Abstract
Disruption of the dopamine D5 receptor gene in mice increases BP and causes salt sensitivity. To determine the role of renal versus extrarenal D5 receptors in BP regulation, we performed cross-renal transplantation experiments. BP was similar between wild-type mice and wild-type mice transplanted with wild-type kidneys, indicating that the transplantation procedure did not affect BP. BP was lower among D5−/− mice transplanted with wild-type kidneys than D5−/− kidneys, demonstrating that the renal D5 receptors are important in BP control. BP was higher in wild-type mice transplanted with D5−/− kidneys than wild-type kidneys but not significantly different from syngenic transplanted D5−/− mice, indicating the importance of the kidney in the development of hypertension. On a high-salt diet, all mice with D5−/− kidneys excreted less sodium than mice with wild-type kidneys. Transplantation of a wild-type kidney into a D5−/− mouse decreased the renal expression of AT1 receptors and Nox-2. Conversely, transplantation of a D5−/− kidney into a wild-type mouse increased the expression of both, suggesting that both renal and extrarenal factors are important in the regulation of AT1 receptor and Nox-2 expression. These results highlight the role of renal D5 receptors in BP homeostasis and the pathogenesis of hypertension.
Dopamine is an important regulator of systemic BP.1–3 In the kidney it regulates fluid and electrolyte balance by its actions on hemodynamics and epithelial transport and by regulation of hormones and humoral agents.1–2,4–6 Dopamine also controls BP by actions on neuronal cardiovascular centers and the heart, as well as arterial and venous vessels,1–4 and modulates fluid and sodium intake via “appetite” centers in the brain and via gastrointestinal transport.7,8
Dopamine is produced locally in the kidney, independent of innervation, and its actions are exerted through five subtypes of receptors: the D1-like receptors comprised of the D1 (D1R) and D5 (D5R) receptor subtypes and the D2-like receptors comprised of the D2, D3, and D4 receptor subtypes.1–3 Renal dopamine receptors are important in the regulation of NaCl transport in almost all segments of the nephron1–3 and are responsible for more than 50% of incremental sodium excretion when NaCl intake is increased.9–11
The D5R has a higher affinity for dopamine than the DlR and is constitutively active.12,13 In the kidney D5R is expressed in proximal and distal tubules and tunica media of arterioles14,15 and together with the D1 receptor may mediate the diuretic and natriuretic effects of D1-like receptors. However, the role of renal D5R in the regulation of BP is not completely understood because of the lack of drugs that selectively stimulate or antagonize this receptor.1–3
We reported that disruption of the D5R in mice resulted in elevated systolic, diastolic, and mean BPs, as well as heart weights. The increased BP in these mice, measured under anesthesia, appears to be, in part, related to increased sympathetic tone primarily attributable to the central nervous system.16 However, further studies suggested that the kidney may play a significant role in the hypertension of D5−/− mice. A high salt diet increases BP further in D5−/− mice, indicating that renal D5Rs are important in the control of BP via regulation of sodium transport.17 The renal expression of angiotensin type I receptor (AT1R) is increased in D5−/− mice relative to D5+/+ littermates,18,19 and chronic intraperitoneal administration of the AT1R antagonist losartan normalizes BP in pentobarbital-anesthetized D5−/− mice but minimally affects BP in D5+/+ littermates.19 Renal and brain reactive oxygen species and oxidative stress are increased in D5−/− mice.17
To determine the role of renal D5R in the regulation of BP, we performed cross-transplantation studies in D5−/− and wild-type mice in which one kidney of a D5−/− mouse was transplanted into a bilaterally nephrectomized wild-type mouse or one kidney of a wild-type mouse was transplanted into a bilaterally nephrectomized D5−/− mouse. Syngenic transplants (wild-type kidney to wild-type mouse and D5−/− kidney to D5−/− mice) were also performed. We studied the effects of renal cross-transplantation on BP on normal and high salt diet and determined the renal expression of D1R and AT1R and NADPH oxidase isoform 2 (Nox-2) and nitro-tyrosine.
RESULTS
BP in Unmanipulated D5−/− Mice and Wild-type Littermates
Systolic and diastolic BPs measured under anesthesia were significantly higher in unmanipulated D5−/− mice than in unmanipulated D5 wild-type littermates (systolic: 124 ± 2 versus 97 ± 3 mmHg; diastolic: 93 ± 4 versus 70 ± 3 mmHg). These results are consistent with our previous studies in anesthetized D5−/− mice.16,17,19
BP in Transplanted Mice
Four groups of mice were generated from the cross-transplantation procedure between genetically matched wild-type (D5+/+) and D5−/− mice. The mice were genotyped for the presence of the wild-type D5R or the D5R knockout (D5−/−) gene that is truncated in the second extracellular loop, resulting in the absence of D5R function.16 Wild-type mice transplanted with wild-type kidneys expressed the wild-type D5R in both renal and nonrenal tissues (WT-WT). Wild-type mice transplanted with a kidney from a D5−/− mouse expressed wild-type D5R only in nonrenal tissues (KO-WT). D5−/− mice transplanted with a wild-type kidney expressed the wild-type D5R only in the kidney (WT-KO). D5−/− mice transplanted with a kidney from D5−/− mouse did not express the wild-type D5R in any tissue (KO-KO).
