Abstract
AIMS
The tolerance to the prolactin response following administration of antipsychotic drugs has been modelled as a depletion of a prolactin pool (pool model) and a model where the tolerance is explained by a feedback loop including the dopamine interaction of prolactin release (agonist-antagonist interaction model, (AAI model)). The AAI model was superior to the pool model when analyzing data from clinical trials of risperidone and paliperidone. Here we evaluated the two models using the remoxipride data, designed to challenge the short-term prolactin response, from which the original pool model was built.
METHODS
The remoxipride data were collected from a study where eight healthy male subjects received two remoxipride infusions on five occasions. The intervals between the first and second dose on each occasion were 2, 8, 12, 24 and 48 h, respectively. The pool and AAI models were fitted using NONMEM.
RESULTS
According to the objective function values the pool model with a circadian rhythm function fitted the data slightly better, while the AAI model was better in describing the circadian rhythm of prolactin. Visual predictive checks revealed that the models predicted the prolactin profiles equally well.
CONCLUSIONS
According to the analysis performed here, a previous analysis of several clinical studies and literature reports on prolactin concentrations, it appears that the dopamine feedback mechanism included in the AAI model is better than the storage depletion mechanism in the pool model to estimate the bio-rhythm of prolactin time-course and the tolerance development across different populations, drugs, treatment schedules and time.
Keywords: agonist-antagonist interaction model, circadian rhythm, pool model, prolactin, remoxipride, tolerance
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Prolactin response is a common side effect of antipsychotic drugs.
The pool model was proposed to describe the effect of remoxipride on prolactin.
The agonist-antagonist interaction (AAI) model, which incorporated a dopamine-prolactin feedback loop mechanism and circadian rhythm, was used to depict the effects of risperidone and paliperidone on prolactin.
The pool model could not characterize prolactin response in clinical studies of risperidone and paliperdone.
WHAT THIS STUDY ADDS
The AAI model and the pool model were both reconstructed based on the remoxipride data set from which the original pool model was built. The pool model was extended with a circadian rhythm component which was employed in the AAI model.
The objective function values calculated by NONMEM revealed that the pool model with a circadian rhythm component best fitted the data. However, predictive checks of both models were satisfactory and the AAI model was superior in describing the circadian rhythm in healthy male volunteers when compared with earlier studies.
This study shows that the AAI model was superior as it worked well across different populations, study designs and drugs.
Introduction
A group of unwanted side effects of antipsychotic drugs, such as galactorrhoea, mammary gland development and sexual dysfunction, is caused by prolactin response. Prolactin release is regulated by dopamine concentration. The mechanism behind these side effects is that antipsychotic drugs block not only the central dopamine2 (D2) receptors in the brain but also the peripheral D2 receptors in the anterior pituitary. Therefore, the inhibition of dopamine on prolactin release from lactotrophs in the adenohypophysis is suppressed by antipsychotic drugs [1].
Pharmacokinetic and pharmacodynamic (PK/PD) models have been developed to describe the relationship between antipsychotic drugs and prolactin response. Prolactin is synthesized and secreted by lactotrope cells. In the prolactin pool model [2], the response and tolerance development is depicted by the processes of generation, storage and release of prolactin. The model was used to characterize the prolactin response in healthy male volunteers to the antipsychotic drug remoxipride after a design aiming to challenge the prolactin system, two short infusions with various administration intervals. The data were described by a model with two compartments [2]. The structure of the pool model is presented in Figure 1, but in contrast to the original publication mass balance is preserved, i.e. the input rate at baseline conditions is set to be equal to elimination rate at baseline conditions, and a circadian function has been added. In the original publication, a linear relationship between remoxipride concentration and prolactin release was found sufficient to describe the response.
Figure 1.
