Abstract
In mammals, Immunoglobulin light chain (IgL) are localized to two chromosomal regions (designated κ and λ). Here we report a genome-wide survey of IgL genes in the zebrafish revealing (VL–JL–CL) clusters spanning 5 separate chromosomes. To elucidate IgL loci present in the zebrafish genome assembly (Zv6), conventional sequence similarity searches and a novel scanning approach based on recombination signal sequence (RSS) motifs were applied. RT-PCR with zebrafish cDNA was used to confirm annotations, evaluate VJ-rearrangement possibilities and show that each chromosomal locus is expressed. In contrast to other vertebrates in which IgL exon usage has been studied, inversional rearrangement between (VL–JL–CL) clusters were found. Inter-cluster rearrangements may convey a selective advantage for editing self-reactive receptors and poise zebrafish by virtue of their extensive numbers of VL, JL and CL to have greater potential for immunoglobulin gene shuffling than traditionally studied mice and human models.
Keywords: Immunoglobulin, Zebrafish, Rearrangement, Genome, RSS
1. Introduction
The diverse array of immunoglobulins (Ig) and T cell receptors (TCR) are generated from a relatively small number of variable (V), diversity (D), joining (J) and constant region (C) gene segments in the genome. It has been conventional to describe the genomic configurations of these segments as either “translocon” or “multi-clustered” assemblages. The single (V–(D)–J–C) translocon cluster arrangement is typified by mouse and human heavy (IgH) and kappa (κ) light (IgL) chain loci where a number of V segments lie upstream of (DH), several J and finally one or more C genes.
A departure from a single cluster can be found in the mouse as lambda (λ) IgL are arrayed in a 2-cluster (V2–(J–C)2)–(V–(J–C)2) configuration. Because mouse Vλ and Jλ are in the same transcriptional polarity, VJ-rearrangement between the first and second clusters would result in a deletion of intervening Vλ and Jλ, thereby reducing the number of gene segments available for secondary rearrangements. This scenario appears to be avoided as the expressed mouse Vλ repertoire demonstrates a strong bias to rearrange with Jλ within a cluster and rearrangements that leapfrog between clusters appear to be extremely rare [1–3].
Extrapolating from the two λ clusters in mice, it has been conventional to broadly define a single Ig “cluster” as any number of V regions upstream of one or more (D), J and C segments [4–6]. To date, the most extensive assemblages of IgH and IgL clusters have been found in cartilaginous fish (sharks and rays) where several hundred (V–(D)–J–C) clusters have been predicted to exist in a single genome [7]. The exact number and arrangement of segments in each cluster, as well as total numbers of clusters are not known. V(D)J-rearrangements in sharks and rays are thought to occur within and not between clusters [5,8]. This within-cluster restriction may be related to the finding that IgH and IgL loci of cartilaginous fishes appear to be in the same transcriptional polarity necessitating that V(D)J-rearrangement is by deletion [9].
Teleost IgL appear to offer a different possibility for VJ-rearrangements. While the IgH segments of bony fish are in a single cluster configuration [10–13], IgL gene segments are multi-clustered [4,14]. Moreover, as VL are often in opposite polarity to JL, teleost IgL might have the capacity to undergo inversional VJ-rearrangements both within and between clusters. Rearrangement by inversion, as opposed to deletion, would preserve and invert intervening VL, JL and CL thereby maximizing the number of gene segments available for secondary rearrangements. Inversional inter-cluster rearrangements would thus appear to constitute a selective advantage for generating immunoglobulin diversity as gene segments available for secondary rearrangements would be retained while the available exon repertoire for VJ–C combinations would be expanded to include all IgL exons on a given chromosome.
It has long been speculated that inversional inter-cluster IgL rearrangements might be possible in teleosts; however, without a genomic reference sequence such data have remained elusive. The rapidly expanding genomic resources for the zebrafish provide a means by which inter-cluster rearrangement possibilities in an animal harboring extensive germline (VL–JL–CL) clusters can be addressed. In this study, we have combined a suite of bioinformatics-based approaches coupled with EST and cDNA-based cloning strategies to annotate and fit VJ–C transcripts to concordant genomic regions. Collectively, these analyses reveal that inversional VJ-rearrangements occur both within and between IgL clusters in zebrafish. To date, zebrafish represent the only animal model in which inversional rearrangements between IgL clusters have been found.
2. Methods
2.1. Initial data mining for zebrafish IgL
TBLASTN alignments with VL, CL, genomic and cDNA sequences from zebrafish, other teleosts, sharks and a variety of mammals were used as queries to scan the zebrafish whole-genome shotgun sequence, trace files, BAC databases, (www.ensembl.org), EST libraries and sequences in NCBI. Identified genes were used in iterative database scans and contigs harboring potential IgL were downloaded from the genome assembly available from The Wellcome Trust Sanger Institute.
2.2. RSS identification
RSS flanking VL found by TBLASTN approaches were readily apparent by manual annotation of the sequence immediately downstream of VL segments. Using the EMBOSS [15] package, a pattern search was implemented to find JL-specific RSS among the initial genomic contigs found to harbor VL and CL. The pattern was a consensus recombination signal sequence (RSS) heptamer and nonamer with a 20–25-base spacer (CACAGTG-N20–25-ACAAAAACC) region. Open reading frames flanking identified RSS36–41 were scanned for the characteristic amino acid sequence T(X)L(X)V found in JL of sturgeon [16], catfish [17] and zebrafish [18], and classified as JL if this sequence was present.
2.3. Genome-wide RSS motif scanning to find zebrafish VL and JL
As the zebrafish genome project nears completion, a battery of ab initio programs are being used to predict putative exons on a genomic level. We obtained a total of 214,814 Ensembl-predicted zebrafish exons from the Ensembl genome browser [19] (Ensembl Build, V.24a) including 100 bp intronic sequence flanking both sides of each exon. A linear discriminant analysis [20] was then used to score the flanking regions of each exon for the presence or absence of an RSS signal motif.
Based on RSS sequences found by initial data mining, two composite signals, RSS28 and RSS39, were generated by position weight matrices [21]. Each was a concatenation of 3 ordered signals: a heptamer; a spacer; and a nonamer. A 12-base spacer separates the heptamer and nonamer in RSS28 and a 23-base spacer in RSS39. Weight matrices consisted of 4 rows (1 for each residue: A, C, G and T) and 1 column for each position tested (n=28 or 39). Each matrix entry is a probability Px(R), of a given residue, R at a given position x, generated from a set of sequences of length L. As a control, the background matrix, B is defined as B(A)=0.3, B(C)=0.2, B(G)=0.2 and B(T)=0.3. The log-odds score (S) of a given sequence (s) of length (L) is defined as follows:
Using this formula, sense and antisense strands of each downloaded sequence were scanned for RSS28 or RSS39. Scores (S) were tabulated for each of the 214,844 sequences and a classification function was used to identify putative RSS. Score cutoffs of greater than 6 were used to identify putative heptamer and nonamer signals, and scores greater than 5 were used to discriminate spacers. Exons scored to flank a potential RSS were analyzed for other salient features (invariant residues, leader sequences, folds, framework regions, etc.) consistent with classification as IgL segments.
2.4. Annotation of zebrafish IgL
The transcriptional polarity and relative positions of VL and CL in genomic contigs were discerned using the Artemis annotation package. Splice sites between leader and VL exons and JL and CL exons were determined using NNSPLICE and exon boundaries of VL, JL and CL were further refined by comparison to known VJ–C cDNA sequences [18].
2.5. Zv6 assembly
In the current (Zv6, build August 2006), and previous zebrafish genome assemblies, a number of gaps have been present within the whole-genome shotgun contigs identified to harbor IgL. Gaps circumvent the exact delineation of gene configurations as in subsequent genome builds additional exons may be inserted, thereby reconfiguring the apparent locus. It is also important to note that Zv6 is a draft assembly based on a large number of individuals as source DNA for whole-genome shotgun sequencing (∼500 embryos were pooled). Haplotype variability is known to cause false duplications of loci or contig dropouts in the assembly [22], meaning that precise distances between individual gene segments cannot be discerned based on the whole-genome shotgun sequence alone. To address this, the genome project is sequencing several BAC libraries, with insert sizes ∼110–175 kb, which when complete will constitute several fold coverage of the zebrafish genome.
