Abstract
Atypical hemolytic uremic syndrome (aHUS) is a rare form of thrombotic microangiopathy that associates, in 70% of cases, with genetic or acquired disorders leading to dysregulation of the alternative pathway of complement. Autoantibody directed against Factor H causes at least 6% to 10% of aHUS cases, but only a few clinical reports are available. Here, we describe the clinical, biologic, genetic features, treatment, and outcome of 45 patients who presented with aHUS associated with anti-FH autoantibody. We found that this form of aHUS primarily affects children between 9 and 13 years old but it also affects adults. It presents with a high frequency of gastrointestinal symptoms and with extrarenal complications and has a relapsing course. Activation of the alternative pathway of complement at the onset of disease portends a poor prognosis. Early specific treatment may lead to favorable outcomes. These data should improve the recognition and diagnosis of this form of aHUS and help identify patients at high risk of a poor outcome.
Atypical hemolytic uremic syndrome (aHUS) is a rare form of thrombotic microangiopathy that occurs in the absence of infection by Shiga toxin–producing bacteria (OMIM#235400). Development of aHUS may result from either genetic or acquired disorders leading to a dysregulation of the complement alternative pathway. In >60% of patients, genetic abnormalities are found in genes encoding the complement alternative pathway regulatory proteins: Factor H (CFH), membrane cofactor protein (MCP or CD46), Factor I (CFI),1 the C3 convertase components (C3), and Factor B (CFB)2,3 or, recently, in the Thrombomodulin gene.4
The presence of autoantibody directed against Factor H (anti-FH IgG), and a resulting Factor H (FH) functional deficiency, was first published in 2005.5 This etiology, reported only in children, is the cause of aHUS in at least 6% to 10% of cases.5–8 The anti-FH IgG have been shown to interfere with the FH binding to the alternative pathway C3 convertase C3bBb5 and associated with defective FH-dependent cell protection.9,10 Most patients lack the circulating complement Factor H–related proteins (CFHR) 1 and 3 because of a homozygous deletion of CFHR1 and CFHR3.7,11,12
Currently, only a few descriptive reports concerning the clinical and biologic data, and the treatment modalities are available.5–8,10,12–16
We report here the clinical, biologic, and genetic features of 45 aHUS patients who presented anti-FH autoantibodies.
RESULTS
Patients
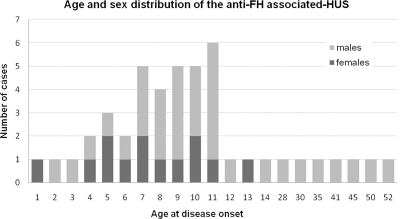
The series comprised 45 patients who fulfilled the clinical criteria of aHUS and were positive for the anti-FH IgG test. The mean follow-up was 39 months (1 to 168 months). Different ethnical origins were observed: 23 Caucasians, 3 Japanese, 3 Koreans, 13 Indians, and 3 Africans. It is composed of 38 children (25 males) and 7 male adults. Median age at disease onset was 8.5 years in children (8 months to 14 years) and 41 years in adults (28 to 52 years) (Figure 1). The diagnosis was retrospective in 19 cases.
Figure 1.
Anti-FH associated-aHUS occurs mainly in late childhood but can affect adults.
Clinical data of 11 patients have been previously reported.5,12,15–17
Disease Description at Onset
Prodromes, Clinical, and Biologic Data at Onset.
Complete clinical data at onset was obtained for 32 of 45 total patients. Four patients developed HUS in a context of infection: one varicella,16 one upper respiratory tract, one STEC, and one norovirus infection.12 Intense abdominal pain and vomiting were present in 84% of patients (27 of 32 patients). In two patients, Mallory-Weiss syndrome was associated. Diarrhea was observed in 53% of patients (17 of 32 patients). Two patients presented urticaria and transient face edema as prodrome. Four patients (three children and one adult) developed seizures at disease onset.
At admission, all patients presented with anemia, schizocytosis, low haptoglobinemia, thrombocytopenia, and acute renal failure. Biologic pancreatitis (increased amylasemia and/or lipasemia) and elevated liver enzymes were observed in 23% and 50% of the documented patients (n = 27), respectively (Table 1). Antinuclear antibodies were present in 7 of 29 tested patients (24%) with titers from 1:80 to 1:1250, and antibodies had a speckle pattern without a specific nuclear antigen identified.
Table 1.