Cross-transplantation of a kidney of a wild-type mouse into a bilaterally nephrectomized wild-type mouse did not affect either systolic or diastolic BP (Figure 1) or heart rate (Supplemental Table 1). Both systolic and diastolic BPs were similar to those in unmanipulated mice (systolic: 98 ± 2 versus 97 ± 2; diastolic: 69 ± 4 versus 70 ± 3). This indicates that the transplantation and its consequences (e.g. renal denervation) do not affect BP.
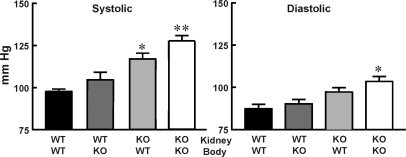
Figure 1.
Transplantation of D5−/− kidneys into wild-type mice increases BP. BP was measured as described under Concise Methods in wild-type mice transplanted with wild-type kidneys (WT-WT; n = 5), D5−/− mice transplanted with wild-type kidneys (WT-KO; n = 6), wild-type mice transplanted with D5−/− kidneys (KO-WT; n = 9), and D5−/− mice transplanted with D5−/− kidneys (KO-KO; n = 4). **P < 0.01 versus WT-WT and WT-KO; *P < 0.05 versus WT-WT and WT-KO. One-way ANOVA followed by Student-Newman-Keul's test were used.
Cross-transplantation of a kidney of a wild-type mouse into a D5−/− mouse did not affect heart rate (Supplemental Table 1) but decreased BP when compared with D5−/− mice transplanted with a syngenic kidney (systolic: 105 ± 5 versus 128 ± 2 mmHg; diastolic: 73 ± 5 versus 94 ± 5 mmHg) (Figure 1) or unmanipulated D5−/− mice. These values were slightly higher but not significantly different from D5+/+ mice transplanted with a syngenic kidney (Figure 1). This shows that D5Rs in nonrenal tissues do not play a major role in the regulation of BP and that renal mechanisms are the main determining factors of chronic BP levels.
Cross-transplantation of a kidney of a D5−/− mouse into a wild-type mouse increased BP (systolic: 117 ± 3 versus 98 ± 2 mmHg; diastolic: 85 ± 4 versus 69 ± 4 mmHg) so that systolic BP in the transplanted mice was no longer different from that in syngenic transplanted D5−/− (Figure 2) or unmanipulated D5−/− mice. Heart rate was not affected (Supplemental Table 1). This indicates that renal D5Rs are important in the regulation of BP and that intact D5Rs in nonrenal tissues do not compensate for their absence in the kidney.
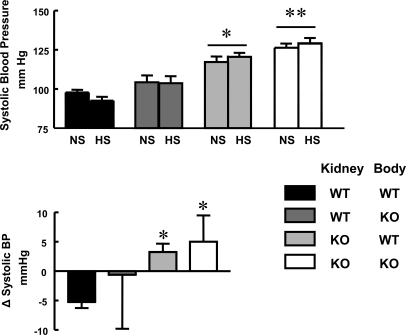
Figure 2.
High-salt diet increases systolic BP in mice with D5−/− kidneys. (Top panel) Systolic BP on normal (0.75% NaCl) and high sodium (6% NaCl) diets. The groups are as described for Figure 1. **P < 0.01 versus WT-WT and WT-KO; *P < 0.05 versus WT-WT and WT-KO. Two-way ANOVA followed by Student-Newman-Keul's test were used. (Bottom panel) BP change from normal to high salt diets in transplanted mice. The groups are as described above. *P < 0.05 versus WT-WT. A Kruskal-Wallis test followed by Dunn's test were used.
Cross-transplantation of a kidney of a D5−/− mouse into a D5−/− mouse also did not affect BP (systolic: 128 ± 2 versus 124 ± 2 mmHg; diastolic: 94 ± 5 versus 93 ± 4 mmHg) (Figure 1) or heart rate when compared with those in unmanipulated D5−/− mice (Supplemental Table 1). This indicates that renal innervation is not involved in the mechanisms by which the absence of D5Rs increases BP.
