Abstract
Monocyte chemoattractant protein 1 (MCP-1) mediates acute ischemic and toxic kidney injury, but whether this can be used as a biomarker of acute kidney injury (AKI) is unknown. We obtained kidney and urine samples from mice with intrarenal (maleate), prerenal (endotoxemia), or postrenal (ureteral obstruction) injury. We also studied the independent effects of uremia without concomitant kidney injury by performing bilateral ureteral transection in mice. Additionally, we obtained urine samples from APACHE II–matched critically ill patients with or without advancing azotemia (n = 10 in each group). We assayed selected samples for MCP-1, MCP-1 mRNA, and for an activating histone mark (H3K4m3) at urinary fragments of the MCP-1 gene and contrasted the results with those obtained for neutrophil gelatinase-associated lipocalin (NGAL), a comparator “AKI biomarker” gene. Maleate increased urinary MCP-1 protein and mRNA more than the corresponding increases in NGAL. Endotoxemia and ureteral obstruction also increased NGAL and MCP-1 gene expression. Uremia, in the absence of renal injury, induced the NGAL gene, but not MCP-1, suggesting the possibility of better specificity of MCP-1 for AKI. Clinical assessments supported the utility of MCP-1 as a biomarker (e.g., nonoverlapping concentrations of urinary MCP-1 in patients with and without AKI). Elevated levels of urinary MCP-1 mRNA and levels of H3K4m3 at the MCP-1 gene supported MCP-1 gene activation in patients with renal injury. In conclusion, these data suggest that MCP-1 has potential as a biomarker of AKI and provide “proof of concept” that urinary histone assessments provide mechanistic insight among patients with kidney disease.
The onset of acute kidney injury (AKI) is typically a silent clinical event, almost always being documented in its aftermath by the onset of progressive azotemia. This is in striking contrast to acute heart or brain injury, whose onsets are clinically apparent (e.g., angina and myocardial infarction; acute neurologic deficits). Given AKI's insidious onset, reliable “biomarkers” for it have been sought. The underlying rationale has been that early detection can lead to early therapy, thereby preventing the onset of acute renal failure (ARF). Toward this end, three general diagnostic approaches have been employed. First, increased excretion of “resident” proximal tubule proteins has been sought. Candidate molecules have included both structural proteins (e.g., renal tubular epithelial, “RTE” antigen; i.e., megalin; see reference 1) and proximal tubular enzymes (e.g., N-acetylglucosaminidase; alkaline phosphatase and γ-glutamyltransferase).2–6 A second approach has been to document proximal tubular dysfunction, as denoted by decreased tubular reabsorption of freely filtered low molecular weight proteins (e.g., β2 microglobin, lysozyme, and cystatin C).7–9 Third, the approach that recently has received the greatest attention has been quantifying the urinary excretion of renal tubular proteins that are acutely overexpressed in response to AKI. Two notable examples within this category are kidney injury molecule-1 (KIM-1) and neutrophil gelatinase–associated lipocalin (NGAL).10–16 However, the latter general approach has a number of potential shortcomings. As examples, (i) neither KIM-1 nor NGAL provide information as to underlying disease pathogenesis; (ii) substantial extrarenal NGAL generation in response to systemic stress can increase urinary NGAL excretion in the absence of AKI; (iii) although NGAL is a presumptive marker of proximal tubule injury, it may be generated in the distal nephron, and thus reflect distal, rather than proximal, tubule events;14 and (iv) NGAL and KIM-1 overexpression may arise from chronic, and not just acute, renal disease.10–16
In a recent experimental study,17 this laboratory tested the hypothesis that the urinary excretion of injury-induced mRNAs, and associated histone alterations at their cognate genes, might also have potential utility as AKI biomarkers, particularly if used in conjunction with the above noted approaches. Toward this end, we quantified the urinary excretion of the mRNAs for monocyte chemoattractant protein-1 (MCP-1), the profibrotic cytokine TGF-β1, and the histone-modifying enzyme BRG-1 (Brahma-related gene) after experimental ischemic AKI.17 In addition, an injury-induced histone alteration was sought (trimethylation of histone 3, at the lysine 4 position; so-called H3K4m3). In each case, increased urinary concentrations of these posited “biomarkers” were observed. What makes this type of approach unique is that quantifying urinary mRNA levels, and corresponding histone modifications at their cognate genes, may provide pathogenetic, as well as diagnostic, insights. Hence, the present study was undertaken to further explore the potential utility of this novel approach with the following specific goals in mind: (1) expand upon the above noted concepts by testing a nephrotoxic (maleate), rather than an ischemic,17 model of experimental AKI; (2) ascertain whether these approaches might have utility in the clinical arena; and (3) given that MCP-1 is uniformly upregulated in nephrotoxic and ischemic forms of AKI (e.g., see references 18–22), test whether it might have diagnostic value as an AKI biomarker, in general, and in comparison to NGAL, the latter being used as a comparator “reference” biomarker gene.
RESULTS
Mouse Investigations
Maleate ARF Model.
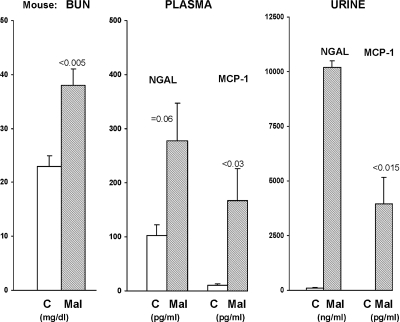
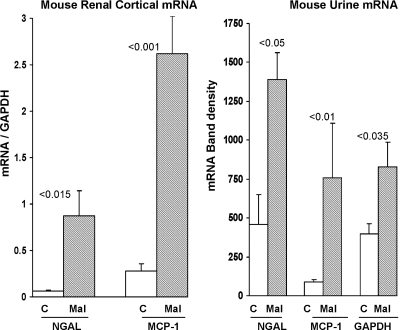
Within 4 hours of maleate injection, a significant increase in blood urea nitrogen (BUN) levels was already apparent (Figure 1, left). Consistent with induction of AKI, increased MCP-1 and NGAL protein levels were observed in both plasma (Figure 1, middle) and in urine (Figure 1 right). As shown in Figure 2, left, the increased plasma and urine NGAL and MCP-1 protein levels were associated with dramatic and comparable percentage increases in their cognate renal cortical mRNA levels, implying that increased renal synthesis was at least partially responsible for the elevated urinary NGAL and MCP-1 protein levels. As shown in Figure 2, right, the NGAL and MCP-1 protein increases were associated with ninefold and threefold increases in urinary MCP-1 mRNA and NGAL mRNA levels, respectively. However, these increases were also associated with a doubling of urinary glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (828 ± 161 versus 406 ± 64 band densities). This implies that at least part of the ninefold and threefold increases in urinary MCP-1 and NGAL mRNAs reflected nonspecific cell/mRNA sloughing.
