Abstract
Adequate respiratory and laryngeal motor control are essential for speech, but may be impaired in Parkinson's disease (PD). Bilateral subthalamic nucleus deep brain stimulation (STN DBS) improves limb function in PD, but the effects on respiratory and laryngeal control remain unknown. We tested whether STN DBS would change aerodynamic measures of respiratory and laryngeal control, and whether these changes were correlated with limb function and stimulation parameters. Eighteen PD participants with bilateral STN DBS were tested within a morning session after a minimum of 12 h since their most recent dose of anti-PD medication. Testing occurred when DBS was on, and again 1 h after DBS was turned off, and included aerodynamic measures during syllable production, and standard clinical ratings of limb function. We found that PD participants exhibited changes with DBS, consistent with increased respiratory driving pressure (n = 9) and increased vocal fold closure (n = 9). However, most participants exceeded a typical operating range for these respiratory and laryngeal control variables with DBS. Changes were uncorrelated with limb function, but showed some correlation with stimulation frequency and pulse width, suggesting that speech may benefit more from low-frequency stimulation and shorter pulse width. Therefore, high-frequency STN DBS may be less beneficial for speech-related respiratory and laryngeal control than for limb motor control. It is important to consider these distinctions and their underlying mechanisms when assessing the impact of STN DBS on PD.
Keywords: Aerodynamics, Air pressure, Air flow, High frequency, Laryngeal resistance, Voice
Background
Parkinson's disease (PD) affects millions of people worldwide, resulting in impairment of limb motor control and substantial deficits of speech motor control, including abnormal respiratory, laryngeal, and supralaryngeal control for breathing and speech [1–11]. The Unified Parkinson's Disease Rating Scale (UPDRS) is commonly employed to assess individuals with PD [12], and is a reasonable limb-assessment tool that correlates well with more specific measures of limb motor function [6, 13]. However, the UPDRS contains only two speech-related items that correlate weakly with more specific measures of speech function [14]. Therefore, the ability to efficiently sample and index specific speech-related respiratory and laryngeal physiology would be valuable in assessing disease severity and the potential influence of intervention on PD.
Adequate respiratory driving pressure is essential for speech, but it is often substantially decreased in PD. These deficits contribute to reduced speech audibility and intelligibility, linguistic confusion, and communicative impairment [15–18]. Respiratory driving pressure during speech may be assessed by measuring subglottal air pressure (PS) directly through a hypodermic needle inserted into the trachea, or indirectly through a polyethylene tube placed in the oral cavity during simple syllable ([ ]) production [19, 20]. Therefore, measuring air pressure in the oral cavity during syllable production provides a non-invasive estimation of respiratory driving pressure.
]) production [19, 20]. Therefore, measuring air pressure in the oral cavity during syllable production provides a non-invasive estimation of respiratory driving pressure.
Respiratory driving pressure during speech results in air flow through the larynx and upper airway. Air flow may be measured directly through a face mask coupled to an air flow sensor placed over the mouth and nose, and provides information regarding respiratory and laryngeal function. For example, the peak in the air flow signal following release of the consonant in the syllable [ ] is influenced by respiratory driving pressure and the degree of vocal fold abduction. The slope of declination in the air flow signal following the peak may provide information on the relative timing of phonatory onset. Mean vocalic air flow during the vowel in [
] is influenced by respiratory driving pressure and the degree of vocal fold abduction. The slope of declination in the air flow signal following the peak may provide information on the relative timing of phonatory onset. Mean vocalic air flow during the vowel in [ ] reflects vocal fold adduction and closure for voicing. Intraoral pressure and vocalic air flow can be used together to estimate laryngeal airway resistance associated with vocal fold closure. Finally, simple time integration of the air flow signal provides a measure of lung air volume expended for each syllable. Therefore, measuring air pressure and air flow during syllable production provides a simple, non-invasive assessment to examine physiological changes in speech respiratory and laryngeal control in PD and response to intervention such as deep brain stimulation (DBS).
] reflects vocal fold adduction and closure for voicing. Intraoral pressure and vocalic air flow can be used together to estimate laryngeal airway resistance associated with vocal fold closure. Finally, simple time integration of the air flow signal provides a measure of lung air volume expended for each syllable. Therefore, measuring air pressure and air flow during syllable production provides a simple, non-invasive assessment to examine physiological changes in speech respiratory and laryngeal control in PD and response to intervention such as deep brain stimulation (DBS).
DBS of the subthalamic nucleus (STN) has rapidly emerged during the past decade as a treatment for advanced PD [21, 22]. The primary goal of STN DBS is to improve limb-related function as defined using the UPDRS. As such, DBS has been associated with significant improvements in limb motor function and quality of life [13, 23]. However, previous speech-related reports on STN DBS demonstrated different outcomes [24–37] (see reviews [32, 38–40]). One possible explanation for these differences may be that high-frequency STN DBS is optimized for limb function as determined by the UPDRS, and the localization and programming of the DBS electrodes are optimized for limb-related somatotopy. However, improved ability to generate larger magnitudes of force, as reported in limb and orofacial force control studies, may be less beneficial for the complex, fine forces required for speech and voice [41–43]. In fact, previous reports suggest that speech may benefit more from low-frequency stimulation than high-frequency stimulation [41, 42]. Therefore, the patterns of fine force control contributing to speech production, including respiratory and laryngeal control, may respond differently than limb function to DBS. However, no studies have examined changes in aerodynamic measures of speech-related respiratory and laryngeal control in PD with STN DBS. Therefore, the primary objective of the present study was to examine the effects of bilateral STN DBS on aerodynamic measures of respiratory and laryngeal control in PD participants. The second objective was to examine the possible association between clinical ratings of limb function (e.g., UPDRS) and stimulation parameters (e.g., frequency, pulse width) with changes in the aerodynamic measures.
Methods
Participants
The protocol was approved by the local institutional ethics committee for the safety of human subjects and written informed consent was obtained from all participants. Data were collected from 18 individuals (15 men, 3 women) with advanced idiopathic PD, an average of 11.5 years since diagnosis and a minimum of 3 months since bilateral STN DBS surgery (Table 1). Mean age was 60 years (range 36–76).
Table 1.