There was no cardiac hypertrophy in the wild-type mice transplanted with D5−/− kidneys, as judged by heart weights. Both groups of wild-type mice transplanted with wild-type or D5−/− kidneys had the same heart weight (WT-WT: 140 ± 7.5 mg; D5-WT: 140 ± 6.7 mg), whereas both groups of D5−/− mice had higher heart weights (WT-D5: 161 ± 4.8 mg; D5-D5: 162 ± 16.2 mg). However, mice were transplanted for only 2 weeks. This period may not be long enough for the mice to develop significant hypertrophy.17
The functional and anatomical viability of the transplant was assessed. Serum creatinines were similar in all groups (Table 1). Histologic study of renal sections showed no gross abnormalities in any of the transplanted kidneys and no evidence of ischemic injury (Supplemental Figure 1).
Table 1.
Systolic and diastolic blood pressures, serum, and urinary sodium and creatinine concentrations in cross renal-transplanted mice on high NaCl diet (6%) for 1 week
| WT-WT | WT-KO | KO-WT | KO-KO | |
|---|---|---|---|---|
| Systolic blood pressure | 92 ± 5 | 104 ± 5 | 120 ± 2a | 129 ± 3b |
| Diastolic blood pressure | 59 ± 2 | 76 ± 8 | 85 ± 3 | 98 ± 4a |
| Serum Na+ (mmol/L) | 154 ± 4 | 155 ± 6 | 147 ± 2 | 146 ± 2 |
| Urinary Na+ (mmol/L) | 271 ± 46 | 237 ± 20 | 273 ± 34 | 270 ± 14 |
| Serum creatinine (mg/dl) | 0.35 ± 0.10 | 0.67 ± 0.08 | 0.40 ± 0.12 | 0.66 ± 0.15 |
| Urinary creatinine (mg/ml) | 0.09 ± 0.03 | 0.07 ± 0.02 | 0.19 ± 0.03 | 0.13 ± 0.04 |
Wild-type mice transplanted with wild-type kidneys (WT-WT; n = 5), D5−/− mice transplanted with wild-type kidneys (WT-KO; n = 6), wild-type mice transplanted with D5−/− kidneys (KO-WT; n = 9), and D5−/− mice transplanted with D5−/− kidneys (KO-KO; n = 4) were used.
aP < 0.05 versus WT-WT and WT-KO. One-way ANOVA and Student-Newman-Keul's test.
bP < 0.01 versus WT-WT and WT-KO.
Effect of Salt Loading on BP and Sodium Excretion in Transplanted Mice
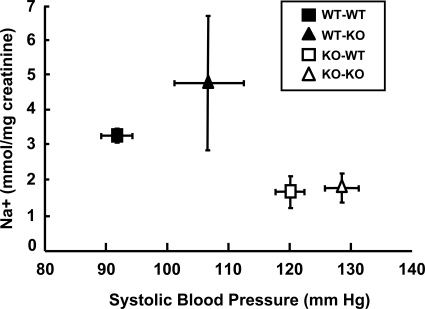
Salt loading did not induce any significant change in absolute BP levels in any of the groups. We have reported that BP in D5−/− mice increases after dietary salt loading.17 However, neither systolic nor diastolic BP increased on high salt diet in either syngenic transplanted D5−/− or wild-type mice transplanted a D5−/− kidney (Figure 2, top panel, and Table 1). In contrast to the apparent absence of an effect of the high salt diet on absolute BP levels, the directional change, i.e. an increase in systolic BP with high salt diet, was significantly different in mice transplanted with D5−/− kidneys, either syngenic or nonsyngenic, than in wild-type mice transplanted with syngenic kidneys (Figure 2, bottom panel). A plot of the relationship between BP and sodium excretion was shifted to the right in mice with D5−/− kidneys, indicating that in these mice higher BPs are necessary to excrete comparatively less sodium (Figure 3).
Figure 3.
The relationship between BP and sodium excretion is shifted to the right in mice with D5−/− kidneys. The groups are as described for Figure 1.