Clinical and biologic characteristics of anti-FH–associated aHUS patients at disease onset
| Age | |
| all patients (n = 45) | Median: 9 years (8 months to 52 years) |
| children (n = 38) | Median: 8.5 years (8 months to 14 years) |
| adults (n = 7) | Median: 41 years (28 to 52 years) |
| Prodromes (n = 32) | |
| gastrointestinal symptoms | 84% (diarrhea: 53%) |
| (2 cases with Mallory-Weiss syndrome) | |
| infection | 4 (1 varicella, 1 upper respiratory tract infection, 1 STEC, 1 norovirus) |
| other | 2 urticaria and face edema |
| Clinical symptoms at onset (n = 32) | |
| hypertension | 68% |
| hematuria | 27% |
| oligo-anuria | 28% |
| seizures | 23.5% |
| Biological features at onset (n = 27) | |
| Hb | Mean: 6.8 g/L (range: 5 to 10) |
| platelets count | Mean: 56.3 × 109/L (range: 9 to 141) |
| creatininemia | Mean: 501 μmol/L (range: 150 to 1593) |
| pancreatitis | 23.1% |
| hepatitis | 50% |
| Autoimmunity at onset (n = 29) | |
| antinuclear antibodies | 24% |
The percentages were calculated according to the number of patients documented. Presence of biologic pancreatitis and hepatitis was defined by elevated levels of amylasemia and/or lipasemia and of liver enzymes, respectively.
Complement Exploration.
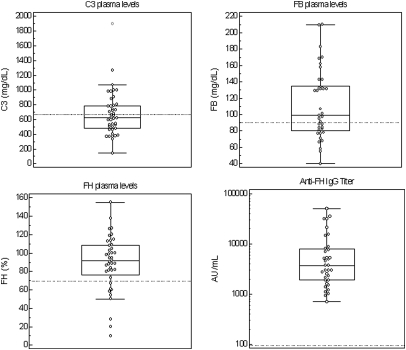
At disease onset, systemic complement alternative pathway activation was noticed in 26 of 45 patients (58%) as indicated by low C3 levels associated with low FB in 13 patients (29%) (Figure 2). C4 levels were within the normal ranges in all cases. A sample collected at the acute phase of the disease was available for 28 patients. The anti-FH IgG titers were established between 1080 and 50,000 AU/ml (positive threshold: 100 AU/ml). Mean plasma C3 antigenic levels in samples exhibiting high titers of anti-FH IgG (>10,000 AU/ml) were statistically lower than those in samples with moderate titers (<10,000 AU/ml), 566 versus 711 mg/L, respectively (P = 0.037).
Figure 2.
At onset, the majority of patients shows complement alternative pathway activation (decreased C3 and/or Factor B) and a subset has decreased FH. The dashed lines represent the lower limit of normal range (for C3, FB, and FH antigenic levels) or the positive threshold (for anti-FH IgG titers).
Antigenic levels of FH were decreased at disease onset in 22% of patients (10 of 45 patients). The values were comprised between 10% and 67% of normal ranges (Figure 2). This decrease was not correlated with the anti-FH IgG titers.
Genetic Exploration.
No abnormality was found by the CFH, CFI, and MCP sequence analysis performed in 26 patients.
The quantity of CFHR1 and CFHR3 copy alleles was determined by MLPA in 43 patients. A homozygous deletion of CFHR1 was found in 40 of 43 tested patients (93%) and was associated with a homozygous deletion of CFHR3 in 39 of 43 patients (91%). One patient exhibited no CFHR1 allele with one CFHR3 allele, and three patients exhibited one allele of each gene.
Treatment and Evolution of the First Flare.
Precise data concerning the treatment administered at the acute phase of the disease were available for 30 patients. Dialysis was necessary in 17 patients (57%). At disease onset, 6 patients were managed conservatively, 6 received fresh frozen plasma (FFP), 15 received plasma exchanges (PE) alone, and 3 received PE associated with immunosuppressive treatments (IS) comprised of steroids associated with cyclophosphamide (n = 2) or mycophenolate mofetil (n = 1). The mean delay between the first symptoms and the treatment initiation of the treatment was 9.5 days. The evolution of the first flare according to treatment modalities is summarized in Table 2. Two patients developed ESRD after the first flare of the disease and one died. Four patients presented no relapse but had chronic renal insufficiency (CRI). Five patients had no relapse and no sequel. Five patients were treatment-resistant such that they had no disease improvement under treatment or presented early relapse (within the first month), requiring a change of the treatment. The 13 remaining patients (43%) presented late relapses such that the disease recurrence occurred >1 month after onset and >15 days after disease remission.
