Abstract
The reasons for inadequate production of erythropoietin (EPO) in patients with ESRD are poorly understood. A better understanding of EPO regulation, namely oxygen-dependent hydroxylation of the hypoxia-inducible transcription factor (HIF), may enable targeted pharmacological intervention. Here, we tested the ability of fibrotic kidneys and extrarenal tissues to produce EPO. In this phase 1 study, we used an orally active prolyl-hydroxylase inhibitor, FG-2216, to stabilize HIF independent of oxygen availability in 12 hemodialysis (HD) patients, six of whom were anephric, and in six healthy volunteers. FG-2216 increased plasma EPO levels 30.8-fold in HD patients with kidneys, 14.5-fold in anephric HD patients, and 12.7-fold in healthy volunteers. These data demonstrate that pharmacologic manipulation of the HIF system can stimulate endogenous EPO production. Furthermore, the data indicate that deranged oxygen sensing—not a loss of EPO production capacity—causes renal anemia.
In adults, the kidneys are the main site of production of the glycoprotein hormone erythropoietin (EPO), which links renal function to erythropoiesis. EPO production is normally inversely related to blood oxygen content, thus establishing an efficient feedback control of red cell production.1 In the presence of chronic kidney disease (CKD), plasma EPO concentrations fail to increase with declining hemoglobin (Hb) levels, causing the development of renal anemia. Peritubular cortical fibroblasts in the kidney have been identified as the sites of EPO synthesis, but why the kidneys lose their ability to produce sufficient EPO in progressive renal disease remains unclear.2,3
EPO is part of a widespread system of hypoxia-inducible gene expression mediated by hypoxia-inducible transcription factors (HIFs).4,5 HIFs are composed of one of two oxygen-regulated α-subunits (HIF-1α and HIF-2α) that form heterodimers with a constitutive HIF-β subunit. HIF-2α is the HIF isoform responsible for regulation of EPO.6–9 Stability and transcriptional activity of HIF-α is regulated by molecular oxygen: Oxygen-dependent hydroxylation of two prolyl residues leads to binding of an E3-ubiquitin–ligase complex that targets HIF for rapid proteasomal degradation.10,11 Hydroxylation of an asparagyl residue interferes with binding of the transcriptional co-activator CBP/p300. These hydroxylation reactions are mediated by a family of prolyl-hydroxylase domain (PHD) enzymes and a related asparagyl-hydroxylase, which require oxygen and oxoglutarate as substrates. Oxoglutarate analogues can therefore function as competitive prolyl-hydroxylase inhibitors (PHD-I). Compounds of this class have been shown to induce HIF-target genes in preclinical in vitro and in vivo models.12–15 Here, we used FG-2216, an orally active small molecule PHD-I, in a single-dose phase I study of nephric and anephric patients who had ESRD and were on regular dialysis to test the ability of diseased kidneys and of extrarenal tissues to produce EPO.
RESULTS
Patient Characteristics
Baseline data of the healthy control subjects and two groups of hemodialysis (HD) patients who were enrolled in the study are shown in Table 1. The reasons for bilateral nephrectomy included chronic urinary tract infection, polycystic kidney disease, refractory hypertension, and bilateral renal carcinoma. The mean weekly epoetin dosage before the study was 9167 mU in nephric HD patients and approximately 7333 mU in anephric HD patients (assuming that 1 μg of darbepoetin is equivalent to 200 mU epoetin).
Table 1.