Individual age (years), months post surgery, and scores from the neurologist's evaluation including the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS, no symptoms = 0), Hoehn and Yahr (H&Y, mild = 1) staging, and the Schwab and England Activities of Daily Living Scale (S&E, completely independent = 100)
| Participant | Sex | Age | Months Post Surgery | Speech item only |
PD motor measures |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPDRS ADL |
UPDRS MOTOR |
UPDRS MOTOR |
H&Y |
S&E |
|||||||||
| Pre (OFF) | Post (ON) | Pre (OFF) | Post (ON) | Pre (OFF) | Post (ON) | Pre (OFF) | Post (ON) | Pre (OFF) | Post (ON) | ||||
| 1 | M | 66 | 6 | 1 | 1 | 2 | 1 | 35 | 26 | 2 | 2 | 70 | 90 |
| 2 | M | 65 | 6 | 2 | 2 | 2 | 2 | 45 | 15 | 3 | 3 | 70 | 80 |
| 3 | M | 64 | 6 | 2 | 1 | 2 | 1 | 42 | 13 | 3 | 3 | 60 | 90 |
| 4 | M | 55 | 3 | 1 | 1 | 2 | 1 | 30 | 14 | 2 | 2 | 70 | 70 |
| 5 | M | 36 | 3 | 3 | 2 | 2 | 2 | 46 | 30 | 3 | 2 | 70 | 90 |
| 6 | F | 38 | 32 | 2 | 1 | 2 | 2 | 39 | 48 | 3 | 4 | 60 | 60 |
| 7 | M | 75 | 3 | 3 | 2 | 2 | 2 | 43 | 27 | 3 | 2 | 70 | 80 |
| 8 | F | 68 | 22 | 2 | 2 | 2 | 1 | 49 | 19 | 3 | 2 | 80 | 90 |
| 9 | M | 76 | 4 | 2 | 2 | 2 | 2 | 25 | 24 | 2 | 2 | 80 | 80 |
| 10 | M | 48 | 25 | 2 | 3 | 2 | 2 | 38 | 32 | 3 | 2 | 80 | 90 |
| 11 | M | 72 | 3 | 2 | 2 | 2 | 1 | 49 | 7 | 2 | 2 | 70 | 100 |
| 12 | M | 42 | 6 | 4 | 3 | 4 | 3 | 36 | 20 | 2 | 2 | 70 | 90 |
| 13 | M | 53 | 12 | 2 | 1 | 2 | 1 | 43 | 17 | 3 | 2 | 70 | 90 |
| 14 | M | 55 | 24 | 2 | 2 | 2 | 2 | 44 | 35 | 4 | 3 | 60 | 90 |
| 15 | M | 44 | 27 | 3 | 2 | 2 | 1 | 28 | 28 | 3 | 2 | 60 | 90 |
| 16 | M | 68 | 4 | 2 | 2 | 2 | 2 | 42 | 22 | 3 | 2 | 60 | 90 |
| 17 | F | 73 | 9 | 2 | 3 | 2 | 3 | 49 | 38 | 3 | 4 | 80 | 80 |
| 18 | M | 74 | 13 | 2 | 3 | 2 | 2 | 34 | 24 | 3 | 2 | 70 | 70 |
Neurological ratings are provided prior to surgical placement (Pre) and following activation of the bilateral STN DBS (Post). Individuals were tested a minimum of 12 h since their last dose of anti-PD medication. UPDRS ADL speech item: 0 = normal; 1 = mildly affected; no difficulty being understood; 2 = moderately affected, sometimes asked to repeat statements; 3 = severely affected, frequently asked to repeat statements; 4 = unintelligible most of the time. UPDRS MOTOR speech item: 0 = normal; 1 = slight loss of expression, diction and/or volume; 2 = monotone, slurred but understandable, moderately impaired; 3 = marked impairment, difficult to understand, 4 = unintelligible
Neurological assessment
Testing was coordinated with the participant's scheduled neurological evaluation. Each PD participant was tested during a single morning session a minimum of 12 h since their most recent dose of anti-PD medication. As part of the scheduled neurological evaluation, each participant underwent post-operative assessment (DBS ON only) by a movement disorder specialist (R.P.) including the motor scale of the UPDRS, Hoehn and Yahr (H&Y) staging, and the Schwab and England Activities of Daily Living Scale (S&E) [12, 44, 45]. This post-operative assessment (DBS ON, no medication) was compared with the pre-operative assessment (no medication). Participants exhibited signifi-cant pre- versus post-operative improvement in clinical ratings of limb-related function with DBS (UPDRS, t = –5.19, p < 0.001; H&Y, t = –2.36, p < 0.04; S&E, t = 5.30, p < 0.001). Assessment results are provided in Table 1. STN DBS stimulation parameters (e.g., frequency, pulse width) are in Table 2.
Table 2.
Individual deep brain stimulation (DBS) parameters and settings for each participant including stimulation frequency (Hz), voltage (V), and pulse width (μs) for left and right internalized pulse generator (IPG), and electrode configuration used for DBS
| Participant | Frequency (Hz) | Voltage (V) | Pulse width (μs) | Electrode configuration (“0” = off, “+” = anode, “–” = cathode) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Left active contacts |
Right active contacts |
||||||||||||
| Left IPG | Right IPG | Left IPG | Right IPG | Left IPG | Right IPG | 0 | 1 | 2 | 3 | IPG Case | 0 | 1 | 2 | 3 | IPG Case | |
| 1 | 170 | 145 | 3.7 | 2.8 | 90 | 60 | 0 | – | – | + | 0 | 0 | 0 | – | 0 | + |
| 2 | 145 | 160 | 2.1 | 3 | 60 | 60 | 0 | 0 | – | 0 | + | 0 | 0 | – | 0 | + |
| 3 | 145 | 145 | 2.4 | 2.3 | 60 | 60 | 0 | – | 0 | 0 | + | 0 | 0 | – | 0 | + |
| 4 | 145 | 145 | 3.2 | 1.6 | 60 | 60 | 0 | – | 0 | 0 | + | 0 | – | 0 | 0 | + |
| 5 | 145 | 145 | 3.5 | 1.5 | 90 | 60 | + | 0 | – | 0 | 0 | 0 | 0 | – | 0 | + |
| 6 | 145 | 145 | 1.5 | 1.8 | 60 | 60 | 0 | 0 | 0 | – | + | 0 | 0 | 0 | – | + |
| 7 | 185 | 170 | 2.9 | 2.3 | 60 | 90 | 0 | – | – | 0 | + | 0 | – | 0 | 0 | + |
| 8 | 145 | 145 | 2.5 | 1.8 | 90 | 90 | 0 | + | – | 0 | 0 | 0 | + | – | 0 | 0 |
| 9 | 145 | 145 | 2.8 | 2.9 | 60 | 60 | 0 | – | – | 0 | + | 0 | 0 | – | – | + |
| 10 | 160 | 185 | 3.2 | 4.1 | 90 | 90 | 0 | 0 | – | – | + | – | – | + | 0 | 0 |
| 11 | 160 | 185 | 3 | 4 | 60 | 60 | 0 | 0 | – | 0 | + | 0 | 0 | – | 0 | + |
| 12 | 145 | 185 | 2.4 | 3.8 | 60 | 90 | 0 | 0 | – | 0 | + | + | 0 | 0 | – | 0 |
| 13 | 170 | 170 | 3.8 | 4.5 | 60 | 90 | 0 | 0 | – | 0 | + | 0 | – | 0 | 0 | + |
| 14 | 170 | 160 | 3.7 | 3.9 | 90 | 90 | + | 0 | 0 | – | 0 | 0 | + | 0 | – | 0 |
| 15 | 185 | 185 | 2.8 | 2.4 | 90 | 60 | 0 | – | 0 | 0 | + | 0 | 0 | – | 0 | + |
| 16 | 145 | 160 | 2.3 | 2.9 | 60 | 90 | 0 | 0 | – | – | + | 0 | + | 0 | 0 | + |
| 17 | 170 | 170 | 3 | 3.6 | 90 | 90 | 0 | 0 | – | – | + | 0 | 0 | – | – | + |
| 18 | 160 | 160 | 3.6 | 2.8 | 60 | 60 | – | + | – | 0 | 0 | 0 | 0 | – | 0 | + |
We carefully considered our participants and their limited availability for testing. Accordingly, we selected non-invasive aerodynamic measures of speech respiratory and laryngeal control, and chose to assess each participant with DBS on, and again 1 h after DBS was turned off. We explored whether changes in aerodynamic measures of speech respiratory and laryngeal control (DBS ON vs. DBS OFF) were associated with clinical ratings of limb function (e.g., UPDRS, pre- vs. post-operative) and DBS stimulation parameters (e.g., frequency, pulse width).
