Abstract
AIMS
The objective of this investigation was to assess the effect of aprepitant on the pharmacokinetics of high-dose melphalan used as conditioning therapy before blood stem cell transplantation in multiple myeloma.
METHODS
Aprepitant (125 mg) or placebo was administered 1 h before melphalan therapy (1 h infusion of 100 mg m−2). Eleven plasma samples were obtained over 8 h and melphalan was quantified using an LC/MS/MS method. Standard pharmacokinetic parameters were calculated and nonparametric testing was applied to assess the differences between aprepitant and placebo treatment.
RESULTS
Twenty patients received placebo and 10 patients aprepitant treatment. There were no differences observed for Cmax at the end of melphalan infusion (placebo 3431 ± 608 ng ml−1vs. aprepitant 3269 ± 660 ng ml−1). In addition, AUC and terminal elimination half-life were not changed by aprepitant. Total clearance of melphalan was 304 ± 58 ml min−1 m−2 (placebo) which was not influenced by aprepitant (288 ± 78 ml min−1 m−2).
CONCLUSIONS
The administration of the NK1 receptor antagonist aprepitant 1 h before a high-dose chemotherapy does not influence the exposure and the elimination of melphalan. Therefore, oral administration of 125 mg aprepitant 1 h before melphalan infusion does not alter the disposition of intravenously administered melphalan.
Keywords: aprepitant, interaction, melphalan
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Nausea and vomiting are the most distressing side-effects of a high dose melphalan regimen.
Aprepitant in addition to an antiemetic standard regimen has been reported to improve significantly both acute and delayed chemotherapy-induced nausea and vomiting.
WHAT THIS STUDY ADDS
Anti-emetic regimens including aprepitant have no clinically relevant effect on the pharmacokinetics of the anticancer agent melphalan when administered 1 h before high dose melphalan infusion.
Introduction
In patients with multiple myeloma high-dose chemotherapy with the nitrogen mustard alkylating agent melphalan followed by autologous peripheral stem cell transplantation is preferred to conventional therapy, since the superiority in respect to complete remission, complete remission duration, event-free survival and overall survival has been proven within well controlled clinical trials [1, 2]. In myeloablative regimens a melphalan dose of 100 mg m−2 on days 1 and 2 (1 h infusion) is recommended. Nausea and vomiting are the most distressing side-effects of a high-dose chemotherapy regimen. The administration of selective 5-HT3-receptor antagonists (5-HT3 RAs) in combination with a corticosteroid ( = conventional standard anti-emetic regimen) is effective for the prevention of those adverse effects in 70 to 80% of patients [3–5]. However, 20 to 30% of the patients still suffer from vomiting and nausea especially in the delayed phase of the chemotherapy. Superior protection could be achieved with the addition of aprepitant to this anti-emetic regimen in acute and delayed phases of these emetogenic chemotherapies. The enhanced anti-emetic protection of aprepitant can be maintained over multiple chemotherapy cycles to an extent superior to that of the standard regimen alone [6]. Furthermore addition of aprepitant to the standard anti-emetic regimen was generally well tolerated and the impact of chemotherapy-induced nausea and vomiting (CINV) on daily life was significantly reduced [7, 8].
Aprepitant is a selective high-affinity receptor antagonist of human substance P/neurokinin-1 (NK1) and has been shown to inhibit emesis induced by cytotoxic chemotherapeutic agents and augments the anti-emetic activity of 5-HT3 RAs (e.g. granisetron, ondansetron) and corticosteroids (e.g. dexamethasone). Thus aprepitant in addition to an anti-emetic standard regimen has been shown to possess powerful superior protection and has been reported in several clinical trials to improve significantly both acute and delayed CINV. The recommended dose of aprepitant according to the Summary of Product Characteristics (SPC) is 125 mg on day 1 and 80 mg on days 2 and 3. The aim of the main protocol was to evaluate an anti-emetic prevention regimen in acute and delayed emesis and nausea in respect to efficacy and safety in patients with multiple myeloma following high-dose melphalan chemotherapy and autologous peripheral blood stem cell transplantation. In a substudy with 30 patients the influence of aprepitant (125 mg) on the pharmacokinetics of high-dose chemotherapy with melphalan (100 mg m−2) was investigated. Given the improvement of aprepitant to inhibit emesis when added to the standard anti-emetic regimen, this study was conducted despite the lack of a mechanistic rationale for a possible pharmacokinetic interaction between the two drugs. A pharmacokinetic interaction of the standard anti-emetic regimen with the DNA alkylating and hence cytotoxic agent melphalan has not been investigated so far.
