Abstract
Soft-tissue calcification is a prominent feature in both chronic kidney disease (CKD) and experimental Klotho deficiency, but whether Klotho deficiency is responsible for the calcification in CKD is unknown. Here, wild-type mice with CKD had very low renal, plasma, and urinary levels of Klotho. In humans, we observed a graded reduction in urinary Klotho starting at an early stage of CKD and progressing with loss of renal function. Despite induction of CKD, transgenic mice that overexpressed Klotho had preserved levels of Klotho, enhanced phosphaturia, better renal function, and much less calcification compared with wild-type mice with CKD. Conversely, Klotho-haploinsufficient mice with CKD had undetectable levels of Klotho, worse renal function, and severe calcification. The beneficial effect of Klotho on vascular calcification was a result of more than its effect on renal function and phosphatemia, suggesting a direct effect of Klotho on the vasculature. In vitro, Klotho suppressed Na+-dependent uptake of phosphate and mineralization induced by high phosphate and preserved differentiation in vascular smooth muscle cells. In summary, Klotho is an early biomarker for CKD, and Klotho deficiency contributes to soft-tissue calcification in CKD. Klotho ameliorates vascular calcification by enhancing phosphaturia, preserving glomerular filtration, and directly inhibiting phosphate uptake by vascular smooth muscle. Replacement of Klotho may have therapeutic potential for CKD.
The high cardiovascular mortality in patients with chronic kidney disease (CKD) is closely associated with vascular calcification (VC).1,2 Risk factors for VC include hypertension, hyperlipidemia, diabetes, plasma phosphate, homocysteine, and osteoprotegerin.3,4 Defects in endogenous anti-calcification factors such as matrix Gla protein, osteoprotegerin, carbonic anhydrase isoenzyme II, fibrillin-1, fetuin-A, fibroblast growth factor 23, and Klotho may play an important role in this dire complication of CKD.5–10 High serum phosphate is associated with significantly increased risk for death.11 Treatment with phosphorus binders improves survival of hemodialysis patients compared with no treatment with matched baseline serum phosphate levels.12
Early diagnosis and treatment is important to retard the progression of CKD. Most biomarkers in current clinical use are not early or sensitive enough.13–16 The need to find a sensitive and early biomarker is of paramount importance for early diagnosis and intervention. Various strategies have been devised to slow progression of renal disease12,17,18 with varying effectiveness.19 We are in dire need of additional new agents in preventing the progression of CKD and in ameliorating VC.
Klotho was originally identified as an aging suppressor.9 Its gene product is a single-pass transmembrane protein9,20 that functions as a coreceptor for fibroblast growth factor (FGF) 23.21–24 Klotho is expressed widely, but its level is highest in the kidney.25,26 Klotho is also secreted into the cerebrospinal fluid, blood, and urine25,27 by ectodomain shedding mediated by membrane-anchored proteases.28,29 Secreted Klotho functions in an endocrine fashion as an enzyme or possibly a hormone. Klotho deficiency in rodents leads to a syndrome of premature aging where ectopic soft tissue calcification is a notable feature.9 Overexpression of Klotho rescues the Klotho-deficient phenotype including ectopic calcification, suggesting that Klotho may be an inhibitor of ectopic calcification.9
Because of the features common to both human CKD and murine experimental Klotho deficiency (Kl−/−), we postulate that Klotho deficiency may be responsible for the VC in CKD. The literature offers suggestive but limited evidence for a pathogenic role of Klotho in CKD. Renal Klotho mRNA is lower in a five-sixths nephrectomy model of CKD and in human nephrectomy samples from end-stage sclerotic kidneys.30–32 A modest amelioration of proteinuria and renal function was observed when Klotho was overexpressed genetically in a chronic glomerulonephritis model33 or via viral delivery in a chronic angiotension II34 and a spontaneous hypertension model.35
We will test three hypotheses: (1) CKD is a state of Klotho deficiency; (2) low Klotho is an early marker of CKD; and (3) Klotho deficiency contributes to VC and Klotho replacement ameliorates CKD via multiple mechanisms.
RESULTS
CKD Is a State of Klotho Deficiency
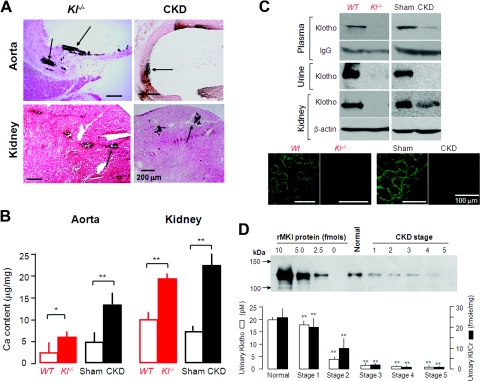
The pattern of calcification of soft tissue in CKD is indistinguishable from that seen in Klotho deficiency in rodents.3,9,36,37 We found similar increases in tissue calcium content in Kl−/− and CKD animals (Figure 1, A and B). We next asked whether CKD is a state of endocrine Klotho deficiency. End-stage CKD patients31 and animals30,33 have reduced Klotho in kidneys, but there is no data on blood or urine Klotho in CKD. Klotho was undetectable in homozygous Klotho deficiency (Kl−/−) (Figure 1C) and was notably decreased in kidney and barely detectable in the blood and urine of CKD mice (Figure 1C), indicating that CKD is a state of “pan deficiency” of Klotho. Because of the lack of a reliable assay for human plasma Klotho at the time of the study, we measured urinary Klotho in CKD patients as a surrogate. Humans (Table 1) with various stages of CKD (National Kidney Foundation classification)14 have lower levels of Klotho in the urine (Figure 1D) extremely early in human CKD stage 1 (Figure 1D and Supplemental Figure 1), and the magnitude of decrease correlates with the severity of decline in estimated GFR (eGFR) in humans.
Figure 1.