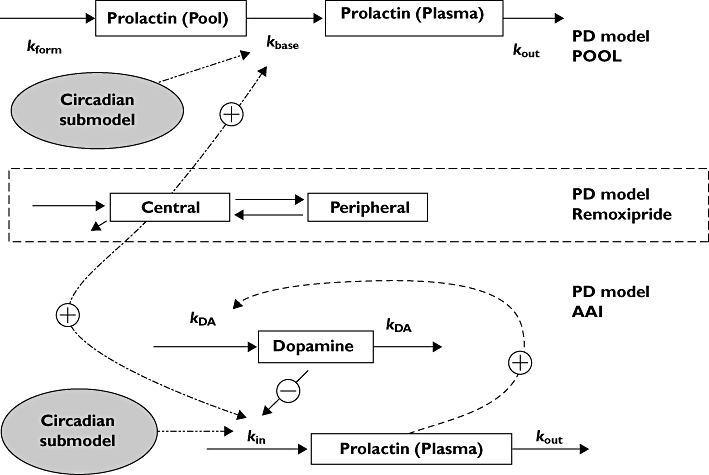
The circadian pool model and the circadian agonist-antagonist interaction model. Rform is the prolactin synthesizing rate; kbase is the rate constant describing prolactin release from the pool compartment to the plasma compartment; kout is the prolactin elimination rate constant. kin is the production rate and kout is the elimination rate constant of prolactin; kDA is the turnover rate constant of the dopamine system
The original pool model was used to describe the prolactin response following an experimental design of the antipsychotic drug remoxipride. Later an agonist-antagonist interaction (AAI) model was proposed to explain the interaction between dopamine concentration and the antipsychotic drug chlorprothixene on prolactin release after a single intravenous or oral dose administration [3]. A phenomenon important to prolactin secretion, the circadian rhythm, was integrated into the AAI model recently [4]. The AAI model with a circadian function described the concentration–time profile of prolactin after single or repeated administration of different formulations of risperidone and paliperidone in both patients and healthy volunteers. In contrast, the pool model, with or without circadian rhythm, could not describe the data from these studies.
By adding knowledge of the mechanism of action and physiology into PK/PD models, the model may not only explain the data at hand better, but it is more likely that the model can be used for extrapolation to other doses, schedules and drugs than those the model was based on. Both the AAI model and the pool model have mechanistic support. However, only the AAI model could successfully extrapolate to prolactin response data from new studies as described above.
The objective of this study was to evaluate the performance of the AAI model, and compare it with that of the pool model, in describing data from the remoxipride study with an experimental design, i.e. the data set the original pool model was developed from in order to determine whether a single mechanism-based model can describe well prolactin data from different study designs and populations.
Methods
The data set, which contained remoxipride and prolactin concentrations from eight healthy male volunteers, was the same as the one used to develop the original pool model [5]. The subjects' age ranged between 29 and 45 years (mean 36 ± 6 years). On each of the five occasions, two repeated doses of remoxipride 50 mg as intravenous infusion during 30 min were administered with varying time intervals. The time intervals between the first dose and the second dose were 2, 8, 12, 24 and 48 h on the five different occasions. Subjects were allocated to sequences of these intervals randomly in a crossover design. On all occasions, the first infusion started between 08.00 h and 09.00 h. For further details regarding material, methods and the study drug, remoxipride, please refer to the original publication [2].
Blood sampling
Blood samples for determination of remoxipride and prolactin concentrations were drawn according to the following schedule: prior to start of infusion and 10, 15, 20, 30, 40, 50, 60, 90 min and 2, 4, 8, 12 and 24 h after the start of the infusion. For the first infusion, this sampling schedule followed up to the start of the second administration.
Pharmacokinetic and pharmacodynamic modeling
Pharmacokinetic and pharmacodynamic models were constructed sequentially. A two compartment pharmacokinetic model was implemented according to the previous analysis [5]. The original parameters were calculated using the two-stage method in PCNONLIN® (Statistical Consultants, Inc., Lexington, KY, USA) and intra-individual variability (IIV) was computed from the estimated individual parameters. In the current analysis the typical pharmacokinetic parameters and IIV were re-estimated using population analysis with the first-order conditional estimation (FOCE) method with INTERACTION in NONMEM® VI (GloboMax/ICON, Inc., Ellicott City, MD, USA). All of the pharmacodynamic models were implemented in NONMEM VI and the individually predicted remoxipride concentration profiles used to drive the drug effects were derived from the two compartment pharmacokinetic model with the population parameter values fixed, keeping the pharmacokinetic data in the data set.
A circadian rhythm model was adopted from the previous circadian AAI model [4] where a double cosine function described the circadian change in release rate of prolactin [6, 7]:
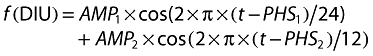 |
(1) |
There are two periods, 24 and 12 h, in the equation. AMP1 and AMP2 are the amplitudes of each cosine functions. PHS1 and PHS2 are the phase shifts relative to time (t). t represents a relative time to the first administration, i.e. 08.00 h, which was set to be the start time of the first infusion in the data set as the exact time was not known (08.00 to 09.00 h).
The original pool model and the circadian pool model are presented in Figure 1. The differential equations describing the time course of prolactin concentration in plasma compartment and the pool compartment are described by:
| (2) |
| (3) |
where CPRL is the prolactin concentration in plasma; Rform is the prolactin synthesis rate; kbase is the rate constant describing prolactin release from the pool compartment to the plasma compartment; kout is the prolactin elimination rate constant; S(t) is a function to describe the relationship between the drug concentration and the release rate of prolactin; f(DIU) is the circadian function which influences kbase. If f(DIU) is assumed to be constant over time and fixed to 0, the model is reduced to the original pool model.