Air pressure and air flow
Participants were comfortably seated in an exam chair and instructed to repeat the syllable [ ] at a rate of two syllables per second at a comfortable pitch and loudness. The air pressure in the oral cavity during the closed phase of the voiceless bilabial plosive [p] equals the air pressure below the glottis (PS). This sampling context provides a reasonable estimate of PS for voice and speech [19, 20]. Air pressure in the oral cavity was measured using a polyethylene catheter (Intramedic PE 260, 1.77 mm ID, 7 cm length) placed in the mouth near the oral angle and oriented perpendicular to the breath stream during speech. The catheter was coupled to a pressure transducer (Honeywell model 164PC01D37). A 10-cm-H2O pressure source (U-tube manometer) was used to calibrate the air pressure transducer. Air flow was measured using a Puritan-Bennett full-face respiratory mask (model 5253) and a Hans Rudolph pneumotachometer (model R4719) instrumented with a pressure transducer (Honeywell model 163PC01D36). A 500-cc/s flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Both air pressure and air flow signals were conditioned by bridge amplifiers (Biocommunication Electronics, model 201, LP –3 dB @ 50 Hz, Butterworth 3-pole). Lip closure and voicing were carefully monitored for each participant. Only syllables with complete seal of the lips during the consonant and continuous voice production during the vowel were included in analysis. Signals were digitized at 3.3 kHz using a custom designed data acquisition and analysis program [46] to compute PS, peak air flow, mean vocalic air flow, and laryngeal airway resistance. Air flow data were processed (Minitab v.13.20) to determine the slope of declination following the peak in the air flow signal associated with phonatory onset, and integrated to determine the lung air volume expended per syllable.
] at a rate of two syllables per second at a comfortable pitch and loudness. The air pressure in the oral cavity during the closed phase of the voiceless bilabial plosive [p] equals the air pressure below the glottis (PS). This sampling context provides a reasonable estimate of PS for voice and speech [19, 20]. Air pressure in the oral cavity was measured using a polyethylene catheter (Intramedic PE 260, 1.77 mm ID, 7 cm length) placed in the mouth near the oral angle and oriented perpendicular to the breath stream during speech. The catheter was coupled to a pressure transducer (Honeywell model 164PC01D37). A 10-cm-H2O pressure source (U-tube manometer) was used to calibrate the air pressure transducer. Air flow was measured using a Puritan-Bennett full-face respiratory mask (model 5253) and a Hans Rudolph pneumotachometer (model R4719) instrumented with a pressure transducer (Honeywell model 163PC01D36). A 500-cc/s flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Both air pressure and air flow signals were conditioned by bridge amplifiers (Biocommunication Electronics, model 201, LP –3 dB @ 50 Hz, Butterworth 3-pole). Lip closure and voicing were carefully monitored for each participant. Only syllables with complete seal of the lips during the consonant and continuous voice production during the vowel were included in analysis. Signals were digitized at 3.3 kHz using a custom designed data acquisition and analysis program [46] to compute PS, peak air flow, mean vocalic air flow, and laryngeal airway resistance. Air flow data were processed (Minitab v.13.20) to determine the slope of declination following the peak in the air flow signal associated with phonatory onset, and integrated to determine the lung air volume expended per syllable.
Statistical analyses
Because of the heterogeneity of participant responses reported previously, we anticipated individual increases and decreases for each of the six aerodynamic measures of respiratory and laryngeal control with DBS such that traditional group analysis (paired t tests DBS ON vs. DBS OFF, α = 0.05/6 measures = 0.008) would not reflect significant changes. However, we hypothesized that individual participants would exhibit significant absolute changes in each measure with DBS. To examine this hypothesis, we used individual change data (see Table 3) to calculate the group mean magnitude of change and the group standard error for each measure. We evaluated whether individuals exhibited an absolute magnitude of change that exceeded two times the group standard error, and used this as our criterion for significance (see Fig. 1, top panels). We then compared our data with typical operating ranges reported in the literature for each of the six aerodynamic measures [19, 20, 47]. We examined the individual baselines, responses to DBS, and examined whether individual values approached, departed from, or stayed within the typical operating range with DBS (see Table 4; Fig. 1, bottom panels). To examine the possible relation between changes in each measure of respiratory and laryngeal control and clinical ratings of limb function (e.g., UPDRS) and stimulation parameters (e.g., frequency, pulse width), we computed Pearson product moment correlation coefficients (α = 0.05).
Table 3.
