Abstract
Gene variants in the alternative pathway of the complement system strongly associate with atypical hemolytic uremic syndrome (aHUS), presumably by predisposing to increased complement activation within the kidney. Complement factor H (CFH) is the major regulator of complement activation through the alternative pathway. Factor H-deficient mice transgenically expressing a mutant CFH protein (Cfh−/−.FHΔ16–20) that functionally mimics the CFH mutations reported in aHUS patients spontaneously develop thrombotic microangiopathy. To investigate the role of complement C5 activation in this aHUS model, we generated C5-deficient Cfh−/−.FHΔ16–20 mice. Both C5-sufficient and C5-deficient Cfh−/−.FHΔ16–20 mice had abnormal C3 deposition within the kidney, but spontaneous aHUS did not develop in any of the C5-deficient mice. Furthermore, although Cfh−/−.FHΔ16–20 animals demonstrated marked hypersensitivity to experimentally triggered renal injury, animals with concomitant C5 deficiency did not. These data demonstrate a critical role for C5 activation in both spontaneous aHUS and experimentally triggered renal injury in animals with defective complement factor H function. This study provides a rationale to investigate therapeutic inhibition of C5 in human aHUS.
The complement system is a major component of innate immunity with diverse functions. Complement functions include host protection against pathogens through cell lysis, recruitment of inflammatory cells, and opsonization, in addition to the physiologic clearance of cell debris and immune complexes.1 Complement activation initially results in deposition of C3, the central complement activation protein, on the triggering surface. On host cell surfaces, deposited C3 is rapidly inactivated. On foreign surfaces, rapid amplification of C3 deposition occurs (termed opsonization) together with activation of the terminal pathway. The latter is triggered by the cleavage of the complement activation protein C5. This initiates the formation of C5b-9 membrane attack complex, which can provoke cell damage2 and the concomitant generation of the anaphylatoxin C5a, a potent inflammatory mediator. The efficiency of this system depends on tight regulation, which restricts complement activation to appropriate sites (e.g. the surface of pathogens), minimizing host tissue damage. Hence, the complement system possesses numerous regulatory proteins distributed between plasma and cell surfaces. Abnormal complement regulation is thought to be important in many different pathologic conditions. In particular, dysregulation of the alternative pathway (AP) of the complement system has been associated with numerous pathologies, including age-related macular degeneration, dense deposit disease, C3 glomerulonephritis,3 and atypical hemolytic uremic syndrome (aHUS).4
The hemolytic uremic syndrome (HUS, MIM 235400) is a renal disease characterized by thrombocytopenia, microangiopathic hemolytic anemia, and acute renal failure caused by glomerular thrombotic microangiopathy.5–7 The majority of HUS episodes are triggered by Escherichia coli 0157:H7 infection.8 However, up to 10% of cases are not associated with infection, and this form (termed atypical HUS) has the poorest long-term prognosis.7 aHUS has been associated with mutations and/or polymorphisms in complement genes encoding the complement regulatory proteins: factor H (CFH),9–14 CD46 (also termed membrane cofactor protein),15,16 and factor I (CFI)17,18 and the complement activation proteins: factor B19 and C3.20 Mutations in complement regulatory genes are loss-of-function mutations, whereas mutations in complement activators are gain-of-function mutations. Hence, aHUS can be viewed as a disorder of AP dysregulation. Missense mutations within the C-terminal surface recognition domains of CFH are among the most frequent genetic alterations found in aHUS patients.4 These mutations specifically reduce the capacity of CFH to bind to surface ligands, an interaction that is required to target this plasma protein to cell surfaces.10,13,21 In contrast, these mutations do not affect the AP regulatory activity of CFH in plasma because this activity is mediated entirely within N-terminal protein domains of CFH. Consequently, in aHUS the presence of mutated CFH results in an inability to regulate AP activation on renal endothelium.
We have previously reported that transgenic mice expressing a mutant CFH protein lacking the C-terminal surface recognition domains (Cfh−/−.FHΔ16–20) developed spontaneous aHUS.22 The FHΔ16–20 mutant protein functionally mimicked CFH mutations reported in aHUS patients as it retained the ability to regulate C3 activation in plasma but did not target to cell surfaces. In this report we demonstrate the key role of complement C5 activation in the development of aHUS in this mouse model. Understanding the role of C5 activation is of major importance because C5 activation is expected to occur within the kidney irrespective of the particular AP mutation underlying aHUS.
