Abstract
Rapid correction of chronic hyponatremia can lead to osmotic demyelination syndrome (ODS), a severe demyelination disease. The microglia that accumulate in the demyelinative lesions may play a detrimental role in the pathogenesis of ODS by producing proinflammatory cytokines, suggesting that they may be a target for therapeutic intervention. Here, we investigated whether minocycline, a selective and potent inhibitor of microglial activation, could protect against ODS in rats. We induced hyponatremia by liquid diet feeding and dDAVP infusion. Rapid correction of the hyponatremia 7 days later resulted in neurologic impairment with severe demyelinative lesions. Activated microglia accumulated at the site of demyelination. Treatment with minocycline within 24 hours of rapid correction, however, was protective: rats exhibited minimal neurologic impairment, and survival improved. Histologic analysis showed that minocycline inhibited demyelination and suppressed the accumulation of microglia at the site of demyelination. Real-time RT-PCR and immunohistochemical analyses showed that minocycline inhibited the activity of microglia and the expression of inflammatory cytokines (e.g. IL-1β, inducible nitric-oxide synthase, and TNF-α), monocyte chemoattractant protein-1, and matrix metalloproteinase-12 in microglia. These results demonstrate that minocycline can protect against ODS by inhibiting the activation and accumulation of microglia at the site of demyelinative lesions, suggesting its possible use in clinical practice.
Hyponatremia is the most common electrolyte disorder encountered in clinical practice. Although hyponatremia is associated with neurologic symptoms, sometimes leading to death in severe cases, too rapid a correction of hyponatremia can produce osmotic demyelination syndrome (ODS), a demyelinating disease that is also associated with neurologic morbidity and mortality.1 In humans, the demyelinative lesion is preferentially found in the central pons and is thus called central pontine myelinolysis.2 There is relative agreement about the rate of correction to avoid ODS (<10 mmol/L in 24 hours and <18 mmol/L in 48 hours).3 However, efforts to improve the neurologic symptoms sometimes lead to unintentional excessive correction.4 In addition, a vasopressin antagonist has been recently approved to treat hyponatremia.5,6 To date, there have been no reported cases of ODS caused by a vasopressin antagonist; however, it is possible that the vasopressin antagonist treatment for severe hyponatremia may be associated with an increased risk for ODS.7 Therefore, understanding the mechanism of ODS associated with the correction of hyponatremia and the development of a therapy, if the correction of hyponatremia was too rapid, to prevent ODS are necessary.
Although its pathogenesis remains to be clarified, we previously reported that dexamethasone could prevent the disruption of the blood-brain barrier (BBB) that is caused by the rapid correction of hyponatremia and the associated demyelination in rats.8 We also reported that the microglia that accumulate in the demyelinative lesions may play a detrimental role in the pathogenesis of ODS by producing proinflammatory cytokines.9
Minocycline is a semisynthetic tetracycline derivative that exerts anti-inflammatory effects independent of its antimicrobial action. It is a highly lipophilic molecule capable of crossing the BBB.10 Minocycline exerts its neuroprotective effect by inhibiting the activation of microglia in experimental models, such as ischemic stroke,11 multiple sclerosis,12 spinal-cord injury,13 Parkinson's disease,14 Huntington's disease,15 and amyotrophic lateral sclerosis.16 Tikka et al.17 reported that the protective effect of minocycline was associated with the reduction of specific cytokines (IL-1β, inducible nitric-oxide synthase [iNOS], and TNF-α) that are mainly expressed by microglia and that the proliferation of microglia was also inhibited by minocycline. Thus, in this study, we investigated whether minocycline could protect against ODS by inhibiting the activation of microglia in rats.