Klotho levels are reduced in CKD mice and CKD patients, and soft tissue calcification is observed in CKD mice. (A) Ectopic calcification in soft tissues by Von Kossa staining and calcification in aortas and kidneys of Kl−/− mice and WT CKD mice (arrows). (B) Calcium content assayed by OCPC in soft tissues (aortas and the kidneys) of Kl−/− mice versus WT littermates and also of WT CKD mice versus WT Sham mice. The data are presented as the means ± SEM (n = 4). *P < 0.05; **P < 0.01 versus WT or Sham mice by unpaired t test. (C) Representative blots of Klotho protein in plasma (n = 3), urine (n = 4), and kidney (n = 5) of Kl−/− mice or WT CKD mice. Immunoprecipitation of Klotho in 100 μl of mouse serum was followed by immunoblot. IgG heavy chain was used as the loading control. Urine Klotho was examined by directly immunoblotting approximately 40 μl of mouse urine with an identical amount of creatinine. Klotho protein in the kidney was analyzed by immunoblotting 30 μg of the total kidney lysate and qualitatively examined by immunohistochemistry. (D) Urinary Klotho protein in humans with normal kidney function and various CKD stages. The upper panel is a representative immunoblot with serial dilutions of known concentration of rMKl and concentrated human urine samples of identical amount of creatinine in same gel. The lower panel is a summary of urinary Klotho protein concentration (depicted in open bars) and of Klotho normalized by creatinine (depicted in solid bars) of normal subjects and CKD patients.
Table 1.
Summary of ages and eGFRs of normal subjects and CKD patients
| Normal | CKD Overall | CKD Stages |
|||||
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | |||
| Age (years) | 47.7 ± 3.1 | 52.9 ± 2.2 | 41.5 ± 3.8 | 49.0 ± 3.7 | 59.6 ± 4.0b | 55.8 ± 5.4 | 60.1 ± 6.0b |
| Gender (male/female) | 6/7 | 18/22 | 1/7 | 5/4 | 4/4 | 6/2 | 2/5 |
| eGFR (ml/min per 1.73 m2) | 105.46 ± 4.81 | 58.80 ± 6.90a | 116.88 ± 3.26 | 75.44 ± 2.97a | 46.00 ± 3.35a | 22.38 ± 4.81a | 10.16 ± 1.26a |
eGFR is calculated with the Modification of Diet in Renal Disease equation.
aP < 0.01 versus normal subjects by one-way ANOVA followed by Student-Newman-Keul's test.
bP < 0.05 versus normal subjects by one-way ANOVA followed by Student-Newman-Keul's test.
Klotho Levels and Progression of CKD and Vascular Calcification in CKD
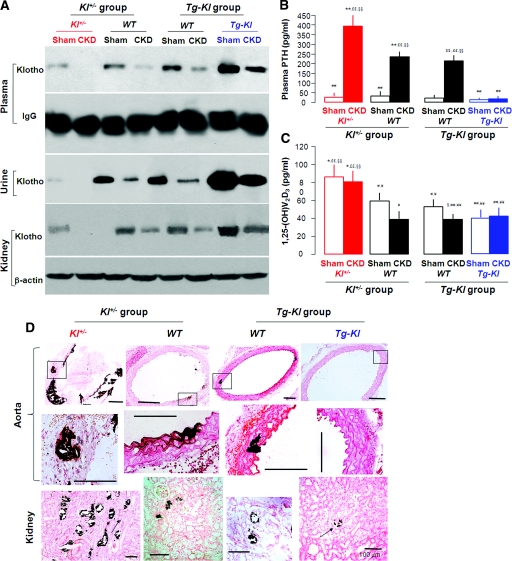
CKD and experimental Klotho deficiency both have low Klotho in blood, kidney, and urine (Figure 1C), high plasma FGF23,38 hyperphosphatemia (Table 2), and ectopic calcification (Figure 1, A and B). A critical question is whether Klotho deficiency is a mere marker or whether it contributes to the pathophysiology of CKD because the latter raises the possibility of therapeutic replacement. To this end, we examined whether Klotho levels affect CKD and its complications. Baseline Klotho was lower in Kl+/− mice compared with WT and was highest in Tg-Kl mice (Figure 2A and Supplemental Figure 2A). Klotho was decreased in all lines of mice when CKD was induced. The Klotho level in Tg-Kl-CKD mice was lower than Tg-Kl-Sham mice but still equivalent to that of WT-Sham mice (Figure 2A and Supplemental Figure 2A). Tg-Kl mice have higher plasma Klotho levels36 and more organs expressing Klotho protein.9 In the kidneys of Tg-Kl mice, almost all of the renal structures express Klotho protein (Supplemental Figure 2B).
Table 2.
Blood Pi and creatinine clearance in Klotho−/− mice and WT CKD mice
|
Klotho−/− Model |
CKD Model in WT Mice |
|||
|---|---|---|---|---|
| WT | Kl−/− | Sham | CKD | |
| Serum Pi (mg/dl) | 5.9 ± 0.4 | 8.1 ± 0.5a | 6.3 ± 0.6 | 7.1 ± 0.7b |
| ClCr (μl/min/g body wt) | 15.41 ± 1.03 | 10.32 ± 2.14b | 10.22 ± 2.74 | 6.15 ± 1.13a |
The data are represented as the means ± SEM (n ≥ 4).
aP < 0.01 versus Sham by unpaired t test.
bP < 0.05 versus WT by unpaired t test.
Figure 2.
Klotho levels and soft tissue calcification in CKD mice are associated with genetic levels of Klotho. (A) Representative blots of Klotho protein in plasma (n = 3), urine (n = 4), and kidney (n = 4) of CKD compared with Sham mice of Kl+/−, Tg-Kl, and their WT littermate mice, respectively. (B and C) Plasma PTH (B) and 1,25-(OH)2 vitamin D3 (C) from Sham and CKD mice with different genetic Klotho background: Kl+/− (red) and Tg-Kl (blue) and their WT (black) littermates for measurement. The data are represented as the means ± SEM (n = 6). *P < 0.05; **P < 0.01 versus Sham WT mice of Kl+/− group; ¥P < 0.05; ¥¥P < 0.01 Sham Kl+/− mice; #P < 0.05; ##P < 0.01 versus CKD Kl+/− mice; $P < 0.05; $$P < 0.01 versus Sham WT mice of Tg-Kl group; §P < 0.05; §§P < 0.01 versus Sham Tg-Kl mice; £P < 0.05; ££P < 0.01 versus CKD Tg-Kl mice by one-way ANOVA followed by Student-Newman-Keul's test. (D) Von Kossa staining of calcification (arrow) in the aortas (low amplification in top panel and high amplification in middle panel) and kidneys (bottom panel) of Kl+/− CKD mice, Tg-Kl CKD mice, and their WT CKD mice, respectively. No Von Kossa staining was found in the tissues of Sham mice (data not shown).
WT-CKD mice had hypertension, anemia, increased plasma creatinine (PCr), declined creatinine clearance (ClCr), increased proteinuria (Supplemental Table 1), and more severe renal histologic damage (Supplemental Figure 3). All of the changes were slightly exaggerated in Kl+/− mice but were much improved in the Tg-Kl mice (Supplemental Table 1 and Supplemental Figure 3). Kl+/− mice had more severe and Tg-Kl mice had milder CKD than WT mice, although all were subjected to the same insult. Hence, amelioration of CKD per se can be a potential factor for less severe soft tissue calcification when Klotho levels are maintained.