Individual mean value, standard error (SE), and change (Δ) for each speech measure with deep brain stimulation (DBS ON vs. DBS OFF) including subglottal air pressure (PS), peak air flow, vocalic air flow, laryngeal resistance (LR), air volume expelled per syllable, and slope of declination in air flow
| Participant |
PS (cm H2O) |
Peak Air Flow (cc/s) |
Vocalic Air Flow (cc/s) |
LR (cm H2O/LPS) |
Volume (cc) |
Slope ((cc/s)/ms) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean |
Mean |
|||||||||||||
| DBS ON | DBS OFF | Δ | DBS ON | DBS OFF | Δ | DBS ON | DBS OFF | Δ | DBS ON | DBS OFF | Δ | DBS ON | DBS OFF | Δ | DBS ON | DBS OFF | Δ | |
| 1 | 11.80 (0.43) | 5.84 (0.16) | 5.96 | 1,084 (60) | 636.8 (18) | 447.20 | 235 (6.9) | 134.9 (6.9) | 100.10 | 52.4 (1.2) | 45.7 (1.9) | 6.75 | 35.87 | 24.77 | 11.10 | 12.42 | 6.77 | 5.65 |
| 2 | 8.30 (0.34) | 2.39 (0.35) | 5.91 | 1,497 (196) | 708 (39) | 789.00 | 236 (43) | 283 (59) | –47.00 | 48.4 (2.7) | 13.4 (2.6) | 34.99 | 57.4 | 44.9 | 12.50 | 8.85 | 3.93 | 4.92 |
| 3 | 13.25 (0.52) | 8.32 (0.26) | 4.93 | 1,034 (233) | 442 (38) | 592.00 | 84.1 (14) | 191.1 (14) | –107.00 | 96.9 (28) | 48.4 (5.5) | 48.50 | 59.93 | 20.09 | 39.84 | 9.47 | 4.72 | 4.75 |
| 4 | 10.39 (0.23) | 7.83 (0.29) | 2.56 | 642.3 (18) | 692 (39) | –49.70 | 261.2 (9.9) | 256.8 (8.7) | 4.40 | 40.5 (1.4) | 30.1 (1.2) | 10.42 | 36.28 | 37.31 | –1.03 | 6.39 | 5.24 | 1.15 |
| 5 | 6.43 (0.23) | 4.42 (0.81) | 2.01 | 595 (42) | 477.1 (34) | 117.90 | 124.6 (2.5) | 273.6 (18) | –148.97 | 49.7 (2.0) | 18.6 (2.8) | 31.10 | 25.72 | 30.44 | –4.72 | 0.21 | 0.40 | –0.19 |
| 6 | 9.93 (0.16) | 8.21 (0.10) | 1.72 | 1,282 (61) | 629 (41) | 653.00 | 112.7 (12) | 100 (9.4) | 12.70 | 86.2 (5.8) | 76.3 (4.6) | 9.90 | 35.38 | 20.49 | 14.89 | 8.84 | 1.83 | 7.01 |
| 7 | 9.96 (0.18) | 8.40 (0.20) | 1.56 | 297.6 (23) | 953 (46) | –655.40 | 87.5 (6.1) | 113.3 (3.6) | –25.80 | 110 (8.8) | 77.2 (2.1) | 32.85 | 10.98 | 27.35 | –16.37 | 6.54 | 2.84 | 3.70 |
| 8 | 12.74 (0.26) | 11.35 (0.15) | 1.39 | 857 (121) | 660.2 (30) | 196.80 | 217.2 (11) | 228.6 (13) | –11.40 | 66.5 (1.7) | 54.4 (1.7) | 12.08 | 30.08 | 25.8 | 4.28 | 2.46 | 0.89 | 1.57 |
| 9 | 8.61 (0.09) | 7.67 (0.19) | 0.94 | 1,163 (30) | 693 (22) | 470.00 | 173 (5.2) | 143.3 (5.9) | 29.70 | 54.9 (1.3) | 56.9 (2.4) | –1.98 | 34.43 | 19.57 | 14.86 | 4.85 | 4.35 | 0.51 |
| 10 | 2.12 (0.36) | 1.81 (0.30) | 0.31 | 665 (66) | 733 (133) | –68.00 | 481 (60) | 378 (86) | 103.00 | 8.8 (4.5) | 3.47 (0.53) | 5.33 | 47.03 | 50.41 | –3.38 | 3.61 | 3.78 | –0.17 |
| 11 | 6.13 (0.31) | 5.86 (0.18) | 0.27 | 392.4 (21) | 468 (36) | –75.60 | 247.1 (16) | 292 (28) | –44.90 | 27.3 (2.3) | 18.8 (0.44) | 8.54 | 21.58 | 30.81 | –9.23 | 2.39 | 3.10 | –0.71 |
| 12 | 5.43 (0.42) | 5.3 (0.46) | 0.13 | 358 (26) | 383 (25) | –25.00 | 262.3 (16) | 249.3 (14) | 13.00 | 21 (1.6) | 24 (3.2) | –2.98 | 21.31 | 26.57 | –5.26 | 0.37 | 1.59 | –1.21 |
| 13 | 9.38 (0.27) | 10.07 (0.20) | –0.69 | 906 (119) | 1,020 (69) | –114.00 | 114 (16) | 238 (13) | –124.00 | 87.2 (12) | 43.8 (2.6) | 43.44 | 35.35 | 47.67 | –12.32 | 7.15 | 5.79 | 1.37 |
| 14 | 8.04 (0.49) | 8.96 (0.34) | –0.92 | 670 (48) | 574 (25) | 96.00 | 169.3 (15) | 204.3 (12) | –35.00 | 60 (3.1) | 49.4 (3.8) | 10.60 | 35.79 | 34.26 | 1.53 | 6.91 | 6.68 | 0.23 |
| 15 | 7.68 (0.97) | 8.63 (0.25) | –0.95 | 612.3 (42) | 886 (55) | –273.70 | 104.8 (7.1) | 132.1 (7.6) | –27.30 | 73.2 (6.3) | 71.5 (3.5) | 1.73 | 13.86 | 27.7 | –13.84 | 4.07 | 7.54 | –3.47 |