When the original pool model was modified to adhere to mass-balance principles, the following constraints were introduced:
| (4) |
where Cpool,0 and CPRL,0 are Cpool and CPRL, respectively, at baseline.
Remoxipride concentrations (C) stimulate the prolactin release rate, kbase, with a linear relationship with slope M[5].
| (5) |
The AAI model and the circadian AAI model are shown in Figure 1. They are described by
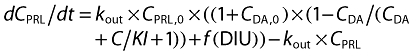 |
(6) |
| (7) |
where kDA is the turnover rate constant of the dopamine system; (CPRL/CPRL,0)γ is a feedback component; γ is a feedback parameter; CDA is the scaled dopamine concentration in a hypothetical dopamine compartment; CDA,0 is the scaled dopamine concentration at baseline, which was fixed to 10 000; KI is the potency parameter; other symbols as for the pool model. If f(DIU) = 0, this model is reduced to the AAI model without circadian rhythm.
The parameters of the original pool model, the pool model with enforced mass-balance, the circadian pool model, the AAI model, and the circadian AAI model were estimated. For all parameters where IIV components were supported by the data, these were estimated.
Model evaluation
Models were evaluated based on standard criteria, including goodness-of-fit (GOF) plots using R (http://www.r-project.org) and Xpose 4.1 [8]. For model selection between nested models the likelihood ratio test was applied, using the objective function values (OFV) provided by the NONMEM program. Numerical predictive checks (NPC) and visual predictive checks (VPCs), based on 1000 simulations for each of the models using PsN 2.2 [9] and in house Perl scripts, were also used to evaluate the models [10]. As the study only included eight patients and thereby little information on inter-subject variability, 40% prediction intervals were used to evaluate the model.
Results
The typical parameters in the pharmacokinetic model when implemented in NONMEM, CL (6.78 l h−1) and Vss (40.1 l), were close to the values (6.84 l h−1 and 41.48 l) estimated earlier by PCNONLIN®[5]. The estimated values of IIV for CL and Vss were 15.3% and 12.2%, respectively, while they were 15.8% and 14.5%, respectively, in the previous investigation where the standard two-stage approach was used. There was no important difference between the two pharmacokinetic models and the VPC presented in Figure 2 shows that predictions based on this pharmacokinetic model were adequate.
Figure 2.
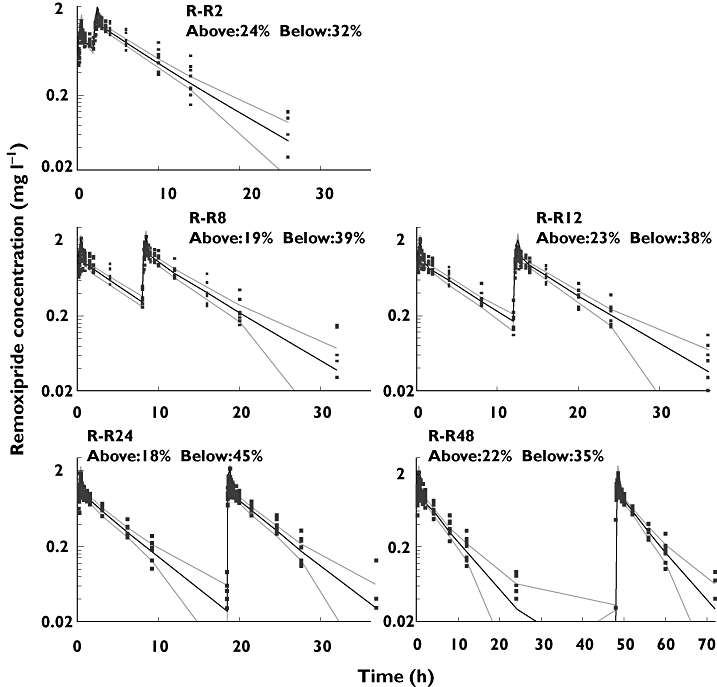
Numerical predictive checks (NPC) and visual predictive checks (VPC) for the two compartment pharmacokinetic model with 40% prediction interval (PI). R-R2 to R-R48 are the five different occasions. R is first dose, R2, R8, R12, R24, and R48 indicate that second doses were given at 2, 8, 14, 24, and 48 h, respectively. Time 0 is 08.00 h. Above and Below indicate the percentages of data points above and below the prediction interval, respectively. 40% Prediction interval ( ); Median prediction (
); Median prediction ( )
)
Original pool model and Circadian pool model
The original pool model and a pool model including mass-balance were both evaluated. The OFVs were 1568.5 and 1651.8, respectively. The original pool model employed more parameters than the pool model with mass-balance. Most importantly, the baseline prolactin concentrations in the plasma compartment and in the pool compartment were predicted to drift over time at the starting time in the original pool model, showing the importance of conserving mass balance in the modeling. In the original pool model the prolactin baseline was predicted to increase systematically from 10 µg l−1 to >13 µg l−1 over the course of the experiment. The prolactin concentrations in the plasma and pool compartment, the prolactin synthesis rate and the prolactin elimination rate estimated in the pool model with mass-balance were all higher than the values from the original pool model (Table 1).The observed median prolactin concentration at the start of the study was 10.05 µg l−1. A nonlinear relationship between remoxipride and prolactin, described by an Emax model, was investigated, but as improvement was limited (decrease in OFV = 3.2) and estimated EC50 was 11.5 mg l−1, considerably higher than the observed concentrations, the linear function was kept. The original pool model suggested a stronger stimulation of remoxipride on prolactin release (M, the slope constant) compared with the pool model with mass-balance.