Table 2.
Disease evolution of the first flare, according to the treatment administered at onset
| Conservative Treatment (n = 6), HD = 2 | FFP Infusion (n = 6), HD = 5 | PE (n = 15), HD = 11 | PE + IS (n = 3), HD = 1 | |
|---|---|---|---|---|
| No sequel | 1 | 0 | 1 | 3 |
| CRI | 1 | 0 | 3 | 0 |
| Late relapse | 2 | 5 | 6 | 0 |
| Treatment resistance | 1 | 1 | 3 | 0 |
| ESRD | 1 | 0 | 1 | 0 |
| Death | 0 | 0 | 1 | 0 |
In total, 30 patients were documented. Follow-up occurred between 5 and 168 months with a mean of 48 months. The number of patients in each group is indicated in parentheses. HD indicates the number of patients needing hemodialysis at the first flare. Treatment resistance comprised cases without disease improvement under treatment and cases with relapse within the first month. Late relapses were defined by disease recurrence >1 month after onset and >15 days after disease remission.
None of the three patients treated by PE and IS relapsed, and all of them fully recovered without relapse up to 12 month follow-up. By contrast, late relapsing patients were treated by conservative treatment (2 of 6 patients, 33%), FFP infusion (5 of 6 patients, 83.3%), or PE (6 of 15 patients, 40%) alone.
Long-Term Evolution and Treatment
Long-term follow-up was documented in 44 patients. Disease relapses occurred in 25 of these patients (58%), who had a mean of 1.5 recurrences. The relapses occurred in 17 of 25 patients (68%) during the first 6 months after onset. However, five patients exhibited a relapse >12 months after onset.
No sequel was noticed in 11 patients (25%), 4 developed extrarenal sequels (3 cardiac insufficiencies and 1 diabetes), and 17 had CRI (39%). Additionally, 12 patients (27%) developed ESRD, 4 died, 3 after the first flare of the disease and 1 adult 2 years after onset from cardiac insufficiency (Table 3). The occurrence of renal sequels (ESRD or CRI) was correlated with high creatininemia (>250 μmol/L, P = 0.01), low C3 levels (<660 mg/L, P = 0.034), and low Factor B levels (<93 mg/L, P = 0.002) at disease onset. The odds ratio (OR) associated with these pejorative prognosis factors were 4.04, 95% confidence interval (CI) [1.1 to 14.78] for low C3 levels, and 12.4, 95% CI [2.65 to 58.99] for low Factor B levels. However, there was no correlation between the risk of renal sequel and the anti-FH IgG titer at the acute phase with mean titers being 13,470 and 8937 AU/ml in the patients with n = 20 or without n = 13 renal sequels, respectively (P > 0.05) (Table 4). We observed that the occurrence of relapses increased the risk of renal sequels (ESRD or CRI). Renal sequels were present in 71% versus 27% of patients with n = 14 or without n = 11 late relapses, respectively (OR = 6.67, 95% CI [1.15 to 38.85]).
Table 3.
Long-term evolution of the disease
| Death, 4 patients | 1 adult at W2 from unknown reason |
| 1 child at M4 from unexplained sudden death after a hemodialysis session | |
| 1 child from pulmonary arterial hypertension before M6 | |
| 1 adult at 2 year follow-up from cardiac insufficiency | |
| Renal sequel | None: 25% |
| CRI: 39% | |
| ESRD: 27% | |
| Other organs involvement | Cardiac insufficiency: 3 (2 adults, 1 child) |
| Non-autoimmune diabetes: 1 (child) | |
| Kidney transplantation | 3 of 3 unfavorable without specific management |
| 3 of 3 favorable with specific management14,15 |
In total, 44 patients were documented during a range of follow-up from 1 to 168 months (mean: 39 months). W2, week 2; M4, month 4; M6, month 6.
Table 4.