Baseline characteristics of healthy control subjects and HD patients who were enrolled in the study
| Patient | Gender | Age (years) | Body Weight (kg) | Time on Dialysis (months) | Cause of ESRD or Time and Reason for Nephrectomy | Regular rhEPO Treatment (Mean Weekly Dosage) | Comorbidity |
|
|---|---|---|---|---|---|---|---|---|
| Diabetes | Hypertension | |||||||
| Healthy control subjects | ||||||||
| 1 | M | 43 | 115 | – | – | – | No | No |
| 2 | M | 37 | 88 | – | – | – | No | No |
| 3 | M | 44 | 82 | – | – | – | No | No |
| 4 | M | 36 | 94 | – | – | – | No | No |
| 5 | M | 47 | 73 | – | – | – | No | No |
| 6 | M | 37 | 100 | – | – | – | No | No |
| HD patients with native kidneys in situ | ||||||||
| 1 | M | 37 | 68 | 20 | Tubulointerstitial nephritis, chronic hypokalemia and anorexia | 18,000 IU Epoetin-β intravenously | No | Yes |
| 2 | M | 35 | 115 | 38 | Membranoproliferative glomerulonephritis | 9000 IU Epoetin-β subcutaneously | No | Yes |
| 3 | M | 43 | 72 | 114a | Suspicion of Alport syndrome | 15,000 IU Epoetin-β intravenously | No | Yes |
| 4 | M | 46 | 83 | 98 | Interstitial nephritis and nephrosclerosis | 2000 IU Epoetin-α intravenously | No | Yes |
| 5 | M | 34 | 76 | 47 | Nephrosclerosis | 9000 IU Epoetin-β intravenously | No | Yes |
| 6 | M | 39 | 84 | 32 | Chronic glomerulonephritis | 2000 IU Epoetin-α intravenously | No | Yes |
| HD patients with bilateral nephrectomy | ||||||||
| 1 | M | 27 | 65 | 87 | Chronic pyelonephritis (r: 8/2000; l: 10/2001) | 7000 IU Epoetin-β subcutaneously | No | Yes |
| 2 | F | 59 | 66 | 39 | ADPKD, recurrent infections of renal cysts (r: 7/2004; l: 9/2005) | 50 μg Darbepoetin alfa intravenously | No | Yes |
| 3 | M | 61 | 61 | 99 | ADPKD (r: 1998 preparation for KTx; l: 9/2004 cyst bleeding) | 40 μg Darbepoetin alfa intravenously | No | Yes |
| 4 | F | 51 | 59 | 71 | Refractory, severe hypertension (bl: 2001) | 9000 IU Epoetin-β intravenously | No | Yes |
| 5 | F | 51 | 68 | 101 | Recurrent UTI, medullary sponge kidneys (bl: 1977) | 6000 IU Epoetin-β subcutaneously | No | No |
| 6 | M | 82 | 60 | 64 | Bilateral RCC (bl: 12/2001) | 4000 IU Epoetin-β intravenously | No | Yes |
ADPKD, autosomal dominant polycystic kidney disease; bl, bilateral nephrectomy; KTx, kidney transplantation; l, left nephrectomy; r, right nephrectomy; RCC, renal cell carcinoma; UTI, urinary tract infection.
aCumulative time on HD interrupted by episodes of KTx, no renal allograft in situ at the time of study participation.
Pharmacodynamic Effects/Plasma EPO Levels
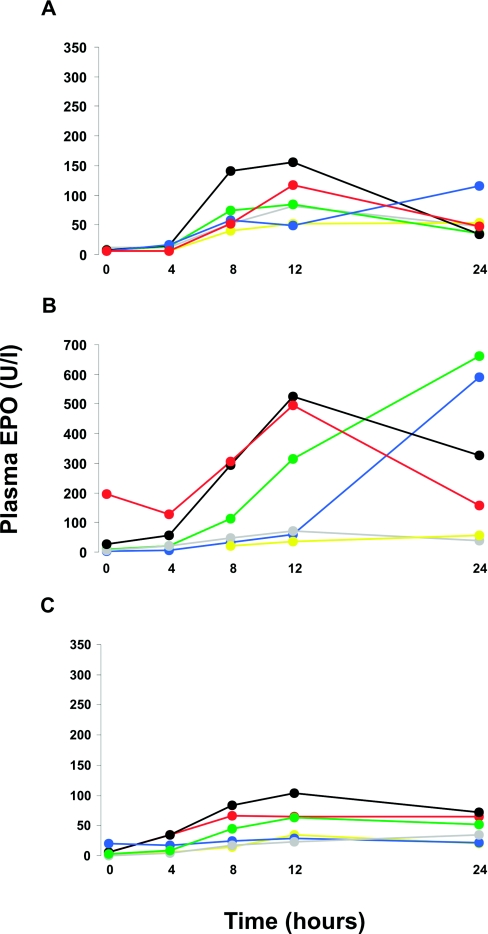
In all participants, FG-2216 significantly increased plasma EPO concentrations. In control subjects, median plasma EPO rose from 6.4 U/L at baseline to a maximum of 81.2 U/L at 12 hours after the dose (Figure 1A). Nephric HD patients showed the largest rise in plasma EPO, with median levels increasing from 7.8 U/L at baseline to 240.6 U/L at 24 hours (Figure 1B). Although the median plasma EPO concentration peaked at 24 hours, plasma EPO of three patients in this group already decreased between 12 and 24 hours. One anephric patient (patient 4) was accidentally underdosed, receiving 250 mg (approximately 4 mg/kg) rather than 1250 mg of FG-2216. When this patient was excluded from the summary analysis, the median plasma EPO concentrations increased from 4.4 to 63.1 U/L in anephric patients (Figure 1C).