Methods
The clinical study (EudraCT 2004-004956-38) including amendments was approved by the University Hospital Ethics committee and the competent authority (BfArM). It was planned and conducted as a prospective, placebo-controlled, randomized (1 : 1), double-blind, single-centre study with two treatment groups (aprepitant or placebo) with a total of 360 patients. However, the substudy to assess the possible alteration of melphalan pharmacokinetics by aprepitant required a separate informed consent sheet. Aprepitant (125 mg) or matched placebo capsules were administered 1 h before melphalan therapy (1 h infusion of 100 mg m−2). The standard anti-emetic regimen of granisetron (Kevatril®) 2 mg once daily on days 1–4, dexamethasone 8 mg once daily on day 1 and 4 mg once daily on days 2 and 3 was applied with placebo treatment, in the aprepitant arm dexamethasone was reduced to 4 mg on day 1 and 2 mg on days 2 and 3, respectively [9]. Previously it has been shown that aprepitant had no effect on the pharmacokinetics of granisetron [10]. In 30 of these patients eleven blood samples were obtained over 8 h, plasma was separated within 15 min and stored at −20°C until analysis. Melphalan was quantified in plasma using a validated LC/MS/MS method [11]. The limit of quantification (LOQ) for melphalan was 10 ng ml−1 with an accuracy of 106.6% and precision of 8.2% (CV). Standard pharmacokinetic parameters were calculated using WinNonlin 5.2 (Pharsight, Mountain View, CA). The unpaired Mann-Whitney test was applied to assess the differences between aprepitant and placebo treatment (Prism 5.00, GraphPad Software, La Jolla, CA). Mean, standard deviations and 95% confidence intervals (CI) of the difference of the means are reported.
Results
In the main study patients were randomized 1 : 1 to placebo and aprepitant. Consecutive patients were asked to participate also in the pharmacokinetic substudy and had to give separate informed consent. Hence, 30 (non-consecutive) patients from the main study were finally included in the substudy with a resulting imbalance of the group size with 20 patients receiving placebo and 10 patients aprepitant treatment (Table 1).
Table 1.
Demographic data of the 30 patients included in the study
| Placebo | Aprepitant | ||||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | P value | |
| Age (years) | 62.1 | 39–71 | 57.4 | 40–69 | NS* |
| Weight (kg) | 79.3 | 59.7–108.0 | 80.7 | 61.0–99.6 | NS* |
| Male | 13 | 6 | NS† | ||
| Female | 7 | 4 | |||
Mann–Whitney U-test.
Chi-square with Yates' correction. NS not significant.
There were no differences observed for Cmax at the end of melphalan infusion (placebo 3431 ± 608 ng ml−1vs. aprepitant 3269 ± 660 ng ml−1). No obvious differences in the measured melphalan plasma concentrations were observed between aprepitant and placebo (see Figure. 1). In addition, AUC and terminal elimination half-life were not significantly changed by aprepitant (Table 1). Total clearance of melphalan was 304 ± 58 ml min−1 m−2 (placebo) which was not influenced by aprepitant (288 ± 78 ml min−1 m−2) (Table 2). In addition, all 95% CIs of the difference between means of the placebo and aprepitant group were narrow and included zero (Table 2).
Figure 1.
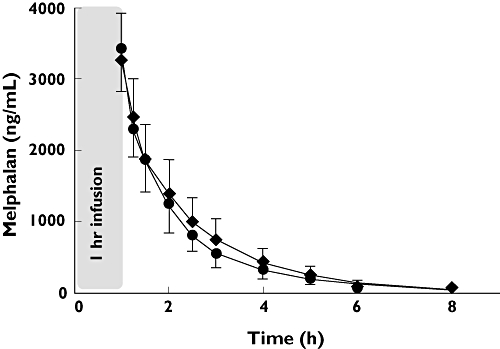
Plasma concentration–time profile of melphalan after a 1 h infusion of 100 mg m−2 in patients with concomitant aprepitant (n = 10) or placebo (n = 20). Placebo ( ); Aprepitant (
); Aprepitant ( )
)
Table 2.