RESULTS
C5 and Spontaneous aHUS in Cfh−/−.FHΔ16–20 Mice
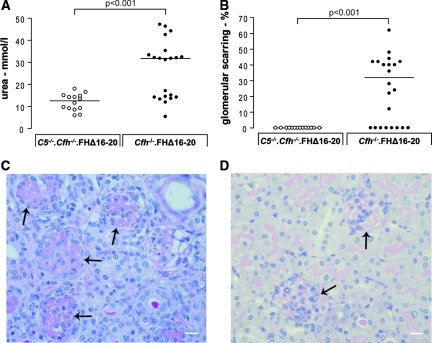
To analyze the role of C5 in aHUS, cohorts of Cfh−/−.FHΔ16–20 mice (n = 26) and Cfh−/−.FHΔ16–20 mice deficient in C5 (C5−/−.Cfh−/−.FHΔ16–20 mice, n = 14) were studied over a 4-month period. At the end of this period, the surviving mice were killed, and renal function, histology, peripheral blood smears, and complement analyses were performed. During this period, no mortality was observed in the C5−/−.Cfh−/−.FHΔ16–20 mice, whereas there were 11 deaths (three animals found dead and eight animals sacrificed humanely because of sickness) in the Cfh−/−.FHΔ16–20 cohort (P = 0.0011, log rank test). Renal function, assessed by blood urea measurement, was normal (Figure 1A) in C5−/−.Cfh−/−.FHΔ16–20 mice. Because of premature mortality and morbidity, serum was available to analyze in only 21 of the 26 Cfh−/−.FHΔ16–20 cohort animals. In 12 of these, the urea level was greater than 20 mmol/L (normal value defined <20 mmol/L). We have previously demonstrated that the spectrum of spontaneous glomerular lesions in Cfh−/−.FHΔ16–20 mice includes glomerular thrombosis, capillary microaneurysms, mesangiolysis, and glomerular scarring.22 In this cohort glomerular scarring was seen in 14 out of the 22 mice (64%) for which renal histology was available (Figure 1, B and C). All of the 12 mice with urea levels >20 mmol/L had glomerular scarring (median = 40%, range = 22 to 62, n = 12). Glomerular thrombosis was also noted in these uraemic animals (median glomerular thrombosis 14%, range = 2.3 to 25, n = 12). In contrast, renal histology was entirely normal in 4-month-old C5−/−.Cfh−/−.FHΔ16–20 mice (Figure 1D). We have also previously demonstrated that Cfh−/−.FHΔ16–20 mice with renal disease developed peripheral blood red cell fragmentation and thrombocytopenia, features typical of human aHUS.22 In this cohort we assessed red cell fragmentation, which was detected in only 11 out of 21 Cfh−/−.FHΔ16–20 mice. All of the Cfh−/−.FHΔ16–20 mice with red cell fragments (median red cell fragments per 200 red cells = 17, range =2 to 51, n = 11) had hematuria and abnormal glomerular histology. In contrast, red cell fragmentation was not present in any of the C5−/−.Cfh−/−.FHΔ16–20 mice, and urinalyses showed no evidence of hematuria at 4 months of age (data not shown). Furthermore, Cfh−/−.FHΔ16–20 mice with hematuria were anemic (median hematocrit = 18.4, range = 6.8 to 54.9, n = 9) in contrast to normal hematocrits in Cfh−/−.FHΔ16–20 mice without hematuria (median hematocrit = 42.3, range = 33.9 to 44.4, n = 8, P < 0.05 versus Cfh−/−.FHΔ16–20 mice with hematuria) and all of the C5−/−.Cfh−/−.FHΔ16–20 mice (median hematocrit = 42.9, range = 31.9 to 48.1, n = 14, P < 0.01 versus Cfh−/−.FHΔ16–20 mice with hematuria).
Figure 1.
C5−/−.Cfh−/−.FHΔ16–20 mice do not develop spontaneous aHUS. (A) Assessment of renal function. Because of the accelerated mortality and morbidity urea, levels could only be assessed in 21 of the 26 Cfh−/−.FHΔ16–20 mice. In these animals the median urea level was 31.8 mmol/L (range = 5.4 to 47.2, n = 21, P < 0.001 versus C5−/−.Cfh−/−.FHΔ16–20 mice). Using 20 mmol/L as the upper limit of normal, 57% of the Cfh−/−.FHΔ16–20 mice were uraemic at the time of analysis. In contrast, serum urea levels in C5−/−.Cfh−/−.FHΔ16–20 mice were all <20mmol/L (median = 12.6 mmol/L, range = 5.9 to 18, n = 14). (B) Assessment of renal pathology. The glomerular histologic appearances in all of the C5−/−.Cfh−/−.FHΔ16–20 mice were normal at 4 months (n = 14). In contrast, evidence of renal thrombotic microangiopathy was evident in the majority of Cfh−/−.FHΔ16–20 mice by 4 months. Of the 22 renal specimens available for analysis, glomerular scarring was evident in 14 mice by 4 months of age. The horizontal bars denote median values. (C and D) Renal histology. (C) Example of glomerular scarring seen in Cfh−/−.FHΔ16–20 mice. (D) In contrast the glomeruli in C5−/−.Cfh−/−.FHΔ16–20 mice were normal. Glomeruli are indicated by arrows. Original magnification, 20. Bar, 10 μm.
To determine whether the absence of C5 had fully protected C5−/−.Cfh−/−.FHΔ16–20 mice against renal damage or just delayed the onset of disease, separate cohorts of C5−/−.Cfh−/−.FHΔ16–20 (n = 14) and Cfh−/−.FHΔ16–20 mice (n = 8) were monitored up to the age of 8 months. No deaths occurred in the C5−/−.Cfh−/−.FHΔ16–20 cohort, and glomerular light microscopy was normal in all of the animals (data not shown). In contrast, in the Cfh−/−.FHΔ16–20 group, three animals died before the 8-month time point, and of the remaining five that were sacrificed at 8 months, two animals had evidence of renal scarring (data not shown).