| 16 | 2.98 (0.62) | 5.41 (0.21) | –2.43 | 234.8 (29) | 396 (15) | –161.20 | 109 (15) | 125.1 (10) | –16.10 | 27.2 (4.8) | 42.5 (1.6) | –15.33 | 11.75 | 17.14 | –5.39 | 4.16 | 6.21 | –2.05 |
| 17 | 8.68 (0.69) | 11.17 (1.10) | –2.49 | 870 (105) | 1,211 (85) | –341.00 | 408.3 (14) | 415 (91) | –6.70 | 19.4 (1.7) | 19.6 (6.6) | –0.18 | 59.1 | 79 | –19.95 | 0.94 | 1.94 | –1.01 |
| 18 | 12.94 (0.92) | 17.94 (0.24) | –5.00 | 889 (116) | 1,013 (74) | –124.00 | 122.2 (17) | 166.2 (8.5) | –44.00 | 131 (15) | 113 (6.9) | 17.90 | 25.46 | 30.57 | –5.11 | 1.74 | 7.64 | –5.89 |
|
Group mean, standard error (SE), minimum (MLN), and maximum (MAX) magnitude of change for each measure | ||||||||||||||||||
| Mean (SE) | 2.23 (0.46) | 291.60 (58.30) | 50.10 (10.70) | 16.37 (3.56) | 10.87 (2.15) | 2.53 (0.53) | ||||||||||||
| MIN | 0.13 | 25.00 | 4.40 | 0.18 | 1.03 | 0.17 | ||||||||||||
| MAX | 5.96 | 789.00 | 149.00 | 48.50 | 39.84 | 7.01 | ||||||||||||
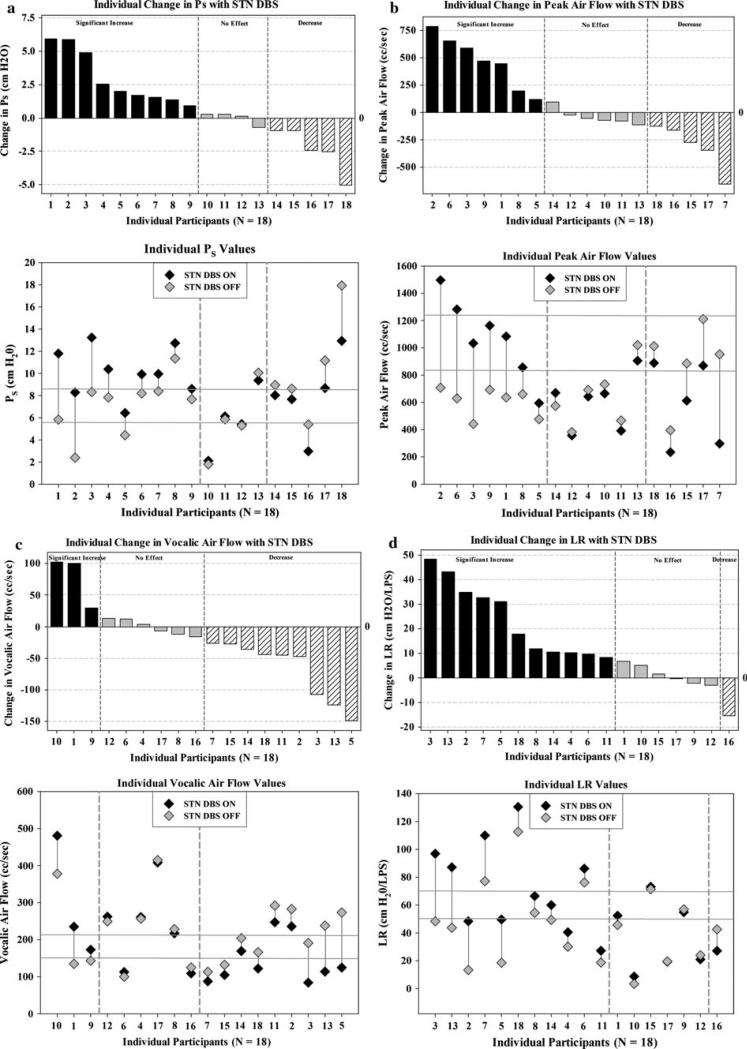
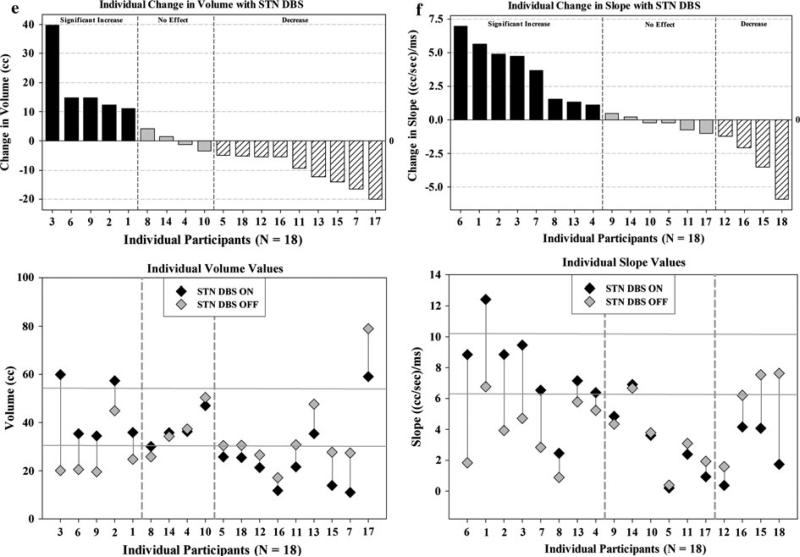
Fig. 1.
Individual values and corresponding change for each aerodynamic measure (a through f). (PS subglottal pressure, LR laryngeal resistance). Top individual change (DBS ON–DBS OFF). Bottom individual values for each participant with DBS ON (black symbols) and DBS OFF (gray symbols). In each graph, dashed vertical reference lines demarcate boundaries between significant increases (left), non-significant changes (middle), and significant decreases (right). Solid horizontal reference lines in the bottom graph demarcate a typical operating range
Table 4.
Direction of change for each speech measure with deep brain stimulation (on minus off) including subglottal pressure, peak air flow, vocalic air flow, laryngeal resistance, volume expelled per syllable, and slope of declination in air flow
| Variable | Number of increases |
Number of decreases |
||||||
|---|---|---|---|---|---|---|---|---|
| Approach | Depart | Stay | Total | Approach | Depart | Stay | Total | |
| Subglottal pressure (cm H2O) | 2 | 7 | 0 | 9 (50%) | 4 | 1 | 0 | 5 (28%) |
| Peak air flow (cc/s) | 7a | 0 | 0 | 7 (39%) | 0 | 3 | 2 | 5 (28%) |
| Vocalic air flow (cc/s) | 2a | 1 | 0 | 3 (17%) | 2a | 6 | 1 | 9 (50%) |
| Laryngeal resistance (cm H2O/LPS) | 7a | 3 | 1 | 11 (61%) | 0 | 1 | 0 | 1 (6%) |
| Volume (cc) | 4 | 1 | 0 | 5 (28%) | 1 | 7 | 1 | 9 (50%) |
| Slope ((cc/s)/ms) | 7 | 1 | 0 | 8 (44%) | 0 | 4 | 0 | 4 (22%) |
Total indicates the number and percentage of participants who exhibited a significant change (increase or decrease) with deep brain stimulation. Approach indicates changes toward an expected operating range. Depart indicates changes away from an expected operating range. Stay indicates a significant change in which the measure was within an expected operating range during both test conditions (on and off)
Includes approaches that overshot typical range
Results
Individual values and corresponding change for each aerodynamic measure of respiratory and laryngeal control are shown in Fig. 1, and summarized in Table 3. As anticipated, we observed individual increases and decreases for each of the six aerodynamic measures of respiratory and laryngeal control with DBS such that traditional group analysis (paired t tests DBS ON vs. DBS OFF, all p values > 0.05) did not reflect significant changes.
