Abstract
Plant plasma membrane intrinsic proteins (PIPs) cluster in two evolutionary subgroups, PIP1 and PIP2, with different aquaporin activities when expressed in Xenopus oocytes. Maize ZmPIP1;1 and ZmPIP1;2 do not increase the osmotic water permeability coefficient (Pf), whereas ZmPIP2;1, ZmPIP2;4, and ZmPIP2;5 do. Here, we show that coexpression of the nonfunctional ZmPIP1;2 and the functional ZmPIP2;1, ZmPIP2;4, or ZmPIP2;5 resulted in an increase in Pf that was dependent on the amount of injected ZmPIP1;2 complementary RNA. Confocal analysis of oocytes expressing ZmPIP1;2–green fluorescent protein (GFP) alone or ZmPIP1;2-GFP plus ZmPIP2;5 showed that the amount of ZmPIP1;2-GFP present in the plasma membrane was significantly greater in coexpressing cells. Nickel affinity chromatography purification of ZmPIP2;1 fused to a His tag coeluted with ZmPIP1;2-GFP demonstrated physical interaction and heteromerization of both isoforms. Interestingly, coexpression of ZmPIP1;1 and ZmPIP2;5 did not result in a greater increase in Pf than did the expression of ZmPIP2;5 alone, but coexpression of the ZmPIP1;1 and ZmPIP1;2 isoforms induced a Pf increase, indicating that PIP1 isoform heteromerization is required for both of them to act as functional water channels. Mutational analysis demonstrated the important role of the C-terminal part of loop E in PIP interaction and water channel activity induction. This study has revealed a new mechanism of plant aquaporin regulation that might be important in plant water relations.
INTRODUCTION
Plant growth and development are dependent on the tight regulation of water movement and homeostasis. Water can pass through cellular membranes not only by diffusion but also via water channels or aquaporins (AQPs). AQPs are small (23- to 34-kD) transmembrane channels that contain six membrane-spanning α-helices with both the N and C termini facing the cytosol. The two halves of the polypeptide are very similar, and each contains a loop with a highly conserved Asn-Pro-Ala motif (loops B and E). Structural analysis of mammalian AQP1 crystals has shown that it forms tetramers in the plasma membrane and that, within a monomer, loops B and E form short helices that dip into the membrane from opposite sides and are involved in the formation of the water-selective channel (Murata et al., 2000; Sui et al., 2001).
Many AQPs have been identified in plants. In maize, 36 AQP genes have been detected in EST databases and sequenced (Chaumont et al., 2001), whereas in the Arabidopsis genome, 35 AQP genes have been identified (Johanson et al., 2001; Quigley et al., 2002). Sequence alignment and comparison has allowed us to classify the 36 AQPs into four subfamilies, two of which contain proteins known to be present in the plasma membrane (plasma membrane intrinsic proteins [PIPs]) or the vacuolar membrane or tonoplast (tonoplast intrinsic proteins [TIPs]).
Evidence that AQPs form water channels is derived mainly from experiments using heterologous expression systems, such as Xenopus laevis oocytes. This system is convenient because the endogenous water permeability coefficient (Pf) of the oocyte membrane is low and water transport into the oocyte can be measured with high accuracy. The expression of AQPs in this heterologous system results in a considerable increase in the Pf of the plasma membrane. Most plant AQPs that have been tested for water transport activity have been found to be water channels (reviewed by Johansson et al., 2000; Tyerman et al., 2002); some plant AQPs also transport glycerol or urea (Biela et al., 1999; Dean et al., 1999; Gerbeau et al., 1999; Guenther and Roberts, 2000; Weig and Jakob, 2000; Ciavatta et al., 2001).
On the basis of their sequences, the plasma membrane PIPs can be divided into two major groups, PIP1 and PIP2. Compared with PIP1 proteins, PIP2 proteins have a shorter N-terminal extension and a longer C-terminal end containing putative phosphorylation sites (Chaumont et al., 2000a, 2001; Johansson et al., 2000; Johanson et al., 2001). All plant PIP2 proteins examined have been shown to have high water channel activity in Xenopus oocytes, whereas PIP1 proteins often are inactive or have low activity (Daniels et al., 1994; Kammerloher et al., 1994; Yamada et al., 1995; Weig et al., 1997; Johansson et al., 1998; Biela et al., 1999; Chaumont et al., 2000a; Marin-Olivier et al., 2000; Dixit et al., 2001; Moshelion et al., 2002). This difference also is seen with the maize PIP1 and PIP2 isoforms. Oocytes expressing ZmPIP2;5 increase rapidly in volume when incubated in hypoosmotic medium, indicating the presence of a facilitated water transport pathway, whereas oocytes expressing ZmPIP1;1 or ZmPIP1;2 swell very slowly and have Pf values similar to those of water-injected oocytes (Chaumont et al., 2000a). The reason for this difference is not known. Control experiments using 35S-labeled amino acids show that ZmPIP1;1 and ZmPIP1;2 are synthesized and incorporated into oocyte membranes, so the failure to detect any water channel activity could indicate either that both proteins are transporters for other substrates or that they need to be positively regulated. Several plant AQPs have been shown to increase the membrane permeability to small neutral solutes, such as glycerol or urea (Rivers et al., 1997; Biela et al., 1999; Gerbeau et al., 1999; Guenther and Roberts, 2000; Weig and Jakob, 2000). However, ZmPIP1;2 did not facilitate the transport of any solute tested (glycerol, choline, ethanol, urea, or amino acids) (Chaumont et al., 2000a).
Multimerization of membrane proteins can regulate their activity and function (Veenhoff et al., 2002). For instance, several mammal α-subunits of the voltage-gated potassium channel cannot generate current as homomers, but they are able to form heterotetrameric channels with active and/or other silent channels, resulting in current with distinguishable properties (Post et al., 1996; Dreyer et al., 1997; Ottschytsch et al., 2002).
To further investigate the function and regulation of ZmPIP1;2, we examined whether nonfunctional ZmPIP1;2 could interact with functional AQPs in the oocyte system. Preliminary experiments showed that oocytes coinjected with complementary RNAs (cRNAs) encoding ZmPIP1;2 and active ZmPIP2;5 showed a Pf increase (Chaumont et al., 2000b). Here, we describe a positive cooperative effect between ZmPIP1;2 and different PIP1 and PIP2 isoforms that results in heteromerization and improved ZmPIP1;2 targeting to the plasma membrane and in a significant increase in Pf.
RESULTS
Coexpression of ZmPIP1;2 and ZmPIP2;5 in Xenopus Oocytes Results in an Increase in Pf
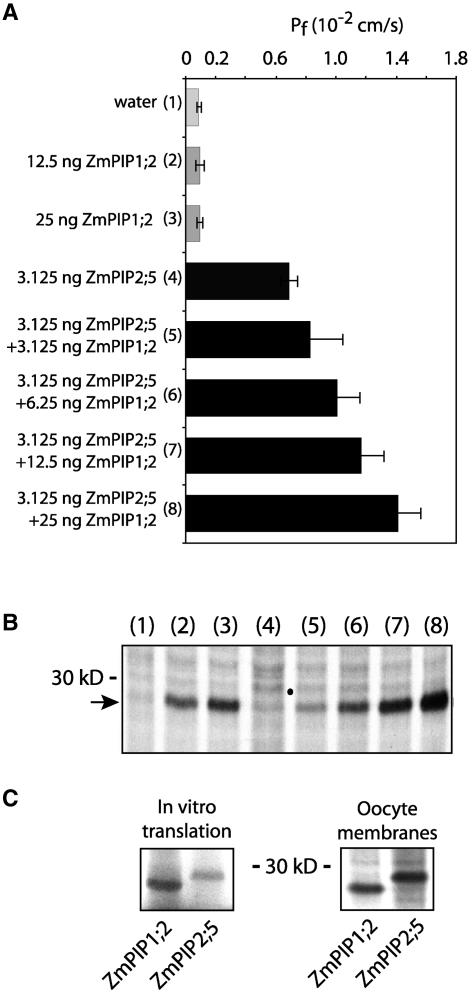
To determine whether nonfunctional ZmPIP1;2 interacted with functional ZmPIP2;5, the Pf was measured in oocytes coinjected with cRNAs encoding the two isoforms. Preliminary experiments indicated that injection of 3.125 ng of ZmPIP2;5 cRNA alone increased the Pf to 0.6 to 0.8 × 10−2 cm/s (i.e., to ∼50% of its maximal value) (Figure 1A, bar 4) (Chaumont et al., 2000a). When this same low amount of ZmPIP2;5 cRNA was coinjected with increasing amounts of ZmPIP1;2 cRNA (3.125 to 25 ng), which, alone, had no effect on Pf, a significant increase in Pf was measured compared with that observed using ZmPIP2;5 cRNA alone (Figure 1A, bars 5 to 8), the increase being dependent on the amount of injected ZmPIP1;2 cRNA. These data suggest that the two isoforms interact positively to increase Pf.
Figure 1.
Pf Values and Protein Labeling of Individual Xenopus Oocytes Expressing ZmPIP1;2 and ZmPIP2;5 Alone and in Combination.
(A) Pf values of oocytes injected with water or ZmPIP1;2 and/or ZmPIP2;5 cRNA. The different amounts of ZmPIP1;2 cRNA injected are indicated at left. The results are expressed as means of measurements from four to nine cells. The bars represent 95% confidence intervals. The numbers in parentheses correspond to the lane numbers in (B).
(B) In vivo labeled proteins in the total membrane fraction prepared from oocytes from the same microinjection shown in (A). The band with an apparent molecular mass of ∼28 kD in lanes 2, 3, and 5 to 8 corresponds to ZmPIP1;2. ZmPIP2;5 (dot in lanes 4 to 8) is barely visible because of the very low amount of cRNA injected. A total of 100,000 cpm was loaded per lane, and the dried gel was exposed for 1 day at −70°C. The position of a 30-kD molecular weight mass is indicated.
(C) Labeled ZmPIP1;2 and ZmPIP2;5 synthesized in vitro in rabbit reticulocyte lysate or in vivo in oocytes injected with 25 ng of cRNA.