Renal Expression of D1 and AT1 Receptors, Nox-2 and Nitro-tyrosine
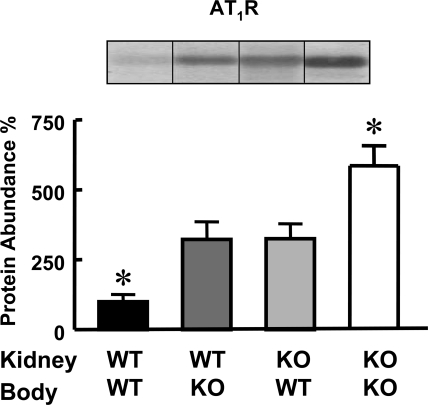
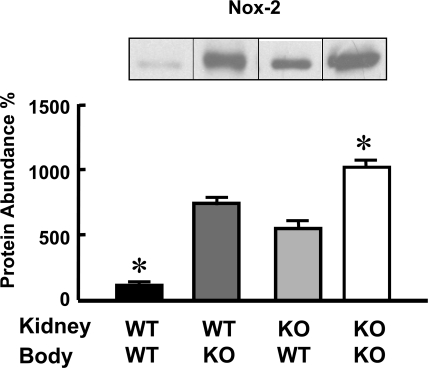
The renal expression of D1Rs was similar in all groups (WT-WT: 100 ± 24; WT-KO: 108 ± 28; KO-WT: 68 ± 20; KO-KO: 96 ± 12 expressed as percentages of WT-WT and corrected for protein loading). The expression of AT1Rs was highest in D5−/− mice with syngenic transplanted kidneys and lowest in wild-type mice with syngenic transplanted kidneys. As mentioned previously we have already reported that renal expression of AT1Rs is increased in D5−/− mice.18,19 Surprisingly nonsyngenic cross-transplanted D5−/− kidneys expressed approximately 50% fewer AT1Rs than kidneys that were syngenic transplanted. Conversely nonsyngenic cross-transplanted wild-type kidneys expressed twice as many AT1Rs relative to those that were syngenic transplanted (Figure 4). The renal expression of Nox-2 showed a pattern similar to that of AT1Rs. It was lowest in syngenic transplanted wild-type mice and highest in syngenic transplanted D5−/− mice. This is in agreement with our previous report showing that renal and brain Nox-2 expression is increased in D5−/− mice.17 However, Nox-2 expression in the kidneys of D5−/− mice transplanted into wild-type mice was lower than in syngenic transplanted D5−/− mice, and its expression in kidneys of wild-type mice transplanted into D5−/− mice was higher than in syngenic transplanted wild-type mice (Figure 5).
Figure 4.
Nonsyngenic transplantation alters renal AT1 expression. Quantification of the immunoblots for AT1 receptors (54-kD band) and Nox-2 (91-kD band) in kidney homogenates of transplanted mice. The groups are as described for Figure 1. The inset shows one immunoblot per group. The values were corrected for protein loading; amounts of protein on the loading gel before and after membrane transfer were quantified. The data are the means ± SEM. *P < 0.05 versus all others. One-way ANOVA followed by Student-Newman-Keul's test were used.
Figure 5.
Nonsyngenic transplantation alters renal Nox2 expression. Quantification of the immunoblots for Nox-2 (91-kD band) in kidney homogenates of transplanted mice. The groups are as described for Figure 1. The inset shows one immunoblot per group. The values were corrected for protein loading; the amounts of protein on the loading gel before and after membrane transfer were quantified. The data are the means ± SEM. *P < 0.05 versus all others. One-way ANOVA followed by Student-Newman-Keul's test were used.
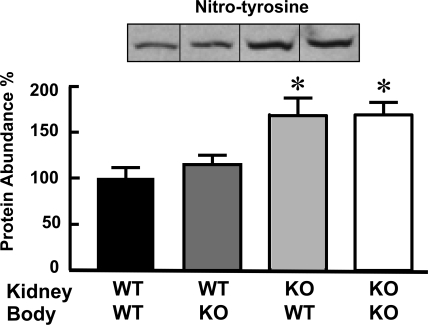
The presence of nitro-tyrosine on proteins, a marker for peroxynitrite formation in vivo, was also determined. Nitro-tyrosine expression was lower in syngenic or congenic transplanted wild-type kidneys than in D5−/− kidneys transplanted into either D5−/− or wild-type mice (Figure 6).
Figure 6.
Renal expression of nitro-tyrosine is not modified by transplantation. The groups are as described for Figure 1. The inset shows one immunoblot per group. The values were corrected for protein loading; the amounts of protein on the loading gel before and after membrane transfer were quantified. The data are the means ± SEM. *P < 0.05 versus all others. One-way ANOVA followed by Student-Newman-Keul's test were used.
DISCUSSION
Our cross-transplantation studies demonstrate an important role for renal D5Rs in the regulation of BP. This is supported by the finding of increased systolic and diastolic BPs when a kidney lacking D5Rs is transplanted into a wild-type mouse and a decrease in BP when a kidney from a wild-type mouse is transplanted into a mouse lacking D5Rs. The rodent kidney expresses D5R in proximal and distal convoluted tubules, cortical collecting ducts, medullary ascending limbs of Henle, and arterioles, but not in the glomeruli, juxtaglomerular cells, or macula densa.20–22 The thick ascending limb of Henle and the cortical collecting duct preferentially express the D5R over the D1R.20–22 Stimulation of D1-like receptors induces diuresis and natriuresis in all of the mammalian species studied, including rats and mice.1–3,6,9–11,23–25 Similarly to the D1R, the D5R also increases cAMP production,26,27 which mediates, in part, the inhibition of renal sodium transport27,28 by decreasing the activities of NHE3, Na+/Pi2, Cl−/HCO3, and Na+/K+ATPase.28–32 Thus, wild-type mice transplanted with kidneys lacking the D5R may have increased renal sodium reabsorption because of a lack of the inhibitory effects of the constitutively active D5R13 on tubular sodium transport. In fact, on a high salt diet, wild-type mice transplanted with a kidney lacking D5Rs or syngenic transplanted D5−/− mice excrete comparatively less sodium than syngenic transplanted wild-type mice. Our data also show that renal D1R cannot compensate for the lack of D5Rs, suggesting that renal D1R and D5R functions are not redundant.16
The D5R may also affect renal tubular sodium reabsorption by interacting with AT1R.33,34 Previous studies have shown that inhibition of renal angiotensin II production or blockade of AT1Rs increases the inhibitory effect of the D1-like receptor agonist fenoldopam on sodium transport.35–38 We have shown that the high BP of D5−/− mice is associated with increased renal AT1R protein and is normalized by AT1R blockade.18,19 This indicates that in basal conditions the constitutively active D5Rs13 can decrease AT1R expression. Furthermore, activation of the D5R decreases the AT1R protein level by increasing AT1R degradation via an ubiquitin/proteasome pathway.19,26 However, renal AT1Rs are lower in D5−/− kidneys transplanted into wild-type mice than in syngenic transplanted D5−/− mice. Conversely, wild-type kidneys transplanted into D5−/− mice express more AT1Rs than syngenic transplanted D5+/+. These suggest that extrarenal factors other than D5R are also involved in the regulation of the renal expression of AT1Rs.