Figure 1.
Maleate induces azotemia and increases urinary and plasma NGAL and MCP-1 levels within 4 hours of injection. Renal injury 4 hours after maleate injection was assessed by BUN, NGAL, and MCP-1 concentrations. Significant azotemia was already apparent at 4 hours after maleate (MAL) injection, versus controls (C) with corresponding increases in plasma NGAL and MCP-1 concentrations. This injury was associated with massive increases in urinary NGAL and MCP-1 concentrations, indicating their extreme sensitivity as early AKI biomarkers. Plasma NGAL and MCP-1 protein levels were also markedly elevated, indicating increased synthesis and possibly a reduction in glomerular filtration.
Figure 2.
Maleate increases NGAL and MCP-1 gene expression within 4 hours of injection. NGAL and MCP-1 mRNAs were assessed in renal cortex and in urine at 4 hours after maleate injection. By 4 hours after maleate injection, marked increases in renal cortical NGAL and MCP-1 mRNAs were apparent (indicating that the increase in urine NGAL and MCP-1 protein levels likely reflected, at least in part, renal production). There were corresponding threefold and ninefold increases in urinary NGAL and MCP-1 mRNAs, compared with control values. However, there was also a doubling of the mRNA for GAPDH, a housekeeping gene. The latter finding suggests that at least part of the urinary NGAL and MCP-1 mRNA increases reflected nonspecific tubular cell sloughing.
NGAL and MCP-1 mRNA Responses to Lipopolysaccharide, Ureteral Obstruction, and Uremia.
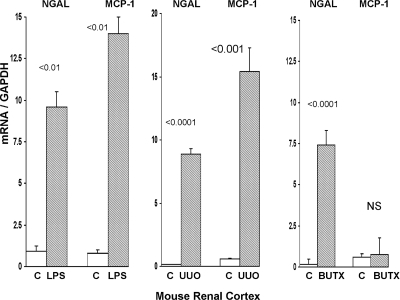
As shown in Figure 3, both lipopolysaccharide (LPS) and unilateral ureteral obstruction-induced striking, and reasonably comparable, increases in renal cortical MCP-1 and NGAL mRNAs. Induction of uremia in the absence of structural renal damage (BUTx) caused an approximate 50-fold increase in renal cortical NGAL mRNA. Conversely, no increase in MCP-1 mRNA was observed in the BUTx uremia model. BUTx raised the BUN from control values of 25 ± 6 to 134 ± 10 mg/dl.
Figure 3.
Endotoxemia, ureteral obstruction, and bilateral ureteral transection affect MCP-1 and NGAL gene expression. NGAL and MCP-1 gene expression were assessed in mouse renal cortex after induction of either a prerenal form of ARF (endotoxemia “LPS”), postrenal ARF (unilateral ureteral obstruction, UUO), or the induction of uremia in the presence of structurally normal kidneys (BUTx). Both LPS and UUO caused marked increases in renal cortical NGAL and MCP-1 mRNAs (assessed at 2 and 18 hours after LPS and UUO). Uremia (BUN 14 ± 14 mg/dl), as induced by BUTx, caused a massive increase in NGAL mRNA. Conversely, BUTx did not impact MCP-1 mRNA expression.
Clinical Proof of Concept Study
Serum Assessments.
Serum Creatinine.
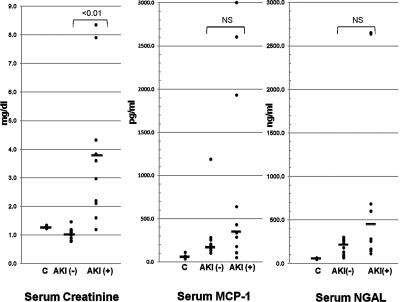
The initial mean serum creatinines for the AKI− and AKI+ patients were 1.0 and 3.8 mg/dl (AKI− versus AKI+ patients; P < 0.01). Individual values for each subject are presented in Figure 4.
Figure 4.
Clinical acute kidney injury increases plasma NGAL and MCP-1 levels. Serum creatinine, serum MCP-1, and serum NGAL values were assessed for individuals within the three test clinical groups. (C = normal individuals; AKI− = ICU patients without ARF; AKI+ = ICU patients with ARF. As expected, the serum creatinines were significantly elevated in the AKI+ group compared with the AKI− group (mean values of 3.8 versus 1.0 mg/dl, respectively). Serum MCP-1 values did not significantly differ between the three groups (although the trend was clearly toward increasing MCP-1 concentrations from controls → to AKI− → AKI+). No significant correlation was observed between serum MCP-1 and serum creatinine concentrations. However, in the case of NGAL, a significant correlation did exist between serum NGAL and serum creatinine (r = 0.5; P < 0.05) concentrations. However, no overt statistical difference between the means for the three groups was observed. Solid horizontal lines = mean values.
Serum MCP-1 and NGAL.
Serum MCP-1 levels did not significantly differ between the three clinical groups (Figure 4). Furthermore, there was no significant correlation between the MCP-1 and serum creatinine concentrations (r = 0.18; comparing all AKI− and AKI+ patients together). As with MCP-1, serum NGAL concentrations also did not statistically differ among the control, AKI−, and AKI+ patient groups (Figure 4). However, unlike MCP-1, a significant correlation (r = 0.50; P < 0.05) did exist between serum NGAL and serum creatinine concentrations.