The zebrafish BAC data currently complement the whole-genome shotgun draft sequence, and as with the human genome, BAC inserts are expected to resolve problems with gaps and haplotypic variability in the assembly. BAC inserts are generally of higher quality than shotgun contigs as a BAC insert is a continuous stretch of DNA from a single individual whereas shotgun contigs are assembled from short (0.5–1.0 kb) overlapping fragments amplified from pooled source DNA. The final zebrafish assembly is projected to consist solely of a BAC-derived sequence with no sequences from the whole-genome shotgun approach (archived information at zebrafish genome project website).
2.6. Reference sequences from BAC clones
Given definitive gene orders and accurate physical distances between IgL gene segments are currently restricted to sequences annotated from BAC inserts, we identified a number of BAC clones screened to harbor IgL and had them prioritized for sequencing by the Sanger Institute. To date, 6 such clones have been fully sequenced, 4 of which contain IgL and 2 extend the sequences of BACs zK158E13 and zC276F18 yet do not contain IgL. The IgL annotated from BACs constitute the most amenable germline reference sequences available for evaluating VJ–C rearrangements from cDNA. As such, we have limited our conclusions on adjacent versus distant rearrangements as well as intra- and inter-cluster recombination to VJ–C cDNA clones that can be fitted to IgL segments anchored to fully sequenced BAC clones.
2.7. Animals/RNA isolation
Zebrafish (Tübingen) were obtained from the Zebrafish International Resource Center (Eugene, Oregon). RNA was isolated from these fish or their offspring. The zebrafish whole-genome shotgun sequence and BACs sequenced for this study are also of the Tübingen line. Whole zebrafish or organs were frozen in liquid N2 and pulverized. RNA was isolated with Trizol (Life Technologies) and reverse-transcribed into cDNA incorporating oligo-dT, random hexamer, or gene-specific primers.
2.8. Cloning VJ–C rearrangements from cDNA
Conventional PCR, 3′/5′ FirstChoice RLM-RACE (Ambion) with cDNA templates were used to evaluate IgL exon usage. Reactions were performed using a series of primers optimized to target VJ–C rearranged sequences. In all cases, forward primers were situated in VL regions and reverse primers in CL. Amplicons of appropriate sizes were purified from agarose gels using Qiaquick Gel Purification kit (QIAgen), ligated into pCRII-TOPO vectors and transformed into TOP10 cells (Invitrogen). Plasmid DNA was purified using a miniprep kit (QIAgen) and VJ–C clones containing inserts by EcoR1 restriction analysis were sequenced.
2.9. Fitting VJ–C cDNA to genomic regions
VJ–C sequences were compared with the VL, JL and CL identified in BAC and whole-genome shotgun databases using the Matrix Global Alignment Tool [23]. Clones were assigned to genomic VL contingent upon global alignments exceeding a 95% threshold identity score. This stringent fitting criterion was employed, as the existence of additional IgL segments cannot be ruled out from the current assembly of the zebrafish genome. As the zebrafish genome project is nearing completion and the percent variability in nucleotide sequence of identified VL ranges between 43% and 93%, a 95% criterion is suitably rigorous. Moreover, a 95% threshold exceeds criteria used to fit germline segments to VJ-transcripts in humans [24].
2.10. DNA sequencing/sequence data deposition
VJ–C inserts were sequenced bi-directionally on an ABI instrument at the Tufts Medical School Core Facility or the Grice Sequencing Core at the College of Charleston using combinations of T7, SP6 or internal primers. GenBank accession numbers for cloned VJ–C cDNA sequences are as follows: Chr 1 (EF222425, EF222423, EF222424); Chr12 (EF222420, EF222431, EF222434, EF222429, EF222430, EF222433); Chr19 (EF222427, EF222428, EF222426); Chr24 (EF222442, EF222437, EF222441, EF222422, EF222440, EF222421, EF222438, EF222439); Chr25 (EF222432). Accession numbers and corresponding locations of germline VL, JL and CL sequences identified from genome shotgun contigs and BAC clones are listed in Table 1.
Table 1.
Genomic contigs and BAC clones harboring zebrafish IgL
| NCBI accession no. | IgL | Location on genomic contigs (Zv6) or BAC clones |
Zv4a | NCBI accession no. | IgL | Location on genomic contigs (Zv6) or BAC clones |
Zv4a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leader | VL, JL or CL exon | RSS | Leader | VL, JL or CL exon | RSS | ||||||
| NW_001511898 | Chr1-V1 | 2175058..2175010 | 2174933..2174623 | 2174630..2174603 | NW_001512699 | Chr24-V1 | 343628..343702 | 343769..344100 | 344093..344120 | ||
| J1 | N/A | 2179292..2179329 | 2179253..2179291 | NW_001512718 | V2 | 48286..48238 | 48136..47808 | 47815..47788 | |||
| V2 | 2183129..2183081 | 2182997..2182687 | 2182694..2182667 | V3 | 54735..54681 | 54575..54250 | 54254..54227 | ||||
| V3 | 2186206.. 2186156 | 2185919..2185609 | 2185616..2185588 | V4 | 56059..56015 | 55935..55579 | 55586..55559 | ||||
| V4 | 2189017..2188969 | 2188879..2188569 | 2188576..2188549 | J1 | N/A | 58089..58126 | 58050..58088 | ||||
| V5 | 2189867..2189819 | 2189739..2189489 | – | C1 | N/A | 59338..59676 | N/A | ||||
| V6 | 2196118..2196070 | 2195992..2195887 | – | BX001030 | V5 | 1207..1255 | 763..1117 | 742..769 | V1a | ||
| J2 | N/A | 2197566..2197527 | 2197526..2197488 | J2 | N/A | 1916..1953 | 1877..1915 | J1a | |||
| C1 | N/A | 2200603..2200922 | N/A | C2 | N/A | 4291..4631 | N/A | C1a | |||
| Orphan-V1 | 2207393..2207342 | 2207268..2206955 | 2206962..2206935 | V6 | 5916..5964 | 5489..5814 | 5469..5496 | V1b | |||
| NW_001510726 | Chr12-V1 | 2043590..2043551 | 2043472..2043141 | 2043148..2043121 | V7 | 7166..7199 | 6640..6983 | 6620..6647 | V1c | ||
| V2 | 2046039..2046002 | 2045900..2045570 | 2045577..2045550 | V8 | 9153..9201 | 8709..9064 | 8689..8716 | V1d | |||
| V3 | – | 2047080..2046721 | 2046729..2046702 | J3 | N/A | 10012..10049 | 9973..10011 | J1b | |||
| V4 | – | 2048935..2048601 | – | C3 | N/A | 12389..12729 | N/A | C1b | |||
| C1 | N/A | 2052109..2051784 | N/A | C2a | CT573356 | V9 | 50403..50451 | 49970..50301 | 49950..49977 | V1k | |
| J1 | N/A | 2053636..2053602 | 2053675..2053637 | J2a | V10 | 48419..48473 | 47932..48263 | 47912..47939 | |||
| J2 | N/A | 2056116..2056082 | 2056155..2056117 | J2b | C4 | N/A | 46427..46752 | N/A | |||
| V5 | 2058504..2058462 | 2058386..2058053 | 2058059..2058032 | V2h | J4 | N/A | 45111..45152 | 45072..45110 | |||
| V6 | – | 2060582..2060242 | 2060249..2060222 | V2j | V11 | 43805..43853 | 43406..43731 | 43386..43413 | |||
| V7 | 2062657..2062611 | 2062524..2062185 | 2062165.. 2062192 | V2c | V12 | 42416..42470 | 41975..42300 | 41952..41979 | |||
