Abstract
Antibodies recognizing plasminogen, a key component of the fibrinolytic system, associate with venous thrombotic events in PR3-ANCA vasculitis. Here, we investigated the prevalence and function of anti-plasminogen antibodies in independent UK and Dutch cohorts of patients with ANCA-associated vasculitis (AAV). We screened Ig isolated from patients (AAV-IgG) and healthy controls by ELISA. Eighteen of 74 (24%) UK and 10/38 (26%) Dutch patients with AAV had anti-plasminogen antibodies compared with 0/50 and 1/61 (2%) of controls. We detected anti-plasminogen antibodies in both PR3-ANCA– and MPO-ANCA–positive patients. In addition, we identified anti-tissue plasminogen activator (tPA) antibodies in 13/74 (18%) patients, and these antibodies were more common among patients with anti-plasminogen antibodies (P = 0.011). Eighteen of 74 AAV-IgG (but no control IgG) retarded fibrinolysis in vitro, and this associated with anti-plasminogen and/or anti-tPA antibody positivity. Only 4/18 AAV-IgG retarded fibrinolysis without harboring these antibodies; dual-positive samples retarded fibrinolysis to the greatest extent. Patients with anti-plasminogen antibodies had significantly higher percentages of glomeruli with fibrinoid necrosis (P < 0.05) and cellular crescents (P < 0.001) and had more severely reduced renal function than patients without these antibodies. In conclusion, anti-plasminogen and anti-tPA antibodies occur in AAV and associate with functional inhibition of fibrinolysis in vitro. Seropositivity for anti-plasminogen antibodies correlates with hallmark renal histologic lesions and reduced renal function. Conceivably, therapies that enhance fibrinolysis might benefit a subset of AAV patients.
The anti-neutrophil cytoplasmic autoantibody (ANCA)-associated small vessel vasculitides (AAV) are systemic disorders that may or may not incorporate a granulomatous component, especially in upper airways, and that may occasionally manifest as renal limited vasculitis. For the classical ANCA types anti-proteinase 3 (PR3) and anti-myeloperoxidase (MPO), cumulative data from clinical and experimental studies strongly support their pathogenic role.1,2 Recently, it was reported that a subset of patients with PR3-ANCA harbor antibodies against human plasminogen.3 These anti-plasminogen antibodies recognize a structure within the protease domain, and their presence correlated with venous thromboembolic events (VTEs), suggesting functional interference with coagulation and/or fibrinolysis.3 The relationship between anti-plasminogen antibodies and coagulation may also be directly relevant to glomerular injury and the evolution of fibrinoid necrosis, a hallmark lesion of AAV. In AAV, vascular injury is mediated by ANCA-induced neutrophil and monocyte activation4 and characterized by the influx of inflammatory cells with fibrinoid necrosis affecting glomeruli and occasionally small arteries. It is possible that fibrinoid necrosis is in fact an aberrant repair mechanism that follows vascular injury. This hypothesis would account for histologic and compositional similarities between fibrinoid necrosis and conventional fibrin clots5,6 and explain the importance of the fibrinolytic system in renal injury, as shown by animal models of rapidly progressive glomerulonephritis.7,8 Under these circumstances, down-regulation of fibrinolysis by anti-plasminogen antibodies in AAV patients would promote persistent or extensive fibrinoid necrosis.
In this study, we investigated the presence and functionality of anti-plasminogen antibodies in independent cohorts of United Kingdom (UK) and Dutch patients with AAV. We show that Ig from AAV patients retards fibrinolysis and that seropositivity for these antibodies correlates with reduced renal function and the presence of hallmark histopathologic renal lesions. We also detected antibodies against the partially homologous serine protease tissue plasminogen activator (tPA) in some patients.
RESULTS
Identification of Anti-Plasminogen Antibodies in AAV-IgG
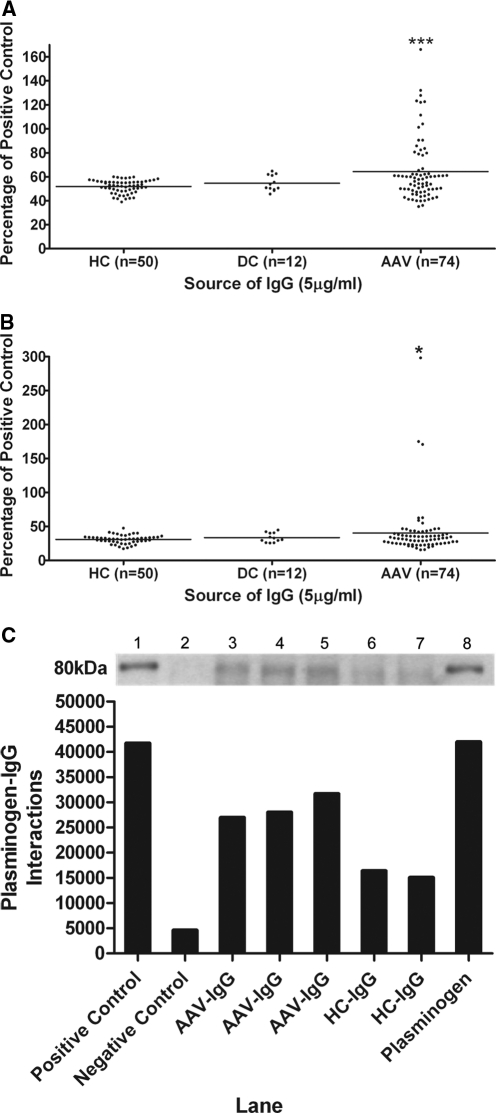
Anti-plasminogen antibodies were identified in 18/74 (24.3%) UK AAV-IgG compared with 0/50 healthy control IgG (HC-IgG) (Figure 1A, ***P < 0.001). The prevalence of anti-plasminogen antibodies was 24.1% (7/29) in MPO-ANCA– and 24.4% (11/45) in PR3-ANCA–positive patients. Only 1/12 (8.3%) disease control IgG (DC-IgG) was positive for anti-plasminogen antibodies (anti-glomerular basement membrane [GBM] disease without concurrent ANCA positivity).
Figure 1.
Identification of anti-plasminogen and anti-plasmin antibodies in AAV patients. (A) Representative plasminogen ELISA for UK AAV patients. ***P < 0.001 AAV IgG compared with healthy control IgG. (B) Representative plasmin ELISA for UK AAV patients. *P < 0.05 AAV IgG compared with healthy control IgG. (C) Presence of anti-plasminogen antibodies was confirmed by immunoprecipitation Western blot with quantitative densitometry. Plasminogen was immunoprecipitated with (lane 1) goat anti-plasminogen–positive control, (lane 2) species-specific IgG-negative control, (lanes 3–5) patient IgG, (lanes 6 and 7) healthy control IgG, and (lane 8) plasminogen control.
To validate this observation, IgG from an independent cohort of Dutch AAV patients was screened: 10/38 (26.3%) harbored anti-plasminogen antibodies compared with 1/61 (2%) HC-IgG (***P < 0.001). Anti-plasminogen antibodies were detected in three MPO-ANCA and six PR3-ANCA patients, as well as in one ANCA-negative patient.