One explanation for these findings might be that an interaction between ZmPIP2;5 and ZmPIP1;2 improves protein folding, leading to better stability and/or targeting of the isoforms to the oocyte plasma membrane. To determine the amount of protein in the oocyte membrane, oocytes injected with the same PIP cRNAs used for Figure 1A were labeled with 35S-amino acids, and the proteins in the total membrane fraction were extracted, separated by SDS-PAGE, and visualized by fluorography. The fluorogram shows the presence of a 28-kD polypeptide corresponding to ZmPIP1;2 (Figure 1B, lanes 2, 3, and 5 to 8) and of a very faint band with a higher molecular mass (29 kD) (Figure 1B, lanes 4 to 8), probably corresponding to ZmPIP2;5. Similar ZmPIP1;2 and ZmPIP2;5 migration patterns were observed after cRNA in vitro translation in a rabbit reticulocyte lysate or in membranes of oocytes injected with 25 ng of cRNA (Figure 1C). These data suggest that the low level of ZmPIP2;5 expression observed in Figure 1B results from the low amount of ZmPIP2;5 cRNA injected.
Interestingly, for a given amount of injected ZmPIP1;2 cRNA, the intensity of the ZmPIP1;2 band was stronger when coexpressed with ZmPIP2;5 (cf. lanes 2 and 7 or 3 and 8 in Figure 1B; the lane 7:lane 2 signal intensity ratio was 1.54, and that for lane 8:lane 3 was 1.84), indicating that ZmPIP2;5 had a positive effect on ZmPIP1;2 expression and/or stability.
ZmPIP1;2 Shows a Positive Interaction with Different PIP2 Isoforms
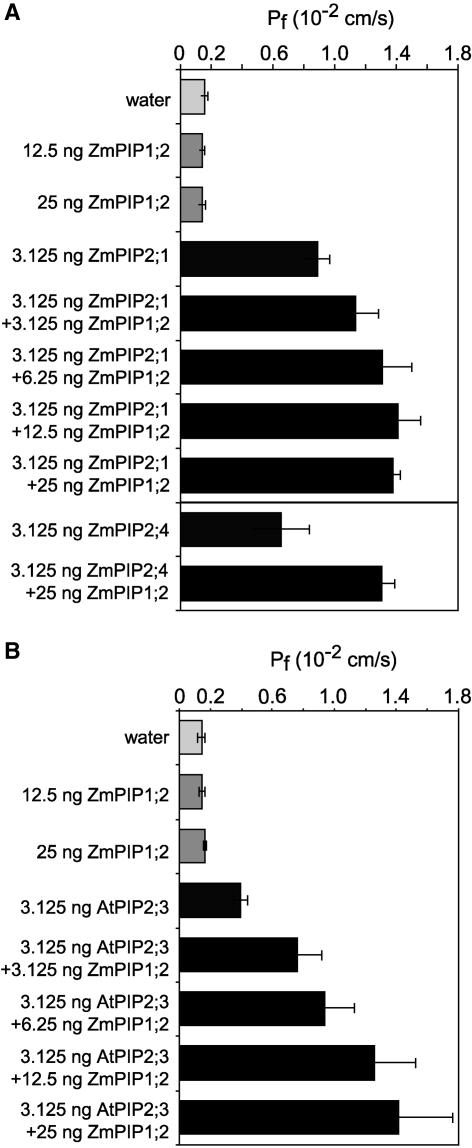
To determine if the observed positive interaction was specific for active ZmPIP2;5, we performed similar coexpression experiments using other PIP2 isoforms. We chose maize ZmPIP2;1, a PIP2 isoform highly expressed in many tissues, including the reproductive organs, in which ZmPIP1;2 transcripts are highly abundant (Chaumont et al., 2000a, 2001), and ZmPIP2;4, an isoform expressed mostly in roots (Chaumont et al., 2001). Both ZmPIP2;1 and ZmPIP2;4 were active AQPs when expressed in Xenopus oocytes (Figure 2A). Coexpression of ZmPIP1;2 and ZmPIP2;1 or ZmPIP2;4 also resulted in a positive cooperative effect, with a significant increase in Pf (Figure 2A). Similar data were obtained using oocytes coexpressing ZmPIP1;2 and Arabidopsis AtPIP2;3 (previously RD28) (Daniels et al., 1994) (Figure 2B), allowing us to conclude that this positive interaction was not specific for ZmPIP2;5 but is a general phenomenon seen with PIP2 proteins.
Figure 2.
Pf Values of Oocytes Coexpressing ZmPIP1;2 and ZmPIP2;1, ZmPIP2;4, or AtPIP2;3.
(A) Pf values for oocytes injected with water or ZmPIP1;2 and/or either ZmPIP2;1 or ZmPIP2;4 cRNAs. The amounts of ZmPIP1;2, ZmPIP2;1, and ZmPIP2;4 cRNAs used are indicated at left. The results are expressed as means of measurements from 5 to 20 cells. The bars represent 95% confidence intervals.
(B) Pf values for oocytes injected with water or ZmPIP1;2 and/or AtPIP2;3 cRNAs. The amounts of ZmPIP1;2 and AtPIP2;3 cRNAs used are indicated at left. The results are expressed as means of measurements from five to nine cells. The bars represent 95% confidence intervals.
Coexpression of ZmPIP1;2-GFP and ZmPIP2;5 Results in Predominant Localization of ZmPIP1;2-GFP in the Oocyte Plasma Membrane
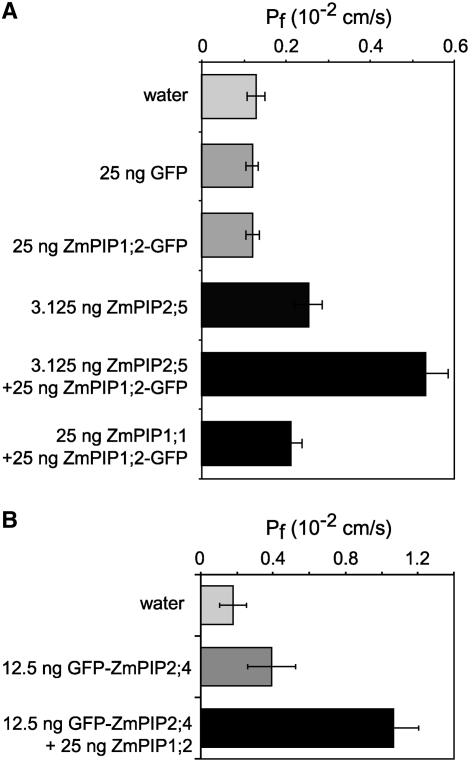
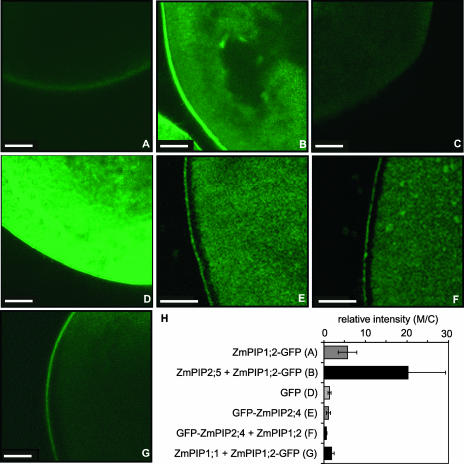
We then investigated whether the increase in the amount of ZmPIP1;2 in crude membranes of oocytes coinjected with ZmPIP1;2 and ZmPIP2;5 cRNAs also was seen in the plasma membrane. Because experiments on the detection of in vivo labeled proteins in purified oocyte plasma membranes (Kamsteeg and Deen, 2001) did not give clear results, we studied the plasma membrane localization of a ZmPIP1;2–green fluorescent protein (GFP) fusion protein (Chaumont et al., 2000a). We first determined whether the presence of the GFP reporter at the C terminus of ZmPIP1;2 affected the positive cooperative effect observed previously. Figure 3A shows that coexpression of ZmPIP1;2-GFP and ZmPIP2;5 resulted in an increase in the Pf similar to that seen in cells coexpressing ZmPIP1;2 and ZmPIP2;5. In these experiments, the Pf values were lower than those measured in Figure 1A (cf. the Pf of oocytes injected with 3.125 ng of ZmPIP2;5 cRNA). These variations could be attributable to differences in oocyte bath solutions and/or the oocyte batches, but they did not modify the significance of the data. In addition, because of the presence of the fused GFP, the protein ratio of ZmPIP1;2-GFP to ZmPIP2;5 resulting from the injection of 25 ng of ZmPIP1;2-GFP cRNA was approximately half of that observed in oocytes injected with the same amount of ZmPIP1;2 cRNA. We then studied the localization of ZmPIP1;2-GFP using confocal microscopy and found that cells expressing ZmPIP1;2-GFP alone showed weak green fluorescence around the cell (Figure 4A), whereas in cells coexpressing ZmPIP1;2-GFP and ZmPIP2;5, the signal was very intense and sharp (Figure 4B). Control cells microinjected with water showed weak autofluorescence, whereas cells expressing GFP alone showed strong green fluorescence throughout the cell (Figures 4C and 4D). The fluorescence intensity in the plasma membrane and the cytosol of different oocytes was quantified using ImageJ (version 1.18; http://rsb.info.nih.gov/ij/) to calculate the relative intensity (Figure 4H). The data showed a significant increase (threefold to fourfold) in the amount of ZmPIP1;2-GFP in the membranes of oocytes coexpressing ZmPIP1;2-GFP and ZmPIP2;5 compared with controls, indicating that ZmPIP2;5 interacts with ZmPIP1;2-GFP to increase the amount of ZmPIP1;2-GFP in the oocyte plasma membrane.
Figure 3.
Pf Values of Oocytes Coexpressing Different Combinations of GFP-Tagged ZmPIP1;2 or ZmPIP2;4 and Either Active ZmPIP2;5, ZmPIP1;2, or ZmPIP1;1.
(A) Pf values for oocytes injected with ZmPIP1;2-GFP and ZmPIP2;5 or ZmPIP1;1 cRNAs. The amounts of cRNA used are indicated at left. The results are expressed as means of measurements from 6 to 12 cells. The bars represent 95% confidence intervals.
(B) Pf values for oocytes injected with GFP-ZmPIP2;4 and ZmPIP1;2 cRNAs. The amounts of cRNA used are indicated at left. The results are expressed as means of measurements from six to eight cells. The bars represent 95% confidence intervals.
Figure 4.
Confocal Microscopic Images of Oocytes Showing the Localization of ZmPIP1;2-GFP or GFP-ZmPIP2;4.
(A) Oocyte injected with 25 ng of ZmPIP1;2-GFP cRNA.