Urine MCP-1 and NGAL.
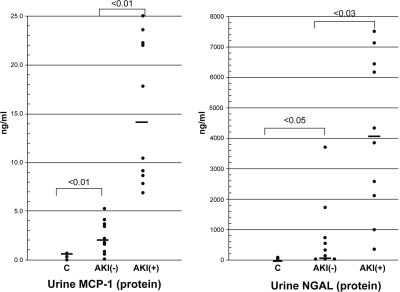
Urinary MCP-1 and NGAL concentrations were not normally distributed, and thus the absolute urine values were first analyzed by Wilcoxon rank sum test. As shown in Figure 5, there was no overlap in the absolute urine MCP-1 concentrations for the AKI− and AKI+ groups (P < 0.01). Conversely, there was substantial overlap in urinary NGAL concentrations for the AKI− and AKI+ groups, although these two groups still showed a statistically significant difference in urine NGAL levels (<0.03). No significant correlation was observed between urine MCP-1 versus urine NGAL, suggesting that these two assays may provide complementary information by reflecting different pathogenic mechanisms. Furthermore, neither urinary MCP-1 nor NGAL significantly correlated with the serum creatinine concentrations (r = 0.02 and 0.27, respectively).
Figure 5.
Clinical acute kidney injury increases urinary NGAL and MCP-1 protein levels. Urinary MCP-1 and NGAL concentrations were measured in the control, AKI−, and AKI+ groups. There was a complete separation (no overlap) of urine MCP-1 concentrations between the AKI− and AKI+ groups (<0.01). Conversely, significant overlap in urinary NGAL values for the AKI− and AKI+ groups was observed. Despite this overlap, the mean NAGL urine values for these two groups were statistically different (P < 0.03). The solid horizontal lines reflect mean values.
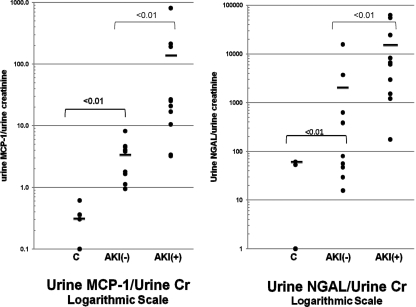
To further analyze urinary MCP-1 and NGAL levels, the values were log-transformed and factored by urine creatinine (Cr) concentrations (Figure 6). Almost a complete separation of these transformed MCP-1 values was observed for the AKI− and AKI+ groups (P < 0.01). Conversely, there was an approximate 50% overlap in the AKI− and AKI+ transformed urine NGAL values (although a statistical difference still existed between the AKI− and AKI+ groups by unpaired t test). Of note, these differences did not merely reflect differences in urine Cr concentrations (used to factor MCP-1 and NGAL values), given that the mean urine Cr values were nearly identical for the AKI− and AKI+ groups (91 ± 20 versus 89 ± 28 mg/dl, respectively).
Figure 6.
Clinical acute kidney injury increases urinary NGAL and MCP-1 protein levels, as assessed following log transformation and factoring by urine creatinine. Log base 10 conversions of urinary MCP-1 and NGAL concentrations for the three study groups were assessed. Because the absolute NGAL and MCP-1 concentrations did not have a Gaussian distribution, the values were converted to log base 10. The values were then factored by corresponding urine creatinine (Cr) values. As shown, using these corrected values, MCP-1 showed less overlap between the AKI+ and AKI− groups, versus substantial overlap with the converted NGAL values. It is noteworthy that even the AKI− patients showed a trend toward higher NGAL and MCP-1 values, compared with the normal control group, suggesting that even the AKI− group had sustained subclinical renal injury.
Total Urine Protein Assessments.
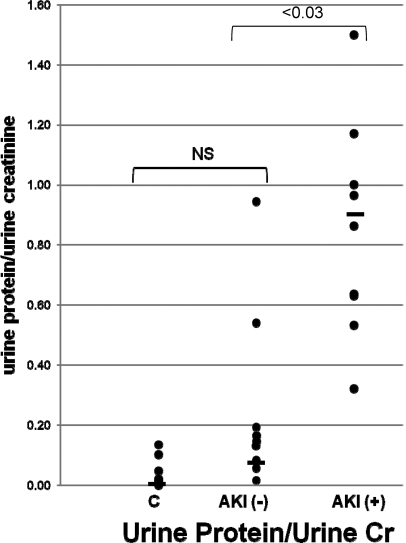
As shown in Figure 7, the AKI+ patients had, on average, an approximate tenfold increase in total urine protein/urine creatinine concentrations, compared with the AKI− patients. Only two of the ten AKI− patients manifested significant overlap with the range that was observed in the AKI+ group. Similarly, urine albumin/creatinine ratios were significantly higher in the AKI+ versus AKI− patients (0.74 ± 0.03 versus 0.1 ± 0.0.1 mg/mg creatinine; P < 0.035 unpaired t test). The correlation coefficient between absolute urine albumin and absolute total protein concentrations was 0.96.
Figure 7.
Clinical acute kidney injury increases urinary protein concentrations. Total urine protein concentrations as determined by the pyrogallol red-molybdate assay method were assessed with the results factored by the corresponding urine creatinine concentrations. As shown, there was no statistical difference between the control and AKI− patient groups. However, an average tenfold increase in urine protein concentrations were observed between the AKI− and AKI+ groups with only two AKI− patients showing overlap with the AKI+ values.
Urinary mRNA Assessments.