Figure 1.
FG-2216 increases plasma-EPO levels in healthy controls and in HD patients with and without remaining renal tissue. Twenty-four-hour kinetics of plasma EPO levels after a single dose of FG-2216. (A through C) Individual values are depicted for control subjects (A), nephric HD patients (B), and anephric HD patients (C). All individuals except one received FG-2216 at a dosage of 20 mg/kg; patient 4 in the anephric group (blue line in C) was accidentally underdosed with approximately 4 mg/kg.
Reticulocytes tended to decrease in HD patients after withdrawal from recombinant human EPO (rhEPO). From dosing until day 7 of the study, the reticulocyte count increased significantly in nephric HD patients, the group with the highest median plasma EPO levels (from 39.37 ± 13.56 to 52.73 ± 27.8/nl; P < 0.05), and they did not decrease further in the anephric HD patients. In all groups, there were no significant changes in Hb levels during the study period (Table 2).
Table 2.
Blood chemistry before (day −7, baseline visit) and 1 week after (day 7, final study visit) administration of FG-2216
| Laboratory Parameter (Normal Range) | Baseline (Day −7) |
End of Study (Day 7) |
||||
|---|---|---|---|---|---|---|
| Control Subjects | Nephric HD Patients | Anephric HD Patients | Control Subjects | Nephric HD Patients | Anephric HD Patients | |
| AST (IU/L; <31) | 24.5 ± 9.5 | 14.8 ± 4.8 | 24.5 ± 13.3 | 27.0 ± 5.6 | 12.8 ± 4.7 | 24.8 ± 15.0 |
| ALT (IU/L; <34) | 35.5 ± 20.9 | 13.7 ± 8.8 | 14.0 ± 3.7 | 34.8 ± 17.7 | 19.2 ± 6.6 | 13.5 ± 4.8 |
| Total bilirubin (mg/dl; 0.1 to 1.1) | 0.65 ± 0.32 | 0.57 ± 0.16 | 0.72 ± 0.23 | 0.60 ± 0.32 | 0.57 ± 0.15 | 0.61 ± 0.20 |
| Albumin (g/L; 35 to 55) | 41.7 ± 1.6 | 42.0 ± 1.3 | 40.8 ± 5.8 | 42.3 ± 1.3 | 40.2 ± 4.7 | 37.6 ± 4.8 |
| AP (U/L; 42 to 98) | 76.7 ± 19.2 | 99.8 ± 14.3 | 88.2 ± 29.5 | 75.8 ± 14.6 | 89.3 ± 15.2 | 87.0 ± 15.8 |
| Lipase (U/L; <60) | 21.2 ± 17.5 | 56.7 ± 25.2 | 66.8 ± 30.2 | 21.2 ± 10.8 | 61.5 ± 24.8 | 76.0 ± 34.4 |
| Hb (g/dl; 12 to 16) | 15.40 ± 0.70 | 13.30 ± 1.60 | 14.00 ± 1.30 | 15.60 ± 1.20 | 12.20 ± 1.51 | 12.20 ± 1.20 |
| Ferritin (22 to 112) | 168 ± 124 | 1108 ± 496 | 975 ± 630 | 173 ± 146 | 1210 ± 615 | 1001 ± 682 |
| Transferrin saturation (%; 16 to 45) | 30.7 ± 13.1 | 35.1 ± 11.6 | 39.9 ± 11.6 | 26.8 ± 7.3 | 41.5 ± 29.1 | 50.1 ± 6.5 |
When baseline levels at screening visit were compared with measurements 1 week after study drug administration, FG-2216 caused no significant changes in liver parameters, Hb, iron parameters, or lipid profile. Data are means ± SD (n = 6 per group). AST, aspartate transaminase; ALT, alanine transaminase; AP, alkaline phosphatase.
