Abstract
Understanding the genetics of behavioral variation remains a fascinating but difficult problem with considerable theoretical and practical implications. We used the genome-tagged mice (GTM) and an extensive test battery of well-validated behavioral assays to scan the genome for behavioral quantitative trait loci (QTLs). The GTM are a panel of ‘speed congenic’ mice consisting of over 60 strains spanning the entire autosomal genome. Each strain harbors a small (B23 cM) DBA/2J donor segment on a uniform C57BL/6J background. The panel allows for mapping to regions as small as 5 cM and provides a powerful new tool for increasing mapping power and replicability in the analysis of QTLs. A total of 97 loci were mapped for a variety of complex behavioral traits including hyperactivity, anxiety, prepulse inhibition, avoidance and conditional fear. A larger number of loci were recovered than generally attained from standard mapping crosses. In addition, a surprisingly high proportion of loci, 63%, showed phenotypes unlike either of the parental strains. These data suggest that epistasis decreases sensitivity of locus detection in traditional crosses and demonstrate the utility of the GTM for mapping complex behavioral traits with high sensitivity and precision.
Keywords: fear, anxiety, learning, memory, mapping, gene
Introduction
Dissecting the mechanisms of behavior represents one of the great challenges for modern genetics. Most behaviors show a complex pattern of inheritance where many genes interact with environment to produce the final phenotype. While this reality poses a daunting problem for analysis in outbred human populations, the mouse is a convenient and powerful model for complex behavioral trait mapping. Combined with the continued refinement of behavioral protocols, the standardization of quantification techniques and the ease of maintaining and manipulating environments, the mouse represents an indispensable tool for analyzing behavioral phenotypes relevant to humans. Consequently, substantial efforts have been devoted to mapping behavioral genes in mice, resulting in the identification of loci for a variety of processes including learning and memory,1,2 fear and anxiety,3-6 sensorimotor gating7 and depression.8
Despite the power of current approaches in the mouse, gene mapping of complex traits is still complicated by several factors. A relatively large number of loci appear to contribute, suggesting that each locus makes only a small contribution to the phenotype. In addition, multiple alleles of a single gene (allelic heterogeneity) can have different effects on the phenotype of interest and any one locus can display multiple interactions with multiple loci in the genome.9-11 Even in experimental organisms, there are substantial environmental contributions to the development of certain phenotypes.12 Accurate modeling of these environmental events is complicated by several issues including an incomplete understanding of the contingencies controlling behavioral traits.13,14
The recent production of genome-tagged mice (GTM) provides a powerful new strategy for increasing both mapping power and replicability in the analysis of complex traits.15 Using marker-assisted breeding, 64 GTM strains were created covering all the autosomes, each with a small (B23 cM) DBA/2J homozygous donor segment introgressed on a uniform C57BL/6J background. The C57BL/6J and DBA/2J strains were chosen because of their many phenotypic differences. Each chromosome was represented by three to four overlapping congenic GTM strains—proximal (p), middle (m) and distal (d). Some of the mice also carried larger contiguous congenic segment with proximal and middle (pm) regions or middle and distal (md) regions. GTM strains were also created that were consomic for the entire chromosomes 1, 9, 12, 14, 16, 17 and 19.
The relatively small genetic segments of the GTM, combined with the opportunity to phenotype multiple individuals of the same genotype, provide marked advantages in resolution and sensitivity compared to traditional mapping strategies. Approximately 30% of the genome is covered by overlaps of introgressed segments, providing additional localization of any quantitative trait locus (QTL) that falls in the overlapping regions. Our investigation used the GTM set to conduct a genome-wide scan on the autosomes for behavioral QTLs. Considering the significant behavioral differences between C57BL/6J and DBA/2J strains, we reasoned the GTM set should provide candidate QTL for a number of behavioral end points. The behavioral test battery consisted of 10 individual assays designed to screen for loci related to affective behaviors, such as fear, anxiety and depression, and cognitive function including spatial representation, learning and memory. A total of 97 loci were identified, some mapped to an interval as small as 5 Mb.
Materials and methods
Subjects
Male mice, approximately 70 days old, were used. All autosomal GTM strains and parental controls were used as subjects. The GTM set contains 64 strains with on average a 23 cM introgressed donor segment in each strain (range 8–58 cM, 0.6–4% of the genome) and covering all 19 autosomes. GTM mice were bred and reared in the University of California at Los Angeles (UCLA) Gonda Research Building vivarium. At the time of weaning, mice were group housed (3–4 per cage) under a 12:12 h light/dark cycle with ad lib access to food and water. Control mice (C57 and DBA), approximately 40 days of age, were obtained from Jackson Laboratories and housed in the same facility for 30 days prior to the beginning of behavioral testing. All experimental procedures were conducted during the light portion of the cycle, between 1000 and 1800 hours. All procedures were performed under NIH Care and Use Guidelines and were approved by the UCLA Animal Research Committee.
Behavioral testing
The behavioral test battery consisted of 10 assays administered serially over 10 days. Mice were tested in batches of 40–50. A total of 832 GTM mice were tested along with 15 mice from each from the B6 and D2 parental strains. The mean number of mice tested per GTM strain was 14 with extremes 37 (3m) and 3 (19m). On each test day, all mice were retrieved from the housing facility and transported to a holding room adjacent to the behavioral laboratory. Following a 30 min acclimation period, behavioral testing began. For each task, mice were either tested in parallel groups of four or individually. Once tested, mice were returned to a holding facility until testing of the entire set was complete. The interval between tests was 24 h. All apparatus were cleaned with a 70% ethanol solution before the introduction of each mouse. No additional odorants were used during testing. Tests were performed in the following order: general activity/auditory habituation, open-field/bright light test, novel object challenge, prepulse inhibition (PPI), tail flick test, elevated plus maze, Porsolt forced swim test, fear conditioning, context fear test and cued fear test.
Data analysis
For each test in which mice were allowed free locomotion, behavior was recorded to videotape from cameras mounted above the apparatus, then digitized at 15 frames per second with the EthoVision Pro tracking system (Noldus Information Technology). The various behavioral end points were calculated automatically from the digitized activity tracks with EthoVision analysis software. For each of these tests we quantified multiple behavioral end points including velocity, multiple path-shape variables (turn angle, angular velocity, meander) and relevant place preference measures (thigmotaxis, closed-arm preference). Interactive variables (distance to object/point, proximity to object/point, speed moving relative to an object/point) and immobility were calculated for selected tests.