Correlation of clinical and biologic parameters with occurrence of renal sequels (CRI, ESRD, and death) or relapses
| Parameter | Renal sequels or relapse (n = 20) | No sequel, no relapse (n = 13) | P |
|---|---|---|---|
| Age at onset (years) | 12 | 13 | 0.87 |
| Hb (g/L) | 6.65 | 6.95 | 0.31 |
| Pt (109/L) | 59 | 53 | 0.29 |
| Creat (μmol/L) | 762 | 285 | 0.01 |
| Schizocytosis (%) | 11 | 4 | 0.07 |
| Anti-FH IgG titer (AU/ml) | 13,470 | 8940 | 0.4 |
| Low C3 (%) | 73 | 41 | 0.034 |
| Low FB (%) | 55.5 | 9.5 | 0.002 |
Means of age, hemoglobinemia (Hb), quantity of platelets (Pt), creatininemia (Creat), schizocytosis, and anti-FH titer at onset are indicated in each group of patients. The percentages of patients exhibiting low C3 (<660 mg/L) and low Factor B levels (<93 mg/L) at onset are also indicated. P values were calculated with a Student t test.
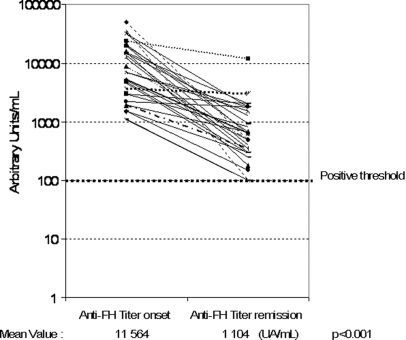
Titers of anti-FH IgG were significantly higher at the acute phase than in remission. Indeed, in the 26 patients tested, the mean of titers at disease onset was significantly higher than that in remission (11,564 versus 1104 AU/ml, respectively, P < 0.0001) (Figure 3). For seven patients, prospective follow-up revealed a correlation between anti-FH titer increase (>2000 UA/ml) and the occurrence of relapse (nine relapses). However, our data remain insufficient to propose a reliable algorithm capable of predicting disease recurrence according to anti-FH titer.
Figure 3.
Anti-FH levels are lower at remission than at disease onset. The difference reaches statistical significance using the t test.
Long-term follow-up showed that the anti-FH IgG remained detectable in most patients (29 of the 33 documented, 88%), even during disease remission. Only one patient exhibited a spontaneous decrease in anti-FH IgG during the 60 months after onset and complete disappearance at 6 years remission, without PE or immunosuppressive treatment. In three other patients, the autoantibodies became undetectable after therapies combining PE and IS.
IVIg were administered in 15 patients. Whether administered alone or in association with PE or steroids, this treatment failed to demonstrate any efficacy. Therefore, an additional immunosuppressant was introduced in all of these patients.
Five patients received anti-CD20 therapy.
In two cases, the treatment was done during the disease's acute phase. One child was treated 1 month after onset. The child relapsed twice at months 2 and 7 after onset, when PE was stopped. He was treated thereafter by cyclophosphamide, which allowed PE cessation.17 The antibodies never disappeared during and up to 3 years of follow-up but his renal function remained normal. One adult received rituximab at month 2 because of PE dependence. Associated with PE and steroids, this allowed the improvement of the renal function and disappearance of the anti-FH IgG, which remained undetectable during the next 10 months without any immunosuppressive treatment.
In the three other patients, rituximab was administered in preparation to renal transplantation. One was reported in reference 14 Currently, at the 4-year follow-up, the graft function was normal and the anti-FH IgG remained under the positive threshold with the immunosuppressive treatment given to prevent graft rejection. One patient was transplanted under similar management of the peritransplantation time with PE and was well at the 8-month follow-up. The last patient is waiting for renal transplantation. In the last two cases, the anti-FH IgG did not disappear after rituximab.
Two patients have been transplanted with undiagnosed anti-FH–associated aHUS. Both had posttransplant recurrence of HUS and one had a favorable outcome under specific management, allowing reduced anti-FH IgG production. Under treatment, both patients exhibited no more detectable anti-FH IgG since the transplantation time at follow-up time points of 42 and 50 months.
No development of another autoimmune disease was noticed in any patient during the follow-up time periods ranging from 5 to 168 months (mean: 41 months).
DISCUSSION
In this study, we report the clinical and biologic data of 45 patients exhibiting anti-FH IgG in a context of aHUS. The earliest known case occurred in 1975.15
To date, this form of aHUS has been reported only in children.5–7 In our study it was diagnosed mainly in children (84%). However, the age of onset is not similar to the ages usually reported in pediatric aHUS, which occurs primarily before the age of 2 years (70% in Sellier-Leclerc et al.18), whereas anti-FH IgG-aHUS occurs mainly in teenagers (Figure 1). In the French cohort, this etiology represents 60% of aHUS cases occurring between 9 and 12 years old. This age is also different from the age of shigatoxin-associated HUS (Stx-HUS), which primarily affects children before 5 years of age.19–21
We report here, for the first time, seven cases of adult onset of anti-FH IgG aHUS. Interestingly, all occurred in males, and we observed a sex ratio of 3 men for every woman among the 45 patients.