Elevation of parathyroid hormone (PTH) in WT-CKD mice was blunted by Klotho overexpression and worsened by Klotho deficiency (Figure 2B). CKD decreased plasma 1,25-(OH)2D3 modestly in WT mice (Figure 2C), which is compatible with the moderate CKD (Supplemental Table 1). The increased 1,25-(OH)2D3 in Kl+/− mice is compatible with Klotho being a potent suppressor of 1,25-(OH)2D3 production39,40 (Figure 2C). Our in vivo data do not exclude the possibility that Klotho's beneficial effect may be through various calciotropic hormones. The direct effect of Klotho will be examined below.
One determinant of soft tissue calcification is plasma phosphate (Pi) concentration.41–44 Both Kl+/− and WT animals with CKD had higher levels of plasma Pi and higher fractional excretion of phosphorus (FEphos) than Sham animals (Supplemental Table 1). In contrast, Tg-Kl-CKD mice did not show much hyperphosphatemia; their FEphos were already high in baseline and did not increase further with CKD (Supplemental Table 1). Therefore, a second mechanism by which Klotho can lessen soft tissue calcification might be by lowering plasma phosphate levels through promotion of phosphaturia.25
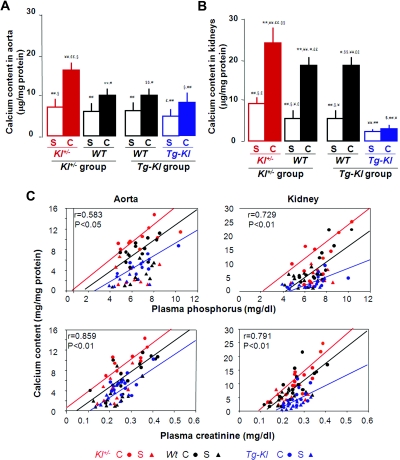
We screened for ectopic calcification in multiple organs. As expected, there was no staining in Sham animals (not shown). In CKD, there was calcification in the kidneys and aortas of both WT and Kl+/− mice (Figure 2D). In contrast, Tg-Kl-CKD animals had very little or no calcification (Figure 2D). The modest and patchy calcification in the vasculature of WT CKD mice might be due to the modest renal failure and/or short duration of follow-up. The percentage of mice with detectable calcification for each CKD group was: Kl+/− mice, 69.2% (9 of 13) versus WT 57.1% (8 of 14); and Tg-Kl, 25% (4 of 16) versus WT 53.3% (8 of 15). Calcium content in aortas (Figure 3A) and kidneys (Figure 3B) was higher in CKD than Sham in both the WT and Kl+/− mice. The calcium content in all organs is inversely related to Klotho levels: highest in Kl+/− mice, intermediate in WT, and lowest in Tg-Kl (Figure 3, A and B).
Figure 3.
The levels of calcium content in the kidneys and the aortas of Sham and CKD mice are correlated with genetic levels of Klotho. (A and B) Calcium content was assayed using OCPC in the aortas (A) and the kidneys (B) of Sham and CKD mice at different genetic Klotho levels: Kl+/− (red) and Tg-Kl (blue) and their WT littermates (black). The data are represented as the means ± SEM (n = 7). *P < 0.05; **P < 0.01 versus Sham WT mice of Kl+/− group; ¥P < 0.05; ¥¥P < 0.01 Sham Kl+/− mice; #P < 0.05; ##P < 0.01 versus CKD Kl+/− mice; $P < 0.05, $$P < 0.01 versus Sham WT mice of Tg-Kl group; §P < 0.05; §§P < 0.01 versus Sham Tg-Kl mice; £P < 0.05; ££P < 0.01 versus CKD Tg-Kl mice by one-way ANOVA followed by Student-Newman-Keul's test. (C) Relationship of calcium content in the aortas and the kidneys with blood Pi and blood Cr, respectively, in Sham (triangles) and in CKD (circles) mice at three different genetic Klotho levels: Kl+/− (red) and Tg-Kl (blue) and their WT littermates (black). C, CKD; S, Sham.
In humans with CKD, both plasma Cr45 and Pi levels41–44 are predictors of soft tissue calcification. Soft tissue calcium content is positively related to plasma Pi and Cr in all mice (Figure 3C). When we divided the animals into subgroups on the basis of their genetic Klotho status, despite the overlap, one could see that for a given plasma Pi and Cr concentration, Tg-Kl mice had the lowest soft tissue calcium content, and Kl+/− had the highest, with WT in between (Figure 3C). Therefore, differences in plasma Pi or Cr are insufficient to explain the different levels of ectopic calcification in the various Klotho background. The data suggest that Klotho has a direct protective effect on soft tissue calcification above and beyond that of the renal effects of phosphaturia and preservation of glomerular filtration.
Pi Uptake and Pi-induced Mineralization and Dedifferentiation in Cultured Cells
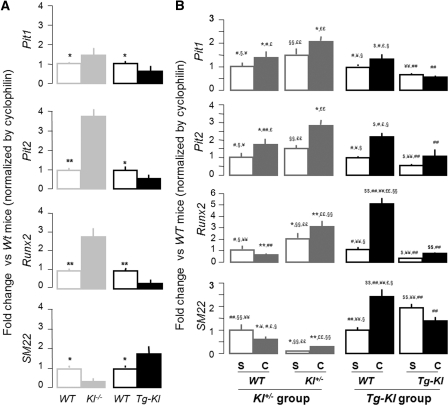
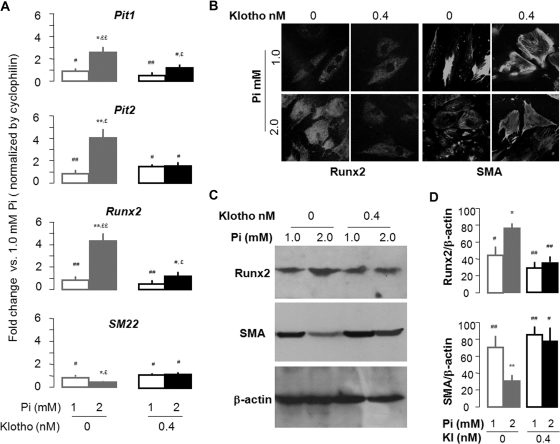
Elevated plasma Pi is associated with VC in experimental animals and in CKD patients.41–44 Pi influx is believed to be mediated by NaPi-3 group of Na+-coupled transporters (Pit-1 and Pit-2) in vascular smooth muscle cells (VSMC).46 Runx2 expression has been postulated to be an early step of mineralization for osteoblasts and may represent ectopic osteogenesis when expressed in other cells.47–49 Pit1 and Pit2 mRNA was increased in Kl−/− and decreased in Tg-Kl compared with WT mice (Figure 4A). Klotho deficiency increased Runx2 and decreased the smooth muscle marker SM22, whereas overexpression of Klotho had the opposite effect. Therefore, Klotho may control the balance between differentiation and dedifferentiation of VMSC. In the aorta, Pit1, Pit2, and Runx2 mRNA was upregulated, and SM22 mRNA was downregulated in CKD in both the Kl+/− and WT background compared with Sham (Figure 4B). Klotho overexpression completely blocked the changes in Pit1, Pit2, Runx2, and SM22 mRNA induced by CKD (Figure 4B), suggesting that Klotho may maintain VSMC differentiation.