RESULTS
Effects of Minocycline on the Neurologic Symptoms
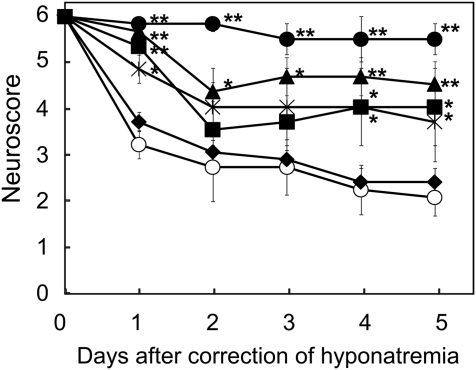
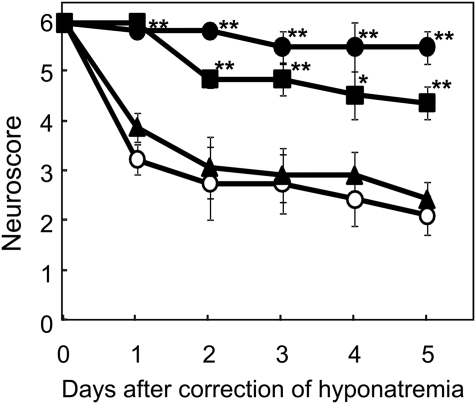
The time-dependent effects of minocycline on the neurologic scores after the rapid correction of hyponatremia are shown in Figure 1. Animals that were not treated with minocycline (MINO (−) group) showed severe neurologic impairments. Minocycline administration within 24 hours after the correction significantly improved the animals' neurologic scores. The administration of minocycline (45 mg/kg body wt) at 0, 12, and 24 hours after the hypertonic saline injection (MINO (0,12,24) group) was the most effective treatment among the groups. In contrast, a minocycline injection at 12 hours before the correction failed to prevent ODS. The dose-dependent effects of minocycline on the neurologic scores after the rapid correction of hyponatremia are shown in Figure 2. The administration of 4.5 as well as 45 mg/kg body wt of minocycline significantly improved the neurologic symptoms after the correction of hyponatremia. The dose of 4.5 mg/kg body wt is similar to the dose commonly used in clinical practice (3 mg/kg body wt). However, 0.45 mg/kg body wt of minocycline failed to improve the neurologic symptoms. These results indicate that minocycline improved the neurologic symptoms in a dose-dependent manner. With regard to the effect of minocycline on mortality, five of six rats died within 8 days after the rapid correction of hyponatremia; meanwhile, all rats (six of six) in the MINO (45 mg/kg body wt) (0,12,24) group and five of six rats in the MINO (4.5 mg/kg body wt) (0,12,24) group survived by 8 days after the correction of hyponatremia. There was a significant correlation between the treatment and mortality by day 8 after the rapid correction of hyponatremia (MINO (45 mg/kg body wt) and MINO (4.5 mg/kg body wt), χ2 test P = 0.003 and P = 0.021, respectively), demonstrating that minocycline significantly reduced mortality after the correction of hyponatremia. Minocycline did not affect serum sodium levels after correction (Supplemental Table 1).
Figure 1.
Minocycline administration within 24 hours after the rapid correction of hyponatremia significantly improves the neurologic symptoms. The neurologic symptoms were assessed using neuroscores as follows: 6, no impairment; 5, poorly grooming; 4, slow or awkward gait; 3, limb weakness and/or paralysis; 2, seizures, severe motor deficits; 1, complete inability to move; and 0, death. MINO (−) group (○), MINO (0,12,24) group (●), MINO (0) group (■), MINO (12) group (▴), MINO (24) group (*), and MINO (−12) group (♦). Minocycline of 45 mg/kg body wt was injected intraperitoneally. The values are expressed as the means ± SEM (n = 6). **P < 0.01; *P < 0.05 compared with the MINO (−) group.
Figure 2.
Minocycline improves the neurologic symptoms in a dose-dependent manner. MINO (−) group (○), MINO (45 mg/kg body wt) group (●), MINO (4.5 mg/kg body wt) group (■), and MINO (0.45 mg/kg body wt) group (▴). The values are expressed as the means ± SEM (n = 6). **P < 0.01; *P < 0.05 compared with the MINO (−) group.
Demyelinative Lesions after the Rapid Correction of Hyponatremia
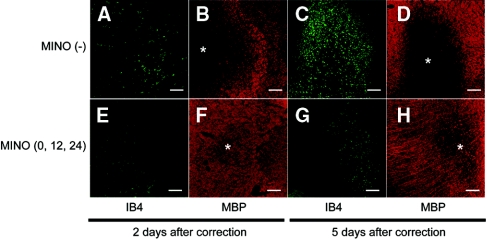
To evaluate the demyelination and the distribution of microglia, we performed double immunostaining for myelin basic protein (MBP), a marker of myelin, and IB4, a marker of microglia. In the MINO (−) group, demyelinative lesions were observed in various regions. The most prominent demyelination was seen in the subcortical white matter and midbrain. Representative lesions in the midbrain are shown in Figure 3. At 2 days after correction in the MINO (0,12,24) group, only mild demyelinative lesions were observed (Figure 3F), and microglia were mildly accumulated at the lesion (Figure 3E). At 5 days after correction, the demyelination (Figure 3H) and the accumulation of microglia (Figure 3G) in the MINO (0,12,24) group were much milder than those of the MINO (−) group (Figure 3, D and C, respectively). These results suggest that minocycline treatment attenuated the subsequent progression of demyelination by inhibiting the migration of microglia to the demyelinative lesions. In the noncorrected group, no demyelinative lesions were observed (data not shown).
Figure 3.
Minocycline treatment attenuates the progression of demyelination by inhibiting the migration of microglia to the demyelinative lesions. Double staining for MBP (B, D, F, and H) and IB4 (A, C, E, and G). (A through D) MINO (−) group. (E through H) MINO (0,12,24) group. (A, B, E, and F) and (C, D, G, and H), 2 and 5 days after the rapid correction of hyponatremia, respectively. Demyelinative lesions were observed (B) and IB4-positive microglia were mildly accumulated at the demyelinative lesion (A) at 2 days after correction in the MINO (−) group. At 5 days after correction in the MINO (−) group, prominent demyelination was observed (D) and markedly increased numbers of microglia accumulated at the lesion (C). In contrast, only mild demyelinative lesions were observed (F), and microglia were mildly accumulated at the lesion (E) at 2 days after correction in the MINO (0,12,24) group. At 5 days after correction, the demyelination (H) and the accumulation of microglia (G) in the MINO (0,12,24) group were much milder than those of the MINO (−) group (D and C, respectively). All of the sections are from the midbrain. The scale bars show 200 μm. *Demyelinative lesion.
