Abstract
AIMS
Dalcetrapib, which targets cholesteryl ester transfer protein activity, is in development for prevention of cardiovascular events. Because dalcetrapib will likely be prescribed with other lipid-modifying therapies such as ezetimibe, a study was performed to investigate potential pharmacokinetic interactions between dalcetrapib and ezetimibe. Lipids changes and tolerability were secondary endpoints.
METHODS
Co-administration of dalcetrapib 900 mg (higher than the phase III dose) with ezetimibe was investigated in a three period, three treatment crossover study in healthy males: 7 days of dalcetrapib, 7 days of dalcetrapib plus ezetimibe, 7 days of ezetimibe alone. A full pharmacokinetic profile was performed on day 7 of each treatment.
RESULTS
Co-administration of dalcetrapib with ezetimibe was associated with minimal changes in dalcetrapib exposure compared with dalcetrapib alone. Least squares mean ratio (LSMR) (90% confidence interval) was 93.6 (87.1, 100.7) for AUC(0,24 h) and 99.0 (85.2, 115.0) for Cmax. Ezetimibe exposure was reduced with co-administration of ezetimibe with dalcetrapib compared with ezetimibe alone: LSMR 80.3 (74.6, 86.4) for AUC(0,24 h) and 88.9 (80.9, 99.9) for Cmax for total ezetimibe. High-density lipoprotein cholesterol increases associated with co-administration of dalcetrapib with ezetimibe (+29.8%) were comparable with those with dalcetrapib alone (+25.6%), while the reduction in low-density lipoprotein cholesterol with co-administration (−35.9%) was greater than with ezetimibe alone (−20.9%). Dalcetrapib was generally well tolerated when administered alone and when co-administered with ezetimibe.
CONCLUSION
Co-administration of dalcetrapib with ezetimibe was not associated with clinically significant changes in pharmacokinetic parameters or tolerability and did not diminish the lipid effects of either drug.
Keywords: cholesteryl ester transfer protein, dalcetrapib, drug–drug interactions, ezetimibe
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Dalcetrapib, which targets cholesteryl ester transfer protein, is currently in phase III development to evaluate its effect on the prevention of cardiovascular events. It will likely be co-administered with other lipid-modifying drugs such as the cholesterol absorption inhibitor ezetimibe. There are currently no studies on the co-administration of dalcetrapib with ezetimibe.
WHAT THIS STUDY ADDS
This study showed no clinically relevant pharmacokinetic interactions when dalcetrapib was co-administered with ezetimibe. The effect of dalcetrapib on raising high-density lipoprotein cholesterol was not compromised, and there was an additive effect on low-density lipoprotein cholesterol lowering with co-administration of dalcetrapib with ezetimibe compared with ezetimibe alone. Dalcetrapib alone or co-administered with ezetimibe was not associated with an increased rate of adverse events compared with ezetimibe alone.
Introduction
As current standard of care reduces the risk of cardiovascular disease (CVD) by 30–40%, a sizeable risk of CVD remains [1]. Low-density lipoprotein cholesterol (LDL-C) lowering with statins, the current standard of care, leads to reductions in CVD morbidity and mortality [2]. Ezetimibe, a cholesterol absorption inhibitor, has also been associated with reduced concentrations of LDL-C when administered either alone or in combination with a statin [3, 4].
Increasing the level of high-density lipoprotein cholesterol (HDL-C) is an alternative way to reduce the residual risk that is not adequately addressed by LDL-C lowering. Pharmacological interventions to raise HDL-C concentrations have been associated with improved CVD outcomes in clinical trials [5–7]. One potential way to increase HDL-C is by inhibiting the activity of cholesteryl ester transfer protein (CETP), as decreased plasma concentrations of CETP have been associated with increased HDL-C [8]. Dalcetrapib, an agent that acts on CETP, is currently in development and has been shown to increase HDL-C by >30% in phase II trials [9, 10].
Clinical development of torcetrapib, the first inhibitor of CETP activity to enter extensive clinical evaluation, was terminated when an increase in cardiovascular events and all-cause mortality was observed compared with atorvastatin alone in the phase III Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial [11]. This effect of torcetrapib is believed to be due, in part, to an increase in blood pressure and electrolytes (serum sodium and bicarbonate), via a compound specific off-target effect on the renin-angiotensin-aldosterone system, and not due to inhibition of CETP. In light of this, it is important to consider whether dalcetrapib has any clinically significant effects on blood pressure and electrolytes. Dalcetrapib, which interacts with cysteine 13 of CETP [12, 13], is structurally dissimilar to torcetrapib and anacetrapib, another potent CETP inhibitor. Dalcetrapib, at a dose of 900 mg day−1, 50% higher than is being used in ongoing clinical development, has shown no clinically relevant effects on mean blood pressure up to 48 weeks' treatment or clinically significant changes in electrolytes or aldosterone concentrations [14].
