because dislocation of the microelectrodes was the most likely reason for the disappearance of the potential difference, two more glomeruli were micropunctured in each animal, and still no potential difference could be detected (Figure 2H4). By transmission electron microscopy, the ultrastructure of the glomerular filtration barrier was not affected by protamine perfusion (Figure 2, I and J, same animal as in H).
In this work, we provided experimental evidence for the existence of electrical potential differences of approximately −0.045 mV/10 cm H2O perfusion pressure in Necturus across the glomerular filtration barrier. In addition, we showed that the potential differences are generated by filtration. The forced passage of an ionic solution across the electrostatically charged mechanical barrier separates charged particles, generating a streaming potential.9,10 However, in Necturus, the filtration-dependent potential exhibited a polarity opposite to that observed in simple in vitro channels with a negatively charged surface. The physical phenomenon called “charged reversal” or “overcharging”11–18 could be one of several possible physical mechanisms to explain why the negatively charged glomerular filter shows the electrokinetic characteristics of a positively charged filter. “Charge reversal” may occur if additional soluble counter ions (e.g., cations) are bound to the negatively charged filter walls by forces other than electrical interactions (e.g., chemical interactions) and thus change the apparent (i.e., the electrokinetic) charge of the filter walls to a net positive charge. However, in a complex biologic barrier, such as the glomerular filtration barrier, multiple physical and chemical effects likely contribute to the generation of streaming potentials, and our understanding of these complex phenomena is far from complete.
Other effects such as a Donnan potential are predicted to form across the glomerular filtration barrier as a result of the high protein concentration within the blood against the low protein concentration within the primary urine. The Donnan potential is a secondary phenomenon occurring only after a low-protein primary filtrate has been formed, it is not directly dependent on filtration pressure, and it is orientated into the opposite direction as the filtration-dependent potential difference observed in Necturus. Therefore, Donnan potentials are predicted to modify the potential difference across the filtration barrier but cannot be the relevant physical effect for the observed potential difference. The fact that the potential difference could be abolished by the cationic polymer protamine supports the notion that (nonclassical) electrokinetic mechanisms are relevant for its generation.
It remains to be determined whether potential differences of only −0.02 mV are sufficient to induce significant electrophoretic fluxes that alter the permeability of the filter to albumin. We refer to the Supplemental Data, where we propose a mathematical hypothesis to consider among others. The mathematical model of the filter includes electrokinetic effects for the first time. These calculations showed that relatively small potential differences (0.02 to 0.05 mV) are sufficient to effectively prevent albumin from passing into the primary filtrate. Note that potential differences of the same order of magnitude (0.045 mV/10 cm H2O) were measured experimentally in Necturus.
Virtually all macromolecules of the plasma are electrically charged and thus will be influenced by the electric field of the filtration-dependent potential difference. Because most of the plasma proteins are negatively charged (http://www.expasy.ch/swiss-2dpage/), they will be electrophoresed out of the filter back into the blood. This amount of protein cannot be removed from the filter by metabolic or cellular processes, which do not have a high capacity.
In our hands, mammalian glomeruli were too small for direct potential measurements. Nevertheless, filtration-dependent potentials likely are also generated in the mammalian kidney. (1) In an isolated perfused mammalian kidney, the sieving coefficient of albumin was observed to depend inversely on filtration pressures,19 which is consistent with a filtration-dependent potential difference in our model. (2) Other experiments in isolated perfused mammalian kidneys are also consistent with our hypothesis. When the pH of the perfusate in an isolated perfused kidney was lowered to 4.0, albumin permeability increased >10-fold20 to a value similar to those in the absence of electrophoretic forces. At pH 4, the electronegative charges of plasma albumin (isoelectric point = 4 to 5) and of the glomerular filtration barrier are at least partially neutralized, and thus, albumin in the glomerular filter would no longer be expected to be moved backward into serum by electrophoresis. Vice versa, at pH 8.75, the sieving coefficient of albumin decreased further, suggesting consistently that electrical effects are increased at this pH. Similarly, in other experiments, neutralizing the negative electrostatic charges of the glomerular filter with protamine (or other polycations) or reversing the charge of albumin by cationization increased the glomerular permeability to albumin.20–22 However, in all of the above-described experiments, other electrical effects likely also play an important role. Changes in the pH will modify the effective pore radius of the charged glomerular filter and thus mediate similar changes of the albumin sieving coefficient as discussed elsewhere.23 At this point, it is not possible to differentiate between the two effects.