Evaluation of BBB Disruption
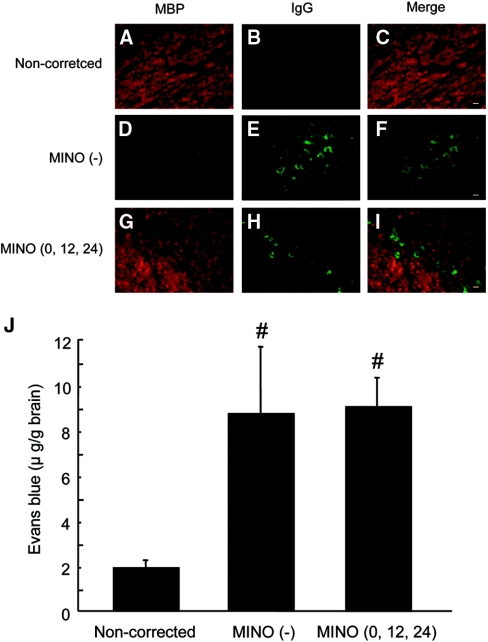
Because we previously reported that dexamethasone could prevent ODS by inhibiting the disruption of the BBB after the correction of hyponatremia, we used IgG immunostaining to determine whether minocycline treatment prevented its disruption. IgG extravasation was observed in the MINO (0,12,24) group (Figure 4H) as well as in the MINO (−) group (Figure 4E). BBB permeability, quantified by Evans blue (EB) dye extravasation, was significantly increased in the MINO (0,12,24) and MINO (−) groups compared with the noncorrected group, and there were no significant differences between the MINO (0,12,24) and MINO (−) groups (Figure 4J). Taken together with the results of the IgG staining, these results indicate that minocycline did not prevent the disruption of the BBB.
Figure 4.
Minocycline does not prevent the disruption of the BBB. Double immunostaining for MBP (A, D, and G) and IgG (B, E, and H). Merged images (C, F, and I). (A through C) noncorrected group. (D through F) MINO (−) group. (G through I) MINO (0,12,24) group. In the noncorrected group, no demyelinative lesions were observed (A), and IgG staining was not detected (B). In the MINO (−) group, severe demyelination was observed (D), and strong IgG staining was detected around the vessels in the demyelinative lesions (E and F). The demyelination observed in the MINO (0,12,24) group (G) was mild in comparison with that of the MINO (−) group (D), whereas IgG extravasation was observed in the MINO (0,12,24) group (H and I). The brains were removed 2 days after the rapid correction of hyponatremia. The scale bars show 20 μm. (J) BBB permeability was quantified 24 hours after correction. All of the values are expressed as the mean ± SEM (n = 5). #P < 0.05 compared with the noncorrected group.
mRNA Expression of Proinflammatory Cytokines
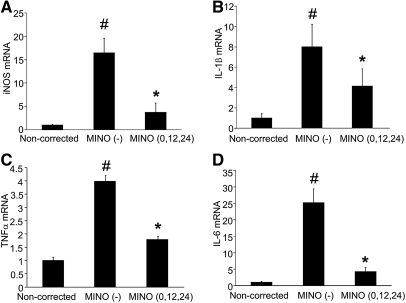
To examine the expression of proinflammatory cytokines, we performed real-time PCR analysis for iNOS, IL-1β, TNF-α, and IL-6. The expression of iNOS, IL-1β, TNF-α, and IL-6 was significantly increased in the MINO (−) group, but they were diminished in the MINO (0,12,24) group, close to the levels observed in the noncorrected animals (Figure 5).
Figure 5.
Minocycline inhibits mRNA expression of proinflammatory cytokines. The mRNA expression of iNOS (A), IL-1β (B), TNF-α (C), and IL-6 (D) was significantly increased in the MINO (−) group compared with the noncorrected group and was significantly decreased by minocycline treatment. The amount of mRNA was determined using quantitative RT-PCR. The values are normalized to β-actin RNA. All of the values are expressed as the means ± SEM (n = 4). #P < 0.05 compared with the noncorrected group. *P < 0.05 compared with the MINO (−) group.
Cell Type-specific Expression of Proinflammatory Cytokines
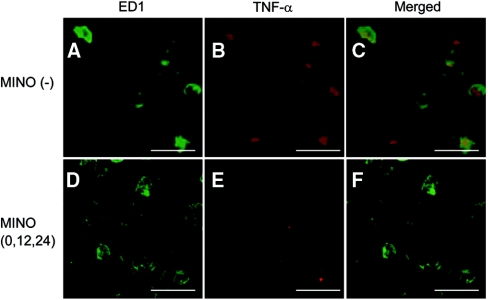
We examined the cell type-specific expression of proinflammatory cytokines using immunohistochemistry. Strong immunoreactivity of TNF-α was present in ED1-positive microglia (Figure 6, A through C). In addition, the immunoreactivity of ED1, a marker of activated rat microglia/monocyte/macrophages, and TNF-α was weaker in the MINO (0,12,24) group compared with the MINO (−) group (Figure 6). The MINO (0,12,24) treatment also inhibited the increased immunoreactivity of iNOS, IL-1β, and IL-6 after rapid correction of hyponatremia (data not shown). Together with the results from the mRNA expression of proinflammatory cytokines, these data indicate that minocycline inhibited the activity of microglia and the expression of inflammatory cytokines by microglia after the rapid correction of hyponatremia.