Because dalcetrapib will likely be prescribed in combination with other lipid-modifying therapies, including statins and agents such as ezetimibe, an understanding of its metabolism and the potential for drug–drug interactions is essential. Dalcetrapib is metabolized through multiple pathways, including hydrolysis, glucuronidation, oxidation and methylation (Report no. JTT705-AD-003, data on file, F. Hoffmann-La Roche Ltd, Basel, Switzerland), with the most prominent degradation products detected in human plasma being the glucuronide and methyl metabolites of dalcetrapib, both of which are inactive [15, 16]. A number of studies have shown that dalcetrapib has no clinically significant effect on the activity of any of the major cytochrome P450 (CYP) isoforms, including CYP3A4, and no clinically relevant drug–drug interactions when co-administered with pravastatin, rosuvastatin or simvastatin [17, 18]. Unlike most of the statins, ezetimibe is not metabolized via the CYP pathway [19] but undergoes extensive conjugation in the intestine and liver, forming the pharmacologically active ezetimibe-glucuronide metabolite [20, 21]. Therefore, this study was performed to investigate potential drug–drug interactions when dalcetrapib is co-administered with ezetimibe.
The primary pharmacokinetic endpoints were area under the concentration–time curve [AUC(0,24 h)] and maximum plasma concentration (Cmax) for dalcetrapib and ezetimibe. Effects on lipids and safety were secondary endpoints. Dalcetrapib was administered at a dose of 900 mg, which was used in earlier studies [9, 10, 14] but is higher than the 600 mg dose chosen for the Phase III dal-OUTCOMES study [22] (ClinicalTrials.gov identifier: NCT00658515).
Methods
Study population
This drug–drug interaction study was performed in healthy male subjects, aged 18–65 years inclusive with body mass index 18–30 kg m−2. Exclusion criteria included clinically significant symptoms of infectious disease, known history of porphyria, myopathy, active liver disease, use of concomitant medication except paracetamol, clinically relevant history of drug or alcohol misuse or abuse, alcohol intake greater than approximately 21 units per week, and positive drugs of abuse test at screening.
The study was conducted in compliance with the principles of the Declaration of Helsinki and performed according to Good Clinical Practice Guidelines. All subjects provided written informed consent. The protocol was reviewed by an independent ethics committee, Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale d'Alsace, Strasbourg, France.
Study medication
Dalcetrapib (300 mg tablets) was provided by Clinical Trial Supplies, F. Hoffmann-La Roche Ltd (Basel, Switzerland) in accordance with Roche standards and local regulations. Ezetimibe (10 mg tablets) was purchased locally by the Roche Clinical Pharmacology Unit (Strasbourg, France).
Study design
The study was a randomized, open label, crossover study with three 7 day treatment periods: dalcetrapib 900 mg alone, dalcetrapib 900 mg plus ezetimibe 10 mg, ezetimibe 10 mg alone. There was a washout period between treatments of 10–14 days (Figure 1).
Figure 1.
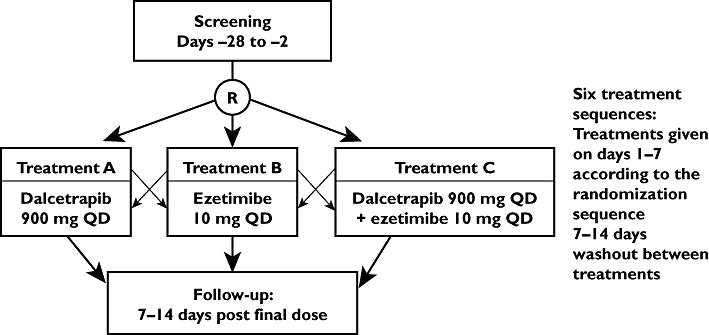
Study design. R, randomization; QD, once daily
Screening was performed between 28 and 2 days prior to dosing and involved a full medical history and complete physical examination including electrocardiogram (ECG), vital signs and clinical laboratory tests. On day −1, blood and urine samples were collected for laboratory safety tests, a medical re-evaluation was performed, and tests were performed for drugs of abuse. These included urinary cotinine and an alcohol breath analyzer test. Clinical assessments performed during the study included measurement of vital signs, ECG, and blood sampling for CETP mass, CETP activity, plasma lipid profile and pharmacokinetic measurements. Full pharmacokinetic profiles were determined on day 7 of each treatment period.
Pharmacokinetic assessments
The primary pharmacokinetic parameters, AUC(0,24 h) and Cmax for dalcetrapib and for ezetimibe at steady state (day 7), were determined from venous blood samples. Plasma samples were treated with dithiothreitol for thiolysis and, after the formation of the N-ethylmaleimide derivative, the concentration of dalcetrapib active form was determined using liquid chromatography-tandem mass spectrometry by Swiss BioAnalytics AG (Birsfelden, Switzerland). The precision of the assay, as determined from the analysis of quality control samples, was ≤7.9% for dalcetrapib active form. The accuracy of the assay ranged from 93.3% to 103.7% for dalcetrapib active form. The lower limit of quantification was 5.00 ng ml−1.