We have reported that the generation of ROS is increased in D5−/− mice. The expression of NADPH oxidase activity and proteins (Nox-2 and p47phox) in the brain and kidney of D5−/− mice is increased, as well as plasma thiobarbituric acid reactive substances, an index of systemic oxidative stress.17 In the transplanted kidneys the pattern of expression of nitro-tyrosine was somewhat different from that of Nox-2. This may indicate the presence of other sources of oxidative stress in the kidneys of D5−/− mice. Oxidative stress and angiotensin II signaling regulate each other by multiple mechanisms, and oxidative stress induces upregulation of AT1Rs in several tissues.39–41 Thus, it is possible that increased systemic oxidative stress in D5−/− mice may increase AT1R expression in the transplanted wild-type kidney. Similarly, increased renal AT1Rs in D5−/− mice may be, in part, caused by increased systemic oxidative stress; thus, transplanting a D5−/− kidney into a wild-type mouse that does not have increased systemic oxidative stress would result in a decrease in the expression of renal AT1Rs.
The diastolic BP was lower and the systolic BP tended to be lower in wild-type mice transplanted with D5−/− kidneys than in syngenic transplanted D5−/− mice. The decreased renal AT1R expression in nonsyngenic transplanted kidneys may be responsible for this effect. Conversely, D5−/− mice transplanted with wild-type kidneys tended to have higher BP levels than syngenic transplanted wild-type mice and showed increased renal AT1R, which may be responsible for the slightly elevated BP. However, the BP levels cannot be completely explained by the changes in the expression of Nox-2 and AT1R, indicating that other D5R actions are just as important, i.e. regulation of sodium transport. Indeed, the pressure-natriuresis plot is shifted to the right in mice with kidneys that lack D5Rs. Regardless of the possible mechanisms by which the absence of the D5R increases BP, our studies show the pre-eminence of the kidney in the long-term regulation of BP. The important role of the kidney in the long-term regulation of BP using cross-transplantation experiments was reported by Crowley et al.42 They showed that the renal expression is more important than the extrarenal expression of the AT1R in the regulation of BP.
We have reported that D5Rs in nonrenal tissues participate in the short-term regulation of BP.16 However, these studies indicate that D5Rs in nonrenal tissues do not seem to have a prominent role in the long-term regulation of BP. In this study BP was measured under isoflurane anesthesia. In mice, isoflurane produces fewer systemic hemodynamic effects than pentobarbital anesthetics43 but may induce a reduction in centrally generated sympathetic activity.44 If this were the case the contribution of D5Rs other than in the kidney may be underestimated. However, this should similarly affect D5−/− mice syngenic or congenic transplanted and wild-type mice transplanted with wild-type or D5−/− kidneys, making unlikely a significant underestimation of the effect of nonrenal D5Rs on BP regulation. In the cardiovascular system D5Rs are expressed in smooth muscle of the tunica media of pial, pulmonary, coronary, and mesenteric artery branches,45–48 and in vivo administration or in vitro application of D1-like receptor agonists induces vasodilation in the cerebral, coronary, and mesenteric vascular beds, reduces vascular resistance, and causes hypotension. Dopamine and its analogs, acting via D1-like receptors, are coronary vasodilators in animals and humans. The vasodilation of coronary arteries, mediated by D5Rs, is attributed, in part, to activation of hyperpolarizing vasorelaxant potassium channels via cAMP/protein kinase G.46,49 It is possible that the lack of the D5R-induced vasodilation is compensated by other vasodilatory systems that may include increased D1R function, although in other organs like brain and kidney, D1R function has not been shown to compensate for the lack of D5R.16,50,51 In our studies, D1R expression is not altered by the absence of the D5R in renal and nonrenal areas.