In contrast, anti-plasmin antibodies were less common: only 5/74 (6.7%) UK AAV-IgG showed anti-plasmin reactivity compared with 1/50 (2%) HC-IgG (*P < 0.05) and 0/12 DC-IgG (Figure 1B). All 5 AAV-IgG positive for anti-plasmin antibodies were among the 18 AAV-IgG positive for antibodies against plasminogen. Anti-plasmin antibodies occurred in three MPO-ANCA and two PR3-ANCA patients. Furthermore, the three most reactive anti-plasmin IgG were also the top three most reactive with plasminogen. In the Dutch cohort, 2/38 (5.3%) AAV-IgG harbored anti-plasmin antibodies compared with 1/61 (2%) HC-IgG (P = 0.31). Both of these AAV-IgG samples were dual positive for anti-plasminogen and anti-plasmin antibodies.
Immunoprecipitation Western blotting corroborated the existence of anti-plasminogen antibodies in AAV-IgG (Figure 1C). Three AAV-IgG that were positive for anti-plasminogen antibodies by ELISA effectively immunoprecipitated plasminogen in contrast to two HC-IgG that did not immunoprecipitate plasminogen.
Specificity of Antibody Binding to Plasminogen
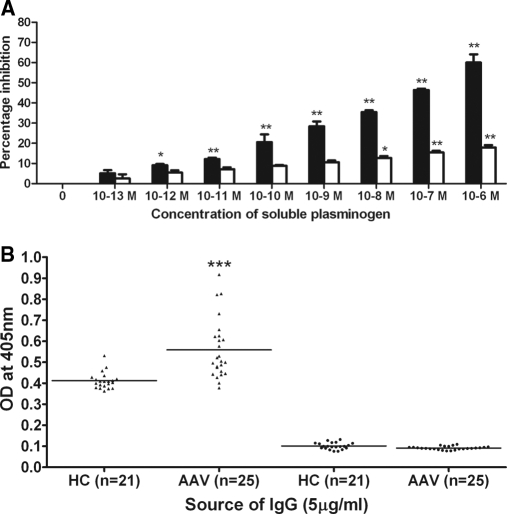
Competitive inhibition assays also supported a specific interaction between AAV-IgG and plasminogen (Figure 2A, solid bars). Premixing anti-plasminogen antibody–positive UK AAV-IgG (n = 4) samples with soluble plasminogen inhibited binding to plasminogen used as the coated antigen in a concentration-dependent manner. Inhibition was 60.1 ± 0.87% using soluble plasminogen at 1 μM (**P < 0.01), which is comparable to the inhibition obtained by premixing affinity purified polyclonal rabbit antibody with 1 μM soluble plasminogen (70.1 ± 5.2%). In contrast, premixing with 1 μM denatured plasminogen inhibited AAV-IgG binding to native plasminogen by only 17.93 ± 0.99% (Figure 2A, open bars, **P < 0.01). Similarly, binding of anti-plasminogen antibody–positive Dutch AAV-IgG (n = 4) to coated plasminogen was inhibited by 58.8 ± 1.59% (**P < 0.01) after preincubation with soluble plasminogen and 11.4 ± 1.07% (*P < 0.05) with denatured plasminogen.
Figure 2.
Specificity of antibody binding to plasminogen. (A) IgG (1.25 μg/ml) from four UK patients harboring antibodies against plasminogen was premixed with increasing concentrations of native (solid bars) or denatured (open bars) soluble plasminogen for 2 hours before the plasminogen assay. Data are means ± SEM (n = 3). *P < 0.05 and **P < 0.01 compared with uninhibited IgG. (B) UK AAV-IgG binding to native (▴) and denatured plasminogen (●). ***P < 0.001 AAV-IgG compared with healthy control IgG binding to native plasminogen.
When denatured plasminogen acted as coat antigen, 0/74 UK and 1/38 Dutch AAV-IgG exhibited significant binding (Figure 2B). In contrast, denatured plasminogen was recognized by the affinity purified polyclonal rabbit antibody compared with negative control rabbit antibody (***P < 0.001). This implies that conformational epitopes predominate in AAV-IgG, whereas the rabbit anti-plasminogen antibody contains some dominant linear epitopes.
As a negative control for AAV-IgG, reactivity toward an unrelated autoantigen was also examined. Only 1/18 (5.5%) anti-plasminogen antibody–positive UK AAV-IgG bound to recombinant α3(IV)NC1 compared with 1/50 (2%) HC-IgG. No anti-plasminogen antibody–positive Dutch AAV-IgG reacted with α3(IV)NC1. In contrast, seven of seven IgG from patients with anti-GBM disease bound α3(IV)NC1 as anticipated (***P < 0.001).
Binding Properties of AAV-IgG to Plasminogen, Plasmin, and Tissue Plasminogen Activator
Previously, anti-cardiolipin antibodies (aCLs) that cross-react with the catalytic domains of several serine proteases involved in coagulation/fibrinolysis have been reported in patients with anti-phospholipid syndrome (APS).9 However, 73/74 UK AAV-IgG and 11/12 DC-IgG samples were negative for aCL.
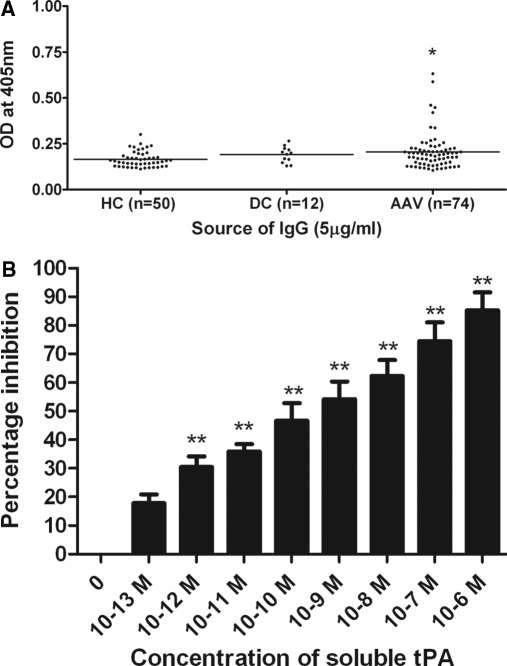
Meanwhile, significant binding to tissue plasminogen activator (tPA) was prevalent among AAV-IgG. Thirteen of 74 (17.6%) UK AAV-IgG were positive for anti-tPA antibodies compared with 1/50 (2%) HC-IgG (Figure 3A, *P < 0.05). The prevalence of anti-tPA antibodies was 20.7% (6/29) and 15.6% (7/45) in MPO-ANCA and PR3-ANCA patients, respectively. One DC-IgG was positive for anti-tPA antibodies; this patient with anti-GBM disease also expressed antibodies reactive with plasminogen. Examining the relationship between anti-plasminogen and anti-tPA antibodies in the UK AAV cohort, 7/18 (39%) anti-plasminogen antibody–positive patients harbored anti-tPA antibodies versus 6/56 (11%) anti-plasminogen–negative patients (P = 0.011, Fisher's exact test). Four of the dual anti-plasminogen and anti-tPA antibody–positive patients were also positive for antibodies against plasmin. Four of 38 (10.5%) Dutch AAV-IgG also tested positive for anti-tPA antibodies.
Figure 3.
Identification of anti-tissue plasminogen activator antibodies in AAV patients. (A) Representative ELISA from UK AAV patients. *P < 0.05 AAV IgG compared with healthy control IgG. (B) Preincubation with increasing concentrations of soluble tPA inhibited IgG binding. **P < 0.01 compared with uninhibited IgG.