Therefore, this etiology of aHUS must be looked at systematically not only in children (particularly during late childhood) but also in adults, primarily in men.
Clinical features at disease onset are more similar to those of Stx-HUS than those of aHUS, linked to complement abnormalities. Indeed, we noted a high frequency of intense abdominal pain and vomiting (84%) and occurrence of diarrhea (53%) in our anti-FH–positive patients, whereas diarrhea was reported by Sellier-Leclerc et al. in only 28% of children presenting with aHUS.18 The acute phase extrarenal complications were also more frequent than previously described in complement mutation–associated HUS, with seizures in 23.5% of anti-FH–positive patients (10.8% in Sellier-Leclerc et al.18), pancreatitis in 23.1%, and hepatitis in 50% of cases. These complications, as well as cardiac involvement, were very rarely reported among the other complement-associated aHUS, including the cases of FH deficiencies. However, such complications were described in Stx-HUS with variable frequencies (pancreas and brain involvement and cardiac complications in 3% to 10%).22,23 Recently, Abarrategui-Garrido et al. also reported death caused by cardiac insufficiency in 1 of 7 children with anti-FH–associated HUS.13 Diabetes was also reported as a complication of childhood Stx-HUS with negative diabetes mellitus autoantibodies.24
We observed comparable symptoms between children and adults but a severer evolution in adults. This must be confirmed by a large cohort of adult cases.
The long-term evolution of anti-FH–associated aHUS is more severe than that in Stx-HUS (frequency of ERSD and/or death is 12%),25 but it is quite similar to the other forms of aHUS, with a high frequency of relapses (59% in anti-FH–associated HUS versus null in Stx-HUS, 38% to 54% in the other forms of aHUS and 66% to 86% in MCP mutation1,18,26). Additionally, when anti-FH–associated HUS is being compared to other forms of aHUS, there are comparable frequencies of CRI (39% versus 31%), ESRD (27% versus 22% to 35%18,26), and death (9.5% versus 11% to 10%18,26). However, anti-FH–associated aHUS has a better prognosis than the FH mutation–associated aHUS in which the frequencies of ESRD and death have been reported to be 42% to 60% and 20% to 48%, respectively.
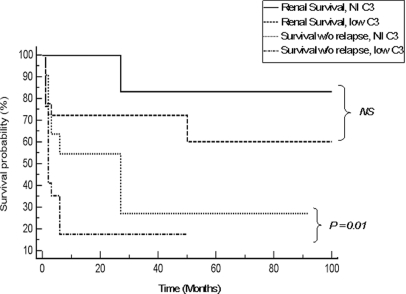
The presence of anti-FH autoantibodies was found to be associated at disease onset with a systemic complement alternative pathway activation leading to the consumption of C3 and FB. The association is comparable to that observed in patients exhibiting CFH or CFI mutations.18,26 However, during disease remission, C3, Factor B, and FH antigenic levels normalized in all patients. This systemic alternative pathway activation as well as the FH haploinsufficiency observed in 20% of patients reveals that alternative pathway dysregulation plays a major physiopathological role in anti-FH autoantibodies–associated HUS. For the first time, we show that low C3 and FB levels at onset represent for this form major pejorative prognosis factors (Figure 4). Renal sequels and relapses were analyzed together because relapse occurrence was associated with an increased frequency of renal sequels and is an indicator of treatment failure.
Figure 4.
Low C3 levels at onset are correlated with a higher risk of disease relapse. The Kaplan-Meier curves calculate the probability of survival without relapse and of renal survival (ESRD or death) according to C3 levels at onset (normal or low levels) and the disease progression parameters of 31 patients from our series of anti-FH–associated aHUS.
The evolution observed after the first flare of the disease according to the treatment administered revealed for the first time that FFP infusion, or even PE, failed to demonstrate significant efficacy at long term. Interestingly, none of the three patients treated by PE and IS relapsed and all fully recovered as seen in a follow-up >12 months later.