We have reported that D5−/− mice have a greater reduction in mean arterial pressure after acute adrenalectomy or acute α-adrenergic blockade compared with D5+/+ mice, suggesting that increased sympathetic activity ascribed to central nervous system mechanisms may be involved in the hypertension of D5−/− mice,16 although plasma and urinary catecholamines were normal in these mice.19 Our cross-transplantation studies demonstrate that increased sympathetic activity does not play a major role in the chronic regulation of BP, such as the BP increase in D5−/− mice because wild-type mice with D5−/− kidneys that should have normal sympathetic activity have BP levels that are indistinguishable from syngenic transplanted D5−/− animals, and D5−/− mice with wild-type kidneys that should have increased sympathetic activity have BP levels similar to syngenic transplanted wild-type mice.
Renal sympathetic innervation may be a major contributor to the increase in BP brought about by high salt intake because renal nerves can modulate sodium handling.52 In salt-sensitive hypertension, increased salt intake results in increased renal sympathetic nerve activity via actions in the central nervous system leading to increased renal sodium retention and increased BP.53,54 Sympathetic innervation is impaired by the surgical procedure in the transplanted kidney.55 This may explain why salt sensitivity is lost in syngenic transplanted D5−/− mice and in wild-type mice transplanted with a D5−/− kidney that would be expected to be salt-sensitive and indicate the need for renal nerves to impart salt sensitivity.53 Nevertheless other renal mechanisms may be involved in the salt sensitivity of D5−/− mice because although not significantly, both groups of mice with D5−/− kidneys have a tendency to higher BP values on high salt diet.
The locus of DRD5, 4p15.1 to 16.1,56,57 and its pseudogenes, 1q21.1 and 2p11.1-p11.2,58 have been linked to human essential hypertension.56,57,59,60 Moreover, humans have single nucleotide polymorphisms in the DRD5 gene with diminished D5R function and abnormal coupling to adenylyl cyclase.61 Our results indicate that diminished renal D5R function may increase the susceptibility to hypertension. Genetic testing for polymorphisms associated with decreased function may be developed and applied for personalized treatment of hypertension.
CONCISE METHODS
Mice
Wild-type and D5−/− mice were bred at the National Institute of Health. The generation of the mouse model is described in the Supplemental Methods. We studied male knockout mice and their wild-type littermates that were at least 8 weeks old. The mice were genotyped using a PCR-based protocol.16 Mouse genomic DNA was isolated from tail biopsies and renal tissue using standard methods.
Mouse Kidney Transplantation
The mice were uninephrectomized 1 week before the transplantation procedure. The remaining native kidney was removed 1 week later. All of the experiments were started 1 week after the last surgery. Creatinine clearances in all of the groups of mice were similar to those of unmanipulated and uninephrectomized control mice. The detailed transplantation procedure is described in the Supplemental Methods.
BP Measurements on Normal and High Salt Diet
The mice were allowed to fully recover and acclimatize for a week, during which they were fed a normal salt diet (0.75% NaCl). At the end of the week, BP levels under isoflurane anesthesia were measured by cannulation of the femoral artery (PE-50 with tip heat stretched to 180 μm). The catheter was advanced to the aorta and then connected to a BP detection equipment (Cardiomax II).17 After a day of full recovery from the femoral arterial cannulation, the normal rodent chow feed was replaced with a high salt diet (6.0% NaCl) for 1 week. The mice were then anesthetized, and the BP was taken as described above through the cannulation of the other femoral artery. A urine sample was collected from the bladder by paracentesis, and blood was collected for measurement of serum and urinary electrolytes and creatinine. The mice were then sacrificed by an overdose of pentobarbital (100 mg/kg body wt), after which the kidney and other organs were collected, flash frozen in isopentane over dry ice, and stored in at −80°C until assayed. BP was also measured in a group of unmanipulated D5−/− mice and D5 wild-type littermates, as described above.
Immunoblotting
Mouse kidney homogenates were subjected to immunoblotting, as previously reported.17–19 The primary antibodies used were rabbit polyclonal directed against the D1R that was generated, affinity purified, and characterized in our laboratory,62 rabbit polyclonal against AT1R (Santa Cruz Biotechnology, Santa Cruz, California), mouse monoclonal anti-Nox-2 (a kind gift of Dr. M. T. Quinn, Department of Veterinary Molecular Biology, Montana State University, Bozeman, Montana), and rabbit polyclonal nitro-tyrosine (Abcam, Cambridge, Massachusetts). The densitometry values were corrected for protein loading (amounts of protein on the loading gel before and after membrane transfers were quantified) and expressed as the mean densities of the syngenic transplanted wild-type mice.
Histopathological Evaluation of the Kidney
Fixed kidney tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. All of the tissues were examined by a pathologist without knowledge of the experimental groups.
