Abstract
The most common cause of hereditary nephrogenic diabetes insipidus is a nonfunctional vasopressin (VP) receptor type 2 (V2R). Calcitonin, another ligand of G-protein–coupled receptors, has a VP-like effect on electrolytes and water reabsorption, suggesting that it may affect AQP2 trafficking. Here, calcitonin increased intracellular cAMP and stimulated the membrane accumulation of AQP2 in LLC-PK1 cells. Pharmacologic inhibition of protein kinase A (PKA) and deficiency of a critical PKA phosphorylation site on AQP2 both prevented calcitonin-induced membrane accumulation of AQP2. Fluorescence assays showed that calcitonin led to a 70% increase in exocytosis and a 20% decrease in endocytosis of AQP2. Immunostaining of rat kidney slices demonstrated that calcitonin induced a significant redistribution of AQP2 to the apical membrane of principal cells in cortical collecting ducts and connecting segments but not in the inner stripe or inner medulla. Calcitonin-treated VP-deficient Brattleboro rats had a reduced urine flow and two-fold higher urine osmolality during the first 12 hours of treatment compared with control groups. Although this VP-like effect of calcitonin diminished over the following 72 hours, the tachyphylaxis was reversible. Taken together, these data show that calcitonin induces cAMP-dependent AQP2 trafficking in cortical collecting and connecting tubules in parallel with an increase in urine concentration. This suggests that calcitonin has a potential therapeutic use in nephrogenic diabetes insipidus.
Hereditary nephrogenic diabetes insipidus is most often associated with expression of a nonfunctional vasopressin receptor type 2 (V2R) mutant (X-linked nephrogenic diabetes insipidus [NDI]).1–3 The V2R signaling pathway that regulates aquaporin 2 (AQP2) trafficking has been extensively studied.2,4–10 When vasopressin (VP) binds V2R, adenylyl cyclase is activated, intracellular cAMP is increased, and protein kinase A (PKA) is stimulated. AQP2 is phosphorylated at serine 256 (S256), which is critical for AQP2 accumulation at the plasma membrane and an increase in collecting duct water permeability.11,12 Although other strategies to bypass the defective V2R signaling pathway in X-linked NDI have been proposed,13 including the use of cGMP selective phosphodiesterase inhibitors such as sildenafil,14,15 the need for more effective treatment remains. Earlier work by De Rouffignac et al.16,17 proposed an antidiuretic effect of calcitonin (CT), and Carney and Thompson18 also suggested a possible role for CT in water reabsorption. These observations suggest that CT might affect water reabsorption via regulation of AQP2 trafficking subsequent to an increase in cAMP levels in target cells.
CT is primarily secreted by thyroid parafollicular cells,19,20 although its precursor form has been detected in liver, kidney, and neutrophils.21,22 CT also has hypocalcemic and hypophosphatemic effects.23–26 CT binds two G-protein–coupled receptors in humans: CT receptor types 1 (CT(A)) and 2 (CT(B)).27–29 CT(A) and CT(B) are both associated with the heterotrimeric G-protein Gs and increase intracellular cAMP, but CT(B) can also affect phosphoinositide-specific phospholipase C.30
Although CT affects several organs, its principal target is the kidney where it is largely degraded.31–36 The distribution of CT-binding sites is slightly different among rat, mouse, rabbit, primate, and human.37,38 CT stimulates adenylyl cyclase activity in human thick ascending limb and in cortical and medullary collecting ducts.39 In rat, CT-binding sites were detected in the cortical collecting duct and distal convoluted tubule (possibly including the connecting segment) and in thick ascending limbs.38,40–42 Quantitative reverse transcription (RT)-PCR detected CT(A) but not CT(B) mRNA in rat kidney,43 supporting previous work showing that rat kidney has only a single class of CT-binding sites.42 The location of both CT and VP receptors in some of the same tubule segments is consistent with the observation that CT has a VP-like effect on these segments.16,17 De Rouffignac et al.16,17 suggested that CT plays a role in the corticomedullary gradient formation by modifying electrolyte transport in the thick ascending limb. Acute treatment with CT confirmed a VP-like effect on electrolytes and suggested an effect on water reabsorption in collecting ducts.18
Here, we investigated the effect of CT on AQP2 trafficking in vitro, in situ, and in vivo using LLC-PK1 kidney epithelial cells, kidney slices in vitro, and CT-infused VP-deficient Brattleboro rats. CT induced an increase of intracellular cAMP in AQP2-expressing LLC-PK1 cells that express endogenous CT receptor,29,44,45 resulting in AQP2 membrane accumulation. Immunocytochemistry on Brattleboro rat kidneys showed a CT-induced increase of AQP2 membrane accumulation in cells from connecting segments and cortical collecting ducts, in parallel with a significant but transient reduction in urine volume and an increase in urine osmolality.
RESULTS
PKA-dependent AQP2 Membrane Accumulation in Response to CT in LLC-PK1 Cells
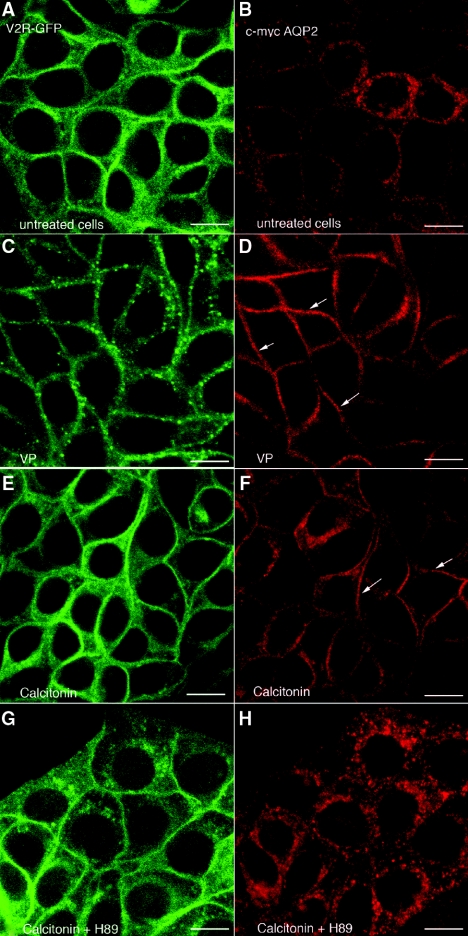
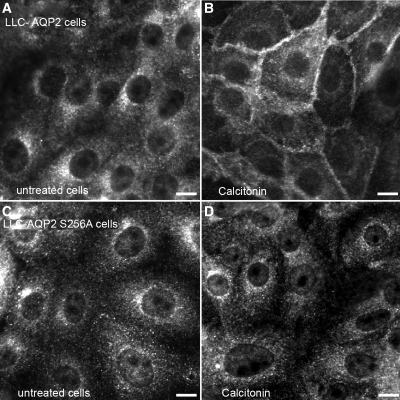
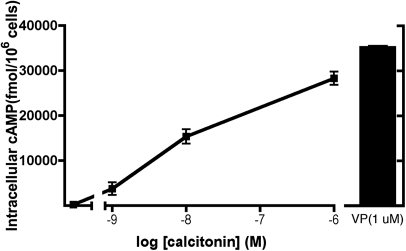
In the absence of ligand, green fluorescence protein (GFP)-tagged V2R (V2R-GFP) is expressed principally at the plasma membrane (Figure 1A), whereas c-myc-tagged AQP2 is in cytoplasmic vesicles (Figure 1B). In the presence of VP (10 nM, 10 minutes), V2R-GFP showed moderate internalization (Figure 1C), whereas AQP2 accumulated at the plasma membrane (Figure 1D). CT (100 nM, 10 minutes) had a similar effect on AQP2 membrane accumulation (Figure 1F) without affecting V2R-GFP localization (Figure 1E). The CT effect was abolished by H89 (30 μM), a PKA inhibitor (Figure 1H), suggesting a role for PKA in the AQP2 response to CT. To determine whether AQP2 phosphorylation at S256 was necessary, LLC-PK1 cells expressing either wild type or AQP2 (S256A), a phosphorylation-deficient mutant, were incubated with CT (Figure 2). Whereas CT (100 nM, 10 minutes) induces cell surface accumulation of wild type AQP2 (Figure 2, A and B), it had no effect on the AQP2 (S256A) mutant (Figure 2, C and D). This suggests that S256 phosphorylation of AQP2 is essential for the CT effect. Furthermore, CT increased intracellular cAMP levels dose-dependently in LLC-VA cells (Figure 3). The effect of CT (1 μM) is almost (approximately 80%) as great as 1 μM VP in LLC-VA cells. CT also increased cAMP in untransfected LLC-PK1 and in LLC-W2 cells that are transfected with AQP2 alone (data not shown).
Figure 1.