Figure 6.
Minocycline inhibits the activity of microglia and the expression of TNF-α in microglia. (A, B, D, and E) Double immunostaining for TNF-α (B and E) and ED1 (A and D). (C and F) Merged images. (A through C) MINO (−) group. (D through F) MINO (0,12,24) group. Immunoreactivity of ED1 (D) and TNF-α (Ε) was reduced in the MINO (0,12,24) group compared with those of the MINO (−) group (A and B, respectively). The scale bars show 20 μm.
Effect of Minocycline on Chemokines in Microglia in Demyelinative Lesions
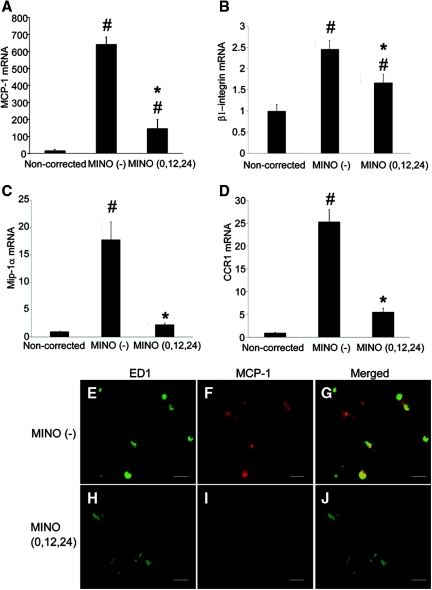
To examine the involvement of chemokines in microglial migration, we performed real-time PCR analysis for monocyte chemoattractant protein-1 (MCP-1), β1-integrin, macrophage inflammatory protein-1α (Mip-1α), and chemokine receptor 1 (CCR1). Their expression was markedly increased in the MINO (−) group as compared with the noncorrected group, and minocycline significantly decreased their expression (Figure 7, A through D). Double immunostaining for ED1 and MCP-1 revealed strong immunoreactivity for ED1 and MCP-1 in microglia of the MINO (−) group (Figure 7, E through G), whereas it was reduced in the MINO (0,12,24) group (Figure 7, H through J). These results suggest that minocycline suppressed microglial migration by inhibiting the activity of microglia and the expression of chemokines.
Figure 7.
Minocycline suppresses the expression of chemokines in microglia. (A through D) The mRNA expression of MCP-1 (A), β1-integrin (B), Mip-1α (C), and CCR1 (D) was significantly increased in the MINO (−) group as compared with the noncorrected group and was significantly decreased by minocycline treatment. The amount of mRNA was determined using quantitative RT-PCR. The values are normalized to β-actin RNA. All of the vals are expressed as the means ± SEM (n = 5). #P < 0.05 compared with the noncorrected group. *P < 0.05 compared with the MINO (−) group. (E through J) Double immunostaining for ED1 (E and H) and MCP-1 (F and I). (E through G) MINO (−) group. (H through J) MINO (0,12,24) group. Immunoreactivity of ED1 (H) and MCP-1 (I) was reduced in the MINO (0,12,24) group compared with those of the MINO (−) group (E and F, respectively). The scale bars show 20 μm.
Effect of Minocycline on Matrix Metalloproteinases (MMPs) in Microglia in Demyelinative Lesions
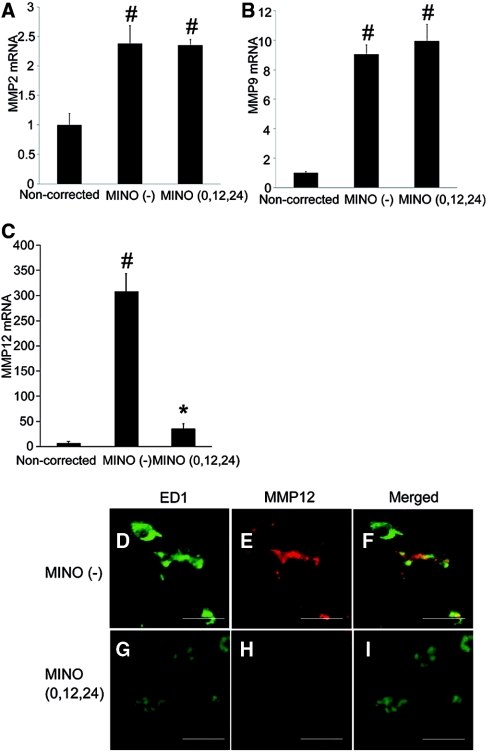
To examine the involvement of MMPs in ODS, we performed real-time PCR analysis for MMP2, MMP9, and MMP12. Their expression was increased in the MINO (−) group as compared with the noncorrected group, and minocycline significantly decreased the expression of MMP12 only (Figure 8, A through C). We performed double immunostaining for ED1 and MMP12. Strong immunoreactivity for MMP12 was detected in microglia in the MINO (−) group (Figure 8, D through F), whereas it was reduced in the MINO (0,12,24) group (Figure 8, G through I). Although minocycline is known to be a direct inhibitor of MMP enzymatic activity, our results suggest that MMP12 is involved in the pathogenesis of ODS, and the beneficial effect of minocycline is, in part, due to the inhibition of MMP12 expression.