The specificity of IgG interactions with tPA was confirmed by competition assays (Figure 3B). Premixing IgG from one anti-tPA antibody–positive UK AAV patient with soluble tPA inhibited binding to coated tPA (**P < 0.01). Inhibition was 85.2 ± 6.3% using soluble tPA at 1 μM.
Cross-inhibition studies were performed to characterize relative affinities of UK AAV-IgG for plasminogen, plasmin, and tPA. All 5 IgG dual positive for anti-plasminogen and anti-plasmin antibodies and the 13 anti-plasminogen–only reactive IgG were tested. Soluble plasminogen (1 μM) inhibited binding of the dual positive AAV-IgG to coated plasminogen by 59.75 ± 1.37% and to coated plasmin by 48.76 ± 0.57% (Table 1, **P < 0.01). Although Nα-tosyl-l-lysine chloromethyl ketone-HCl (TLCK)-inactivated plasmin significantly inhibited binding of dual positive AAV-IgG to coated plasmin by 43.35 ± 1% (**P < 0.01), binding to plasminogen was only reduced by 24.39 ± 1% (**P < 0.01). Pretreating IgG that was positive for only anti-plasminogen antibodies had a similar effect: soluble plasminogen inhibited binding to coated plasminogen by 61.37 ± 0.63%, whereas soluble plasmin inhibited binding to plasminogen by 23.49 ± 0.54% (Table 1).
Table 1.
Binding properties of IgG to plasminogen, plasmin, and tissue plasminogen activator
| AAV-IgG | PLG (Coat) and PLG (Soluble) | PLG (Coat) and PLM (Soluble) | PLM (Coat) and PLG (Soluble) | PLM (Coat) and PLM (Soluble) | Number of Samples Tested |
|---|---|---|---|---|---|
| Anti-plg Ab + ve and anti-plm Ab + ve | 59.75 ± 1.37% | 24.39 ± 1% | 48.76 ± 0.57% | 43.35 ± 1% | 5 |
| Anti-plg Ab + ve and anti-plm Ab − ve | 61.37 ± 0.63% | 23.49 ± 0.54% | NA | NA | 13 |
| PLG (Coat) and PLG (Soluble) | PLG (Coat) and tPA (Soluble) | tPA (Coat) and PLG (Soluble) | tPA (Coat) and tPA (Soluble) | ||
| Anti-plg Ab + ve and anti-tPA Ab + ve | 59.01 ± 0.59% | 63.29 ± 0.91% | 47.23 ± 0.58% | 70.75 ± 0.97% | 7 |
| Anti-plg Ab + ve and anti-tPA Ab − ve | 58.13 ± 0.53% | 63.36 ± 0.68% | NA | NA | 11 |
| Anti-plg Ab -ve and anti-tPA Ab + ve | NA | NA | 70.87% ± 0.61% | 74.74% ± 0.69% | 6 |
IgG was preincubated with 1 μM soluble plasminogen (plg), plasmin (plm), or tissue plasminogen activator (tPA) for 2 hours at 25°C before loading onto coat antigen as indicated. Data shown are mean percentage inhibition in IgG binding to the coat antigen ± SEM (n = 4). NA, not applicable.
Soluble tPA (1 μM) effectively inhibited binding of anti-tPA antibody–positive AAV-IgG to coated tPA (**P < 0.01). Similarly, soluble tPA reduced binding of anti-tPA and anti-plasminogen dual positive AAV-IgG to coated plasminogen by 63.29 ± 0.91% (Table 1, **P < 0.01). In contrast, soluble plasminogen was less efficient in reducing dual positive IgG binding to tPA (47.23 ± 0.58%; **P < 0.01). Collectively, these data suggest that AAV-IgG from the patients tested in these experiments bound to the fibrinolysis-associated serine proteases with the differing relative affinities: tPA > plasminogen > plasmin.
Anti-Plasminogen Antibodies in AAV-IgG Compromise Fibrinolysis
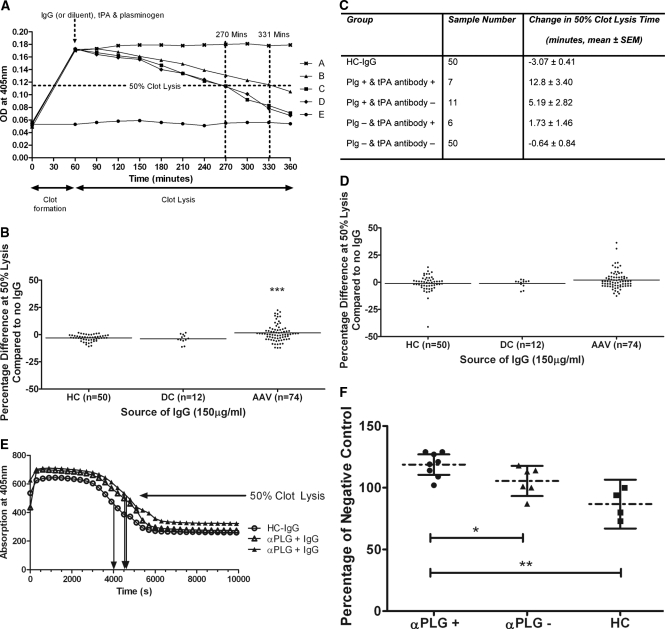
UK AAV-IgG also retarded in vitro fibrinolysis compared with HC-IgG (Figure 4A). IgG were classified as significantly retarding fibrinolysis if they extended time to 50% fibrinolysis by more than the mean + 2 SD effect of the HC-IgG cohort. Accordingly, 18/74 (24.3%) UK AAV-IgG delayed fibrinolysis (Figure 4B, ***P < 0.001). Four fibrinolysis-retarding IgG were from MPO-ANCA and 14 were from PR3-ANCA–positive IgG samples. None of the HC or DC-IgG significantly delayed fibrinolysis. A total of 11/18 (61%) UK AAV-IgG positive for anti-plasminogen antibodies significantly retarded clot lysis versus 7/56 (12.5%) of anti-plasminogen–negative patient IgG (P = 0.0001, Fisher's exact test). Furthermore, 7/13 (54%) anti-tPA–positive UK AAV-IgG retarded clot lysis compared with 11/61 (18%) anti-tPA–negative IgG (P = 0.01, Fisher's exact test). Five of 18 fibrinolysis retarding AAV-IgG were positive for both anti-plasminogen and anti-tPA antibodies. Only 4/18 IgG that significantly retarded fibrinolysis were negative for antibodies against plasminogen or tPA.
Figure 4.