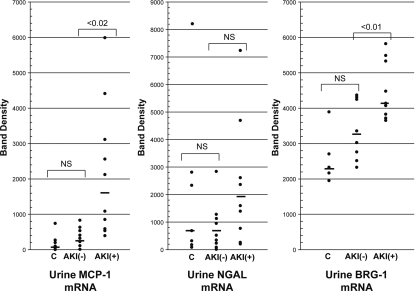
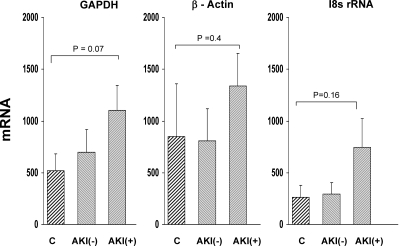
The band densities for urinary MCP-1, NGAL, and BRG-1 mRNAs are presented in Figure 8. As shown, the AKI+ group manifested significant increases in MCP-1 and BRG-1 mRNAs, compared with the AKI− group (P < 0.02 and P < 0.01, respectively). However, substantial overlap in individual values was apparent. In contrast to MCP-1 and BRG-1 mRNAs, no significant difference in urinary NGAL mRNA values was observed between the groups. As shown in Figure 9, housekeeping (reference) mRNAs for GAPDH, β-actin, and 18 sRNA showed stepwise increases from control to AKI− and to AKI+ groups. However, none of these differences achieved statistical significance.
Figure 8.
Clinical acute kidney injury increases urinary concentrations of NGAL, MCP-1, and BRG-1 mRNAs. Band densities for MCP-1, NGAL, and BRG-1 mRNAs were determined in urines obtained from the three clinical groups (controls, C; AKI− and AKI+). Statistically significant differences were observed for MCP-1 and BRG-1 mRNAs between the AKI− and AKI+ groups. Conversely, no significant differences for urinary NGAL mRNA levels were observed.
Figure 9.
Clinical acute kidney injury can increase the urinary concentrations of GAPDH mRNA, actin mRNA, and 18s rRNA. Urinary mRNA levels were determined for two housekeeping genes (GAPDH and β-actin) and for 18s rRNA, used an RNA reference for nonspecific RNA sloughing). As can be seen, although there were no significant differences among the three groups, there was a trend toward increasing housekeeping RNA excretion into urine across the three patient groups (AKI+ > AKI− > controls, albeit NS). This suggests that at least some of the mRNA differences shown in Figure 8 may have reflected nonspecific RNA sloughing.
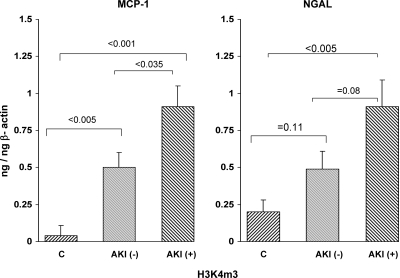
H3K4m3.
As shown in Figure 10, the AKI+ patients manifested significantly greater amounts of urinary H3K4m3 levels specifically located at exon 1 of both the MCP-1 and the NGAL gene. This was true whether or not these values were factored by H3K4m3 levels that were found at the β-actin housekeeping gene (as depicted in Figure 10). As with many of the other biomarkers discussed above, the increased levels of H3K4m3 at the MCP-1 and NGAL gene fragments demonstrated stepwise increases from control to AKI− and to AKI+ groups, suggesting that these patients showed a stepwise progression of injury, rather than an absolute presence (AKI+ patients) or absence of injury (“AKI−”), per se.
Figure 10.
Detection of H3K4m3 in urine from patients with acute kidney injury. Amounts of H3K4m3 recovered in urine that were specifically associated with exon 1 of the MCP-1 and NGAL genes were assessed. The values were factored by simultaneously obtained amounts of H3K4m3 at exon 1 of β-actin. The AKI+ patients manifested highly significant increases in H3K4m3 at both the MCP-1 and the NGAL genes. However, even the AKI− negative patients showed a trend toward increased H3K4m3 at both genes, consistent with intermediate gene activation, compared with the AKI− and normal volunteer groups.
DISCUSSION
Nephrotoxic and ischemic proximal tubule injury remain the two leading causes of AKI. Thus, considerable effort has been devoted to identifying urinary biomarkers for them, both for differential diagnostic purposes and for permiting early disease detection. Well-recognized “diagnostic markers,” such as FeNa and urine sediment analysis, continue to have important roles in differential diagnosis. Indeed, a recent study by Perazella et al. underscored the importance of urine microscopy as an AKI biomarker.23 However, urinalysis lacks sensitivity, and hence, more sophisticated tests, such as NGAL, KIM-1, and cystatin C, have been introduced into the experimental and clinical arenas. However, as previously discussed (Introduction), shortcomings to each of these approaches exist. Hence, the present study has sought some alternative ways to document AKI, while at the same time providing specific pathogenic insights. Toward this end, the following questions were addressed: First, because renal MCP-1 production is an important mediator of toxic and ischemic ARF, might its urinary concentration be reflective of this pathogenic pathway, and at the same time, serving as an acceptable AKI biomarker? Second, might the presence of MCP-1 mRNA in the urine provide some support for the concept that injury-induced increases in urinary MCP-1 concentrations likely reflect, at least in part, increased intrarenal production (as opposed to arising simply from extrarenal generation with subsequent urinary excretion)? And third, might it be possible to identify “gene-activating” chromatin changes at specific gene fragments in urine? If so, this approach might offer a unique opportunity to study potential mechanisms for gene activation and disease pathogenesis in the clinical setting.
As an initial inquiry into these issues, we conducted experiments in the maleate model of nephrotoxic ARF. Specifically, the goal was to assess the potential utility of MCP-1 as an AKI biomarker, compared with a current AKI biomarker “gold standard,” NGAL. As shown in Figure 1, within 4 hours of maleate injection, an approximate 15-fold increase in plasma MCP-1 concentrations was observed. This contrasted with only a twofold increase in plasma NGAL levels. Given that maleate causes proximal tubule specific injury in vivo, this preferential increase in plasma MCP-1 versus NGAL indicates MCP-1's high sensitivity as an AKI biomarker. This is underscored by the marked corresponding increases in renal cortical MCP-1 mRNA and urinary MCP-1 protein excretion, comparable to the corresponding NGAL assessments. However, to put these results into context, it should also be noted that, by 4 hours after maleate injection, a significant increase in BUN concentrations had occurred (38 versus 22 mg/dl for controls; P < 0.005). It is commonly stated that endogenous markers of glomerular filtration, most notably, BUN and creatinine, are neither sensitive nor early AKI biomarkers. At least in the case of experimental AKI, the present results suggest that this is not necessarily the case.
