Abstract
Although cystatin C is a stronger predictor of clinical outcomes associated with CKD than creatinine, the clinical role for cystatin C is unclear. We included 11,909 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) and assessed risks for death, cardiovascular events, heart failure, and ESRD among persons categorized into mutually exclusive groups on the basis of the biomarkers that supported a diagnosis of CKD (eGFR <60 ml/min per 1.73 m2): creatinine only, cystatin C only, both, or neither. We used CKD-EPI equations to estimate GFR from these biomarkers. In MESA, 9% had CKD by the creatinine-based equation only, 2% had CKD by the cystatin C-based equation only, and 4% had CKD by both equations; in CHS, these percentages were 12, 4, and 13%, respectively. Compared with those without CKD, the adjusted hazard ratios (HR) for mortality in MESA were: 0.80 (95% CI 0.50 to 1.26) for CKD by creatinine only; 3.23 (95% CI 1.84 to 5.67) for CKD by cystatin C only; and 1.93 (95% CI 1.27 to 2.92) for CKD by both; in CHS, the adjusted HR were 1.09 (95% CI 0.98 to 1.21), 1.78 (95% CI 1.53 to 2.08), and 1.74 (95% CI 1.58 to 1.93), respectively. The pattern was similar for cardiovascular disease (CVD), heart failure, and kidney failure outcomes. In conclusion, among adults diagnosed with CKD using the creatinine-based CKD-EPI equation, the adverse prognosis is limited to the subset who also have CKD according to the cystatin C-based equation. Cystatin C may have a role in identifying persons with CKD who have the highest risk for complications.
Chronic kidney disease (CKD) affects millions of adults in the United States, and its prevalence is rising, particularly in the elderly.1 Decreased GFR (GFR < 60 ml/min per 1.73 m2) has been associated with increased mortality, cardiovascular adverse events, hospitalizations, fractures, and unsuccessful aging.2–5 International guidelines recommend using creatinine-based equations to estimate GFR, particularly the Modification of Diet in Renal Disease equation.6,7 Recently, a new creatinine-based equation was developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) that reported better accuracy than the Modification of Diet in Renal7 Disease study equation, especially at estimated GFR levels above 60 ml/min per 1.73 m2.8 However, all creatinine-based estimating equations have limitations due to non-GFR determinants of serum creatinine, largely muscle mass, which cannot be accounted for entirely by age, sex, and race. This is a particular problem among the elderly, among non-white populations, and in the range of mildly reduced GFR, where equations have bias. Therefore, the clinician's reliance on creatinine-based equations for estimating GFR and the risk associated with low GFR could cause misclassification of patients who may be at high risk of CKD and its complications.
Recently, cystatin C has emerged as an alternative marker of kidney function that is less influenced by muscle mass.9–12 However, the clinical role for cystatin C measurement has not been elucidated. Cystatin C can be used to estimate GFR, and it has been associated with subsequent adverse clinical events. In prior studies in the general population and in the elderly, cystatin C has been shown to be a better predictor of mortality and adverse cardiovascular events than serum creatinine.13–15 In epidemiologic studies, an elevated cystatin C level (>1 mg/L) in persons with eGFRcreat >60 ml/min per 1.73 m2 has been used to classify persons as having preclinical kidney disease, which portends an increased risk of cardiovascular disease, incident CKD, and death.16 Several equations to estimate GFR on the basis of cystatin C have now been developed, including a CKD-EPI cystatin C equation.17 The utility of estimating reduced GFR by cystatin C versus creatinine-based estimates for predicting clinical outcomes has not been well studied.
We designed this study to compare CKD classification by the estimated GFR values of creatinine (eGFRcreat) and cystatin C (eGFRcys) in ambulatory adults. Specifically, we: (1) determined the proportions with eGFR <60 ml/min per 1.73 m2 on the basis of creatinine, cystatin C, both, and neither; (2) compared the risks for mortality, cardiovascular events, heart failure, and kidney failure among the four groups; (3) evaluated the ability of eGFRcys to detect additional cases of decreased GFR among persons with eGFRcreat ≥60; and (4) evaluated the capacity of eGFRcys to distinguish a group at higher risk for CKD complications among those with GFRcreat <60 ml/min per 1.73 m2.
RESULTS
Study Cohort Characteristics
Overall, there were 6749 Multi-Ethnic Study of Atherosclerosis (MESA) participants, with a mean age 62 ± 10 years. MESA had four major racial/ethnic groups: 39% white, 28% black, 12% Chinese, and 22% Hispanic. There was no prevalent cardiovascular disease at baseline in MESA. There were 5160 Cardiovascular Health Study (CHS) participants, with a mean age of 72 ± 5 years. CHS participants were predominantly white (84%) and 16% black. Prevalent cardiovascular disease was present in 24% of the CHS participants. We classified the cohorts into four mutually exclusive groups using cystatin C and creatinine as described above. (Table 1). In MESA and CHS, those with decreased GFR both were older and had higher prevalence of diabetes and hypertension. In MESA and CHS, the group with decreased GFR both had the lowest eGFRcreat (Table 1).