In the literature, data regarding the treatment modalities were available for 22 patients.5,7,8,10,12,13,15,16,27 Three patients had supportive treatment and developed ESRD. Two received FFP with one recovering, whereas the other reached ESRD. One patient, treated by plasmapheresis (with 5% albumin), died. Seven patients were treated by PE with five patients recovering, whereas one developed CRI and one reached ESRD. Eight of 9 patients treated by PE and immunosuppressant recovered.
Our data and those from literature, strongly suggest altogether a good efficacy of PE associated with immunosuppressant at the anti-FH–associated HUS onset. Among the immunosuppressive treatments used, the rituximab's efficiency cannot be definitively assessed by our data and those from literature.10,27 The anti-C5 antibody treatment should also be considered in patients identified as at high risk of pejorative outcome (with low C3 and FB levels and high creatininemia levels at onset) to block the complement activation.
In the literature and in our series, 14 renal transplantations have been performed in anti-FH–positive patients.8,13–15,28 Among the anti-FH–positive patients at the transplantation time, a rapid aHUS recurrence with graft loss was observed in 2 of 3 patients who had no specific treatment (Table 5). However, the immunosuppressive treatments administered for allograft rejection prevention might also play a role in controlling disease recurrence.
Table 5.
Outcome of 14 renal transplantations (9 patients) from the literature and our series
| Outcome | No. Cases | Posttransplant Follow-up | Anti-FH Documented at Transplantation Time |
|---|---|---|---|
| Graft loss due to aHUS recurrence, no specific treatment | 3 (21%) | <1 month | Positive in 2a15 |
| Not documented in 1a15 | |||
| Graft loss not due to recurrence, no specific treatment | 2 (14%) | 13 years (chronic allograft nephropathy) | Negativea15 |
| 11 years (chronic TMA) | Negativea15 | ||
| Favorable without specific treatment | 6 (43%) | 1 month, 8 months, | Not documented in 58,13 |
| 2 years, 3 years, | Positive in 128 | ||
| 6 years, 15 years | |||
| Favorable under PE | 1 (7%) | 5 years | Positivea15 |
| Favorable under PE + pretransplant anti-CD20 | 2 (14%) | 8 months and 5 years | Positive in 2a14 |
aData included in our series.
Regardless of the ethnic origins, the genetic background includes the homozygous deletion of at least CFHR1 gene found in >90% of the patients. All deleted patients presented a homozygous deletion of both CFHR1 and CFHR3 genes, except one patient, who is likely to be a compound heterozygote with a CFHR1/CFHR3 heterozygous deletion and a CFHR1/CFHR4 heterozygous deletion as recently reported.13 The absence of complete CFHR1 deficiency must not exclude the diagnosis of anti-FH–associated HUS as 10% of anti-FH–positive patients exhibit circulating CFHR1 protein. Thus, the term DEAP-HUS (deficiency of CFHR proteins and CFH autoantibody positive)7 is not appropriate to describe all cases of anti-FH–associated-HUS.
In conclusion, our study describes the specific features of the anti-FH autoantibodies–associated HUS. It identifies alternative pathway activation as major pejorative prognostic factors for relapse and renal survival. These data could help to better diagnose this form of aHUS and to identify patients at high risk of pejorative outcome.
CONCISE METHODS
Participants
All included patients were positive for the anti-FH IgG test and presented the criteria for the diagnosis of HUS: acute anemia, fragmented red cells on blood film, thrombocytopenia (<150 g/L), and renal dysfunction. Renal dysfunction was defined by one or several of the following criteria: (1) serum creatininemia greater than normal values according to their age; (2) urine protein/creatinine ratio >0.2 g/g, proteinuria >0.02 g/mm, or characteristic histopathological findings on renal biopsy.
Thirty-one patients were screened for the biologic criteria of a shigatoxin-productive bacterial infection. A PCR for shigatoxin (STX1 and STX2 were the targeted genes) was conducted on DNA isolated from the stools, and detection of serum antibodies against lipopolysaccharides (LPS) were both found to be negative. Among the patients not tested, one was secondarily diagnosed as having a STEC infection.
CRI was defined by a decreased creatinine clearance calculated by the Schwartz or the MDRD methods, or by a creatininemia superior to normal value according to the age. The absence of renal sequel was defined by normal creatinine clearance or a normal serum creatininemia with no proteinuria.
An informed consent was obtained from each patient or parents of children, and the study was approved by the Ethics Committee (CPP Ile de France V, IDRCB2008-A00144-51).