Pharmacokinetic parameters of melphalan after intravenous administration of 100 mg m−2 over 1 h with co-administration of aprepitant (125 mg) or placebo
| Aprepitant (n = 10) | Placebo (n = 20) | Mann–Whitney test | Difference of means (95% CI) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value | ||
| t1/2 (h) | 1.28 | 0.19 | 1.30 | 0.18 | 0.9825 | −0.022 (−0.166, 0.123) |
| tmax (h) | 0.04 | 0.03 | 0.03 | 0.03 | 0.3811 | 0.008 (−0.14, 0.029) |
| Cmax (ng ml−1) | 3431 | 608 | 3269 | 660 | 0.5234 | 163 (−348, 673) |
| AUC (ng ml−1 h) | 5711 | 1342 | 6213 | 1724 | 0.5526 | −501 (−1779, 777) |
| AUCextrapolated (%) | 1.84 | 1.94 | 2.21 | 2.00 | 0.5526 | − |
| Vz (l m−2) | 33.3 | 6.8 | 32.1 | 8.2 | 0.5824 | 1.24 (−4.96, 7.43) |
| CL (l h−1 m−2) | 18.2 | 3.5 | 17.3 | 4.7 | 0.5526 | 0.92 (−2.53, 4.37) |
| MRT (h) | 1.57 | 0.24 | 1.75 | 0.25 | 0.0903 | −0.174 (−0.371, 0.024) |
| Vss (l m−2) | 28.3 | 5.1 | 29.5 | 6.6 | 0.6760 | −1.28 (−6.18, 3.64) |
t1/2 terminal elimination half-life, tmax time where the maximum plasma concentration after the end of infusion was observed, Cmax maximum plasma concentration, AUC area under the plasma concentration time curve from zero to infinity, AUCextrapolated, percentage of AUC due to extrapolation from the last measured concentration to infinity, Vz volume of distribution based on the terminal phase, CL total body clearance, MRT mean residence time, Vss, volume of distribution at steady-state.
Discussion
Aprepitant, an orally available, selective NK1 receptor antagonist is mainly metabolized by CYP3A4 and was shown to be a moderate inhibitor and weak and transient inducer of CYP3A4 [12]. Cytochrome P450 3A4 (CYP3A4) is abundantly expressed in the liver and small intestine and involved in the metabolism of numerous exogenous compounds, including anticancer agents. Several cytotoxic agents like cyclophosphamide and thiotepa are known to be metabolized by CYP3A4. It has been shown that aprepitant inhibits metabolism of these two agents [13] though others stated that no clinically relevant interaction has been observed [14, 15]. In addition no detectable inhibitory or inductive effect on the pharmacokinetics of the CYP3A4 substrate vinorelbine was observed, when aprepitant was added to an anti-emetic regimen consisting of ondansetron and dexamethasone [16]. Aprepitant at clinically recommended doses may therefore have a low potential to affect the pharmacokinetics of intravenous chemotherapeutic agents metabolized by CYP3A4. This view has recently been confirmed by a review on aprepitant drug–drug interactions [17].
Melphalan, however, is not reported to be a substrate of CYP3A4. It is therefore unlikely that a drug–drug interaction between melphalan and aprepitant might occur on the basis of CYP3A4 inhibition. Recently it was suggested, that melphalan is a substrate of the P-glycoprotein transporter [16, 18]. P-glycoprotein plays a major role in the renal elimination of digoxin. However, no effect of aprepitant on the pharmacokinetics of the P-glycoprotein model drug digoxin has been observed [19]. A limitation of this study was the use of oral digoxin. In order to avoid major intestinal influences intravenous digoxin should have been used [20]. Other influx drug transporters of melphalan have also been investigated in vitro but no correlation between expression of any influx transporters (LAT1, LAT2, TAT1) and melphalan toxicity has been found [18].