However, individual participants exhibited changes in each measure (Table 4; Fig. 1, top panels): subglottal air pressure (n = 14), peak air flow (n = 12), mean vocalic air flow (n = 12), laryngeal airway resistance (n = 12), lung air volume expended per syllable (n = 14), and slope of declination in air flow (n = 12). Of these participants, most exhibited increases in subglottal air pressure (n = 9), peak air flow (n = 7), laryngeal airway resistance (n = 11), and slope (n = 8), with decreases in mean vocalic air flow (n = 9) and lung air volume expended per syllable (n = 9). These changes were primarily consistent with increased respiratory driving pressure, increased vocal fold closure for voicing, associated changes in timing of phonatory onset, and reduced expenditure of lung air volume per syllable. However, participants demonstrated individual responses to DBS. For example, of the 14 individuals who demonstrated changes in subglottal air pressure with DBS, nine exhibited increased pressure while five demonstrated decreased pressure.
We then compared our data with typical operating ranges reported in the literature for each of the six aerodynamic measures [19, 20, 47] to evaluate the individual baselines, responses to DBS, and whether individual values approached, departed from, or stayed within the typical operating range with DBS (see Table 4; Fig. 1, bottom panels). Eight PD participants that exhibited values beyond the typical range (OFF) approached but overshot the typical range for peak air flow (participants 2 and 6), vocalic air flow (participant 1, 5, and 13), laryngeal resistance (participants 3 and 13), and volume (participant 3) with DBS ON. Not all PD participants exhibited values beyond the typical range with DBS OFF, and most participants exhibited values that exceeded the typical range for each measure with DBS ON. Therefore, although most PD participants exhibited changes with DBS, most exhibited measures that deviated from a typical operating range.
Next, we examined the degree to which aerodynamic measures of respiratory and laryngeal control were associated with clinical ratings of limb function (e.g., UPDRS) and stimulation parameters (e.g., frequency, pulse width). These measures were not significantly correlated with changes in the UPDRS, H&Y, or S&E (Pearson product moment correlation coefficients, all p values > 0.09). However, we did find a moderate positive correlation between vocalic air flow and the single item index of functional communication from the UPDRS Activities of Daily Living (ADL) subscale (r = 0.46, p = 0.05). We also found moderate negative correlations (all p ≤ 0.05) between stimulation frequency of the left STN with peak air flow (r = –0.61) and volume (r = –0.56), between stimulation frequency of the right STN with peak air flow (r = –0.59), volume (r = –0.63), and slope (r = –0.48), and between stimulation pulse width of the right STN with peak air flow (r = –0.52) and volume (r = –0.46). Therefore, changes in aerodynamic measures of respiratory and laryngeal control did not parallel clinical ratings of limb function, but demonstrated some relation to DBS parameters (i.e., frequency and pulse width), and vocalic air flow was correlated with degree of functional communication deficit.
Discussion
This study presents the first data examining the effects of bilateral STN DBS on aerodynamic measures of respiratory and laryngeal control in PD. We found that PD participants exhibited changes in respiratory and laryngeal control with STN DBS. These findings are consistent with the heterogeneity of speech responses to STN DBS reported previously. As anticipated, we found individual increases and decreases for each of the six aerodynamic measures with STN DBS. Most of the changes with DBS were consistent with increased respiratory driving pressure, increased vocal fold closure for voicing, associated changes in timing of phonatory onset, and reduced expenditure of lung air volume per syllable. In the present study, changes in aerodynamic measures of respiratory and laryngeal control with DBS may represent changes in central scaling among neural networks involved in speech-related respiratory and laryngeal control. When improvements were observed, it may be that abnormally altered patterns of neural firing presumed to occur throughout the basal ganglia-thalamo-cortical network may approximate a more normal pattern of oscillation with STN DBS, or these patterns may be more amenable to beneficial compensation by remaining healthy brain tissue in the presence of STN DBS. However, it should be noted most participants exhibited speech values that exceeded the typical range with DBS, consistent with respiratory over-drive and excessive vocal fold closure.
We observed a moderate positive correlation between vocalic air flow and the single-item index of functional communication from the UPDRS ADL subscale, suggesting an association between breathiness (consistent with hypophonia in PD) and functional communication deficit. However, changes in aerodynamic measures of respiratory and laryngeal control were uncorrelated with changes in clinical ratings of limb function (e.g., UPDRS Motor sub-scale). This finding is not surprising given that the UPDRS Motor subscale is focused almost entirely on limb function, and lacks the construct validity to accurately and reliably assess the speech motor subsystems [14, 48, 49]. The heterogeneity of participant responses and the lack of association between the speech and limb-related measures may relate to potential variability in the localization of the active DBS electrodes, stimulation fields, and potential current spread beyond the STN target (e.g., internal capsule), individual variability in somatotopic representation in STN, hemispheric interactions [35–37], differences in pre-treatment severity [25, 26], and optimization of DBS settings for limb function rather than for speech-related motor control.
The negative correlations we found between stimulation frequency and aerodynamic measures of respiratory and laryngeal control suggest a differential effect of high-frequency DBS for speech and limb-related control, and indicate that lower frequency stimulation may generally be associated with greater respiratory driving pressure, associated changes in the timing of phonatory onset, and increased expenditure of lung air volume for each syllable. These correlations accord with previous reports, lending further support that speech may benefit more from lower-frequency DBS than high-frequency DBS [41–43]. Therefore, high-frequency stimulation may be of less benefit for speech-related respiratory and laryngeal control than for functions that require larger forces, such as limb movements. In some cases, a differential effect may also be evident for stimulation voltage [50]. We observed an association between right hemisphere pulse width with peak air flow and expended lung air volume. This association may also confirm previously reported hemispheric effects of STN DBS [35–37]. Again, it is important to note that most participants exhibited values that exceeded the typical range with DBS, consistent with respiratory over-drive and excessive vocal fold closure, and that all participants were receiving high-frequency stimulation, with the lowest stimulation frequency at 145 Hz. Therefore, our findings of respiratory over-drive and excessive vocal fold closure with DBS further suggest that high-frequency stimulation may be of less benefit for the fine force requirements of speech respiratory and laryngeal control than for the large magnitudes of force required for limb-related function.