Table 1.
Estimated pharmacodynamic parameters for the evaluated prolactin models. For the agonist-antagonist interaction (AAI) model the relative standard errors (RSE) could be estimated
| Original pool model[3] | Pool model | AAI model | Previous AAI model [4]‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Software | PCNONLIN | NONMEM VI | NONMEM VI | NONMEM VI | ||||||||
| Mass-balance | No | No | Yes | Yes | Yes | Yes | Yes | |||||
| Circadian | No | No | No | Yes | No | Yes | Yes | |||||
| TV | TV | IIV% | TV | IIV% | TV (RSE%) | IIV% (RSE%) | TV (RSE%) | IIV% (RSE%) | TV (RSE%) | IIV% (RSE%) | TV | |
| CPRL (µg l−1) | 9.4 | 9.88 | 28 | 12.7 | 23 | 14.1(7.1) | 24(14) | 9.13 (5.5) | 14(23) | 8.80(6.6) | 12(26) | 7.67 |
| Cpool (µg l−1) | 144 | 97.5 | 53 | 246* | – | 196* | – | |||||
| Mslope (l mg−1) | 4.7 | 9.45 | – | 4.08 | 13 | 2.92(12.5) | 12(34) | |||||
| Rform (µg l−1 h−1) | 16 | 13.5 | 34 | 26.5* | – | 34.7* | – | |||||
| kbase (h−1) | 0.105 | 0.11 | 34 | 0.11 | – | 0.177(15.1) | – | |||||
| kout (h−1) | 1.3 | 1 | 18 | 2.09 | – | 2.46(5.93) | – | |||||
| KI (mg l−1 | 0.0687(21.3) | 48(24) | 0.111(15.7) | 54(23) | ||||||||
| kout (h−1) | 0.803(10.5) | 20(19.7) | 1.19 (9.8) | 25(29) | 0.664 | |||||||
| kDA (h−1) | 0.134(8.9) | – | 0.145(8.4) | – | 0.156 | |||||||
| γ | 2.31(6.3) | – | 1.86 (9.8) | – | 1.44 | |||||||
| AMP1† | 0.250(44.4) | – | 0.192(42) | – | 0.532 | |||||||
| PHS1† (h) | 5.20(24) | – | 20.8 (1.5) | – | 20.1 | |||||||
| AMP2† | −0.563(16.8) | – | −0.32(22.9) | – | −0.314 | |||||||
| PHS2† (h) | −0.872(20.1) | – | 1.5(1.3) | – | 1.7 | |||||||
| Number of typical values | 6 | 4 | 8 | 5 | 9 | |||||||
| Number of IIV parameters | 5 | 2 | 2 | 3 | 3 | |||||||
| Residual variability | ||||||||||||
| Additive error (µgl−1) | 1.77 | 2.74 | 3.05(19) | 2.41(34.4) | 2.70(26.4) | |||||||
| Proportional error (%) | 19.7 | 19 | 15.4(10.8) | 18.6(9.8) | 16.7(12) | |||||||
| OFV | 1568.5 | 1651.8 | 1479.6 | 1566.9 | 1498.6 | |||||||
The values calculated from mass-balance equations.
08.00 h was used as reference time.
Reported values are for those estimated in healthy males.