Plasma concentrations for ezetimibe were determined by Anapharm Inc. (Quebec, Canada) using a validated liquid chromatography-tandem mass spectrometry method. The precision of this assay, as determined from the analysis of quality control samples, was ≤6.8% for unconjugated ezetimibe (parent compound) and ≤4.4% for total ezetimibe (parent and conjugated ezetimibe). The accuracy of the assay ranged from 100.2–109.1% for unconjugated ezetimibe and from 97.8–101.6% for total ezetimibe. The lower limit of quantification was 39.9 pg ml−1 for unconjugated and 201 pg ml−1 for total ezetimibe. Conjugated ezetimibe, which was assumed to be ezetimibe glucuronide, was calculated from the measured plasma concentrations of total ezetimibe and unconjugated ezetimibe (conjugated ezetimibe = total ezetimibe – unconjugated ezetimibe).
Pharmacodynamic assessments
Pharmacodynamic parameters included CETP activity, CETP mass and fasting lipid profiles (HDL-C, LDL-C, total cholesterol, triglycerides and very low-density lipoprotein cholesterol). Analysis of CETP activity and CETP mass was done by Pacific Biometrics Inc. (Seattle, WA, USA). Lipid profile analysis was performed by Eurofins Medinet BV (Breda, the Netherlands).
Safety assessments
Safety assessments included monitoring for adverse events (AEs), laboratory tests, vital signs including blood pressure and pulse rate and 12-lead ECG measurements. For AEs, the investigator determined and recorded the intensity of the AE (mild, moderate or severe) as well as its relationship to study treatment (probable, possible, remote or unrelated). Laboratory tests included haematology, electrolytes, liver enzymes, coagulation, urinalysis, alcohol breath test and tests for drugs of abuse.
Statistical methods
A sample size of 18 was selected to ensure that the AUC(0,24 h) and Cmax for dalcetrapib and for ezetimibe would show co-efficients of variation below 25%. This sample size would ensure approximately 90% power for the 90% confidence interval (CI) for a given parameter to not vary by more than 33% of the true value. According to guidance from the US Food and Drug Administration, least squares mean ratios (LSMRs) of combination therapy to single therapy along with 90% CIs were used [23]. Analysis of variance was used to determine differences in pharmacokinetic parameters between the treatments. No formal statistical modelling was applied to pharmacodynamic or safety parameters.
Results
Baseline demographic characteristics
A total of 27 healthy male participants were randomized, and 22 participants completed the study. A total of five participants were withdrawn for the study: three due to AEs and two due to protocol violations (consumption of alcohol and positive drugs of abuse test, respectively). Baseline demographic characteristics for the participants are shown in Table 1. The study was conducted from 24 January 2006 to 29 March 2006.
Table 1.
Baseline characteristics
| Characteristic | All periods |
|---|---|
| n | 27 |
| Gender, male, n (%) | 27 (100) |
| Race: | |
| White, n (%) | 25 (93) |
| Asian, n (%) | 2 (7) |
| Age (years) mean ± SD | 30.7 ± 8.7 |
| Weight (kg) mean ± SD | 73.6 ± 11.2 |
| Height (cm) mean ± SD | 177.5 ± 6.4 |
| Body mass index (kg m−2) mean ± SD | 23.3 ± 3.0 |
Pharmacokinetic results
Effect of co-administration with ezetimibe on the pharmacokinetic profile of dalcetrapib
No significant change in dalcetrapib exposure was observed with co-administration of ezetimibe compared with dalcetrapib alone. The LSMRs (90% CI) for AUC(0,24 h) and Cmax for co-administration vs. dalcetrapib alone were 93.6 (87.1 100.7) and 99.0 (85.2, 115.0), respectively (Table 2). The median concentration–time profile for dalcetrapib 900 mg co-administered with ezetimibe is shown in Figure 2A.
Table 2.
Effect of co-administration of ezetimibe with dalcetrapib on the pharmacokinetic parameters of dalcetrapib and ezetimibe and its metabolites
| Analyte/parameter | Single therapy | Combination therapy | Least squares mean ratio* (%) | 90% CI |
|---|---|---|---|---|
| Dalcetrapib | ||||
| AUC(0,24 h) (ng ml−1 h) | 12 100 ± 3120 | 11 500 ± 3500 | 93.6 | 87.1, 100.7 |
| Cmax (ng ml−1) | 1 670 ± 515 | 1 660 ± 526 | 99.0 | 85.2, 115.0 |
| Total ezetimibe | ||||
| AUC(0,24 h) (ng ml−1 h) | 682 ± 380 | 542 ± 272 | 80.3 | 74.6, 86.4 |
| Cmax (ng ml−1) | 91.9 ± 46.2 | 80.2 ± 32.5 | 89.9 | 80.9, 99.9 |
| Conjugated ezetimibe | ||||
| AUC(0,24 h) (ng ml−1 h) | 612 ± 359 | 474 ± 255 | 77.5 | 72.3, 83.2 |
| Cmax (ng ml−1) | 86.2 ± 43.5 | 71.8 ± 29.9 | 85.4 | 77.3, 94.4 |
| Unconjugated ezetimibe | ||||
| AUC(0,24 h) (ng ml−1 h) | 69.1 ± 44.7 | 68.1 ± 28.9 | 104.9 | 91.9, 119.8 |
| Cmax (ng ml−1) | 6.20 ± 4.19 | 8.50 ± 5.18 | 134.5 | 111.4, 162.4 |
Ratio of combination therapy to single therapy.