Calculation of all locomotor, path-shape and place preference end points with EthoVision began with the identification of a subject's center of gravity through an analysis of its surface area. Values for the various end points were then estimated continuously across pairs of consecutive samples. Velocity was defined as the distance moved in cm by the center of gravity across samples per unit time. Turn angle was defined as the average change in heading, in degrees, across two consecutive samples, where heading equaled the direction of movement relative to a reference line established over the preceding two samples. Angular velocity was defined as the average turn angle per unit time. Meander was defined as the average turn angle per distance moved. Distance to a point was defined as the average distance, in cm, between the subject's center of gravity and a reference point, either the center of the object in the novel object test or the center of the apparatus in open-field and elevated plus maze tests. Proximity was defined as the percent time a subject's center of gravity was located less than 8 cm from a reference point. Speed moving to a point, a measure of approach intensity, was defined as the time, in seconds, during which a subject was moving toward the point. Immobility was defined as the total time in which less than 3% of the subjects surface area moved across consecutive samples. For all place preference measures, the presence of mice in a predefined region was determined according to the location of its center of gravity. Thigmotaxis in the general activity test was calculated as the total time spent in the periphery of the apparatus, defined as the area within 5 cm of the wall. For both open-field tests the periphery was defined as the area within 6 cm of the wall. Closed-arm preference in the elevated plus maze was calculated as percent time mice were located within the closed arms. Total fecal boli per session were quantified manually from videotape.
For all continuous measures, data were acquired at 20 s intervals. However, this level of resolution provided no unique insights and the data were grouped into 1 min bins for presentation and analysis. Significant GTM strain differences were analyzed with reference to the B6 background parental strain using Dunnett's correction for multiple hypotheses. Significance was set at the P < 0.05 level for all tests. Statistical calculations were made with JMP 5.0.1.2 Statistical Discovery Software.
General activity/auditory novelty test
Mice were placed into an activity monitor chamber consisting of a rectangular box (l, 50 cm; w, 25 cm; h, 25 cm) with white laminated flooring and white plexiglass walls. A ventilation fan provided background noise (58 dB). Total test length was 15 min. Following an initial 5 min stimulus-free baseline period, a 10 min continuous white noise auditory stimulus (70 dB) was delivered from an overhead speaker mounted 1.8 m above the chamber. Five min after noise onset, three 20 s white noise bursts (80 dB) were delivered with an intertrial interval (ITI) of 60 s from in-chamber speakers mounted 10 cm above the floor on one end of the chamber. A single white light bulb provided indirect illumination (~10 lux). We calculated velocity, turn angle, angular velocity, meander, thigmotaxis and fecal boli count as a function of the three auditory conditions.
Open-field/bright light test
A modified version of the open-field test was used to assess anxiety and innate fear.3,4,16 All mice were placed in a circular open field (d, 70 cm; h, 30 cm) with white laminated flooring and white plastic walls. Illumination was provided by floodlights mounted 50 cm above the floor of the field. A ventilation fan provided background noise (50 dB). Total test length was 12 min. The initial 4 min period was conducted under dark lighting conditions (~5 lux). For minutes 5–8, the lighting level was increased to ~400 lux. For the final 4 min period, lighting was returned to the original level (~5 lux). We calculated velocity, turn angle, angular velocity, meander, thigmotaxis and fecal boli count as a function of lighting condition.
Novel object challenge
To assess approach and avoidance tendencies we exposed mice to a novel object challenge.17 Mice were returned to the same apparatus used for the previous open-field test, except a black plastic cylinder (d, 10 cm; h, 10 cm) was placed on the floor of the test chambers, 8 cm from the wall. Lighting was provided by overhead floodlights and was held constant throughout the test at ~200 lux. Total test duration was 8 min. In addition to velocity, turn angle, angular velocity, meander and thigmotaxis, we also calculated average distance from the object and duration of proximity.
Elevated plus maze
The plus maze is a widely employed and pharmacologically validated test of anxiety.18,19 The plus-shaped maze had white laminate flooring with two opposite open arms and two arms enclosed with black plastic walls (h, 30 cm; l, 35 cm). The maze center was open. The apparatus was elevated 1.6 m above the floor. Indirect lighting (~200 lux) was provided by an overhead floodlight positioned 15 cm below the ceiling. Mice were placed in the center of the apparatus for a single 5 min trial. We calculated the standard activity measures of velocity, turn angle, angular velocity and meander along with the test-specific measures of distance from the maze center and speed of moving to the ends of the open arms.
Fear conditioning
Learning and memory were assessed through a Pavlovian fear conditioning procedure. This multiphase assay has proven a reliable measure of multiple processes including elemental and spatial learning, short-term memory, long-term memory, memory extinction and pain sensitivity.20-23 Additionally, this procedure has been used to link various psychological processes to activity in discrete neural regions.24-26 Fear conditioning was conducted in a modified Gemini Avoidance System (San Diego Instruments, San Diego, CA, USA). The chamber had white plexiglass walls and a clear plexiglass ceiling (h, 25 cm; w, 22 cm; d, 18 cm). The floor of the chamber consisted of 12 stainless steel rods (2 mm diameter, 1.2 cm apart) connected to the shock generator. Tones were presented from a speaker mounted on the wall of each chamber. Indirect lighting (~200 lux) was provided by a white floodlight placed 6 inches above the apparatus and 4 ft below the ceiling. Mice were placed in the chamber and given a 3 min habituation period followed by a delay conditioning procedure in which three tone (2 kHz, 80 dB, 15 s)–shock (0.6 mA, 1 s) pairings were delivered at 1 min intervals. Mice were removed from the chamber 1 min after the final pairing. Velocity, turn angle, angular velocity, meander, thigmotaxis, immobility and fecal boli count were assessed across the session as a function of the three distinct test phases: pre-CS, conditioning and post-shock. The pre-CS phase consisted of the 3 min period prior to conditional stimulus (CS)–unconditional stimulus (US) pairings. The conditioning phase consisted of the 2.75 min period encompassing the three CS–US pairings. The post-shock period consisted of the 1 min period following the termination of the final footshock.
Context fear test
Mice were placed in the conditioning chamber for an 8 min extinction test 24 h after conditioning. No stimuli were presented during this period. Velocity, turn angle, angular velocity, meander, thigmotaxis, immobility and fecal boli count were assessed across the session. This test assesses the ability of mice to form both a representation of the conditioning chamber and a memory of its association with shock,27 processes that appear to rely on intact hippocampal function.24,25
Cued fear test
Mice were placed in the chamber previously used to assess general activity for a 15 min cued extinction test. After a 3 min exploration period, 10 CS (2 kHz, 80 dB, 20 s) presentations were delivered with a 1 min ITI. Mice were removed from the chamber 2 min after the final cue presentation. Velocity, turn angle, angular velocity, meander, thigmotaxis and immobility were assessed independently during both the 3 min exploration period and across the CS presentations.
Prepulse inhibition
Prepulse inhibition of the acoustic startle response was used to assess sensorimotor gating.16,28 The Startle Reflex Lab controlled by SR-Lab software (San Diego Instruments) was employed for this test. Mice were confined to a cylindrical restraint tube inside a sound-attenuating chamber for the duration of testing. Background white noise (65 dB) was provided by an overhead speaker mounted inside the chamber. Total test duration was 18 min. Sessions began with a 5 min acclimation period. Next, five pulse-alone trials (P), consisting of a single white noise burst (120 dB, 40 ms), were delivered. The prepulse–pulse trials (PP74P, PP77P and PP80P) consisted of a prepulse of white noise (20 ms at 74, 77 or 80 dB, respectively), followed 100 ms after onset of prepulse by a white noise pulse (120 dB, 40 ms). Data from pulse and prepulse trials were recorded at the onset of the 120 dB pulse for 65 ms in 1 ms increments. The maximum force intensity of startle for each trial was recorded using a piezoelectric transducer in the floor of the cylindrical holder. The average percent reduction in startle intensity between pulse and prepulse trials at all three prepulse levels constituted PPI. Ten blocks, each containing all five trials in pseudorandom order (P, PP74P, PP77P, PP80P and NS), were delivered. ITIs were pseudorandomly distributed between 10 and 20 s.