When a circadian function was incorporated into the pool model with mass-balance, OFV reduced significantly (P < 0.001), from 1651.8 to 1479.6. An on average higher baseline of prolactin concentration in plasma, prolactin synthesizing rate, rate of prolactin release from pool compartment to plasma compartment, prolactin elimination rate and stronger stimulation were estimated by the circadian pool model. Residual variability was reduced by inclusion of the circadian sub-model, from 19% to 15%.
AAI model and circadian AAI model
The circadian sub-model also reduced the OFV of the AAI model, from 1566.9 to 1498.6 when it was added on prolactin release. A sensitivity analysis was conducted on the baseline of dopamine concentration, CDA,0, which was fixed to 10 000. When CDA,0 was larger than 100, it had no significant influence on OFV and parameter estimation. When CDA,0 was larger than 10 000 NONMEM could not complete the computation successfully. The baseline prolactin concentrations in plasma estimated by the AAI model and the circadian AAI model were similar, 9.2 µg l−1 and 8.8 µg l−1, respectively. The parameter that changed the most between the AAI model and the circadian AAI model was the remoxipride potency parameter, KI. KI in the AAI model, 0.0687 mg l−1, was much lower than in the circadian AAI model (0.111 mg l−1). In these two models, KI was estimated to have a relatively large IIV, close to 50%. As for the pool model, addition of a circadian sub-model reduced the residual error.
Pool model and AAI model comparison
OFV reflects how well the model fits the data. When mass-balance was preserved but no circadian rhythm function was allowed, the AAI model fitted the data better than the pool model. When circadian rhythm was allowed, the pool model with a circadian submodel explained the relationship between remoxipride concentration and prolactin release slightly better than the AAI model with a circadian rhythm based on OFV (ΔOFV = 19). The amplitude and average level of the circadian prolactin predicted profile in the absence of drug were clearly larger and higher for the pool model than for the AAI model, but peak times were similar (Figure 3). Peaks were predicted to occur close to 04.00 h and 16.00 h, while troughs occurred around noon and evening (Figure 3).
Figure 3.
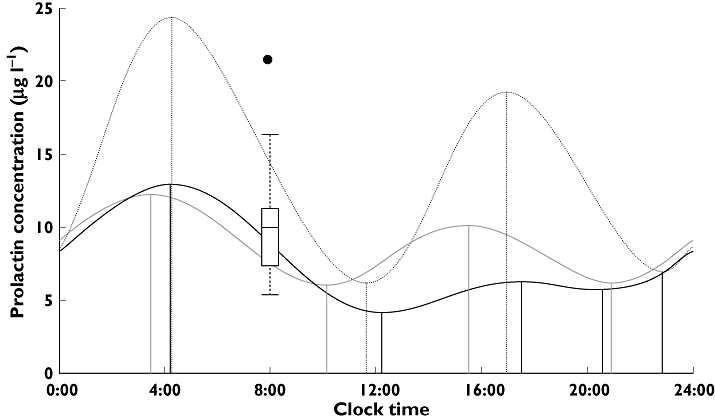
Daily variations in prolactin concentrations in the absence of drug predicted by the circadian agonist-antagonist interaction (AAI) model and the circadian pool model in the remoxipride data set or the risperidone and paliperidone data set [4]. The box-and-whisker plot represents the distribution of the measured prolactin concentrations at 08.00 h before treatment started. Circadian AAl (risperidone and paliperidone) ( ); Circadian AAl (remoxipride) (
); Circadian AAl (remoxipride) ( ); Circadian pool (remoxipride) (
); Circadian pool (remoxipride) ( )
)
The VPCs performed to inspect the simulation ability of these two models revealed that they were equivalent to predict prolactin concentrations (Figure 4). For a perfect model approximately 60% of the observations are expected to fall outside the 40% prediction intervals. On all five occasions, the observations outside of the 40% prediction intervals were close to 60% for these two models. A common shortcoming of these two models revealed by the NPC is that the percentages of points below the prediction interval are larger than those above the prediction interval (Figure 4). The NPC of the pharmacokinetic model (Figure 2) is also asymmetrical, which indicates the prediction bias may come from the pharmacokinetic model.
Figure 4.