Figure 4.
Klotho inhibits dedifferentiation of smooth muscle cells in the aortas of CKD mice. (A) Pit1, Pit2, Runx2, and SM22 transcripts were assessed by qPCR in the aortas of Kl−/− and Tg-Kl mice and their respective WT littermate controls. The results are represented by folds of change of target genes normalized by cyclophilin in Kl−/− or Tg-Kl mice compared with their WT littermates. The data are shown as the means ± SEM (n = 4). *P < 0.05; **P < 0.01 versus WT mice by unpaired t test. (B) The levels of Pit1, Pit2, Runx2, and SM22 transcripts were assessed by qPCR in the aortas of Kl+/− and Tg-Kl mice and their WT littermate mice of Sham and CKD. The results were represented by folds of changes of target genes normalized by cyclophilin compared with their WT Sham mice. The data are shown as the means ± SEM (n = 6). *P < 0.05; **P < 0.01 versus Sham WT mice of Kl+/− group; ¥P < 0.05; ¥¥P < 0.01 Sham Kl+/− mice; #P < 0.05; ##P < 0.01 versus CKD Kl+/− mice; $P < 0.05; $$P < 0.01 versus Sham WT mice of Tg-Kl group; §P < 0.05; §§P < 0.01 versus Sham Tg-Kl mice; £P < 0.05; ££P < 0.01 versus CKD Tg-Kl mice by one-way ANOVA followed by Student-Newman-Keul's test.
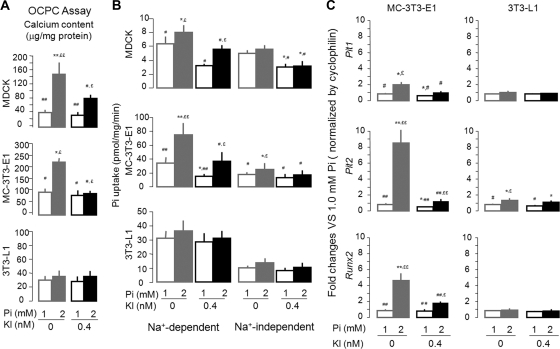
To illustrate whether Klotho directly inhibits VC, we used rat VSMC (A10 cells) to examine for mineralization induced by high ambient Pi. Treatment of A10 cells with recombinant soluble Klotho protein slightly decreased calcium content of A10 cells in 1 mM Pi (Figure 5, A through C, and Supplemental Figure 4A) but significantly reduced the mineralization induced by 2 mM Pi (Figure 5, A through C, and Supplemental Figure 4A) in a dose-dependent fashion (Figure 5, B and C). To examine whether high Pi and Klotho also influence mineralization in other cells, a kidney (MDCK), osteoblast (MC-3T3-E1), and adipocyte cell line (3T3-L1) were used. Calcium content was increased by high Pi in cultured MDCK and MC-3T3-E1 but not in 3T3-L1 (Figure 6A and Supplemental Figure 4, B through D). These in vitro results indicate that Klotho directly inhibits high Pi-induced calcification in a cell type-specific fashion.
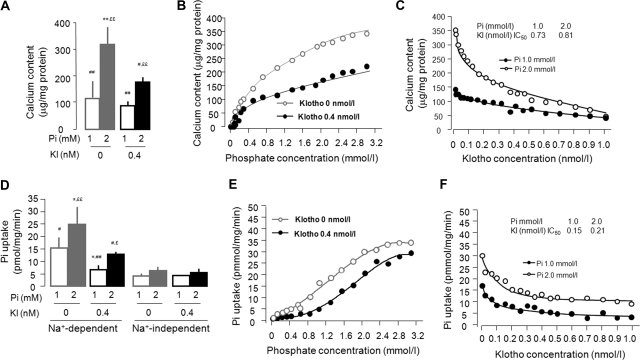
Figure 5.
Klotho regulates Pi-induced mineralization and Pi uptake but not calcium uptake in cultured rat VMSC (A10). (A) A10 cells in a six-well plate were incubated in medium containing 1.0 or 2.0 Pi mM with or without 0.4 nM Klotho for 10 days to examine the Klotho effect on Pi modulated calcium content in A10 cells measured by OCPC assay. The data are presented as the means ± SEM (n = 8). *P < 0.05; **P < 0.01 versus Pi 1.0 mM + Kl 0 nM; #P < 0.05; ##P < 0.01 versus Pi 2.0 mM + Kl 0 nM; £P < 0.05; ££P < 0.01 versus Pi 1.0 mM + Kl 0.4 nM by one-way ANOVA followed by Student-Newman-Keul's test. (B) Effect of Pi on calcium content in A10 cells: dose dependence. The half-maximal effect was achieved at 0.72 mM Pi in the absence of Klotho and at 1.15 mM in 0.4 nM Klotho. (C) Effect of Klotho effect on calcium content in A10 cells: dose dependence. (D) A10 cells were incubated in medium containing 1.0 or 2.0 Pi mM with or without 0.4 nM Klotho for 3 days. Na+-dependent and Na+-independent isotopic Pi uptake was determined. The data are presented as the means ± SEM (n = 6). *P < 0.05; **P < 0.01 versus Pi 1.0 mM + Kl 0 nM; #P < 0.05; ##P < 0.01 versus Pi 2.0 mM + Kl 0 nM; £P < 0.05; ££P < 0.01 versus Pi 1.0 mM + Kl 0.4 nM by one-way ANOVA followed by Student-Newman-Keul's test. (E) Effect of Pi on Na+-dependent uptake on A10 cells: dose dependence. Vmax = 35.2 pmol/mg/min and Km = 12.2 mM Pi in the absence of Klotho, and Vmax = 31.3 pmol/mg/min and Km = 17.6 mM with 0.4 nM Klotho. (F) Effect of Klotho effect on Pi uptake in A10 cells: dose dependence. A10 cells were incubated in medium containing 1.0 or 2.0 Pi mM with or without 0.4 nM Klotho for 3 days.