Tail flick test
This assay utilizes the tail flick reflex to assess pain sensitivity.16 Mice were loosely restrained by hand in a paper towel on a Columbus Instruments Tail-Flick Apparatus. The tail of the animal was placed in a groove in the apparatus above a shuttered lamp. A foot trigger opened the shutter and started a timer. The heat from the lamp provided a nociceptive stimulus eventually causing the mouse to flick its tail away from the groove. Latency was determined by the instrument's autodetection capability and provided a measure of pain sensitivity.
Porsolt forced swim
The Porsolt test is a commonly used test of behavioral despair or depressive-like behaviors.29,30 Mice were placed in a plastic cylinder (d, 35 cm; h, 45 cm), containing water at a temperature of 22–24 °C and a depth of 35 cm so that mice could neither escape nor touch the bottom. Total test duration was 5 min. Velocity, turn angle, angular velocity, meander and immobility were calculated throughout the session.
Results
General activity/auditory novelty test
This test involved exposure to a rectangular open-field for the quantification of general behavioral patterns. The initial 5 min stimulus-free period was followed by two consecutive 5 min periods consisting of continuous white noise and three phasic noise bursts, respectively. Genome-wide data for selected behavioral end points are presented on the left hand of Figure 1. For all tests, Dunnett's correction for multiple hypotheses was employed, meaning that only one significant GTM is expected for every 20 tests at P < 0.05, and every 100 tests at P < 0.01. Thigmotaxis is preference for the periphery of the apparatus and high levels of this behavior indicate high levels of anxiety. Analysis of thigmotaxis revealed significant differences between B6 mice and a number of GTM strains, but no significant differences between the B6 and D2 mice. Strains 3d, 11d, 13d and 15m all demonstrated a reduced preference for the periphery relative to B6 (P < 0.01, all strains; Figure 1a), suggestive of a low-anxiety phenotype. These effects apparently reflect general deficits in thigmotaxis as the pattern was observed consistently across the entire test session independent of the variable auditory stimulus conditions. Four additional strains (2m, 11p, 12m and 16m) displayed significant reductions in thigmotaxis during the initial stimulus-free period relative to B6 mice (P < 0.01, all strains), but not during the presentation of ambient or phasic noise (Figure 1a, right). These data suggest multiple loci for thigmotaxis scattered throughout the genome. Additionally, the results demonstrate an apparent sensitivity of the thigmotaxis response to auditory stimulus conditions.
Figure 1.
General locomotor activity. (a) Thigmotaxis expressed as percent time spent in the periphery. (b) Activity level indexed by mean velocity. (c) Path shape measured by average turn angle. All tests employed comparisons with B6, using Dunnett's correction for multiple hypotheses. (Left) Performance of individual mice (black dots), group mean (open circle) and +/− standard error of the mean (gray rectangle). Black * indicates genome-tagged mice (GTM) strains that were significantly different from B6 across the entire test. Red * indicates strains that were significantly different from B6 in at least one of the three test periods. (Right) Time courses for identified GTM strains and parental strains. Schematic graph depicts prevailing stimulus conditions for each test phase.
Analysis of velocity profiles identified one strain, 2d, that displayed significant hyperactivity relative to B6 controls across the entire test session (P < 0.01), while B6 and D2 parental strains did not significantly differ during any time period. The hyperactivity observed in 2d mice appears to be a stable trait in this strain as it was observed consistently across each stimulus period. Five additional strains (1p, 8p, 11m, 15d and 19p) displayed hyperactivity during the stimulus-free period (P < 0.03, all strains), but did not differ from B6 during the remaining two stimulus periods (Figure 1b, right). As was the case with thigmotaxis, velocity phenotypes in some strains appear to be sensitive to changes in auditory environment.
Analysis of path shape revealed multiple strains displaying increases in turn angle in response to phasic noise stimuli. Strains 11p, 14d, 16c and 18d all displayed significant increases in turn angle relative to B6 mice (P < 0.05, all strains). These strains did not significantly differ during either the stimulus-free baseline period or the presentation of ambient noise. B6 and D2 mice did not differ during phasic noise presentation, but were significantly different during the initial stimulus-free period, with D2 mice displaying significantly larger average turn angle (P < 0.01; Figure 1c). These data suggest that path-shape differences in the GTM panel do not reflect a general characteristic of any strain, but rather an event-related trait. No strain differences were observed for angular velocity, meander or fecal boli count.
Interestingly, three end points (thigmotaxis, velocity and noise-dependent turn angle) revealed multiple loci in the GTM panel despite no significant differences between parental strains. This phenomenon was a recurring theme throughout the behavioral screen, suggesting that epistasis may mask many potential behavior differences between inbred strains.
Open-field/bright light test and novel object challenge
The open-field is a widely accepted assay of anxiety. Anxious animals avoid the periphery of the brightly lit field and show restricted movements. There were three phases to the test: a dark phase of 4 min followed by a brightly lit phase of 4 min and a final dark phase of 4 min. Open-field exposure revealed significant strain differences on both locomotor and place preference measures. Thigmotaxis analysis identified one strain, 11d, which differed significantly from B6 across the test session (Figure 2a). This effect appeared to be driven primarily by performance during the bright phase, where 11d mice spent significantly more time in the center of the field than B6 mice (P < 0.02). While this strain displayed a trend toward thigmotaxis deficits during the initial dark phase, they did not significantly differ from B6 during either the initial or final dark phase (P > 0.05, both phases). D2 mice showed a trend toward enhanced thigmotaxis during the bright phase but they did not significantly differ from B6 during any test phase (P > 0.50, all phases; Figure 2a, right).
Figure 2.
Open-field and novel object challenge. (a) Thigmotaxis expressed as percent time spent in the periphery during open-field test. (b) Activity level expressed as mean velocity during open-field test. (c) Object interaction expressed as average distance from object during novel object challenge. (d) Activity level expressed as mean velocity during novel object challenge. For both tests, black * indicates genome-tagged mice (GTM) strains that were significantly different from B6 across the entire test. For the open-field test, red * indicates strains that were significantly different from B6 in at least one of the three lighting conditions.