Glomerular C3 and C9 Staining in Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 Mice
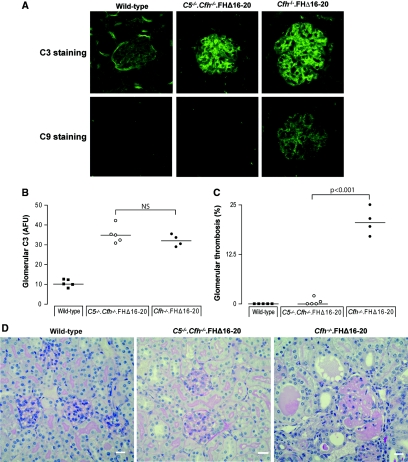
Cfh−/−.FHΔ16–20 mice developed abnormal glomerular C3 deposition.22 Immunofluorescence analysis of 4-month-old C5−/−.Cfh−/−.FHΔ16–20 mice showed a similar pattern of C3 deposition within the kidney to that observed in Cfh−/−.FHΔ16–20 mice (Figure 2A). C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice that did not have histologic evidence of aHUS at the time of sacrifice had similar degrees of abnormal glomerular C3 deposition. However, in Cfh−/−.FHΔ16–20 mice that had developed aHUS, the intensity of glomerular C3 staining was significantly increased (Figure 2B). Cfh−/−.FHΔ16–20 mice had evidence of glomerular C9 deposition, a marker of terminal complement activation. In contrast, in C5−/−.Cfh−/−.FHΔ16–20 and wild-type mice, no glomerular C9 staining was detectable (Figure 2A). The C9 staining pattern in Cfh−/−.FHΔ16–20 mice was similar to that observed for C3 staining. As for C3 staining, the intensity of C9 staining was higher in the animals that had developed histologic renal changes compared with the mice that were still normal (Figure 2C). The increased glomerular deposition of C3 seen in the Cfh−/−.FHΔ16–20 mice with aHUS was associated with increased C9 deposition (Figure 2, B and C).
Figure 2.
Glomerular complement C9 is present in Cfh−/−.FHΔ16–20 mice. (A) Glomerular C3 and C9 staining in 4-month-old wild-type, C5−/−.Cfh−/−.FHΔ16–20, and Cfh−/−.FHΔ16–20 mice with and without aHUS. C3 deposition along the glomerular renal endothelium and within the mesangium was observed in both Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 mice but not in wild-type animals. As expected, glomerular C9 deposition was absent in C5−/−.Cfh−/−.FHΔ16–20 mice. In contrast, glomerular C9 deposition was detectable in Cfh−/−.FHΔ16–20 mice, with or without aHUS, following the same pattern as C3. (B) Quantification of the glomerular C3 deposition in C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice. An equivalent level of glomerular C3 deposition was obtained in C5−/−.Cfh−/−.FHΔ16–20 (n = 14) and Cfh−/−.FHΔ16–20 mice without aHUS (n = 8), whereas glomerular C3 staining was more intense in the Cfh−/−.FHΔ16–20 mice that had developed aHUS at the time of the analysis (n = 12). (C) C9 deposition was absent in the C5−/−.Cfh−/−.FHΔ16–20 mice but present in Cfh−/−.FHΔ16–20 animals with the greatest staining intensity seen in the Cfh−/−.FHΔ16–20 mice with aHUS. The values for glomerular C3 and C9 deposition are given in AFU.
Plasma C3 and FHΔ16–20 Levels in Cfh−/−.FHΔ16–20 Mice and C5−/−.Cfh−/−.FHΔ16–20 Mice
We have previously shown that the degree of plasma C3 activation is one factor that can influence the development of aHUS in the Cfh−/−.FHΔ16–20 mouse model.22 In these animals the plasma C3 regulation is mediated by the circulating FHΔ16–20 protein. Therefore, to determine whether Cfh−/−.FHΔ16–20 mice and C5−/−.Cfh−/−.FHΔ16–20 mice regulated plasma C3 levels to a comparable extent, we measured both plasma FHΔ16–20 and C3 levels in these animals. Given that there were no differences in either plasma C3 or FHΔ16–20 levels with respect to age of the mice, data from the 4- and 8-month age groups were pooled for the respective cohorts. This analysis showed firstly that FHΔ16–20 levels did not differ between Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 mice (Figure 3A). Second, FHΔ16–20 levels did not differ between Cfh−/−.FHΔ16–20 mice with aHUS and Cfh−/−.FHΔ16–20 without aHUS (Figure 3A). Similarly, plasma C3 levels were not significantly different between the C5−/−.Cfh−/−.FHΔ16–20 mice and the Cfh−/−.FHΔ16–20 mice, irrespective of the presence of aHUS in the latter group (Figure 3B). Hence the phenotypic differences could not be attributed to differences in either the expression of the transgene or the degree of plasma C3 regulation.
Figure 3.