Anti-plasminogen antibodies in AAV patient IgG compromise fibrinolysis. (A) Fibrin clot formation was stimulated by mixing thrombin with fibrinogen and monitored spectrophotometrically. No clot was formed in the absence of thrombin (●). Sample diluent (■), healthy control IgG (♦), or UK AAV-IgG (▴) were incubated with plasminogen (PLG) before the addition of tissue plasminogen activator (tPA). The tPA-containing solution was immediately loaded onto the fibrin clot. Fibrinolysis was measured by monitoring the reduction in absorbance at 405 nm. Plasminogen alone did not initiate fibrinolysis (x), whereas AAV-IgG retarded fibrinolysis compared with sample diluent and control IgG (▴). (B) Time to 50% fibrinolysis was retarded by 18/74 (24.3%) UK patient IgG compared with none of the healthy or disease controls. ***P < 0.001 AAV-IgG compared with healthy control IgG. (C) Average change in 50% clot lysis times (UK patients). (D) When IgG was premixed with plasmin (PLM) on ice before loading onto a fibrin clot, 5/74 (6.7%) UK AAV-IgG retarded fibrinolysis (2 from MPO-ANCA and 3 from PR3-ANCA patients). (E) In separate experiments, Dutch AAV-IgG samples were preincubated with PLG and mixed with fibrinogen, factor XIII, and tPA. This mixture was subsequently added to thrombin and CaCl2. Fibrin clot formation and lysis, monitored by change in absorbance at 405 nm, are shown for two anti-plasminogen antibody–positive AAV-IgG and one healthy control. Arrows indicate 50% lysis times. (F) Half-lysis times are given as percentage of a negative control with AAV-IgG grouped by anti-plasminogen antibody status (mean and 95% confidence intervals).
The dual presence of anti-plasminogen and anti-tPA antibodies in UK AAV-IgG caused the greatest inhibition, retarding lysis by 12.8 ± 3.4 minutes. Anti-plasminogen antibodies alone were shown to delay fibrinolysis by 5.19 ± 2.8 minutes, whereas anti-tPA antibodies alone reduced fibrinolysis by 1.73 ± 1.5 minutes (Figure 4C).
When UK AAV-IgG was mixed with plasmin and loaded onto a preformed fibrin clot, only 5/74 (6.7%) IgG delayed fibrinolysis compared with none of the HC or DC-IgG (P = 0.09; Figure 4D). Four of the 5 (80%) AAV-IgG positive for anti-plasmin antibodies retarded plasmin-mediated clot lysis compared with 1/69 (1.4%) antibody-negative patients (P = 0.0003, Fisher's exact test). These IgG were in fact also positive for anti-plasminogen and anti-tPA antibodies. No IgG degradation was observed when IgG was preincubated with plasmin (data not shown).
Confirming results obtained with UK AAV-IgG, Dutch anti-plasminogen antibody–positive AAV-IgG delayed in vitro fibrinolysis significantly compared with anti-plasminogen antibody–negative patient IgG and HC-IgG (Figure 4, E and F).
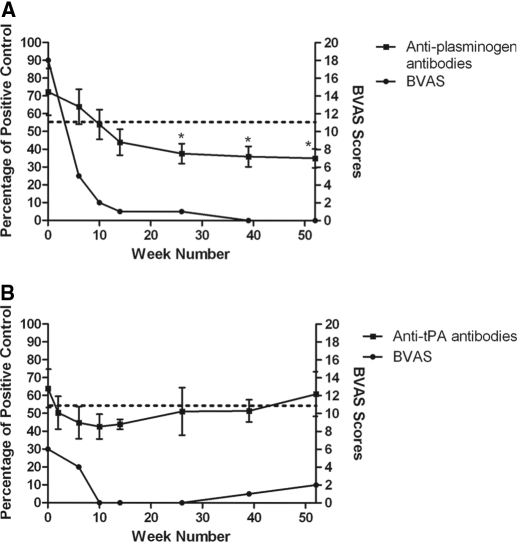
Transient Nature of Anti-Plasminogen Antibodies
The UK AAV-IgG were all obtained during acute disease. Longitudinal testing of these patients was not possible but, intriguingly, serial samples from two other AAV patients suggested that anti-plasminogen antibodies may disappear with treatment (Figure 5). However, in the Dutch cohort, one patient tested positive for anti-plasminogen antibodies while in clinical remission. These observations merit further examination.
Figure 5.
Transient nature of anti-plasminogen and anti-tissue plasminogen activator antibodies. Serial serum samples (diluted 1:100) from two patients collected over 52 weeks were screened for antibodies against plasminogen (A) and tissue plasminogen activator (B). Antibody titers were correlated with disease activity using the Birmingham Vasculitis Activity Scoring system. Data are means ± SEM (n = 3). The dotted line is the positive cut-off representing the mean + 2 SD of healthy controls. *P < 0.05 compared with the antibody titer at presentation.
Seropositivity for Anti-Plasminogen Antibodies Correlates to Reduced Renal Function
Serum creatinine measurements made at the same time as serum samples were collected for this study were available for 36/38 Dutch patients. The 10 patients who tested positive for anti-plasminogen antibodies had a significantly higher serum creatinine and a lower estimated GFR (eGFR; four-variable Modification of Diet in Renal Disease equation10,11) compared with patients who tested negative for anti-plasminogen antibodies (Table 2). At 12-month follow-up, patients with anti-plasminogen antibodies in their initial serum samples still had a higher serum creatinine, and a lower eGFR, compared with patients who tested negative for anti-plasminogen antibodies, whereas there was no difference in corrected eGFR at 12 months, suggesting that the effect of differences in baseline eGFR persisted.
Table 2.
Clinical characteristics of Dutch cohort by anti-plasminogen status
| Dutch Cohort (n = 38) | Anti-PLG Antibodies (n = 10) | No Anti-PLG Antibodies n = 28 | P |
|---|---|---|---|
| Age (mean ± SD) (years) | 68 ± 5 | 62 ± 13 | NS |
| Gender | NS | ||
| male | 7 (70%) | 14 (50%) | |
| female | 3 (30%) | 14 (50%) | |
| ANCA | NS | ||
| PR3 | 6 (60%) | 19 (68%) | |
| MPO | 3 (30%) | 8 (29%) | |
| negative | 1 (10%) | 1 (∼4%) | |
| Diagnosis | NS | ||
| WG | 6 (60%) | 25 (89%) | |
| MPA | 1 (10%) | 2 (7%) | |
| RLV | 3 (30%) | 1 (4%) | |
| ENT | NS | ||
| yes | 4 (40%) | 16 (57%) | |
| no | 6 (60%) | 9 (32%) | |
| 3 unknown (11%) | |||
| Renal disease | NS | ||
| yes | 9 (90%) | 20 (∼71%) | |
| no | 1 (10%) | 6 (∼21%) | |
| 2 unknown (7%) | |||
| Lung disease | NS | ||
| yes | 4 (40%) | 12 (43%) | |
| no | 6 (60%) | 13 (46%) | |
| 3 unknown (11%) | |||
| DVT | NS | ||
| yes | 1 (10%) | 0 | |
| no | 9 (90%) | 24 (86%) | |
| 4 unknown (14%) | |||
| Clinical state | NS | ||
| new, active | 8 (80%) | 19 (68%) | |
| remission | 1 (10%) | 2 (7%) | |
| relapse | 1 (10%) | 4 (14%) | |
| 3 unknown (11%) | |||
| Creatinine (mean ± SD) | 503.6 ± 325.2 | 147.7 ± 139.2 | a |
| MDRD | 20.4 ± 19.4 | 59.7 ± 28.5 | a |
| Creatinine 12 months | 172.9 ± 79.7 | 106.6 ± 31.3 | b |
| MDRD 12 months | 42.4 ± 20.0 | 61.9 ± 17.1 | b |
| Corrected MDRD 12 months | 2.1 ± 14.2 | −1.1 ± 11.3 | NS |
| Follow-up | NS | ||
| death within 12 months | (1 (10%) | (5 (18%) | |
| alive at 12 months | 9 (90%) | 17 (61%) | |
| 6 lost to follow-up (21%) |
Clinical baseline and outcome parameters are given for Dutch patients who are anti-plasminogen antibody (anti-PLG) negative compared with patients who are anti-plasminogen antibody positive at baseline. ENT, ear nose throat disease; DVT, deep venous thrombosis; MDRD, renal function estimated according to the Modification of Diet in Renal Disease equation; NS, not significant.
aP < 0.001.
bP < 0.05.