In a previous publication, we noted that, by 18 hours after renal ischemia-reperfusion injury in the mouse, it was possible to detect an increase in urinary MCP-1 mRNA.17 To further explore this potential diagnostic approach, we have now measured both MCP-1 and NGAL mRNAs in urine at 4 hours after maleate injection. Approximate ninefold and threefold MCP-1 and NGAL mRNAs increases were observed, respectively, roughly paralleling the changes in renal cortical mRNA and their plasma protein levels. This suggests that urinary mRNA levels could conceivably serve as a semiquantitative surrogate marker of intrarenal gene transcription. However, it is important to note that at least some of these ninefold and threefold urinary mRNA increases likely reflected nonspecific tubular cell sloughing, given that twofold increases in urinary GAPDH mRNA levels were also observed.
Because an AKI biomarker should ideally reflect proximal tubular-specific injury, we assessed the relative degrees to which MCP-1 and NGAL were induced by well-established “prerenal” (endotoxemia) and postrenal (ureteral obstruction) ARF models in which proximal tubular necrosis typically does not occur.24,25 As shown in Figure 3, in both of these models, marked renal cortical MCP-1 and NGAL mRNA increases were observed. We next tested whether merely the presence of uremia, independent of direct tubular injury, might also impact NGAL and MCP-1 expression. In this regard, the BUTx model (which raised BUNs to approximately 140 mg/dl) evoked massive renal cortical NGAL mRNA increments, whereas no change in MCP-1 mRNA was observed. In concert, these findings underscore a relative lack of NGAL and MCP-1 specificity as markers of structural proximal tubular injury. However, MCP-1 might have a potential advantage in this regard, given that it was not independently induced by uremia. The cellular sources of these MCP-1 and NGAL increases remain to be defined. For example, they could arise from injured glomeruli, proximal tubules, distal nephron segments, or infiltrating inflammatory cells. However, it is noteworthy that proximal tubular cells do respond to ischemic and toxic injury with brisk activation of the TNF-α and MCP-1 genes (e.g., see reference 29). Thus, it seems highly likely that injured proximal tubules, per se, contribute to AKI− induced urinary cytokine elevations.
A major hypothesis derived from the above experimental studies was that MCP-1 has at least comparable utility to NGAL as an AKI biomarker. Hence, we next assessed whether clinical support for this hypothesis might exist. Toward this end, urine samples were obtained from ten intensive care unit (ICU) patients who manifested progressive azotemia and who ultimately required hemodialysis (AKI+ group). A unique feature of this clinical study was the inclusion of a comparator ICU patient group: ten critically ill patients (comparable APACHE II scores to those of the AKI+ group) who did not manifest azotemia. An additional control group consisted of urine samples from normal volunteers. A number of important findings emerged from these clinical studies. First, in agreement with the mouse studies, it appeared that urinary MCP-1 is at least comparable to urine NGAL as a marker of AKI. Notably, there was no overlap in urine MCP-1 concentrations between the AKI− and the AKI+ groups, compared with NGAL where substantial data overlap existed. Second, the AKI+ group manifested a ninefold increase in urinary MCP-1 mRNA levels, compared with the AKI− group (quantitatively mirroring the mouse study results). Conversely, no significant difference in urinary NGAL mRNA was observed between the AKI+ and AKI− patient cohorts. Third, again as seen in the mouse maleate model, at least some of the urinary MCP-1 mRNA increases likely reflected nonspecific cell sloughing, given that increased (albeit insignificant) levels of urinary GAPDH mRNA, β-actin mRNA, and 18s RNA levels were observed; and fourth, total urine protein concentrations, typically viewed as a standard biomarker of glomerulopathy, also appeared to have substantial utility as an AKI biomarker, rivaling both urinary NGAL or MCP-1 protein levels. Urinary albumin concentrations were also significantly higher in the AKI+ versus AKI− group, suggesting albumin (or total urine protein) assessments also have potential AKI biomarker utility. This concept was also recently advanced by Devarajan et al.26 Indeed, we suggest that, given their widespread availability, urine total protein and/or albumin levels can serve as important reference tests when interpreting urinary levels of more specific AKI protein biomarkers.
A number of important caveats need to be considered when interpreting the above clinical results. First, with regard to mRNA analysis, no attempts were made to prevent urinary mRNA degradation, other than simple freezing of the urinary samples. Nevertheless, significant differences in mRNA values between the groups could still be detected. This strongly suggests that the application of mRNA preservation techniques, such as the addition of RNase inhibitors, or perhaps isolating and quantitating mRNAs in specific urinary compartments (e.g., exosomes), might substantially increase the utility of urinary mRNA analyses. Second, it is notable that the AKI− group tended to have higher urinary MCP-1, urinary NGAL, and urinary mRNA values than did the normal control group. This suggests that the distinction of “AKI+” versus “AKI−” is somewhat arbitrary. Rather, a spectrum of renal injury likely exists in critically ill (ICU) patients, which may confound biomarker data interpretation. In this regard, it is notable that 70% of AKI− patients had sepsis syndrome without AKI. As shown by the animal data, sepsis syndrome/LPS injection can independently raise NGAL and MCP-1 urine concentrations, and this may account for the NGAL/MCP-1 elevations observed in the AKI− patient group. Third, we observed that there was no advantage to factoring urinary NGAL or urinary MCP-1 protein values by their corresponding urine creatinine concentrations. With regard to the current patient cohorts, this lack of diagnostic benefit may simply reflect the fact that the urine creatinine concentrations were essentially identical for the AKI+ and AKI− groups. However, it may also be that factoring by urine creatinine simply introduces additional clinical variables (e.g., creatinine excretion and urine concentrations), thereby complicating data interpretation; and fourth, dividing urinary mRNA values by a housekeeping gene product decreased, rather than increased, their apparent utility. We assume that this is due to the fact that nonspecific urinary mRNA sloughing occurs, impacting both housekeeping and biomarker gene mRNA levels. Furthermore, differences in individual mRNA stabilities in urine exist, which can also impede data interpretation. For example, housekeeping gene mRNAs likely have greater stability in urine than do NGAL and MCP-1 mRNAs; thus, factoring one mRNA by another can lead to erroneous conclusions.