Complement Assays
Measurement of CH50 activity in EDTA plasma samples was performed as described previously.18 Plasma concentrations of the complement components C4, C3, and Factor B (FB) antigens were measured by nephelometry (Dade Behring, Paris La Defense, France). FH and FI antigen concentrations were measured by sensitive ELISA methods.18 CD46 membrane expression was determined by flow cytometry.18
Anti-Factor H Antibody Assessment
Presence of anti-FH IgG was detected by using an enzyme-linked immunosorbent assay (ELISA) method as described previously with some modifications.5
Titers of positive samples were expressed as arbitrary units per mL (AU/ml) and calculated using a calibration curve obtained with serial dilutions of a reference positive plasma given an arbitrary titer from 100 to 2000 AU/ml. The positive threshold was calculated by the mean + 5 SD of those obtained in 100 individual healthy donors' plasma. This titer was determined to be 100 AU/ml, and titers above this value were considered as positive.
Genomic CFH, FI, and MCP DNA Sequencing
For genetic analysis, genomic DNA was extracted from peripheral blood cells. Uncloned genomic DNA was amplified by means of a PCR using oligonucleotides flanking each exon of each CFH, FI, and MCP genes. Primer sequences, length of the PCR amplified fragments, and temperatures of hybridization used for each reaction in addition to the direct DNA sequencing procedure have been previously described.18
Multiplex Ligation–Dependent Probe Amplification
The Multiplex Ligation–Dependent Probe Amplification (MLPA) reaction was performed as described previously.11 Sequences of probes were designed to determine dosage for exon 23 of CFH, exon 5 of CFHR1, and exon 3 of CFHR3 along with the control probe C1INH exon 8. Reagents of MLPA reaction were purchased from MRC Holland (Amsterdam, The Netherlands), and the reaction was carried out according to the manufacturer's recommended protocol.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by grants from the Direction de la Recherche Clinique (DRC) of the Assistance Publique-Hôpitaux de Paris (APHP) (PHRC AOM05130; AOM08198; CIRC 06037).
We thank C. Hautreux, N. Poulain, F. Marliot, S. Roncelin, and F. Hodskin for their technical assistance.
We thank the “Société de Néphrologie Pédiatrique,” the “Société Française de Néphrologie,” and all of the clinicians who referred the patients: H. Nivet and S. Cloarec, Service de Néphrologie, Tours, France; A. Lionet and C. Noel, Lille, France; A. Wynckel and P. Rieu, Service de Néphrologie, and T. Tabary, Service d'Immunologie, Reims, France; G. Landthaler, Service de Néphrologie pédiatrique, Rouen, France; V. Baudet-Bonneville and A. Bensman, Service de Néphrologie pédiatrique, Hôpital Trousseau, Paris, France; A. Karras, Service de Néphrologie, Hôpital Foch, Suresnes, France; E. Siomou, University Hospital of Ioannina, Greece; M. Jadoul, Clinique Universitaire St Luc, Brussels, Belgium; N. Plant, Royal Manchester Children's Hospital, United Kingdom; G. Smith, Children's Kidney Center, Cardiff, United Kingdom; S. Johnson and M. Taylor, Birmingham Children Hospital, United Kingdom; R. Cleper and M. Davidovits, Schneider Children's Medical Center, Petah Tiqua, Israel.