Our findings are also compatible with other, partially unexplained characteristics of the glomerular filtration barrier. First, patients with orthostatic proteinuria excrete about 1 g of protein per day in the upright position but not when supine. This proteinuria is the consequence of a drop in renal perfusion pressure and thus in the GFR in the upright position.24 In experiments, proteinuria was prevented when the legs were compressed in the upright position, and vice versa, when obstructing venous blood returning from the legs with a tourniquet, renal perfusion dropped and patients were proteinuric in the supine position. Our theory provides the first pathophysiologic explanation for this condition, because it implies that the drop in the GFR in the upright position results in a decrease of the filtration-dependent potential difference and thus in significant proteinuria.
Second, the essential requirements for the generation of a filtration-dependent potential difference are conserved in all animal species that form glomeruli, down to the most “primitive” vertebrates. The entire filtering surface is covered with interdigitating podocyte foot processes, allowing filtration to occur homogenously (Figure 3, A and B). The model proposed here implies that a potential difference is only generated where filtration occurs. Therefore, filtration must occur across the entire filter. Remarkably, even the podocyte cell bodies are submerged in the primary urine—a unique feature of these cells. If podocyte cell bodies sat flat on the capillary surface, filtration and separation of charge would be prevented, but because the glomerular filter remains electrically conductive, the potential difference should be short-circuited immediately underneath and around the podocyte cell bodies, resulting in low-grade proteinuria at this specific site (Figure 3C, dotted arrows). Most glomerular diseases with significant proteinuria are associated with fusion of the podocyte foot processes and podocyte cell bodies to sit flat on the outer capillaries. In minimal change nephrotic syndrome, ubiquitous foot process effacement is the only, yet striking, histologic abnormality, leading to a complete breakdown of the potential difference and to nephrotic range proteinuria. Our hypothesis thus provides an explanation for the pathogenesis of nephrotic range proteinuria and also for the loss of charge selectivity in this glomerulopathy (Figure 3D).
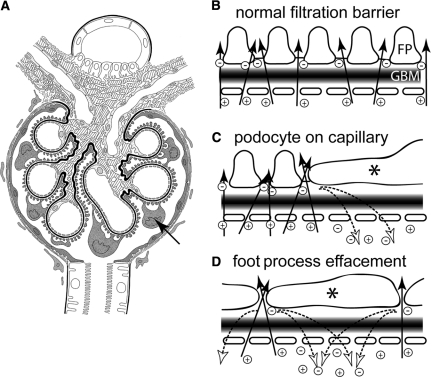
Figure 3.
The filtration-dependent potential difference and its consequences on glomerular permeability. (A) The microanatomy of the glomerulus is optimized to allow the generation of a filtration-dependent potential difference. Podocytes (arrow) cover the entire filtration surface with interdigitating foot processes, allowing filtration to occur homogeneously. Note that the podocyte cell bodies are submerged in the primary urine and do not sit flat on the filtering surface of the capillary (arrow). The parietal cells form a tight epithelial sheet along Bowman's capsule. (B) Hypothetical schematic of the flux of the ionic solution across the glomerular filtration barrier. Arrows indicate the laminar flow of the ions across the three layers of the filter (GBM, glomerular basement membrane; FP, podocyte foot processes). Physicochemical interactions with the glomerular filter affect the individual charged ions (Na+, K+, Cl−, and HCO3−), differently separating them as they are driven across the filter creating the filtration-dependent potential difference (indicated by the [−], anions and [+], cations). (C) Hypothetical model if podocytes sat immediately on the capillary surface. Because the filtration barrier is permeable to small ions, it is electrically conductive. Underneath the podocyte cell body (asterisk), a large nonfiltering area exists that does not generate a potential difference. Next to the podocyte cell bodies, separated ion travel underneath the podocytes back into the capillary (dotted arrow), neutralizing the potential difference in this area. (D) In minimal changes nephropathy, podocyte foot processes are effaced so that they cover almost the entire filtering surface (asterisk). Filtration and charge separation still occurs between the cells (arrows), but the ions will immediately fall back across the filter along the nonfiltering but electrically conductive areas underneath the effaced podocyte cellular extensions (dotted arrows), short circuiting the potential difference throughout the glomerulus.