CT stimulates AQP2 accumulation in the plasma membrane of kidney epithelial cells without affecting V2R-GFP distribution. Confocal immunofluorescence microscopy shows V2R-GFP and AQP2 in LLC-VA cells before and after treatment with VP and CT with or without the PKA inhibitor H89. (A and B) In nonstimulated cells, V2R-GFP (green) is concentrated in the plasma membrane (A), whereas AQP2 (red) remains in cytoplasmic vesicles localized mainly around the nucleus (B). (C through F) In contrast, AQP2 shows plasma membrane localization (arrows) after incubation for 10 minutes with 10 nM VP (D) or 100 nM CT (F). V2R-GFP was partially internalized into cytoplasmic vesicles at this early time point after VP treatment (C), but its distribution was not changed by CT treatment (E). (G and H) The presence of H89 did not effect V2R distribution (G), but the effect of CT on the distribution of AQP2 was completely abolished in the presence of this inhibitor (H). These images are representative of three independent experiments. The bar indicates 10 μm.
Figure 2.
Wild-type AQP2, but not S256A mutant AQP2, accumulates at the plasma membrane in the presence of CT. Indirect immunofluorescence microscopy of AQP2 staining in LLC-AQP2 wild type and S256A mutant expressing cells incubated in the presence or absence of CT. The AQP2 (S256A) mutant is located on intracellular vesicles similar to wild type AQP2 in basal conditions (A and C, respectively). After CT treatment (100 nM, 10 minutes) AQP2 wild type accumulates at the plasma membrane (B), whereas localization of the AQP2 (S256A) mutant is unchanged (D). These images are representative of three independent experiments. The bar indicates 10 μm.
Figure 3.
CT increases the intracellular levels of cAMP in LLC-VA cells. The cells were incubated for 10 minutes with several concentrations of CT (1, 10, and 1000 nM). After incubation, the cells were solubilized, and supernatants were used to measure intracellular cAMP using an ELISA assay. CT stimulation was compared with the elevation of cAMP (35,196 ± 471 fmol/106 cells) determined in the presence of a saturating concentration of VP (1 μM). Each point represents the mean ± SD of triplicate determinations. This figure is representative of three independent experiments.
Effect of CT on Exocytosis and Endocytosis in LLC-PK1 Cells
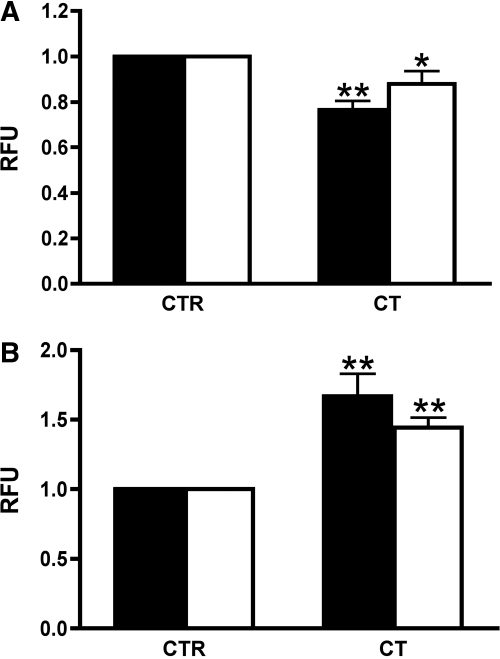
To address the mechanism of AQP2 cell surface accumulation in response to CT, exocytosis and endocytosis were analyzed in LLC-PK1 cells using a fluorescence-based soluble yellow fluorescent protein (ssYFP) secretion assay and a Texas Red-dextran internalization assay as described previously (Figure 4).46 Endocytosis was moderately but significantly inhibited in both cell lines by CT (Figure 4A). CT also significantly increased exocytosis in both LLC-AQP2-ssYFP and LLC-ssYFP cells (Figure 4B), indicating that CT stimulates exocytosis in renal epithelial cells, even if they do not express AQP2.
Figure 4.
CT increases exocytosis and reduces endocytosis in LLC-AQP2-ssYFP and LLC-ssYFP cells. (A) The endocytosis assay shows a reduction of Texas Red dextran internalization into LLC-AQP2-ssYFP (solid bar) and LLC-ssYFP cells (open bar) incubated with CT (100 nM) compared with untreated cells (CTR). (B) In the exocytosis assay, an increased secretion of ssYFP from both LLC-AQP2-ssYFP (solid bar) and LLC-ssYFP cells (open bar) was stimulated by CT. This figure is representative of three different experiments (means ± SD; *P < 0.05; **P < 0.01).
CT Effect on AQP2 Membrane Accumulation in Principal Cells
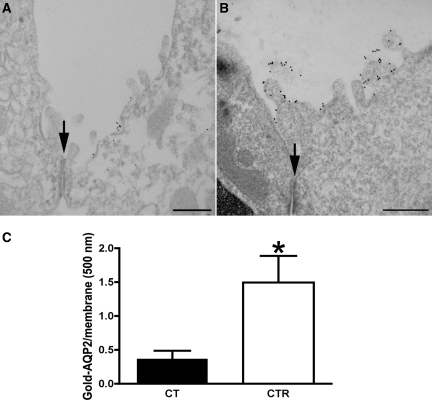
Electron microscopy was used to study AQP2 accumulation in the apical plasma membrane of cortical principal cells (Figure 5). We observed a striking increase of gold-labeled apical AQP2 in tubules from CT-treated Brattleboro rats (Figure 5B) compared with controls (Figure 5A). Quantification is shown in Figure 5C. Apical AQP2 accumulation was 4.3 times higher in CT-treated animals. The technique used here detects only apical plasma membrane AQP2 and not intracellular AQP2.
Figure 5.
Immunogold electron microscopy shows apical plasma membrane insertion of AQP2 induced by CT in vivo. AQP2 was localized by pre-embedding labeling of thick, nonpermeabilized vibratome sections kidney cortical tissue with an antibody against an external epitope of AQP2. Only plasma membrane AQP2 is detected using this procedure. CT treatment (B) showed a significant amount of AQP2 plasma membrane associated with the apical membrane and microvilli, whereas AQP2 in the apical plasma membrane was much less abundant in cortical kidney sections of the untreated rats (A). These results support the epifluorescence microscopy data shown in Figures 6 and 9. The number of gold particles labeling AQP2 is expressed per micrometer of apical membrane length (C). Images of two to three tubules from each tissue were analyzed with ImageJ software (National Institutes of Health). The density of AQP2 at the apical plasma membrane (open bar) was compared with the density of AQP2 in the apical membranes of untreated rats (solid bar) (means ± SEM; n = 3; *P < 0.05). The position of the cell junction between a principal cell (B) and an AQP2-negative intercalated cell (A) is indicated with an arrow in each figure. The bar indicates 0.5 μm.
CT Induces AQP2 Trafficking in Situ
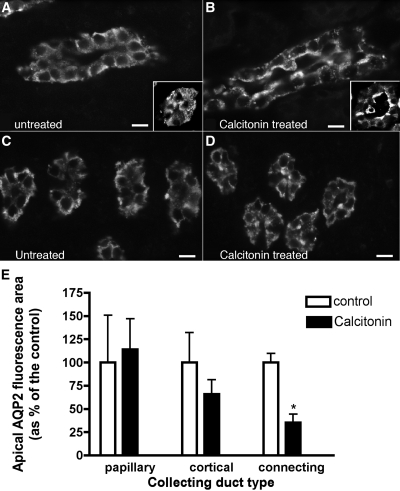
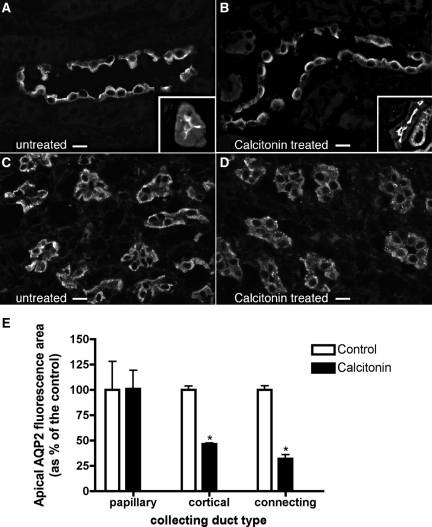
AQP2 trafficking was also followed by immunofluorescence in rat kidney slices incubated in the presence or absence of CT (100 nM) for 1 hour in vitro (Figure 6, A through D). CT significantly increased AQP2 at the apical plasma membrane of cortical collecting ducts (Figure 6, A and B), but more prominent apical accumulation was seen in connecting segments (Figure 6, A and B, insets), identified by calbindin double staining (not shown). In the outer (not shown) and the inner medulla, however, AQP2 was localized throughout the cytoplasm in the presence or absence of CT (Figure 6, C and D). Quantification of these effects is shown in Figure 6E. Some differences in the intensity of the AQP2 response were noted between cells and the kidney in situ, possibly related to different CT affinities among species or the availability/efficiency of the machinery for CT signaling. Nevertheless, these results support our cell culture observations that CT induces AQP2 trafficking, but this effect is restricted mainly to cortical tubules in situ.
Figure 6.