Figure 6.
Klotho suppresses Pi-induced mineralization, Pi uptake, and dedifferentiation in cultured kidney cells and osteoblasts but not in adipocytes. (A) Renal epithelial (MDCK), osteoblasts (MC-3T3-E1), and adipocyte (3T3-L1) cell lines in six-well plates were incubated in medium containing 1.0 or 2.0 Pi mM with or without 0.4 nM Klotho for 10 days followed by measurement of calcium content. The data are presented as the means ± SEM (n = 6). (B and C) The cells were coincubated with Pi (1.0 or 2.0 mM) and/or Klotho (0 or 0.4 nM) for 3 days followed by Pi transport assay (B) or by measurement of mRNA levels of Pit1, Pit2, and Runx2 and assayed by qPCR (C). The data are presented as the means ± SEM (n = 4). *P < 0.05; **P < 0.01 versus Pi 1.0 mM + Kl 0 nM; #P < 0.05; ##P < 0.01 versus Pi 2.0 mM + Kl 0 nM; £P < 0.05; ££P < 0.01 versus Pi 1.0 mM + Kl 0.4 nM by one-way ANOVA followed by Student-Newman-Keul's test.
We next examined the effect of Klotho on Pi influx in cultured cells. Phosphate transport in A10 cells is primarily Na+-dependent (85 to 95% of Pi influx) (Figure 5D). The ambient Pi effect on Pi uptake is dose-dependent (Figure 5E). Klotho significantly suppressed Na+-dependent but not Na+-independent Pi transport (Figure 5, D through F). High ambient Pi did not affect calcium influx in A10 cells, and Klotho did not modulate calcium influx either in normal or high Pi culture medium (Supplemental Figure 5). This finding is compatible with the model proposed by Giachelli42 where Pi uptake activates a series of cellular processes that result in extracellular calcium phosphate deposition. There was inhibition of Pi influx in kidney and osteoblastic cells but not in adipocytes (Figure 6B). Both Pit1 and Pit2 (∼10× lower abundance than Pit1) transcripts are present in A10 cells (NaPi-2, NaPi-2a, and NaPi-2c are not detectable by reverse transcription-PCR; data not shown). High Pi treatment increased both Pit1 and Pit2 mRNA in A10 cells, and Klotho blocked this increase (Figure 7A). A similar inhibition by Klotho was also found in osteoblast cells and adipocytes except for Pit1 in adipocytes (Figure 6C).
Figure 7.
Klotho suppresses Pit1/2 expression and Pi-induced dedifferentiation in cultured rat VMSC (A10). (A) A10 cells were incubated in medium containing 1.0 or 2.0 Pi mM with or without 0.4 nM Klotho for 3 days, and mRNA levels of Pit1, Pit2, Runx2, and SM22 were assayed by qPCR. The data are presented as the means ± SEM (n = 6). (B) The representative immunohistochemistry for Runx2 and smooth muscle actin (SMA) was shown from three independent experiments in A10 cells. (C) A representative immunoblot for Runx2, SMA, and β-actin is displayed from four independent experiments of A10 cells. (D) A summary of densitometric quantification of all samples is shown. The data are presented as the means ± SEM (n = 4). *P < 0.05; **P < 0.01 versus Pi 1.0 mM + Kl 0 nM; #P < 0.05; ##P < 0.01 versus Pi 2.0 mM + Kl 0 nM; £P < 0.05; ££P < 0.01 versus Pi 1.0 mM + Kl 0.4 nM by one-way ANOVA followed by Student-Newman-Keul's test for (A) and (D).
High Pi induced upregulation of Runx2 and downregulation of SM22 mRNA and protein in A10 cells (Figure 7), suggesting that dedifferentiation of smooth muscle occurred with high Pi. Klotho reversed these changes suggests and blocked Pi-induced dedifferentiation of A10.
DISCUSSION
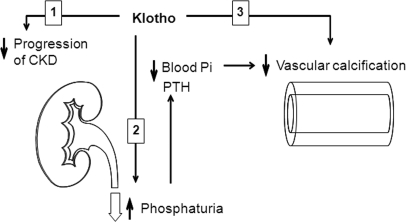
This is the first report that CKD is a state of Klotho deficiency in the kidney, plasma, and urine and that Klotho downregulation is not merely an early biomarker for kidney damage but also plays a pathogenic role in the progression of CKD as well as one of the principal complications of CKD, namely VC. Klotho bestows its anti-calcification effect possibly via at least three mechanisms: a phosphaturic hormone, the preservation of GFR, and a direct effect on soft tissues including the vascular smooth muscle (Figure 8). The potential utility of Klotho in clinical practice is at least two-fold. First, Klotho can serve as an early and sensitive biomarker of CKD. Second, Klotho replacement therapy may be in the horizon in slowing progression of CKD as well as preventing and reversing complications.
Figure 8.
Proposed model of potential effects of Klotho on vascular calcification. Klotho can protect the vasculature against calcification in CKD probably by three actions: (1) slowing progression of CKD; (2) maintenance of normophosphatemia through induction of phosphaturia; and (3) direct inhibition of Pi influx into VSMC, which in turn suppresses the dedifferentiation of VSMC.
CKD Is a State of Klotho Deficiency
Our animals have disease equivalent to human CKD stage 3 to 4, which comprises up to approximately 85% of human CKD.2,50–53 Decreased renal Klotho expression was shown in human renal tissue from end stage kidneys31 and animals with five-sixths nephrectomy.30 Because secreted Klotho exerts multiple effects on distant sites, it is crucial to explore Klotho protein levels in blood and urine. We found commensurate Klotho deficiency in the kidney, plasma, and urine in rodent CKD and in urine in human CKD. The mechanism of how kidney disease lowers Klotho expression is unknown presently but can potentially involve ischemia, oxidative stress, angiotensin II, and inflammation.30,54–56 CKD lowered Klotho even in the Tg-Kl mice, despite the fact that the transgene was driven by a constitutive promoter. This can be due to the fact that endogenous renal Klotho expression actually constitutes 50% of the renal Klotho in the transgenic animals.36 In addition, CKD may have a translational or post-translational effect on the transgenic Klotho.