CONCISE METHODS
All chemicals were purchased from either Sigma-Aldrich (Taufkirchen, Germany) or Merck (Darmstadt, Germany) or as indicated in the text. Standard methodologies, such as electron microscopy studies, were performed as described.25,26
Micropuncture Experiments in Necturus
Adult male Necturi obtained from Nasco (Fort Atkinson, WI) were kept in aquaria with tap water at 19°C. All kidney perfusion experiments were carried out as described8 and were approved by Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen 50.203.2-AC 7, 44/06, Germany. In short, the Necturi were anesthetized by immersion in 0.66 g/L tricaine methanesulfonate (MS222). Abdominal organs were exposed by incising both flanks and cutting the medial flap of the body wall (and ventral abdominal vein) between ligatures. The aorta was cannulated proximal to the kidneys with a polyethylene cannula (inner diameter of 0.6 to 0.8 mm) and perfused from a reservoir at a pressure of 20 cm water column (i.e., 3.6 ml/min; 8.2 ml/min at 80 cm). The perfusate contained 90 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl, 5 mM Hepes, pH 7.4, 5.5 mM glucose, 0.5% bovine serum albumin fraction V (Roche, Mannheim, Germany), and 2% hydroxyethyl starch (Fresenius Kabi, Bad Homburg, Germany) and was bubbled with 100% O2. Fifty milligrams of protamine was used for each animal. Outflow was established by an incision in the postcaval vein. Adequate perfusion was checked during experiments by monitoring the flow rate with a drop counter and postcaval vein outflow rates. In addition, lissamine green B dye was given at intervals into the perfusion solution line to visualize kidney and glomerular perfusion (Figure 1D).
Micropuncture of glomerular capillaries, Bowman's space, or the periglomerular space was performed by two microelectrodes controlled by micromanipulators. Borosilicate glass micropipettes were filled with perfusion solution (outer tip diameter of 5 to 10 μm; resistance, 30 to 50 MΩ), stained with lissamine green B, and connected by Ag/AgCl electrodes to the amplifier (Turbo TEC-05X or EXT-02F amplifier and the CellWorks Lite 5.1 software, NPI Electronics, Tamm, Germany). Before micropuncture, electrode offset was performed in perfusion solution applied to the kidney surface. The position of the microelectrode was verified by short pressure-driven boluses of lissamine green B–stained perfusion solution from the pipette (Figure 1, E and F; Supplemental Movies 1 and 2).