Table 1.
Characteristics of study participants in CHS and MESA
| Characteristics | MESA |
CHS |
||||||
|---|---|---|---|---|---|---|---|---|
| GFR Not Decreased | Decreased GFRcreat Only | Decreased GFRcys Only | Decreased GFR Both | GFR Not Decreased | Decreased GFRcreat Only | Decreased GFRcys Only | Decreased GFR Both | |
| n | 5759 | 614 | 107 | 269 | 3639 | 605 | 227 | 689 |
| Age | 61 (10) | 70 (8) | 67 (10) | 73 (8) | 72 (5) | 73 (5) | 74 (6) | 76 (7) |
| Male | 2738 (48) | 257 (42) | 59 (55) | 132 (49) | 1322 (38) | 264 (44) | 93 (41) | 335 (49) |
| Race | ||||||||
| white | 2127 (37) | 306 (50) | 58 (54) | 107 (40) | 3032 (83) | 511 (85) | 198 (87) | 576 (84) |
| black | 698 (12) | 64 (10) | 2 (2) | 34 (13) | 607 (17) | 94 (16) | 29 (13) | 113 (16) |
| Chinese | 1616 (28) | 148 (24) | 27 (25) | 75 (28) | ||||
| Hispanic | 1318 (23) | 96 (16) | 20 (19) | 53 (20) | ||||
| eGFRcys ml/min per 1.73 m2 | 97 (19) | 76 (12) | 55 (5) | 48 (10) | 86 (15) | 71 (9) | 54 (6) | 47 (10) |
| eGFR-CKD-EPI ml/min per 1.73 m2 | 82 (13) | 55 (5) | 71 (11) | 44 (11) | 81 (12) | 54 (5) | 72 (10) | 44 (11) |
| Diabetes | 690 (12) | 71 (12) | 17 (16) | 70 (26) | 551 (15) | 82 (14) | 46 (20) | 140 (20) |
| Hypertension | 2374 (41) | 387 (63) | 58 (54) | 214 (80) | 1446 (40) | 285 (47) | 126 (56) | 456 (66) |
| Systolic blood pressure | 125 (21) | 133 (21) | 126 (20) | 139 (26) | 135 (21) | 136 (22) | 138 (23) | 140 (24) |
| Body mass index | 28.3 (5.5) | 28.1 (4.9) | 29.8 (6.5) | 29.4 (5.9) | 26.6 (4.7) | 26.4 (4.2) | 28.1 (5.8) | 27.2 (4.9) |
The values are the means (SD) or n (%).
Death, Cardiovascular Events, and Kidney Failure by eGFR Group
Overall, there were 223 deaths and 212 CVD events in MESA after an average follow-up of 4.7 years; 3345 deaths, 2249 CVD events, 1407 incident heart failure events, and 84 confirmed ESRD cases occurred during an average of 12.2 years of CHS follow-up.
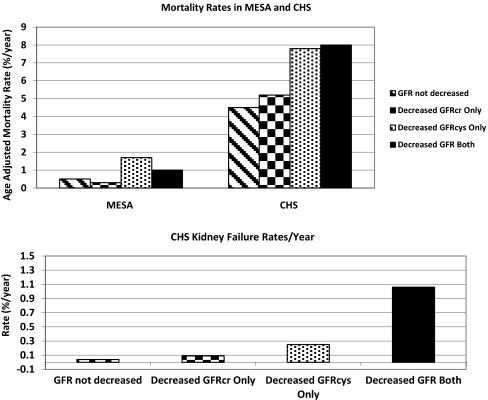
Participants with decreased GFRcys only or decreased GFR both had the highest rates of death and kidney failure, whereas those with decreased GFRcreat only had rates of death and kidney failure comparable to those with GFR not decreased. (Figure 1). In MESA, participants with decreased GFRcys only and decreased GFR both also had the highest mortality rates (3.2 and 2.7% per year, respectively), whereas participants with decreased GFRcreat only had rates of death similar to those with GFR not decreased (0.8 and 0.6%, respectively) (Figure 1).
Figure 1.
Age-adjusted rate of death was highest for those with decreased GFRcys only and decreased GFR both, but not among those with decreased GFRcreat only.
In multivariable models for MESA, the risk of death was elevated for those participants with decreased GFRcys only and decreased GFR both compared with participants with GFR not decreased. MESA participants with decreased GFRcreat only had risks of death similar to those with GFR not decreased. The risk of CVD was highest for those with decreased GFRcys only and decreased GFR both compared with those with GFR not decreased in demographic adjusted models in MESA. This effect was attenuated after full adjustment for those with decreased GFRcys only but remained significant for those with decreased GFR both (Table 2).
Table 2.