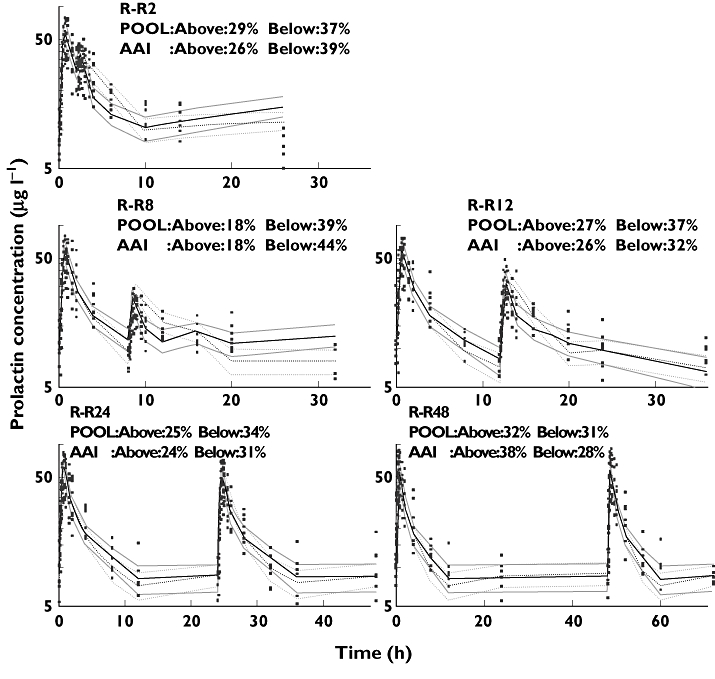
Numerical predictive checks (NPC) and visual predictive checks (VPC) for the circadian pool model and the circadian agonist-antagonist interaction (AAI) model with 40% prediction interval (PI). R-R2 to R-R48 are the five different occasions. R is first dose; R2, R8, R12, R24, and R48 indicate that second doses were given at 2, 8, 12, 24 and 48 h, respectively. Time 0 is 08.00 h. Above and Below indicate the percentages of data points above and below the prediction interval, respectively. Circadian pool model, POOL ( ); Median prediction (
); Median prediction ( ); Circadian AAl model, AAl (
); Circadian AAl model, AAl ( ); Median prediction (
); Median prediction ( )
)
Discussion
In the present study, two PK/PD models for prolactin release after antipsychotic drug administration were compared for the remoxipride data set from which the pool model was originally developed. This data set is particularly informative on the tolerance aspects of remoxipride on prolactin release. When the first and second doses are given with only a 2 h interval, tolerance is pronounced. With increasing dose intervals, the tolerance gradually disappears until, at a 48 h interval, no tolerance can be observed. By incorporating observations across all hours of the day, the design was also informative on the circadian rhythms of prolactin release.
The pool model incorporates the synthesis of prolactin, a prolactin pool and prolactin release into and elimination from blood. A linear relationship was used to describe the increased prolactin release from the anterior pituitary because of the drug-induced reduction in dopamine inhibition [2] and the pool model is based on the assumption that the tolerance development of prolactin release observed after repeated administration is caused by depletion of the prolactin storage pool. Although the circadian sub-model improved the fit of the pool model, in the absence of drug, the circadian rhythm predicted by the pool model was not in agreement with relative magnitudes of trough and peak values earlier described in healthy volunteers (approximately 40% and 160%, respectively, of the 24 h mean value) [11]. In addition, the absolute typical values in this population was predicted to be above the laboratory reference values of 2.1–17.7 ng ml−1 for healthy males [12] during parts of the day.
AAI models extended the dopamine-prolactin relationship from a linear equation to a dopamine-prolactin feedback loop supported by the literature [3]. Three critical concepts contribute to this feedback loop: 1) prolactin release is inhibited by dopamine; 2) antipsychotic drugs block dopamine to bind at dopamine2 receptor; 3) prolactin stimulates the production of dopamine. In AAI models, the tolerance is described by a drug-dopamine agonist-antagonist interaction mechanism to prolactin release. A further development was based on the circadian behaviour of prolactin [4].
The circadian AAI model to describe prolactin response was first implemented for risperidone and paliperidone in both patients and healthy volunteers [4]. KI following risperidone and paliperidone administration was estimated to 0.00196 mg l−1 (4.8 and 4.6 nmol l−1, respectively), while it was estimated to 0.111 mg l−1 (302 nmol l−1) for remoxipride in the present study. The lower potency parameter, KI, for risperidone and paliperidone indicates that the dopamine2 receptor is more effectively blocked by these drugs compared with remoxipride. The inhibition of the dopamine2 receptor by antipsychotic drugs is often quantified by KIrat, the inhibition constant measured from rat striatum. Paliperidone is a metabolite of risperidone and they have a similar capacity to inhibit the dopamine2 receptor [13–15]. The measured inhibition constants KIrat are 113 nmol l−1 and 1.4 nmol l−1 for remoxipride [16] and risperidone [17], respectively. The ratios between in vivo potency parameter from the AAI model and in vitro inhibition constant for these two drugs are 0.37 and 0.29, respectively, suggesting that the estimated in vivo potency parameter in the AAI model and the KIrat are correlated. Also, dopamine2-receptor occupancy of about 64% was achieved when dosing with risperidone 3–8 mg day−1[18] or paliperidone (extended release) 6 mg [19], while the occupancy was 73% when dosing with remoxipride 100 mg three times daily [20], i.e. for a daily total dose approximately 50 times higher than a typical paliperidone dose. The estimated KI was 57 times higher for remoxipride and thus in accordance with the difference in dose amount and the similar apparent clearance values [21, 22] for the compounds.