Indirect immunofluorescence images of kidney tissue slices showing AQP2 in principal cells of cortical or inner medullary collecting ducts. One rat kidney was cut into thin slices and incubated in vitro with buffer (A and C) or with CT (B and D) for 1 hour before fixation by immersion, sectioning, and immunostaining to detect AQP2. Under control conditions, AQP2 was mainly localized throughout the cytoplasm and showed little apical membrane staining in collecting ducts from the cortex (A) and inner medulla (C), as well as in cortical connecting segments (inset). In the presence of CT, AQP2 staining of the apical membrane region was increased in principal cells of the cortical collecting duct (B) and in connecting segments (inset), but AQP2 remained localized in the cytoplasm in the inner medulla (D). Quantification of the effect of CT on AQP2 in principal cells (E) showed that CT induced a significant apical redistribution that was most apparent in the connecting segment but also was significant in the cortical collecting duct. No effect on AQP2 distribution was detectable in the inner medulla. The images are representative of three independent experiments. The quantification shows the means of more than 100 cells taken from the three different experiments (means ± SEM; n = 3; *P < 0.05). The bar indicates 10 μm.
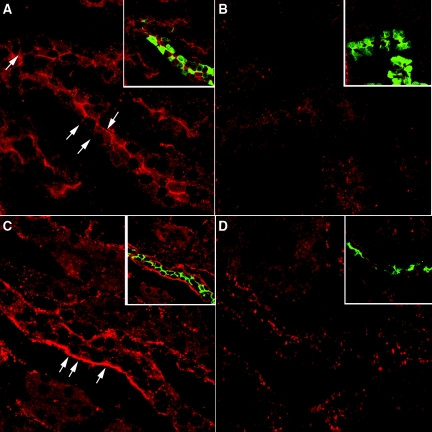
Immunostaining showed that CT receptor is expressed in both the cortical connecting segment and collecting duct (Figure 7, A and C). It is located at both the apical and lateral membranes of connecting segments, identified by calbindin staining (Figure 7A, arrows), whereas it is found mainly at the lateral membrane of cortical collecting ducts, identified by AQP2 staining (Figure 7C). Both staining patterns were abolished in the presence of the immunizing peptide (Figure 7, B and D).
Figure 7.
Indirect immunofluorescence images of kidney showing CT receptor in cortical collecting ducts and connecting segments. One rat kidney was fixed by immersion, sectioned and immunostained to detect the CT receptor as well as AQP2 and calbindin. The CT receptor is located in the apical membrane in cortical tubules (A) that costain for calbindin (A, inset, green), a marker of cortical connecting segments. Apical staining is completely abolished in the presence of directed antibody peptide (B) in calbindin-positive tubules (B, inset). Mainly basolateral CT receptor staining was observed in cortical collecting ducts (C). Both the CT receptor (red) and AQP2 (green) colocalized in the same cells (C, inset). This CT receptor staining was abolished in the presence of peptide inhibitor (D, inset), although some spots of nonspecific fluorescence remain scattered throughout the section. The images are representative of three independent experiments.
CT Has an Antidiuretic Effect in Vivo
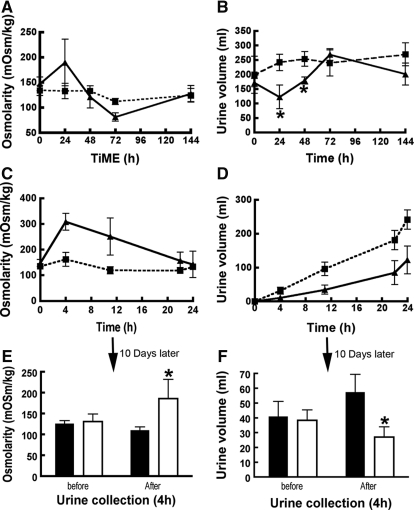
Osmotic pumps containing CT or vehicle were implanted into Brattleboro rats, which have no endogenous VP and display polydipsia and polyuria. All of the animals were initially drinking an average of approximately 237 ml of water/d and excreting most as hypoosmotic urine (approximately 197 ml/d, approximately 134 mOsm/kg). During the first 12 hours of exposure, urine volume from CT-treated rats was three times lower and twice as concentrated as that from saline-treated rats (Figure 8). After 24 hours, these differences were less than after 12 hours. After 48 hours, a difference of 40% in urine volume was found, whereas urine osmolality remained low (approximately 126 mOsm/kg). The effect of CT was lost after 72 hours of continuous infusion (Figure 8, A and B). Consistent with reduced urine output, CT-treated rats drank 116 ± 41 ml of water compared with 290 ± 36 ml in the control group during the first 24 hours. This effect disappeared after the third day of treatment (302 ± 20 versus 277 ± 54 ml). After pump removal and 10 days of rest, the rats were again infused with CT using a new minipump. After 4 hours, CT-treated rats urinated less, and the urine was more concentrated than in the control group (Figure 8, E and F). Serum osmolality was similar in both groups (299 ± 3 mOsmol/kg), and blood profile analysis (Table 1) showed no difference in sodium, potassium, chloride, calcium, glucose, or creatinine. CT did not affect plasma oxytocin levels after 4 hours (13.2 ± 0.6 versus 14.0 ± 1.9 ng/ml) or 24 hours of infusion (16.5 ± 2.9 versus 19.9 ± 5.1 ng/ml). These values are similar to those (4.3 to 18.8 pg/ml) reported by Edwards et al.47
Figure 8.
Urine volume decreases and urine osmolarity increases in CT-treated rats. Metabolic cage analysis of the volume and urine osmolality from Brattleboro rats infused with CT or saline. Brattleboro rats were implanted with osmotic minipumps that deliver saline or CT (2 mU/min/100 g rat) for 7 days. Urine osmolality and volume were not modified in animals treated with saline (■) (A and B, respectively). In contrast, the urine osmolality was significantly increased and its volume was reduced during the first 24 hours in CT-perfused animals (▴) (A and B, respectively). Osmolality of the urine from both groups was analyzed after 4, 11, 22, and 24 hours (C). The osmolality of urine from CT-treated rats (▴) increased rapidly and then declined over time, whereas the osmolality of saline-treated animals was low and stable (■). In parallel, the urine volume of saline-treated groups (■) was greater than animals treated with CT (▴) (D). After removing the pumps for 10 days, the same rats were once again challenged with CT, and their urine osmolality and volume were analyzed (E and F, respectively). A 4-hour urine collection was performed before and after reperfusion of animals with saline (solid bar) or CT (open bar), and the rats were then perfusion-fixed for analysis of AQP2 distribution in their kidneys (see Figure 9). Those results represent the average values obtained from three different animals (means ± SD; *P < 0.05). The bar indicates 10 μm.
Table 1.
Plasma solute and electrolyte profile of calcitonin-treated and control Brattleboro rats
| Control Rat (n = 3) | CT-treated Rat (n = 3) | |
|---|---|---|
| Sodium (mmol/L) | 150.0 ± 3.6 | 148.3 ± 0.6 |
| Potassium (mmol/L) | 4.2 ± 0.4 | 3.9 ± 0.5 |
| Chloride (mmol/L) | 109.0 ± 3.0 | 108.0 ± 2.0 |
| Calcium (mmol/L) | 1.11 ± 0.32 | 1.21 ± 0.33 |
| Glucose (mg/dl) | 208.3 ± 18.3 | 225.6 ± 12.6 |
| Bun (mg/dl) | 16.0 ± 4.0 | 7.7 ± 0.6 |
| Creatine (mg/dl) | 0.3 ± 0.1 | 0.4 ± 0.0 |
Kidneys from both groups of retreated animals were harvested after 4 hours and immunostained for AQP2 (Figure 9). As observed in kidney slices, AQP2 accumulated at the apical pole of cortical principal cells of CT infused rats, in contrast to the more diffuse distribution in controls (Figure 9, A and B). Apical redistribution of AQP2 (Figure 9, A and B, insets) was also prominent in cortical connecting segments, identified by calbindin staining (not shown). CT had no detectable effect on AQP2 distribution in the outer (not shown) or inner medulla (Figures 9D and 6D). Quantification of the CT effect is shown in Figure 9E.
Figure 9.
Indirect immunofluorescence of sections from rat kidney showing AQP2 in principal cells of cortical and inner medullary collecting ducts with and without CT treatment in vivo. After implantation of minipumps containing either saline or CT for 4 hours, the animals were anesthetized, and the kidneys were fixed by perfusion, followed by sectioning and immunostaining to detect AQP2. (A through C) Under control conditions, AQP2 is localized toward the apical pole of collecting ducts from the cortex (A) and inner medulla (C), as well as the cortical connecting segment (A, inset), but in the presence of CT, AQP2 is more sharply concentrated at the apical pole of principal cells from the cortical collecting duct (B) and in cells of the connecting segment (B, inset), reflecting a reduction in cytoplasmic vesicle staining and an increase in apical membrane staining (see also Figure 6). (D) CT had no effect in the inner medulla, where AQP2 remained localized throughout the cytoplasm. (E) Quantification of the effect of CT on AQP2 redistribution in principal cells showed a significant increase in apical staining in both cortical collecting ducts and, to an even greater extent, connecting segments. No effect was detected in the inner medulla. The images are representative of three independent incubations, and quantification shows the mean of more then 100 cells taken from the three different experiments (means ± SEM; *P < 0.05). The bar indicates 10 μm.