Pathogenic Role of Klotho in Progression of CKD and Its Complications
The data clearly show that Klotho is more than a mere marker for CKD. Klotho overexpression lessens progression of CKD, improves Pi metabolism, and protects the vasculature from calcification. Previous studies showed that overexpressing Klotho by viral-based gene transfer34 or genetic manipulation33 attenuated progressive renal injury, but the Klotho status and systemic complications of CKD were not studied. Because Klotho is present in multiple body fluids,25,27,36,57 the restoration of Klotho clearly exerts multiple systemic effects in addition to renoprotection.
Disturbed mineral metabolism is implicated in hyperparathryoidism, osteodystrophy, and vascular calcification in CKD.58–61 Pi overload accelerates calcification in CKD, and control of Pi reduces calcification in CKD.62–64 Klotho exerts its phosphaturic effects by inhibiting renal NaPi-2a and NaPi-2c in the renal proximal tubule.25,38 Maintenance of high Klotho in CKD preserves phosphaturia and lessens phosphate retention. Hyperphosphatemia is also an important contributor to VC observed in Klotho-deficient mice.65
Tg-Kl animals have better renal function when subjected to the same renal insult. The mechanisms whereby Klotho protects kidney from injury are unknown but potentially include anti-oxidation, anti-apoptosis,55–56 and anti-senescence.33
In addition to preservation of phosphaturia and GFR, Klotho has a direct effect on the vasculature. Pathologic calcium phosphate deposition in the blood vessels and heart is found in aging,66 diabetes,67,68 hyperlipidemia,69 and CKD.3–4,7 NaPi-3 proteins (Pit1 and Pit2) are broadly expressed and believed to play housekeeping as well as pathologic roles in different cells.70–73 Extracellular Pi stimulates calcification in VSMC through inorganic Pi influx by NaPi-373–75 and cell-surface Pit2 reorganization.73,76 High ambient Pi accelerates mineralization44,49 and stimulates surrogate markers of osteogenesis in VSMC,49,77 spawning the hypothesis that high Pi stimulates “ossification” of VMSC.77 Soluble Klotho not only suppresses baseline NaPi-3 activity but also abrogates high Pi-induced upregulation of NaPi-3 mRNA and activity and suppresses calcification and maintains differentiation of VMSC. Pit1 might also act through mechanisms independent of Pi influx,78 and suppression of Pit1 may affect cell proliferation.79 One minor caveat of the A10 in vitro model is that we do not know whether cultured rat cells mimic all features of human VSMC in vivo.
FGF23 signal transduction generally requires transmembrane Klotho as a coreceptor.21,80 CKD subjects have high levels of full length FGF23 and upregulation of this signal pathway.81 We did not measure FGF23 levels in our animals. It is possible that part of the beneficial effects of Klotho on soft tissue in CKD result from improvement of FGF23 signal transduction.
Potential Clinical Utility
These preclinical studies lay the foundation for two major potential applications. Extensive effort has been devoted to search for early biomarker for kidney diseases focusing mostly on acute kidney disease82 and less on CKD.83 Proteins such as adiponectin,84 γ-glutamyltransferase,85 cystatin C,16 N-acetyl-β-d-glucosaminidase,86 fatty acid-binding protein 1,87 and endothelin-188 were proposed as biomarkers. We documented that patients with early stage CKD (stage 1 and 2) already have significantly lower urinary Klotho, and urinary Klotho is progressively lowered with declining eGFR. Urinary Klotho protein might be an ideal early biomarker for CKD. One important goal of CKD treatment is to prevent or postpone the progression to end stage. Klotho supplementation might be a good strategy not only to preserve remnant kidney function but also to minimize complications of CKD through multiple mechanisms.
CONCISE METHODS
Human Study
A total of 53 human subjects were included in this study: 13 normal volunteers and 40 CKD patients (Table 1) at different stages according to the National Kidney Foundation.14 This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. All of the human subjects were given and accepted an informed consent form. None of the human subjects were receiving treatment with Pi binder, calcium, or active vitamin D or analogs or renal replacement therapy when their urine samples were collected. For measurement of urinary Klotho protein, 4 ml of fresh urine was concentrated to 0.2 ml through Amicon Ultra-4 filters with 100-kD cutoff (Millipore, Billerica, Massachusetts). The concentrated urine were immediately mixed with Laemmli sample buffer and stored at −80°C. Concentrated urines (with identical urine creatinine) along with recombinant murine Klotho (rMKl) protein of known concentration were subjected to immunoblot. Specific signals on the basis of bands on films were obtained with free Image J program (National Institutes of Health), and the Klotho protein concentrations in urine samples were quantified using the rMKl as a standard curve.
Animal Models
All of the work on mice was conducted following the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. The mice used for preparation of CKD model were: (1) one line of transgenic mice with overexpression of Klotho, EFmKL46 (Tg-Kl),9,36 whose genetic background is a mixture of C57BL/6J and 129 and (2) heterozygous for Klotho-deficient mice (Kl+/−),9 whose genetic background for is C57BL/6J and C3H/J. WT littermates were generated during cross-breeding for Tg-Kl and Kl+/− mice. Kl−/− mice and WT littermates used in this study ranged from 6 to 8 weeks; Kl+/−, Tg-Kl, and WT mice were approximately 12 weeks in age. CKD model was generated using uninephrectomy plus ischemia-reperfusion injury in contralateral kidney. Sham mice underwent laparotomy and manual manipulation of the kidneys. After recovery, the mice were housed in normal cages and fed with 1.0% phosphorus diet for 4 weeks with free access to tap water followed by 2.0% phosphorus diet for 8 weeks. For the metabolic study, the mice were transferred to individual metabolic cages. After acclimatization, 24-hour urine was collected, blood was drawn, and tissues were harvested. Plasma and urine chemistry were analyzed by Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis, Rochester, New York) by the Animal Core Facility in University of Texas Southwestern Medical Center. BP was measured by a computerized tail-cuff system (BP-2000; Visitech Systems, Apex, North Carolina) in conscious animals throughout our study. Plasma intact PTH and 1,25-(OH)2 vitamin D3 were measured using ELISA kits from Alpco (Salem, New Hampshire) and Immunodiagnostic Systems (Scottsdale, Arizona), respectively.
Von Kossa Staining and Calcium Content
The calcium content in soft tissues was measured using the o-cresolphthalein complexone (OCPC) method (Sigma, St. Louis, Missouri).89 Quantitation (μg/mg protein) was normalized to protein concentration determined by Bradford protein assay. Kidney, heart, and aorta were stained with Von Kossa and counterstained with nuclear Fast Red for evaluation of calcium precipitation.90 Positive signal for calcium precipitation would be seen in black or brown-black.