Plasma C3 regulation is comparable in C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice. (A) Plasma FHΔ16–20 levels in 4- and 8-month-old C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice. No significant differences were observed in the levels of the FHΔ16–20 protein between the Cfh−/−.FHΔ16–20 (median = 61.9% of pooled wild-type sera, range = 38 to 97, n = 29) and C5−/−.Cfh−/−.FHΔ16–20 (median = 62.2% of pooled wild-type sera, range = 48 to 79, n = 28) animals. Furthermore, the FHΔ16–20 protein level did not differ between the Cfh−/−.FHΔ16–20 mice with (median = 64% of pooled wild-type sera, range = 38 to 96, n = 18) and without (median = 53% of pooled wild-type sera, range = 42 to 97, n = 11) signs of aHUS. The values are expressed as percentages of normal CFH level determined from pooled wild-type mouse sera. (B) Plasma C3 levels in the Cfh−/−.FHΔ16–20 and the C5−/−.Cfh−/−.FHΔ16–20 mice. The median plasma C3 levels did not differ between the C5−/−.Cfh−/−.FHΔ16–20 mice (median = 108 mg/L, range = 61 to 228, n = 28, Figure 3B) and the Cfh−/−.FHΔ16–20 mice irrespective of disease status (median value for mice with aHUS = 120 mg/L, range = 54 to 176, n = 18, median value for mice without aHUS = 141 mg/L, range = 81 to 221, n = 11, P = NS, Figure 3B). The horizontal bars denote median values.
Plasma FHΔ16–20 and C5 Levels in Cfh−/−.FHΔ16–20 Mice
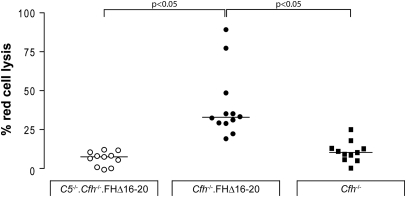
We have previously shown that FHΔ16–20 was able to regulate plasma C3 activation in vivo by comparing C3 levels in factor H-deficient animals that did or did not express the FHΔ16–20 protein.22 C3 levels were markedly reduced in Cfh−/− mice but increased in proportion to the degree of expression of the FHΔ16–20 protein in Cfh−/−.FHΔ16–20 mice.22 In view of our data indicating an essential role for C5 in the development of renal disease in the Cfh−/−.FHΔ16–20 mice, we next examined whether the expression of the FHΔ16–20 protein influenced C5 levels by comparing C5 levels in Cfh−/− and Cfh−/−.FHΔ16–20 mice (Figure 4). There is no reliable ELISA assay to quantify mouse plasma C5 levels, so we utilized an assay that indirectly detects the presence of mouse C5. The assay tests the ability of mouse C5 to reconstitute lytic activity of human C5-depleted sera. As expected, no reconstitution was seen when serum from C5−/−.Cfh−/−.FHΔ16–20 mice was added because these animals are genetically deficient in C5 (Figure 4). Strikingly, sera from Cfh−/− mice demonstrated negligible lytic activity in this assay, indicating that the uncontrolled complement activation associated with factor H deficiency results in plasma C5 depletion. In contrast, hemolytic activity was restored using Cfh−/−.FHΔ16–20 sera. This demonstrated that these mice have sufficient circulating mouse C5 to reconstitute the hemolytic activity of human C5 deficient sera and provides indirect evidence that Cfh−/−.FHΔ16–20 mice can mediate C5 effector functions (C5a generation, terminal pathway activation) in vivo.
Figure 4.
Cfh−/−.FHΔ16–20 mice have functionally active plasma C5. Cfh−/−.FHΔ16–20 sera was able to reconstitute the lytic activity of human C5-depleted sera (median = 33.4, range = 19.2 to 89.3, n = 12) in contrast to negligible lysis seen using sera from Cfh−/− mice (median = 12.5, range = 3.8 to 25.1, n = 11). As expected in genetic C5 deficiency, sera from the C5−/−.Cfh−/−.FHΔ16–20 animals could not restore lytic activity of the human C5-depleted sera (median = 7.5, range = 2.8 to 12.1, n = 11). The horizontal bars denote median values.