To study the effect of food intake and CT on water reabsorption, we performed a second experiment where food (and, therefore, solute) intake was controlled. Both groups were deprived of food for 4 hours, and then food was monitored until the end of the experiment. After 4 hours, urine volume was not significantly different between the treated and nontreated groups, but the urine of CT-treated rats was significantly more concentrated (204 ± 49 mOsM/Kg) than the urine of untreated rats (124 ± 27 mOsm/kg). A significant difference in urine volume became more apparent with time. After 12 hours, the treated rat urine volume was ∼30% lower than untreated groups, although their food consumption was not significantly different. After 24 hours, CT-treated rats produced 39% less urine (128 ± 20 versus 208 ± 10 ml, n = 3, P < 0.05), and it was more hypertonic (172 ± 21 versus 132 ± 14 mOsM/Kg, n = 3, P < 0.05), whereas their food consumption was less than the controls at this later time point (19 ± 2 versus 11 ± 1 g, n = 3, P < 0.05).
CT Effect on AQP2 Expression
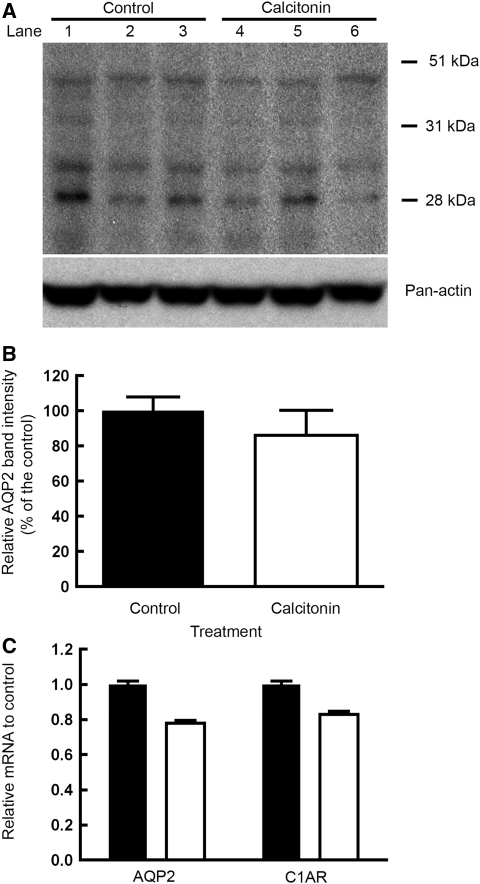
The amount of AQP2 protein was not significantly changed after a 24-hour infusion of CT, as detected by Western blot analysis (Figure 10, A and B). Furthermore, levels of AQP2 and CT receptor mRNA were not affected by CT treatment (Figure 10C).
Figure 10.
CT has no effect on either the level of AQP2 protein or mRNA expression in kidney cortex. The effect of CT on AQP2 expression in the kidney cortex was investigated at the protein (A and B) and gene level (C) using Western blot and real-time PCR techniques. Cortex of the untreated and treated rats (24 hours CT) were homogenized and separated by SDS-PAGE. Each lane represents a single animal. Western blot analysis (A) was performed on three different control tissues (lanes 1 to 3) and CT-treated rat kidneys (lanes 4 to 6). AQP2 was detected by Western blot using an anti-AQP2 antibody directed against the second extracellular region of AQP2. Densitometric analysis of Western blot (B) of immunodetected AQP2 (28 kD) and the glycosylated band around 40 kD was performed. The band intensities were normalized by loading controls. The average intensity of three untreated rats (solid bar) was NS compared with the average band intensity of CT-treated rats (open bar). The data are the means ± SD (n = 3). Real-time PCR (C) showed that CT did not affect AQP2 or CT receptor mRNA. Real-time PCR analysis showed no effect on the level of AQP2 and CT receptor mRNA in rats treated for 24 hours with calcitonin (open bar) compared with the levels of AQP2 mRNA in untreated rats (solid bar). The data are the means ± SD (n = 3).
DISCUSSION
Earlier work from the group of De Rouffignac16 showed that CT has a VP-like antidiuretic activity in hormone-depleted Brattleboro rats, but evidence of an effect on principal cells was lacking, and determining the effects of CT on AQP2 trafficking was not possible. Our data confirm that CT-treated Brattleboro rats show a significant increase in urine osmolality and show that CT stimulates an increase in cell surface accumulation of AQP2 in vitro, in situ, and in vivo. The increase in plasma membrane AQP2 after CT stimulation, in parallel with decreased urine output and increased urine osmolality, supports the idea that CT plays a direct role in the urinary concentrating mechanism at the level of the collecting duct (and connecting segment), in addition to its effect on NaCl transport in the thick ascending limb that could affect the medullary concentration gradient.16 Brattleboro rats treated with CT drank less water than controls, as expected when urinary output is reduced. Previous studies showed that a reduction of urinary output is also correlated with reduced water intake in VP-treated Brattleboro rats.48,49 Furthermore, although acute treatment of Brattleboro rats with physiologic doses of VP had no reported effect on GFR,50,51 direct measurements of GFR were not performed in these studies, and a contribution of a change in GFR to the observed effect of CT cannot be ruled out.
The signaling pathway by which CT increases AQP2 membrane accumulation seems similar to the cAMP-dependent mechanism of VP action. CT binding to its receptor triggers an increase in cAMP that activates PKA and leads to AQP2 phosphorylation at S256, which is required for AQP2 membrane accumulation.11,12 The absence of any effect of CT on trafficking of an AQP2(S256A) mutant in LLC-PK1 cells confirms that phosphorylation of S256 is essential for AQP2 membrane accumulation in response to CT as well as VP.11,12 At present, the effect of CT on phosphorylation of other target residues in the AQP2 protein52,53 is not known. The mechanism regulating AQP2 trafficking upon CT and VP binding to their respective receptors seems similar; CT signals mainly via cAMP in the nephron,40,42 whereas VP affects both intracellular cAMP and calcium levels, and increased intracellular calcium has been implicated in the response to VP54 including AQP2 trafficking.55 However, CT might effect intracellular calcium levels in cortical collecting duct cells as a result of cAMP elevation,56 but this effect was believed to occur in intercalated cells.57 Further comparative studies may reveal a role of calcium in fine-tuning the cAMP signaling pathway in renal epithelial cells.
Functional differences that distinguish CT and VP action are, however, suggested by these and prior studies on their relative effects on exo- and endocytosis in LLC-PK1 cells. CT stimulated exocytosis of ssYFP both in native LLC-PK1 cells and LLC-PK1 cells that expressed AQP2. In contrast, VP increases exocytosis only in LLC-PK1 cells that express AQP2.46 Furthermore, CT had a small but significant inhibitory effect on endocytosis in both cell types whether or not they express AQP2. These data suggest that CT may have a general effect on exo- and endocytosis, whereas the effect of VP is more specifically related to the presence of AQP2 in transporting vesicles.46 Finally, because CT acts via cAMP, its effect could involve both short term trafficking as well as long term stimulation of AQP2 transcription via activation of a cAMP-response element in the 5′-flanking region of the AQP2 gene.58–60 However, in these studies, no significant alteration in levels of either AQP2 protein or mRNA were detectable after CT treatment for 24 hours, at least at the level of detection afforded by our techniques. Interestingly, another G-protein–coupled receptor ligand, glucagon, also has antidiuretic effects, although its effect on AQP2 trafficking in vivo remains to be determined.41,61
As expected from the distribution of CT receptors along the urinary tubule,37,40 the effect of CT on AQP2 membrane accumulation was region-dependent. The effect was greater in the connecting segment and cortical collecting duct, where CT receptors are most abundant, than in the medullary collecting duct. Because considerable water reabsorption occurs in the cortex, preventing excessive fluid delivery to the medulla, increasing water permeability of only cortical tubule segments would have a significant (but only partial) antidiuretic effect. Our in vivo data show that this is the case. A combination of agents that stimulate AQP2 apical accumulation in both cortical and medullary collecting ducts will be required to elicit a more substantial antidiuretic response in the absence of defective V2R signaling.
Salmon CT has been examined as a potential therapeutic agent for metabolic bone diseases with minimal side effects.62 A high concentration of CT affected calcium and magnesium excretion and even had a diuretic effect.18,63,64 However, the concentration of CT used in our study was equivalent to the plasma concentration of endogenous thyrocalcitonin that does not cause phosphaturia and natriuresis.16,18 At this lower concentration, we show that AQP2 membrane accumulation in the kidney is stimulated, indicating that CT affects principal cells and not only intercalated cells, as suggested earlier.57 Although we attribute reduced drinking in CT-treated rats to changes in water balance caused by decreased urinary output, a partial direct effect of CT on the drinking response cannot be completely ruled out. Salmon CT, an amylin agonist, reduces mainly prandial drinking, whereas it does not affect the drinking-inducing response to angiotensin II.65,66 Salmon CT may directly stimulate neurons in the subfornical organ in rat, and it has a dipsogenic effect in starved pygmy goats.65,67 It is also possible that reduction of water intake may participate in increasing urine concentration via the release of oxytocin either indirectly or by direct activation of CT family receptors in the brain.68–70 Indeed, intracerebroventricular injection of oxytocin inhibits water intake.71,72 It has also been shown previously that oxytocin-induced AQP2/water channel mobilization occurs, in parallel with an oxytocin-induced antidiuresis in Brattleboro rats.73–75 However, we were unable to detect any significant change in plasma oxytocin levels in CT-treated rats. Likewise, GFR is slightly but not significantly increased by acute CT treatment of “hormone-deprived” Brattleboro rats,16 suggesting that changes in GFR are not responsible for the CT effect on urine concentration. In our study, GFR was not directly measured, but serum creatinine was not significantly different between control and CT-treated rats.