Cell Culture
Rat vascular muscle cell (A10), mouse osteoblasts (MC-3T3-E1), mouse adipocytes (3T3-L1), and canine kidney cell (MDCK) were cultured and maintained in condition as described previously.46,91,92 The cells were treated with Pi and/or soluble Klotho protein (amino acid number 31 to 982) as previous described.36 At given time points, the cells were harvested for Von Kossa staining, OCPC assay, RNA extraction, and for 32P-phosphate and 45Ca uptake (detailed protocol in Supplemental Methods).
Kidney Histology and Immunohistochemistry
Four-μm sections of frozen kidney tissues were stained with hematoxylin and eosin and observed and photographed by a renal pathologist (JZ) blinded to the experimental conditions using an Axioplan 2 Imaging System (Carl Zeiss, Thornwood, New York). For immunofluorescence study, a monoclonal rat antibody (KM2076) against human Klotho (1:250)26 was used for staining and followed by secondary antibodies conjugated to fluorescin isothiocyanate (detailed methods in the supplemental materials). Rhodamine-phalloidin (1:50) (Molecular Probes, Eugene, Oregon) for staining β-actin filaments was applied for double staining. The sections were visualized with a Zeiss LSM-510 laser scanning microscope.
Quantifying Klotho in the Kidney, Urine, and Blood of Mice
Kidney total lysates were prepared as described.25 Thirty μg of protein of kidney lysate was solubilized in Laemmli sample buffer; approximately 40 μl of fresh urine were immediately mixed in Laemmli sample buffer after collection. Urine samples with identical amounts of urine creatinine were subjected to SDS-PAGE. One hundred μl of mouse serum were subjected to immunoprecipitation with 4 μl of rabbit anti-serum of human Klotho,26 followed by immunoblot analysis with anti-Klotho antibody (KM2076) (1:2500),26 goat anti-rabbit antibody conjugated with horseradish peroxidase (1/5000 dilution) for IgG heavy chain, and monoclonal mouse antibody for β-actin (1/5000 dilution; Sigma). Specific signal was visualized using the ECL kit (PerkinElmer LAS, Inc., Boston, Massachusetts).
PCR
For real time PCR, total RNA was extracted from mouse tissues (kidney and aorta) and cell lines (rat vascular smooth muscle cells, A10; mouse osteoblast-like cells, MC-3T3-E1; mouse adiopocytes, 3T3-L1). Complimentary DNA was generated. Primers for quantitative PCR (qPCR) are shown in Supplemental Table 4 with conditions described previously.93 The detailed methods are described in the supplemental materials.
Statistical Analyses
The data are expressed as the means ± SEM (n = 8 or more unless indicated otherwise). The detailed analyses were described in the supplemental materials.
DISCLOSURES
None.
Acknowledgments
This work was supported primarily by the Simmons Family Foundation. The authors also received supported from the National Institutes of Health (AG-19712, AG-25326, DK-48482, and DK-54392), the George M. O'Brien Kidney Research Core Center/University of Texas Southwestern Medical Center at Dallas (NIH P30DK-07938), the American Heart Association (0865235F), Eisai Research Fund, Ellison Medical Foundation, Ted Nash Long Life Foundation, and a Grant from the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research. Parts of this work was published in abstract form in the Journal of the American Society of Nephrology (18: 7A–8A, 2007). The authors are grateful to Mr. Lei Wang for breeding and maintaining Kl+/− and Tg-Kl mice and their wild-type littermates, to Ms. Olga Sineshchekova for Klotho protein preparation, and to Ms. Rebecca Aricheta for assistance in measurement of PTH and 1,25-(OH)2 vitamin D3.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Klotho: An Elixir of Youth for the Vasculature?” on pages 5–7.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Parikh NI, Hwang SJ, Larson MG, Levy D, Fox CS: Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 102: 47–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, Hedayati SS: Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int 73: 615–621, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Wallin R, Wajih N, Greenwood GT, Sane DC: Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med Res Rev 21: 274–301, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Proudfoot D, Shanahan CM: Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology 11: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Mellgren RL, Huang X: Fetuin A stabilizes m-calpain and facilitates plasma membrane repair. J Biol Chem 282: 35868–35877, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD: Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18: 2116–2124, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Isakova T, Gutierrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M: Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20: 388–396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Damsgaard EM, Mogensen CE: Microalbuminuria in elderly hyperglycaemic patients and controls. Diabet Med 3: 430–435, 1986 [DOI] [PubMed] [Google Scholar]
- 16. Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ: Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med 147: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ingelfinger JR: Blood-pressure control and delay in progression of kidney disease in children. N Engl J Med 361: 1701–1703, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Vilayur E, Harris DC: Emerging therapies for chronic kidney disease: What is their role? Nat Rev Nephrol 5: 375–383, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Tokumoto M, Mizobuchi M, Finch JL, Nakamura H, Martin DR, Slatopolsky E: Blockage of the renin-angiotensin system attenuates mortality but not vascular calcification in uremic rats: Sevelamer carbonate prevents vascular calcification. Am J Nephrol 29: 582–591, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y: Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J Biol Chem 279: 9777–9784, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS: In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J 23: 433–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Razzaque MS, Lanske B: The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol 194: 1–10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Shawkat Razzaque M, Rosenblatt KP, Baum MG, Kuro OM, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C: Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 249: 865–871, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Deng M, Zhao J, Huang L: Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 391: 261–266, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Sun Z: Klotho Gene Delivery Prevents the Progression of Spontaneous Hypertension and Renal Damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cozzolino M, Brancaccio D, Gallieni M, Slatopolsky E: Pathogenesis of vascular calcification in chronic kidney disease. Kidney Int 68: 429–436, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K: Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol 292: F769–F779, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, Chihara Y, Rui X, Rakugi H, Ogihara T: Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression. Endocrine 25: 229–234, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Yoshida T, Fujimori T, Nabeshima Y: Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 143: 683–689, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC: Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol 15: 1392–1401, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Caudarella R, Vescini F, Buffa A, Francucci CM: Hyperphosphatemia: Effects on bone metabolism and cardiovascular risk. J Endocrinol Invest 30: 29–34, 2007 [PubMed] [Google Scholar]
- 44. Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA: The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 19: 1092–1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asselbergs FW, Mozaffarian D, Katz R, Kestenbaum B, Fried LF, Gottdiener JS, Shlipak MG, Siscovick DS: Association of renal function with cardiac calcifications in older adults: The cardiovascular health study. Nephrol Dial Transplant 24: 834–840, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishiwaki-Yasuda K, Suzuki A, Kakita A, Sekiguchi S, Asano S, Nishii K, Nagao S, Oiso Y, Itoh M: Vasopressin stimulates Na-dependent phosphate transport and calcification in rat aortic smooth muscle cells. Endocr J 54: 103–112, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E: Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res 44 [Suppl 1]: 109–116, 2003 [PMC free article] [PubMed] [Google Scholar]