Accelerated Serum Nephrotoxic Nephritis in Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 Mice
aHUS in humans is frequently associated with an environmental trigger. To mimic this experimentally, we assessed the accelerated serum nephrotoxic nephritis (ANTN) model in 2-month-old Cfh−/−.FHΔ16–20 mice, C5−/−.Cfh−/−.FHΔ16–20 mice, and wild-type animals. Cfh−/−.FHΔ16–20 mice without any evidence of renal impairment (normal urea levels and absence of hematuria on urinalysis) were used in these experiments. Three days after the administration of the nephrotoxic serum, all of the Cfh−/−.FHΔ16–20 mice developed anasarca, and hence the experiment was terminated at this time point. In contrast, at this stage both wild-type and C5−/−.Cfh−/−.FHΔ16–20 mice appeared healthy. Hematuria and uraemia was present in all of the Cfh−/−.FHΔ16–20 mice but not in either C5−/−.Cfh−/−.FHΔ16–20 or wild-type animals (Table 1). Cfh−/−.FHΔ16–20 mice also had proteinuria at this time point (Table 1). Red cell fragmentation was evident on blood films of Cfh−/−.FHΔ16–20 mice exclusively (Table 1). Glomerular C3 deposition was observed to a comparable extent in both the Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 mice, whereas no abnormal C3 staining was seen in the wild-type animals (Figure 5, A and B). Glomerular C9 deposition was detectable only in the Cfh−/−.FHΔ16–20 mice (Figure 5A). The pattern of C3 and C9 deposition after ANTN in Cfh−/−.FHΔ16–20 mice was similar to the pattern observed in the spontaneous aHUS phenotype. Renal histology showed marked glomerular thrombosis (Figure 5, C and D) and glomerular neutrophil accumulation (Table 1) in Cfh−/−.FHΔ16–20 mice, whereas appearances in the wild-type and C5−/−.Cfh−/−.FHΔ16–20 mice were normal. In summary, Cfh−/−.FHΔ16–20 mice demonstrated a uniformly hypersensitive response to ANTN that was dependent on the presence of C5.
Table 1.
Renal function in C5−/−.Cfh−/−.FHΔ16–20, Cfh−/−.FHΔ16–20, and wild-type mice during ANTN
| Wild-type (n = 5) | Cfh−/−.FHΔ16–20 (n = 4) | C5−/−.Cfh−/−.FHΔ16–20 (n = 5) | |
|---|---|---|---|
| Urea (mmol/L) | 8.1 (7.2 to 11.7) | 41 (37.5 to 41.5)a | 8.7 (7.1 to 12.2) |
| Grade of haematuria (0 to 3) | negative | 3 (2 to 3)a | negative |
| Grade of proteinuria (0 to 3) | 2 (1 to 3) | 3 (2 to 3) | 1 (1 to 3) |
| Red cell fragments (per 200 RBC) | negative | 12.5 (10 to 17)a | negative |
| Glomerular neutrophils | 0.24 (0.04 to 0.52) | 3.27 (2.48 to 3.89)a | 0.17 (0 to 0.3) |
Numbers represent median with range of values shown in parentheses.
aP < 0.001 in Cfh−/−.FHΔ16–20 mice versus wild-type or C5−/−.Cfh−/−.FHΔ16–20 mice, determined by Bonferroni multiple comparison test. The results shown are representative of three independent experiments.
Figure 5.
Cfh−/−.FHΔ16–20 but not C5−/−.Cfh−/−.FHΔ16–20 mice are hypersensitive to ANTN. (A) Glomerular C3 and C9 staining. Complement dysregulation at the level of C3, as demonstrated by the presence of abnormal glomerular C3 staining, was evident only in C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice. Glomerular C9 staining was only evident in the Cfh−/−.FHΔ16–20 animals. (B) Quantification of the glomerular C3. Glomerular C3 deposition was similar in C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 animals. In both groups the degree of staining was significantly greater than that seen in wild-type mice (Bonferroni multiple comparison test). The values for glomerular C3 deposition are given in AFU. (C) Scoring of glomerular thrombosis. Significant glomerular thrombosis was evident in all of the Cfh−/−.FHΔ16–20 mice, whereas thrombosis was absent in both C5−/−.Cfh−/−.FHΔ16–20 and wild-type animals. Glomerular thrombosis was expressed as percentages of PAS-positive material per 50 glomerular cross-sections examined. (D) Representative light microscopy images of renal sections. Original magnification, 20. Bar, 10 μm.
C3 Is Required for Spontaneous aHUS to Develop in Cfh−/−.FHΔ16–20 Mice
Recently a C3-independent C5 activation pathway has been described.23 To analyze whether such a pathway was relevant to our mouse model, we generated Cfh−/−.FHΔ16–20 mice deficient in C3 (C3−/−.Cfh−/−.FHΔ16–20 mice). An experimental cohort was then monitored over an 8-month period (n = 30). At 8 months, all of the animals remained well, and there was no evidence of hematuria. Serum urea levels were also normal (median urea = 10.8 mmol/L, range = 3.3 to 17.5, n = 30). Consistent with these observations the renal histology of the C3−/−.Cfh−/−.FHΔ16–20 mice did not show any light microscopic abnormalities (data not shown).