Our final goal was to test a hypothesis that was derived from our prior experimental studies: that is, that injury-induced gene-activating histone modifications at specific genes can be detected in urine and provide a glimpse into clinical disease pathogenesis. Because increases in urinary MCP-1 protein and mRNA levels were observed in the AKI+ patient population, compared with their AKI− controls, we sought to determine whether increased amounts of a gene-activating histone change, H3K4 trimethylation,27 could also be detected at exon 1 of urinary MCP-1 gene fragments. Toward this end, H3K4m3 was immunoprecipitated from urine, and then the associated chromatin was sheared and probed for MCP-1 exon 1 by qPCR. The values obtained were then corrected for nonspecific H3K4m3 sloughing by factoring them by H3K4m3 levels at the β-actin “housekeeping” gene. As shown in Figure 10, a significant increase in H3K4m3-MCP levels could be documented in AKI+ patients, versus the AKI− controls. These elevations persisted despite factoring by the levels at the β-actin gene. This implies that the results were not merely a reflection of nonspecific nucleoprotein excretion. Indeed, histone proteins are far more likely to be stable in urine than is mRNA, and thus assessments of them may provide more accurate information concerning in vivo gene activity. Of note, increased amounts of H3K4m3 could also be detected at urinary NGAL gene fragments. This further suggests the potential utility of urinary chromatin immunoprecipitation (ChIP) assay for assessing in vivo gene activity. Finally, it is notable that urinary mRNA levels for the histone-modifying enzyme BRG-1 were elevated in AKI+ patients, compared with AKI− controls. We recently observed that BRG-1 plays an important mechanistic role in MCP-1 gene activation after renal tubular injury.28 Thus, the present result further suggests a role for BRG-1 as a potential mediator of AKI− initiated inflammatory pathways in the clinical arena.
In conclusion, using a combination of mouse studies and human urine assessments, we suggest the following: First, urinary MCP-1 may be a useful biomarker of AKI, and possibly provide complementary information to that arising from NGAL analysis; second, urinary mRNA assessments may be useful adjuncts for interpreting urinary “biomarker protein” levels; and third, we provide the first “proof of concept” evidence that urinary histone assessments, directed at “activating histone marks” at specific target genes, may be a highly useful, and novel, tool for studying pathogenetic mechanisms in patients with kidney disease.
CONCISE METHODS
Maleate Model of AKI
When injected into rodents, maleate undergoes selective proximal tubule cell uptake via organic anion transporters.29 Upon its intracellular metabolism, severe ATP and glutathione depletion result, culminating in apoptotic or necrotic cell death.29 With use of this model to complement previous studies with ischemia/reperfusion,17 the following questions were addressed: (1) Does the maleate model rapidly induce MCP-1 and NGAL mRNA in renal cortex? (2) If so, is there an associated increase in urinary MCP-1 and NGAL mRNAs? (3) What are the relative utilities of urinary MCP-1 versus NGAL protein levels as “biomarkers” of these processes? All mouse experiments were conducted following Fred Hutchinson Cancer Research Center IACUC guidelines, using 25- to 30-g male CD-1 mice (Charles River Laboratories, Wilmington, MA). Twelve mice were place in restraining tubes and then injected with either sodium maleate (800 mg/kg intraperitoneally [ip]; n = 6) or its vehicle (1 ml of saline; n = 6). Four hours later, they were deeply anesthetized with pentobarbital (50 mg/kg ip), the abdominal cavities were opened, and urine was expressed from the urinary bladder. Terminal plasma samples were obtained from the vena cava by venipuncture, and then the kidneys were excised. The cortices were dissected and total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). The urine samples were centrifuged, and the pellets were extracted for total RNA. The cortical RNA samples were assayed for MCP-1 and NGAL mRNAs by competitive reverse transcription-polymerase chain reaction (RT-PCR).30 The results were expressed as ratios to a simultaneously determined GAPDH cDNA product. Urine samples were assayed in Singleplex RT-PCR reactions for MCP-1, NGAL, and GAPDH. Plasma and urine samples were assayed for NGAL and MCP-1 protein levels using commercially available ELISAs (R&D Systems, Minneapolis, MN). BUN levels were also assessed.
Models of Prerenal and Postrenal Acute Renal Insufficiency
Ideally, a urinary AKI biomarker would specifically identify acute structural proximal tubular injury (the primary lesion in both ischemic and toxic forms of ARF). In rodents, Gram negative sepsis and endotoxemia primarily induce renal vasoconstriction and, hence, a hemodynamic form of ARF (i.e., in the absence of tubular necrosis24,31). Acute urinary tract obstruction also results in ARF in the absence of early proximal tubule cell death.25 Hence, to gain insights as to the relative specificities of NGAL and MCP-1 as markers of structural proximal tubule injury, their mRNAs were assessed in renal cortex after either endotoxemia or urinary tract obstruction.
Endotoxemia.
Five mice were placed into restraining tubes and injected via the tail vein with 2 mg/kg of Escherichia coli (0111B4) LPS (Sigma L-2630; stock solution, 4 mg/ml saline). Five mice received an equal amount of saline vehicle. Two hours later, the mice were anesthetized and the kidneys were extracted and analyzed for MCP-1 and NGAL mRNAs as noted above.
Urinary Tract Obstruction.
Five mice were deeply anesthetized, the abdominal cavity was opened, and the left ureter was ligated at its approximate midpoint. The abdominal cavities were then sutured and the mice were allowed to recover from anesthesia. Free food and water access were provided. Approximately 18 hours later, the mice were re-anesthetized and both the obstructed (left kidney) and the nonobstructed right kidney were removed. The relative impacts of the left ureteral obstruction on renal cortical MCP-1 and NGAL mRNAs was assessed by comparison to values observed in nonobstructed right kidneys.
Independent Effects of Uremia on MCP-1/NGAL mRNA Expression.