AUC(0,24 h), area under the concentration–time curve; CI, confidence interval; Cmax, maximum plasma concentration.
Figure 2.
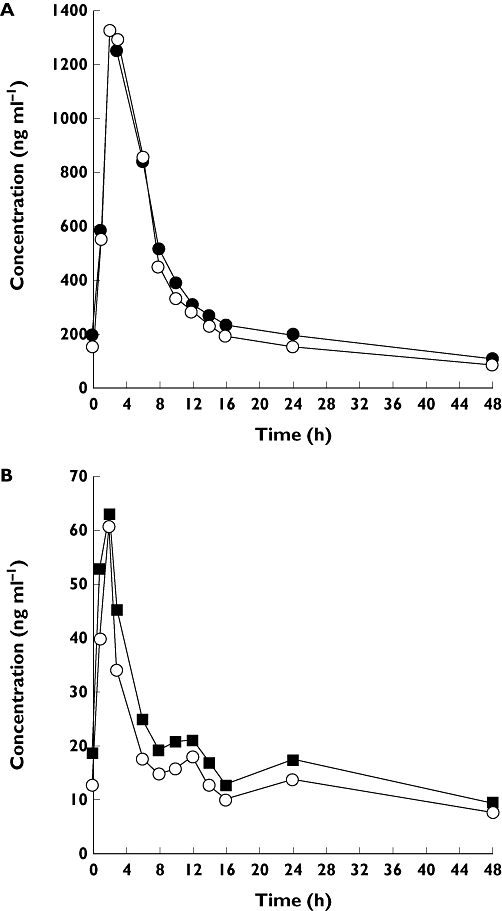
(A) Median dalcetrapib plasma concentrations following administration alone or in combination with ezetimibe. Dalcetrapib ( ); Dalcetrapib + ezetimibe (
); Dalcetrapib + ezetimibe ( ). (B) Median total ezetimibe plasma concentrations following administration alone or in combination with dalcetrapib. Ezetimibe (
). (B) Median total ezetimibe plasma concentrations following administration alone or in combination with dalcetrapib. Ezetimibe ( ); Ezetimibe + dalcetrapib (
); Ezetimibe + dalcetrapib ( )
)
Effect of co-administration with dalcetrapib on the pharmacokinetic profile of ezetimibe
Co-administration of ezetimibe with dalcetrapib resulted in reduced exposure of total ezetimibe. The LSMRs (90% CI) for AUC(0,24 h) and Cmax for total ezetimibe (co-administration vs. ezetimibe alone) were 80.3 (74.6, 86.4) and 89.8 (80.9, 99.9), respectively (Table 2, Figure 2B). The exposure of conjugated ezetimibe was also reduced by co-administration of dalcetrapib: the LSMRs (90% CI) for co-administration vs. ezetimibe alone were 77.5 (72.3, 83.2) and 85.4 (77.3, 94.4) for AUC(0,24 h) and Cmax, respectively (Table 2). Mean AUC(0,24 h) for unconjugated ezetimibe was unchanged by co-administration with dalcetrapib, whilst Cmax was increased compared with administration of ezetimibe alone: LSMR (90% CI) was 104.9 (91.9, 119.8) for AUC(0,24 h) and 134.5 (111.4, 162.4) for Cmax.
Pharmacodynamic results
Effect of co-administration of ezetimibe with dalcetrapib on CETP activity and mass
Dalcetrapib 900 mg was associated with a 37.8% decrease in CETP activity and a 44.6% increase in CETP mass when administered alone for 7 days (Table 3). The effect of dalcetrapib 900 mg on CETP activity and mass was comparable when administered alone or when co-administered with ezetimibe (Table 3). Administration of ezetimibe alone had little effect on CETP activity, but there was an 18.0% decrease in CETP mass following 7 days of ezetimibe alone (Table 3).
Table 3.