Analysis of velocity data identified four strains (1pm, 2d, 18pm and 19p) that displayed significant hyperactivity across the three test phases relative to B6 (P < 0.01, all strains; Figure 2b, left). Collectively, behavior in these strains mirrored that of B6 mice, displaying rapid velocity habituation during the initial dark phase, brief velocity acceleration following light onset and general activity suppression across the light phase. Independent analysis of each test phase revealed two additional strains (10m and 19d) that displayed significantly elevated velocity selectively during bright light exposure (P < 0.03, both strains). These two strains exhibited normal performance across both dark phases. D2 mice did not significantly differ from B6 during any test phase (P > 0.40, all phases; Figure 2b, right). No strain differences were observed for turn angle, angular velocity, meander or fecal boli count.
Open-field exposure in the presence of a novel object identified multiple potential loci related to approach tendency (Figure 2c). Analysis of average distance from the object identified eight strains (2m, 2d, 3m, 5d, 6p, 8pm, 8d and 12d) that maintained significantly closer proximity to the object than B6 mice (P < 0.02, all strains). Behavior in these strains was similar to D2 mice, which gradually increased proximity across the test session. In contrast, B6 mice displayed the opposite tendency (Figure 2c, right). It is possible that the strain differences are due to a mnemonic or learning effect rather than neophobia. This is rendered less likely by the small overlap between GTM significant for the novel object test and context and cued fear tests (see below).
Analysis of velocity data in the presence of the novel object confirmed previously identified loci in the open field, and identified additional, object-dependent loci (Figure 2d, left). With the exception of strain 19p, all strains identified as hyperactive in the initial open-field test (1pm, 2d and 18pm) also displayed elevated velocity relative to B6 (P < 0.03, all strains). D2 mice did not differ significantly from B6 mice (P > 0.60). We also identified six novel hyperactive strains (1d, 3m, 6p, 10p, 12d and 16p; P < 0.03, all strains). As all these strains displayed normal activity levels during the previous open-field test, the observed hyperactivity appears to be directly related the presence of the novel object. Consistent with this characterization, three of these hyperactive strains (3m, 6p and 12d) also maintained closer proximity to the object (Figure 2d, right). No strain differences were observed for turn angle, angular velocity, meander thigmotaxis, duration of proximity or fecal boli count.
Elevated plus maze
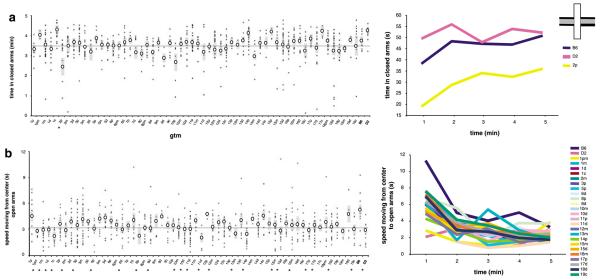
The elevated plus maze elicits a range of defensive responses and has been widely used to assess anxiety behavior. The two open and two closed arms of the maze are thought to be differentially anxiogenic, with the open arms being more anxiety provoking. This results in divergent behavioral profiles on place preference and locomotor variables. Accordingly, a strong preference for the closed arms was observed, with B6 controls collectively spending ~75% of the session in these areas. A single GTM strain, 2p, exhibited significant reductions in this preference (P < 0.005), while D2 mice were statistically indistinguishable from B6 (P > 0.90; Figure 3a, right). The increasing degree of dark arm preference for strain 2p mirrored other GTM and parental strains across the test.
Figure 3.
Elevated plus maze. (a) Place preference expressed as total time spent in closed arms. (b) Open-arm exploration expressed as speed moving from center. Asterisks (*) indicate genome-tagged mice (GTM) strains that were significantly different from B6.
Differential defensive behavior between the open and closed arms was reflected in a number of additional variables including velocity (P < 0.001), speed of moving to the ends of the open arms (P < 0.001) and distance from center (P < 0.001). Analysis of exploratory forays directed from the maze center into the anxiogenic open arms identified numerous GTM strains (1pm, 1m, 1d, 1c, 2m, 3p, 5p, 6d, 8p, 8d, 10m, 10d, 11p, 11d, 12m, 13m, 14p, 15m, 15d, 16m, 17p, 17d, 18d and 19c) that, like D2, displayed significant reductions in exploration speed time relative to B6 (P < 0.01, all strains; Figure 3b, right). Reductions in exploration time are consistent with increased anxiety for the open arms. The similarity between the identified GTM strains and D2 parental mice suggests multiple, independent loci for this anxiety phenotype. Temporally, a qualitatively similar pattern was observed in the parental and GTM strains, with the open-arm exploration time rapidly declining over the initial 2 min period followed by a more gradual decline across the remainder of the test. No strain differences were observed for turn angle, angular velocity, meander, distance from center or fecal boli count.
Fear conditioning
Fear conditioning is a series of tests used to assess multiple processes including short term-memory, long-term memory, fear response and memory extinction. Training consisted of three pairings of an auditory cue CS with footshock US, presented in a distinctive training chamber. After 24 h, mice were returned to the training chamber, with increased immobility indicating intact fear memory. To assess cued fear, mice were placed in a novel context for 3 min prior to 10 nonreinforced CS presentations. Levels of immobility and its decrease across CS presentations served as measures of cued fear and memory extinction, respectively.
All strains showed comparable levels of activity and immobility prior to CS–US pairings. Immediately following CS–US pairings, overall activity levels declined while immobility increased (data not shown). While immobility data did not identify significant GTM strain differences, consideration of velocity revealed five strains (8pm, 9c, 12p, 12d and 14c) that maintained significantly elevated velocity relative to B6 (P < 0.02, all strains; Figure 4a, right). A similar elevation was also observed in D2 mice (P < 0.02). No strain differences were observed for turn angle, angular velocity, meander, thigmotaxis or fecal boli count. The observed velocity elevations are consistent with deficits in either short-term memory or the expression of this particular fear response.
Figure 4.
Fear conditioning. (a) Post-shock activity suppression expressed as mean velocity. (b) Contextual fear indexed by percent immobility. (c) Cued fear indexed by percent immobility during the 10 min conditional stimulus (CS) presentation phase. Inset depicts timing of CS presentations. Asterisks (*) indicate genome-tagged mice (GTM) strains that were significantly different from B6.
Contextual fear test
Contextual fear testing identified 16 strains (1pm, 1m, 1c, 2d, 5p, 5d, 7d, 8d, 9m, 10d, 11p, 12m, 14p, 15p, 16m and 18d) that maintained significantly enhanced immobility across the test session (P < 0.005, all strains; Figure 4b, right). Notably all the significant GTM strains showed enhancements of freezing relative to B6, while D2 mice displayed the typically observed trend toward immobility deficits (P > 0.10).31,32 The immobility elevations observed in multiple GTM strains were consistent with enhancements of representational or associational processes involved in fear memory formation or retrieval. Interestingly, none of the five strains exhibiting apparent deficits during the training session differed significantly from B6 in immobility during the contextual fear test, suggesting normal fear response production in these strains. No strain differences were observed for turn angle, angular velocity, meander or thigmotaxis or fecal boli count.