The observed melphalan pharmacokinetics are well in agreement with the previously known data after high-dose intravenous melphalan [21]. In the 30 patients investigated the coefficient of variation of the obtained pharmacokinetic parameters (Table 1) was always below 30% showing relatively low variability.
Due to the study design there are several limitations. This was not a classical drug–drug interaction study with cross-over design in healthy volunteers where all conditions can be adjusted to maximize the inhibition. Instead, this drug–drug interaction was evaluated under the recommended therapeutic conditions in patients with multiple myeloma. Aprepitant was administered 1 h before start of the melphalan infusion, Cmax was reached within 4 h, and the terminal elimination half-life ranged from 9 to 13 h. Therefore, sufficient concentrations of aprepitant were present during the melphalan pharmacokinetic observation period. Aprepitant was administered in this study according to the recommended dose: once daily 125 mg on day 1 and 80 mg on days 2, 3 and 4. Melphalan was given on days 1 and 2 (same dose) and the interaction was studied on day 1 only where the higher aprepitant dose was administered. Despite these limitations of drug–drug interaction studies carried out in patients the conclusion of this trial is clinically relevant for such patient populations.
In conclusion, our study demonstrates that anti-emetic regimens including aprepitant (125 mg) will have no clinically relevant effect on the pharmacokinetics of the intravenously administered anticancer agent melphalan, especially Cmax and total body clearance, when administered 1 h before high dose melphalan infusion.
Acknowledgments
This investigator initiated study was partly sponsored by a grant from MSD Sharp & Dohme.
There was no involvement of the study sponsor in study design, collection, analysis, interpretation of data, writing of the manuscript and in the decision to submit for publication.
The skilful technical assistance of Magdalena Longo is greatly acknowledged.
Competing interests
GE and GM received speakers fee from MSD Sharp & Dohme and GE received consultancy fees from MSD Sharp & Dohme.
REFERENCES
- 1.Fassas AB, Spencer T, Desikan R, Zangari M, Anaissie E, Barlogie B, Tricot G. Cytotoxic chemotherapy following tandem autotransplants in multiple myeloma patients. Br J Haematol. 2002;119:164–8. doi: 10.1046/j.1365-2141.2002.03772.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldschmidt H, Sonneveld P, Cremer FW, van der Holt B, Westveer P, Breitkreutz I, Benner A, Glasmacher A, Schmidt-Wolf IG, Martin H, Hoelzer D, Ho AD, Lokhorst HM. Joint HOVON-50/GMMG-HD3 randomized trial on the effect of thalidomide as part of a high-dose therapy regimen and as maintenance treatment for newly diagnosed myeloma patients. Ann Hematol. 2003;82:654–9. doi: 10.1007/s00277-003-0685-2. [DOI] [PubMed] [Google Scholar]
- 3.Gralla RJ, Navari RM, Hesketh PJ, Popovic W, Strupp J, Noy J, Einhorn L, Ettinger D, Bushnell W, Friedman C. Single-dose oral granisetron has equivalent antiemetic efficacy to intravenous ondansetron for highly emetogenic cisplatin-based chemotherapy. J Clin Oncol. 1998;16:1568–73. doi: 10.1200/JCO.1998.16.4.1568. [DOI] [PubMed] [Google Scholar]
- 4.Noble A, Bremer K, Goedhals L, Cupissol D, Dilly SG. A double-blind, randomised, crossover comparison of granisetron and ondansetron in 5-day fractionated chemotherapy: assessment of efficacy, safety and patient preference. The Granisetron Study Group. Eur J Cancer. 1994;30A:1083–8. doi: 10.1016/0959-8049(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 5.Italian Group for Antiemetic Research. Ondansetron plus dexamethasone versus metoclopramide plus dexamethasone plus diphenhydramine in cisplatin-treated patients with ovarian cancer. Support Care Cancer. 1994;2:167–70. [PubMed] [Google Scholar]