Effect of co-administration of dalcetrapib 900 mg with ezetimibe on cholesteryl ester transfer protein (CETP) activity and mass
| Mean ± SD CETP activity (%) | Mean ± SD CETP mass (µg ml−1) | |||||
|---|---|---|---|---|---|---|
| Dalcetrapib 900 mg alone | Dalcetrapib 900 mg + ezetimibe | Ezetimibe alone | Dalcetrapib 900 mg alone | Dalcetrapib 900 mg + ezetimibe | Ezetimibe alone | |
| n | 25* | 27† | 22 | 25* | 27† | 22 |
| Pre-dose day 1 | 84.5 ± 14.6 | 87.4 ± 14.2 | 85.0 ± 12.6 | 1.9 ± 0.4 | 1.9 ± 0.4 | 2.1 ± 0.4 |
| Day 7‡ | 54.8 ± 16.3 | 51.6 ± 12.2 | 87.7 ± 13.4 | 2.8 ± 0.8 | 2.8 ± 0.5 | 1.7 ± 0.3 |
| Percent change | −37.8 | −41.5 | 3.1 | 44.6 | 46.5 | −18.0 |
n = 24 at day 7.
n = 26 at day 7.
CETP activity measured 6 h post-dose; CETP mass measured pre-dose.
Effect of co-administration of ezetimibe with dalcetrapib on plasma lipids
There was no clinically significant change in the observed increase in HDL-C when dalcetrapib was co-administered with ezetimibe compared with dalcetrapib administered alone (Table 4). Co-administration of dalcetrapib with ezetimibe was associated with a greater reduction in LDL-C compared with ezetimibe alone. The reduction in mean LDL-C was 35.9% for ezetimibe co-administered with dalcetrapib compared with 20.9% for ezetimibe alone. When dalcetrapib 900 mg was co-administered with ezetimibe the reduction in total cholesterol was comparable to the reduction in total cholesterol with ezetimibe alone (13.1% vs. 14.3%). The reduction in total cholesterol with dalcetrapib administered alone was minimal (−1.24%) (Table 4).
Table 4.
Effect of co-administration of dalcetrapib 900 mg with ezetimibe on changes in plasma lipids
| Dalcetrapib 900 mg alone | Dalcetrapib 900 mg + ezetimibe | Ezetimibe alone | |
|---|---|---|---|
| n | 25* | 27† | 22 |
| HDL-C, mean ± SD (mg dl−1) | |||
| Day 1 | 48.3 ± 11.1 | 48.2 ± 9.8 | 49.1 ± 9.7 |
| Final day of dosing | 62.7 ± 12.8 | 62.7 ± 10.7 | 46.7 ± 9.0 |
| Percent change | 25.6 | 29.8 | −4.8 |
| LDL-C, mean ± SD (mg dl−1) | |||
| Day 1 | 102 ± 27.5 | 106 ± 30.5 | 108 ± 21.3 |
| Final day of dosing | 87.7 ± 26.3 | 70.2 ± 25.4 | 86.2 ± 22.6 |
| Percent change | −16.1 | −35.9 | −20.9 |
| nonHDL-C : HDL-C ratio, mean ± SD | |||
| Day 1 | 2.5 ± 0.7 | 2.6 ± 0.7 | 2.5 ± 0.7 |
| Final day of dosing | 1.7 ± 0.5 | 1.4 ± 0.4 | 2.1 ± 0.5 |
| Percent change | −32.5 | −46.6 | −14.1 |
| Total cholesterol (mg dl−1), mean ± SD | |||
| Day 1 | 164 ± 29.3 | 167 ± 33.1 | 168 ± 25.4 |
| Final day of dosing | 164 ± 31.4 | 146 ± 28.4 | 145 ± 28.0 |
| Percent change | −1.24 | −13.1 | −14.3 |
| Triglyceride, mean ± SD (mg dl−1) | |||
| Day 1 | 80.4 ± 39.3 | 79.7 ± 35.4 | 66.0 ± 21.6 |
| Final day of dosing | 69.0 ± 30.4 | 65.0 ± 22.4 | 72.8 ± 33.1 |
| Percent change | −10.1 | −13.2 | 6.87 |
| VLDL-C, mean ± SD (mg dl−1) | |||
| Day 1 | 13.4 ± 6.9 | 13.2 ± 4.0 | 11.2 ± 4.1 |
| Final day of dosing | 13.5 ± 5.2 | 13.5 ± 3.7 | 11.9 ± 5.2 |
| Percent change | 8.9 | 6.1 | 1.8 |
n = 24 at day 7.
n = 26 at day 7.
Percent change was calculated from day 1 (pre-dose) to day 7 (post-dose).
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
Safety
Dalcetrapib alone and in combination with ezetimibe was generally well tolerated (Table 5). Headache was the most commonly reported AE and more common with ezetimibe alone (23%) than with dalcetrapib alone (12%) or with dalcetrapib co-administered with ezetimibe (8%). The majority of the AEs were mild in intensity and were considered either unrelated or remotely related to treatment. There were no serious AEs.
Table 5.
Overview of adverse events (AEs)
| Dalcetrapib 900 mg alone | Dalcetrapib 900 mg + ezetimibe | Ezetimibe alone | |
|---|---|---|---|
| n | 25 | 26 | 22 |
| Participants with ≥1 AE, n (%) | 10 (40) | 7 (27) | 9 (41) |
| AEs, n | 13 | 9 | 12 |
| Participants with ≥1 severe AE, n (%) | 0 | 0 | 0 |
| Participants with ≥1 treatment-related AE*, n (%) | 5 (20) | 4 (15) | 6 (27) |
| Withdrawals due to AEs, n (%) | 2 (8)† | 1 (4) | 0 |
| Withdrawals due to treatment-related AEs*, n (%) | 0 | 0 | 0 |
Assessed as possibly or probably related to study treatment.