DISCUSSION
There is now overwhelming evidence that dysregulation of the AP of complement is involved in the pathogenesis of aHUS.24 Although activation of complement along the renal endothelium is considered to be the initiating event, the relative importance of C3 and C5 activation in the pathology is not known. In this study we have demonstrated that renal histology remained normal in cohorts of C5−/−.Cfh−/−.FHΔ16–20 animals at 4 and 8 months of age, indicating that the absence of C5 had protected Cfh−/−.FHΔ16–20 mice from the development of spontaneous aHUS. Previously our studies in Cfh−/−.FHΔ16–20 mice have shown that the ability to regulate C3 in plasma is critical for the development of renal thrombotic microangiopathy: one line of Cfh−/−.FHΔ16–20 mice expressed only low levels of the FHΔ16–20 protein and consequently did not regulate plasma C3 effectively and did not develop renal disease.22 This suggested that aHUS would only develop if there was defective protection of renal endothelium in the setting of a certain level of circulating intact C3. Therefore, a critical consideration in this study was whether the phenotypic differences between the Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 mice could be explained by differential expression of the FHΔ16–20 protein and consequently plasma C3 levels. Importantly, plasma levels of both FHΔ16–20 and C3 did not differ between the two experimental mouse lines, allowing us to conclude that the phenotypic differences could not be explained by differential degrees of C3 regulation. Additional aspects of this study supported a role for C5 activation in the development of aHUS in the Cfh−/−.FHΔ16–20 mice. First, these animals have detectable circulating plasma C5 as demonstrated by the ability of their sera to reconstitute the lytic activity of human C5-deficient sera. Second, the presence of glomerular C9 staining suggested that terminal pathway activation within the kidney occurs in this model.
Interestingly, abnormal glomerular C3 deposition was evident in C5−/−.Cfh−/−.FHΔ16–20 mice, whereas, as would be expected with complete genetic deficiency of C5, glomerular C9 staining was absent. In C5−/−.Cfh−/−.FHΔ16–20 and Cfh−/−.FHΔ16–20 mice that had not developed aHUS, the degree of abnormal glomerular C3 deposition was similar. In contrast, the abnormal glomerular C3 staining was substantially greater in the Cfh−/−.FHΔ16–20 mice that had developed aHUS. Similarly, glomerular C9 deposition was also most marked in the Cfh−/−.FHΔ16–20 with aHUS. The most likely explanation for this is that the damaged renal endothelium in the animals with aHUS provided a permissive surface for complement activation. It also suggested that a certain threshold of complement activation needed to be reached in order for renal inflammation and thrombosis to develop.
Cfh−/−.FHΔ16–20 animals developed spontaneous renal disease as we have previously reported.22 However, the development of aHUS in these animals was clearly variable: aHUS was not present in eight of the 4-month cohort (n = 26) and three of the 8-month cohort (n = 8) at the respective time points. We speculate that this variability is likely to be driven by background genetic factors because all of the animals were housed in an identical specific pathogen-free facility, thereby minimizing environmental variability. However, at this stage we cannot exclude that stochastic events may also affect the onset of the disease, a phenomenon that is seen in other spontaneous models of renal disease.
Incomplete penetrance of aHUS is a common event in carriers of CFH, MCP, CFI, C3, and factor B aHUS-associated mutations.13,19,20,24,25 Hence, it is generally accepted that a combination of multiple risk factors (i.e. genetic or environmental) is needed for disease to develop. Human aHUS is frequently triggered by an environmental insult that in most cases remain unclear. We therefore explored the response of the Cfh−/−.FHΔ16–20 and C5−/−.Cfh−/−.FHΔ16–20 animals to ANTN. Our data demonstrated that Cfh−/−.FHΔ16–20 mice were uniformly hypersensitive to renal injury triggered by ANTN, developing rapid glomerular thrombosis and glomerular neutrophil accumulation. This hypersensitive response was dependent on complement dysregulation because wild-type animals remained healthy at this time point. Importantly, the hypersensitive response of the Cfh−/−.FHΔ16–20 mice to ANTN was specifically dependent on the ability to activate C5 because the C5−/−.Cfh−/−.FHΔ16–20 animals did not develop glomerular thrombosis or neutrophil accumulation, despite demonstrating a similar degree of glomerular C3 activation. This observation also indicated that abnormal glomerular C3 deposition alone was not sufficient to trigger aHUS in this model.
Taken together, these phenotypic data demonstrate that C5 is required for aHUS to develop in Cfh−/−.FHΔ16–20 mice. Presently, we cannot determine the relative importance of the membrane attack complex and C5a in aHUS pathogenesis because both of these important mediators of inflammation are generated upon activation of C5.
Activation of C3 and C5 can theoretically be mediated by noncomplement enzymes present within inflammatory reactions. Neutrophil elastase,26 macrophage serine protease,27 as well as proteins from the coagulation system such as factors FXa and FXIa, plasmin, and thrombin have been shown to activate C3 and C5 in vitro.28 Recently, using an immune complex-mediated lung injury model, tissue damage in C3-deficient mice has been shown to be dependent on the generation of C5a by thrombin.23 However, in this study C3−/−.Cfh−/−.FHΔ16–20 mice did not develop aHUS. This demonstrated that canonical C5 activation mediated by C5 convertases is required for the development of aHUS in the Cfh−/−.FHΔ16–20 mouse model.
The strong association between AP dysregulation and aHUS makes complement inhibition an obvious therapeutic strategy. Understanding the role of C5 activation is of key importance because this is expected to occur irrespective of the particular AP mutation. We have shown that in a spontaneous and experimentally-triggered renal injury model of aHUS C5, dysregulation plays a key role. Eculizumab, an anti-C5 antibody that prevents C5 activation, has been successfully used in two aHUS patients.29,30 Although these are only anecdotal reports, our murine data strongly support the rationale that C5 inhibition should be tested in aHUS patients.