Association of decreased GFR (<60 ml/min per 1.73 m2) by cystatin C and creatinine with adverse events in MESA and CHS
| MESA |
CHS |
|||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) |
n | HR (95% CI) |
|||
| Demographic Adjusteda | Fully Adjustedb | Demographic Adjusteda | Fully Adjustedb | |||
| All-cause mortalityc | ||||||
| GFR not decreased | 5759 | 1.00 (ref) | 1.00 (ref) | 3639 | 1.00 (ref) | 1.00 (ref) |
| decreased GFRcreat only | 614 | 0.76 (0.48, 1.20) | 0.80 (0.50, 1.26) | 605 | 1.10 (0.98, 1.22) | 1.09 (0.98, 1.21) |
| decreased GFRcys only | 107 | 3.43 (1.96, 5.98) | 3.23 (1.84, 5.67) | 227 | 1.94 (1.67, 2.25) | 1.78 (1.53, 2.08) |
| decreased GFR both | 269 | 1.97 (1.31, 2.96) | 1.93 (1.27, 2.92) | 689 | 1.96 (1.78, 2.16) | 1.74 (1.58, 1.93) |
| Cardiovascular diseased | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| decreased GFRcreat only | 1.18 (0.78, 1.78) | 1.22 (0.80, 1.85) | 1.13 (0.99, 1.29) | 1.05 (0.92, 1.20) | ||
| decreased GFRcys only | 2.22 (1.13, 4.39) | 1.92 (0.97, 3.82) | 1.83 (1.52, 2.21) | 1.52 (1.26, 1.84) | ||
| decreased GFR both | 2.07 (1.32, 3.24) | 1.67 (1.06, 2.63) | 1.86 (1.65, 2.09) | 1.46 (1.29, 1.65) | ||
| Heart failuree | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | ||||
| decreased GFRcreat only | 1.08 (0.91, 1.27) | 0.99 (0.84, 1.18) | ||||
| decreased GFRcys only | 2.12 (1.68, 2.66) | 1.69 (1.33, 2.13) | ||||
| decreased GFR both | 1.91 (1.64, 2.23) | 1.43 (1.22, 1.67) | ||||
| Kidney failuref | ||||||
| GFR not decreased | 1.00 (ref) | 1.00 (ref) | ||||
| decreased GFRcreat only | 2.67 (1.03, 6.90) | 2.60 (1.00, 6.75) | ||||
| decreased GFRcys only | 7.69 (2.78, 21.25) | 6.14 (2.18, 17.29) | ||||
| decreased GFR both | 30.95 (17.0, 56.34) | 23.82 (12.68, 44.76) | ||||
ref, referent group.
aAdjusted for age, race, and gender.
bAdjusted for age, race, gender, diabetes, hypertension, LDL, HDL, CRP, and prevalent CVD for CHS (persons with baseline CVD were excluded for incident CVD analyses).
c223 deaths for MESA and 3345 deaths for CHS.
d212 events for MESA and 2249 events for CHS.
e1407 events for CHS.
f84 events for CHS.
In CHS, participants identified as having decreased GFRcys only or decreased GFR both had similarly elevated risk of death, cardiovascular events, and heart failure compared with those with GFR not decreased (Table 2). In contrast, those with decreased GFRcreat only had risks similar to those with GFR not decreased for death, CVD, and heart failure. The risk of kidney failure was the highest for those with decreased GFR both (24-fold higher), followed by decreased GFRcys only (six-fold higher) and decreased GFRcr only (two-fold) compared with those with GFR not decreased (referent) (Table 2).
Prevalence of eGFRcys <60 ml/min per 1.73 m2 among Those with eGFR Creatinine ≥ and <60 ml/min per 1.73 m2
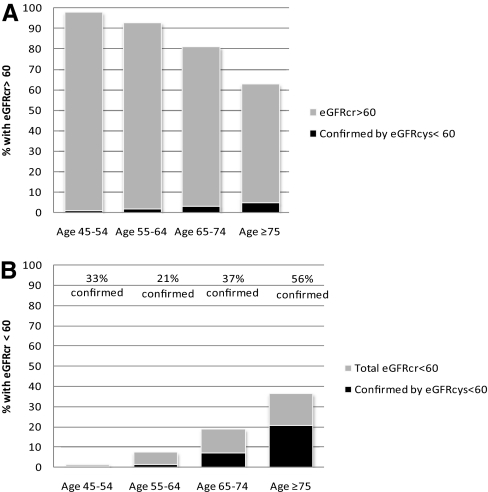
Due to the striking differences in prognosis observed among these groups, we estimated the proportion of participants classified differently by eGFRcys and eGFRcreat. Among those with an eGFRcreat ≥60 ml/min per 1.73 m2, only 4% of CHS participants had eGFRcys <60 ml/min per 1.73 m2 (n = 227), and 2% in MESA (n = 107). The prevalence varied by age, with increasing proportions in the elderly (Figure 2A).
Figure 2.
(A) The prevalence of eGFRcr ≥60 ml/min per 1.73 m2 and the proportion missed by creatinine but detected by cystatin C varies by age. (B) The overall prevalence of eGFR <60 ml/min per 1.73 m2 by creatinine and proportion confirmed by cystatin C varies by age.