Pharmacokinetics of FG-2216
The pharmacokinetic data of FG-2216 are summarized in Table 3. The urinary excretion of unchanged FG-2216 in healthy control subjects amounted to 11.0 ± 3.5% of the administered dose within 48 hours. Compared with healthy control subjects, HD patients had comparable FG-2216 Tmax and Cmax levels but lower apparent clearance (Cl/F), longer half-life (T½) values, and a higher area under the curve (AUC; Table 3).
Table 3.
Pharmacokinetics of FG-2216. Tmax and Cmax are not different among groups
| Group | Tmax (hours) | Cmax (μg/ml) | AUC (hr/μg per ml) | T1/2 (hours) | Clearance (ml/h) |
|---|---|---|---|---|---|
| Control subjects | 3.5 ± 1.8 | 179.0 ± 31.0 | 3660 ± 813 | 15.7 ± 1.1 | 530 ± 172 |
| Nephric HD patients | 3.0 ± 1.1 | 156.0 ± 17.1 | 5730 ± 2420 | 29.8 ± 11.4 | 311 ± 125 |
| Anephric HD patients | 4.0 ± 2.5 | 159.0 ± 18.8 | 6572 ± 2349 | 33.3 ± 8.4 | 208 ± 91 |
Data are means ± SD. The AUC is significantly increased and the plasma T1/2 is twice as long in HD patients in comparison with healthy control subjects (P = 0.015 for nephric HD and P = 0.004 for anephric HD versus control subjects but not different between nephric and anephric HD patients).
No change of FG-2216 plasma levels was observed during the dialysis period, and the dialysis procedure removed only a small fraction of the administered FG-2216 dose (1.01 ± 0.53 and 1.77 ± 0.45% in nephric and anephric HD patients, respectively). The patient who received only one fifth of the intended dose (patient 4 in the anephric group) had a lower Cmax and AUC, but all other pharmacokinetic parameters were within the range of all HD patients. All HD patients except one had anuria during the collection period; the patient with residual urine production excreted 2% of the administered dose (27 mg) in 1900 ml urine in 48 hours.
Safety
There was no clinically significant change in laboratory and clinical findings. In total, 17 adverse events were recorded: Two in control subjects, six in nephric HD patients, and nine in anephric HD patients. These included diarrhea, abdominal discomfort, nausea, lesion of a radial nerve branch caused by venipuncture, rash, headache, hyperkalemia, dizziness, sweating, arterial hypotension, an accidental scalp wound. Two serious adverse events occurred, both of which were considered not to be drug related. One nephric HD patient required an additional HD session because of hyperkalemia attributed to dysfunction of his arteriovenous fistula. One anephric patient was hospitalized after a transplant offer, but transplantation could not be performed for donor reasons.
During the study period, repetitive blood samples were drawn for clinical chemistry (day −7, day 0, day 1, day 3 or 4, and day 7). Overall, we did not observe a significant change in all parameters tested, including iron parameters, liver function tests, and lipase levels, in all groups after administration of FG-2216. Values at baseline (day −7) and at day 7 are shown in Table 2.
DISCUSSION
This proof-of-concept study provides unexpected novel insights into the pathogenesis of renal anemia. Unused production capacity for EPO, presumably as a result of desensitization of the oxygen-sensing mechanism rather than destruction of its cellular production sites, seems to cause the inappropriately low production of the hormone in patients with CKD.
Previous observations showed that plasma EPO concentrations in dialysis patients can increase in response to acute episodes of hypoxia, despite not responding adequately to the chronic reduction in Hb levels.16,17 Moderate inverse responses of plasma EPO to changes in Hb levels induced by phlebotomy and transfusion have also been reported.18 Moreover, it was recently found that with increasing altitude, HD patients receive lower doses of rhEPO and yet achieve higher hematocrit levels, which could also indicate residual hypoxia-stimulated EPO production.19 The source of EPO under all of these circumstances, however, remains unclear. Our comparative analysis of nephric and anephric HD patients clearly indicates that both the diseased kidneys and extrarenal sites can contribute to a marked rise in plasma EPO concentrations with pharmacologic activation of HIF. Besides the kidneys, the liver is a site of relevant EPO production, producing most EPO during fetal life and contributing up to one third to total EPO production in animals who are exposed to severe hypoxia.20,21 EPO production in anephric patients is therefore highly likely to be of hepatic origin, although it is obviously impossible to prove this assumption.