Statistical Analyses
The data are the means ± SEM, except as indicated. Comparison between two groups was performed using a t test. Statistical differences among the four groups were performed using one-way ANOVA followed by post hoc analysis using the Student-Newman-Keul's multiple comparison test. Comparisons between normal and high salt diet were performed using two-way repeated-measure ANOVA followed by Student-Newman-Keul's multiple comparison test. Comparisons of the change in systolic BP from normal to high salt were done using a Kuskal-Wallis test followed by a Dunn multiple comparison test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (HL068686 HL023081, HL074940, HL092196, and DK039308).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA: Functional genomics of the dopaminergic system in hypertension. Physiol Genomics 19: 233–246, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Banday AA, Lokhandwala MF: Dopamine receptors and hypertension. Curr Hypertens Rep 10: 268–275, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Aperia A: Intrarenal dopamine: A key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 [DOI] [PubMed] [Google Scholar]
- 4. van den Buuse M: Role of the mesolimbic dopamine system in cardiovascular homeostasis: Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin Exp Pharmacol Physiol 25: 661–668, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Sowers JR, Viosca SP, Windsor C, Korenman SG: Influence of dopaminergic mechanisms on 24-hour secretory patterns of prolactin, luteinizing hormone and testosterone in recumbent men. J Endocrinol Invest 6: 9–15, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Carey RM, Sen S: Recent progress in the control of aldosterone secretion. Recent Prog Horm Res 42: 251–296, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Roitman MF, Schafe GE, Thiele TE, Bernstein IL: Dopamine and sodium appetite: Antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci 111: 606–611, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Vieira-Coelho MA, Teixeira VA, Finkel Y, Soares-Da-Silva P, Bertorello AM: Dopamine-dependent inhibition of jejunal Na+-K+-ATPase during high-salt diet in young but not in adult rats. Am J Physiol 275: G1317–G1323, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA: Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 15: 560–569, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM: Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol 257: F469–F477, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Chen CJ, Lokhandwala MF: An impairment of renal tubular DA 1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens A 14: 615–628, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB: Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350: 614–661, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Tiberi M, Caron MG: High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem 269: 27925–27931, 1994 [PubMed] [Google Scholar]
- 14. Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA: Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension 41: 604–610, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Amenta F: Light microscope autoradiography of peripheral dopamine receptor subtypes. Clin Exp Hypertens 19: 27–41, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR: Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci 22: 10801–10810, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA: D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regulatory Integrative Comp Physiol 290: R96–R104, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA: Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 45: 804–810, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA: Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118: 2180–2189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amenta F, Ricci A, Tayebati SK, Zaccheo D: The peripheral dopaminergic system: morphological analysis, functional and clinical applications. Ital J Anat Embryol 107: 145–167, 2002 [PubMed] [Google Scholar]
- 21. Amenta F, Barili P, Bronzetti E, Ricci A: Dopamine D1-like receptor subtypes in the rat kidney: A microanatomical study. Clin Exp Hypertens 21: 17–23, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Yao LP, Huque E, Baraniuk J, Carey RM, Felder RA, Jose PA: Dopamine receptor subtype expression (D1A and D1B) in rat nephron segments [Abstract]. J Investig Med. 44: 305A, 1996 [Google Scholar]
- 23. Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA: Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol 297: R1660–R1669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsen NV, Hansen JM, Ladefoged SD, Fogh-Andersen N, Leyssac PP: Renal tubular reabsoption of sodium and water during infusion of low-dose dopamine in normal mam. Clin Sci 78: 503–507, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Vieira-Coelho MA, Gomes P, Serrão MP, Soares-da-Silva P: D1-like dopamine receptor activation and natriuresis by nitrocatechol COMT inhibitors. Kidney Int 59: 1683–1694, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Gildea JJ, Wang X, Jose PA, Felder RA: Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension 51: 360–366, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Demchyshyn LL, McConkey F, Niznik HB: Dopamine D5 receptor agonist high affinity and constitutive activity profile conferred by carboxyl-terminal tail sequence. J Biol Chem 275: 23446–23455, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Baines AD, Ho P, Drangova R: Proximal tubular dopamine production regulates basolateral Na-K-ATPase. Am J Physiol 262: F566–F571, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Aperia A, Bertorello A, Seri I: Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 252: F39–F45, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW: Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 64: 2133–2141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grider JS, Ott CE, Jackson BA: Dopamine D1 receptor-dependent inhibition of NaCl transport in the rat thick ascending limb: Mechanism of action. Eur J Pharmacol 473: 185–190, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Pedrosa R, Jose PA, Soares-Da-Silva P: Defective D1-like receptor mediated inhibition of Cl−/HCO3-exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol 286: F1120–F1126, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Chen C-J, Apparsundaram S, Lokhandwala MF: Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam in rats. J Pharmacol Exp Ther 256: 486–491, 1991 [PubMed] [Google Scholar]