Among those with eGFRcreat <60 ml/min per 1.73 m2, the proportion confirmed by eGFRcys <60 ml/min per 1.73 m2 also increased by age (Figure 2B). Among persons under age 75, less than 40% were confirmed, whereas over half of cases of eGFRcreat <60 ml/min per 1.73 m2 were confirmed by eGFRcys in the oldest age group.
Number Needed to Screen and Number Needed to Confirm
Among those with eGFRcreat ≥60 ml/min per 1.73 m2, we found that the number needed to screen to detect a single case of eGFRcys <60 ml/min per 1.73 m2 varied over 10-fold by age. Among those aged 45 to 54 years, 135 tests (95% CI 89, 283) would be needed, 60 tests (95% CI 44, 94) among those 55 to 64, 25 tests (95% CI 22,29) among those 65 to 74, and 10 tests (95% CI 9, 11) among those ≥75 years.
Among those with eGFRcreat <60 ml/min per 1.73 m2, the number needed to confirm by cystatin C was very low and decreased by age: 2.6 tests (1.8, 4.6) for those aged 45 to 54 years, 4.6 tests (95% CI 3.5, 6.8) for ages 55 to 64, 2.5 tests (95% CI 2.4, 2.8) for ages 65 to 74, and 1.5 tests (95% CI 1.4, 1.6) tests for those aged ≥75 years.
Net Reclassification Improvement
Overall, we found that the addition of eGFRcys was useful in reclassifying mortality risk among persons initially defined by eGFRcreat alone. In CHS, the annualized risk of death for persons classified by eGFRcreat as having 10 to 20% risk in 10 years was 2.7%/year. Persons who were reclassified into the lower risk category had an annual risk of 1.8%, whereas persons classified into the higher risk had an annualized rate of death of 4%. In MESA, the annual rate of death for those classified as having a 5 to 10% risk of death in 5 years was 1.3% in the CKD-EPI model. When using cystatin C, persons reclassified to the higher risk category had an annualized death rate of 2.5%, and those reclassified to the lower risk category had an annualized death rate of 1%.
In general, cystatin C was most useful in reclassifying persons to lower risk categories, particularly among CHS participants (elderly). In CHS, among persons whose risk of death was determined to be <10% by eGFRcreat, only 22 persons were reclassified as higher risk (net reclassification improvement [NRI] 1%, P value 0.46). Among persons at 10 to 20% risk of death, 213 persons were reclassified as being at higher risk for death, and 160 were reclassified as being at lower risk (NRI 15%, P value <0.001), whereas among persons classified as having a >20% risk of death by eGFRcreat, 329 persons were reclassified as having a lower risk (NRI 14%, P value <0.001). In MESA, among persons whose risk of death was determined to be <5% by eGFRcreat, 220 persons were reclassified as having higher risk (NRI 6%, P value 0.04). Among persons at 5 to 10% risk of death, 132 persons were reclassified as being at higher risk for death, and 262 were reclassified as being at lower risk (NRI 17%, P value 0.01), whereas among persons classified as having a >10% risk of death by eGFRcreat, 105 persons were reclassified as having a lower risk (NRI 8%, P value 0.07).
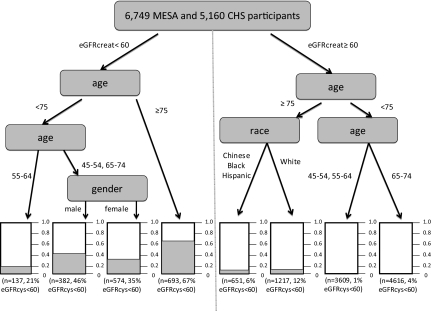
Predictors of High Risk CKD (eGFRcys <60 ml/min per 1.73 m2)
Due to the findings that the decreased GFRcreat only group had similar rates of death, CVD and heart failure to those with GFR not decreased, we defined “high risk CKD” as eGFRcys <60 ml/min per 1.73 m2. Of the variables evaluated by classification and regression tree analysis (CART), we found that an eGFRcreat <60 ml/min per 1.73 m2 and age were the best discriminators of likelihood of high risk CKD (Figure 3). Importantly, among younger participants with eGFRcreat <60 ml/min per 1.73 m2, only 21 to 46% had high risk CKD. Among persons aged >75 years with eGFRcreat >60 ml/min per 1.73 m2, 12% of white patients and 6% of non-white patients had high risk CKD that was missed by creatinine (Figure 3).
Figure 3.
CART tree for detection of eGFRcys <60 ml/min per 1.73 m2 by age, gender, and race in MESA and CHS.