One of the two withdrawals occurred during washout after dalcetrapib alone.
No participants withdrew due to laboratory abnormalities. However, an elevated alanine aminotransferase (ALT) concentration was reported in one participant during the washout following administration of ezetimibe alone. The ALT concentration in this participant became a marked laboratory abnormality (≥110 U l−1 with a 50% increase from baseline) on study day 26, and peaked at 135 U l−1 on day 30, then gradually decreased to just outside the standard reference range on day 59 (56 U l−1). In addition, elevated bicarbonate concentrations were reported in three participants (two during co-administration of dalcetrapib plus ezetimibe and one during the washout following co-administration of dalcetrapib plus ezetimibe, during the administration of ezetimibe alone and at follow-up), and isolated elevated phosphate concentrations were reported in two participants during administration of dalcetrapib alone.
There was a low frequency of blood pressure changes [a total of seven diastolic blood pressure and systolic blood pressure elevations in five participants (Table 6)]. Two participants receiving ezetimibe alone had prolonged QTcB intervals (450–480 ms). Three participants were withdrawn due to abnormal ECG readings: one during combination treatment, one during the washout period following dalcetrapib and one during dalcetrapib administration.
Table 6.
Blood pressure elevations
| Participants with elevated blood pressure | |||
|---|---|---|---|
| Dalcetrapib 900 mg alone | Dalcetrapib 900 mg + ezetimibe | Ezetimibe alone | |
| n | 25 | 26 | 22 |
| Diastolic blood pressure >90 mmHg, n (%) | 0 | 1 (4) | 2 (9) |
| Systolic blood pressure >140 mmHg, n (%) | 2 (8) | 1 (4) | 1 (5) |
Discussion
Because dalcetrapib, an agent that acts on CETP, is being investigated for the prevention and regression of atherosclerosis and the prevention of CVD events, it will likely be co-administered with other lipid-modifying drugs such as ezetimibe. It is therefore important to determine whether there are any effects on pharmacokinetic parameters, pharmacodynamic parameters, or safety when dalcetrapib is co-administered with ezetimibe. Taken together, the pharmacokinetic, pharmacodynamic and safety results showed no clinically relevant drug–drug interactions between dalcetrapib and ezetimibe. The safety results supported that the combination of dalcetrapib and ezetimibe was well tolerated, and co-administration of dalcetrapib with ezetimibe was not associated with an increased rate of AEs compared with either drug alone. In addition, the incidence of blood pressure elevations was low, with no discernable difference between ezetimibe or dalcetrapib or their combination.
Dalcetrapib exposure was unchanged with co-administration of ezetimibe. The exposure of unconjugated ezetimibe as expressed by AUC(0,24 h) was unchanged by dalcetrapib co-administration, with 90% CIs within the default no-effect boundary of 80–125%. The Cmax for unconjugated ezetimibe was increased by 34.5%. In contrast, the exposure of conjugated ezetimibe was decreased by −22.5% and −10.1% for AUC(0,24 h), and Cmax, respectively. Consequently the exposure of total ezetimibe was significantly decreased with dalcetrapib co-administration, by −19.7% and −14.6% for AUC(0,24 h) and Cmax, respectively. Nonetheless, the effect of dalcetrapib-ezetimibe co-administration on lipid concentrations suggests these pharmacokinetic interactions have little clinical relevance. The rise in HDL-C associated with dalcetrapib alone (25.6% increase) was maintained by co-administration of dalcetrapib with ezetimibe (29.8% increase). Compared with either dalcetrapib or ezetimibe alone, co-administration had an additive effect on LDL-C lowering (16%, 21% and 36%, respectively). The 21% reduction in LDL-C with ezetimibe alone reported here is smaller than the 34% reduction reported with ezetimibe alone (10 mg) in a drug interaction study of 24 healthy men treated for 14 days [24], but consistent with the approximate 16–19% reductions reported in trials of patients with hypercholesterolaemia treated for 8–12 weeks with ezetimibe 10 mg [25, 26].
There are likely to be a number of reasons for the pharmacokinetic and pharmacodynamic findings of the current study. Dalcetrapib and ezetimibe are metabolized, at least in part, by glucuronidation in the gut and liver [15, 27]. However this is only one of a number of pathways by which dalcetrapib is degraded, whereas it appears to be the primary pathway for ezetimibe metabolism. Thus, competition for glucuronidation would be expected to have more of an impact on ezetimibe pharmacokinetics than on dalcetrapib pharmacokinetics, as suggested by the results of our study. Unlike dalcetrapib glucuronide, ezetimibe glucuronide is pharmacologically active and at least equipotent to the parent compound. However ezetimibe also has a flat dose–response curve [28] and therefore the impact of a modest, albeit statistically significant, decrease in exposure to the glucuronide in this case would translate into minimal clinical effects.