(B) Oocyte injected with 25 ng of ZmPIP1;2-GFP and 3.125 ng of ZmPIP2;5 cRNAs.
(C) Oocyte injected with water.
(D) Oocyte injected with 25 ng of GFP cRNA.
(E) Oocyte injected with 12.5 ng of GFP-ZmPIP2;4 cRNA.
(F) Oocyte injected with 12.5 ng of GFP-ZmPIP2;4 and 25 ng of ZmPIP1;2 cRNAs.
(G) Oocyte injected with 25 ng of ZmPIP1;2-GFP and 25 ng of ZmPIP1;1 cRNAs.
The oocytes were observed 3 days after injection as described in Methods. Bars = 100 μm.
(H) Relative fluorescence signal intensity in the membrane (M) compared with the cytosol (C) measured with the ImageJ program. The results are expressed as means from four to seven oocytes. The bars represent 95% confidence intervals.
To determine if the amounts of active PIP2 in the plasma membrane also increased upon coexpression with PIP1;2, we performed a converse experiment in which tagged GFP-ZmPIP2;4 was coexpressed with ZmPIP1;2. The presence of GFP at the N terminus of ZmPIP2;4 decreased the water channel activity of ZmPIP2;4, but the protein remained active and a positive interaction with ZmPIP1;2 was observed, as reported previously (Figures 2A and 3B). In this experiment, we injected 12.5 ng of GFP-ZmPIP2;4 RNA to have enough protein to detect the GFP fluorescence. Even if intense green fluorescence was observed in the cytosol of the oocytes expressing GFP-ZmPIP2;4 (Figure 4E) alone or together with ZmPIP1;2 (Figure 4F), a sharp signal was detected in the plasma membranes of both cell types. Quantification of the relative signal intensity detected in the membrane showed no significant differences, indicating that the amount of GFP-ZmPIP2;4 is not increased when coexpressed with ZmPIP1;2 (Figure 4H).
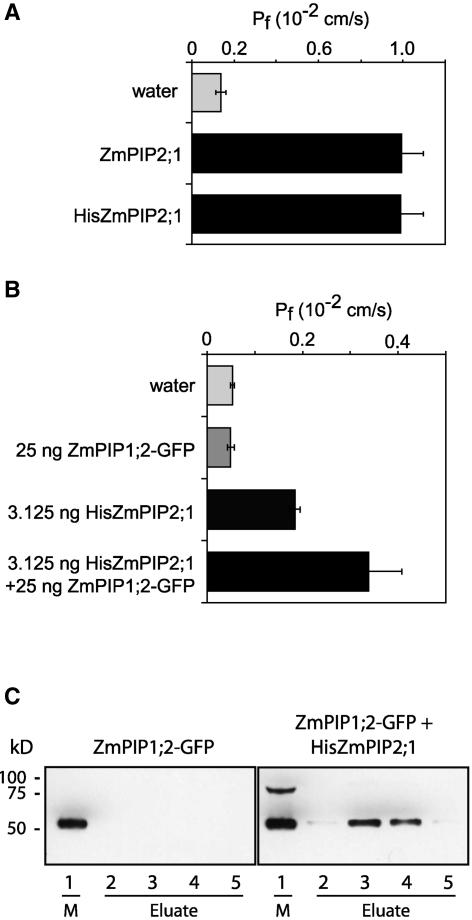
Heteromerization of ZmPIP1;2-GFP and His-ZmPIP2;1
To demonstrate that the ZmPIP1;2 and ZmPIP2 proteins interact physically, we used a chromatography copurification approach. A His tag was added to the N terminus of ZmPIP2;1, and we demonstrated that the presence of the His tag did not modify the water channel activity of ZmPIP2;1 (Figure 5A). Coexpression of His-ZmPIP2;1 and ZmPIP1;2-GFP also resulted in a positive cooperative effect (Figure 5B). Crude membranes from oocytes coexpressing both isoforms were extracted, and His-ZmPIP2;1 was solubilized using octyl-β-d-thioglucopyranoside and purified on a nickel column. The presence of ZmPIP1;2-GFP in the eluted fractions was checked by immunodetection (Figure 5C). A band with a molecular mass of 54 kD, corresponding to ZmPIP1;2-GFP, was present in the eluted fractions (Figure 5C, lanes 2 to 5), demonstrating that it copurified with His-ZmPIP2;1. As a negative control, we performed the same procedure with crude membranes from oocytes expressing ZmPIP1;2-GFP alone (Figure 5C). The latter protein was not detected after protein elution, indicating that ZmPIP1;2-GFP did not bind to the nickel column by itself. In addition, a band with a molecular mass of ∼85 kD was detected only in crude membranes from oocytes coexpressing His-ZmPIP2;1 and ZmPIP1;2-GFP. This signal could correspond to a dimeric form that includes both isoforms.
Figure 5.
Pf Values of Oocytes Expressing ZmPIP2;1, His-ZmPIP2;1, ZmPIP1;2-GFP, or Combinations of These and Copurification of ZmPIP1;2-GFP with His-ZmPIP2;1.
(A) Pf values of oocytes injected with water or 50 ng of cRNA encoding ZmPIP2;1 and His-ZmPIP2;1. The results are expressed as means of measurements from 9 to 16 cells. The bars represent 95% confidence intervals.
(B) Pf values of oocytes injected with water or cRNA encoding ZmPIP1;2-GFP, His-PIP2;1, or a combination of these. The results are expressed as means of measurements from 6 to 16 cells. The bars represent 95% confidence intervals.
(C) Immunodetection of ZmPIP1;2-GFP in oocyte crude membrane (M; lane 1) or in eluted fractions (lanes 2 to 5) obtained after protein purification on a nickel column. The left and right gels show fractions obtained from oocytes expressing ZmPIP1;2-GFP alone or coexpressing ZmPIP1;2-GFP and His-ZmPIP2;1, respectively. The positions of molecular mass markers are indicated at left.
ZmPIP1;2 and ZmPIP2;5 Interact in the Xenopus Oocyte Plasma Membrane
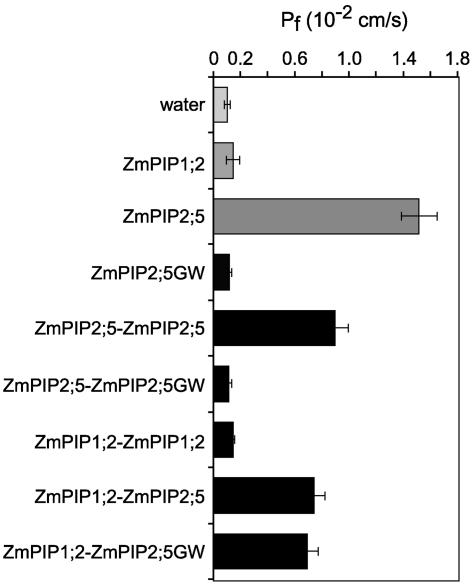
ZmPIP1;2 and ZmPIP2 proteins interact physically in the oocyte, leading to an increase of ZmPIP1;2 plasma membrane targeting. These data suggest that both proteins form heterotetramers in the membrane that stabilize ZmPIP1;2 and/or ZmPIP2s and induce a Pf increase. To check the water channel activity of heterotetramers, we forced ZmPIP1;2 and ZmPIP2;5 monomers to associate by expressing cDNA chimeric dimers, as reported previously for mammalian wild-type and mutated AQP1 (Jung et al., 1994; Shi et al., 1994).
We first tested if the positive cooperative effect was dependent on the activity of ZmPIP2;5. We prepared an inactive ZmPIP2;5 by replacing Gly-104, located in the putative short α-helix (loop B) of the aqueous pore, with a larger amino acid, Trp (ZmPIP2;5GW). The corresponding mutation in mammal AQP4 inactivated its water channel activity in oocytes even though this protein appeared to be expressed at the plasma membrane (Shi and Verkman, 1996). Injection of different amounts of ZmPIP2;5GW cRNA into oocytes did not increase Pf, indicating that ZmPIP2;5GW was not functional (Figure 6). Contrary to what was observed with active ZmPIP2;5, coexpression of ZmPIP1;2 with inactive ZmPIP2;5GW did not cause any Pf activation and induced no increase of ZmPIP1;2 expression at the plasma membrane level (data not shown). This observation could be attributable to a misfolding and/or misrouting of ZmPIP2;5GW, which prevents its interaction with ZmPIP1;2. Nevertheless, this mutated ZmPIP2;5GW was included in the construction of chimeric dimers.
Figure 6.
Pf Values and Protein Labeling of Xenopus Oocytes Expressing Single Polypeptide Chain Dimeric ZmPIP Proteins.
Pf values for oocytes injected with 50 ng of cRNA encoding ZmPIP2;5 monomers or chimeric dimers as indicated at left. The results are expressed as means of measurements from 9 to 17 cells. The bars represent 95% confidence intervals.
When the Pf values of oocytes injected with the same amount of ZmPIP2;5-ZmPIP2;5 or ZmPIP2;5-ZmPIP2;5GW dimer or ZmPIP2;5 monomer cRNA were compared (Figure 6), those expressing the ZmPIP2;5-ZmPIP2;5 dimer showed a significant increase in Pf, but only to 60% of the level seen using the ZmPIP2;5 monomer. Surprisingly, no Pf increase was detected in oocytes expressing the ZmPIP2;5-ZmPIP2;5GW dimer. The decreased efficiency of the ZmPIP2;5-ZmPIP2;5 dimer compared with that of the ZmPIP2;5 monomer might indicate differences in plasma membrane expression and/or inactivation of a ZmPIP2;5 monomer in the dimer. Both possibilities could explain the absence of Pf increase in oocytes expressing the ZmPIP2;5-ZmPIP2;5GW dimer. Oocytes expressing the ZmPIP1;2-ZmPIP1;2 homodimer had the same Pf as water-injected or ZmPIP1;2 monomer cRNA–injected oocytes, whereas cells expressing the ZmPIP1;2-ZmPIP2;5 or ZmPIP1;2-ZmPIP2;5GW heterodimer had a high Pf similar to that in cells expressing the ZmPIP2;5-ZmPIP2;5 homodimer. The Pf increase observed in oocytes expressing ZmPIP1;2 fused to the inactive ZmPIP2;5GW suggests that ZmPIP1;2 was activated when associated with ZmPIP2;5.