| J3 | – | 2066768..2066734 | 2066807..2066769 | C5 | N/A | 38757..39082 | N/A | C1c | |||
| V8 | 2067870..2067831 | 2067752..2067418 | 2067425..2067398 | J5 | N/A | 37456..37494 | 37456..37494 | J1c | |||
| V9 | 2069527..2069485 | 2069395..2069061 | – | V13 | 36216..36264 | 35808..36142 | 35788..35815 | V1i | |||
| V10 | 2071974..2071928 | 2071841..2071502 | 2071509..2071482 | V14 | 35040..35094 | 34599..42300 | 34576..34603 | V1h | |||
| V11 | 2073924..2073885 | 2073806..2073472 | 2073479..2073452 | V15 | 33105..33155 | 32692..33021 | 32672..32699 | V1g | |||
| NW_001513144 | C2 | N/A | 120680..120365 | N/A | C2b | V16 | 31479..31533 | 31032..31363 | 31012..31039 | V1f | |
| BX571825 | Chr19-V1 | 158539..158575 | 158653..159017 | 159010..159037 | V1l, V1o | NW_001512845 | Chr25-V1 | 39800..39752 | 39665..39355 | 39359..39332 | |
| J1 | N/A | 157776..157813 | 157814..157852 | V2 | 42256..42228 | 42108..41778 | 41785..41758 | V3h | |||
| C1 | N/A | 156244..156560 | N/A | C1f | V3 | 52525..52553 | 52674..52982 | 52995..53024 | |||
| V2 | 154213..154258 | 154348..154703 | 154692..154723 | V1p | V4 | 54947..54976 | 55116..55420 | 55413..55440 | |||
| V3 | – | 153237..153560 | 153579..153606 | C1 | N/A | 57069..56774 | N/A | C3a | |||
| J2 | N/A | 152981..153018 | 153019..153059 | J1 | N/A | 58626..58588 | 58665..58627 | ||||
| C2 | N/A | 151374.. 151610 | N/A | V5 | 59340..59382 | 59475..59815 | 59808..59835 | V3f | |||
| V4 | 149032..149080 | 149250..149525 | 149518..149545 | V6 | 60934..60982 | 61093..61418 | 61411..61438 | V3e | |||
| V5 | 136657..136705 | 137113..137520 | 137469..137498 | V1r | V7 | 61955..62000 | 62054..62442 | 62435..62462 | V3d | ||
| J3 | N/A | 121221..121260 | 121261..121299 | V8 | 66844..66816 | 66783..66464 | 66473..66444 | ||||
| C3 | N/A | 119649..119968 | N/A | C1h | NW_001512858 | J2 | N/A | 708..742 | 669..707 | ||
| V6 | 86840..86892 | 86934..87295 | 87300..87327 | C2 | N/A | 3680..3975 | N/A | ||||
| J4 | N/A | 86400..86437 | 86438..86477 | V9 | 5961..5933 | 5848..5484 | 5491..5464 | ||||
| C4 | N/A | 81554..81769 | N/A | V10 | 9108..9060 | 8971..8657 | 8661..8634 | ||||
| V7 | 80568..80616 | 80750..81051 | 81044..81071 | V1m, V1t | |||||||
| J5 | N/A | 80204..80241 | 80241..80279 | ||||||||
| C5 | N/A | 77129..77448 | N/A | C1d, C1j | |||||||
| V8 | – | 72491..72840 | 72817..72847 | ||||||||
IgL previously reported [25] are from whole-genome shotgun contigs in Zv4 (September 2004). Zv4 was the first zebrafish genome build to map sequence data to chromosomes and several misalignments were present. IgL on chromosomes 1 and 5 in Zv4 have been reassigned to 24 and 25 in Zv5 (November 2005) and Zv6 (August 2006).
3. Results
3.1. A genome-wide IgL annotation spans 5 chromosomes
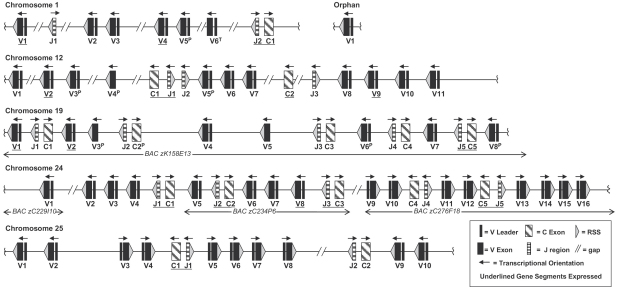
A total of 84 IgL gene segments were located in the zebrafish genome assembly Zv6 (Fig. 1). VL were classified functional if they contained leader exons and a downstream RSS. VL and CL were considered pseudogenes if they contained frame shifts or in-frame stop codons. Zebrafish IgL had previously been located to 3 separate chromosomes [25]. Here we provide an extended annotation of zebrafish IgL to include 2 additional chromosomes and considerably more VL and CL. With the exception of a single VL (Orphan V1), all 84 IgL gene segments can be anchored to 1 of 5 zebrafish chromosomes. This arrangement in zebrafish is very different from κ and λ IgL loci of mammals as at least 5 as opposed to 2 chromosomes harbor multiple IgL gene segments including CL regions.
Fig. 1.
Zebrafish IgL span 5 chromosomes. Overall configurations drawn approximately to scale with exon sizes exaggerated. VP/T designates pseudogene or truncated exon, other notations defined in box. Arrangements are based on Ensembl genome build Zv6 (August 2006). Regions with gaps constitute tentative IgL assemblages as with subsequent genome builds additional exons may be inserted. Where indicated annotation discerned from fully sequenced BAC clone inserts.
3.2. Efficacy of RSS motif scanning
The RSS scan revealed the same contigs to harbor zebrafish IgL as conventional TBLASTN approaches. These results indicate the efficacy of RSS scanning to identify VL or JL from an automatically annotated Ensembl Build and validate 2 independent methods to locate IgL in an emerging genome sequence. Since RSS are more highly conserved than VL, the RSS scanning approach may prove especially useful in situations where limited exon coding information is available for use as queries in TBLASTN searches. The RSS approach is also more expedient and represents to our knowledge the first use of a motif signal to comprehensively scan for immunoglobulin segments in a whole-genome context.
3.3. Additional genes identified with flanking RSS
The RSS scan in addition to locating VL (with associated RSS28) and JL (RSS39) revealed numerous VH (RSS39) and TCR (RSS39) gene segments. Retrieval of VH and TCR sequences was somewhat surprising as the weighted RSS motifs used in our analysis were based on VL (RSS28) and JL (RSS39) sequences. These findings indicate that RSS scanning is appropriate for surveying emerging genomes for Ig or TCR exons regardless of specific knowledge concerning Ig or TCR coding regions or even lineage-specific RSS motifs. The RSS scan also revealed ortholog of cytochrome C reductase and several immune receptor translocation-associated (IRTA) genes flanked by RSS. Interestingly, IRTA genes have been implicated in translocations into the IgH locus in human B cell malignancies [26], facilitated by an RSS heptamer (CTTAAC) flanking both IRTA and CH regions [27]. The presence of intact RSS flanking IRTA in zebrafish may represent a possible genomic predisposition for Ig translocations involving these genes in a teleost model.
3.4. Zebrafish VL
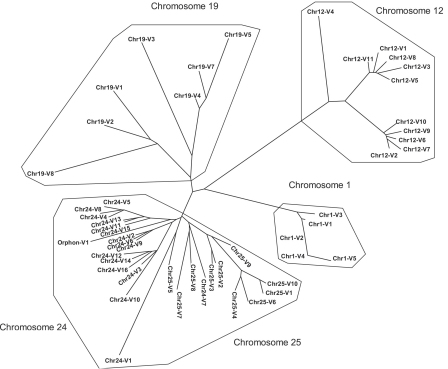
Segments encoding the variable regions of Ig are often grouped by percent identities, with the implication that those most similar descended from a common ancestor [28]. In all but one instance (chr24-V1 vs. chr25-V5), the most similar VL are located on the same chromosome (Fig. 2), suggesting a chromosome-specific pattern of VL evolution with those on chromosomes 24 and 25 having diverged most recently. Zebrafish VL also group by chromosome by percent matrix analysis (Supplementary Fig. 1 online), amino acid alignments (Fig. 3) and RSS logos (Fig. 4). Comparisons of translated VL with sequences in NCBI revealed highest similarities to those of carp, a species phylogenetically close to zebrafish (both species belong to the Cyprinidae family), which is in agreement with previous analyses of VL regions in fish [32].
Fig. 2.