Albuminuria and Cystatin C among CHS Participants with eGFRcreat <60 ml/min per 1.73 m2
At year 7 of follow-up, the mean age for CHS participants was 78 ± 5 years, and 789 participants had eGFRcreat <60 ml/min per 1.73 m2. Of those, 239 (30%) had an albumin to creatinine ratio >30 mg/g, whereas 580 (73%) had decreased eGFRcys, and 170 (22%) had neither albuminuria nor decreased eGFRcys. Only 5% (n = 39) of these participants with eGFRcreat <60 ml/min per 1.73 m2 had microalbuminuria without eGFRcys <60 ml/min per 1.73 m2. Both albuminuria and eGFRcys <60 ml/min per 1.73 m2 were associated with higher mortality rates, and participants with both had the highest risk. After multivariable adjustment, both albuminuria and GFRcys <60 ml/min per 1.73 m2 were independently associated with mortality risk, and the presence of both retained the highest risk of death compared with those with eGFRcys ≥60 ml/min per 1.73 m2 and no albuminuria (Table 3).
Table 3.
All-cause mortality among CHS participants with eGFRcreat <60 ml/min per 1.73 m2 at year 7 by albuminuria and eGFRcys
| n | Events | HR (95% CI) |
||
|---|---|---|---|---|
| Demographic Adjusteda | Fully Adjustedb | |||
| eGFRcys ≥60 and alb/cr <30 | 170 | 71 | 1.00 (ref) | 1.00 (ref) |
| eGFRcys ≥60 and alb/cr >30 | 39 | 29 | 2.01 (1.29, 3.14) | 1.94 (1.23, 3.04) |
| eGFRcys <60 and alb/cr <30 | 380 | 262 | 1.74 (1.30, 2.29) | 1.71 (1.30, 2.25) |
| eGFRcys <60 and alb/cr >30 | 200 | 181 | 3.72 (2.80, 4.96) | 3.41 (2.54, 4.59) |
Median follow-up time was 10.14 years. ref, referent group.
aAdjusted for age, gender, and race.
bAdjusted age, gender, race, diabetes, smoking, total cholesterol, body mass index, prevalent CVD, and C-reactive protein.
Sensitivity Analyses
We repeated all of the analyses using the cystatin C-based equation with demographic coefficients to estimate GFR, and we found very similar results. The primary difference was that the prevalence of eGFRcysc <60 ml/min per 1.73 m2 increased from 11 to 17% (n = 1965) in the combined MESA and CHS participants. Although the prevalence of the decreased GFRcys only group increased, the risk estimates remained similar to the results provided.
DISCUSSION
CKD is increasingly recognized as a risk factor for adverse events, including death, cardiovascular disease, and kidney failure.2,18,19 Thus, efforts to increase detection and recognition of decreased GFR have been the focus of national and international campaigns. In these analyses, we found that the presence of eGFR <60 ml/min per 1.73 m2 was only associated with elevated risk of death, cardiovascular events, and heart failure if it was confirmed by cystatin C. Persons with decreased GFR by creatinine alone had equivalent risks to persons without decreased GFR. Thus, cystatin C may have an important clinical role in distinguishing between “higher risk” and “lower risk” individuals for CKD complications with creatinine-based eGFR <60 ml/min per 1.73 m2 on the basis of this differential risk for cardiovascular and mortality outcomes.
National and international efforts have advocated improved detection of CKD by using creatinine-based equations to estimate GFR.7 Early detection requires screening tests that balance sensitivity and specificity. The creatinine-based eGFR equations may be sensitive for detecting persons with risk for adverse outcomes related to kidney disease; however, in these cohorts of multi-ethnic and older adults, specificity was greatly improved with cystatin C. This suggests a potential role for cystatin C as a confirmation test for diagnosing CKD.
Automatic reporting of eGFR by creatinine-based equations has now been widely adopted by many medical centers in the United States and abroad, which may lead to improved CKD detection. However, some have suggested that automatic reporting of eGFR labels certain persons as having kidney disease who may be at low risk for complications. Recently, automatic reporting of eGFR was shown to be associated with an increase in referrals to nephrologists,20,21 which in some studies has been associated with improved survival but may also result in unnecessary testing, treatments, and cost. If confirmed by future studies, cystatin C may be a useful test to determine which patients warrant prioritization for nephrology referral or additional testing.
We were surprised to find that the prevalence of decreased GFR on the basis of cystatin C only was relatively low in this diverse cohort of ambulatory subjects, particularly in the nonelderly. However, this group was at very high risk for adverse events. The number needed to screen to detect such individuals was high overall but decreased to about 10 among persons over the age of 75. Even although the screening yield for detecting decreased GFR <60 ml/min per 1.73 m2 may be relatively low, cystatin C has also been shown to detect preclinical kidney disease, which is associated with higher risk of mortality, cardiovascular disease, heart failure, and kidney disease progression.16 As demonstrated by our risk stratification tree, the likelihood of high risk CKD in adults can be stratified from 1 to 67% on the basis of the creatinine-based GFR, age, sex, and race. These proportions can be used to estimate the yield for cystatin C testing as either a confirmatory test or as a screening test in high risk groups. Since the presence of albuminuria can also detect persons at high risk for adverse events who have an eGFRcreat >60 ml/min per 1.73 m2,22 future studies should focus on evaluating the cost effectiveness of a triple screen of renal markers strategy to include creatinine, albuminuria, and cystatin C measurements.