A particularly striking feature of our observations is the magnitude of the rise in plasma EPO evoked by FG-2216. The increase in anephric individuals was similar to that in healthy volunteers, although one might have expected a fraction of the normal response, corresponding to the hepatic contribution to EPO formation. In HD patients with their diseased kidneys in situ, the rise in plasma EPO was approximately fourfold higher than in normal individuals. The systemic exposure to FG-2216 was significantly enhanced in patients with kidney failure, with a 1.65-fold increase in the AUC of its plasma concentrations; however, this increase was primarily due to an increase in plasma T½. Tmax and Cmax were similar in dialysis patients and healthy individuals, so FG-2216 exposure in the first hours after dosing was similar in both groups. Nevertheless, plasma EPO concentrations in nephric HD patients tended to be higher and in anephric HD patients were similar to those in control subjects after 4 hours. Therefore, the increased response cannot be explained on the basis of pharmacokinetics only; rather, it may indicate an increased sensitivity to the drug, the molecular basis of which remains to be elucidated.
Despite significant progress in understanding the oxygen-sensing mechanisms that regulate HIF stability and transcriptional activity,4,5 the overall control of EPO production in the kidney and its relationship to tissue oxygen tensions, oxygen supply, and oxygen consumption by tubular cells remain incompletely understood. Observations in the early posttransplantation period indicate that excretory kidney function and the ability to produce EPO may dissociate.22 Tubular function is probably a prerequisite for the adaptation of EPO formation to changes in blood oxygen content.23,24 In fact, it is possible that, as a result of the lack of tubular oxygen consumption, renal oxygen tensions in diseased kidneys are higher than in intact kidneys25; however, other evidence points toward an important role of hypoxia in the progression of kidney disease.26
Irrespective of the mechanisms that impair the normal feedback control of erythropoiesis in patients with CKD, stimulation of endogenous EPO production through HIF stabilization offers a potential novel opportunity for the treatment of anemia,27–29 and our results indicate that this approach could potentially be extended to patients who are on dialysis. The magnitude of the increase in plasma EPO observed in this study for nephric HD patients is comparable to plasma EPO concentrations achieved after subcutaneous administration of rhEPO, using dosages that are typically administered for anemia correction.30 Moreover, the pharmacokinetic data obtained here indicate that accumulation of FG-2216 can be avoided if dosing intervals are adjusted according to its prolonged plasma T½ in kidney failure.
In summary, this study provides proof of principle that pharmacologic inhibition of HIF PHD enzymes with an oral agent can induce endogenous EPO in dialysis patients; however, the safety of any therapeutic long-term activation of the HIF system requires careful consideration, given the broad biological potential of HIF in mediating hypoxia-driven processes.31 Interestingly, genetic causes of impaired degradation of HIF, potentially comparable to chronic pharmacologic inhibition of HIF degradation, have been identified as causes of rare polycythemias.32,33
CONCISE METHODS
Patients
Twelve long-term HD patients and six healthy control subjects were enrolled after informed consent. Six HD patients were anephric, and six had their native kidneys in situ; none carried a renal transplant. Major exclusion criteria were a history of malignancy within 5 years, recent thromboembolic events, evidence of active infection or inflammatory disease, active bleeding, and severe congestive heart failure.
Study Protocol
The study was an open-label, single-dose phase I study to investigate the pharmacokinetics, biological activity, and safety of the novel, orally active PHD-I FG-2216 (FibroGen Inc., South San Francisco, CA; Eudra cat. no. 2005-003664-29). In all HD patients, treatment with rhEPO was paused 7 days before study initiation. All participants were to receive a single dose of 20 mg/kg body wt FG-2216 on day 1 and were followed up for 7 days. In HD patients, FG-2216 was administered on the morning after their first HD of the week. Pharmacokinetic plasma levels of FG-2216 were determined before dosing and between 0.5 and 24 hours (all groups) and after 48 hours (control subjects) or 72 hours (HD patients) using mass spectroscopy. The dialyzer clearance of FG-2216 was determined in HD patients at day 2 (24 hours after dosing), using a standardized HD protocol: Low-flux dialyzer (Hemoflow F6HPS; Fresenius Medical Care, Bad Homburg, Germany), 4-hour dialysis time, blood flow of 200 to 250 ml/min, and dialysate flow of 500 ml/min. EPO concentrations were determined before dosing and 4, 8, 12, and 24 hours after dosing, using a commercially available ELISA-kit (R&D Systems).