Zebrafish VL group by chromosome. Gene segments aligned in ClustalX and plotted with DrawGram utility of PHYLIP in TreeView.
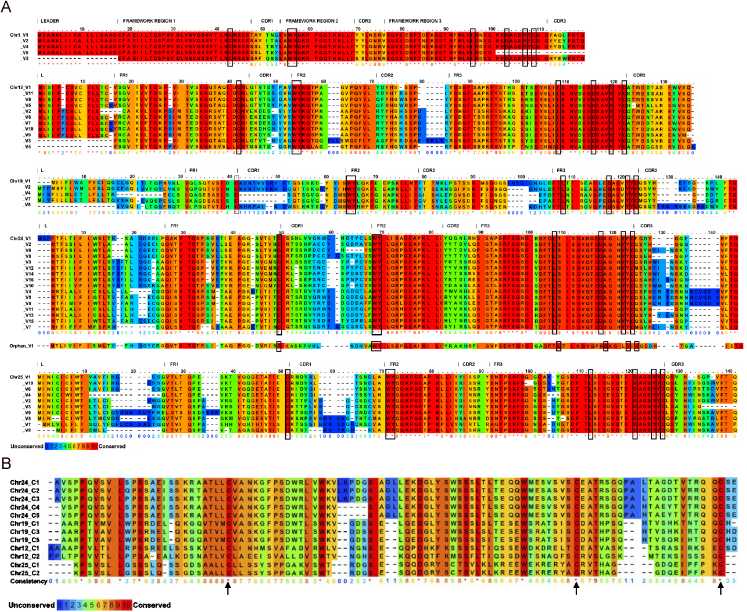
Fig. 3.
Alignments of inferred amino acid sequences show zebrafish IgL group by chromosome. (A) Alignment of zebrafish VL. Conservation (0–10) calculated using PRALINE [29]. Fully conserved positions (score 10) within chromosomes indicated by asterisks and positions invariant among all VL outlined in boxes. Cysteines involved in intra-chain disulfide bridges depicted by arrows on Chr 25. Framework (FR) and complementarity determining regions (CDR) are labeled according to Kabat delineation [30]. (B) Alignment of zebrafish CL. Invariant cysteines (indicated by arrows) at residues 28 and 91 are predicted to form intra-chain disulfide bridges whereas cysteine at position 102 is consistent with an inter-chain disulfide bridge with an immunoglobulin heavy chain.
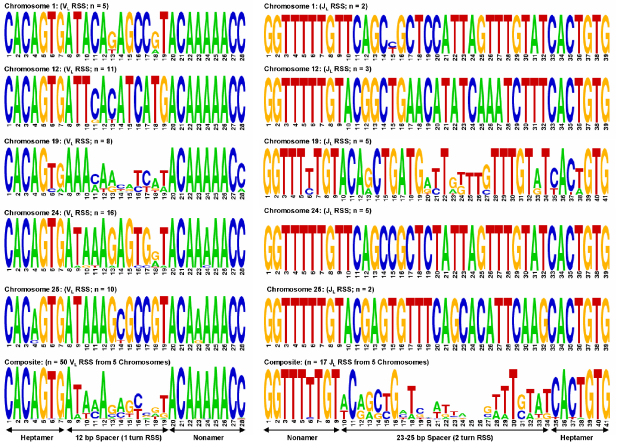
Fig. 4.
Zebrafish IgL RSS. Sequence logos for VL and JL RSS aligned by chromosome and as composites. Each logo consists of stacks of nucleotides; the overall height of each indicates conservation at that position, while the height of the nucleotides within each stack reflects the relative contribution of each nucleotide to the consensus. Logos constructed using applet available at www.weblogo.berkley.edu and are based on statistical methods previously described [31].
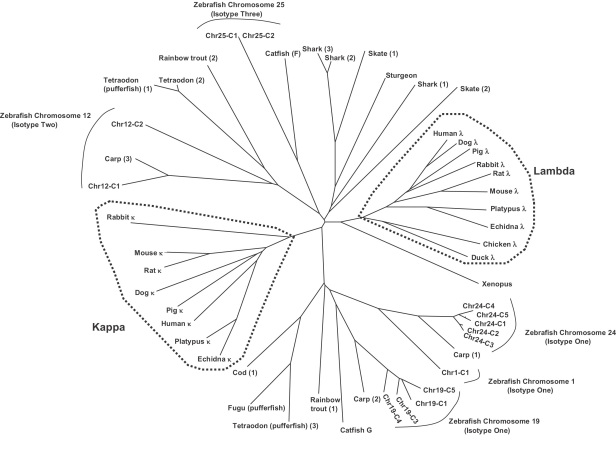
3.5. Zebrafish CL
Zebrafish CL were compared on a phylogenetic tree to evaluate CL relationships among vertebrates (Fig. 5). This analysis revealed none of the zebrafish CL group with mammalian λ or κ isotypes. The large phylogenic distances and rapid rates of evolution of antigen receptors appear to preclude a single scheme of IgL classification among vertebrates. Zebrafish CL do however group with CL of other fish and in several cases a common lineage is apparent. For example: zebrafish CL (chr 25) with catfish [33] F; zebrafish CL (chr 19) with catfish G; and CL on chromosomes 24, 1 and 12 group with carp [32] light chain types 1, 2 and 3, respectively (Fig. 5). Collectively, these findings indicate 3 or more CL may have been present in a teleost ancestor and selective pressures have maintained each type in extant species.
Fig. 5.
Zebrafish CL are diverged from mammalian IgL. Mammalian kappa (κ) and lambda (λ) regions are outlined to emphasize the clear divergence of teleost and elasmobranch sequences from traditional IgL classification schemes. Zebrafish CL classified as isotypes designated by Haire et al. [24]. Accession numbers for sequences from GenBank are as follows: Mouse, Mus musculus, AC140201, BC080787; Rat, Rattus norvegicus, DZ394090, DQ402471; Pig, Sus domesticus, M59321, M59322; Human, Homo sapiens, NG_000002, BC063599; Dog, Canis familiaris, XM_845215, XM_532962; Rabbit, Oryctolagus cuniculus, X00231, M25621; Platypus, Ornithorhynchus anatinus, AF525122, AF491640; Echidna, Tachyglossus aculeatus, AY113112, AF491643; Chicken, Gallus gallus, XM_415219; Duck, Anas platyrhynchos, X82069; Xenopus, Xenopus laevis, BC082892; Skate, Raja erinacea, JI9209, L25566; Sandbar shark, Carcharhinus plumbeus, U35008, U34992; Horn shark, Heterodontus francisci, L25563; Sturgeon, Acipencer baeri, X90557; Fugu, Takifugu rubripes, AB126061; Tetraodon, Tetraodon nigroviridis, BX572609, CR701925, CR720937; Rainbow trout, Oncorhynchus mykiss, X68521, AJ231628; Carp, Cyprinus carpio, AB035729, AB091120; Crucian carp, Carassius auratus, AB201791; Cod, Gadus morhua, AF104899; Catfish, Ictalurus punctatus, AY165790S2, IPU25704. Alignments were carried out in ClustalW and plotted with DrawGram utility of PHYLIP in TreeView.
3.6. VJ–C expression from 5 chromosomes
In total, 23 in-frame (designated as productive) and 3 out-of-frame VJ–C sequences (designated sterile) were cloned. Relationships between these VJ–C clones and their closest match germline segments are shown in Table 2. The upper portion of Table 2 lists clones exceeding 95% threshold criteria for fitting cDNA to germline VL. As shown in this table, the CL of clones (EF222427, EF222421, EF222434, EF222432 and EF222433) were fitted in their entirety (100%) to germline segments, suggesting limited polymorphism or somatic mutation in CL among fish of the Tübingen line. Also shown in Table 2, at least one VJ–C clone was fitted to each of the 5 chromosomes depicted in Fig. 1.
Table 2.