Mathematical estimations and figures were generated using Prism 4 for Mac, Adobe Photoshop CS, Illustrator CS, and Adobe 6.0 professional software.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by TP17 SFB/Transregio 57 of the German Research Foundation (DFG), the NephCure Foundation (to M.J.M.), an ERC-OPEN Pathfinder Grant and Boost Fund OPBo45 of the Excellence Initiative by the DFG (to M.J.M., R.H., and H.E.), and a START grant by the Medical Faculty of the RWTH Aachen (to G.B. and M.J.M.). We thank Profs. J. Greven and H. Osswald for advice. We apologize for not having cited all relevant papers because of size limitations.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorials, “The Glomerular Filtration Barrier and Molecular Segregation: Guilty as Charged?” on pages 2009–2011, and “A New Role for Charge of the Glomerular Capillary Membrane,” on pages 2011–2013.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Rippe B, Haraldsson B: Transport of macromolecules across microvascular walls: The two-pore theory. Physiol Rev 74: 163–219, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Deen WM, Bohrer MP, Brenner BM: Macromolecule transport across glomerular capillaries: Application of pore theory. Kidney Int 16: 353–365, 1979 [DOI] [PubMed] [Google Scholar]
- 3. Wolgast M, Kallskog O, Wahlstrom H: Characteristics of the glomerular capillary membrane of the rat kidney as a hydrated gel. II. On the validity of the model. Acta Physiol Scand 158: 225–232, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Schaffner A, Rodewald R: Glomerular permeability in the bullfrog Rana catesbeiana. J Cell Biol 79: 314–328, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haraldsson B, Nystrom J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chang RL, Deen WM, Robertson CR, Brenner BM: Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int 8: 212–218, 1975 [DOI] [PubMed] [Google Scholar]
- 7. Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, Brenner BM: Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest 61: 72–78, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanner GA, Kinter WB: Reabsorption and secretion of p-aminohippurate and Diodrast in Necturus kidney. Am J Physiol 210: 221–231, 1966 [DOI] [PubMed] [Google Scholar]
- 9. Delgado AV, Gonzalez-Caballero F, Hunter RJ, Koopal LK, Lyklema J: Measurement and interpretation of electrokinetic phenomena. Pure Appl Chem 77: 1753–1805, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Yang C, Li D: Electrokinetic effects on pressure-driven liquid flows in rectangular microchannels. J Colloid Interface Sci 194: 95–107, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Lyklema J: Quest for ion-ion correlations in electric double layers and overcharging phenomena. Adv Colloid Interface Sci 147–148: 205–213, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Lyklema J: Overcharging, charge reversal: Chemistry or physics? Colloids Surfaces Physicochem Engineer Aspects 291: 3–12, 2006 [Google Scholar]
- 13. Quesada-Perez M, Martin-Molina A, Galisteo-Gonzalez F, Hidalgo-Alvarez R: Electrophoretic mobility of model colloids and overcharging: Theory and experiment. Molec Physics 100: 3029–3039, 2002 [Google Scholar]
- 14. Freundlich H: Kapillarchemie, Frankfurt am Main, Germany, Akademische Verlagsgesellschaft, 1922, p 588 ff. [Google Scholar]
- 15. Knecht V, Risselada HJ, Mark AE, Marrink SJ: Electrophoretic mobility does not always reflect the charge on an oil droplet. J Colloid Interface Sci 318: 477–486, 2008 [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A: Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol 77: 445–473, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen PB, Saykally RJ: Is the liquid water surface basic or acidic? Macroscopic vs. molecular-scale investigations. Chem Phys Lett 458: 255–261, 2008 [Google Scholar]
- 18. Vacha R, Buch V, Milet A, Devlin JP, Jungwirth P: Autoionization at the surface of neat water: Is the top layer pH neutral, basic, or acidic? Phys Chem Chem Phys 9: 4736–4747, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B: Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol Renal Physiol 284: F1226–F1234, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Ciarimboli G, Schurek HJ, Zeh M, Flohr H, Bokenkamp A, Fels LM, Kilian I, Stolte H: Role of albumin and glomerular capillary wall charge distribution on glomerular permselectivity: Studies on the perfused-fixed rat kidney model. Pflugers Arch 438: 883–891, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Kelley VE, Cavallo T: Glomerular permeability: Transfer of native ferritin in glomeruli with decreased anionic sites. Lab Invest 39: 547–553, 1978 [PubMed] [Google Scholar]
- 22. Vehaskari VM, Chang CT, Stevens JK, Robson AM: The effects of polycations on vascular permeability in the rat. A proposed role for charge sites. J Clin Invest 73: 1053–1061, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pujar NS, Zydney AL: Electrostatic and electrokinetic interactions during protein transport through narrow pore membranes. Ind Engin Chem Res 33: 2473–2482, 1994 [Google Scholar]
- 24. Robinson RR, Lecocq FR, Phillippi PJ, Glenn WG: Fixed and reproducible orthostatic proteinuria. III. Effect of induced renal hemodynamic alterations upon urinary protein excretion. J Clin Invest 42: 100–110, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hahnel B, Grone HJ, Koesters R, Kriz W: Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol 175: 1883–1895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.