Unlike ezetimibe, statins, with the exception of pravastatin and rosuvastatin, are metabolized by CYP3A4. In studies in healthy subjects, no clinically relevant effects of dalcetrapib on any of the major CYP isoforms, including CYP3A4, have been observed [17]. Studies of dalcetrapib have shown no clinically significant drug–drug interactions when co-administered with pravastatin, rosuvastatin or simvastatin, and no decreases in the efficacy of dalcetrapib or statins on HDL-C or LDL-C, respectively, were observed [18]. In addition, effective LDL-C lowering was demonstrated in patients in phase II studies of dalcetrapib co-administered with atorvastatin, pravastatin or simvastatin [10, 14, 29]. Ezetimibe and statins lower LDL-C by different mechanisms, and co-administration of dalcetrapib with either drug resulted in enhanced decreases in LDL-C. Whilst published data on drug–drug interactions with torcetrapib are limited, anacetrapib, which is predominantly metabolized by CYP3A4 [30–32] does not appear to inhibit or induce CYP3A4 activity [33].
In conclusion, dalcetrapib co-administered with ezetimibe in healthy participants was not associated with any clinically significant changes in pharmacokinetic parameters or CETP activity. There was no change in the treatment effect of dalcetrapib towards increasing HDL-C when co-administered with ezetimibe and an additive effect on LDL-C reduction with ezetimibe. Dalcetrapib alone and in combination with ezetimibe was not associated with an increased incidence of AEs compared with ezetimibe alone.
Acknowledgments
Editorial assistance was provided by Prime Medica Inc., New York, NY and was funded by F. Hoffmann-La Roche Ltd.
Competing interests
MD and MA are employees of and own shares in F. Hoffmann-La Roche Ltd, Basel, Switzerland. MP is an employee of F. Hoffman La-Roche Ltd.
REFERENCES
- 1.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102(Suppl.):1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 3.Pandor A, Ara RM, Tumur I, Wilkinson AJ, Paisley S, Duenas A, Durrington PN, Chilcott J. Ezetimibe monotherapy for cholesterol lowering in 2722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265:568–80. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 4.Mikhailidis DP, Sibbring GC, Ballantyne CM, Davies GM, Catapano AL. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin. 2007;23:2009–26. doi: 10.1185/030079907x210507. [DOI] [PubMed] [Google Scholar]
- 5.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB, VA-HIT Study Group Veterans Affairs High-Density Lipoprotein Intervention Trial. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 6.Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2006;97:477–9. doi: 10.1016/j.amjcard.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg I, Goldbourt U, Boyko V, Behar S, Reicher-Reiss H, BIP Study Group Relation between on-treatment increments in serum high-density lipoprotein cholesterol levels and cardiac mortality in patients with coronary heart disease (from the Bezafibrate Infarction Prevention trial) Am J Cardiol. 2006;97:466–71. doi: 10.1016/j.amjcard.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 8.Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990;323:1234–8. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 9.de Grooth GJ, Kuivenhoven JA, Stalenhoef AF, de Graaf J, Zwinderman AH, Posma JL, van Tol A, Kastelein JJ. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 2002;105:2159–65. doi: 10.1161/01.cir.0000015857.31889.7b. [DOI] [PubMed] [Google Scholar]
- 10.Stein E, Stroes E, Steiner G, Buckley BM, Capponi AM, Burgess T, Niesor EJ, Kallend D, Kastelein JJ. Safety and tolerability of dalcetrapib. Am J Cardiol. 2009;104:82–91. doi: 10.1016/j.amjcard.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Yonemori F, Wakitani K, MInowa T, Maeda K, Shinkai H. A cholesterol ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–7. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]
- 13.Niesor EJ, von der Mark E, Brousse M, Maugeais C. lnhibition of cholesteryl ester transfer protein (CETP): different in vitro characteristics of RO4607381/JTT-705 and torcetrapib (TOR) Atherosclerosis. 2008;199:231. Abstract. [Google Scholar]
- 14.Stein EA, Roth EM, Rhyne JM, Burgess T, Kallend D, Robinson JG. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur Heart J. 2010;31:480–8. doi: 10.1093/eurheartj/ehp601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derks M, Busse-Reid R, Kuhlmann O, Paehler A. [14C]-dalcetrapib ADME following a single oral dose in healthy male subjects. Clin Pharmacol Ther. 2010;87:PI–47. [Google Scholar]