VJ–C cDNA clones and concordant germ-line gene segments
| V(J)–C clone |
Most similar germline VL |
Most similar germline CL |
Next closest match |
ORFa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession no. | Insert (bp) | % Identity |
% Identity |
||||||||
| VL | w/o CDR3 | Lengthb | CL | Lengthb | VL (%) | CL (%) | |||||
| EF222425 | 647 | Chr1-V1 | 98.7 | 99.3 | 308 | Chr1-C1 | 99.3 | 300 | Chr1-V2 (91.3) | Chr24-C5 (78.8) | P |
| EF222424 | 626 | Chr1-V4 | 95.9 | 97.6 | 297 | Chr1-C1 | 97.9 | 299 | Chr1-V5 (92.6) | Chr24-C5 (78.2) | P |
| EF222427 | 713 | Chr19-V1 | 98.9 | 100 | 362 | Chr19-C5 | 100 | 309 | Chr19-V2 (69.5) | Chr19-V2 (93.0) | P |
| EF222428 | 714 | Chr19-V1 | 99.2 | 100 | 362 | Chr19-C5 | 99.0 | 309 | Chr19-V2 (69.8) | Chr19-C3 (94.4) | P |
| DV593802 | 750 | Chr19-V1 | 98.6 | 100 | 365 | Chr19-C5 | 100 | 276 | Chr19-V2 (69.2) | Chr19-C3 (93.4) | P |
| EF222426 | 698 | Chr19-V2 | 98.9 | 100 | 353 | Chr19-C5 | 99.3 | 309 | Chr19-V1 (69.5) | Chr24-C3 (94.1) | P |
| EF222441 | 574 | Chr24-V2 | 98.8 | 100 | 324 | Chr24-C1 | 99.0 | 195 | Chr19-V9 (88.6) | Chr24-C3 (95.8) | P |
| EF222421 | 673 | Chr24-V8 | 98.3 | 100 | 351 | Chr24-C3 | 100 | 283 | Chr24-V5 (88.5) | Chr24-C2 (99.2) | P |
| VJ–C below 95% threshold criteria for fitting germline VL; indicative of somatic mutation, allelic variation or unidentified IgL in genome | |||||||||||
| EF222420 | 683 | Chr12-V9 | 92.5 | 95.5 | 332 | Chr12-C1 | 99.6 | 324 | Chr12-V2 (90.4) | Chr12-C2 (59.3) | P |
| EF222431 | 714 | Chr12-V9 | 91.6 | 95.5 | 332 | Chr12-C1 | 99.6 | 324 | Chr12-V2 (91.3) | Chr12-C2 (59.3) | P |
| EF222434 | 517 | Chr12-V5P | 94.6 | 97.2 | 111 | Chr12-C1 | 100 | 324 | Chr12-V11 (91.1) | Chr12-C2 (59.6) | S |
| EF222429 | 663 | Chr12-V8 | 89.8 | 93.1 | 332 | Chr12-C2 | 99.7 | 307 | Chr12-V11 (89.8) | Chr12-C1 (59.0) | P |
| EF222430 | 668 | Chr12-V9 | 94.9 | 96.6 | 332 | Chr12-C2 | 97.7 | 307 | Chr12-V6 (92.8) | Chr12-C1 (58.8) | P |
| EF222433 | 694 | Chr12-V9 | 92.4 | 94.9 | 330 | Chr12-C2 | 100 | 307 | Chr12-V6 (91.6) | Chr12-C1 (59.2) | P |
| EF222442 | 318 | Chr24-V2 | 93.7 | 95.7 | 80 | Chr24-C2 | 99.5 | 204 | Chr19-V11 (76.9) | Chr24-C3 (98.5) | P |
| EF222437 | 582 | Chr24-V2 | 89.2 | 89.7 | 324 | Chr24-C1 | 97.4 | 192 | Chr19-V6 (82.7) | Chr24-C3 (92.3) | P |
| EF222422 | 688 | Chr24-V5 | 82.2 | 85.5 | 305 | Chr24-C2 | 99.0 | 320 | Chr24-V4 (79.1) | Chr24-C3 (98.7) | P |
| EF222440 | 341 | Chr24-V5 | 91.0 | 95.7 | 110 | Chr24-C3 | 99.0 | 204 | Chr24-V3 (89.2) | Chr24-C2 (98.5) | P |
| DT318541 | 666 | Chr24-V6 | 90.4 | 94.3 | 318 | Chr24-C5 | 99.6 | 323 | Chr24-V9 (89.7) | Chr24-C4 (99.1) | P |
| EF222438 | 326 | Chr24-V12 | 87.4 | 100 | 86 | Chr24-C1 | 98.5 | 204 | Chr24-V13 (87.2) | Chr24-C5 (97.6) | S |
| EF222439 | 330 | Chr24-V7 | 91.5 | 93.0 | 86 | Chr24-C3 | 99.0 | 230 | Chr24-V6 (88.3) | Chr24-C2 (97.1) | S |
| EF222432 | 650 | Chr25-V9 | 86.8 | 90.0 | 327 | Chr25-C1 | 100 | 273 | Chr25-V2 (87.2) | Chr25-C2 (100) | P |
Single open reading frame=productive (P); lack of ORF=sterile (S).
Length of global alignments in bp. Sizes of inserts contingent upon primer locations in VL and CL, and junctional flexibility.
The potential to generate IgL from 5 haploid chromosomes presents a conceptually intriguing scenario and implies that if allelic exclusion is to occur in zebrafish, feedback mechanisms are in place to silence a considerable number of IgL segments widely scattered throughout the genome. With functional IgL loci on essentially 10 autosomes, each with multiple VL and JL (zebrafish being diploid and chromosomes 1, 12, 19, 24, 25 do not appear sex-linked [34]), it is plausible that zebrafish have a greater need for gene silencing than κ and λ systems of mammals.
Although mechanisms underlying allelic exclusion have yet to be fully elucidated in mammals, changes in chromatin, methylation and replication timing are all considered critical to ensure that each B cell can elaborate an antigen receptor of a single type [35]. In mammals, Ig-κ positive B cells retain λ in a germline configuration [36], whereas Ig-λ positive B cells have rearranged Ig-κ alleles in addition to rearranged Ig-λ allele(s) [37]. These findings imply a hierarchical process starting with κ-rearrangement events followed by λ if self-reactive or sterile Ig-κ receptors are formed.
In Ig-λ positive B cells, Ig-κ alleles are often inactivated by rearrangements involving the κ-deleting element (Kde) in humans [38] or rearranging sequence (RS) in mice [39]. Kde/RS are 3′ to Cκ and recombine to Vκ upstream of a rearranged VJ or to an RSS heptamer between Jκ and Cκ [40]. Recombination to a Jκ–C heptamer deletes the Cκ, while rearrangement to a 5′ Vκ deletes the entire Jκ–Cκ region [41]. As Kde/RS rearrangements render a κ locus inoperative, they appear central in κ/λ isotypic exclusion in mammals.
To see if zebrafish might have Kde/RS elements, we searched zebrafish whole-genome sequence and BAC databases by conventional BLAST approaches, and performed pattern searches of regions 3′ to each CL yet did not find putative Kde/RS homologs. We did find RSS-like heptamers and nonamers (data not shown) within several JL–CL intronic regions. It remains to be seen if these RSS are involved in deleting nonproductive VJ-rearrangements or if zebrafish use other means to facilitate allelic exclusion.
3.7. VJ-rearrangements in zebrafish
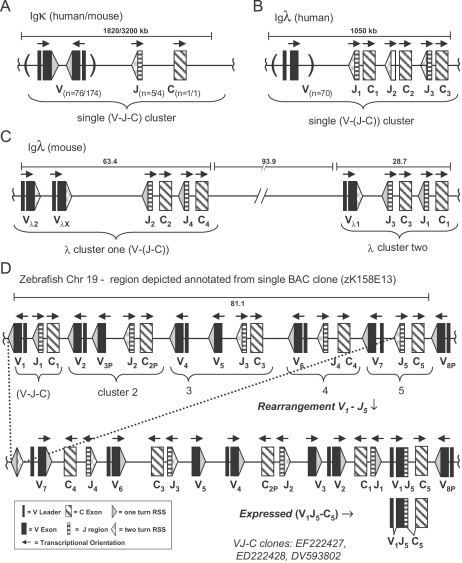
As depicted in Fig. 1, three patterns of transcriptional polarity are evident among zebrafish IgL: VL, JL and CL in the same orientation (chr12); VL opposite to JL and CL (chr1,19); and VL in both orientations to JL and CL (chr24, 25). Transcriptional polarities dictate either deletional or inversional rearrangement. For example, given the tentative gene order depicted on chromosome 12, rearrangement between V7 and J1 would result in deletion of (J2, V5P, V6). In contrast, an inversional VJ-rearrangement between Chr19-V1 and Chr19-J5 would reposition the intervening gene segments upstream of the rearranged V5–J5 and in opposite transcriptional orientation of the original germline configuration (Fig. 6).