Anti-Plasminogen Antibodies Are Correlated with the Extent of Hallmark Renal Histologic Lesions
For 19 Dutch patients tested for anti-plasminogen antibodies, contemporaneous renal biopsies were available. The average number of glomeruli per biopsy was 18 (range, 6 to 35). Seven of 19 biopsied patients were positive for anti-plasminogen antibodies: renal biopsies from these 7 patients showed higher percentages of glomeruli with fibrinoid necrosis (P < 0.05) and cellular crescents (P < 0.001; Table 3). No difference in globally sclerosed glomeruli or fibrous crescents was found. These results show that patients with anti-plasminogen antibodies show more active renal lesions than patients without anti-plasminogen antibodies.
Table 3.
Anti-plasminogen antibodies and renal histology
| Renal Histology Cohort (n = 19) | Anti-PLG Antibodies (n = 7) | No Anti-PLG Antibodies (n = 12) | P |
|---|---|---|---|
| Glomeruli with fibrinoid necrosis | |||
| percent of total glomeruli ± SD | 21 ± 18 | 5 ± 7 | a |
| Glomeruli with crescents | |||
| percent of total glomeruli ± SD | 52 ± 21 | 20 ± 17 | b |
| Glomeruli with cellular crescents | |||
| percent of total glomeruli ± SD | 37 ± 23 | 9 ± 7 | c |
| Glomeruli with fibrous crescents | |||
| percent of total glomeruli ± SD | 15 ± 15 | 11 ± 17 | NS |
| Glomeruli with global sclerosis | |||
| percent of total glomeruli ± SD | 13 ± 14 | 13 ± 11 | NS |
The percentage of glomeruli (mean ± SD) showing hallmark renal lesions of ANCA-associated glomerulonephritis is reported in biopsies of anti-plasminogen antibody (anti-PLG)–negative patients compared with anti-plasminogen antibody positive–patients. NS, not significant.
aP < 0.05.
bP < 0.01.
cP < 0.001.
DISCUSSION
We identified anti-plasminogen antibodies in approximately 25% of patients in two independent AAV cohorts. Furthermore, anti-tPA antibodies were detected in 17.6% of AAV patients from one cohort, and the majority of AAV-IgG that inhibited in vitro fibrinolysis harbored either anti-plasminogen and/or anti-tPA antibodies. Importantly, there was also a correlation between anti-plasminogen antibodies and the proportion of glomeruli exhibiting fibrinoid necrosis and cellular crescents.
Antibodies that react with plasminogen/plasmin and other serine proteases of the coagulation/fibrinolysis cascade were previously detected in patients with autoimmune disease, particularly as a subspecies of aCL antibodies.9,12–14 Notably, high affinity monoclonal anti-plasmin antibodies isolated from patients with APS bind plasminogen, thrombin, factor X, and activated protein C with considerably lower affinity.12,14 Typically, these autoantibodies seem to cross-react with epitopes within the catalytic domains of serine proteases and conformational epitopes in β2 glycoprotein 1.
In contrast, IgG derived from AAV patients preferentially bind plasminogen with infrequent binding to plasmin. Cross-inhibition studies also suggest lower affinity binding to plasmin than plasminogen in dual positive samples, and aCL antibodies did not account for anti-plasminogen antibodies in AAV patients. We examined total IgG rather than affinity-purified IgG, but collectively, the data suggest that anti-plasminogen antibodies in AAV are likely to be distinct from related antibodies in other autoimmune diseases.
The epitope(s) recognized by anti-plasminogen antibodies in AAV are not yet fully defined. A lack of reactivity toward denatured plasminogen suggests conformational epitope(s). The failure of most anti-plasminogen–positive AAV-IgG to recognize plasmin suggests that major epitopes are not preserved in the active serine protease. Anti-plasminogen antibodies isolated from PR3-ANCA vasculitis patients in a North American cohort seem to recognize an epitope within the protease domain, but again, these antibodies did not bind to plasmin or thrombin for reasons that are unclear.3
The detection of anti-tPA antibodies in AAV is intriguing. tPA is a serine protease that exhibits an overall 35% shared identity with plasminogen. In addition to homologous protease domains, tPA and plasminogen contain kringle domains. Anti-tPA antibodies characterized in two APS patients bound to its protease domain but did not cross-react with plasminogen or thrombin.15 AAV-IgG that display dual reactivity against plasminogen and tPA may comprise distinct immunoglobulins that bind exclusively to one or other serine protease or, alternatively, cross-reactive immunoglobulins recognizing common epitopes. The cross-inhibition studies favor the existence of cross-reactive immunoglobulins in at least some AAV patients. The previously proposed core epitope for anti-plasminogen antibodies isolated from PR3-ANCA patients is a PHxQ motif, whereas alignment of plasminogen and tPA shows that of these three residues; only glutamic acid (Q) is conserved.3
How might the observations in this study be reconciled with previous findings in the North American AAV cohort? If the development of anti-plasminogen autoantibodies is driven by exposure to exogenous antigens such as microbial proteins, divergent immunologic memory in patients from distinct geographic areas could influence autoantibody development. Notably, anti-plasminogen antibodies in North American AAV patients are also anti-complementary proteinase 3 (anti-cPR3) antibodies. A range of microbial proteins show homology to cPR3 and might initiate an immune response accounting for anti-cPR3 antibodies that cross-react with plasminogen in a cohort-specific manner. We did not examine anti-cPR3 antibodies in European patients, but the occurrence of anti-plasminogen antibodies in both PR3-ANCA– and MPO-ANCA–positive AAV patients indicates differences from the North American cohort.
This study showed the capacity of anti-plasminogen and anti-tPA antibodies to delay fibrinolysis, which supports the hypothesis that they have pathogenic significance. Studies in four distinct AAV patient cohorts have identified an increased incidence of venous thromboembolic events (VTE), which does not seem to be attributable to conventional prothrombotic risk factors.16,17 Meanwhile, anti-plasminogen antibodies correlated with VTE episodes in PR3-ANCA patients.3 Although one of the Dutch patients with particularly high titers of anti-plasminogen antibodies had a history of deep venous thrombosis, we do not have comprehensive data on VTE episodes in the cohorts. Nevertheless, we hypothesized that anti-plasminogen antibodies might also influence glomerular pathology through a disturbance of fibrinolysis in the microvasculature.
In AAV, vascular injury is mediated by ANCA-induced neutrophil and monocyte activation and characterized histopathologically by an influx of inflammatory cells with fibrinoid necrosis in small vessel walls.18 In the renal biopsy, fibrinoid necrosis is apparent in glomeruli. Although fibrinoid necrosis is a ubiquitous feature of ANCA-associated vascular injury,19 little is known about its pathogenesis.20 Meanwhile, there are striking parallels in composition between fibrinoid necrosis and the fibrin clot in vascular repair. Both lesions consist primarily of fibrin, platelets, leukocytes, and extracellular matrix molecules such as fibronectin.5,6,21–23 Conceivably, fibrinoid necrosis may therefore originate from a flaw in the physiologic vascular repair mechanism wherein the fibrin clot fails to be removed after its scaffold function for angiogenesis is completed. This disturbance may be initiated or accelerated by anti-plasminogen antibodies.