The strength of our study is that it is the first to demonstrate a potentially important role for cystatin C in the clinical setting of CKD detection and confirmation. Our sample size is large, ethnically diverse, and fairly representative of the U.S. population. Moreover, CHS and MESA are very well characterized cohorts with standardized measures of kidney function and rigorously adjudicated outcomes. However, our study lacks information on kidney failure in MESA, thus limiting some of our findings to the elderly. Moreover, cystatin C has been shown to be associated with factors other than kidney function.23 However, our results are robust after adjustment for most of these factors, suggesting that cystatin C's associations with adverse events are predominantly due to its approximation of kidney filtration. Most importantly, like all population-based epidemiologic studies, our study is limited by the lack of measured GFR. Thus, we cannot determine which participants actually have a decreased GFR, as measured by the clearance of an exogenous filtration marker. Although we are limited by the lack of albuminuria measures at baseline, our secondary analyses in CHS suggest that cystatin C has utility for risk stratification, even when combined with albuminuria among elderly participants with decreased eGFR by creatinine.
Detection of CKD is likely an important step for preventing complications associated with this disease. We believe that our findings suggest a possible targeted approach whereby cystatin C confirmation can be used in a step-wise manner to identify individuals at higher risk for CKD complications among those with eGFR creatinine <60 ml/min per 1.73 m2 with or without albuminuria. We found that only subjects with confirmed decreased GFR by cystatin C had elevated risk of death, cardiovascular disease, and heart failure, and they had an extremely elevated risk of kidney failure. In addition, only a limited number of cystatin C tests would need to be used, with high-yield results, to confirm the diagnosis in ambulatory adults. Participants classified as decreased GFR by cystatin C but not by creatinine were also at increased risk for adverse events, although the number needed to screen to detect these individuals was an order of magnitude higher than the number needed to confirm decreased GFR. Now that cystatin C is Food and Drug Administration approved and more readily available across United States laboratories, this strategy could greatly improve the specificity of CKD screening programs and limit the resulting burden of nephrology referrals, disease-labeling, and additional diagnostic tests and treatments.
CONCISE METHODS
Study Subjects
We used data from the MESA and the CHS that together provide a wide range of age, kidney function, and race/ethnicity. The MESA is an NHLBI, National Institutes of Health-sponsored study to understand subclinical cardiovascular disease and its progression in a racially diverse cohort. Details on recruitment and design have been previously published.24 MESA recruited 6814 men and women who were between 45 and 84 years old, who were free of cardiovascular disease, and who self-identified as white, African American, Hispanic, or Chinese American. Subjects were recruited from Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota between July 2000 and August 2002. Participants were excluded from the study if they had physician diagnosed heart attack, angina, heart failure, stroke, transient ischemic attack, or atrial fibrillation; had undergone coronary artery bypass grafting, angioplasty, valve replacement, or pacemaker; or weighed >300 lbs.24
The CHS is a community-based longitudinal study designed to understand risk factors for the development and progression of cardiovascular disease. CHS recruited community-dwelling adults who were 65 years of age or older using Medicare eligibility lists in four sites: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Participants were excluded if they were not expected to remain in the current community for 3 years or longer, were receiving treatment for cancer, or were unable to provide informed consent. The initial 5201 participants were enrolled from January 1989 to June 1990; an additional 687 black participants (with race self-reported) were recruited and enrolled by June 1993. The study details and design have been published previously.25 The institutional review boards at all participating centers approved these studies, and all participants gave informed consent. For these analyses, we included participants from CHS and MESA who had a serum creatinine and a serum cystatin C measured at baseline in MESA (n = 6749) or at the initial visit for CHS (i.e. 1989–1990 for original CHS cohort or 1992–1993 for the CHS black participants, n = 5160).
Kidney Function Measurements
All of the assays were performed in frozen serum specimens that were stored at −70°C. Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring) with a nephelometer (BNII; Dade Behring). Serum cystatin C was calibrated to Cleveland Clinic using internal standards supplied by the manufacturer to both sites. Serum creatinine was measured by a colorimetric method (Ektachem 700; Eastman Kodak) in CHS. In MESA, serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, New York) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, Minnesota). Serum creatinine was calibrated directly to Cleveland Clinic in MESA and indirectly in CHS.3,26 We estimated the GFR with the use of the newly developed CKD-EPI creatinine equation8 and the CKD-EPI cystatin C equation without demographic coefficients: eGFRcys = 76.7 × cystatin C−1.19.17 Both formulae were developed from the pooling of several cohorts with GFR measured from iothalamate clearance. Urine albumin and creatinine were not available at baseline in CHS but were measured at year 7 in CHS using nephelometry.