Fig. 6.
Comparative configuration of IgL loci. (A) Igκ rearrangements are restricted to a single IgL cluster. (B/C) The single transcriptional orientation of λ segments of man/mouse necessitates VJ-rearrangement by deletion within and between clusters. (D) Zebrafish have extensive (V–J–C) clusters, those of Chr19 are shown. A potential rearrangement for VJ–C clones (EF222427, EF222428) is depicted. Inversional inter-cluster rearrangements preserve intervening DNA, thus maximizing VL and JL available for subsequent rearrangements. Numbers of segments and physical distances (given in kb) for mouse/human loci are from IMGT [42]. All zebrafish VL and JL identified have 1 and 2 turn RSS similar to Igκ loci of humans/mouse, respectively.
The VJ–C clone (EF222427, Table 2, line 3) is indicative of a VJ-rearrangement between Chr19-V1 and Chr19-J5/C5. This clone (EF222427) was fitted with percent identities of 98.9% and 100% (Table 2), with the next best match being Chr19-V2 (69%) and Chr19-C1 (93%), indicating that assignment of this clone to concordant germline gene segments is sound. Because IgL segments on Chr19 are annotated from a BAC insert (representing an intact section of DNA from a single fish), conclusions concerning distances of the rearrangement can also be made. Of all the VJ–C clones anchored to BACs, this clone represents the most distant recombination as Chr19-V1 and Chr19-J5 are located 81 kb apart (Fig. 6D). This VJ–C clone and others (EF222427, DV593802 and EF222426) show inversional rearrangements that leapfrog CL and as such are indicative of rearrangement between zebrafish IgL clusters.
3.8. Inference of selection on VL
For VJ–C clones fitted with less than 5% deviation from germline VL, assessments of the number of replacement (R) and silent (S) mutations in framework (FR) and complementary determining regions (CDR) were made. The distribution of mutations in corresponding VL regions was analyzed using a multinomial distribution model [43] JAVA applet available at: www.stat.stanford.edu/immunoglobulin. Theoretical probabilities of an excess or scarcity of R and S mutations occurring by chance were computed as accumulation of replacement as opposed to silent mutations in CDRs would indicate antigen selection of variants with improved binding properties [44]. As shown in Table 3, the majority of the VL show statistically significant evidence of selection. These findings indicate CDRs are more plastic, while mutations in FR regions are more likely to be selected against. While these results are not unexpected, they do suggest that VL mutations observed in zebrafish are a product of the antigen-driven somatic hypermutation of Ig loci common in traditionally studied vertebrate animals [45,46].
Table 3.
Inference of selection on zebrafish VL genes
| Clone accession no. | Most similar germline VL | FR/CDR | Observed mutationsa |
PMb | |
|---|---|---|---|---|---|
| R | S | ||||
| EF222425 | Chr1-V1 | FR | 0 | 1 | 0.01236 |
| CDR | 2 | 1 | 0.07169 | ||
| EF222424 | Chr1-V4 | FR | 3 | 2 | 0.00805 |
| CDR | 6 | 1 | 0.00373 | ||
| EF222420 | Chr12-V2 | FR | 0 | 2 | 0.00003 |
| CDR | 10 | 1 | 0.00009 | ||
| EF222431 | Chr12-V2 | FR | 2 | 1 | 0.00112 |
| CDR | 12 | 0 | 0.00001 | ||
| EF222434 | Chr12-V5P | FR | 0 | 0 | 0.04010 |
| CDR | 2 | 1 | 0.03863 | ||
| EF222430 | Chr12-V9 | FR | 3 | 3 | 0.02144 |
| CDR | 5 | 1 | 0.08865 | ||
| EF222433 | Chr12-V9 | FR | 4 | 3 | 0.00330 |
| CDR | 10 | 3 | 0.00614 | ||
| EF222427 | Chr19-V1 | FR | 0 | 0 | 0.20319 |
| CDR | 1 | 0 | 0.08465 | ||
| DV593802 | Chr19-V1 | FR | 0 | 0 | 0.20060 |
| CDR | 2 | 0 | 0.08303 | ||
| EF222426 | Chr19-V2 | FR | 0 | 0 | 0.15765 |
| CDR | 2 | 0 | 0.05997 | ||
| EF222442 | Chr24-V2 | FR | 0 | 0 | 0.02731 |
| CDR | 5 | 0 | 0.00313 | ||
| EF222441 | Chr24-V2 | FR | 0 | 0 | 0.03714 |
| CDR | 1 | 1 | 0.81578 | ||
| EF222440 | Chr24-V5 | FR | 0 | 0 | 0.00408 |
| CDR | 8 | 5 | 0.00108 | ||
| DT318541 | Chr24-V6 | FR | 1 | 1 | 0.00084 |
| CDR | 8 | 3 | 0.00505 | ||
| EF222421 | Chr24-V8 | FR | 0 | 0 | 0.13431 |
| CDR | 2 | 0 | 0.04538 | ||
Statistically significant values in bold. PFR is selection to preserve FR and PCDR infers antigen selection of CDR variants.
Replacement (R) and silent (S) mutations from germline VL (over global alignment lengths reported in Table 1).
PM; multinomial probability calculated that excess (for CDR) or scarcity (for FR) of mutations occurred by chance.
4. Discussion
IgL gene segments have undergone major evolutionary transitions in genomic configurations during vertebrate phylogeny. At one extreme is the chicken, where a single IgL cluster harbors a solitary VL that can undergo primary rearrangement [47,48]. The mouse λ locus contains a small number of VL in a (V–V–(J–C)2)–(V–(J–C)2) configuration, whereas human κ, human λ and mouse κ contain larger numbers of VL in a single discrete cluster per locus (Fig. 6). Herein, we show that zebrafish occupy an entirely different configuration with multiple (VL–JL–C) clusters arrayed on at least 5 different chromosomes (Fig. 1).
Efforts to evaluate VJ-rearrangements in the context of genomic cluster/exon usage have been largely limited to species for which concordant germline information is available. To date, complete genome-wide annotations of IgL loci are available for only mouse and human [49,50]. Early findings by Southern blotting indicated that the mouse VJλ repertoire is strongly biased to VJ-rearrangement within each of the 2 clusters [2,3,51]. Recent sequencing of mouse VJ–C cDNA [52] linked to genomic analyses also indicates that VJ-rearrangement is constrained to a single cluster. Intra-cluster restriction in mice may be due to the large (∼1.75 Mb) distance between the 2 λ clusters [42]. Thus, a mouse B cell with a λ-rearrangement yielding a self-reactive receptor may be in a potentially dangerous position because of its inability to delete the λ rearrangement [53].
In mammals, the potential of generating self-reactive λ receptors is abated by timing (κ rearrangements occur before λ); secondary κ rearrangements (facilitated by nested Vκ and Jκ); or unknown mechanisms that limit λ expression. The mechanisms underlying the disparate κ : λ ratio of approximately 10:1 in mice [54] and 3:1 in man [55] remain unresolved. Nevertheless, that a VJ-rearrangement can become fixed constitutes a potential liability as a self-reactive receptor could trigger an autoimmune response. Given that each mouse/human κ can sustain a total of 5/4 successive VJ-rearrangements (providing sequential Jκ usage), the probability of a B cell generating a self-reactive λ receptor is likely quite small. However, as a λ receptor rescues κ-deleted B cells from oblivion, there appears an evolutionary tradeoff for sustaining B cells at the expense of generating a final λ-rearrangement that cannot be deleted.