Figure 8.
Minocycline suppresses the expression of MMP12 in microglia. (A through C) The mRNA expression of MMP2 (A), ΜΜP9 (B), and MMP12 (C) was significantly increased in the MINO (−) group as compared with the noncorrected group. The mRNA expression of MMP12 was significantly decreased by minocycline treatment (C). The amount of mRNA was determined using quantitative RT-PCR. The values are normalized to β-actin RNA. All of the values are expressed as the means ± SEM (n = 5). #P < 0.05 compared with the noncorrected group. *P < 0.05 compared with the MINO (−) group. (D through I) Double immunostaining for ED1 (D and G) and MMP12 (E and H). (D through F) MINO (−) group. (G through I) MINO (0,12,24) group. Immunoreactivity of ED1 (G) and MMP12 (Η) was reduced in the MINO (0,12,24) group compared with those of the MINO (−) group (D and E, respectively). The scale bars show 20 μm.
DISCUSSION
Minocycline is a potent inhibitor of microglial activation. In vitro, minocycline attenuates LPS-stimulated inflammatory cytokines in BV-2 microglia cultures. Recently, minocycline has been demonstrated to have neuroprotective effects in animal models of neurologic dysfunction. Minocycline can penetrate the BBB. Yrjanheikki et al.11 reported that minocycline prevented microglial activation; the expression of caspase 1 and inducible nitric-oxide synthase was reduced after treatment with minocycline in a model of forebrain ischemia. Tikka et al.17 reported that minocycline was protective against N-methyl-d-aspartate neurotoxicity by inhibiting microglia, but not astrocytes, in vitro using primary mixed glial and microglial cultures. However, the pathogenesis of ODS remains unclear; it has been reported that microglia accumulate at the site of the demyelinative lesions in humans and in animal models, and we previously reported that these microglia expressed proinflammatory cytokines and may play a detrimental role in the pathogenesis of ODS.9 These findings prompted us to test the beneficial effect of minocycline on ODS. In this study, minocycline markedly improved the neurologic symptoms and mortality after the rapid correction of hyponatremia. Minocycline prominently reduced the expression of proinflammatory cytokines, suggesting that the enhanced expression of proinflammatory cytokines in microglia plays a critical role in the pathogenesis of ODS and that minocycline prevents ODS by inhibiting the expression of these cytokines.
In this study, we found that the expression of chemokines, including MCP-1, β1-integrin, Mip-1α, and CCR1, was increased in microglia at the site of demyelination after the rapid correction of hyponatremia, and their expression was decreased by minocycline treatment. Chemokines and β1-integrin play a role in inflammation and cell migration. MCP-1 recruits and enhances the activation of monocytes.18 The anti-inflammatory actions of minocycline in the brain are produced by the modulation of chemokine function.19 Minocycline treatment decreases Mip-1α and CCRs mRNA levels in hypoxic-ischemic neonatal rat brain and prevents the recruitment of microglia to injury sites in the brain. In vitro, treating cells with minocycline significantly decreases MCP-1 secretion.20 Minocycline has also been reported to decrease microglial motility and β1-integrin expression.21 Taken together, our data suggest that minocycline reduced the activation and accumulation of microglia at the site of demyelinative lesions by inhibiting the expression of chemokines and β1-integrin, thereby preventing the progression of ODS.
Although it has been reported that MMPs are upregulated and involved in the pathogenesis of neurologic disorders including multiple sclerosis, an autoimmune demyelinative disease, the role of MMPs in ODS is not known. In this study, the expression of MMPs (i.e. MMP2, MMP9, and most notably MMP12) was increased after the rapid correction of hyponatremia, and minocycline treatment significantly decreased MMP12 expression in microglia at the site of demyelinative lesions. MMP12 is not expressed in the healthy brain and is highly expressed by microglia in an multiple sclerosis mouse model,22 and minocycline decreases MMP12 activity;12 therefore, our results suggest that the expression of MMP12 in active microglia is involved in the pathogenesis of ODS, and the beneficial effect of minocycline is, in part, due to the inhibition of MMP12 expression. Meanwhile, MMP9 is associated with the disruption of the BBB and MMP9 gene knockout reduced the proteolytic degradation of the BBB.23 In this study, the disruption of the BBB occurred after the rapid correction of hyponatremia, and minocycline did not prevent its disruption; therefore, it is possible that MMP9 expression is relevant to BBB disruption after the correction of hyponatremia. Clearly, more work needs to be done in this area.