To this end, we scored histopathologic lesions in patients with biopsy-proven ANCA-associated glomerulonephritis and found that the occurrence of anti-plasminogen antibodies was associated with higher percentages of glomeruli with fibrinoid necrosis and cellular crescents, accompanied by more severely reduced renal function.
Fibrinoid necrosis is often encountered in glomeruli with cellular crescents, and there are indications that fibrin is an important mediator of extracapillary proliferation. Fibrinogen leakage through damaged glomerular basement membranes could potentially trigger the formation of crescents. Several experimental models support a role of coagulation, and fibrin in particular, in the formation of fibrinoid necrosis and crescents.24–26 Experimental anti-GBM glomerulonephritis has a more severe course in tPA and plasminogen knockout mice, with more fibrin deposition, tuft necrosis, and crescents, in addition to more severe renal failure.8 Other knockout models show that fibrinogen deficiency is protective in experimental crescentic glomerulonephritis.7
The role of glomerular fibrin deposition in crescent formation is underlined by experimental data that showed that defibrinating agents substantially prevented crescent formation and renal failure.27–29 In AAV, large studies on the efficacy of anti-coagulant therapies have not been performed, but case reports suggest that heparin and defibrotide can be therapeutically beneficial.30–34 Similarly, systemic plasminogen concentrates or recombinant tPA could be of interest. Recombinant tPA treatment reduced fibrin deposition and crescent formation and improved renal function in experimental crescentic glomerulonephritis,35 whereas streptokinase reduced glomerular fibrin deposition and preserved renal function when used early after disease onset.36
In conclusion, approximately 25% of AAV patients harbor anti-plasminogen antibodies. Anti-plasminogen antibodies, particularly in combination with anti-tPA antibodies, delay fibrinolysis in vitro and are related to hallmark renal histologic lesions that are closely associated with disturbances in coagulation. Therapies aiming at enhancing or replacing fibrinolytic activity may be of benefit in AAV patients with anti-plasminogen and anti-tPA antibodies.
CONCISE METHODS
Study Population
UK Cohort.
Stored frozen plasma exchange (PEX) fluid from 74 consenting AAV patients treated at University Hospital Birmingham was studied. All patients satisfied Chapel Hill Conference consensus criteria37 and received PEX for severe renal disease (serum creatinine ≥ 500 μmol/L or dialysis dependency) and/or pulmonary hemorrhage. Pulsed intravenous steroids were not routinely used, and the majority of patients started plasma exchange concurrently with oral corticosteroids. Cyclophosphamide (daily oral or pulsed intravenous) was initiated at the same time or immediately after corticosteroids and plasma exchange. Plasma exchange effluent was collected at the time of the first exchange. ANCA positivity and specificity of paired serum from these patients were determined at the time of diagnosis by indirect immunofluorescence and ELISA, respectively, at the Clinical Immunology Laboratory, University of Birmingham, Birmingham, UK. Twelve ANCA-negative patients with other renal diseases (anti-GBM disease, n = 7; SLE, n = 3; myeloma, n = 1; antibody-mediated renal allograft rejection, n = 1) who had also undergone PEX were used as a disease control cohort. Laboratory personnel (n = 50) served as a healthy control cohort, and serum was separated from peripheral blood collected after the study started.
IgG was purified from patient PEX fluid and healthy control serum by protein G affinity chromatography (GE Healthcare, Buckinghamshire, UK) and eluted with 0.1 M glycine (pH 2.7).38 Concentrations were determined by spectrophotometry. IgG samples were purified at the time of conducting this study, and results of the original ANCA testing were verified by retesting derived IgG samples in the Clinical Laboratory. AAV-IgG were also screened by ELISA for anti-cardiolipin antibodies using a kit available from the Binding Site (Birmingham, UK).
For separate assays using serial samples collected over time, serum from two AAV patients was available. For these experiments, sera from 127 healthy blood donors were used as the control population.
Dutch Cohort.
A total of 38 patients with AAV of whom serum or PEX fluid was available were recruited. The majority of patients (33/38) were seen in one nephrology unit (Meander Medical Center, Amersfoort, The Netherlands). All patients met the Chapel Hill criteria for diagnosis of AAV.37 The ANCA specificity of each patient was determined at Leiden University Medical Centre using an ELISA kit from Wieslab (Lund, Sweden). IgG was isolated from patient sera/PEX using the Melon Gel IgG Purification Kit (Pierce Protein Research Products; Thermo Scientific). The integrity of isolated IgG was confirmed through SDS-PAGE and visualization through Coomassie blue staining.
Glomerular filtration rates were estimated (eGFR) at the time of serum/PEX (baseline) and at 1 year using the four-variable Modification of Diet in Renal Disease equation.10,11 To evaluate an independent predictive effect of anti-plasminogen antibodies on eGFR at 1-year follow-up, we corrected for the eGFR at baseline. The corrected eGFR at 1 year was defined as the difference between the observed eGFR at 1 year and its linear prediction on the basis of baseline eGFR.39,40
Nineteen patients had a serum sample taken or underwent PEX on the same day a renal biopsy was performed. Two independent observers, who were unaware of the clinical data, reviewed all biopsies for glomerular lesions according to a previously designed scoring protocol.41 For each biopsy silver, periodic acid-Schiff and Martius Scarlet blue trichrome stainings were evaluated. The number of glomeruli with specific lesions was calculated as a percentage of the total number of glomeruli for each individual biopsy.
ELISA
High binding microtiter plates (Costar, Appleton Woods, Birmingham, UK) were coated overnight with 2.5 μg/ml plasminogen, plasmin (Cambridge Biosciences, Cambridge, UK), tPA (Merck Chemicals, Nottingham, UK), or 5 μg/ml recombinant α3(IV)NC1 (purified from 293-F cells using the FreeStyle Expression System; Invitrogen, Paisley, UK) at 4°C. Plates were blocked for 60 minutes at 25°C with Stabilcoat (Diarect AG, Freiburg, Germany) diluted to 50% with sterile water. After washing with buffer containing 1% Tris 1 M (pH 8.0), 2.5% NaCl 3 M, and 0.5% Tween-20, test IgG was pipetted (5 μg/ml unless stated) in duplicates into appropriate microtiter wells and incubated at 25°C for 2 hours. Wells were washed and incubated (60 minutes at 25°C) with a 1:50,000 dilution of alkaline phosphatase–conjugated donkey anti-human IgG (Jackson ImmunoResearch, Cambridge, UK). All dilutions were performed using sample diluent (1% BSA and 0.1% Tween-20). After washing, 4-nitrophenyl phosphate (Sigma-Aldrich, Gillingham, UK) was used as the substrate, and the 96-well microtiter plate was analyzed spectrophotometrically at 405 nm after 120 minutes. Each IgG was tested six times, and where data are expressed as a percentage of positive control, IgG from a separate cohort was used. In assays requiring the use of sera, a 1:100 dilution was used.
For competition assays, dose–response studies were performed to select the lowest concentration of IgG reactive on each antigen (1.25 μg/ml). IgG was preincubated (2 hours at 25°C) with increasing concentrations (0 to 1 μM) of native soluble plasminogen or plasminogen denatured by heating with 5% β-mercaptoethanol (5 minutes at 100°C). Before use, denatured plasminogen was diluted to the required concentration, and residual levels of β-mercaptoethanol were calculated at 0.05%. The integrity of IgG after preincubation with denatured plasminogen was confirmed by SDS-PAGE and visualization through Coomassie blue staining. The subsequent ELISA was performed as described above. For separate assays, plasminogen denatured in this way was also used as the coat antigen.