Cued fear test
Behaviors during the initial 3 min baseline period and the 10 min CS presentation period were analyzed separately. Immobility gradually increased over the 3 min baseline period in all strains, displaying peak immobility near the placement-to-shock interval during training. However, no GTM strains differed significantly from B6 mice (P > 0.05, all strains; data not shown), indicating normal contextual fear generalization across the GTM panel. Analysis of behavior during the CS presentation period revealed a single strain (1c) that displayed significantly elevated immobility relative to B6 (P < 0.01; Figure 4c, right). D2 mice showed significantly reduced immobility (P < 0.01). No strain differences were observed for turn angle, angular velocity, meander or thigmotaxis or fecal boli count during either the pre-cue or cue presentation period. Notably, strain 1c also exhibited enhanced immobility during the contextual fear test, but no differences in activity suppression during the training post-shock period. This pattern demonstrates selective effects on long-term fear memory rather than general effects on the fear response. Because none of the other chromosome 1 GTM showed an effect on cued fear, the phenotype displayed by strain 1c must represent a synthetic effect of multiple loci on chromosome 1.
Tail flick test
Pain sensitivity was assessed with the tail flick test, in which radiant heat is applied to the tail eliciting the tail withdrawal reflex. Longer withdrawal latency indicates decreased nociception. Three GTM strains (2d, 16m and 19m) and D2 parental mice showed significant elevations in withdrawal latency relative to B6 mice (P < 0.01, all strains; Figure 5a). Similar to the parental strains, the three GTM strains collectively displayed significant decreases in latency across the three trial sessions suggesting general differences in pain sensitivity rather than sensitization of the withdrawal reflex (data not shown). The similarity in phenotypes between the identified GTM strains and D2 parental mice is consistent with codominant loci for pain sensitivity within the introgressed segments of strains 2d, 16m and 19m.
Figure 5.
Tail flick, prepulse inhibition (PPI) and forced swim tests. (a) Pain sensitivity expressed as mean withdrawal latency in second. (b) Mean PPI (percent of startle-alone response) across three prepulse intensities. (c) Percent forced swim immobility. Asterisks (*) indicate genome-tagged mice (GTM) strains that were significantly different from B6.
Prepulse inhibition
Prepulse inhibition was used to assess sensorimotor gating, an attentional mechanism often abnormal in neuropsychiatric patients, who have decreased PPI.33,34 The response is quantified by the extent to which the startle response to an unexpected acoustic stimulus is inhibited by a brief auditory prepulse. Three strains (8pm, 11p and 15p) exhibited significant enhancements in PPI relative to B6 mice (P < 0.03, all strains). D2 mice showed a trend toward reduced PPI but were not significantly different from B6 controls (P > 0.06; Figure 5b). Prepulse intensity was positively correlated with PPI in both parental strains and the implicated GTM strains, suggesting normal sensory processing throughout the panel. All GTM strains displayed normal startle amplitude, despite significant reductions in D2 parental mice (P < 0.005; data not shown).
Porsolt forced swim test
The swim test is a commonly used assay of behavioral despair or depressive-like behaviors. Overall activity and immobility levels serve as the primary behavioral measures. Despite pronounced immobility deficits in D2 mice (P < 0.005), no GTM strains differed significantly from B6 (Figure 5c). Similarly, no strain differences were observed for turn angle, angular velocity, meander or thigmotaxis. The failure to detect loci for behavioral despair, despite pronounced differences in the parental inbred strains, suggests that the behavioral profiles elicited by this test are under the control of a very large number of loci.
Discussion
This study employed the GTM mice, a panel of B6 × D2 congenics, to scan the genome for loci related to a number of complex behavioral traits. In comparison to existing chromosome substitution strains that are congenic for full chromosomes, the GTM contain smaller segments of chromosomes and therefore represent a significant increase in mapping resolution. Each GTM strain contains donor segments ranging from 0.4 to 6% of the genome. The existence of numerous overlapping regions between GTM strains further enhances potential mapping power. The GTM library thus represents a powerful resource for mapping complex traits including behavior. Combined with a multi-test behavioral screen consisting of 10 well-validated assays, we fully characterized within-test performance across a number of locomotor and behavioral variables. This approach mapped 97 loci for many task-specific behaviors to specific genetic regions.
The congenic approach to mapping behavioral loci assumes that genes in the donor region contribute directly to any observed variability between the congenic and background strains. Thus, congenic strains might be expected to behave similarly to one of the two parental strains, with donor-like behavior in a strain indicating a potential locus for the trait. Some GTM strain differences observed in the current study did fit this profile, where significant GTM strain variations generally mirrored the parental differences in both magnitude and direction. However, many (~63%) strain effects were observed outside the phenotypic range defined by the parental strains or in the absence of any parental phenotypic divergence. These GTM strain differences likely reflect epistatic effects, which mask phenotypic differences between the parental inbred strains. Consistent with this notion, inter- or backcross mapping experiments, which result in much more complex genetic structures than the GTM, usually result in phenotypes intermediate between parental strains. Epistasis probably also explains why many more loci were uncovered by the GTM compared to conventional mapping crosses. For example, we uncovered 16 loci for contextual fear conditioning compared to 7 using a C3H × B6 back-cross strategy.1
The current data provided a complex picture of the genetics underlying hyperactivity. As indexed by velocity, strain 2d displayed consistent hyperactivity in open environment tests (general activity test, open-field test and novel object challenge). An additional strain, 19p, exhibited significant hyperactivity across the first two tests and a similar trend in the novel object challenge. These strains correspond to previously reported loci for open-field hyperactivity identified through mapping with B × D RI strains.35,36 Elevated activity levels were also observed in a task-dependent manner, with several strains displaying elevations selectively during a subset of these tests or during a particular test phase. Strains 1pm and 18pm were hyperactive across all open-field test phases and the novel object challenge, while multiple strains (1d, 3m, 6p, 10p, 12d and 16p) exhibited hyperactivity selectively during the novel object challenge. Interpretation of task-specific hyperactivity is not straightforward, but may reflect the anxiogenic properties of the respective assays. As such, the lack of overlap in GTM strains exhibiting hyperactivity in these tasks may suggest the existence of independent, genetically dissociable anxiety phenotypes.
Consideration of place preference behavior yielded multiple strains deficient in thigmotaxis. Of these, only strain 11d exhibited consistent deficits across both the general activity and open-field tests. Previous work with consomic strains has implicated chromosome 11 as a locus for thigmotaxis.37 A strong locus in the middle region of chromosome 16 has also been previously mapped with B6 × D2 RI strategy.38 GTM strains corresponding to previously reported loci on chromosomes 1, 6 and 14 in a A/J × B6 intercross5 did not differ from B6. With respect to loci identified in the activity test, most effects did not manifest as a general trait but rather as an event-related response to the presentation of ambient noise. Surprisingly, strain differences in thigmotaxis were observed almost exclusively during the general activity test. Open-field testing, for which thigmotaxis is a defining trait, identified only the aforementioned strain 11d while the novel object challenge yielded no strain differences. The apparent normalization of thigmotaxis deficits between general activity and open-field testing in the remaining strains was an unexpected result. This pattern does not appear to reflect a long-term habituation of the response, as overall level of thigmotaxis in the open-field test was normal. Any effect of habituation on response levels should also be dishabituated by the pronounced shift in context between activity and open-field testing. Given the insufficiency of nonassociative interpretations, this pattern may reflect learning acquired during the activity test.