The antidiuretic effect of CT persisted for over 24 hours but diminished thereafter. This tachyphylaxis may be due to receptor downregulation or tolerance. After removal of the minipump, re-exposure of the same animals to CT once again induced an antidiuretic response. Such downregulation might be diminished by fine-tuning the dose of CT or by giving periodic treatments (e.g. once per 24 hours) rather than a continuous infusion of CT. In summary, we have shown that CT modulates AQP2 trafficking in a manner similar to VP and that its infusion results in a significant increase of urine osmolality and a significant reduction in urine output in Brattleboro rats. Additional studies will be required to determine whether CT might also be beneficial to patients suffering from X-linked NDI.
CONCISE METHODS
Experimental Animals and Statistical Analyses
Animal experiments were approved by the Institutional Committee on Research Animal Care, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Brattleboro rats (Harlan, Indianapolis, IN), a model of central diabetes insipidus, were used for the in situ and in vivo portions of this study (see below) and were supplied by the National Rat Resource Research Center (Madison, WI). All of the results were statistically analyzed using the t test. The differences were considered significant at P values less than 0.05.
Cell Culture
Unless otherwise stated, all of the chemicals were purchased from Sigma (St. Louis, MO), and all of the cell culture reagents were purchased from Invitrogen (Carlsbad, CA). LLC-PK1a cells stably expressing both GFP-tagged V2R and c-myc-tagged AQP2 (referred to here as LLC-VA cells, to indicate V2R-GFP and AQP2 expression) were cultured in DMEM, 10% FBS, and neomycin (1 mg/ml) as described previously.15,76 LLC-PK1 cells stably expressing wild type c-myc-tagged aquaporin-2 (LLC-W2 cells) and serine 256 mutant to alanine (LLC-AQP2 (S256A)) were grown as described previously.14
cAMP Assays
Measurements of intracellular cAMP levels in the constant presence of 3-isobutyl-1-methyl-xanthine (1 mM), a phosphodiesterase inhibitor (Amersham Biosciences Corp., Piscataway, NJ), were performed using Biotrak kit as described previously.14,77,78 Intracellular cAMP accumulation was evaluated in LLC-VA cells after incubation in the presence of 1 μM VP or CT for 10 minutes at 37°C. All of the cAMP assays were performed in triplicate.
Fluorescence Exocytosis and Endocytosis Assays
The fluorescence exocytosis assay was performed as described previously46 using either LLC-PK1 cells expressing AQP2 and a soluble yellow fluorescence protein (LLC-AQP2-ssYFP) or with LLC-PK1 cells expressing ssYFP only (LLC-ssYFP cells). Briefly, the cells were grown to confluence on 24-well cell culture plates. The cells were washed twice and incubated for 1 hour in 250 μl of Hanks' balanced salt solution, 20 mM HEPES, and 2 g/L glucose (HBSS). The cells were then incubated in 250 μl of control HBSS or in HBSS containing CT (10 μM) for 30 minutes at 37°C. At the end of the incubation, 150 μl of medium was transferred from each well to a black half-area 96-well plate (Corning, Corning, NY). The ssYFP fluorescence in each well was read immediately after collection using a multimode plate reader (model DTX880; Beckman-Coulter, Fullerton, CA). The values represent at least three independent experiments, measured in triplicate. Background fluorescence values were obtained from empty wells, and fluorescence values are reported as a ratio of each background- and zero-subtracted value to the corresponding LLC-AQP2-ssYFP and LLC-ssYFP cell 30-minute control value.
The endocytosis assay was also performed on LLC-AQP2-ssYFP and LLC-ssYFP cells. The cells were grown to confluence on 96-well cell culture plates. The cells were rinsed once and incubated for 1 hour in HBSS and then for 30 minutes in 0.5 mg/ml of dialyzed Texas Red dextran (10,000 kD) diluted in HBSS in the presence or absence of CT (10 μM). After incubation, the cells were washed twice in PBS and then lysed in 50 μl of lysis buffer (20 mM Tris/HCl, pH 7.4, 5 mM EDTA, 5 mM EGTA, 30 mM NaF, 30 mM Na4P2PO7, 2 mM Na3VO4, 1% Triton X-100, 0.1% SDS. Fluorescence released into the medium was read at 590 nm/650 nm using a multimode plate reader (model DTX880; Beckman-Coulter, Fullerton, CA). The values represent at least three independent experiments, measured in triplicate. The background fluorescence values were obtained from empty wells, and fluorescence values are reported as a ratio of each background- and zero-subtracted value to the corresponding LLC-AQP2-ssYFP and LLC-ssYFP cells 30-minute control values.
Immunocytochemistry of Cell Cultures
LLC-VA, LLC-AQP2 or LLC-AQP2 (S256A) cells were plated on 12 × 12-mm glass coverslips (Fisher Scientific, Pittsburgh, PA). The cells were incubated at 37°C in DMEM with or without VP (10 nM) or CT (100 nM). The effect of CT on AQP2 trafficking was also examined in the presence of N-(2[[3-(4-bromophenyl)-2-propenyl]-amino]-ethyl)-5-isoquinolinesulfonamide (H89) (30 μM), a PKA inhibitor. After incubation, the cells were rinsed with cold PBS and fixed in PBS containing 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and 5% sucrose for 20 minutes at room temperature. After fixation, LLC-AQP2, LLC-AQP2 (S256A), and LLC-VA cells were stained with nondiluted ascites fluid containing a monoclonal anti-c-myc antibody (A9E10) as described previously.14,79 The c-myc antibody was detected using donkey anti-mouse IgG conjugated to indocarbocyanine (CY3) at a final concentration of 1.8 μg/ml (Jackson ImmunoResearch, West Grove, PA) for 1 hour. The coverslips were washed in PBS and mounted in Vectashield (Vector Labs, Burlingame, CA). Immunofluorescence in LLC-VA cells was examined using a Zeiss 63 × 1.4NA Plan Apo objective mounted on a Bio-Rad Radiance 2000 confocal microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY), whereas fluorescence of LLC-AQP2 and LLC-AQP2 (S256A) was examined using a Nikon Eclipse E800 epifluorescence microscope equipped with a 60 × 1.4NA Plan Apo objective and a Hamamatsu Orca CCD Camera (MVI, Avon, MA).
In Situ Kidney Tissue Slices: Preparation and Treatment
The effect of CT on AQP2 trafficking was studied in thin slices of kidney in vitro, which were prepared as described previously.14,77 Briefly, adult male homozygous Brattleboro rats (350 g) were anesthetized using sodium pentobarbital (65 mg/kg body wt intraperitoneally). The blood was washed out from the kidneys by intraventricular perfusion using HBSS, pH 7.4, at 37°C equilibrated with 5% CO2, 95% O2. The thin transversally sliced kidneys (approximately 0.5 mm) were cut using a Stadie-Riggs slicer (Thomas Scientific, Swedesboro, NJ). All of the slices were incubated at 37°C for 15 minutes in equilibrated HBSS only. After equilibration, the slices were distributed into new vials containing either HBSS or CT (100 nM). After 60 minutes, all of the slices were fixed by immersion in paraformaldehyde-lysine-periodate (PLP) fixative at 4°C overnight. The slices were then rinsed several times in 10 mM sodium phosphate buffer containing 0.9% NaCl, pH 7.4, and stored in the same buffer plus 0.02% NaN3 at 4°C before use for immunostaining and quantification as described below.