Covariates
Age, gender, race, income, education, past or present smoking, and alcohol use were ascertained by questionnaire at the baseline visit. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index was calculated as weight in kilograms divided by height in meters squared. Fasting blood was collected and stored at −70°F until needed for the appropriate assays, including HDL cholesterol, triglycerides, glucose, and C-reactive protein. LDL cholesterol was calculated using the Friedewald equation. Hypertension was defined as the use of antihypertensive medications, a self-report of hypertension, or a BP of >140/90 mmHg at the baseline visit. Diabetes was defined as a self-report of diabetes, the use of insulin or oral hypoglycemic agents, or a fasting glucose of ≥126 mg/dl. Three BP measurements were obtained 5 minutes apart in the seated position. The mean of the second two measurements was used for analysis. For CHS only, prevalent cardiovascular disease was defined as having a history of coronary heart disease, heart failure, or stroke.
Ascertainment of Outcomes
Outcomes of interest included: (1) death from all causes; (2) incident cardiovascular event (CVD), defined as myocardial infarction, cardiac arrest, stroke, or cardiovascular death; (3) incident heart failure (for CHS only); and (4) incident kidney failure (for CHS only). Each of the four outcomes was considered separately.
In MESA, in addition to the follow-up visits, an interviewer contacted each participant or family member by telephone every 9 to 12 months. A trained interviewer inquired about interim hospital admissions, outpatient diagnoses of cardiovascular disease, and deaths. To verify self-reported diagnoses, MESA requested copies of medical records for participants who had been hospitalized or received an outpatient diagnosis of cardiovascular disease. Records were obtained on 98% of reported cardiovascular events associated with hospitalization. For participants who had died of cardiovascular causes outside the hospital, MESA conducted interviews with the next of kin and requested copies of death certificates. Two physicians who were members of the MESA mortality and morbidity review committee independently classified events and assigned incidence dates. If they disagreed, the full committee made the final classification. Ascertainment of stroke was on the basis of clinical symptoms and brain imaging. A more detailed description of the MESA follow-up methods is available at www.mesa-nhlbi.org, and the methods have been previously published.27,28 Median follow-up time was 4.7 years.
In CHS, follow-up visits were conducted by telephone every 6 months and in person annually. All of the events were adjudicated by a CHS outcome-assessment committee. Deaths were identified by a review of obituaries, medical records, death certificates, and the Centers for Medicare and Medicaid Services health care-utilization database for hospitalizations and from household contacts; 100% complete follow-up for ascertainment of mortality status was achieved. Cases of CVD events were ascertained from hospital records that included clinical histories, elevated cardiac enzyme levels, electrocardiographic changes, and brain imaging studies. Incident heart failure was adjudicated on the basis of diagnosis from a physician and consideration of symptoms, signs, chest radiographic findings, and treatment of heart failure. More details on event adjudication have been previously published.14 Kidney failure was identified by linking the CHS cohort to the United States Renal Data System (USRDS) in 2005 (which includes data through March 31, 2003) to identify individuals who initiated dialysis or underwent kidney transplantation. In addition to linking the CHS cohort to the USRDS, a chart review was performed to determine which CHS participants met the criteria for initiation of dialysis or transplantation, elected not to undergo these therapies, died before receiving them, or died before being enrolled in the USRDS. Median follow-up time was 12.2 years.
Statistical Analyses
Participant characteristics were summarized by cohort. We first estimated GFR using equations on the basis of creatinine and by cystatin C separately for each individual. We then categorized individuals into four groups defined by presence or absence of eGFR <60 ml/min per 1.73 m2 on the basis of cystatin C and creatinine: (1) those with eGFRcreat <60 ml/min per 1.73 m2, and eGFRcys <60 ml/min per 1.73 m2 herein described as decreased GFR both; (2) those with eGFRcreat ≥60 ml/min per 1.73 m2 but eGFRcys <60 ml/min per 1.73 m2, herein described as decreased GFRcys only; (3) those with eGFRcreat <60 ml/min per 1.73 m2 but eGFRcys ≥60 ml/min per 1.73 m2, herein described as decreased GFRcreat only; and (4) those with eGFRcreat ≥60 ml/min per 1.73 m2, and eGFRcys ≥60 ml/min, herein described as GFR not decreased. We estimated the prevalence of each group in CHS and MESA separately.
For each of the above four groups, we estimated the incidence rates of death and cardiovascular disease in MESA and CHS, and the rates of heart failure and kidney failure in CHS only. Then, using Cox proportional hazard models, we determined their association with the risks for death, cardiovascular events, incident heart failure, and kidney failure in separate models. We adjusted for the above-mentioned covariates chosen a priori from the literature as potential confounders of the association of eGFR <60 ml/min per 1.73 m2 with adverse outcomes.