The other system-related parameters of the AAI models were similar across the two studies, except that kout and the amplitude of the first cosine function were here estimated to be approximately two and three times higher, respectively (Table 1). The elimination half-life of prolactin (ln(2)/kout) has been experimentally determined to 30 min in male healthy volunteers [23] and was here estimated to 35 min by the circadian AAI model. A similar value was found when healthy volunteers were allowed to have a different kout from patients in the risperidone and paliperidone analysis [4]. When mass balance was introduced kout increased in the pool model resulting in an estimated half-life of 17–21 min.
The predicted prolactin concentrations and circadian rhythm should in the absence of drug be independent of which antipsychotic drug is administered. Although the parameters of the two circadian AAI models for healthy volunteers were estimated based on different drugs, different administration schedules and different experimental sites, Figure 3 reveals that the two prolactin profiles were similar. The peak prolactin plasma concentrations have been reported to occur 4 h after the onset of sleep [11], or 1–2 h before wakening [1], and similar values were predicted by both the circadian AAI model and the circadian pool model (Figure 3). The trough concentrations predicted by these two models were around 11.00 h, while they have earlier been reported to be observed around 6 h after wakening [11]. The observed peak prolactin concentration occurred at around 04.00 h [24], which was predicted by both models, while the circadian pool model predicted an additional high peak at 16.00 h. As discussed above, the noteworthy difference between the circadian pool model and the circadian AAI model was the predicted amplitude of peaks and troughs.
Based on the present study and the prolactin study on risperidone and paliperidone [4], it can be concluded that AAI models are more able to describe prolactin response to antipsychotic drugs than pool models. The major difference between these two types of models was the assumption on the key mechanism for observed tolerance development. The plausibility of a drug-dopamine feedback for prolactin response, also following a unique experimental design such as the one conducted in the remoxipride study, [25] was shown here. There was also a drawback of AAI models when compared with pool models. The computation times of circadian AAI models (63 h) were much longer than for circadian pool models (1 h) when the computation environment was CPU Intel(R) Xeon(TM) 2.80GHz, 2GB memory with G77 Fortran compiler.
In conclusion, the evaluation with NPC and VPC showed that the circadian pool model and the circadian AAI model were approximately equivalent in describing prolactin concentrations from the experimental study where two short infusions of remoxipride had been administered to challenge the development of tolerance. Prolactin circadian rhythm in healthy male volunteers was estimated by the circadian AAI model to be consistent with prolactin concentrations reported in the literature and with the previous analysis of prolactin from large clinical studies following placebo and risperidone/paliperidone treatment. The analysis presented here also showed that the circadian AAI model worked across drugs and study situations successfully. The estimation of prolactin rhythm by the AAI model supported the importance of a feedback mechanism to describe tolerance to prolactin release following antipsychotic drug administration. The circadian AAI model appeared promising to predict prolactin release across different antipsychotic drugs and across a wide range of administration schedules.
Acknowledgments
Lena Friberg would like to thank the Knut and Alice Wallenberg Foundation for financial support.
Competing interests
GM-O is an employee of AstraZeneca AB, Sweden. No other authors have competing interests to declare.