- 48. Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA: Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 1117: 40–50, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM: Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89: 1147–1154, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Westerberg PA, Linde T, Wikstrom B, Ljunggren O, Stridsberg M, Larsson TE: Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant 22: 3202–3207, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Kohagura K, Tomiyama N, Kinjo K, Takishita S, Iseki K: Prevalence of anemia according to stage of chronic kidney disease in a large screening cohort of Japanese. Clin Exp Nephrol 13: 614–620, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Bakris GL, Ritz E: Hypertension: The message of World Kidney Day 2009. Nat Rev Nephrol 5: 188–190, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI: Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R: Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun 251: 920–925, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H: Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 101: e67–e74, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, Tsuchiya K: Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 25: 60–68, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP: A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A 105: 3455–3460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L: Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18: 2996–3003, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Parfitt AM: Renal bone disease: A new conceptual framework for the interpretation of bone histomorphometry. Curr Opin Nephrol Hypertens 12: 387–403, 2003 [DOI] [PubMed] [Google Scholar]
- 60. de Francisco AM, Ellis HA, Owen JP, Cassidy MJ, Farndon JR, Ward MK, Kerr DN: Parathyroidectomy in chronic renal failure. Q J Med 55: 289–315, 1985 [PubMed] [Google Scholar]
- 61. Albaaj F, Hutchison A: Hyperphosphataemia in renal failure: Causes, consequences and current management. Drugs 63: 577–596, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH, 2nd: A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 75: 176–184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Levin NW, Hoenich NA: Consequences of hyperphosphatemia and elevated levels of the calcium-phosphorus product in dialysis patients. Curr Opin Nephrol Hypertens 10: 563–568, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Salusky IB, Goodman WG: Managing phosphate retention: Is a change necessary? Nephrol Dial Transplant 15: 1738–1742, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Ohnishi M, Nakatani T, Lanske B, Razzaque MS: In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet 2: 583–590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nicita-Mauro V, Maltese G, Nicita-Mauro C, Basile G: Vascular aging and geriatric patient. Minerva Cardioangiol 55: 497–502, 2007 [PubMed] [Google Scholar]
- 67. Qunibi WY, Abouzahr F, Mizani MR, Nolan CR, Arya R, Hunt KJ: Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int 68: 271–277, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Chen NX, Moe SM: Arterial calcification in diabetes. Curr Diab Rep 3: 28–32, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Awan Z, Alrasadi K, Francis GA, Hegele RA, McPherson R, Frohlich J, Valenti D, de Varennes B, Marcil M, Gagne C, Genest J, Couture P: Vascular calcifications in homozygote familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 28: 777–785, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Abraham KA, Brault JJ, Terjung RL: Phosphate uptake and PiT-1 protein expression in rat skeletal muscle. Am J Physiol Cell Physiol 287: C73–C78, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Yoshiko Y, Candeliere GA, Maeda N, Aubin JE: Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 27: 4465–4474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zoidis E, Ghirlanda-Keller C, Gosteli-Peter M, Zapf J, Schmid C: Regulation of phosphate (Pi) transport and NaPi-III transporter (Pit-1) mRNA in rat osteoblasts. J Endocrinol 181: 531–540, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Li X, Yang HY, Giachelli CM: Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 98: 905–912, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Villa-Bellosta R, Bogaert YE, Levi M, Sorribas V: Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol 27: 1030–1036, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Ravera S, Virkki LV, Murer H, Forster IC: Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol 293: C606–C620, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Salaun C, Marechal V, Heard JM: Transport-deficient Pit2 phosphate transporters still modify cell surface oligomers structure in response to inorganic phosphate. J Mol Biol 340: 39–47, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Mizobuchi M, Ogata H, Hatamura I, Koiwa F, Saji F, Shiizaki K, Negi S, Kinugasa E, Ooshima A, Koshikawa S, Akizawa T: Up-regulation of Cbfa1 and Pit-1 in calcified artery of uraemic rats with severe hyperphosphataemia and secondary hyperparathyroidism. Nephrol Dial Transplant 21: 911–916, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Villa-Bellosta R, Levi M, Sorribas V: Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-alpha, and Pi. Pflugers Arch 458: 1151–1161, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Beck L, Leroy C, Salaun C, Margall-Ducos G, Desdouets C, Friedlander G: Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J Biol Chem 284: 31363–31374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H: Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab 95: 578–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 83. Lemley KV: An introduction to biomarkers: Applications to chronic kidney disease. Pediatr Nephrol 22: 1849–1859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D: Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant 20: 129–134, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Ryu S, Chang Y, Kim DI, Kim WS, Suh BS: gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 53: 71–77, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Lapointe C, Belanger MC, Dunn M, Moreau M, Bedard C: N-acetyl-beta-D-glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 22: 1103–1110, 2008 [DOI] [PubMed] [Google Scholar]
- 87. Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T, Portilla D, Sugaya T: Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol 296: F669–F679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dhaun N, Lilitkarntakul P, Macintyre IM, Muilwijk E, Johnston NR, Kluth DC, Webb DJ, Goddard J: Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am J Physiol Renal Physiol 296: F1477–F1483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang CC, Sorribas V, Sharma G, Levi M, Draznin B: Insulin attenuates vascular smooth muscle calcification but increases vascular smooth muscle cell phosphate transport. Atherosclerosis 195: e65–e75, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cozzolino M, Dusso AS, Liapis H, Finch J, Lu Y, Burke SK, Slatopolsky E: The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol 13: 2299–2308, 2002 [DOI] [PubMed] [Google Scholar]
- 91. Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T: Klotho protein promotes adipocyte differentiation. Endocrinology 147: 3835–3842, 2006 [DOI] [PubMed] [Google Scholar]
- 92. Lundquist P, Murer H, Biber J: Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate. Cell Physiol Biochem 19: 43–56, 2007 [DOI] [PubMed] [Google Scholar]
- 93. Zhang Q, Moe OW, Garcia JA, Hsia CC: Regulated expression of hypoxia-inducible factors during postnatal and postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 290: L880–L889, 2006 [DOI] [PubMed] [Google Scholar]