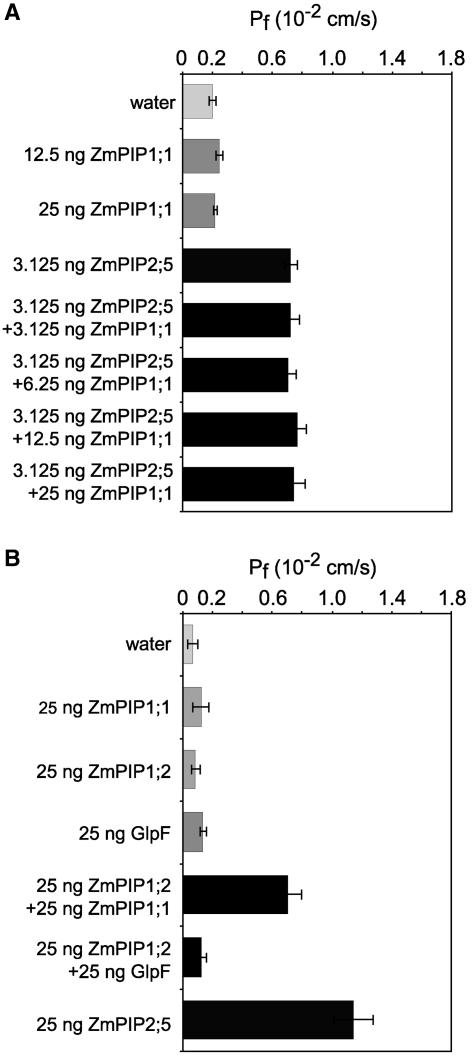
ZmPIP1;1 Shows a Positive Interaction with ZmPIP1;2 but Not with ZmPIP2;5
Because ZmPIP1;2 and ZmPIP2 proteins interacted positively to increase the Pf of the oocyte plasma membrane, we examined whether similar synergy was seen with ZmPIP1;1, which is 96% identical in sequence to ZmPIP1;2 (Chaumont et al., 2000a). When increasing amounts of ZmPIP1;1 cRNA were coinjected with 3.125 ng of ZmPIP2;5 cRNA, as in the previous coexpression experiments, the Pf increase was no greater than that in oocytes expressing ZmPIP2;5 alone (Figure 7A). These results indicated that ZmPIP1;1 did not interact functionally with ZmPIP2;5 in Xenopus oocytes.
Figure 7.
Pf Values of Oocytes Coexpressing ZmPIP1;1 and ZmPIP2;5, ZmPIP1;2, or GlpF.
(A) Pf values of oocytes injected with water or ZmPIP1;1 and/or ZmPIP2;5 cRNAs. The amounts of ZmPIP1;1 cRNA injected are indicated at left. The results are expressed as means of measurements from 7 to 17 cells. The bars represent 95% confidence intervals.
(B) Pf values of oocytes injected with water or ZmPIP1;1, ZmPIP1;2, or ZmPIP1;2 plus either ZmPIP1;1 or GlpF cRNAs. The amounts of cRNAs injected are indicated at left. The results are expressed as means of measurements from 8 to 11 cells. The bars represent 95% confidence intervals.
We then tested whether ZmPIP1;1 and ZmPIP1;2 could interact and function as a water channel when coexpressed. Abundant ZmPIP1;1 and ZmPIP1;2 transcripts are found in the same maize tissues, and both are highly expressed in tassels (Chaumont et al., 2000a). As a negative control, we used the glycerol facilitator, GlpF, from Escherichia coli (Maurel et al., 1994). As reported previously (Maurel et al., 1994; Chaumont et al., 2000a), oocytes injected with ZmPIP1;1, ZmPIP1;2, or GlpF cRNA did not swell more rapidly than water-injected cells (Figure 7B). However, coexpression of ZmPIP1;1 and ZmPIP1;2 resulted in a significant increase in Pf (approximately sixfold) compared with that in ZmPIP1;1-expressing oocytes, indicating that the two PIP1 isoforms could act in synergy to form functional water channels (Figure 7B).
Because the positive interaction between ZmPIP1;2 and ZmPIP2;5 resulted in an increased amount of ZmPIP1;2 in the oocyte plasma membrane (Figures 1B and 4B), we used the same approach for ZmPIP1;2-GFP and ZmPIP1;1 coexpression to study the levels of ZmPIP1;2 in the plasma membrane and found that coexpression of ZmPIP1;2-GFP and ZmPIP1;1 resulted in a twofold increase in the Pf compared with that in cells expressing ZmPIP1;2-GFP alone (Figure 3A). In general, the green fluorescent signal seemed more intense and sharper in the plasma membranes of oocytes coexpressing ZmPIP1;1 and ZmPIP1;2-GFP than in ZmPIP1;2-GFP–expressing oocytes (cf. Figures 4A and 4G). However, because a more intense signal was observed in the cytosols of cells coexpressing ZmPIP1;2-GFP and ZmPIP1;1 than in those expressing ZmPIP1;2-GFP alone, the relative intensity signal in the plasma membrane was lower (Figure 4H). We also cannot exclude the notion that the Pf increase was caused by the interaction and stabilization of the two PIP1 isoforms.
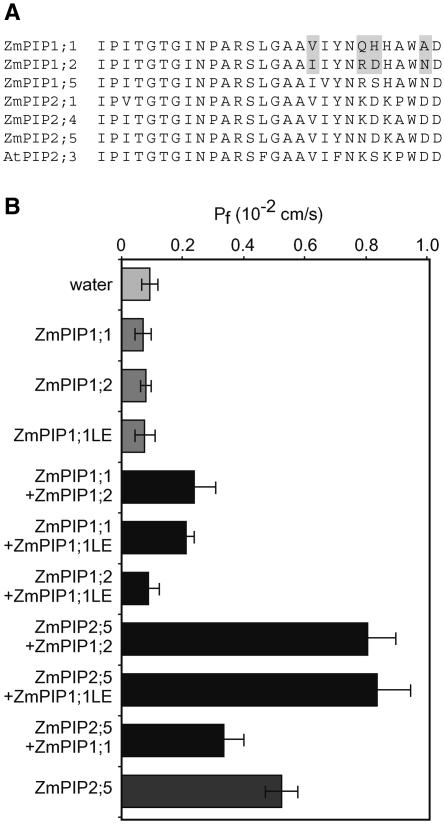
Loop E of ZmPIP1;2 Plays an Essential Role in the Protein Interaction
ZmPIP1;2, but not ZmPIP1;1, interacted positively with PIP2s, resulting in an increase in the Pf that correlated with an increased amount of ZmPIP1;2 in the oocyte plasma membrane. Amino acid sequence comparison of ZmPIP1;1 and ZmPIP1;2 revealed significant amino acid residue substitutions in loop E, in which V246, Q250, H251, and A255 of ZmPIP1;1 are replaced, respectively, by I247, R250, D251, and N256 of ZmPIP1;2 (Figure 8A). The important role of loop E in AQP function and in the interaction between monomers in the tetramer has been reported (Jung et al., 1994; Duchesne et al., 2002). To investigate whether loop E of ZmPIP1;2 was involved in its function and its interaction with another PIP, a chimeric protein, ZmPIP1;1LE, consisting of ZmPIP1;1 in which loop E was replaced with loop E from ZmPIP1;2 (amino acid residues 247 to 256), was constructed. As expected, the expression of this protein alone in oocytes did not increase the Pf (Figure 8B). Interestingly, coexpression of ZmPIP1;1LE with ZmPIP1;2 did not result in an increase in Pf, but a positive cooperative effect was seen in oocytes coexpressing ZmPIP1;1LE and either ZmPIP1;1 or ZmPIP2;5 (Figure 8B). This experiment demonstrated the essential role of loop E in ZmPIP1 interaction. The replacement of loop E of ZmPIP1;1 by that from ZmPIP1;2 thus converts nonfunctional ZmPIP1;1 to a functional isoform when coexpressed with other PIPs.
Figure 8.
Sequence Comparison of Loop E, and Pf Values for Oocytes Coexpressing Various Combinations of ZmPIP1;1LE, ZmPIP1;1, ZmPIP1;2, and ZmPIP2;5.
(A) Loop E sequences from ZmPIP1;1, ZmPIP1;2, ZmPIP1;5, ZmPIP2;1, ZmPIP2;4, ZmPIP2;5, and AtPIP2;3. Amino acid residues that differ between ZmPIP1;1 and ZmPIP1;2 are indicated by gray boxes.
(B) Pf values of oocytes expressing ZmPIP1;1, ZmPIP1;1LE, ZmPIP1;2, or ZmPIP2;5 and combinations of these. The amounts of cRNAs injected were 3.125 ng for ZmPIP2;5 and 25 ng for the other ZmPIP1 cRNAs. The results are expressed as means of measurements from 4 to 10 cells. The bars represent 95% confidence intervals.
Colocalization of ZmPIP1;2 and ZmPIP2;5 mRNA in Xylem Parenchyma Cells
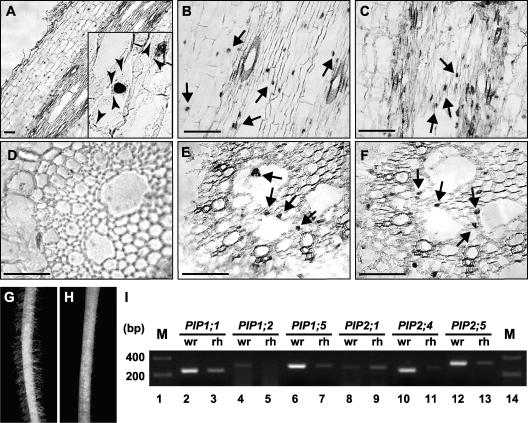
For the plasma membrane aquaporin interaction to be physiologically relevant in plants, both the PIP1 and PIP2 isoforms must be expressed in the same cell. ZmPIP1;2 is expressed abundantly in reproductive organs and, to a lesser extent, in embryos, shoots, and roots (Chaumont et al., 2000b). By contrast, ZmPIP2;5 transcripts are much less abundant and have only been detected in roots by RNA gel blot hybridization, although ESTs also are found in vegetative tissues (Chaumont et al., 2000a, 2001). The expression of ZmPIP1;2 and ZmPIP2;5 at the cellular level in maize roots was analyzed by in situ reverse transcriptase–mediated (RT) PCR (Figure 9). Positive staining for the ZmPIP2;5 RT-PCR product was detected clearly in cortex cells and the stele (Figures 9A and 9B). Cross-sections of the stele allowed us to localize the staining to parenchyma cells surrounding the late metaxylem vessels (Figure 9E). The signals were observed mostly in the nucleus and some labeling was seen in the cytoplasm (Figure 9A, inset), but no signal was detected in non-reverse-transcribed maize root, confirming that DNase treatment prevented the amplification of genomic DNA (Figure 9D). ZmPIP1;2 RT-PCR signals were observed mostly in the stele (Figure 9C). The restricted signal localization to certain cell types indicated successful in situ RT-PCR. Interestingly, as with ZmPIP2;5 RT-PCR products, signals also were detected in the xylem parenchyma cells surrounding the late metaxylem vessels, demonstrating that both isoforms were expressed in the same cell type (Figure 9F). Plasma membrane PIP expression also was investigated in root hair cells by RT-PCR. Root hair cells were shaved easily from liquid N2–frozen roots using forceps (Figures 9G and 9H). RT-PCR experiments using specific PIP primers showed that ZmPIP1;1, ZmPIP1;5, ZmPIP2;1, and ZmPIP2;5 transcripts were present in root hair (Figure 9I), demonstrating that both PIP1 and PIP2 isoforms were expressed in the same cell type. Few ZmPIP1;2 and ZmPIP2;4 transcripts were detected in root hair, confirming the in situ RT-PCR data.