For alternative competition assays, IgG was preincubated with tPA or plasmin that had been inactivated through mixing with TLCK (Sigma) according to a protocol previously described.14 Briefly, 12 μM plasmin was incubated with 6 mM TLCK in PBS (pH 7.4) for 100 minutes at 25°C.14 Using this method, the proteolytic activity of plasmin is reportedly inhibited in excess of 99%.14 The inactivation of plasmin was confirmed by SDS-PAGE and Coomassie blue staining after mixing inactivated plasmin with IgG under the conditions required for the ELISA.
Immunoprecipitation Western
Two micrograms of plasminogen was immunoprecipitated with 10 μg IgG and 100 μl of protein A (Sigma) and G Sepharose (Pierce, Northumberland, UK) beads (50-μl bead volume) in nondenaturing lysis buffer. For control experiments, goat anti-plasminogen antibody (Abcam, Cambridge, UK) and irrelevant goat IgG (Bethyl Laboratories, Cambridge, UK) were used. Equal volumes of immunoprecipitates were separated on a 10% SDS-PAGE gel, and the membrane was probed with rabbit anti-plasminogen antibody (Abcam) at a dilution of 1:1000 in 5% marvel/Tris-buffered saline Tween-20 overnight at 4°C. The membrane was incubated for 1 hour with a horseradish peroxidase–conjugated donkey anti-rabbit secondary antibody (GE Healthcare) diluted 1:10,000. Visualization of bound proteins was carried out using an enhanced chemiluminescence kit (GE Healthcare), and the image of each band was scanned with a densitometer using Genetools by Syngene (Cambridge, UK).
Fibrinolysis Assays
UK AAV-IgG Samples.
The functional effects of anti-plasminogen antibodies were determined in vitro using a slight modification of a method previously described.14 Clot formation was initiated by mixing 10 μM fibrinogen with 1 nM thrombin (both from Merck Chemicals). Immediately, 100 μl was dispensed into each well of a 96-well microtiter plate. Increases in turbidity during clot formation were measured spectrophotometrically at 405 nm over 60 minutes (25°C). Sample diluent or IgG (final concentration, 150 μg/ml) was simultaneously incubated with plasminogen (final concentration, 10 μg/ml) for 60 minutes at 25°C before mixing with tPA (final concentration, 10 nM). Clot dissolution was initiated by the addition of the IgG/plasminogen and tPA solution and measured by reductions in absorbance at 405 nm. All samples were loaded in duplicates, and dilutions were performed in diluent containing 50 mM Tris-HCl, 150 mM NaCl, and 5 mM CaCl2 (pH 7.5). In separate experiments, IgG (final concentration, 150 μg/ml) was incubated with plasmin (final concentration, 50 nM) for 60 minutes on ice before loading onto the preformed fibrin clots. Each IgG was tested four times. During analysis, background absorbance and the time required for clot formation (60 minutes) were subtracted from the final values. Based on the clot lysis curves, we chose to analyze the data when 50% fibrinolysis had occurred. The integrity of IgG premixed with plasmin on ice was confirmed through SDS-PAGE and visualization through Coomassie blue staining.
Dutch AAV-IgG Samples.
To determine the effect of anti-plasminogen antibodies on fibrinolysis in vitro, clot lysis experiments were performed. IgG isolated from patient sera/PEX was dialyzed against triethanolamine (TEA) buffer (0.1% Tween, 50 mM TEA, 100 mM NaCl in MQ). Healthy control IgG (final concentration, 0.4 mg/ml), patient IgG (final concentration, 0.4 mg/ml), or buffer was preincubated with human Glu-plasminogen (final concentration, 0.1 μM; Enzyme Research Laboratories) for 30 minutes at 31°C. After incubation, fibrinogen (final concentration, 2.6 mg/ml; Sigma-Aldrich), factor XIII (final concentration, 2 U/ml; Fibrogammin, Behring), and tPA (final concentration, 1.5 U/ml; kind gift of Dr. F. Havekate, Gaubius TNO, Leiden, The Netherlands) were added, and subsequently, the mixture was added to a 96-well plate containing thrombin (final concentration, 10 nM; Sigma-Aldrich) and CaCl2 (final concentration, 10 mM). Clot formation and dissolution were monitored by absorbance at 405 nm. Each reaction was performed in duplicate, and measurements took place overnight. In each experiment, the same healthy control IgG and a negative sample only containing TEA buffer were tested. Half-lysis times were calculated for each patient IgG sample and expressed as percentages of the healthy control sample.
Statistical Analysis
A strict analysis criterion was used to determine whether patient samples reacted with plasminogen or other given antigens and also whether IgG was able to interfere with fibrinolysis. Any values exceeding the mean + 2 SD of the healthy control population were considered positive. These criteria had to be met on >50% of the occasions tested. Differences between groups were analyzed using one-way ANOVA followed by Bonferroni or Dunnett's post hoc tests. A Mann-Whitney U test (two-tailed) was performed to assess differences in renal function and the percentage of glomerular lesions according to anti-plasminogen antibody subgroup. P ≤ 0.05 was considered significant. All statistical tests were performed using GraphPad Prism (version 4; GraphPad, San Diego, CA) and the SPSS statistical software package for Windows (version 16.0; SPSS, Chicago IL).
DISCLOSURES
P.H. was supported by a fellowship provided by the Wellcome Trust, United Kingdom.