We used data from MESA and CHS combined to estimate the prevalence of eGFRcys <60 ml/min per 1.73 m2 among persons with eGFRcreat <60 or ≥60 ml/min per 1.73 m2, overall and by decade of age. Using these proportions, we estimated the number needed to screen to detect additional cases of CKD, which was calculated as (1/(# with decreased cys only/# with eGFRcreat ≥60 ml/min per 1.73 m2). We also calculated the number needed to confirm CKD, which was calculated as (1/ (# with decreased GFR both /# eGFRcreat <60 ml/min per 1.73 m2). We stratified these analyses by ages.
We also estimated the net reclassification improvement for eGFRcys.29 To this end, we constructed a risk prediction model for death including eGFRcreat <60 ml/min per 1.73 m2 as the primary predictor and adjusting for age, gender, race, diabetes, hypertension, LDL and HDL cholesterol, smoking status, body mass index, and C-reactive protein. We classified persons into three categories of mortality risk in CHS (10 years risk of death <10%, 10 to 20%, or >20%) and MESA (5 years risk of death <5%, 5 to 10%, or >10%) separately. We replaced eGFRcreat with eGFRcys in the model and estimated the reclassification of persons into higher or lower risk strata. We calculated the net reclassification improvement as the sum of the proportions of correctly reclassified subjects with and without death.
Based upon the results of the multivariate analyses, we used CART to determine the likelihood of eGFRcys <60 ml/min per 1.73 m2, on the basis of combinations of age, gender, race, and serum creatinine. CART is on the basis of recursive partitioning and is an empirical method that can generate decision trees with binary splits for best model fit.30
In a secondary analysis, we compared the prevalences of albuminuria (defined as albumin to creatinine ratio >30 mg/g) and eGFRcys <60 ml/min per 1.73 m2 among CHS participants with GFRcreat <60 ml/min per 1.73 m2. We used data from the CHS year 7 exam, because urine albumin and creatinine were not available at baseline. We categorized the CHS participants with eGFRcreat <60 ml/min per 1.73 m2 into four mutually exclusive groups: (1) eGFRcys ≥60 ml/min per 1.73 m2 and no albuminuria; (2) eGFRcys ≥60 ml/min per 1.73 m2 and albuminuria; (3) eGFRcys <60 ml/min per 1.73 m2 and no albuminuria; and (4) eGFRcys <60 ml/min per 1.73 m2 and albuminuria. We estimated the age adjusted mortality rates for each group and used Cox proportional hazards to determine adjusted associations with mortality risk for each group.
Finally, we performed a sensitivity analysis using the cystatin C eGFR estimating equation that includes demographic coefficients.17 We divided the MESA and CHS participants into the four mutually exclusive groups on the basis of eGFRcys and eGFRcr, and we replicated our primary analyses.
All of the analyses were performed using S-Plus (release 8.0; Insightful Inc, Seattle, WA) and SPSS statistical software (release 16.0.1; SPSS Inc, Chicago, IL). Two-tailed P values <0.05 were considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute for MESA. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
For CHS, the research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133; grant number U01 HL080295 from the National Heart, Lung, and Blood Institute; with additional contributions from the National Institute of Neurologic Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
This work was also funded by the NIDDK (1K23DK082793-01 to C.P.) and R01DK 066488 (to M.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Is Cystatin C the Answer to Detecting Progression in CKD?” on pages 9–11.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Sarnak MJ, Katz R, Fried LF, Siscovick D, Kestenbaum B, Seliger S, Rifkin D, Tracy R, Newman AB, Shlipak MG: Cystatin C and aging success. Arch Intern Med 168: 147–153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J: Cystatin C: A new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101: 875–881, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Shlipak MG, Sarnak MJ, Katz R, Fried L, Seliger S, Newman A, Siscovick D, Stehman-Breen C: Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol 45: 268–271, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J: Method of Glomerular Filtration Rate Estimation Affects Prediction of Mortality Risk. J Am Soc Nephrol 20: 2214–2222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 19. Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Kritchevsky SB, Harris TB, Satterfield S, Cummings SR, Shlipak MG: Renal function and heart failure risk in older black and white individuals: The Health, Aging, and Body Composition Study. Arch Intern Med 166: 1396–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 20. den Hartog JR, Reese PP, Cizman B, Feldman HI: The costs and benefits of automatic estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol 4: 419–427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jain AK, McLeod I, Huo C, Cuerden MS, Akbari A, Tonelli M, van Walraven C, Quinn RR, Hemmelgarn B, Oliver MJ, Li P, Garg AX: When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney Int 76: 318–323, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Rifkin DE, Katz R, Chonchol M, Fried LF, Cao J, de Boer IH, Siscovick DS, Shlipak MG, Sarnak MJ: Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly. Nephrol Dial Transplant 25: 1560–1567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS: Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP: Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 156: 871–881, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Kramer H, Palmas W, Kestenbaum B, Cushman M, Allison M, Astor B, Shlipak M: Chronic kidney disease prevalence estimates among racial/ethnic groups: The Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol 3: 1391–1397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS: Cardiovascular events with absent or minimal coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 158: 554–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM: Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172 and 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Breiman L: Classification and Regression Trees, New York, Kluwer Academic Publishers, 1984 [Google Scholar]