One of the interesting findings in this study is that the administration of minocycline at 24 hours after the rapid correction of hyponatremia significantly improved the neurologic symptoms. We previously reported that dexamethasone treatment effectively prevented ODS when it was administered soon after the correction; however, dexamethasone administration at 6 hours after the correction, when the disruption of the BBB occurred, did not prevent ODS, suggesting that dexamethasone prevented ODS by inhibiting the disruption of the BBB. The disruption of the BBB as a consequence of the rapid correction of hyponatremia has been thought to be involved in the pathogenesis of ODS.24,25 However, the extravasation of IgG and EB dye as well as the efficacy of minocycline administration after the disruption of the BBB demonstrate that BBB permeability is not involved in the preventive mechanism of minocycline on ODS. Alternatively, as Kengne et al.26 reported, the increased permeability of BBB after the rapid correction of hyponatremia may not be essential in the pathogenesis of ODS.
Few therapeutic interventions are available for ODS; however, the reinduction of hyponatremia after the rapid correction of hyponatremia reduces mortality in rats.26,27 Other interventions that reduced mortality after the rapid correction of hyponatremia include the administration of myoinositol28 or urea,29 which affect the brain's organic osmolyte content. However, it is sometimes difficult to control the serum sodium levels or administer compounds such as myoinositol and urea in clinical practice. As discussed in the previous paragraph, because minocycline is considered to be more effective than dexamethasone in terms of its broad time range of efficacious action after the correction of hyponatremia, has an established history of safety, and has been well tolerated by patients, minocycline may be a better choice than dexamethasone to prevent ODS when the correction of severe hyponatremia is planned in clinical practice.
In conclusion, our data suggested that proinflammatory cytokines, chemokines, adhesion molecules, and MMPs are implicated in the activation and accumulation of microglia at the site of demyelinative lesions after the rapid correction of hyponatremia. Minocycline markedly prevented ODS by inhibiting the activation and accumulation of microglia. Thus, minocycline may represent a beneficial therapeutic strategy for ODS in clinical practice.
CONCISE METHODS
Animal Experiments
Male Sprague-Dawley rats (body weight, 250–300 g; Chubu Science Materials, Nagoya, Japan) were housed in a standard animal facility under conditions of constant temperature (23°C) and a 12-hour/12-hour light/dark cycle. The rats had ad libitum access to standard chow and tap water until the induction of hyponatremia. Hyponatremia was induced in rats using the previously described methods.8,30 Briefly, osmotic minipumps (Alzet model 2002; Alza, Palo Alto, California) containing the vasopressin V2 receptor agonist, 1-deamino-8-d-arginine vasopressin (dDAVP, 10 μg/ml; Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) were implanted subcutaneously under ether anesthesia, and dDAVP was injected continuously at a rate of 5 ng/h. After 2 days of dDAVP administration, the rats were water loaded by substituting their daily feed with a liquid formula (Isocal plus; Mead-Johnson, Evansville, Indiana). Seven days after the start of liquid-formula feeding, the serum sodium levels were rapidly corrected by a bolus injection of 1.0 m NaCl (1.5 ml/100 g body weight) administered intraperitoneally. Minocycline (45, 4.5, and 0.45 mg/kg body wt; Sigma Chemicals, St. Louis, Missouri) was injected intraperitoneally at 0, 12, and 24 hours (MINO (0,12,24) group), at 0 hours (MINO (0) group), at 12 hours (MINO (12) group), or at 24 hours (MINO (24) group) after hypertonic saline injection or at 12 hours before hypertonic saline injection (MINO (−12) group). Control animals (MINO (−) group) were injected with the equivalent volume of saline. Because microglial activation was observed at 2 days but not at 5 days after the rapid correction of hyponatremia in this study, we hypothesized that early treatment with minocycline would inhibit the early activation and accumulation of microglia in the demyelinative lesions, thereby preventing the demyelination. The neurologic symptoms in all animals were independently evaluated by three experienced individuals. One person knew the experimental status of the animals; however, the other two individuals were blinded to the treatment conditions. The neurologic symptoms after the rapid correction of hyponatremia were assessed using neurologic scores as previously reported8: 6, no impairment; 5, poorly grooming; 4, slow or awkward gait; 3, limb weakness and/or paralysis; 2, seizures, severe motor deficits; 1, complete inability to move; and 0, death. Blood samples for the determination of sodium serum levels were obtained from the tail vein under light ether anesthesia. All of the procedures were performed in accordance with the institutional guidelines for animal care at Nagoya University, which conform to the National Institutes of Health animal care guidelines.
Determination of BBB Permeability by EB Dye Extravasation
EB is a diazo dye that binds to albumin in vivo and is used as a tracer for serum albumin. To quantify the permeability of the BBB, EB dye extravasation was determined as described previously.8 Briefly, EB (2% in saline; 0.3 ml/100 mg body weight) was injected intravenously 24 hours after the correction of hyponatremia. After EB administration, the rats were perfused transcardially with saline to wash out the intravascular EB dye, under ether anesthesia. The brains were then removed, and each hemisphere was quickly frozen. EB was extracted from brain homogenates by precipitation with TCA and verified spectrophotometrically.