REFERENCES
- 1.Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–73. doi: 10.1016/s0920-9964(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 2.Movin-Osswald G, Hammarlund-Udenaes M. Prolactin release after remoxipride by an integrated pharmacokinetic-pharmacodynamic model with intra- and interindividual aspects. J Pharmacol Exp Ther. 1995;274:921–7. [PubMed] [Google Scholar]
- 3.Bagli M, Suverkrup R, Quadflieg R, Hoflich G, Kasper S, Moller HJ, Langer M, Barlage U, Rao ML. Pharmacokinetic-pharmacodynamic modeling of tolerance to the prolactin-secreting effect of chlorprothixene after different modes of drug administration. J Pharmacol Exp Ther. 1999;291:547–54. [PubMed] [Google Scholar]
- 4.Friberg LE, Vermeulen AM, Petersson KJ, Karlsson MO. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85:409–17. doi: 10.1038/clpt.2008.234. [DOI] [PubMed] [Google Scholar]
- 5.Movin-Osswald G, Hammarlund-Udenaes M, Von Bahr C, Eneroth P, Walton-Bowen K. Influence of the dosing interval on prolactin release after remoxipride. Br J Clin Pharmacol. 1995;39:503–10. doi: 10.1111/j.1365-2125.1995.tb04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francheteau P, Steimer JL, Dubray C, Lavene D. Mathematical model for in vivo pharmacodynamics integrating fluctuation of the response: application to the prolactin suppressant effect of the dopaminomimetic drug DCN 203–922. J Pharmacokinet Biopharm. 1991;19:287–309. doi: 10.1007/BF03036252. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Twenty-four-hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. J Clin Endocrinol Metab. 1990;71:1616–23. doi: 10.1210/jcem-71-6-1616. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit – a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins J, Karlsson MO, Jonsson E. Patterns and power for the visual predictive check. PAGE. 2006:15. Abstr 1028. [Google Scholar]
- 11.Frantz AG. Prolactin. N Engl J Med. 1978;298:201–7. doi: 10.1056/NEJM197801262980408. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler MJ. Infertility. In: Wild D, editor. The Immunoassay Handbook. 3rd edn. New York: Elsevier Ltd; 2005. p. 571. [Google Scholar]
- 13.Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 14.Leysen JE, Schotte A, Janssen PMF, Gommeren W, Van Gompel P, Lesage AS, De Backer MD, Luyten WHML, Amlaiky N, Megens AAHP. Interaction of new antipsychotics with neurotransmitter receptors in vitro and in vivo: pharmacological to therapeutic significance. In: Fog R, Gerlach J, Hemningsen R, editors. Schizophrenia, an integrated view. Munskgard, Copenhagen. Denmark: Alfred Benson Symposium. 1995;38:344–56. [Google Scholar]
- 15.Leysen JE, Janssen PM, Megens AA, Schotte A. Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994;55(Suppl):5–12. [PubMed] [Google Scholar]
- 16.Mohell N, Sällemark M, Rosqvist S, Malmberg Å, Högberg T, Jackson DM. Binding characteristics of remoxipride and its metabolites to dopamine D2 and D3 receptors. Eur J Pharmacol. 1993;238:121–5. doi: 10.1016/0014-2999(93)90515-j. [DOI] [PubMed] [Google Scholar]
- 17.Leysen JE, Janssen PM, Gommeren W, Wynants J, Pauwels PJ, Janssen PA. In vitro and in vivo receptor binding and effects on monoamine turnover in rat brain regions of the novel antipsychotics risperidone and ocaperidone. Mol Pharmacol. 1992;41:494–508. [PubMed] [Google Scholar]
- 18.Tauscher J, Kufferle B, Asenbaum S, Tauscher-Wisniewski S, Kasper S. Striatal dopamine-2 receptor occupancy as measured with [123I]iodobenzamide and SPECT predicted the occurrence of EPS in patients treated with atypical antipsychotics and haloperidol. Psychopharmacology (Berl) 2002;162:42–9. doi: 10.1007/s00213-002-1082-6. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson P, Dencker E, Nyberg S, Mannaert E, Boom S, Talluri K. Pharmacokinetics, dopamine D2 and serotonin 5-HT2A receptor occupancy and safety profile of paliperidone extended-release in healthy subjects. Schizophr Res. 2006;81:85–6. [Google Scholar]
- 20.Farde L, von Bahr C. Distribution of remoxipride to the human brain and central D2-dopamine receptor binding examined in vivo by PET. Acta Psychiatr Scand Suppl. 1990;358:67–71. doi: 10.1111/j.1600-0447.1990.tb05292.x. [DOI] [PubMed] [Google Scholar]
- 21.Movin-Osswald G, Hammarlund-Udenaes M. Remoxipride: pharmacokinetics and effect on plasma prolactin. Br J Clin Pharmacol. 1991;32:355–60. doi: 10.1111/j.1365-2125.1991.tb03911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeulen A, Piotrovsky V, Ludwig EA. Population pharmacokinetics of risperidone and 9-hydroxyrisperidone in patients with acute episodes associated with bipolar I disorder. J Pharmacokinet Pharmacodyn. 2007;34:183–206. doi: 10.1007/s10928-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DS, Ridgway EC, Kliman B, Kjellberg RN, Maloof F. Metabolic clearance and production rates of prolactin in man. J Clin Invest. 1979;64:1669–80. doi: 10.1172/JCI109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 25.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]