A number of strains exhibited significant elevations in turn angle, which were primarily observed during the presentation of ambient noise. Path-shape variables have not been widely scrutinized within the context of anxiety tests and the relevant psychological construct is unclear. The observation of phasic path-shape changes in distinct test phases suggests that this end point may mirror an increase in an anxiety state resulting from novelty detection.
Further phenotyping of the novel object challenge identified eight GTM strains that maintained significantly closer proximity than B6, a trait associated with low-anxiety performance on more traditional tests.17 The striking similarity in behavioral time courses between the identified GTM strains and the D2 parental mice strongly suggests that these strains harbor codominant loci contributing directly to the behavior. Additional measures such as approach or withdrawal speeds relative to the object did not identify additional loci. This test has not been previously employed in anxiety-mapping studies and hence no loci have been previously mapped for variability in object interaction. However, a QTL for defensive behavior has been previously identified on the distal region of chromosome 8,39 consistent with a locus for influencing object interaction on GTM strain 8d.
The collection of traits quantified in the elevated plus maze test identified loci for both high and low anxiety. A traditional index of plus maze performance, closed-arm preference, identified a single strain (2p), demonstrating a significant reduction in preference presumably indicating loci for reduced anxiety. Loci corresponding to this strain have not been previously mapped for any phenotype associated with the elevated plus maze. However, this strain effect was observed in the absence of any significant differences between parental strains, suggesting that an effect of this locus could have been masked through epistatic effects in previous mapping studies. An additional measure, open-arm exploration speed, identified a large number of loci that mirrored performance of D2 parental mice. This trait measures the intensity of movements from the relatively safe center to the more anxiogenic regions toward the ends of the open arms. Given the lack of parametric work done on this end point, the psychological construct underlying behavior is unclear. Performance on this measure does not appear to be related to differences in open-arm preference, as none of the identified strains differed from B6 in the percentage of time spent in the open arms. As this trait measures behavior solely in the more anxiogenic regions of the maze, it likely reflects levels of fear or anxiety. Interestingly, multiple GTM strains from chromosome 1, including the consomic 1c, all showed performance deficits, consistent with the widely reported loci for fear and emotionality on chromosome 1.40
The multi-phase fear conditioning procedure revealed many potential loci for both short- and long-term fear memory. While data from the training session identified no strain differences during the post-shock period as indexed by immobility, multiple strains did exhibit significantly elevated velocity, as did D2 parental mice. Reduction in post-shock activity levels has been proposed as a model of short-term fear memory.20 By this view, the elevated velocity observed in these GTM strains can be interpreted as a short-term memory deficit and represents the initial mapping of loci for short-term fear memory.
A number of potential loci were observed for contextual fear. This test measures the ability of mice to form a representation of the training context and associate that representation with the footshock US,27 a process convincingly linked to hippocampal-dependent processes.41 Notably, all the identified GTM strains showed enhanced immobility relative to B6, while D2 mice displayed typical immobility deficits.31,32 Loci affecting contextual fear have previously been reported in many of the regions represented by these strains. Using both intercross and backcross approaches, QTL have been observed in regions corresponding to GTM strains 1pm, 1m, 2d and 16m.1,2 Mapping with B6 × D2 RI strains has identified similar effects on chromosomes 1 and 2 as well as loci represented by GTM strains 5p, 8d and 9m.36 Additionally, a large collection of new loci was identified with strains 5d, 7d, 10d, 11p, 12m, 14p, 15p and 18d.
Contextual generalization was assessed during the 3 min baseline period prior to CS presentation. Exposure to a novel context following fear conditioning typically produces fear responses, presumably reflecting the generalization of conditional fear from the training context to the new and not entirely dissimilar context. This phenotype may prove to be of some clinical interest due to the prevalence of excessive generalization in many human anxiety disorders.42,43 No strain differences were found for immobility prior to the CS, although a trend toward reduced velocity, suggestive of a low-level fear response, was apparent in several strains. The failure to observe significant strain differences was unexpected given the presumed dependence of context generalization on context fear memory and the large number of loci identified for context fear in this study. The lack of loci does not appear to stem from a failure of generalization. The two test contexts differed substantially, yet we observed steady recruitment of immobility across the initial 3 min interval, a pattern typical in tests of context fear. It remains possible that strain differences were masked due to sharpening of generalization gradients for contextual stimuli following exposure to various apparatus across the screen.44 This would reduce overall immobility levels, effectively producing a floor effect and masking any potential loci. Additional work would be required to address this possibility. Alternatively, the failure to detect loci for context generalization may indicate that this behavior is controlled by a very large number of loci. Demonstrating a genetic dissociation between contextual fear and context generalization would have important implication for theoretical accounts of fear expression and therapeutic strategies.
A single strain, 1c, displayed enhanced CS immobility across the test period. All other GTM strains showed comparable levels of CS fear with no appreciable extinction over the 10 trials. Loci for fear-related behavior on chromosome 1 have been consistently observed with a variety of mapping strategies.1,2,5,38,45 As strain 1c represents the entire chromosome, the observed enhancement in immobility, without appreciable effects in the remaining chromosome 1 congenic strains, suggests that interactions between independent chromosome 1 loci govern the final phenotype. Other previously reported loci, such as those identified through a B6 × D2 intercross in the middle regions of chromosomes 10 and 16,2 were not observed in the corresponding GTM strains.
The lack of concordance between strains identified in the context and cued fear tests in our study was somewhat surprising given the results of previous mapping studies that found several overlapping QTLs.1,2 As plasticity critical for both context and cued fear learning occurs in common neural regions and requires similar molecular processes,24,46,47 congruent loci might be expected. However, context and cued fear appear to be mediated by somewhat independent neural pathways, with the latter capable of acquisition through multiple pathways.48 This property may render cued fear relatively difficult to disrupt with the relatively simple congenic manipulations inherent in the GTM strains. Despite a general lack of strain effects for cued fear, our data demonstrate that the observed enhancements in context fear do not reflect an increase in fear response production but rather a specific effect on processes mediating aspects of contextual fear learning or expression.
One of the aims of our multi-test approach was to assess the reliability of certain phenotypes across measures that presumably tap similar psychological constructs. Thigmotaxis, object interaction and closed-arm preference in the elevated plus maze have all been offered as measures of anxiety. However, a consideration of the performance of GTM strains across all tests (Table 1) shows that individual strains did not always express consistent enhancements or deficits across these related measures. As discussed above, strain 11d expressed consistent deficits in thigmotaxis in both the general activity and open-field tests. However, all other strains deficient in thigmotaxis in the initial test showed normal performance on that same measure in the open-field test and either normal performance or behavior indicative of increased anxiety on the other related measures. Similar dissociations were observed in strains displaying both normal thigmotaxis and low-anxiety phenotypes in the novel object challenge. These patterns underscore the complexity of the genetics underlying defensive behavior and the difficulties associated with modeling complex behavioral traits.