Because clinical neurological assessment scales (e.g., UPDRS) focus almost exclusively on limb motor function and possess limited ability to accurately identify speech changes in PD, we employed aerodynamic measures of speech respiratory and laryngeal function. As demonstrated in the present study, non-invasive measures of speech physiology, including speech aerodynamics, may be helpful to assess even subtle changes in speech-related function that may be unidentified during a standard neurological evaluation. A limitation of our study was that we only were able to perform post-operative speech testing (DBS ON vs. DBS OFF). In planning future experiments, we would suggest inclusion of pre- and post-operative testing for comparison, to examine the impact of pre-operative speech severity, and a more comprehensive task set including phrases and sentences. Future study designs would also benefit from the integration of aerodynamics with other assessment tools (e.g., acoustics, perceptual scales). Finally, we would suggest repeating clinical ratings of limb function (e.g., UPDRS) with DBS turned off to directly compare limb function (DBS ON vs. DBS OFF), and to include electrode localization coordinates with speech and limb measures for additional correlative analyses.
Conclusions
The effects of STN DBS on speech-related respiratory and laryngeal control in PD were previously unknown. In this study, we used non-invasive aerodynamic measures to examine speech respiratory and laryngeal function. We found that bilateral STN DBS may change respiratory and laryngeal function during syllable production in PD. The effects on respiratory and laryngeal control were not uniform across participants and did not correlate with changes in limb-related function. We found that high-frequency STN DBS often resulted in respiratory over-drive and excessive vocal fold closure, suggesting that high-frequency STN DBS may be less beneficial for speech-related respiratory and laryngeal control than for limb control, and that speech may benefit more from low-frequency stimulation than high-frequency stimulation. It is important to consider the differences between speech versus limb function, and between high- versus low-frequency stimulation when planning and optimizing the stimulation parameters for individuals with PD.
Acknowledgments
Dr. Hammer's research is supported through NIH grants DC007260, RR025012, and RR023268. Dr. Barlow's research is supported through NIH grants DC003311, DC005803, and HD02528. Drs. Pahwa and Lyons are consultants for and have received honoraria from Medtronic and St. Jude Medical (Advanced Neuromodulation Systems).
Contributor Information
Michael J. Hammer, Department of Surgery, Division of Otolaryngology, University of Wisconsin, Room K4/769 Clinical Sciences Center, 600 Highland Avenue, Madison, WI 53792, USA hammer@surgery.wisc.edu
Steven M. Barlow, Speech-Language-Hearing, Neurosciences, Human Biology and Bioengineering, University of Kansas, Lawrence, KS, USA
Kelly E. Lyons, Department of Neurology, Parkinson Disease and Movement Disorder Center, University of Kansas Medical Center, Kansas City, KS, USA
Rajesh Pahwa, Department of Neurology, Parkinson Disease and Movement Disorder Center, University of Kansas Medical Center, Kansas City, KS, USA.
References
- 1.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 2.DeLong MR. Basal ganglia. In: Kandel E, Schwartz J, Jessel T, editors. Principles of neural science. McGraw Hill; New York: 2000. pp. 853–867. [Google Scholar]
- 3.Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson's disease. Brain. 2004;127:1755–1773. doi: 10.1093/brain/awh206. [DOI] [PubMed] [Google Scholar]
- 4.Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson's disease. Mov Disord. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- 5.Pfann KD, Robichaud JA, Gottlieb GL, Comella CL, Brandabur M, Corcos DM. Muscle activation patterns in point-to-point and reversal movements in healthy, older subjects and in subjects with Parkinson's disease. Exp Brain Res. 2004;157:67–78. doi: 10.1007/s00221-003-1821-x. [DOI] [PubMed] [Google Scholar]
- 6.Robichaud JA, Pfann KD, Vaillancourt DE, Comella CL, Corcos DM. Force control and disease severity in Parkinson's disease. Mov Disord. 2005;20:441–450. doi: 10.1002/mds.20350. [DOI] [PubMed] [Google Scholar]
- 7.Barlow SM, Iacono RP, Paseman LA, Biswas A, D'Antonio LD. The effects of experimental posteroventral pallidotomy on force and speech aerodynamics in Parkinson's disease. In: Cannito MP, Yorkston KM, Beukelman DR, editors. Speech motor control. Paul H. Brookes Publishing Company; Baltimore: 1998. pp. 117–156. [Google Scholar]
- 8.Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. J Speech Lang Hear Res. 2001;44:1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- 9.Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51:1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- 10.Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J Neurophysiol. 1999;81:2131–2139. doi: 10.1152/jn.1999.81.5.2131. [DOI] [PubMed] [Google Scholar]
- 11.Hunker CJ, Abbs JH, Barlow SM. The relationship between Parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:755–761. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton R, Members of the UPDRS Development Committee . In: Recent developments in Parkinson's disease. Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Vol. 2. Macmillian Health Care Information; Florham Park: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 13.Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RAE, Ver-hagen LV, Crocos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson's disease. Mov Disord. 2006;21:50–58. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards M, Marder K, Cote L, Mayeaux R. Interrater reliability of the Unified Parkinson's Disease Rating Scale motor examination. Mov Disord. 1994;9:89–91. doi: 10.1002/mds.870090114. [DOI] [PubMed] [Google Scholar]
- 15.Bunton K. Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson disease. J Commun Disord. 2005;38:331–348. doi: 10.1016/j.jcomdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Solomon NP, Hixon TJ. Speech breathing in Parkinson's disease. J Speech Hear Res. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- 17.Tamaki A, Matsuo Y, Yanagihara T, Abe K. Influence of thoracoabdominal movement on pulmonary function in patients with Parkinson's disease: comparison with healthy subjects. Neurorehabil Neural Repair. 2000;14:43–47. doi: 10.1177/154596830001400105. [DOI] [PubMed] [Google Scholar]
- 18.Tzelepis GE, McCool DF, Friedman JH, Hoppin FG. Respiratory muscle dysfunction in Parkinson's disease. Am Rev Respir Dis. 1988;138:266–271. doi: 10.1164/ajrccm/138.2.266. [DOI] [PubMed] [Google Scholar]