In Vivo CT Treatment of Rats and Perfusion Fixation
Before experiments, all adult Brattleboro rats (weighting approximately 350 g) were installed in individual metabolic cages for 24 hours. Urine volume and osmolality were analyzed using a vapor pressure osmometer (Vapro 5520; Wescor Inc., Logan, UT). The rats were divided into two groups of three animals. After anesthesia with isofluorane (Hospira Inc., Lake Forest, IL), Alzet osmotic pumps (Durect Corp., Cupertino, CA) filled with saline solution were implanted into the first group, whereas the second group received pumps loaded with salmon CT that released 2 mU (312 pg)/min/100 g body weight. The rats were placed back into metabolic cages. Urine was collected at 4, 11, 22, and 24 hours, and after the first day, 24-hour urine samples were collected over the next 6 days. Throughout the study the individual animals were handled for approximately 5 minutes every day. The rats were kept under normal conditions of light cycling (12 hours of dark/12 hours of light). The animals were allowed to rest in their previous cage for 10 days and then were placed for 24 hours into metabolic cages before the implantation of a second mini pump. The rats were then put back into metabolic cages, where the effects of CT on both urine volume and osmolality were followed for 4 hours. The rats were anesthetized with isofluorane at the end of the experiment. Blood samples were collected by cardiac puncture. All of the animals were perfused with PBS for 1 minutes followed by PLP perfusion fixation for 5 minutes. After perfusion, the kidneys were cut into 5-mm slices, immersed overnight in PLP solution, and stored in PBS containing 0.02% NaN3 at 4°C before use for immunostaining and quantification as described below. Serum and urine and profiles were analyzed at the Massachusetts General Hospital Clinical Pathology Laboratory facility. Oxytocin plasma concentration was determined by enzyme immunoassay following manufacturer's protocol (Phoenix Pharmaceuticals, Inc., Burlingame, CA).
This study was performed with food ad libitum. However, we also carried out a second experiment with regulated food intake. After 24 hours in metabolic cages, the rats were anesthetized. One kidney was harvested for RT-PCR and Western blot analysis. Then the animals were perfused as described above.
Immunocytochemistry of Kidney Tissues
Before immunofluorescence staining and quantification, kidney samples were first cryoprotected in PBS containing 30% sucrose for 2 hours. The slices were mounted on a cutting block covered with OCT compound 4583 (Tissue-Tek; Miles Inc., Elkhart, IN). Four-μm sections were cut from the frozen tissue using a Leica cryostat (Leica, Deerfield, IL). The sections were attached to Superfrost-plus glass slides (Fisher Scientific) for immunofluorescence staining of AQP2 or colocalization of CT receptor with either calbindin or AQP2. First, to quantify the effect of CT on AQP2 trafficking, the kidney sections were rehydrated in PBS for 20 minutes and then incubated in PBS containing 1% bovine serum albumin for 15 minutes to block nonspecific staining. The sections were incubated with a previously characterized antiserum raised against the second extracellular loop of AQP280 (diluted 1:100 in PBS) for 1 hour at room temperature, followed by a 15-minute wash in high-salt PBS (containing 2.7% NaCl) to reduce background staining and two 5-minute washes in PBS. The sections were then incubated with donkey anti-rabbit IgG conjugated to fluorescein (FITC) at a final concentration of 11 μg/ml (Jackson ImmunoResearch) for 1 hour at room temperature and then washed as for the primary antibody. Kidney tissues were then incubated with ascites fluid (diluted 1:600 in PBS) containing mouse monoclonal anti-calbindin antibodies (Sigma). The sections were washed and incubated with 1.5 μg/ml of donkey anti-mouse IgG conjugated to CY3 (Jackson ImmunoResearch) and then mounted in Vectashield (Vector Labs) diluted 2:1 in 0.1 M Tris-HCl, pH 8.0. Mounted slides were examined using a Nikon Eclipse E800 epifluorescence microscope equipped with a 40 × 1.0NA Plan Apo objective, and the images were captured digitally using a Hamamatsu Orca CCD camera and IPLab Spectrum software (Scanalytic, Vienna, VA).
To confirm the site of action of CT, we performed an immunostaining essay to observe colocalization of the CT receptor with either calbindin, a marker of the cortical connecting segment or AQP2, a marker of collecting duct principal cells. After rehydration, the tissue sections were incubated for 10 minutes at 90°C in EDTA buffer pH 9.0 (Thermo Fisher Scientific, Waltham, MA). One hour after the heat-induced antigen retrieval treatment, the tissue sections were incubated with PBS containing 1% donkey serum for 15 minutes to block nonspecific staining. The sections were incubated with antibodies against the second extracellular loop of AQP2 or the mouse monoclonal anti-calbindin antibody as described above. The sections were then incubated for 1 hour with either donkey anti-rabbit IgG or anti-mouse IgG conjugated to fluorescein (FITC) (11 μg/ml) (Jackson ImmunoResearch). The sections were washed and then incubated for 12 hours at 4°C with goat anti-CT receptor (2 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) in the presence or the absence of immunizing peptide peptide as a control (20 μg/ml). After incubation, the sections were washed and incubated with 1.5 μg/ml of donkey anti-goat IgG conjugated to CY3 and then mounted in Vectashield (Vector Labs) diluted 2:1 in 0.1 M Tris-HCl, pH 8.0. The mounted slides were examined using a confocal microscope.
Apical AQP2 Fluorescence Quantification
To quantify the fluorescence of AQP2 labeling at the apical membrane, the images from sections incubated with antibodies at the same time were collected using the same exposure time, which was set so that the brightest images were not saturated. After collection, the images were analyzed with IPLab Spectrum software. All of the cells used for quantification had a complete spherical nucleus, and apical and basolateral membranes were clearly delimited. The apical area occupied by AQP2-associated fluorescence in the principal cells was measured in a defined region of interest. The apical AQP2-associated fluorescence pixels were taken into account if they had an intensity that was twice the background value. Background intensity levels were defined as twice the mean pixel intensity of the nucleus. The total area of the highlighted pixels at the apical pole of the cells was quantified from at least 20 cells. As we have previously described,77 a reduction in total area occupied by AQP2 fluorescence reflects a concentration of the AQP2 protein in the apical pole and apical membrane of the cell caused, in this case, by the action of CT. Between 30 and 60 measurements were taken for each sample, and the samples were taken from at least three different controls and experimental sets of tissue. The results are expressed as the means ± SEM of the pixel area occupied by the fluorescence signal. Statistical analyses were performed using the t test. The differences were considered significant at P values less than 0.05.
Immunogold Labeling of AQP2 at the Plasma Membrane: Effect of CT
Fifty-μm vibratome sections of PLP perfused kidneys were cut and hydrated 5 for minutes in PBS and then incubated 5 minutes in PBS containing 1% SDS. The sections were washed four times in PBS and then blocked for 30 minutes in PBS, 1% BSA. The sections were incubated for 12 hours at 4°C with the AQP2 extracellular domain antibody followed by three washes in PBS and then incubated 12 hours at 4°C with goat-anti-rabbit antibody conjugated to 15-nm gold particles (Ted Pella, Redding, CA; 1.4 μg/ml). After incubation, the sections were washed three times with PBS, rinsed twice with 0.1 M cacodylate buffer, and then fixed in 1% glutaraldehyde diluted in 0.1 M cacodylate buffer. The sections were postfixed in 1% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 M cacodylate buffer, dehydrated in a series of ascending graded alcohols, pre-embedded in alcohol:Epon (1:1) for 12 hours to 100% EPON (Ted Pella), then flat-embedded in 100% EPON on slides and coverslips coated with liquid release agent, and polymerized for 12 hours at 60°C.
The cortical regions of the flat-embedded sections were cut out and re-embedded in Epon in flat molds again for 12 hours at 60°C. Ninety-nm sections were cut on a Reichert (Depew, NY) ultramicrotome and collected on Formvar-coated grids. The cells were stained with 2% uranyl acetate, rinsed in distilled water, and further contrasted with lead citrate for 5 minutes. The samples were examined and photographed at 80 kv with a JEOL (Tokyo, Japan) 1011 electron microscope equipped with an AMT (Danvers, MA) digital camera. Image analysis was performed as described previously81 with ImageJ software (National Institutes of Health, Bethesda, MD).
Electrophoresis and Western Blot Analysis
The effect of 24 hours of CT perfusion on AQP2 expression in the rat kidney cortex was studied by Western blot analysis. The kidneys of anesthetized Brattleboro rats with subcutaneous pumps containing buffer or CT for 24 hours were harvested, and the cortex was separated for analysis. Protein electrophoresis and Western blotting was performed as described previously.77 In brief, approximately 20 mg of cortical proteins were solubilized in radioimmune precipitation assay buffer (Boston Bioproducts, Boston, MA), 4 mM EDTA, and protease inhibitor (Roche Diagnostics), and the lysates were separated on 4 to 12% Bis-Tris-PAGE gel (Invitrogen) and then transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). The presence of AQP2 was detected using a polyclonal rabbit anti-AQP2 antibody (1:500) and revealed using an Amdex goat anti-rabbit IgG-horseradish peroxidase (1:100,000 dilution; Amersham, Little Chalfont, United Kingdom). The proteins were visualized using a Western Lightning chemiluminescence reagent plus system (PerkinElmer Life Sciences, Boston, MA). For reblotting, acid-stripped membranes were incubated with a mouse anti-pan-actin antibody (0.2 μg/ml; Chemicon International, Temecula, CA) and used as loading control. All of the Kodak BioMax XAR films (Fisher Scientific) were scanned, and the band intensities were quantified using IPLab software (BD Biosciences, San Jose, CA).