Figure 9.
Expression of ZmPIPs in Maize Roots.
(A) to (F) Localization of ZmPIP2;5 ([A], [B], and [E]) and ZmPIP1;2 ([C] and [F]) mRNA by in situ RT-PCR on the maize root tip. Longitudinal sections ([A] to [C]) and cross-sections ([D] to [F]) are shown. (D) shows a control section for DNase treatment efficiency using the same conditions except that no reverse transcriptase was added. Arrows indicate expression in the parenchyma cells of the late xylem vessels. Arrowheads in the inset in (A) indicate expression in the cytoplasm. Bars = 100 μm.
(G) and (H) A maize root before (G) and after (H) shaving the root hair.
(I) RT-PCR analysis of the expression of ZmPIP1;1, ZmPIP1;2, ZmPIP1;5, ZmPIP2;1, ZmPIP2;4, and ZmPIP2;5 in the root hair. M, molecular mass markers; rh, root hair; wr, whole root.
DISCUSSION
The plant plasma membrane PIP1 and PIP2 subgroups differ not only in the length of their N and C termini and in several single amino acid residues but also in terms of their water channel activity (Chaumont et al., 2000a). Maize ZmPIP1;1 and ZmPIP1;2 are inactive as aquaporins when expressed in oocytes but might be transporters for other substrates; for example, it was shown recently that ZmPIP1;1 slightly stimulates boron uptake by oocytes (Dordas et al., 2000). Another possibility is that ZmPIP1;1 and ZmPIP1;2 function as a water channel but need to be positively regulated. The water transport activity of certain plant AQPs is regulated by phosphorylation (Maurel et al., 1995; Johansson et al., 1998). However, in oocytes expressing ZmPIP1;2, no change was seen in the Pf after the addition of protein kinase A activators or phosphatase inhibitors (Chaumont et al., 2000a). Because decreasing the pH increases the Pf of oocytes expressing AQP0 or AQP6 (Yasui et al., 1999; Nemeth-Cahalan and Hall, 2000), we investigated the swelling rate at different pH levels (pH 5.0 to 7.5) of oocytes injected with ZmPIP1;2 or ZmPIP2;5 cRNAs, but no change in the Pf was seen (data not shown).
A positive interaction might result from the tetrameric structure of AQPs. Mammalian AQP1 and bacterial GlpF have been crystallized and their three-dimensional structures determined (Fu et al., 2000; Murata et al., 2000; Sui et al., 2001). The functional unit in both proteins is a homotetramer, each monomer providing an independent channel. The AQP1 monomer interacts with two neighboring monomers through the spanning α-helices and the loops that contribute to tetramer stability (Murata et al., 2000). The N terminus of AQP1 also is very close to the C terminus of the adjacent monomer. These structural data indicate that, within a tetramer, interaction between monomers is essential for the protein to adopt a functional conformation. In addition, the folding and correct assembly of most membrane proteins in the endoplasmic reticulum is a prerequisite for their transport to the cell surface (Green and Millar, 1995).
Coexpression of nonfunctional ZmPIP1;2 and functional ZmPIP2s led to an increase in Pf that was dependent on the amount of ZmPIP1;2 cRNA injected (Figures 1 and 2) and correlated with increased ZmPIP1;2 expression and/or stability (Figure 1B) and improved targeting to the oocyte plasma membrane, as shown using the ZmPIP1;2-GFP fusion protein (Figure 4). Inversely, no increase in GFP-ZmPIP2;4 was detected in the plasma membrane when coexpressed with ZmPIP1;2. These data indicate that the interaction between ZmPIP2s and ZmPIP1;2 leads to better folding, stability, and/or assembly of the latter and to more efficient trafficking to the plasma membrane. The observation that the threefold to fourfold increased amount of ZmPIP1;2-GFP in the plasma membrane results in only a twofold increase of Pf (Figure 3A and 4) suggests that some proteins might not be activated.
The positive cooperation, resulting in an increase in the Pf, is attributable to the formation of heteromers containing both ZmPIP1;2 and ZmPIP2s. Direct physical interaction between ZmPIP1;2 and His-ZmPIP2;1 was demonstrated clearly by copurification on a nickel affinity chromatography column (Figure 5). Such a positive interaction has been described in experiments in which nonfunctional AQP1 proteins mutated in loop B or E complemented a truncated AQP1 mutant (D237Z) by forming mixed oligomers, which then were targeted to the plasma membrane (Jung et al., 1994). The opposite effect is seen in dominant nephrogenic diabetes insipidus in mammals, in which the routing of wild-type AQP2 to the oocyte plasma membrane is inhibited by heterotetramerization with a functional, but misrouted, AQP2-R187C mutant (Kamsteeg et al., 1999). However, a major difference between the present report and these AQP1 or AQP2 oligomerization studies is that the heteromerization occurs between two monomers that differ significantly at the amino acid level throughout the sequence (66.8% identity). Such heteromerization of different subunits is known to occur in other transporters, including sucrose transporters and ion channels (Dreyer et al., 1997; Zhang et al., 1999; Veenhoff et al., 2001). Examples of protein coexpression and interaction leading to their activation in Xenopus oocytes have been reported. For instance, K+ channel α-subunits from Arabidopsis and potato are silent as homomers but form an active K+ channel when coexpressed (Dreyer et al., 1997). Similarly, coexpression of the α- and β-subunits of the Arabidopsis K+ channel increases the amplitude of whole-cell currents, suggesting stabilization of the channel by the β-subunit (Zhang et al., 1999). In addition, the protein interaction of sucrose transporters of different affinities expressed in the same enucleate sieve element was demonstrated recently using the yeast system (Reinders et al., 2002).
The expression of ZmPIP1;2 and ZmPIP2;5 dimer cDNAs allowed us to determine whether individual monomeric subunits interact at the functional level in a mixed oligomer (Figure 6). Membranes from oocytes expressing the ZmPIP2;5 homodimer were found to have a lower Pf than those from oocytes expressing the ZmPIP2;5 monomer. It is possible that the introduction of too short a linker (three amino acid residues) between the monomers could influence protein folding, leading to lower expression at the plasma membrane, lower water channel activity, and/or full inactivation of a monomer in the dimer. Inactivation of the N-terminal monomer could explain the absence of Pf increase in oocytes expressing the ZmPIP2;5-ZmPIP2;5GW dimer, even if we cannot exclude a misrouting of the protein. However, chimeric dimers of mammal AQP1 containing a single amino acid linker are targeted to the oocyte plasma membrane, and each monomer in the fusion is functional (Shi et al., 1994). Oocytes expressing the ZmPIP1;2-ZmPIP2;5 and ZmPIP2;5-ZmPIP2;5 dimers showed similar Pf increases. If we assume that similar amounts of protein are present in the plasma membrane, these data can indicate either that ZmPIP1;2 is an active aquaporin when associated with ZmPIP2;5 or that only the C-terminal ZmPIP2;5 monomer is active in both dimers. However, the observation that the fusion of ZmPIP1;2 with inactive ZmPIP2;5GW increased the Pf to a level similar to that in ZmPIP1;2-ZmPIP2;5 favors the hypothesis that ZmPIP1;2 is activated by ZmPIP2;5 isoforms.
Surprisingly, no synergetic interaction was seen between ZmPIP1;1 and ZmPIP2;5, but ZmPIP1;1 and ZmPIP1;2 interacted positively to increase the Pf (Figure 7). These data clearly demonstrate that ZmPIP1 heteromerization results in the activation of the water channels in Xenopus oocytes. The different behavior of ZmPIP1;1 and ZmPIP1;2 on coinjection with ZmPIP2;5, together with the positive cooperative effect of both ZmPIP1s, is intriguing, because the amino acid sequences of the two isoforms are 96% identical. Sequence comparison of ZmPIP1;1 and ZmPIP1;2 allowed us to detect eight substitutions, four of which are located in the extracytosolic E loop immediately after the short α-helix containing the second Asn-Pro-Ala motif (Figure 8A). Replacement of the E-loop C-terminal part of ZmPIP1;1 with the corresponding part from ZmPIP1;2 converted ZmPIP1;1 to a ZmPIP1;2-like isoform that interacted positively with ZmPIP1;1 or ZmPIP2;5 (Figure 8B). These data demonstrate that loop E is important for aquaporin interaction and functionality, confirming reports suggesting that loops B and E do not have exactly equivalent functions. Loop B of AQP1 is more hydrophobic than loop E, and mutations in loop B result in a smaller decrease in Pf than corresponding mutations in loop E (Jung et al., 1994). The replacement of loop E in a “weak” aquaporin, AQP0, with loop E from a more active aquaporin, AQP2, increases the Pf of oocytes, and this correlates with an increase in the amount of the protein in the plasma membrane (Kuwahara et al., 1999). Another study showed that exchanging loop E of GlpF and AQPcic modified channel solute specificity and targeting to the plasma membrane (Duchesne et al., 2002). The effect of single, double, or triple mutations in ZmPIP1;1 loop E on Pf induction when coexpressed with ZmPIP2;5 should reveal the roles of specific residues.