Table 1.
GTM phenotype summary
| GTM | General activity |
Open field |
Novel object |
Plus maze |
Fear conditioning |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THG | VEL | TA | THG | VEL | ODST | VEL | CAP | SFC | P-VEL | CT-IM | C-IM | TF | PPI | FS-IM | |
| 1p | — | ▲ | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 1pm | — | — | — | — | ▲ | — | ▲ | — | ▼ | — | ▲ | — | — | — | — |
| 1m | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | |
| 1d | — | — | — | — | — | — | ▲ | — | ▼ | — | — | — | — | — | — |
| 1c | — | — | — | — | — | — | — | — | ▼ | — | ▲ | — | — | — | |
| 2p | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — | — |
| 2m | ▼ | — | — | — | — | ▼ | — | — | ▼ | — | — | — | — | — | — |
| 2d | — | ▲ | — | — | ▲ | ▼ | ▲ | — | — | — | ▲ | — | ▲ | — | — |
| 3p | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 3m | — | — | — | — | — | ▼ | ▲ | — | — | — | — | — | — | — | — |
| 3d | ▼ | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 5p | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | |
| 5m | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 5d | — | — | — | — | — | ▼ | — | — | — | — | ▲ | — | — | — | — |
| 6p | — | — | — | — | — | ▼ | ▲ | — | — | — | — | — | — | — | — |
| 6pm | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6d | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 7p | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 7d | — | — | — | — | — | — | — | — | — | — | ▲ | — | — | — | — |
| 8p | — | ▲ | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 8pm | — | — | — | — | — | ▼ | — | — | — | ▲ | — | — | — | ▲ | — |
| 8d | — | — | — | — | — | ▼ | — | — | ▼ | — | ▲ | — | — | — | — |
| 9m | — | — | — | — | — | — | — | — | — | — | ▲ | — | — | — | — |
| 9d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 9c | — | — | — | — | — | — | — | — | — | ▲ | — | — | — | — | — |
| 10p | — | — | — | — | — | — | ▲ | — | — | — | — | — | — | — | — |
| 10m | — | — | — | — | ▲ | — | — | — | ▼ | — | — | — | — | — | — |
| 10d | — | — | — | — | — | — | — | — | ▼ | — | ▲ | — | — | — | — |
| 11p | ▼ | — | ▲ | — | — | — | — | — | ▼ | — | ▲ | — | — | ▲ | — |
| 11m | — | ▲ | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 11d | ▼ | — | — | ▼ | — | — | — | — | ▼ | — | — | — | — | — | — |
| 12p | — | — | — | — | — | — | — | — | — | ▲ | — | — | — | — | — |
| 12m | ▼ | — | — | — | — | — | — | — | ▼ | — | ▲ | — | — | — | — |
| 12d | — | — | — | — | — | ▼ | ▲ | — | — | ▲ | — | — | — | — | — |
| 12c | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 13p | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 13m | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 13d | ▼ | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 14p | — | — | — | — | — | — | — | — | ▼ | — | ▲ | — | — | — | — |
| 14m | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 14d | — | — | ▲ | — | — | — | — | — | — | — | — | — | — | — | — |
| 14c | — | — | — | — | — | — | — | — | — | ▲ | — | — | — | — | — |
| 15p | — | — | — | — | — | — | — | — | — | — | — | — | — | ▲ | — |
| 15m | ▼ | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 15d | — | ▲ | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 16p | — | — | — | — | — | — | ▲ | — | — | — | — | — | — | — | — |
| 16m | ▼ | — | — | — | — | — | — | — | ▼ | — | ▲ | — | ▲ | — | — |
| 16d | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 16c | — | — | ▲ | — | — | — | — | — | — | — | — | — | — | — | — |
| 17p | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 17d | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| 17c | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 18pm | — | — | — | — | ▲ | — | ▲ | — | — | — | — | — | — | — | — |
| 18d | — | — | ▲ | — | — | — | — | — | ▼ | — | ▲ | — | — | — | — |
| 19p | — | ▲ | — | — | ▲ | — | — | — | — | — | — | — | — | — | — |
| 19m | — | — | — | — | — | — | — | — | — | — | — | — | ▲ | — | — |
| 19d | — | — | — | — | ▲ | — | — | — | — | — | — | — | — | — | — |
| 19c | — | — | — | — | — | — | — | — | ▼ | — | — | — | — | — | — |
| B6 | |||||||||||||||
| D2 | — | — | ▲ | — | — | — | — | — | ▼ | ▲ | ▼ | ▼ | ▲ | — | ▼ |
Abbreviations: CAP, closed-arm preference; CT-IM, context immobility; FS-IM, forced swim test immobility; ODST, distance from object; PPI, prepulse inhibition; P-VEL, pretraining velocity; TA, turn angle; TF, tail flick latency; THG, thigmotaxis; VEL, velocity.
▲ and ▼ arrows indicate strains that showed enhancements or deficits relative to B6. Dashes indicate strains that did not differ from B6.
As the current study employed a multi-test screen featuring assays related to anxiety and fear, the results may have been affected by potential carryover effects. Repeated exposure to anxiogenic contexts and events may represent significant psychological stressors capable of significantly shaping subsequent performance. With respect to the current study, this influence is at least partially controlled by the fact that the progenitor strains underwent the same test battery. However, some GTM strains may be particularly susceptible to these experiences resulting in phenotypes that are not directly controlled by the genotype but are instead a function of an interaction between the testing regimen and genotype. The design of the experiment precludes any firm conclusions on this issue. A direct assessment of this point would require a between-groups design involving independent groups of GTM and control mice for each test, which would be very labor intensive. Though by no means conclusive, the control data provide evidence of normal performance on the assays despite the previous behavioral testing. Well-documented behavioral differences between C57 and DBA, including enhanced closed-arm preference and deficits in conditional fear, were observed. This suggests at a minimum that the influence of previous testing in the two parental strains was comparable.
The use of DBA/2J donor segments for the GTM panel may have implications for loci identified in tests involving auditory stimuli, as this strain is known to undergo progressive hearing loss with age. While no rigorous examination of hearing capacity in the GTM has been conducted, inspection of time course data for individual mice in both the general activity test and cued fear test revealed normal stimulus-dependent changes for multiple end points across all GTM strains and DBA/2J control mice. Previous work has demonstrated normal cued fear conditioning and auditory fear-potentiated startle in similarly aged DBA/2J mice.49 As such, a systematic deficit in hearing does not appear to account for the observed strain differences in these tests.
The GTM panel, in which defined segments covering the entire donor genome are propagated on a uniform background, represents a powerful new approach for QTL mapping. Compared to traditional F2 intercross mapping techniques, and outbred mouse populations, the GTM panel offers increased sensitivity and power. While outbred mice offer potentially better mapping resolution relative to the GTM panel,50 this disadvantage can be partially overcome through overlapping GTM strains. Additional concerns with the GTM panel include the presence of unwanted heterozygosity in individual strains. This issue is somewhat mitigated by the low rate of heterozygosity, an average of 2%.51
Unraveling the genetics of complex behavioral traits remains a daunting task. Significant progress in identifying behavior genes will ultimately depend on the expansion of environmental modeling and further refinement in behavioral protocols and phenotype quantification combined with increased refinement of genetic reagents. Our results suggest that the GTM represent a useful step in this direction, giving improved sensitivity and mapping power for behavioral traits.