- 34. Clark KL, Hilditch A, Robertson MJ, Drew GM: Effects of dopamine DA1-receptor blockade and angiotensin converting enzyme inhibition on the renal actions of fenoldopam in the anesthetized dog. J Hypertens 9: 1143–1150, 1991 [PubMed] [Google Scholar]
- 35. Sheikh-Hamad D, Wang Y-P, Jo OD, Yanagawa N: Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol 264: F737–F743, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH: Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28719–28726, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Gesek FA, Schoolwerth AC: Hormone responses of proximal Na+-H+ exchanger in spontaneously hypertensive rats. Am J Physiol 261: F526–F536, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A: Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 295: F1110–F1116, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Banday AA, Lokhandwala MF: Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol 295: F698–F706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bagi Z, Erdei N, Koller A: High intraluminal pressure via H2O2 upregulates arteriolar constrictions to angiotensin II by increasing the functional availability of AT1 receptors. Am J Physiol Heart Circ Physiol 295: H835–H841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu D, Gao L, Roy SK, Cornish KG, Zucker IH: Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res 103: 186–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17989, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL: Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287: H1618–H1624, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Tank J, Obst M, Diedrich A, Brychta RJ, Blumer KJ, Heusser K, Jordan J, Luft FC, Gross V: Sympathetic nerve traffic and circulating norepinephrine levels in RGS2-deficient mice. Auton Neurosci 136: 52–57, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amenta F, Barili P, Bronzetti E, Felici L, Mignini F, Ricci A: Localization of dopamine receptor subtypes in systemic arteries. Clin Exp Hypertens 22: 277–288, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Natarajan A, Han G, Chen SY, Yu P, White R, Jose P: The d5 dopamine receptor mediates large-conductance, calcium- and voltage-activated potassium channel activation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther 332: 640–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ricci A, Amenta F, Bronzetti E, Felici L, Hussain T, Lokhandwala MF: Age-related changes of dopamine receptor protein immunoreactivity in the rat mesenteric vascular tree. Mech Ageing Dev 123: 537–546, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Ricci A, Mignini F, Tomassoni D, Amenta F: Dopamine receptor subtypes in the human pulmonary arterial tree. Auton Autacoid Pharmacol 26: 361–369, 2006 [DOI] [PubMed] [Google Scholar]
- 49. White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO: cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86: 897–905, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Karlsson RM, Hefner KR, Sibley DR, Holmes A: Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology 200: 117–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hernández-Echeagaray E, Cepeda C, Ariano MA, Lobo MK, Sibley DR, Levine MS: Dopamine reduction of GABA currents in striatal medium-sized spiny neurons is mediated principally by the D1 receptor subtype. Neurochem Res 32: 229–240, 2007 [DOI] [PubMed] [Google Scholar]
- 52. DiBona GF, Sawin LL: Renal nerves in renal adaptation to dietary sodium restriction. Am J Physiol 245: F322–F328, 1983 [DOI] [PubMed] [Google Scholar]
- 53. DiBona GF: Renal neural mechanisms in salt-sensitive hypertension. Blood Press Suppl 2: 81–87, 1995 [PubMed] [Google Scholar]
- 54. Fujita M, And K, Nagae A, Fujita T: Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 50: 360–367, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Crowley SD, Gurley SB, Oliiverio MI, Pazmino AK, Griffiths RM, Flannery PJ, Spurney RF, Kim H-S, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and the systemic tissues in blood pressure regulation by the renin-angiotensin system, J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allayee H, de Bruin TW, Dominguez KM, Cheng LS, Ipp E, Cantor RM, Krass KL, Keulen ET, Aouizerat BE, Lusis AJ, Rotter JI: Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension 38: 773–778, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Casari G, Barlassina C, Cusi D, Zagato L, Muirhead R, Righetti M, Nembri P, Amar K, Gatti M, Macciardi F, Binelli G, Bianchi G: Association of the alpha-adducin locus with essential hypertension. Hypertension 25: 320–326, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Grandy DK, Allen LJ, Zhang Y, Magenis RE, Civelli O: Chromosomal localization of three human D5 dopamine receptor genes. Genomics 13: 968–973, 1992 [DOI] [PubMed] [Google Scholar]
- 59. Cohn DH, Shohat T, Yahav M, Ilan T, Rechavi G, King L, Shohat M: A locus for an autosomal dominant form of progressive renal failure and hypertension at chromosome 1q21. Am J Hum Genet 67: 647–651, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, Bouzekri N, Ward R, Rorimi C: Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension 40: 629–633, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Cravchik A, Gejman PV: Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharmacogenetics 9: 199–206, 2002 [PubMed] [Google Scholar]
- 62. Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA: Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension 43: 673–679, 2004 [DOI] [PubMed] [Google Scholar]