M.-A.D.-D. designed the research, analyzed data, and wrote the paper. B.R., C.L., W.H.F., and V.F.B analyzed data and wrote the paper. S.K.S, A.B., C.B., J.B., J.L.A, N.T., H.C., P.J., M.L.Q., and P.N. performed research. All the authors read and approved the submission of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roumenina LT, Jablonski M, Hue C, Blouin J, Dimitrov JD, Dragon-Durey MA, Cayla M, Fridman WH, Macher MA, Ribes D, Moulonguet L, Rostaing L, Satchell SC, Mathieson PW, Sautes-Fridman C, Loirat C, Regnier CH, Halbwachs-Mecarelli L, Fremeaux-Bacchi V: Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood 114: 2837–2845, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM: Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med 361: 345–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V: Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 555–563, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Jozsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, Skerka C, Zipfel PF: Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 110: 1516–1518, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Jozsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C: Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111: 1512–1514, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ: Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zipfel PF, Edey M, Heinen S, Jozsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C: Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3: e41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strobel S, Hoyer PF, Mache CJ, Sulyok E, Liu WS, Richter H, Oppermann M, Zipfel PF, Jozsi M: Functional analyses indicate a pathogenic role of factor H autoantibodies in atypical haemolytic uraemic syndrome. Nephrol Dial Transplant 25: 136–144, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Dragon-Durey MA, Blanc C, Marliot F, Loirat C, Blouin J, Sautes-Fridman C, Fridman WH, Fremeaux-Bacchi V: The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet 46: 447–450, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Lee BH, Kwak SH, Shin JI, Lee SH, Choi HJ, Kang HG, Ha IS, Lee JS, Dragon-Durey MA, Choi Y, Cheong HI: Atypical hemolytic uremic syndrome associated with complement factor H autoantibodies and CFHR1/CFHR3 deficiency. Pediatr Res 66: 336–340, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Abarrategui-Garrido C, Martinez-Barricarte R, Lopez-Trascasa M, de Cordoba SR, Sanchez-Corral P: Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood 114: 4261–4271, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Kwon T, Dragon-Durey MA, Macher MA, Baudouin V, Maisin A, Peuchmaur M, Fremeaux-Bacchi V, Loirat C: Successful pre-transplant management of a patient with anti-factor H autoantibodies-associated haemolytic uraemic syndrome. Nephrol Dial Transplant 23: 2088–2090, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Le Quintrec M, Zuber J, Noel LH, Thervet E, Fremeaux-Bacchi V, Fridman WH, Legendre C, Dragon-Durey MA: Anti-Factor H autoantibodies in a fifth renal transplant recipient with atypical hemolytic and uremic syndrome. Am J Transplant 9: 1223–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kwon T, Belot A, Ranchin B, Baudouin V, Fremeaux-Bacchi V, Dragon-Durey MA, Cochat P, Loirat C: Varicella as a trigger of atypical haemolytic uraemic syndrome associated with complement dysfunction: Two cases. Nephrol Dial Transplant 24: 2752–2754, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Boyer O, Balzamo E, Charbit M, Biebuyck-Gouge N, Salomon R, Dragon-Durey MA, Fremeaux-Bacchi V, Niaudet P: Pulse cyclophosphamide therapy and clinical remission in atypical hemolytic uremic syndrome with anti-complement factor H autoantibodies. Am J Kidney Dis 55: 923–927, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C: Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Cummings KC, Mohle-Boetani JC, Werner SB, Vugia DJ: Population-based trends in pediatric hemolytic uremic syndrome in California, 1994–1999: Substantial underreporting and public health implications. Am J Epidemiol 155: 941–948, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Scheiring J, Andreoli SP, Zimmerhackl LB: Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol 23: 1749–1760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Espie E, Grimont F, Mariani-Kurkdjian P, Bouvet P, Haeghebaert S, Filliol I, Loirat C, Decludt B, Minh NN, Vaillant V, de Valk H: Surveillance of hemolytic uremic syndrome in children less than 15 years of age, a system to monitor O157 and non-O157 Shiga toxin-producing Escherichia coli infections in France, 1996–2006. Pediatr Infect Dis J 27: 595–601, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Siegler RL, Pavia AT, Christofferson RD, Milligan MK: A 20-year population-based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics 94: 35–40, 1994 [PubMed] [Google Scholar]
- 23. Pomajzl RJ, Varman M, Holst A, Chen A: Hemolytic uremic syndrome (HUS)-incidence and etiologies at a regional Children's Hospital in 2001–2006. Eur J Clin Microbiol Infect Dis 28: 1431–1435, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Nesmith JD, Ellis E: Childhood hemolytic uremic syndrome is associated with adolescent-onset diabetes mellitus. Pediatr Nephrol 22: 294–297, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF: Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: A systematic review, meta-analysis, and meta-regression. JAMA 290: 1360–1370, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wigger M, Drückler E, Jozsi M, Skerka C, Zipfel PF, Haffner D: Course of atypical HUS due to Factor H autoantibodies and CFHR1/CFHR3 deficiency during 1 year treatment with mycophenolate mofetil. Pediatr Nephrol 24: 1777–1899, abstr FRI-M-1117, 2009 [Google Scholar]
- 28. Waters AM, Pappworth I, Marchbank K, Bockenhauer D, Tullus K, Pickering MC, Strain L, Sebire N, Shroff R, Marks SD, Goodship TH, Rees L: Successful renal transplantation in factor H autoantibody associated HUS with CFHR1 and 3 deficiency and CFH variant G2850T. Am J Transplant 10: 168–172, 2010 [DOI] [PubMed] [Google Scholar]