Acknowledgments
We acknowledge funding from the National Institutes of Health RO1 MH071779.
References
- 1.Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L. Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet. 1997;17:335–337. doi: 10.1038/ng1197-335. [DOI] [PubMed] [Google Scholar]
- 2.Wehner JM, Radcliffe RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW, et al. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]
- 3.Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, et al. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- 4.Gershenfeld HK, Neumann PE, Mathis C, Crawley JN, Li X, Paul SM. Mapping quantitative trait loci for open-field behavior in mice. Behav Genet. 1997;27:201–210. doi: 10.1023/a:1025653812535. [DOI] [PubMed] [Google Scholar]
- 5.Gershenfeld HK, Paul SM. Mapping quantitative trait loci for fear-like behaviors in mice. Genomics. 1997;46:1–8. doi: 10.1006/geno.1997.5002. [DOI] [PubMed] [Google Scholar]
- 6.Talbot CJ, Nicod A, Cherny SS, Fulker DW, Collins AC, Flint J. High-resolution mapping of quantitative trait loci in outbred mice. Nat Genet. 1999;21:305–308. doi: 10.1038/6825. [DOI] [PubMed] [Google Scholar]
- 7.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 8.Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2005;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N. Identification of multiple genetic loci linked to the propensity for ‘behavioral despair’ in mice. Genome Res. 2002;12:357–366. doi: 10.1101/gr.222602. [DOI] [PubMed] [Google Scholar]
- 10.Flint J. The genetic basis of cognition. Brain. 1999;122:2015–2032. doi: 10.1093/brain/122.11.2015. [DOI] [PubMed] [Google Scholar]
- 11.Wehner JM, Radcliffe RA, Bowers BJ. Quantitative genetics and mouse behavior. Annu Rev Neurosci. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- 12.Wahlsten D, Rustay NR, Metten P, Crabbe JC. In search of a better mouse test. Trends Neurosci. 2003;26:132–136. doi: 10.1016/S0166-2236(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 13.Wahlsten D, Metten P, Phillips TJ, Boehm SL, II, Burkhart-Kasch S, Dorow J, et al. Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- 14.Kafkafi N, Benjamini Y, Sakov A, Elmer GI, Golani I. Genotype–environment interactions in mouse behavior: a way out of the problem. Proc Natl Acad Sci. 2005;102:4619–4624. doi: 10.1073/pnas.0409554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iakoubova OA, Olsson CL, Dains KM, Ross DA, Andalibi A, Lau K, et al. Genome-tagged mice (GTM): two sets of genome-wide congenic strains. Genomics. 2001;74:89–104. doi: 10.1006/geno.2000.6497. [DOI] [PubMed] [Google Scholar]
- 16.Sayah DM, Khan AH, Gasperoni TL, Smith DJ. A genetic screen for novel behavioral mutations in mice. Mol Psychiatry. 2001;5:369–377. doi: 10.1038/sj.mp.4000742. [DOI] [PubMed] [Google Scholar]
- 17.Kazlauckas V, Schuh J, Dall'Igna OP, Pereira GS, Bonan CD, Lara DR. Behavioral and cognitive profile of mice with high and low exploratory phenotypes. Behav Brain Res. 2005;162:272–278. doi: 10.1016/j.bbr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Pellow S, Chopin P, File SE, Briley M. Validation of open, closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2001;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 23.Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 25.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 26.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 27.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 28.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 29.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- 30.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Singh RP, Khan AH, Bhavsar K, Lusis AJ, Davis RC, et al. Identifying loci for behavioral traits using genome-tagged mice. J Neurosci Res. 2003;74:562–569. doi: 10.1002/jnr.10765. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Singh RP, Khan AH, Lusis AJ, Davis RC, Smith DJ. Mapping behavioral traits by use of genome-tagged mice. Am J Geriatr Psychiatry. 2004;12:158–165. [PubMed] [Google Scholar]
- 33.Grillon C, Ameli R, Charney DS, Krystal J, Braff D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry. 1992;32:939–943. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- 34.Waldo M, Myles-Worsley M, Madison A, Byerley W, Freedman R. Sensory gating deficits in parents of schizophrenics. Am J Med Genet. 1995;60:506–511. doi: 10.1002/ajmg.1320600605. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Demarest K, Hitzemann R, Sikela JM. Gene coding variant in Cas1 between the C57BL/6J and DBA/2J inbred mouse strains: linkage to a QTL for ethanol-induced locomotor activation. Alcohol Clin Exp Res. 2002;26:1–7. [PubMed] [Google Scholar]
- 36.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 37.Singer JB, Hill AE, Nadeau JH, Lander ES. Mapping quantitative trait loci for anxiety in chromosome substitution strains of mice. Genetics. 2005;169:855–862. doi: 10.1534/genetics.104.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Williams RW, Manly KF. WebQTL: web-based complex trait analysis. Neuroinformatics. 2003;1:299–308. doi: 10.1385/NI:1:4:299. [DOI] [PubMed] [Google Scholar]
- 39.Valentinuzzi VS, Kolker DE, Vitaterna MH, Shimomura K, Whiteley A, Low-Zeddies S, et al. Automated measurement of mouse freezing behavior and its use for quantitative trait locus analysis of contextual fear conditioning in (BALB/cJ × C57BL/6J) F2 mice. Learn Mem. 1998;5:391–403. [PMC free article] [PubMed] [Google Scholar]
- 40.Willis-Owen SA, Flint J. Identifying the genetic determinants of emotionality in humans: insights from rodents. Neurosci Biobehav Rev. 2006;31:115–124. doi: 10.1016/j.neubiorev.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 43.Marshall RD, Garakani A. Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatr Clin N Am. 2002;25:385–395. doi: 10.1016/s0193-953x(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 44.Bouton ME, Nelson JB, Rosas JM. Stimulus generalization, context change, and forgetting. Psychol Bull. 1999;125:171–186. doi: 10.1037/0033-2909.125.2.171. [DOI] [PubMed] [Google Scholar]
- 45.Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- 46.Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romanski LM, LeDoux JE. Equipotentiality of thalamo–amygdala and thalamo–cortico–amygdala circuits in auditory fear conditioning. J Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav Brain Res. 2004;154:567–576. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, et al. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet. 2006;38:879–8870. doi: 10.1038/ng1840. [DOI] [PubMed] [Google Scholar]
- 51.Iakoubova OA, Olsson CL, Dains KM, Ross DA, Andalibi A, Lau K, et al. Genome-tagged mice (GTM): two sets of genome-wide congenic strains. Genetics. 2001;74:89–104. doi: 10.1006/geno.2000.6497. [DOI] [PubMed] [Google Scholar]