Statistical and Analysis
All results are expressed as mean ± SD with the exception of plasma EPO levels, which are expressed as median. Descriptive statistical analyses were performed using t test and Mann-Whitney U test for pharmacokinetic data and reticulocyte count, using SPSS 15.0 for Windows. P < 0.05 was considered significant.
DISCLOSURES
P.S. was working as a consultant for FibroGen, and V.G. and J.C. are employees of FibroGen.
Acknowledgments
The expert support of U. Heinritz and other staff members of the clinical research unit at the Department of Nephrology and Hypertension at FAU is gratefully acknowledged. Drs. B. Bueschges-Seraphin and J. Nikolay (Fürth), J. Wopperer (Neumarkt), J. Mann (Munich), and D. Soreth-Rieke (Miesbach) provided invaluable support in recruiting the patients and cooperated in the study performance. Gert Kochendoerfer from FibroGen helped to develop the study protocol.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Jelkmann W: Erythropoietin: Structure, control of production, and function. Physiol Rev 72: 449–489, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Bachmann S, Le HM, Eckardt KU: Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ: Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Hirota K, Semenza GL: Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun 338: 610–616, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU: Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 17: 271–273, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU: Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA: HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105: 3133–3140, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, Flippin LA, Arend M, Klaus SJ, Bachmann S: Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int 77: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG, Jr: Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A 99: 13459–13464, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Bernhardt WM, Gottmann U, Doyon F, Buchholz B, Campean V, Schodel J, Reisenbuechler A, Klaus S, Arend M, Flippin L, Willam C, Wiesener MS, Yard B, Warnecke C, Eckardt KU: Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci U S A 106: 21276–21281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warnecke C, Griethe W, Weidemann A, Jurgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt KU: Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 17: 1186–1188, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Safran M, Kim WY, O'Connell F, Flippin L, Gunzler V, Horner JW, Depinho RA, Kaelin WG, Jr: Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A 103: 105–110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Blumberg A, Keller H, Marti HR: Effect of altitude on erythropoiesis and oxygen affinity in anaemic patients on maintenance dialysis. Eur J Clin Invest 3: 93–97, 1973 [DOI] [PubMed] [Google Scholar]
- 17. Chandra M, Clemons GK, McVicar MI: Relation of serum erythropoietin levels to renal excretory function: evidence for lowered set point for erythropoietin production in chronic renal failure. J Pediatr 113: 1015–1021, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Walle AJ, Wong GY, Clemons GK, Garcia JF, Niedermayer W: Erythropoietin-hematocrit feedback circuit in the anemia of end-stage renal disease. Kidney Int 31: 1205–1209, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Rothman KJ, Fischer M, Mehta J, Winkelmayer WC: The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol 19: 1389–1395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zucali JR, Mirand EA: Extrarenal erythropoietin and erythrogenin production in the anephric rat. Am J Physiol 229: 1094–1097, 1975 [DOI] [PubMed] [Google Scholar]
- 21. Eckardt KU, Ratcliffe PJ, Tan CC, Bauer C, Kurtz A: Age-dependent expression of the erythropoietin gene in rat liver and kidneys. J Clin Invest 89: 753–760, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eckardt KU, Frei U, Kliem V, Bauer C, Koch KM, Kurtz A: Role of excretory graft function for erythropoietin formation after renal transplantation. Eur J Clin Invest 20: 563–572, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Eckardt KU, Kurtz A, Bauer C: Regulation of erythropoietin production is related to proximal tubular function. Am J Physiol 256: F942–F947, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Donnelly S: Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv Exp Med Biol 543: 73–87, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Priyadarshi A, Periyasamy S, Burke TJ, Britton SL, Malhotra D, Shapiro JI: Effects of reduction of renal mass on renal oxygen tension and erythropoietin production in the rat. Kidney Int 61: 542–546, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF: HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood 110: 2140–2147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macdougall IC, Eckardt KU: Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet 368: 947–953, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Bunn HF: New agents that stimulate erythropoiesis. Blood 109: 868–873, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Macdougall IC, Roberts DE, Neubert P, Dharmasena AD, Coles GA, Williams JD: Pharmacokinetics of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Lancet 1: 425–427, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Pouyssegur J, Dayan F, Mazure NM: Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT: Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32: 614–621, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS: A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A 103: 654–659, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]