Quantitative Reverse Transcription (RT)-PCR
After decapitation, the brains were removed and stored at −80°C until use. Total RNA was extracted from coronal brain sections (150 μm) containing demyelinative lesions using an RNeasy Mini Kit (Qiagen, Valencia, California), following the manufacturer's instructions. One μg of RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, California) in a final volume of 25 μl. Real-time PCR was performed on the reverse transcription products using SYBR Premix Ex TaqII (Takara Bio Inc., Otsu, Japan) within an ABI PRISM 7900 HT (Applied Biosystems, Foster City, CA), with cycling parameters (40 cycles of 95°C for 5 seconds and 60°C for 30 seconds), following the manufacturer's instructions. The primers used for this experiment were designed and synthesized by Takara Bio Inc. (Otsu, Japan). These sequences are indicated in Table 1. All of the samples were run in duplicate. The mRNA levels were compared using the Ct method (Applied Biosystems), which was normalized by β-actin. The experiments were repeated independently at least three times. In each experiment, a negative (RT minus) control was included.
Table 1.
Primer sequences for real-time PCR analysis
| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| iNOS forward | CTGTGTGCCTGGAGGTTCTAGATG |
| iNOS reverse | AAGTAGGTGAGGGCTTGCCTGA |
| IL-1β forward | GCTGTGGCAGCTACCTATGTCTTG |
| IL-1β reverse | AGGTCGTCATCATCCCACGAG |
| TNF-α forward | AACTCGAGTGACAAGCCCGTAG |
| TNF-α reverse | GTACCACCAGTTGGTTGTCTTTGA |
| IL-6 forward | CCACTTCACAAGTCGGAGGCTTA |
| IL-6 reverse | GTGCATCATCGCTGTTCATACAATC |
| MCP-1 forward | CTATGCAGGTCTCTGTCACGCTTC |
| MCP-1 reverse | CAGCCGACTCATTGGGATCA |
| β1-Integrin forward | TGCGATAGGTCCAACGGCTTA |
| β1-Integrin reverse | CATTTGTCGCTACGCATGGAAC |
| Mip-1α forward | GGATGGTGGAGCCATCAAGAA |
| Mip-1α reverse | AGATGCTTGGCCCAGATTTGTTAC |
| CCR1 forward | CAGCTGGACCTGGCCATACA |
| CCR1 reverse | GGTACTTCCGGAACCGCTCA |
| MMP2 forward | CCAAGAACTTCCGACTATCCAATGA |
| MMP2 reverse | CAGTGTAGGCGTGGGTCCAGTA |
| MMP9 forward | TCCAGTAGACAATCCTTGCAATGTG |
| MMP9 reverse | CTCCGTGATTCGAGAACTTCCAATA |
| MMP12 forward | CTGGGCAACTGGACACCTCA |
| MMP12 reverse | ATCCGCACGCTTCATGTCTG |
| β-Actin forward | GGAGATTACTGCCCTGGCTCCTA |
| β-Actin reverse | GACTCATCGTACTCCTGCTTGCTG |
iNOS, inducible nitric-oxide synthase; TNF-α, tumor necrosis factor-α; IL-1β, IL-6, interleukin-1β, −6; MCP-1, monocyte chemoattractant protein-1: Mip-1α, macrophage inflammatory protein-1α; CCR1 chemokine receptor 1; MMP matrix metalloproteinases.
Histologic Evaluations
The rats were sacrificed, under deep ether anesthesia, by transcardial perfusion with 4% paraformaldehyde in PBS at 2 or 5 days after the rapid correction of hyponatremia. The brains were removed, postfixed in 4% paraformaldehyde in PBS, and cryoprotected, and cryostat sections (7 μm) were made as described previously.8 The following primary antibodies were used for immunohistochemistry: monoclonal anti-bovine myelin basic protein antibody (1:10; Chemicon International, Inc., Temecula, California), rabbit polyclonal anti-TNF-α antibody (1:250; PEPROTECH EC Ltd., London, United Kingdom), rabbit polyclonal anti-MCP-1 antibody (1:100; Abcam Inc.), rabbit monoclonal anti-MMP12 antibody (1:100; Epitomics, Inc.), and mouse monoclonal anti-ED1 antibody (1:100; Serotec, Oxford, United Kingdom), a marker specific for activated rat microglia/monocytes/macrophages, following the manufacturer's guidelines. After washing, the sections were incubated for 1 hour at room temperature with the secondary antibodies: A11031 Alexa Fluor 568 goat anti-mouse IgG (H+L) highly cross-adsorbed (1:500; Molecular Probes, Inc., Invitrogen), A11036 Alexa Fluor 568 goat anti-rabbit IgG (H+L) highly cross-adsorbed (1:500; Molecular Probes, Inc., Invitrogen), and A11029 Alexa Fluor 488 goat anti-mouse IgG (H+L) highly cross-adsorbed (1:500; Molecular Probes, Inc., Invitrogen). Isolectin B4 (IB4)-FITC (Sigma) was also used for detection of microglia after incubation with the secondary antibody. IgG immunostaining was performed to evaluate the disruption of the BBB at 2 days after the correction of hyponatremia. The sections were immunostained with an anti-rat IgG-FITC antibody (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, California) as described previously.8 All fluorescently stained sections were examined with a fluorescence microscope (BZ-8000; Keyence, Osaka, Japan).