- 16.Derks M, Fowler S, Kuhlmann O. A single-center, open-label, one-sequence study of dalcetrapib coadministered with ketoconazole, and an in vitro study of the S-methyl metabolite of dalcetrapib. Clin Ther. 2009;31:586–99. doi: 10.1016/j.clinthera.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Derks M, Fowler S, Kuhlmann O. In vitro and in vivo assessment of the effect of dalcetrapib on a panel of CYP substrates. Curr Med Res Opin. 2009;25:891–902. doi: 10.1185/03007990902790928. [DOI] [PubMed] [Google Scholar]
- 18.Derks M, Abt M, Phelan M, Turnbull L, Meneses-Lorente G, Bech N, White A-M, Parr G. Coadministration of dalcetrapib with pravastatin, rosuvastatin, or simvastatin: no clinically relevant drug–drug interactions. J Clin Pharmacol. 2010 doi: 10.1177/0091270009358709. OnlineFirst, published on May 20, 2010 as doi: 10.1177/0091270009358709. [DOI] [PubMed] [Google Scholar]
- 19.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Simard C, Turgeon J. The pharmacokinetics of ezetimibe. Can J Clin Pharmacol. 2003;10(Suppl. A):13A–20A. [PubMed] [Google Scholar]
- 21.Oswald S, Haenisch S, Fricke C, Sudhop T, Remmler C, Giessmann T, Jedlitschky G, Adam U, Dazert E, Warzok R, Wacke W, Cascorbi I, Kroemer HK, Weitschies W, von Bergmann K, Siegmund W. Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate-glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin Pharmacol Ther. 2006;79:206–17. doi: 10.1016/j.clpt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJV, Shah PK, Tardif J-C, Chaitman BR, Duttlinger-Maddux R. Mathieson J on behalf of the dal-OUTCOMES Committees and Investigators. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901. doi: 10.1016/j.ahj.2009.09.017. e3. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: in vivo drug metabolism/drug interaction studies – study design, data analysis, and recommendations for dosing and labeling, 1999. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072119.pdf (last accessed 1 July 2009)
- 24.Kosoglou T, Meyer I, Veltri EP, Statkevich P, Yang B, Zhu Y, Mellars L, Maxwell SE, Patrick JE, Cutler DL, Batra VK, Affrime MB. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol. 2002;54:309–19. doi: 10.1046/j.1365-2125.2002.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, Lipka LJ, Lebeaut AP, Yang B, Mellars LE, Cuffie-Jackson C, Veltri EP, Ezetimibe Study Group Effectiveness and tolerability of ezetimibe in subjects with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–30. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 26.Knopp RH, Gitter H, Truitt T, Lipka LJ, LeBeaut AP, Suresh R, Veltri EP, The Ezitimibe Study Group Ezetimibe reduces low-density lipoprotein cholesterol: results of a Phase III, randomized, double-blind, placebo-controlled trial. Atheroscler Suppl. 2001;2:38. Abstract. [Google Scholar]
- 27.Schmitz G, Schmitz-Madry A, Ugocsai P. Pharmacogenetics and pharmacogenomics of cholesterol-lowering therapy. Curr Opin Lipidol. 2007;18:164–73. doi: 10.1097/MOL.0b013e3280555083. [DOI] [PubMed] [Google Scholar]
- 28.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–94. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kuivenhoven JA, de Grooth GJ, Kawamura H, Klerkx AH, Wilhelm F, Trip MD, Kastelein JJ. Effectiveness of inhibition of cholesteryl ester transfer protein by JTT-705 in combination with pravastatin in type II dyslipidemia. Am J Cardiol. 2005;95:1085–8. doi: 10.1016/j.amjcard.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Tan EY, Hartmann G, Biddle Z, Bergman AJ, Dru J, Ho JZ, Jones AN, Staskiewicz SJ, Braum MP, Karanam B, Dean DC, Gendrano IN, Graves MW, Wagner JA, Krishna R. Metabolism and excretion of anacetrapib, a novel inhibitor of the cholesterol ester transfer protein, in humans. Drug Metab Dispos. 2010;38:474–83. doi: 10.1124/dmd.109.028704. [DOI] [PubMed] [Google Scholar]
- 31.Krishna R, Bergman AJ, Jin B, Garg A, Roadcap B, Chiou R, Dru J, Cote J, Laethem T, Wang RW, Didolkar V, Vets E, Gottesdiener K, Wagner JA. Assessment of the CYP3A-mediated drug interaction potential of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy volunteers. J Clin Pharmacol. 2009;49:80–7. doi: 10.1177/0091270008326718. [DOI] [PubMed] [Google Scholar]
- 32.Garg A, Maes A, Corr C, Jin B, Wadhwa T, Handa N, Van Dyck K, De Lepeleire I, Shah J, Wagner JA, Krishna R. Effect of diltiazem, a moderate CYP3A inhibitor, on the pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein inhibitor, in healthy subjects. J Clin Pharmacol. 2010 doi: 10.1177/0091270010368676. OnlineFirst, published on April 23, 2010 as doi: 10.1177/009127001036867610.1177/0091270010368676. [DOI] [PubMed] [Google Scholar]
- 33.Krishna R, Garg A, Jin B, Keshavarz SS, Bieberdorf FA, Chodakewitz J, Wagner JA. Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2009;67:520–6. doi: 10.1111/j.1365-2125.2009.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