Total RNA Extraction and Quantitative RT-PCR
The extraction and quantitative RT-PCR was performed as described previously.82In brief, the tissues were homogenized in Ambion TRI reagent, and RNA was isolated following the RiboPure kit protocol (Applied Biosystems, Foster City, CA). Genomic DNA contamination was eliminated using the Ambion DNA-free kit. DNA-free total RNA samples were aliquoted and stored at −80°C. The samples were reverse-transcribed for 1 hour at 42°C in a final volume of 50 μl with 1× buffer II, 5 mM MgCl2, 1.0 mM each dNTP, 1 unit/μl RNase inhibitor, 2.5 μM random hexamers, and 2.5 units/μl murine leukemia virus reverse transcriptase (all RT reagents were from Applied Biosystems). Reverse transcription products were used as templates for quantitative PCR. The sequences of PCR primer sets (synthesized by Invitrogen) are as follows: RC1AR-F1 (GTGGCCCTTGGATACTGAGA) and RC1AR-R1 (TTGTACCAGAGCTGCCTGAA) for the CT receptor; RAQP2-TMF1 (CCCTCTCCATTGGTTTCTCTGT) and RAQP2-TMR1 (GGCTGGATTCATGGAGCAA) for aquaporin 2; and RGAPDH-TMF1 (AGAGAGAGGCCCTCAGTTGCT) and RGAPDH-TMR1 (TGGAATTGTGAGGGAGATGCT) for glyceraldehyde-3-phosphate dehydrogenase. Quantitative PCR analysis was performed with a 7300 Real-Time PCR System (Applied Biosystems). Each reaction was performed in triplicate by using the iTaq SYBR Green Supermix with ROX reagent (Bio-Rad). A dissociation curve was generated after each SYBR Green PCR run to confirm the specificity of the amplification, and relative quantification was performed using the “ΔΔCt” method.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grant PO1DK38452 (DB). R. Bouley received an investigator award from the National Kidney Foundation. P. Nunes was supported by a Doctoral Level Postgraduate Scholarship from the Natural Sciences and Engineering Research Council of Canada, and H. A. J. Lu was supported by NIDDK, National Institutes of Health Grant K08 DK-075940-01. The Microscopy Core facility of the Massachusetts General Hospital Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (grant DK57521) and the Center for the Study of Inflammatory Bowel Disease (grant DK43341). This work had been partially presented at the American Society of Nephrology meeting 2009.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Bichet DG, Razi M, Arthus MF, Lonergan M, Tittley P, Smiley RK, Rock G, Hirsch DJ: Epinephrine and dDAVP administration in patients with congenital nephrogenic diabetes insipidus: Evidence for a pre-cyclic AMP V2 receptor defective mechanism. Kidney Int 36: 859–866, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Canfield MC, Tamarappoo BK, Moses AM, Verkman AS, Holtzman EJ: Identification and characterization of aquaporin-2 water channel mutations causing nephrogenic diabetes insipidus with partial vasopressin response. Hum Mol Genet 6: 1865–1871, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Vargas-Poussou R, Forestier L, Dautzenberg MD, Niaudet P, Dechaux M, Antignac C: Mutations in the vasopressin V2 receptor and aquaporin-2 genes in 12 families with congenital nephrogenic diabetes insipidus. J Am Soc Nephrol 8: 1855–1862, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Brown D: The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA: Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264: 92–95, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Deen PM, Brown D: Trafficking of native and mutant mammalian MIP proteins. In: Aquaporins: Current Topics in Membranes, edited by Hohmann S. NSAAP, New York, Academic Press, 2001, pp 235–276 [Google Scholar]
- 7. Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E: Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 133–157, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Procino G, Mastrofrancesco L, Mira A, Tamma G, Carmosino M, Emma F, Svelto M, Valenti G: Aquaporin 2 and apical calcium-sensing receptor: New players in polyuric disorders associated with hypercalciuria. Semin Nephrol 28: 297–305, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Sasaki S, Kuwahara M, Yamashita Y, Marumo F: Structure and function of AQP2. Nephrol Dial Transplant 15[Suppl 6[: 21–22, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Fushimi K, Sasaki S, Marumo F: Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Katsura T, Gustafson CE, Ausiello DA, Brown D: Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 13. Bouley R, Hasler U, Lu HA, Nunes P, Brown D: Bypassing vasopressin receptor signaling pathways in nephrogenic diabetes insipidus. Semin Nephrol 28: 266–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D: Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouley R, Lin HY, Raychowdhury MK, Marshansky V, Brown D, Ausiello DA: Downregulation of the vasopressin type 2 receptor after vasopressin-induced internalization: Involvement of a lysosomal degradation pathway. Am J Physiol Cell Physiol 288: C1390–C401, 2005 [DOI] [PubMed] [Google Scholar]
- 16. de Rouffignac C, Elalouf JM: Effects of calcitonin on the renal concentrating mechanism. Am J Physiol 245: F506–F511, 1983 [DOI] [PubMed] [Google Scholar]
- 17. de Rouffignac C, Imbert-Teboul M: Effects of antidiuretic hormone on renal reabsorption of electrolytes. Adv Nephrol Necker Hosp 13: 297–317, 1984 [PubMed] [Google Scholar]
- 18. Carney S, Thompson L: Acute effect of calcitonin on rat renal electrolyte transport. Am J Physiol 240: F12–F16, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Copp DH, Cheney B: Calcitonin: A hormone from the parathyroid which lowers the calcium-level of the blood. Nature 193: 381–382, 1962 [DOI] [PubMed] [Google Scholar]
- 20. Hirsch PF, Gauthier GF, Munson PL: Thyroid Hypocalcemic Principle and Recurrent Laryngeal Nerve Injury as Factors Affecting the Response to Parathyroidectomy in Rats. Endocrinology 73: 244–252, 1963 [DOI] [PubMed] [Google Scholar]
- 21. Koszegi T: Immunoluminometric detection of human procalcitonin. J Biochem Biophys Methods 53: 157–164, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Morgenthaler NG, Struck J, Chancerelle Y, Weglohner W, Agay D, Bohuon C, Suarez-Domenech V, Bergmann A, Muller B: Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. Horm Metab Res 35: 290–295, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Evanson JM, Garner A, Holmes AM, Lumb GA, Stanbury SW: Interrelations between thyrocalcitonin and parathyroid hormone in rats. Clin Sci 32: 271–278, 1967 [PubMed] [Google Scholar]
- 24. Gudmundsson TV, MacIntyre I, Soliman HA: The isolation of thyrocalcitonin and a study of its effects in the rat. Proc R Soc Lond B Biol Sci 164: 460–477, 1966 [DOI] [PubMed] [Google Scholar]
- 25. Morii H, DeLuca HF: Relationship between vitamin D deficiency, thyrocalcitonin, and parathyroid hormone. Am J Physiol 213: 358–362, 1967 [DOI] [PubMed] [Google Scholar]
- 26. Munson PL, Hirsch PF: Thyrocalcitonin: newly recognized thyroid hormone concerned with metabolism of bone. Clin Orthop Relat Res 49: 209–232, 1966 [PubMed] [Google Scholar]
- 27. Gorn AH, Lin HY, Yamin M, Auron PE, Flannery MR, Tapp DR, Manning CA, Lodish HF, Krane SM, Goldring SR: Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest 90: 1726–1735, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuestner RE, Elrod RD, Grant FJ, Hagen FS, Kuijper JL, Matthewes SL, O'Hara PJ, Sheppard PO, Stroop SD, Thompson DL, Whitmore TE, Findlay DM, Houssami S, Sexton PM, Moore EE: Cloning and characterization of an abundant subtype of the human calcitonin receptor. Mol Pharmacol 46: 246–255, 1994 [PubMed] [Google Scholar]
- 29. Lin HY, Harris TL, Flannery MS, Aruffo A, Kaji EH, Gorn A, Kolakowski LF, Jr, Lodish HF, Goldring SR: Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science 254: 1022–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Nussenzveig DR, Thaw CN, Gershengorn MC: Inhibition of inositol phosphate second messenger formation by intracellular loop one of a human calcitonin receptor: Expression and mutational analysis of synthetic receptor genes. J Biol Chem 269: 28123–28129, 1994 [PubMed] [Google Scholar]
- 31. Blower PJ, Puncher MR, Kettle AG, George S, Dorsch S, Leak A, Naylor LH, O'Doherty MJ: Iodine-123 salmon calcitonin, an imaging agent for calcitonin receptors: synthesis, biodistribution, metabolism and dosimetry in humans. Eur J Nucl Med 25: 101–108, 1998 [DOI] [PubMed] [Google Scholar]
- 32. de Luise M, Martin TJ, Greenberg PB, Michelangeli V: Metabolism of porcine, human and salmon calcitonin in the rat. J Endocrinol 53: 475–482, 1972 [DOI] [PubMed] [Google Scholar]
- 33. Marx SJ, Woodward C, Aurbach GD, Glossmann H, Keutmann HT: Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem 248: 4797–4802, 1973 [PubMed] [Google Scholar]
- 34. Newsome FE, O'Dor RK, Parkes CO, Copp DH: A study of the stability of calcitonin biological activity. Endocrinology 92: 1102–1106, 1973 [DOI] [PubMed] [Google Scholar]
- 35. Scarpace PJ, Neuman WF, Raisz LG: Metabolism of radioiodinated salmon calcitonin in rats. Endocrinology 100: 1260–1267, 1977 [DOI] [PubMed] [Google Scholar]
- 36. Scarpace PJ, Parthemore JG, Deftos LJ: The distribution of biologically active and inactive radioiodinated human calcitonin in the rat. Endocrinology 103: 128–132, 1978 [DOI] [PubMed] [Google Scholar]