Uremia, per se, could conceivably represent a sufficient stress to independently impact renal cortical NGAL and MCP-1 gene expression. The following experiment addressed this possibility. Five mice were subjected to bilateral ureteral transection, as described previously.32 This diverts urine flow to the retroperitoneum and peritoneal cavity, thereby inducing severe renal failure (BUNs approximately 120 to 140 mg/dl) in the presence of normal kidneys (i.e., no structural damage.). Eighteen hours later, the mice were anesthetized and the kidneys were removed and harvested for RNA. They were then assayed for NGAL and MCP-1 mRNAs, as noted above.
Clinical Proof of Concept Study
Study Population.
Twenty human serum and urine samples were obtained from a matched nested case-controlled study performed at Vanderbilt University Medical Center (the VALID study; a single-center, prospective observational study of critically ill adult patients treated in multiple ICUs33). The research project was reviewed and approved by the Vanderbilt University institutional review board (IRB). Informed consent was obtained from the patient or surrogate, whenever possible; however, given the minimally invasive nature of the study, a waiver of consent was permissible as deemed by the Vanderbilt IRB. The samples were collected between February 2006 and March 2009. They were collected on the morning of ICU day 2 from ten critically ill patients who developed AKI (AKI+), defined as ≥50% or ≥26.5 μmol/L increase in serum creatinine from the value measured closest to enrollment and who ultimately required hemodialysis. ICU control samples were collected from ten critically ill patients who did not develop AKI (AKI−), but who had comparable overall illness severity as judged by APACHE II scores (see Table 1). The AKI + and AKI− populations were also matched for age, race, gender, and sepsis status. As normal controls, eight urine and five serum samples were obtained from healthy volunteers (data not included in Table 1). Thus, a total of 28 urine and 25 serum samples were used for the clinical portion of this study. All patients in the study population had a presumptive nephrologic diagnosis of “acute tubular necrosis,” as judged by consulting nephrologists.
Table 1.
Patient demographics for the study AKI+ and AKI− populations
| Patient Demographics | AKI− Population (n = 10) | AKI+ Population (n = 10) |
|---|---|---|
| Patient characteristics | ||
| age (average) | 28 to 70 (46.6) | 23 to 65 (45.5) |
| gender | 60% Women | 60% Women |
| race | 90% Caucasian | 90% Caucasian |
| 10% African American | 10% African American | |
| Comorbidities | ||
| CKD | 0 | 0 |
| diabetes | 2 | 1 |
| hypertension | 3 | 3 |
| cardiovascular disease (CHF and CAD) | 1 | 2 |
| PVD | 0 | 0 |
| chronic liver disease | 0 | 2 |
| Clinical data | ||
| admission diagnosis (N) | Sepsis (7) | Sepsis + ATN (5) |
| Lymphoma + ATN (1) | ||
| Tumor lysis + ATN (1) | ||
| ATN (2) | ||
| ESLD, hypotension + ATN (1) | ||
| AKIN stage (N) | I (4) | I (0) |
| based on creatinine measured in research laboratory compared to baseline or admission | II (1) | II (0) |
| III (0) | III (10) | |
| APACHE II score (average) | 20 to 41 (29.8) | 20 to 45 (32.7) |
| RRT requirement | 0% | 100% |
| overall mortality | 0% | 30% |
| sepsis | 70% | 70% |
| Laboratory data | ||
| ICU admission creatinine (mg/dl) (average) | 0.5 to 1.0 (0.7) | 0.5 to 7.96 (3.0) |
| sample creatinine as measured in research laboratory | 0.8 to 1.5 (1.0) | 1.2 to 8.3 (3.8) |
| peak creatinine (average) | 0.57 to 1.23 (0.83) | 3.9 to 9.58 (6.3) |
| baseline creatinine (average) | 0.5 to 0.96 (0.7) | 0.5 to1.1 (0.8) |
MCP-1/NGAL/Creatinine Measurements.
Matching serum and urine samples were assayed for (1) creatinine (Jaffe method), (2) NGAL (urine levels measured in the Shared Resource Cytokine laboratory, Fred Hutchinson Cancer Research Center, by ELISA; serum levels measured at the Kidney Research Institute, Seattle, WA (BioPorto Diagnostics, Denmark), (3) MCP-1 (measured by ELISA; R&D Systems), (4) total urine protein concentrations (pyrogallol red-molybdate assay method34), and (5) urine albumin concentrations by the bromocresol green method (Bioassays, Hayward, CA).
Urine mRNA Analysis.
Total RNA was extracted from centrifuged urine pellets using the RNeasy Plus Kit according to manufacturer's instructions (Qiagen). The mRNAs for MCP-1, NGAL, BRG-1, GAPDH, and β-actin, and also 18s rRNA were assessed in all urine samples by RT-PCR and expressed as band densities.29 The employed primers are presented in Table 2.
Table 2.
Primers used to quantify human mRNAs in urine samples
| Human Primers RT-PCR | Product Length (bp) | |
|---|---|---|
| MCP-1 | 5′-TCG CTC AGC CAG ATG CAA TCA ATG-3′ (sense) | 250 |
| 5′-AGT TTG GGT TTG CTT GTC CAG GTG-3′ (antisense) | ||
| NGAL | 5′-TCT CAG AGA AGA CAA AGA CCC GCA-3′ (sense) | 321 |
| 5′-AGT TCC GAA GTC AGC TCC TTG GTT-3′ (antisense) | ||
| GAPDH | 5′-CTC TTC ACC ACC ATG GAG AAG-3′ (sense) | 490 |
| 5′-GCT TCA CCA CCT TCT TGA TGT CAT-3′ (antisense) | ||
| b-actin | 5-ACC AAC TGG GAC GAC ATG GAG AAA′-3′ (sense) | 856 |
| 5′-ACT CCT GCT TGC TGA TCC ACA TCT-3′ (antisense) | ||
| 18s | 5′-CCA GAG CGA AAG CAT TTG CCA AGA-3′ (sense) | 499 |
| 5′-TAT TGC TCA ATC TCG GGT GGC TGA-3′ (antisense) | ||
| BRG-1 | 5-TCC GGC AGA AGA AAT CAT CAC GGA′-3′ (sense) | 224 |
| 5′-TCC ACT GCT GCT CTC CTT GTA CTT-3′ (antisense) |
ChIP Analysis
We have previously shown that trimethylation of lysine 4 of histone (converting H3K4 to H3K4m3) is a gene-activating chromatin modification that occurs in response to both nephrotoxic and ischemic renal injury in mice.17,28 Thus, urinary levels of H3K4m3 were sought at the MCP-1 and NGAL genes (exon 1) using previously described ChIP methodologies. Briefly, chromatin samples were obtained from formalin-fixed urinary pellets and immunoprecipitated with H3K4m3 antibody.17,28 The pellets were formalin-fixed and sheared, and the fragmented immunoprecipitates were then subjected to real time PCR (qPCR) using the primers presented in Table 3. The results were expressed as the amount of H3K4m3 detected at exon 1 of MCP-1 and NGAL genes, factored by H3K4m3 at exon 1 of the β-actin reference housekeeping gene. The primer sets used are presented in Table 3.