- 6.de Wit R, Herrstedt J, Rapoport B, Carides AD, Carides G, Elmer M, Schmidt C, Evans JK, Horgan KJ. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol. 2003;21:4105–11. doi: 10.1200/JCO.2003.10.128. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin - the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 8.Dando TM, Perry CM. Aprepitant: a review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2004;64:777–94. doi: 10.2165/00003495-200464070-00013. [DOI] [PubMed] [Google Scholar]
- 9.McCrea JB, Majumdar AK, Goldberg MR, Iwamoto M, Gargano C, Panebianco DL, Hesney M, Lines CR, Petty KJ, Deutsch PJ, Murphy MG, Gottesdiener KM, Goldwater DR, Blum RA. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24. doi: 10.1016/S0009-9236(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 10.Blum RA, Majumdar A, McCrea J, Busillo J, Orlowski LH, Panebianco D, Hesney M, Petty KJ, Goldberg MR, Murphy MG, Gottesdiener KM, Hustad CM, Lates C, Kraft WK, Van Buren S, Waldman SA, Greenberg HE. Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects. Clin Ther. 2003;25:1407–19. doi: 10.1016/s0149-2918(03)80128-5. [DOI] [PubMed] [Google Scholar]
- 11.Davies ID, Allanson JP, Causon RC. Rapid determination of the anti-cancer drug Melphalan (Alkeran™) in human serum and plasma by automated solid phase extraction and liquid chromatography tandem mass spectrometry. Chromatographia. 2000;52:S92–S97. doi: 10.1016/s0378-4347(99)00280-7. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez RI, Wang RW, Newton DJ, Bakhtiar R, Lu P, Chiu SH, Evans DC, Huskey SE. Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004;32:1287–92. doi: 10.1124/dmd.104.000216. [DOI] [PubMed] [Google Scholar]
- 13.de Jonge ME, Huitema AD, Holtkamp MJ, van Dam SM, Beijnen JH, Rodenhuis S. Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolism. Cancer Chemother Pharmacol. 2005;56:370–8. doi: 10.1007/s00280-005-1005-4. [DOI] [PubMed] [Google Scholar]
- 14.Bubalo JS, Leis JF, Curtin PT, Maziarz RT, Kovascovics TJ, Meyers G, McCune JS, Munar MY, Hayes-Lattin B, Jones N. A randomized, double-blinded, pilot trial of aprepitant added to standard antiemetics during conditioning therapy for hematopoietic stem cell transplant (HSCT) J Clin Oncol. 2007;25:9112. [Google Scholar]
- 15.Walko CM, Yu YA, Bhushan S, Spasojevic I, Carey L, Collichio F, Armstrong D, Dees EC. Effect of aprepitant on cyclophosphamide pharmacokinetics in early breast cancer patients. J Clin Oncol. 2009;27:15S. [Google Scholar]
- 16.Loos WJ, de Wit R, Freedman SJ, Van Dyck K, Gambale JJ, Li S, Murphy GM, van Noort C, de Bruijn P, Verweij J. Aprepitant when added to a standard antiemetic regimen consisting of ondansetron and dexamethasone does not affect vinorelbine pharmacokinetics in cancer patients. Cancer Chemother Pharmacol. 2007;59:407–12. doi: 10.1007/s00280-006-0359-6. [DOI] [PubMed] [Google Scholar]
- 17.Aapro MS, Walko CM. Aprepitant: drug-drug interactions in perspective. Ann Oncol. 2010 doi: 10.1093/annonc/mdq149. May 20; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Kühne A, Tzvetkov MV, Hagos Y, Lage H, Burckhardt G, Brockmöller J. Influx and efflux transport as determinants of melphalan cytotoxicity: Resistance to melphalan in MDR1 overexpressing tumor cell lines. Biochem Pharmacol. 2009;78:45–53. doi: 10.1016/j.bcp.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Feuring M, Lee Y, Orlowski LH, Michiels N, De Smet M, Majumdar AK, Petty KJ, Goldberg MR, Murphy MG, Gottesdiener KM, Hesney M, Brackett LE, Wehling M. Lack of effect of aprepitant on digoxin pharmacokinetics in healthy subjects. J Clin Pharmacol. 2003;43:912–7. doi: 10.1177/0091270003256113. [DOI] [PubMed] [Google Scholar]
- 20.Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, Haefeli WE. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76:73–84. doi: 10.1016/j.clpt.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99. doi: 10.1200/JCO.1995.13.7.1786. [DOI] [PubMed] [Google Scholar]