It is important to determine whether this synergy is seen only in Xenopus oocytes or if it has physiological significance, allowing tight regulation of water membrane permeability, in plant cells. Analysis of Arabidopsis plants with reduced expression of PIP1 and/or PIP2 showed that the osmotic hydraulic conductivity is reduced significantly compared with that in wild-type plants but is similar regardless of which PIP subgroup is downregulated, suggesting that the aquaporin subgroup that is not downregulated is less active when the expression of the other PIP subgroup is reduced and that the PIP1 and PIP2 isoforms may function cooperatively (Martre et al., 2002). Five PIP1 and eight PIP2 proteins have been identified in the Arabidopsis genome (Johanson et al., 2001), and six PIP1 and seven PIP2 proteins have been found in maize ESTs libraries (Chaumont et al., 2001). Several of these, including ZmPIP1;1, ZmPIP1;2, and ZmPIP2;1, are highly expressed in most tissues, and we have demonstrated that coexpression of these isoforms increases the Pf in oocytes. In situ RT-PCR experiments in roots showed the presence of both ZmPIP1;2 and ZmPIP2;5 transcripts in xylem parenchyma cells (Figure 9), demonstrating that these two isoforms are expressed in the same cell type. Interestingly, high expression of the tonoplast AQP, ZmTIP1;1, also was detected in these cells, which play an active role in building the water potential gradient necessary for the control of water movement into and out of the xylem vessels (Barrieu et al., 1998). Simultaneous expression of ZmPIP1;2 and ZmPIP2;5 in xylem parenchyma cells could increase the Pf of the plasma membrane via a positive protein–protein interaction.
Root hair was found to express several ZmPIP genes, including ZmPIP1;1, ZmPIP2;1, ZmPIP2;4, and ZmPIP2;5 (Figure 9). Very low expression of ZmPIP1;2 was detected, but ZmPIP1;5 transcripts were present. ZmPIP1;5 has a loop E that is more similar to that of ZmPIP1;2 than that of ZmPIP1;1 (Figure 8A), suggesting that it might interact positively with other ZmPIPs. However, even if PIP1 and PIP2 proteins are expressed in the same cell type, they do not necessarily interact functionally. The purification and protein analysis of plasma membrane vesicles from red beet have shown that two prominent PIPs belonging to the PIP1 and PIP2 subfamilies (BvMIP2 and BvMIP3) have distinct biochemical and topological properties and form homodimeric aggregates, as observed with antibodies specific to each isoform (Barone et al., 1998). PM28A (PIP2) and PM28C (PIP1), the major constituents of spinach leaf plasma membranes, were copurified recently and shown to form tetramers by scanning transmission electron microscopy mass analysis (Fotiadis et al., 2001). Reconstitution into lipid bilayers has shown that the two isoforms segregate into two different crystal types and that no mixed crystals are seen. On the other hand, heterooligomers of two tonoplast aquaporins (25- and 26-kD proteins) from lentil seed have been detected in cross-linking experiments. Incubation of TIP-enriched membranes in the presence of dithiobis-succinimidylpropionate induced the formation of dimers, trimers, and tetramers made of both 25- and 26-kD proteins (Harvengt et al., 2000). Thus, additional experiments are necessary to reveal PIP interactions and oligomerization in plant cells and to elucidate the physiological importance of such regulation mechanisms.
METHODS
Plasmid Construction, in Vitro RNA Synthesis, and Translation
Constructs containing maize (Zea mays) ZmPIP1;2 or ZmPIP2;5 (pXZmPIP1;2 or pXZmPIP2;5) cDNA were prepared as described previously (Chaumont et al., 2000a). The cDNAs encoding ZmPIP2;1 and ZmPIP2;4 (Chaumont et al., 2001) were amplified by PCR using the specific primers ZmPIP2;1-1 (5′-CGGCAGATCTAGAAGACGATGGGC-3′), ZmPIP2;1-2 (5′-CTCCAGATCTAAGCCCCGCTCATCACG-3′), ZmPIP2;4-1 (5′-CAGCAGATCTGACTGACATGGCGAA-3′), and ZmPIP2;4-2 (5′-AAACAGATCTGGGCGTCATGTGGAGG-3′). The cDNA encoding ZmPIP2;1, fused with six His residues (His-ZmPIP2;1), was amplified by PCR using the specific primers PIP2;1-His (5′-AGCGGATCCAGCCATGGCGCACCATCACCATCACCATGGCAAGGACGACGTGATCGA-3′) and ZmPIP2;1-2. Mutated cDNA encoding ZmPIP2;5GW was obtained by recombinant PCR using specific primers (ZmPIP2a-1 and ZmPIP2a-2 [Chaumont et al., 2000a], ZmPIP2a-3 [5′-GATGTGCCAACCCGACACCC-3′], and ZmPIP2a-4 [5′-GGGTGTCGGGTTGGCACATC-3′]). A new amplification of ZmPIP1;2 cDNA was performed with ZmPIP1b-4 (Chaumont et al., 2000a) and ZmPIP1;2-14 (5′-GGGGAGATCTACAATGGAGGGG-3′) to generate pX2ZmPIP1;2 for use in the preparation of dimer constructs. To generate the two GFP vectors, pXZmPIP1;2-GFP and pXGFP, ZmPIP1;2-GFP and GFP cDNAs were amplified by PCR using the 35SC4PPDK-ZmPIP1;2sGFP(S65T) and 35SC4PPDK-sGFP(S65T) plasmids, respectively, as templates (Chaumont et al., 2000a) and the specific primers ZmPIP1;2-12 (5′-CGCGGATCCATGGAGGGGAAAG-3′), GFP1 (5′-CGCGGATCCTGCAGCCCGGGCGGCCGCT-3′), and GFP2 (5′-CGCGGATCCATGGTGAGCAAG-3′), thus incorporating a BamHI site at both ends. To generate pXGFP2, mGFP5 cDNA was amplified by PCR using N-ST-GFP plasmid as a template (Batoko et al., 2000) and the specific primers MGFP5-L (5′-GCGCAGATCTATGAGTAAAGGAGAAGAAC-3′) and MGSPS-A (5′-CAGGATCCCGGGAGCTCGAGTTTGTATAGTTCT-3′), incorporating BglII (MGFP5-L) and BamHI, SmaI, and XhoI (MGSPS-A) sites at the ends. All PCR-amplified fragments containing BamHI and/or BglII sites at both ends were subcloned into the BglII site of a pSP64T-derived BS vector (Preston et al., 1992), generating plasmids pXZmPIP2;1, pX2ZmPIP1;2, pXZmPIP2;5GW, pXZmPIP1;2-GFP, pXGFP, and pXGFP2.
To construct pXGFP-ZmPIP2;4, ZmPIP2;4 cDNA (Chaumont et al., 2001) was amplified by PCR using the specific primers ZAORF2;4-L (5′-CCCGCTCGAGATGGCGAAGGACATCGAG-3′) and ZAORF2;4-R (5′-GGGCCTCGAGTTAGGCGTTGCTCCGGTA-3′), incorporating XhoI sites at both ends, and subcloned into the XhoI site of pXGFP2, generating pXGFPZmPIP2;4.
To construct the plasmids encoding the dimers (ZmPIP1;2-ZmPIP1;2, ZmPIP2;5-ZmPIP2;5, ZmPIP2;5-ZmPIP2;5GW, ZmPIP1;2-ZmPIP2;5, and ZmPIP1;2-ZmPIP2;5GW), ZmPIP1;2 and ZmPIP2;5 cDNAs were amplified by PCR using the primers ZmPIP1;2-14, ZmPIP1;2-15 (5′-CGGCAGATCTAGACCTGCTCTTGAAC-3′), ZmPIP2;5-1 (5′-GGCTAGATCTAGAATGGCCAAG-3′), and ZmPIP2;5-10 (5′-CTACAGATCTGCGGCTGAAGGAGGCA-3′), thus incorporating BglII sites at both ends and removing the stop codon for both PIPs. These PCR-amplified fragments then were inserted into partially BglII-digested and dephosphorylated pX2ZmPIP1;2, pXZmPIP2;5, and pXZmPIP2;5GW. These constructs resulted in the fusion of two cDNA open reading frames linked by three codons coding for Arg-Ser-Thr (pXZmPIP1;2-ZmPIP1;2) or Arg-Ser-Arg (pXZmPIP2;5-ZmPIP2;5, pXZmPIP1;2-ZmPIP2;5, and pXZmPIP1;2-ZmPIP2;5GW). The fidelity of these constructs was checked by restriction and sequence analysis.
Capped complementary RNAs encoding monomers and dimers of ZmPIPs were synthesized in vitro as described previously (Preston et al., 1992). ZmPIP1;2 and ZmPIP2;5 complementary RNAs were translated in vitro using a rabbit reticulocyte lysate from the Linked in Vitro SP6/T7 Transcription/Translation Kit-Radioactive (Roche Molecular Biochemicals, Mannheim, Germany) in the presence of l-35S-Met and l-35S-Cys (PRO-MIX; Amersham Pharmacia).
Oocyte Isolation and Injection and Osmotic Water Permeability Assay
Xenopus laevis oocytes were isolated and defolliculated by digestion at room temperature for 1.5 h with 4 mg/mL collagenase A (Roche Diagnostics, Mannheim, Germany) in Barth's solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM Hepes-NaOH, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, pH 7.4, and 200 mosm/kg]. In vitro transcripts or distilled water were injected in a volume of 50 nL, and the oocytes were incubated at 18°C in Barth's solution supplemented with 50 μg/mL gentamycin sulfate. To measure the osmotic water permeability coefficient (Pf), oocytes were transferred at 2 to 3 days after injection from Barth's solution to the same solution diluted fivefold with distilled water, and changes in the cell volume were followed using a microscope linked to a black-and-white digital camera. Images were captured at 5-s intervals using a LG-3 Grayscale Scientific PCI Frame Grabber (Scion Corporation, Frederick, MD), and the surface area of the oocytes, analyzed using Scion Image software, was used to calculate the relative volume of the oocyte. The Pf was calculated using the equation Pf = V0[d(V/V0)/dt]/[SxVw(Osmin − Osmout)], with an initial volume (V0) of 9 × 10−4 cm3, an initial oocyte surface area (S) of 0.045 cm2, and a molar volume of water (Vw) of 18 cm3/mol (Zhang and Verkman, 1991).