- 37. Chai SY, Christopoulos G, Cooper ME, Sexton PM: Characterization of binding sites for amylin, calcitonin, and CGRP in primate kidney. Am J Physiol 274: F51–F62, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Morel F: Sites of hormone action in the mammalian nephron. Am J Physiol 240: F159–F164, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Chabardes D, Gagnan-Brunette M, Imbert-Teboul M, Gontcharevskaia O, Montegut M, Clique A, Morel F: Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest 65: 439–448, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chabardes D, Imbert-Teboul M, Montegut M, Clique A, Morel F: Distribution of calcitonin-sensitive adenylate cyclase activity along the rabbit kidney tubule. Proc Natl Acad Sci U S A 73: 3608–3612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morel F, Chabardes D, Imbert-Teboul M, Le Bouffant F, Hus-Citharel A, Montegut M: Multiple hormonal control of adenylate cyclase in distal segments of the rat kidney. Kidney Int Suppl 11: S55–S62, 1982 [PubMed] [Google Scholar]
- 42. Sexton PM, Adam WR, Moseley JM, Martin TJ, Mendelsohn FA: Localization and characterization of renal calcitonin receptors by in vitro autoradiography. Kidney Int 32: 862–868, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Firsov D, Bellanger AC, Marsy S, Elalouf JM: Quantitative RT-PCR analysis of calcitonin receptor mRNAs in the rat nephron. Am J Physiol 269: F702–F709, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Dayer JM, Vassalli JD, Bobbitt JL, Hull RN, Reich E, Krane SM: Calcitonin stimulates plasminogen activator in porcine renal tubular cells: LLC-PK1. J Cell Biol 91: 195–200, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jans DA, Resink TJ, Wilson LE, Reich E, Hemmings BA: Isolation of a mutant LLC-PK1 cell line defective in hormonal responsiveness: A pleiotropic lesion in receptor function. Eur J Biochem 160: 407–412, 1986 [DOI] [PubMed] [Google Scholar]
- 46. Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D: A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol 295: C1476–C1487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edwards BR, LaRochelle FT, Jr, Gellai M: Concentration of urine by dehydrated Brattleboro homozygotes: Is there a role for oxytocin? Ann N Y Acad Sci 394: 497–502, 1982 [DOI] [PubMed] [Google Scholar]
- 48. Laycock JF, Lee J, Lewis AF: The effect of chlorpropamide on water balance in pitressin-treated Brattleboro rats. Br J Pharmacol 52: 253–263, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Valtin H, Schroeder HA: Familial Hypothalamic Diabetes Insipidus in Rats (Brattleboro Strain). Am J Physiol 206: 425–430, 1964 [DOI] [PubMed] [Google Scholar]
- 50. Forsling ML, Judah JM, Windle RJ: The effect of vasopressin and oxytocin on glomerular filtration rate in the conscious rat: contribution to the natriuretic response. J Endocrinol 141: 59–67, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Gellai M, Silverstein JH, Hwang JC, LaRochelle FT, Jr, Valtin H: Influence of vasopressin on renal hemodynamics in conscious Brattleboro rats. Am J Physiol 246: F819–F827, 1984 [DOI] [PubMed] [Google Scholar]
- 52. Brown D, Hasler U, Nunes P, Bouley R, Lu HA: Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moeller HB, MacAulay N, Knepper MA, Fenton RA: Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: Evidence against channel gating. Am J Physiol Renal Physiol 296: F649–F657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nickols HH, Shah VN, Chazin WJ, Limbird LE: Calmodulin interacts with the V2 vasopressin receptor: Elimination of binding to the C terminus also eliminates arginine vasopressin-stimulated elevation of intracellular calcium. J Biol Chem 279: 46969–46980, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA: Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct: Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Siga E, Champigneulle A, Imbert-Teboul M: cAMP-dependent effects of vasopressin and calcitonin on cytosolic calcium in rat CCD. Am J Physiol 267: F354–F365, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Siga E, Mandon B, Roinel N, de Rouffignac C: Effects of calcitonin on function of intercalated cells of rat cortical collecting duct. Am J Physiol 264: F221–F227, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Hozawa S, Holtzman EJ, Ausiello DA: cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol 270: C1695–C702, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F: Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Yasui M, Zelenin SM, Celsi G, Aperia A: Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol 272: F443–F450, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Yano Y, Cesar KR, Araujo M, Rodrigues AC, Jr, Andrade LC, Magaldi AJ: Aquaporin 2 expression increased by glucagon in normal rat inner medullary collecting ducts. Am J Physiol Renal Physiol 296: F54–F59, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Chesnut CH, 3rd, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L: Salmon calcitonin: A review of current and future therapeutic indications. Osteoporos Int 19: 479–491, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Keeler R, Walker V, Copp DH: Natriuretic and diuretic effects of salmon calcitonin in rats. Can J Physiol Pharmacol 48: 838–841, 1970 [DOI] [PubMed] [Google Scholar]
- 64. Twery MJ, Obie JF, Cooper CW: Ability of calcitonins to alter food and water consumption in the rat. Peptides 3: 749–755, 1982 [DOI] [PubMed] [Google Scholar]
- 65. Del Prete E, Schade B, Riediger T, Lutz TA, Scharrer E: Effects of amylin and salmon calcitonin on feeding and drinking behavior in pygmy goats. Physiol Behav 75: 593–599, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Plata-Salaman CR, Oomura Y: Calcitonin effect on the dipsogenic response to intra-cerebroventricular administration of angiotensin II. Physiol Behav 40: 515–521, 1987 [DOI] [PubMed] [Google Scholar]
- 67. Riediger T, Schmid HA, Young AA, Simon E: Pharmacological characterisation of amylin-related peptides activating subfornical organ neurones. Brain Res 837: 161–168, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Otsubo H, Hyodo S, Hashimoto H, Kawasaki M, Suzuki H, Saito T, Ohbuchi T, Yokoyama T, Fujihara H, Matsumoto T, Takei Y, Ueta Y: Centrally administered adrenomedullin 5 activates oxytocin-secreting neurons in the hypothalamus and elevates plasma oxytocin level in rats. J Endocrinol 202: 237–247, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Serino R, Ueta Y, Hara Y, Nomura M, Yamamoto Y, Shibuya I, Hattori Y, Kitamura K, Kangawa K, Russell JA, Yamashita H: Centrally administered adrenomedullin increases plasma oxytocin level with induction of c-fos messenger ribonucleic acid in the paraventricular and supraoptic nuclei of the rat. Endocrinology 140: 2334–2342, 1999 [DOI] [PubMed] [Google Scholar]
- 70. Ueta Y, Serino R, Shibuya I, Kitamura K, Kangawa K, Russell JA, Yamashita H: A physiological role for adrenomedullin in rats: A potent hypotensive peptide in the hypothalamo-neurohypophysial system. Exp Physiol 85: 163S–169S, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Arletti R, Benelli A, Bertolini A: Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48: 825–830, 1990 [DOI] [PubMed] [Google Scholar]
- 72. Fitts DA, Thornton SN, Ruhf AA, Zierath DK, Johnson AK, Thunhorst RL: Effects of central oxytocin receptor blockade on water and saline intake, mean arterial pressure, and c-Fos expression in rats. Am J Physiol Regul Integr Comp Physiol 285: R1331–R1339, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Edwards BR, LaRochelle FT, Jr: Antidiuretic effect of endogenous oxytocin in dehydrated Brattleboro homozygous rats. Am J Physiol 247: F453–F465, 1984 [DOI] [PubMed] [Google Scholar]
- 74. Lencer WI, Brown D, Ausiello DA, Verkman AS: Endocytosis of water channels in rat kidney: Cell specificity and correlation with in vivo antidiuresis. Am J Physiol 259: C920–C932, 1990 [DOI] [PubMed] [Google Scholar]
- 75. Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW: Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol 19: 225–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bouley R, Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D: Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in “endocytosis-resistant” membrane domains after vasopressin treatment. Biol Cell 98: 215–232, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D: Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Bouley R, Sun T-X, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA: Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol 285: C750–C762, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Evan GI, Lewis GK, Ramsay G, Bishop JM: Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol 5: 3610–3616, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gustafson CE, Levine S, Katsura T, McLaughlin M, Aleixo MD, Tamarappoo BK, Verkman AS, Brown D: Vasopressin regulated trafficking of a green fluorescent protein-aquaporin 2 chimera in LLC-PK1 cells. Histochem Cell Biol 110: 377–386, 1998 [DOI] [PubMed] [Google Scholar]
- 81. Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D: cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Da Silva N, Silberstein C, Beaulieu V, Pietrement C, Van Hoek AN, Brown D, Breton S: Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod 74: 427–438, 2006 [DOI] [PubMed] [Google Scholar]