Receptor editing (replacing receptors on B cells by continued gene rearrangement) is the principal means by which immature bone marrow B cells become self-tolerant. The potential for receptor editing appears optimized in κ as in contrast to λ exons, approximately half the Vκ in mouse and human are in opposite transcriptional polarity to Jκ. This flip-flop potential allows κ to undergo inversional VJ-rearrangements that preserve the intervening VL, JL and associated RSS, between the Vκ and Jκ to be joined. Thus, VL available for a secondary rearrangement is maximized. In the case of mouse and human, inversional VJ-rearrangements precede a single Cκ limiting rearrangement within a single cluster. However, zebrafish with multiple CL on a chromosome are poised to reconfigure an Ig locus by inversional VJ-rearrangements, which place VL from one cluster into another (Fig. 6D). Zebrafish also have more JL (14; haploid) than mouse/man (8/8; haploid) suggesting enhanced potential for IgL receptor editing overall.
Inversional VJ-rearrangements that leapfrog CL, as found in zebrafish (Table 2, Fig. 6D), have yet to be documented in any other animal model. While such rearrangements are not possible in mice/humans (each harbor a single Cκ and λ loci are limited to deletional rearrangements), it is plausible that inversional VJ-rearrangements between clusters may occur in other animals. For example, rabbits have 2 Cκ isotypes (Cκ1 and Cκ2) each with its own set of Jκ [56]. This combined with the recent finding of rabbit Vκ are in both transcriptional orientations to Jκ preceding Cκ1 [57] may mean that inversional VJ-rearrangements that leapfrog Cκ1 are possible. However, it is unknown whether each rabbit Cκ has its own set of VL and efforts to evaluate rearrangements in the context of cluster/exon usage in rabbits have been limited to VL clustered with Cκ1 and not the downstream Cκ2 [58].
As hundreds of CL have been predicted to exist in cartilaginous fish, it might also appear possible that inter-cluster VJ-rearrangements could also occur in sharks. However, evidence obtained to date suggests that V(D)J rearrangement in cartilaginous fish occurs within and not between clusters [5,8]. Although sharks and teleosts both have multiple clustered IgL loci, differences are evident in the configuration of IgL gene segments in these groups of animals. For example, (VL–JL–C) clusters are thought to be physically closer to one another in teleosts than in sharks and rays [59]. Additionally, teleost VL are often in opposite polarity to JL and CL, whereas IgL segments in cartilaginous fishes are in the same orientation [9]. Thus, inter-cluster rearrangement may be absent in sharks as a result of distance constraints and inversional rearrangement may be lacking as the existence of IgL in the same transcriptional polarity dictates that VJ-recombination is by deletion.
With ongoing efforts to sequence additional genomes it will be interesting to discern whether inversional inter-cluster rearrangements are teleost specific or commonplace in other extant animal lineages. That zebrafish IgL span at least 5 haploid chromosomes with VL and CL grouping by chromosome also supports the notion that gene duplications of IgL loci are a relatively common phenomenon in vertebrate evolution. The finding of appreciably more CL upstream and downstream from arrays of VL and JL (in both transcriptional polarities) in zebrafish raises the possibility that zebrafish B cells may have a greater potential for IgL gene shuffling than traditionally studied mice and human models.
In conclusion, we provide the first evidence of inversional inter-cluster IgL rearrangement in any animal model. This finding and the implication that zebrafish B cells have potential for extensive editing to ablate expression of self-reactive receptors enhances the utility of zebrafish as an emerging immunological model system. In addition, as zebrafish IgL appear to undergo antigen-driven somatic hypermutation, they represent a meaningful branch point in vertebrate phylogeny for further investigations of IgL loci.
Clarifying how allelic exclusion might occur over essentially 10 autosomes in zebrafish may provide considerable insight into elucidating unresolved mechanisms that underlie how B cells elaborate an antigen receptor of a single type while maintaining a genomic reservoir for subsequent diversification.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dci.2007.08.005.
Appendix A. Supporting Information
Fig. S1. Comparison of zebrafish VL segments. Amino acids percent identity matrix calculated according to Spalding and Lammers (2004). Identities ⩾70% designation for Ig “V families” (Matsuda, 2004) depicted as shaded boxes. At least 5 distinct families were identified, each by chromosome as shown. Apparent pseudogenes and truncated VL sequences were omitted from analyses.Spalding JB, Lammers (2004) Nucleic Acids Res 32:26–32.Matsuda F (2004) Molecular Biology of B Cells. eds Honjo T, Alt F, Neuberger M (Elsevier) Pp: 1–17.
References
- 1.Elliott B.W., Eisen H.N., Steiner L.A. Unusual association of V, J and C regions in a mouse immunoglobulin lambda chain. Nature. 1982;299:559–561. doi: 10.1038/299559a0. [DOI] [PubMed] [Google Scholar]
- 2.Reilly E.B., Blomberg B., Imanishi-Kari T., Tonegawa S., Eisen H.N. Restricted association of V and J–C gene segments for mouse lambda chains. Proc Natl Acad Sci USA. 1984;81:2484–2488. doi: 10.1073/pnas.81.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez P., Nadel B., Cazenave P.A. V lambda–J lambda rearrangements are restricted within a V–J–C recombination unit in the mouse. Eur J Immunol. 1991;21:907–911. doi: 10.1002/eji.1830210408. [DOI] [PubMed] [Google Scholar]
- 4.Litman G.W., Anderson M.K., Rast J.P. Evolution of antigen binding receptors. Annu Rev Immunol. 1999;17:109–147. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- 5.Dooley H., Flajnik M.F. Antibody repertoire development in cartilaginous fish. Dev Comp Immunol. 2006;30:43–56. doi: 10.1016/j.dci.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Jones J.C., Ghaffari S.H., Lobb C.J. Patterns of gene divergence and VL promoter activity in immunoglobulin light chain clusters of the channel catfish. Immunogenetics. 2004;56:448–461. doi: 10.1007/s00251-004-0700-3. [DOI] [PubMed] [Google Scholar]
- 7.Cannon J.P., Haire R.N., Rast J.P., Litman G.W. The phylogenetic origins of the antigen-binding receptors and somatic diversification mechanisms. Immunol Rev. 2004;200:12–22. doi: 10.1111/j.0105-2896.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg A.S., Avila D., Hughes M., McKinney E.C., Flajnik M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 9.Pilstrom L., Lundqvist M.L., Wermenstam N.E. The immunoglobulin light chain in poikilothermic vertebrates. Immunol Rev. 1998;166:123–132. doi: 10.1111/j.1600-065x.1998.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 10.Danilova N., Bussmann J., Jekosch K., Steiner L.A. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J.D., Landis E.D., Phillips R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci USA. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savan R., Aman A., Sato K., Yamaguchi R., Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005;35:3320–3331. doi: 10.1002/eji.200535248. [DOI] [PubMed] [Google Scholar]
- 13.Bengtén E., Quiniou S., Hikima J., Waldbieser G., Warr G.W., Miller N.W. Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene. Immunogenetics. 2006;58:831–844. doi: 10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- 14.Daggfeldt A., Bengtén E., Pilström L. A cluster type organization of the loci of the immunoglobulin light chain in Atlantic cod (Gadus morhua L.) and rainbow trout (Oncorhynchus mykiss Walbaum) indicated by nucleotide sequences of cDNAs and hybridization analysis. Immunogenetics. 1993;38:199–209. doi: 10.1007/BF00211520. [DOI] [PubMed] [Google Scholar]
- 15.Sarachu M., Colet M. wEMBOSS: a web interface for EMBOSS. Bioinformatics. 2005;21:540–541. doi: 10.1093/bioinformatics/bti031. [DOI] [PubMed] [Google Scholar]
- 16.Lundqvist M., Bengten E., Stromberg S., Pilstrom L. Ig light chain gene in the Siberian sturgeon (Acipenser baeri) J Immunol. 1996;157:2031–2038. [PubMed] [Google Scholar]
- 17.Ghaffari S.H., Lobb C.J. Structure and genomic organization of a second class of immunoglobulin light chain genes in the channel catfish. J Immunol. 1997;159:250–258. [PubMed] [Google Scholar]