Labeling of Oocyte Proteins and Isolation of Crude Membranes
Microinjected oocytes were incubated in Barth's solution for 4 to 8 h, and then 5 to 10 cells were transferred to 1 mL of Barth's solution supplemented with 12.6 μL of PRO-MIX (14.3 mCi/mL; Amersham Pharmacia) containing 70% l-35S-Met and 30% l-35S-Cys and incubated overnight at 18°C.
They were placed on ice for 10 min, washed three times with ice-cold Barth's solution, and homogenized in 0.5 mL of homogenization buffer (10 mM KH2PO4, 5 mM EDTA, 5 mM EGTA, and 1 mM phenanthroline, pH 7.6) containing 2 μg/mL each of leupeptin, aprotinin, antipain, pepstatin, and chymostatin. The homogenate was centrifuged at 4°C for 5 min at 110g to remove yolk proteins, then for 30 min at 20,800g, and the final pellet containing crude membranes was washed with 0.5 mL of homogenization buffer and resuspended in Laemmli buffer containing 50 mM DTT (Laemmli, 1970).
After counting the samples in a scintillation counter, proteins (100,000 cpm) were analyzed by SDS-PAGE. After migration, the gels were equilibrated with DMSO, incubated in 20% (w/v) 2,5-diphenyloxazole in DMSO, rinsed with tap water, dried, and exposed to Kodak Bio-Max film at −70°C. Bands were quantified using a Molecular Imager System GS-25 (Bio-Rad Laboratories, Hercules, CA).
Solubilization, Purification of His-ZmPIP2;1, and Immunodetection
Crude membranes were resuspended in buffer A (20 mM Hepes-NaOH, pH 7.8, 50 mM NaCl, 10% glycerol, and 2 mM β-mercaptoethanol). One milligram of proteins was solubilized with 3.5% (w/v) octyl-β-d-thioglucopyranoside in a final volume of 1 mL and incubated overnight on a rotary wheel at room temperature. Unsolubilized material was removed by centrifugation at 169,000g for 40 min, and the supernatant was added to 500 μL of Ni2+-nitriloacetic acid agarose matrix (Qiagen, Valencia, CA) preequilibrated with buffer B (20 mM Hepes-NaOH, pH 7.8, 300 mM NaCl, 10% [w/v] glycerol, 2 mM β-mercaptoethanol, and 0.4% [w/v] octyl-β-d-thioglucopyranoside) containing 10 mM imidazole. The mixture was incubated for 2 h on a rotary wheel at room temperature, loaded on a column, and washed three times with 1 mL of buffer B containing 30 mM imidazole. Bound proteins were eluted with 6 × 200 μL of buffer B containing 200 mM imidazole. Proteins (same volumes) were electrophoresed (SDS-PAGE), transferred to a nitrocellulose membrane, and immunodetected with antibodies directed against GFP (Duby et al., 2001) by the enhanced bioluminescence method.
Maize Section Preparation
Maize (B73) root tips (2 cm) were excised from 4-day-old seedlings and fixed for 24 h at 4°C in 60% (v/v) ethanol, 5% (v/v) acetic acid, and 2% (v/v) formaldehyde. After fixation, the roots were dehydrated through an alcohol series and incubated at 60°C in melted Paraplast Plus (Sherwood Medical, St. Louis, MO). After five changes of Paraplast over 72 h, the tissues were embedded in Paraplast Plus blocks, sectioned into 6- to 8-μm slices, and floated on diethyl pyrocarbonate (DEPC)–treated water at 42°C. The sections were loaded onto SuperFrost Plus glass slides (Menzel-Glaser, Braunschweig, Germany), dried for 36 h at 42°C, dewaxed for 3 × 3 min in Histoclear (National Diagnostics, Atlanta, GA), and then gradually hydrated by passing through an alcohol series to DEPC-treated water. After rinses for 20 min in 0.02 M HCl and for 30 min in 2 × SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate), the sections were incubated for 5 min at 37°C in pectinase buffer (1% [w/v] pectinase [Sigma-Aldrich], 0.1 M sodium acetate, and 5 mM EDTA, pH 4), rinsed with PBS for 10 min, incubated for 7 min at 37°C with 2 mg/mL proteinase K (Roche Molecular Biochemicals) in PBS, rinsed successively with 2 mg/mL Gly for 1 min, PBS for 5 min, and DEPC-treated water for 5 min, and then dried for 30 min at room temperature. Each section was covered with 5 μL of DNase solution containing 8 units of DNase (Roche Molecular Biochemicals) and 20 units of RNase inhibitor (Roche Molecular Biochemicals), incubated in a humid chamber at 37°C overnight, and washed successively for 10 min with 0.5 M EDTA, 2 × SSC, 1 × SSC, and DEPC-treated water.
In Situ Reverse Transcriptase–Mediated PCR
Single-stranded cDNAs were synthesized on the slide in a 5-μL reaction mixture containing 40 units of Moloney murine leukemia virus reverse transcriptase (Promega), 0.5 mM deoxynucleotide triphosphates, 4 units of RNase inhibitor, and 1 μM primers at 42°C for 1 h. PCR was performed on the slide in a 50-μL reaction mixture containing 1.2 units of Taq Polymerase (Biotools B&M Labs, Madrid, Spain), 0.2 mM dATP, dCTP, dGTP, and dTTP, 0.25 nmol of DIG-11-dUTP (digoxigenin-11-2′-uridine-5′-triphosphate; Roche Molecular Biochemicals), and 1 μM primers (see above). The PCR conditions were 94°C for 2.5 min; 29 cycles of 94°C for 30 s, 61°C for 45 s, and 72°C for 35 s; and a final elongation step of 7 min at 72°C. PCR products were detected using the DIG Nucleic Acid Detection Kit (Roche Molecular Biochemicals).
Root Hair RNA Isolation
Maize seeds were incubated for 12 h at room temperature in 1 mM CaCl2 and germinated on a nylon net (mesh size, 1 mm) stretched across the top of a 1000-mL glass beaker containing 150 mL of Murashige and Skoog (1962) medium and a stirring magnet, leaving ∼15 cm of air between the medium and the top of the beaker. A 500-mL glass beaker was placed upside down on top of the 1000-mL beaker, and the two were sealed together with Parafilm. The beakers then were covered with aluminum foil, except for the upper end, placed at 30°C for 72 h on a magnetic stirrer (750 rpm) in a dark room, and transferred to a growing chamber on an 8-h-dark/16-h-light regime at 23°C. After 4 days, the roots (8 to 12 cm) were cut off directly into liquid nitrogen, and the root hairs were gently shaved off with forceps. The tissue was ground up using a glass rod in Trizol reagent (Roche Molecular Biochemicals), and RNA was extracted according to the manufacturer's protocol. Reverse transcriptase–mediated PCR was performed as described above, except that 5 μM oligo(dT)15-18 was used for single-stranded cDNA synthesis. The primers used for PCR were ZmPIP1;1-1 (5′-TAAAGGAGCCGATGCTGCTG-3′), ZmPIP1;1-2 (5′-GGATGAACTCTTAAAGCTTGAC-3′), ZmPIP1;2-1 (5′-GCGTCTTCCTGTGATGTCTTCT-3′), ZmPIP1;2-2 (5′-AAATCAAGAAAACCCTGAATCG-3′), ZmPIP1;5-1 (5′-ATTACCAACAGCAACCATGCAG-3′), ZmPIP1;5-2 (5′-CTTCACCGTACCAAAACCCAAG-3′), ZmPIP2;1-1 (5′-CGGCCTTCTACCACCAGTACAT-3′), ZmPIP2;1-2 (5′-CATGATTACATTGCAGGGGAAC-3′), ZmPIP2;4-3 (5′-CTACCGGAGCAACGCCTAA-3′), ZmPIP2;4-4 (5′-ATCGGATAAAAACTCACGCAAT-3′), ZmPIP2;5-1 (5′-GCGCTGCTGTCATCTACAACAA-3′), and ZmPIP2;5-2 (5′-GCAAGCAAAATGCAGTGGAAAT-3′).
Microscopy
Oocytes expressing GFP constructs were fixed and sliced as described previously (Sayers et al., 1997). GFP fluorescence was visualized using a confocal laser scanning microscope (MRC-1024; Bio-Rad Laboratories) with excitation at 488 nm and detection between 506 and 538 nm using a ×20 objective lens. Photographs of the root sections were taken using a Leica DMR microscope (Wetzlar, Germany).
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact François Chaumont, chaumont@fysa.ucl.ac.be.
Acknowledgments
This work was begun in the laboratory of M.J. Chrispeels at the University of California, San Diego. We thank F. Barrieu for his contribution to the early work. We are very grateful to M.J. Chrispeels and M. Boutry (Université Catholique de Louvain) for helpful comments and the critical reading of the manuscript, to R. Jung (Pioneer Hi-Bred International, Johnston, IA) for providing ZmPIP1;5, ZmPIP2;1, ZmPIP2;4, and ZmPIP2;5 cDNAs, and to C. Maurel (Université Montpellier 2) for providing the GlpF clone. This work was supported by grants from the Belgian Fund for Scientific Research and the Interuniversity Poles of Attraction Program (Belgian State, Prime Minister's Office, Federal Office for Scientific, Technical, and Cultural Affairs), a grant from the Université Catholique de Louvain to K.F., an individual Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture fellowship to V.V.W., and an individual Marie Curie European fellowship to M.M.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017194.
References
- Barone, L.M., Mu, H.H., Shih, C.J., Kashlan, K.B., and Wasserman, B.P. (1998). Distinct biochemical and topological properties of the 31- and 27-kilodalton plasma membrane intrinsic protein subgroups from red beet. Plant Physiol. 118, 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrieu, F., Chaumont, F., and Chrispeels, M.J. (1998). High expression of the tonoplast aquaporin ZmTIP1 in epidermal and conducting tissues of maize. Plant Physiol. 117, 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biela, A., Grote, K., Otto, B., Hoth, S., Hedrich, R., and Kaldenhoff, R. (1999). The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 18, 565–570. [DOI] [PubMed] [Google Scholar]
- Chaumont, F., Barrieu, F., Jung, R., and Chrispeels, M.J. (2000. a). Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 122, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont, F., Barrieu, F., Wojcik, E., Chrispeels, M.J., and Jung, R. (2001). Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 125, 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont, F., Van Wilder, V., Fetter, K., Barrieu, F., and Chrispeels, M.J. (2000b). Characterization of plasma membrane MIP proteins in maize. In Molecular Biology and Physiology of Water and Solute Transport, S. Hohmann and S. Nielsen, eds (New York: Kluwer Academic/Plenum Publishers), pp. 269–274.