Statistical Analyses
The results are expressed as the means ± SEM, and statistical analyses were performed using one-way ANOVA followed by Fisher's projected least significant difference test. The Mann-Whitney nonparametric test was used to evaluate the neurologic scores. P values less than 0.05 were considered to be significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Michiko Yamada and Keiko Inazumi for excellent technical assistance. This work was supported in part by a Grant-in-aid for Scientific Research from Japanese Society for the Promotion of Science 21500349 (to Y. Sugimura) and by Grants-in-Aid for Scientific Research (Research on Hypothalamo-hypophyseal Disorders) from the Ministry of Health, Labor and Welfare, Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Managing Overly Rapid Correction of Chronic Hyponatremia: An Ounce of Prevention or a Pound of Cure?” on pages 2015–2016, and related article, “Minocycline Protects against Neurologic Complications of Rapid Correction of Hyponatremia,” on pages 2099–2108.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Norenberg MD, Leslie KO, Robertson AS: Association between rise in serum sodium and central pontine myelinolysis. Ann Neurol 11: 128–135, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Wright DG, Laureno R, Victor M: Pontine and extrapontine myelinolysis. Brain 102: 361–385, 1979 [DOI] [PubMed] [Google Scholar]
- 3. Sterns RH, Cappuccio JD, Silver SM, Cohen EP: Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J Am Soc Nephrol 4: 1522–1530, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH: Hypertonic saline for hyponatremia: Risk of inadvertent overcorrection. Clinical J Am Soc Nephrol 2: 1110–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. SALT Investigators: Tolvaptan, a selective oral vasopressin V-2-receptor antagonist, for hyponatremia. N Engl J Med. 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, Czerwiec FS: Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol 21: 705–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sterns RH, Nigwekar SU, Hix JK: The Treatment of Hyponatremia. Semin Nephrol. 29: 282–299, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, Murata Y: Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol 192: 178–183, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Takefuji S, Murase T, Sugimura Y, Takagishi Y, Hayasaka S, Oiso Y, Murata Y: Role of microglia in the pathogenesis of osmotic-induced demyelination. Exp Neurol 204: 88–94, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM: The promise of minocycline in neurology. Lancet Neurol 3: 744–751, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J: Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 95: 15769–15774, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW: Targeting leukocyte MMPs and transmigration: Minocycline as a potential therapy for multiple sclerosis. Brain 125: 1297–1308, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W: Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24: 2182–2190, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S: Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22: 1763–1771, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen M, Ona VO, Li MW, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JHJ, Friedlander RM: Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6: 797–801, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Zhu S, Stavrovskaya IG, Drozda M, Kim BYS, Ona V, Li MW, Sarang S, Liu AS, Hartley DM, Du CW, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM: Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417: 74–78, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Tikka TM, Koistinaho JE: Minocycline provides neuroprotection against N-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol 166: 7527–7533, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M: Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 144: 110–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremlev SG, Roberts RL, Palmer C: Minocycline modulates chemokine receptors but not interleukin-10 mRNA expression in hypoxic-ischemic neonatal rat brain. J Neurosci Res 85: 2450–2459, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Mitchell RM, Lee SY, Randazzo WT, Simmons Z, Connor JR: Influence of HFE variants and cellular iron on monocyte chemoattractant protein-1. J Neuroinflamm 6: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nutile-McMenemy N, Elfenbein A, DeLeo JA: Minocycline decreases in vitro microglial motility, beta(1)-integrin, and Kv1.3 channel expres sion. J Neurochem 103: 2035–2046, 2007 [DOI] [PubMed] [Google Scholar]
- 22. DaSilva AG, Yong VW: Matrix Metalloproteinase-12 Deficiency Worsens Relapsing-Remitting Experimental Autoimmune Encephalomyelitis in Association with Cytokine and Chemokine Dysregulation. Am J Pathol 174: 898–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asahi M, Sumii T, Fini ME, Itohara S, Lo EH: Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport 12: 3003–3007, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Baker EA, Tian Y, Adler S, Verbalis JG: Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp Neurol 165: 221–230, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Rojiani AM, Prineas JW, Cho ES: Electrolyte-induced demyelination in rats: 1. Role of the blood-brain-barrier and edema. Acta Neuropathol 88: 287–292, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Kengne FG, Soupart A, Pochet R, Brion JP, Decaux G: Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int 76: 614–621, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G: Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int 45: 193–200, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Silver SM, Schroeder BM, Sterns RH, Rojiani AM: Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J Neuropathol Exp Neurol 65: 37–44, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Soupart A, Silver S, Schroeder B, Sterns R, Decaux G: Rapid (24-hour) reaccumulation of brain organic osmolytes (particularly myo-inositol) in azotemic rats after correction of chronic hyponatremia. J Am Soc Nephrol 13: 1433–1441, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Verbalis JG, Gullans SR: Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res 567: 274–282, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.