CONCISE METHODS
Animals
Cfh−/−.FHΔ16–20 mice were developed as previously reported on the CBAxC57BL/6 genetic background.22 Cfh−/−.FHΔ16–20 mice deficient in C5 (C5−/−.Cfh−/−.FHΔ16–20) were generated by intercrossing Cfh−/−.FHΔ16–20 mice with naturally DBA/J2 C5-deficient mice31 that had been back-crossed on to the C57BL/6 genetic background for 10 generations. C3−/−.Cfh−/−.FHΔ16–20 mice were generated by interbreeding Cfh−/−.FHΔ16–20 mice with C3-deficient mice32 that had been back-crossed on to the C57BL/6 genetic background for 10 generations. C57BL/6 mice were used as wild-type animals. All of the procedures were performed in accordance with institutional guidelines.
Quantification of Plasma FHΔ16–20 and C3 Levels
FHΔ16–20 levels were measured by ELISA using a goat anti-rat CFH antibody (a gift from M. Daha, Leiden University Medical Center, Leiden, The Netherlands) and a rabbit anti-mouse CFH antibody (a gift from S. Rodriguez de Cordoba, Centro de Investigaciones Biologicas, Madrid, Spain). Samples were quantified by reference to a standard curve generated using normal wild-type serum. C3 levels were measured by ELISA, and the results were quantified by reference to a standard curve generated from acute-phase sera containing a known quantity of C3 (Calbiochem) as described previously.33
Assessment of Renal Function and Blood Films
Proteinuria and hematuria levels were determined using Hema-Combistix (Bayer). Serum urea was measured by using a UV method kit (R-Biopharm Rhone) according to the manufacturer's instructions. Blood films were manually prepared using EDTA whole blood and stained using a rapid staining kit (Diff-Quik, Dade Behring). The presence of red blood cell fragments in the blood films were determined by counting the number of fragments per 200 red blood cells counted.
Hemolytic Assay
This assay was used to measure the extent to which mouse C5 was able to restore hemolytic activity to human C5-depleted sera.34 Briefly, sensitized sheep erythrocytes were prepared with subagglutinating amount of rabbit IgM antibodies. The intermediate EAC1–3b was prepared by incubating sheep erythrocytes with 1/10 dilution of human serum deficient in C5 in glucose veronal-buffered saline. Hemolytic activity was performed by mixing dilutions of mouse test sera in glucose veronal-buffered saline with 50 μl of 1% EAC1–3b suspended in 1/20 dilution of human C5-deficient serum to a final volume of 250 μl. After incubation at 37°C for 30 minutes, red cell lysis was calculated by measuring the OD415. Hemolytic activity was expressed as a percentage of lysis induced by water.
Histologic Studies
For light microscopy, the kidneys were fixed in Bouin's solution and embedded in paraffin, and the sections were stained with periodic acid Schiff (PAS) reagent. Glomerular scarring was determined by analyzing 50 glomeruli/section. The number of scarred glomeruli identified was expressed as a percentage of the total number of glomeruli examined. Glomerular thrombosis was scored by assessing the amount of PAS-positive material per glomerular cross-section. Scores of 0, 1, 2, 3, and 4 were recorded when the amount of PAS-positive material was zero, 0 to 25%, 25 to 50%, 50 to 75%, and 75 to 100% of the glomerular cross-section, respectively. A total of 50 glomeruli were examined, and the recorded totals were expressed as percentages of the maximum possible score (200 maximum). Glomerular neutrophils were identified by their characteristic morphology. The total number of neutrophils identified was expressed as a percentage of the number of glomeruli examined (50 glomeruli total). All histologic analysis was done in a blinded fashion. For immunofluorescence studies, the kidneys were snap-frozen. For C3 staining, FITC-conjugated anti-mouse C3 (ICN) was used on snap-frozen sections. C9 staining was done using rabbit IgG against mouse C9 (a gift from Prof M. Daha) followed by a secondary FITC-conjugated mouse antibody against rabbit IgG (Sigma). The staining with FITC-conjugated antibodies was quantified as described previously35 and expressed as arbitrary fluorescence units (AFU).
Induction of ANTN
To induce renal damage 200 μg of sheep IgG (Sigma, I-5131) in complete Freund's adjuvant was injected intraperitoneally 5 days before intravenous administration of sheep nephrotoxic serum. This was prepared as described previously.35
Statistical Analyses
The data were analyzed by using Mann-Whitney test for the comparison of two groups and Bonferroni's multiple comparison test for the analysis of three groups. Mortality curves were analyzed by the log rank test. The differences were considered significant for P values <0.05. The data were analyzed by using PRISM 3.0 software for Windows (GraphPad, San Diego, CA).
DISCLOSURES
None.
Acknowledgments
We thank the staff of the Biologic Services Unit at Imperial College (London, United Kingdom) for the care of the animals involved in this study and Ms. Lorraine Lawrence (Imperial College) for the processing of the histologic specimens. M.C.P. is a Wellcome Trust Senior Fellow in Clinical Science (WT082291MA). D.P.-C. was funded by a Marie Curie Fellowship (IMDEMI, 005632). F.T. was supported by grant of Ministero dell'Università e della Ricerca (PRIN).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Targeting Complement C5 in Atypical Hemolytic Uremic Syndrome,” on pages 7–9.