- Ciavatta, V.T., Morillon, R., Pullman, G.S., Chrispeels, M.J., and Cairney, J. (2001). An aquaglyceroporin is abundantly expressed early in the development of the suspensor and the embryo proper of loblolly pine. Plant Physiol. 127, 1556–1567. [PMC free article] [PubMed] [Google Scholar]
- Daniels, M.J., Mirkov, T.E., and Chrispeels, M.J. (1994). The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol. 106, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R.M., Rivers, R.L., Zeidel, M.L., and Roberts, D.M. (1999). Purification and functional reconstitution of soybean nodulin 26: An aquaporin with water and glycerol transport properties. Biochemistry 38, 347–353. [DOI] [PubMed] [Google Scholar]
- Dixit, R., Rizzo, C., Nasrallah, M., and Nasrallah, J. (2001). The Brassica MIP-MOD gene encodes a functional water channel that is expressed in the stigma epidermis. Plant Mol. Biol. 45, 51–62. [DOI] [PubMed] [Google Scholar]
- Dordas, C., Chrispeels, M.J., and Brown, P.H. (2000). Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 124, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer, I., Antunes, S., Hoshi, T., Muller-Rober, B., Palme, K., Pongs, O., Reintanz, B., and Hedrich, R. (1997). Plant K+ channel α-subunits assemble indiscriminately. Biophys. J. 72, 2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne, L., Pellerin, I., Delamarche, C., Deschamps, S., Lagree, V., Froger, A., Bonnec, G., Thomas, D., and Hubert, J.F. (2002). Role of C-terminal domain and transmembrane helices 5 and 6 in function and quaternary structure of major intrinsic proteins: Analysis of aquaporin/glycerol facilitator chimeric proteins. J. Biol. Chem. 277, 20598–20604. [DOI] [PubMed] [Google Scholar]
- Duby, G., Oufattole, M., and Boutry, M. (2001). Hydrophobic residues within the predicted N-terminal amphiphilic α-helix of a plant mitochondrial targeting presequence play a major role in in vivo import. Plant J. 27, 539–549. [DOI] [PubMed] [Google Scholar]
- Fotiadis, D., Jeno, P., Mini, T., Wirtz, S., Muller, S.A., Fraysse, L., Kjellbom, P., and Engel, A. (2001). Structural characterization of two aquaporins isolated from native spinach leaf plasma membranes. J. Biol. Chem. 276, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Fu, D., Libson, A., Miercke, L.J., Weitzman, C., Nollert, P., Krucinski, J., and Stroud, R.M. (2000). Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290, 481–486. [DOI] [PubMed] [Google Scholar]
- Gerbeau, P., Guclu, J., Ripoche, P., and Maurel, C. (1999). Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J. 18, 577–587. [DOI] [PubMed] [Google Scholar]
- Green, W.N., and Millar, N.S. (1995). Ion-channel assembly. Trends Neurosci. 18, 280–287. [PubMed] [Google Scholar]
- Guenther, J.F., and Roberts, D.M. (2000). Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 210, 741–748. [DOI] [PubMed] [Google Scholar]
- Harvengt, P., Vlerick, A., Fuks, B., Wattiez, R., Ruysschaert, J.M., and Homble, F. (2000). Lentil seed aquaporins form a hetero-oligomer which is phosphorylated by a Mg2+-dependent and Ca2+-regulated kinase. Biochem. J. 352, 183.–190. [PMC free article] [PubMed] [Google Scholar]
- Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjovall, S., Fraysse, L., Weig, A.R., and Kjellbom, P. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 126, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, I., Karlsson, M., Johanson, U., Larsson, C., and Kjellbom, P. (2000). The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta 1465, 324–342. [DOI] [PubMed] [Google Scholar]
- Johansson, I., Karlsson, M., Shukla, V.K., Chrispeels, M.J., Larsson, C., and Kjellbom, P. (1998). Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J.S., Preston, G.M., Smith, B.L., Guggino, W.B., and Agre, P. (1994). Molecular structure of the water channel through aquaporin CHIP: The hourglass model. J. Biol. Chem. 269, 14648–14654. [PubMed] [Google Scholar]
- Kammerloher, W., Fischer, U., Piechottka, G.P., and Schaffner, A.R. (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 6, 187–199. [DOI] [PubMed] [Google Scholar]
- Kamsteeg, E.J., and Deen, P.M. (2001). Detection of aquaporin-2 in the plasma membranes of oocytes: A novel isolation method with improved yield and purity. Biochem. Biophys. Res. Commun. 282, 683–690. [DOI] [PubMed] [Google Scholar]
- Kamsteeg, E.J., Wormhoudt, T.A., Rijss, J.P., van Os, C.H., and Deen, P.M. (1999). An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J. 18, 2394–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara, M., Shinbo, I., Sato, K., Terada, Y., Marumo, F., and Sasaki, S. (1999). Transmembrane helix 5 is critical for the high water permeability of aquaporin. Biochemistry 38, 16340–16346. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Marin-Olivier, M., Chevalier, T., Fobis-Loisy, I., Dumas, C., and Gaude, T. (2000). Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant J. 24, 231–240. [DOI] [PubMed] [Google Scholar]
- Martre, P., Morillon, R., Barrieu, F., North, G.B., Nobel, P.S., and Chrispeels, M.J. (2002). Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 130, 2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, C., Kado, R.T., Guern, J., and Chrispeels, M.J. (1995). Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. EMBO J. 14, 3028–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, C., Reizer, J., Schroeder, J.I., Chrispeels, M.J., and Saier, M.H., Jr. (1994). Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 269, 11869–11872. [PubMed] [Google Scholar]
- Moshelion, M., Becker, D., Biela, A., Uehlein, N., Hedrich, R., Otto, B., Levi, H., Moran, N., and Kaldenhoff, R. (2002). Plasma membrane aquaporins in the motor cells of Samanea saman: Diurnal and circadian regulation. Plant Cell 14, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473.–497. [Google Scholar]
- Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J.B., Engel, A., and Fujiyoshi, Y. (2000). Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605. [DOI] [PubMed] [Google Scholar]
- Nemeth-Cahalan, K.L., and Hall, J.E. (2000). pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 275, 6777–6782. [DOI] [PubMed] [Google Scholar]
- Ottschytsch, N., Raes, A., Van Hoorick, D., and Snyders, D.J. (2002). Obligatory heterotetramerization of three previously uncharacterized Kv channel α-subunits identified in the human genome. Proc. Natl. Acad. Sci. USA 99, 7986–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, M.A., Kirsch, G.E., and Brown, A.M. (1996). Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current. FEBS Lett. 399, 177–182. [DOI] [PubMed] [Google Scholar]
- Preston, G.M., Carroll, T.P., Guggino, W.B., and Agre, P. (1992). Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387. [DOI] [PubMed] [Google Scholar]
- Quigley, F., Rosenberg, J.M., Shachar-Hill, Y., and Bohnert, H.J. (2002). From genome to function: The Arabidopsis aquaporins. Genome Biol. 3, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders, A., Schulze, W., Kuhn, C., Barker, L., Schulz, A., Ward, J.M., and Frommer, W.B. (2002). Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 14, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers, R.L., Dean, R.M., Chandy, G., Hall, J.E., Roberts, D.M., and Zeidel, M.L. (1997). Functional analysis of nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem. 272, 16256–16261. [DOI] [PubMed] [Google Scholar]
- Sayers, L.G., Miyawaki, A., Muto, A., Takeshita, H., Yamamoto, A., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1997). Intracellular targeting and homotetramer formation of a truncated inositol 1,4,5-trisphosphate receptor-green fluorescent protein chimera in Xenopus laevis oocytes: Evidence for the involvement of the transmembrane spanning domain in endoplasmic reticulum targeting and homotetramer complex formation. Biochem. J. 323, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L.B., Skach, W.R., and Verkman, A.S. (1994). Functional independence of monomeric CHIP28 water channels revealed by expression of wild-type mutant heterodimers. J. Biol. Chem. 269, 10417–10422. [PubMed] [Google Scholar]
- Shi, L.B., and Verkman, A.S. (1996). Selected cysteine point mutations confer mercurial sensitivity to the mercurial-insensitive water channel MIWC/AQP-4. Biochemistry 35, 538–544. [DOI] [PubMed] [Google Scholar]
- Sui, H., Han, B.G., Lee, J.K., Walian, P., and Jap, B.K. (2001). Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878. [DOI] [PubMed] [Google Scholar]
- Tyerman, S.D., Niemietz, C.M., and Bramley, H. (2002). Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25, 173–194. [DOI] [PubMed] [Google Scholar]
- Veenhoff, L.M., Heuberger, E.H., and Poolman, B. (2001). The lactose transport protein is a cooperative dimer with two sugar translocation pathways. EMBO J. 20, 3056–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhoff, L.M., Heuberger, E.H., and Poolman, B. (2002). Quaternary structure and function of transport proteins. Trends Biochem. Sci. 27, 242–249. [DOI] [PubMed] [Google Scholar]
- Weig, A., Deswarte, C., and Chrispeels, M.J. (1997). The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 114, 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig, A.R., and Jakob, C. (2000). Functional identification of the glycerol permease activity of Arabidopsis thaliana NLM1 and NLM2 proteins by heterologous expression in Saccharomyces cerevisiae. FEBS Lett. 481, 293–298. [DOI] [PubMed] [Google Scholar]
- Yamada, S., Katsuhara, M., Kelly, W.B., Michalowski, C.B., and Bohnert, H.J. (1995). A family of transcripts encoding water channel proteins: Tissue-specific expression in the common ice plant. Plant Cell 7, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui, M., Kwon, T.H., Knepper, M.A., Nielsen, S., and Agre, P. (1999). Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 96, 5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R.B., and Verkman, A.S. (1991). Water and urea permeability properties of Xenopus oocytes: Expression of mRNA from toad urinary bladder. Am. J. Physiol. 260, C26–C34. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Ma, J., and Berkowitz, G.A. (1999). Evaluation of functional interaction between K+ channel α- and β-subunits and putative inactivation gating by co-expression in Xenopus laevis oocytes. Plant Physiol. 121, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]