Table 3.
Primers used to detect exon 1 of the MCP-1, NGAL, and β-actin gene in human urine samples by chromatin immunoprecipitation assay
| Human Primers ChIP | |
|---|---|
| MCP-1 | 5′-GAA TGA AGG TGG CTG CTA TG-3′ (sense) |
| 5′-AAC CCA GAA ACA TCC AAT TCT C-3′ (antisense) | |
| NGAL | 5′-CTT CTT CGG CCC TGA AAT-3′ (sense) |
| 5′-GGT TGT CCT GGA AGT TCT GC-3′ (antisense) | |
| β-actin | 5′-GCT ATT CTC GCA GCT CAC CA-3′ (sense) |
| 5′-CAC GAT GGA GGG GAA GAC-3′ (antisense) | |
Calculations and Statistical Analysis.
All values are presented as means ± 1 SEM. Statistical comparisons between unpaired data were made by unpaired t test. Urine MCP-1 and NGAL protein values were also contrasted by Wilcoxon rank sum test (for nonparametric data). Significance was judged by a P value of <0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK384342; R21-DK083315; HL081332) and from nonrestricted research funds from the Kidney Research Institute, Seattle, Washington.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Zager RA, Carpenter CB: Radioimmunoassay for urinary renal tubular antigen: A potential marker of tubular injury. Kidney Int 13: 505–512, 1978 [DOI] [PubMed] [Google Scholar]
- 2. Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, Philipp T, Kribben A: Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem 50: 552–558, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Blaikley J, Sutton P, Walter M, Lapsley M, Norden A, Pugsley W, Unwin R: Tubular proteinuria and enzymuria following open heart surgery. Intensive Care Med 29: 1364–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Kharasch ED, Frink EJ, Jr., Zager R, Bowdle TA, Artru A, Nogami WM: Assessment of low-flow sevoflurane and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology 86: 1238–1253, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Zager RA: Urinary protein markers of tubulointerstitial nephritis. Invest Urol 8: 197–202, 1980 [PubMed] [Google Scholar]
- 6. Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL: A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 28: 486–494, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Zager RA: Lysozyme and albumin radioimmunoassays. New techniques for the study of proteinuria. Invest Urol 17: 526–528, 1980 [PubMed] [Google Scholar]
- 8. Ma Y, Li Q, Wang J, Xu Z, Song C, Zhuang R, Yang K, Yang A, Jin B: Cystatin C, a novel urinary biomarker for sensitive detection of acute kidney injury during haemorrhagic fever with renal syndrome. Biomarkers 15: 410–417, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Parikh CR, Lu JC, Coca SG, Devarajan P: Tubular proteinuria in acute kidney injury: A critical evaluation of current status and future promise. Ann Clin Biochem 47(Pt 4): 301–312, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Bonventre JV: Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 241: 78–83, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV: Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devarajan P: NGAL in acute kidney injury: From serendipity to utility. Am J Kidney Dis 52: 395–399, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J: Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A: GAL Meta-analysis Investigator Group: Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Zager RA, Johnson AC: Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol 296: F1032–F1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, Nath KA: Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: Implications for chronic kidney disease. Am J Physiol Renal Physiol 292: F837–F844, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA: MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kanakiriya SK, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA: Heme: A novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol 284: F546–F554, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Rice JC, Spence JS, Yetman DL, Safirstein RL: Monocyte chemoattractant protein-1 expression correlates with monocyte infiltration in the post-ischemic kidney. Renal Fail 24: 703–723, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Zager RA, Johnson AC, Lund S, Hanson S: Acute renal failure: Determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Perazella MA, Coca SH, Hall IE, Iyanam U, Koraishy, Parikh CR: Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 5: 402–408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK: Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol 290: F1453–F1462, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Zager RA: Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int 47: 628–637, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Devarajan P, Krawczeski CD, Nguyen MT, Wang Z, Parikh CR: Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis 56: 632–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zager RA, Johnson AC: Progressive histone alterations and proinflammatory gene activation: Consequences of heme protein/iron-mediated proximal tubule injury. Am J Physiol Renal Physiol 298: F827–F837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naito M, Zager RA, Bomsztyk K: BRG1 increases transcription of proinflammatory genes in renal ischemia. J Am Soc Nephrol 20: 1787–1796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zager RA, Johnson AC, Naito M, Bomsztyk K: Maleate nephrotoxicity: Mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294: F187–F197, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Johnson AC, Becker K, Zager RA: Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Physiol Renal Physiol 299: F426–F435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang W, Falk SA, Jittikanont S, Gengaro PE, Edelstein CL, Schrier RW: Protective effect of renal denervation on normotensive endotoxemia-induced acute renal failure in mice. Am J Physiol Renal Physiol 283: F583–F587, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Zager RA: Uremia induces proximal tubular cytoresistance and heme oxygenase-1 expression in the absence of acute kidney injury. Am J Physiol Renal Physiol 296: F362–F368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA: Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 20: 1823–1832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orsonneau JL, Coiuet P, Masssoubre P, Lustenerger P, Bernard S: An improved pyrogallol red-molybdate method for determining total urinary protein. Clin Chem 35: 2233–2236, 1989 [PubMed] [Google Scholar]