- 19.Hammer M, Barlow SM. Laryngeal somatosensory deficits in Parkinson's disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201:401–409. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smitheran JR, Hixon TJ. A clinical method for estimating laryngeal airway resistance during vowel production. J Speech Hear Disord. 1981;46:138–146. doi: 10.1044/jshd.4602.138. [DOI] [PubMed] [Google Scholar]
- 21.Breit S, Schulz JB, Benabid A-L. Deep brain stimulation. Cell Tissue Res. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- 22.Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol. 2004;21(1):6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Vaillancourt DE, Prodoehl J, Metman LV, Bakay RA, Crocos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson's disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 24.D'Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on Parkinsonian speech impairment. J Voice. 2008;22:365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Dromey C, Kumar R, Lang AC, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Farrell A, Theodoros D, Ward E, Hall B, Silburn P. Effects of neurosurgical management of Parkinson's disease on speech characteristics and oromotor function. J Speech Lang Hear Res. 2005;48:5–20. doi: 10.1044/1092-4388(2005/002). [DOI] [PubMed] [Google Scholar]
- 27.Gentil M, Chauvin P, Pinto S, Pollak P, Benabid AL. Effect of bilateral stimulation of the subthalamic nucleus on Parkinsonian voice. Brain Lang. 2001;78:233–240. doi: 10.1006/brln.2001.2466. [DOI] [PubMed] [Google Scholar]
- 28.Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of stimulation of the subthalamic nucleus on oral control of patients with Parkinsonism. J Neurol Neurosurg Psychiatry. 1999;67:329–333. doi: 10.1136/jnnp.67.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman-Ruddy B, Schulz G, Vitek J, Evatt M. A preliminary study of the effects of subthalamic nucleus (STN) deep brain stimulation (DBS) on voice and speech characteristics in Parkinson's disease (PD). Clin Linguist Phon. 2001;15:97–101. doi: 10.3109/02699200109167638. [DOI] [PubMed] [Google Scholar]
- 30.Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, Curio G, Sappok T. Effects of subthalamic deep brain stimulation in dysarthrophonia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- 31.Pinto S, Gentil M, Fraix V, Benabid AL, Pollak P. Bilateral subthalamic stimulation effects on oral force control in Parkinson's disease. Neurology. 2003;250:179–187. doi: 10.1007/s00415-003-0966-7. [DOI] [PubMed] [Google Scholar]
- 32.Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson's disease. Lancet Neurol. 2004;3:547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- 33.Pinto S, Gentil M, Krack P, Sauleau P, Fraix V, Benabid AL, Pollak P. Changes induced by levodopa and subthalamic nucleus stimulation on Parkinsonian speech. Mov Disord. 2005;20:1507–1515. doi: 10.1002/mds.20601. [DOI] [PubMed] [Google Scholar]
- 34.Rousseaux M, Krystkowiak P, Kozlowski O, Ozsancak C, Blond S, Destee A. Effects of subthalamic nucleus stimulation on Parkinsonian dysarthria and speech intelligibility. Neurology. 2004;251:327–334. doi: 10.1007/s00415-004-0327-1. [DOI] [PubMed] [Google Scholar]
- 35.Santens P, De Letter M, Van Borsel J, De Reuck J, Caemaert J. Lateralized effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson's disease. Brain Lang. 2003;87:253–258. doi: 10.1016/s0093-934x(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang E, Metman LV, Bakay R, Arzbaecher J, Bernard B. The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson's disease—a preliminary report. Clin Linguist Phon. 2003;17:283–289. doi: 10.1080/0269920031000080064. [DOI] [PubMed] [Google Scholar]
- 37.Wang EQ, Metman LV, Bakay RAE, Arzbaecher J, Bernard B, Corcos DM. Hemisphere-specific effects of subthalamic nucleus deep brain stimulation on speaking rate and articulatory accuracy of syllable repetitions in Parkinson's disease. J Med Speech Lang Pathol. 2006;14:323–333. [PMC free article] [PubMed] [Google Scholar]
- 38.Barlow SM, Hammer M. Pallidotomy and deep brain stimulation in Parkinson's disease: effects on speech and voice. In: McNeil M, editor. The clinical management of sensorimotor speech disorders. Thieme Medical Publishers; New York: 2009. pp. 362–364. [Google Scholar]
- 39.Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson's disease: a review of the literature. J Commun Disord. 2000;33:59–88. doi: 10.1016/s0021-9924(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 40.Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson's disease. NeuroRehabilitation. 2005;20:205–221. [PubMed] [Google Scholar]
- 41.Montgomery EB. Deep brain stimulation and speech: a new model of speech function and dysfunction in Parkinson's disease. J Med Speech Lang Pathol. 2007;15:9–25. [Google Scholar]
- 42.Tornqvist AL, Schalen L, Rehncrona S. Effects of different electrical parameter settings on the intelligibility of speech in patients with Parkinson's disease treated with subthalamic deep brain stimulation. Mov Disord. 2004;20:416–423. doi: 10.1002/mds.20348. [DOI] [PubMed] [Google Scholar]
- 43.Bunton K, Weismer G. Evaluation of a reiterant force-impulse task in the tongue. J Speech Hear Res. 1994;37:1020–1031. doi: 10.1044/jshr.3705.1020. [DOI] [PubMed] [Google Scholar]
- 44.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 45.Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson's disease. In: Gillingham FJ, Donaldson IML, editors. Third symposium on Parkinson's disease, Royal College of Surgeons in Edinburgh. E. & S. Livingstone; Edinburgh: 1968. pp. 152–157. [Google Scholar]
- 46.Barlow SM, Suing G, Andreatta RD. Handbook of Clinical Speech Physiology. Singular Publishing Group, Inc; San Diego: 1999. Speech aerodynamics using AEROWIN. pp. 165–190. [Google Scholar]
- 47.Netsell R, Hixon TJ. A noninvasive method for clinically estimating subglottal pressure. J Speech Hear Disord. 1978;43:326–330. doi: 10.1044/jshd.4303.326. [DOI] [PubMed] [Google Scholar]
- 48.Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, Burn DJ. Prevalence and pattern of perceived intelligibility changes in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:1188–1190. doi: 10.1136/jnnp.2006.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller N, Noble E, Jones D, Allcock L, Burn DJ. How do I sound to me? Perceived changes in communication in Parkinson's disease. Clin Rehabil. 2008;22:14–22. doi: 10.1177/0269215507079096. [DOI] [PubMed] [Google Scholar]
- 50.Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, Hariz MI, Limousin P. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008;16:2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]