Acknowledgments
We thank Dr. Matthew Morgan for permitting the use of the serial serum samples and providing clinical information relating to these patients. We also thank Dr. Gloria Preston (University of North Carolina, Chapel Hill, NC) for providing IgG from an anti-plasminogen antibody–positive patient for use as a positive control while setting up the ELISA. Additionally, we thank Timothy Plant for screening patient IgG for anti-cardiolipin antibodies. We thank Dr. J. Eikenboom (Department of Hematology, LUMC, Leiden) for kindly providing fibrogammin and Dr. F. Havekate (Gaubius TNO, Leiden) for providing tPA. Furthermore, we thank A. Bakker for performing the ANCA ELISAs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Jennette JC, Falk RJ: New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr Opin Rheumatol 20: 55–60, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bautz DJ, Preston GA, Lionaki S, Hewins P, Wolberg AS, Yang JJ, Hogan SL, Chin H, Moll S, Jennette JC, Falk RJ: Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J Am Soc Nephrol 19: 2421–2429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heeringa P, Tervaert JW: Pathophysiology of ANCA-associated vasculitides: Are ANCA really pathogenic? Kidney Int 65: 1564–1567, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Novak RF, Christiansen RG, Sorensen ET: The acute vasculitis of Wegener's granulomatosis in renal biopsies. Am J Clin Pathol 78: 367–371, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Collen A: Fibrin Matrix Structure and Angiogenesis [PhD thesis], Leiden, The Netherlands, Leiden University, 2000 [Google Scholar]
- 7. Hertig A, Rondeau E: Role of the coagulation/fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol 15: 844–853, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Kitching AR, Holdsworth SR, Ploplis VA, Plow EF, Collen D, Carmeliet P, Tipping PG: Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med 185: 963–968, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin WS, Chen PC, Yang CD, Cho E, Hahn BH, Grossman J, Hwang KK, Chen PP: Some antiphospholipid antibodies recognize conformational epitopes shared by beta2-glycoprotein I and the homologous catalytic domains of several serine proteases. Arthritis Rheum 56: 1638–1647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levey AS, Greene T, Kusek JW, G.J.B: A simplified equation to predict glomerular filtration rate from serum creatinine. [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Chen XX, Gu YY, Li SJ, Qian J, Hwang KK, Chen PP, Chen SL, Yang CD: Some plasmin-induced antibodies bind to cardiolipin, display lupus anticoagulant activity and induce fetal loss in mice. J Immunol 178: 5351–5356, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Lu CS, Horizon AA, Hwang KK, FitzGerald J, Lin WS, Hahn BH, Wallace DJ, Metzger AL, Weisman MH, Chen PP: Identification of polyclonal and monoclonal antibodies against tissue plasminogen activator in the antiphospholipid syndrome. Arthritis Rheum 52: 4018–4027, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang CD, Hwang KK, Yan W, Gallagher K, FitzGerald J, Grossman JM, Hahn BH, Chen PP: Identification of anti-plasmin antibodies in the antiphospholipid syndrome that inhibit degradation of fibrin. J Immunol 172: 5765–5773, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Cugno M, Cabibbe M, Galli M, Meroni PL, Caccia S, Russo R, Bottasso B, Mannucci PM: Antibodies to tissue-type plasminogen activator (tPA) in patients with antiphospholipid syndrome: Evidence of interaction between the antibodies and the catalytic domain of tPA in 2 patients. Blood 103: 2121–2126, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Sebastian JK, Voetsch B, Stone JH, Romay-Penabad Z, Lo GH, Allen NB, Davis JC, Jr, Hoffman GS, McCune WJ, St Clair EW, Specks U, Spiera R, Loscalzo J, Pierangeli S, Merkel PA: The frequency of anticardiolipin antibodies and genetic mutations associated with hypercoagulability among patients with Wegener's granulomatosis with and without history of a thrombotic event. J Rheumatol 34: 2446–2450, 2007 [PubMed] [Google Scholar]
- 17. Tomasson G, Monach PA, Merkel PA: Thromboembolic disease in vasculitis. Curr Opin Rheumatol 21: 41–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jennette JC, Charles LA, Falk RJ: Antineutrophil cytoplasmic autoantibodies: Disease associations, molecular biology, and pathophysiology. Int Rev Exp Pathol 32: 193–221, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Jennette JC: Antineutrophil cytoplasmic autoantibody-associated diseases: A pathologist's perspective. Am J Kidney Dis 18: 164–170, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Bajema IM, Bruijn JA: What stuff is this! A historical perspective on fibrinoid necrosis. J Pathol 191: 235–238, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Bajema IM: Aspects of ANCA-Associated Glomerulonephritis. PhD Thesis, Leiden, The Netherlands, Leiden University, 2000 [Google Scholar]
- 22. Jennette JC, Wilkman AS, Falk RJ: Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol 135: 921–930, 1989 [PMC free article] [PubMed] [Google Scholar]
- 23. Serra A, Cameron JS, Turner DR, Hartley B, Ogg CS, Neild GH, Williams DG, Taube D, Brown CB, Hicks JA: Vasculitis affecting the kidney: Presentation, histopathology and long-term outcome. Q J Med 53: 181–207, 1984 [PubMed] [Google Scholar]
- 24. Channing AA, Kasuga T, Horowitz RE, Dubois EL, Demopoulos HB: An ultrastructural study of spontaneous lupus nephritis in the NZB-BL-NZW mouse. Am J Pathol 47: 677–694, 1965 [PMC free article] [PubMed] [Google Scholar]
- 25. Silva FG, Hoyer JR, Pirani CL: Sequential studies of glomerular crescent formation in rats with antiglomerular basement membrane-induced glomerulonephritis and the role of coagulation factors. Lab Invest 51: 404–415, 1984 [PubMed] [Google Scholar]
- 26. Vassalli P, McCluskey RT: The pathogenic role of the coagulation process in rabbit masugi nephritis. Am J Pathol 45: 653–677, 1964 [PMC free article] [PubMed] [Google Scholar]
- 27. Naish P, Penn GB, Evans DJ, Peters DK: The effect of defibrination on nephrotoxic serum nephritis in rabbits. Clin Sci 42: 643–646, 1972 [DOI] [PubMed] [Google Scholar]
- 28. Thomson NM, Simpson IJ, Peters DK: A quantitative evaluation of anticoagulants in experimental nephrotoxic nephritis. Clin Exp Immunol 19: 301–308, 1975 [PMC free article] [PubMed] [Google Scholar]
- 29. Thomson NM, Moran J, Simpson IJ, Peters DK: Defibrination with ancrod in nephrotoxic nephritis in rabbits. Kidney Int 10: 343–347, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Crummy CS, Perlin E, Moquin RB: Microangiopathic hemolytic anemia in Wegener's granulomatosis. Am J Med 51: 544–548, 1971 [DOI] [PubMed] [Google Scholar]
- 31. Juncos LI, Alexander RW, Marbury TC: Intravascular clotting preceding crescent formation in a patient with Wegener's granulomatosis and rapidly progressive glomerulonephritis. Nephron 24: 17–20, 1979 [DOI] [PubMed] [Google Scholar]
- 32. Fuller T, Olsen N, Block A, Cade J: Wegener's granulomatosis. Treatment with heparin in addition to azathioprine and corticosteroids. Nephron 9: 225–234, 1972 [DOI] [PubMed] [Google Scholar]
- 33. Whitaker AN, Emmerson BT, Bunce IH, Nicoll P, Sands JM: Reversal of renal failure in Wegener's granulomatosis by heparin. Am J Med Sci 265: 399–406, 1973 [DOI] [PubMed] [Google Scholar]
- 34. Frasca GM, Zoumparidis NG, Borgnino LC, Neri L, Neri L, Vangelista A, Bonomini V: Combined treatment in Wegener's granulomatosis with crescentic glomerulonephritis—Clinical course and long-term outcome. Int J Artif Organs 16: 11–19, 1993 [PubMed] [Google Scholar]
- 35. Zoja C, Corna D, Macconi D, Zilio P, Bertani T, Remuzzi G: Tissue plasminogen activator therapy of rabbit nephrotoxic nephritis. Lab Invest 62: 34–40, 1990 [PubMed] [Google Scholar]
- 36. Tipping PG, Holdsworth SR: Fibrinolytic therapy with streptokinase for established experimental glomerulonephritis. Nephron 43: 258–264, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG: Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 37: 187–192, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Williams JM, Pettitt TR, Powell W, Grove J, Savage CO, Wakelam MJ: Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J Am Soc Nephrol 18: 1112–1120, 2007 [DOI] [PubMed] [Google Scholar]
- 39. de Lind van Wijngaarden RAF, Hauer HA, Wolterbeek R, Jayne DRW, Gaskin G, Rasmussen N, Noel LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM. for the European Vasculitis Study Group (EUVAS): Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Senn S, Stevens L, Chaturvedi N: Repeated measures in clinical trials: Simple strategies for analysis using summary measures. Stat Med 19: 861–877, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Bajema IM, Hagen EC, Hansen BE, Hermans J, Noel LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996 [DOI] [PubMed] [Google Scholar]