REFERENCES
- 1. Walport MJ: Complement: First of two parts. N Engl J Med 344: 1058–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Muller-Eberhard HJ: The membrane attack complex of complement. Annu Rev Immunol 4: 503–528, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Pickering MC, Cook HT: Translational mini-review series on complement factor H: Renal diseases associated with complement factor H: Novel insights from humans and animals. Clin Exp Immunol 151: 210–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Cordoba SR, de Jorge EG: Translational mini-review series on complement factor H: Genetics and disease associations of human complement factor H. Clin Exp Immunol 151: 1–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noris M, Bucchioni S, Galbusera M, Donadelli R, Bresin E, Castelletti F, Caprioli J, Brioschi S, Scheiflinger F, Remuzzi G: Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol 16: 1177–1183, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Richards A, Goodship JA, Goodship TH: The genetics and pathogenesis of haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Curr Opin Nephrol Hypertens 11: 431–435, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Ruggenenti P, Noris M, Remuzzi G: Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 60: 831–846, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Remuzzi G, Ruggenenti P: The hemolytic uremic syndrome. Kidney Int Suppl 66: S54–S57, 1998 [PubMed] [Google Scholar]
- 9. Caprioli J, Bettinaglio P, Zipfel PF, Amadei B, Daina E, Gamba S, Skerka C, Marziliano N, Remuzzi G, Noris M: The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol 12: 297–307, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann HP, Remuzzi G, Zipfel PF: Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest 111: 1181–1190, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez-Caballero D, Gonzalez-Rubio C, Gallardo ME, Vera M, Lopez-Trascasa M, Rodriguez de Cordoba S, Sanchez-Corral P: Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet 68: 478–484, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richards A, Buddles MR, Donne RL, Kaplan BS, Kirk E, Venning MC, Tielemans CL, Goodship JA, Goodship TH: Factor H mutations in hemolytic uremic syndrome cluster in exons 18–20, a domain important for host cell recognition. Am J Hum Genet 68: 485–490, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanchez-Corral P, Perez-Caballero D, Huarte O, Simckes AM, Goicoechea E, Lopez-Trascasa M, de Cordoba SR: Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am J Hum Genet 71: 1285–1295, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA: Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 53: 836–844, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, Porrati F, Gamba S, Remuzzi G: Familial haemolytic uraemic syndrome and an MCP mutation. Lancet 362: 1542–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Muslumanoglu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH: Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U.S.A. 100: 12966–12971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH: Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41: e84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kavanagh D, Kemp EJ, Mayland E, Winney RJ, Duffield JS, Warwick G, Richards A, Ward R, Goodship JA, Goodship TH: Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 2150–2155, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, Lopez-Trascasa M, Sanchez-Corral P, Morgan BP, Rodriguez de Cordoba S: Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U.S.A. 104: 240–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pangburn MK: Cutting edge: localization of the host recognition functions of complement factor H at the carboxyl-terminal: Implications for hemolytic uremic syndrome. J Immunol 169: 4702–4706, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Pickering MC, de Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, de Cordoba SR, Botto M: Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med 204: 1249–1256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA: Generation of C5a in the absence of C3: A new complement activation pathway. Nat Med 12: 682–687, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Esparza-Gordillo J, Jorge EG, Garrido CA, Carreras L, Lopez-Trascasa M, Sanchez-Corral P, de Cordoba SR: Insights into hemolytic uremic syndrome: Segregation of three independent predisposition factors in a large, multiple affected pedigree. Mol Immunol 43: 1769–1775, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, Carreras Berges L, Lopez-Trascasa M, Sanchez-Corral P, Rodriguez de Cordoba S: Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet 14: 703–712, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ward PA, Hill JH: C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol 104: 535–543, 1970 [PubMed] [Google Scholar]
- 27. Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA: Generation of C5a by phagocytic cells. Am J Pathol 161: 1849–1859, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M: Interaction between the coagulation and complement system. Adv Exp Med Biol 632: 71–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gruppo RA, Rother RP: Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360: 544–546, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Nurnberger J, Philipp T, Witzke O, Opazo Saez A, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M: Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360: 542–544, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Wetsel RA, Fleischer DT, Haviland DL: Deficiency of the murine fifth complement component (C5): A 2-base pair gene deletion in a 5′-exon. J Biol Chem 265: 2435–2440, 1990 [PubMed] [Google Scholar]
- 32. Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC: Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U.S.A. 92: 11490–11494, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC: Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest 118: 608–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pontoglio M, Pausa M, Doyen A, Viollet B, Yaniv M, Tedesco F: Hepatocyte nuclear factor 1alpha controls the expression of terminal complement genes. J Exp Med 194: 1683–1689, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robson MG, Cook HT, Botto M, Taylor PR, Busso N, Salvi R, Pusey CD, Walport MJ, Davies KA: Accelerated nephrotoxic nephritis is exacerbated in C1q-deficient mice. J Immunol 166: 6820–6828, 2001 [DOI] [PubMed] [Google Scholar]