- 18.Haire R.N., Rast J.P., Litman R.T., Litman G.W. Characterization of three isotypes of immunoglobulin light chains and T-cell antigen receptor alpha in zebrafish. Immunogenetics. 2000;51:915–923. doi: 10.1007/s002510000229. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard T. The Ensembl genome database project. Nucleic Acids Res. 2002;30:38–41. doi: 10.1093/nar/30.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda R., Hart P., Stork D. 2nd ed. Wiley; New York: 2001. Pattern classification. [Google Scholar]
- 21.Staden R. Computer methods to aid the determination and analysis of DNA sequences. Biochem Soc Trans. 1984;12:1005–1008. doi: 10.1042/bst0121005. [DOI] [PubMed] [Google Scholar]
- 22.Guryev V. Genetic variation in the zebrafish. Genome Res. 2006;16:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campanella J.J., Bitincka L., Smalley J. MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinform. 2003;4:1–4. doi: 10.1186/1471-2105-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giudicelli V., Chaume D., Lefranc M.P. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V–J and V–D–J rearrangement analysis. Nucleic Acids Res. 2004;32:435–440. doi: 10.1093/nar/gkh412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu E., Criscitiello M.F. Diverse immunoglobulin light chain organizations in fish retain potential to revise B cell receptor specificities. J Immunol. 2006;177:2452–2462. doi: 10.4049/jimmunol.177.4.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuppers R., Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 27.Roth D.B., Wilson J.H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodeur P.H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984;14:922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- 29.Simossis V.A., Heringa J. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 2005;33:289–294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabat E.A., Wu T.T., Perry H.M., Gottesman K.S., Foeller C. 5th ed. National Institutes of Health; Bethesda, MD: 2001. Sequences of proteins of immunological interest. [Google Scholar]
- 31.Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa J., Etsuou I., Moritomo T., Nakao M., Yano T., Tomana M. Characterization of a fourth immunoglobulin light chain isotype in common carp. Fish Shellfish Immunol. 2004;16:369–379. doi: 10.1016/j.fsi.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Jones J.C., Ghaffari S.H., Lobb C.J. Patterns of gene divergence and VL promoter activity in immunoglobulin light chain clusters of the channel catfish. Immunogenetics. 2004;56:448–461. doi: 10.1007/s00251-004-0700-3. [DOI] [PubMed] [Google Scholar]
- 34.Von Hofsten J., Olsson P.E. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod Biol Endocrinol. 2005;10:3–11. doi: 10.1186/1477-7827-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corcoran A.E. Immunoglobulin locus silencing and allelic exclusion. Semin Immunol. 2005;17:141–154. doi: 10.1016/j.smim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Gorman J.R., Alt F.W. Regulation of immunoglobulin light chain isotype expression. Adv Immunol. 1998;69:113–181. doi: 10.1016/s0065-2776(08)60607-0. [DOI] [PubMed] [Google Scholar]
- 37.Retter M.W., Nemazee D. Receptor editing occurs frequently during normal B cell development. J Exp Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siminovitch K.A., Moore M.W., Durdik J., Selsing E. The human kappa deleting element and the mouse recombining segment share DNA sequence homology. Nucleic Acids Res. 1987;15:2699–2705. doi: 10.1093/nar/15.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunda O., Corcos D. Recombining sequence recombination in normal kappa-chain-expressing B cells. J Immunol. 1997;159:4362–4366. [PubMed] [Google Scholar]
- 40.Klobeck H.G., Zachau H.G. The human CK gene segment and the kappa deleting element are closely linked. Nucleic Acids Res. 1986;14:4591–4603. doi: 10.1093/nar/14.11.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langerak A.W. Unraveling the consecutive recombination events in the human IGK locus. J Immunol. 2004;173:3878–3888. doi: 10.4049/jimmunol.173.6.3878. [DOI] [PubMed] [Google Scholar]
- 42.Lefranc M.P. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999;27:209–212. doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lossos I.S., Tibshirani R., Narasimhan B., Levy R. The inference of antigen selection on Ig genes. J Immunol. 2000;165:5122–5126. doi: 10.4049/jimmunol.165.9.5122. [DOI] [PubMed] [Google Scholar]
- 44.Shlomchik M.J., Marshak-Rothstein A., Wolfowicz C.B., Rothstein T.L., Weigert M.G. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 45.Wilson M., Hsu E., Marcuz A., Courtet M., Du Pasquier L., Steinberg C. What limits affinity maturation of antibodies in Xenopus—the rate of somatic mutation or the ability to select mutants? EMBO J. 1992;11:4337–4347. doi: 10.1002/j.1460-2075.1992.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S.S., Tranchina D., Ohta Y., Flajnik M., Hsu E. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity. 2002;16:571–582. doi: 10.1016/s1074-7613(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 47.Reynaud C.A., Anquez V., Grimal H., Weill J.C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- 48.Reynaud C.A., Dahan A., Anquez V., Weill J.C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 49.Brekke K.M., Garrard W.T. Assembly and analysis of the mouse immunoglobulin kappa gene sequence. Immunogenetics. 2004;56:490–505. doi: 10.1007/s00251-004-0659-0. [DOI] [PubMed] [Google Scholar]
- 50.Johnston C.M., Wood A.L., Bolland D.J., Corcoran A.E. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 51.Boudinot P., Drapier A.M., Cazenave P.A., Sanchez P. Mechanistic and selective constraints act on the establishment of V lambda J lambda junctions in the B cell repertoire. J Immunol. 1994;152:2248–2255. [PubMed] [Google Scholar]
- 52.Da Sylva T.R., Fong I.C., Cunningham L.A., Wu G.E. RAG1/2 re-expression causes receptor revision in a model B cell line. Mol Immunol. 2007;44:889–899. doi: 10.1016/j.molimm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 53.Doyle C.M., Han J., Weigert M.G., Prak E.T. Consequences of receptor editing at the lambda locus: multireactivity and light chain secretion. Proc Natl Acad Sci USA. 2006;103:11264–11269. doi: 10.1073/pnas.0604053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woloschak G.E., Krco C.J. Regulation of kappa/lambda immunoglobulin light chain expression in normal murine lymphocytes. Mol Immunol. 1987;24:751–757. doi: 10.1016/0161-5890(87)90058-7. [DOI] [PubMed] [Google Scholar]
- 55.Goto T., Kosaka M., Wolfenbarger D., Weiss D.T., Solomon A. Clin Exp Immunol. 1988;111:457–462. doi: 10.1046/j.1365-2249.1998.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hole N.J., Harindranath N., Young-Cooper G.O., Garcia R., Mage R.G. Identification of enhancer sequences 3′ of the rabbit Ig kappa L chain loci. J Immunol. 1991;146:4377–4384. [PubMed] [Google Scholar]
- 57.Ros F., Reichenberger N., Dragicevic T., van Schooten W.C., Buelow R., Platzer J. Sequence analysis of 0.4 megabases of the rabbit germline immunoglobulin kappa1 light chain locus. J Anim Genet. 2005;36:51–57. doi: 10.1111/j.1365-2052.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 58.Sehgal D., Johnson G., Wu T.T., Mage R.G. Generation of the primary antibody repertoire in rabbits: expression of a diverse set of Igk–V genes may compensate for limited combinatorial diversity at the heavy chain locus. Immunogenetics. 1999;50:31–42. doi: 10.1007/s002510050683. [DOI] [PubMed] [Google Scholar]
- 59.Ventura-Holman T., Jones J.C., Ghaffari S.H., Lobb C.J. Structure and genomic organization of VH gene segments in the channel catfish: members of different VH gene families are interspersed and closely linked. Mol Immunol. 1994;31:823–832. doi: 10.1016/0161-5890(94)90020-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of zebrafish VL segments. Amino acids percent identity matrix calculated according to Spalding and Lammers (2004). Identities ⩾70% designation for Ig “V families” (Matsuda, 2004) depicted as shaded boxes. At least 5 distinct families were identified, each by chromosome as shown. Apparent pseudogenes and truncated VL sequences were omitted from analyses.Spalding JB, Lammers (2004) Nucleic Acids Res 32:26–32.Matsuda F (2004) Molecular Biology of B Cells. eds Honjo T, Alt F, Neuberger M (Elsevier) Pp: 1–17.