Abstract
Including the true tissue kallikrein KLK1, kallikrein-related peptidases (KLKs) represent a family of fifteen mammalian serine proteases. While the physiological roles of several KLKs have been at least partially elucidated, their activation and regulation remain largely unclear. This obscurity may be related to the fact that a given KLK fulfills many different tasks in diverse fetal and adult tissues, and consequently, the timescale of some of their physiological actions varies significantly. To date, a variety of endogenous inhibitors that target distinct KLKs have been identified. Among them are the attenuating Zn2+ ions, active site-directed proteinaceous inhibitors, such as serpins and the Kazal-type inhibitors, or the huge, unspecific compartment forming α2-macroglobulin. Failure of these inhibitory systems can lead to certain pathophysiological conditions. One of the most prominent examples is the Netherton syndrome, which is caused by dysfunctional domains of the Kazal-type inhibitor LEKTI-1 which fail to appropriately regulate KLKs in the skin. Small synthetic inhibitory compounds and natural polypeptidic exogenous inhibitors have been widely employed to characterize the activity and substrate specificity of KLKs and to further investigate their structures and biophysical properties. Overall, this knowledge leads not only to a better understanding of the physiological tasks of KLKs, but is also a strong fundament for the synthesis of small compound drugs and engineered biomolecules for pharmaceutical approaches. In several types of cancer, KLKs have been found to be overexpressed, which makes them clinically relevant biomarkers for prognosis and monitoring. Thus, down regulation of excessive KLK activity in cancer and in skin diseases by small inhibitor compounds may represent attractive therapeutical approaches.
Keywords: Tissue kallikrein, Specificity pockets, Inhibitory compound, Zinc, Rule of five
1. Introduction
Regulation of protease activity in the living organism is a highly complex task that involves all levels of cellular organization. Control and timing of protease activity starts with gene expression, transcription and translation, and continues with protein targeting and zymogen activation. Once activated, the protease is often kept in check by endogenous inhibitors, while the last steps of protease regulation may be limited proteolysis and final degradation. The emerging research in the field of tissue kallikrein-related peptidases (KLKs) provides many diverse examples for nearly all aspects of protease regulation by inhibitors.
Tissue kallikrein (Kallikrein 1, KLK1) and the kallikrein-related peptidases are (chymo)trypsin-like serine proteases, belonging to family S1A of clan PA(S) according to the MEROPS classification [1]. Prior to the introduction of a new nomenclature in 2006, the KLKs were often referred to as hKs or rKs for human and rat proteins, respectively [2]. Fully sequenced genomes of placental mammals, such as primates or rodents, and even of marsupials (e.g. the opossum), exhibit at least eleven KLK genes, but usually lack the counterparts of human KLK2 and KLK3 [3], [4]. However, the numbers of corresponding proteases vary from ten KLKs in cows, eleven in dogs, and 26 ones in mice. The latter possess a series of functional KLK1 paralogs [2], [5], [6]. Kallikrein 1 and the kallikrein-related peptidase genes are organized in a single cluster on chromosome locus 19q13.4 [7], [8]. The 15 human KLK members are only distantly related to plasma kallikrein, which shares 38% identical residues with KLK1 in the catalytic domain, while KLK1 and trypsin share 46% identity [9], [10].
One or more KLK genes are expressed in nearly all tissues and fluids of the human body. They fulfill a diverse range of tasks throughout one’s lifetime from embryonic development to processes in adulthood [8], [11], [12], [13]. KLKs are intracellulary synthesized as precursors with a signal peptide (15–34 amino acids) that is cleaved off upon secretion into the endoplasmatic reticulum. The proform or zymogen of the KLK protease is extracellularly activated by the removal of the propeptide (3–37 amino acids), resulting in active proteases of 223–238 residues (Fig. 1), and in some cases reaching molecular weights of up to 50 kDa due to heavy glycosylation [14]. The activation process of KLKs may involve autoactivation [15], [16], [17], KLK activation cascades [18], [19], [20], serine proteases from the thrombostasis axis, such as plasmin, plasma kallikrein, and factor Xa [21], or the proteolytic activity of other proteases, such as urokinase-type plasminogen activator (uPA), matrix metalloproteinases (MMPs), and dipeptidyl peptidase I [22], [23], [24]. However, the KLK activity is not restricted to regulation by steroid-dependent expression [25], [26], [27] or by fine-tuned zymogen activation. In the case of KLKs 6, 12, and 14, from example, regulation is likely, at least in part, achieved by autolysis [17], [28], [29], [30], [31]. Furthermore, in some cases an interplay of KLKs and their corresponding natural inhibitors has been established, even with pathophysiological significance [32]. However, many potential inhibitors of KLKs have not yet been unambiguously assigned to a given KLK. Another unusual feature of certain KLKs is the timescale of their activity, which can reach months, if not years, as seen with KLK4 in tooth development, which was also observed in a comparison of Klk4/lacZ knockin mice and the wild type [33], [34].
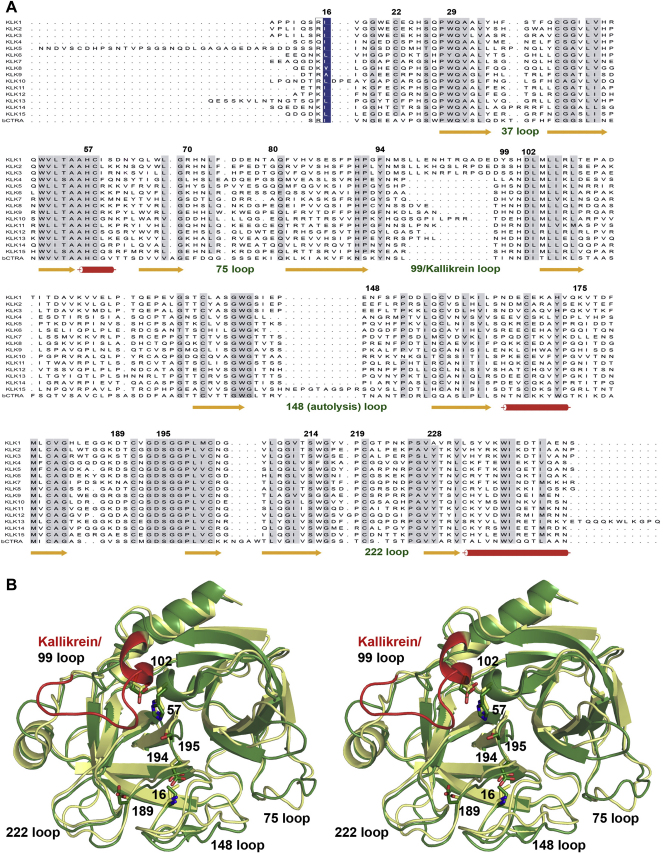
Fig. 1.
Primary and tertiary structure of KLKs A: Sequence alignment of kallikrein 1 (KLK1) and the human kallikrein-related peptidases KLKs 2–15 with bovine chymotrypsin (bCTRA). Secondary structure is shown for KLK3/KLK5 for β-strands (arrows) and α-helices (cylinders). Propeptides are included and the numbering is derived from chymotrypsinogen [247]. The “classical” KLKs 1, 2, and 3 share 61–79% identical residues, while the conservation of the “new” KLKs 4–15 ranges from 38% to 57% [130], [300]. Highly conserved residues are displayed with a grey background, while residue 16 that is located in the P1, position when the propeptide is cleaved off is shown with blue background. B: Overlay of KLK3 (beige) and KLK5 (green) as ribbon representation in stereo. The catalytic triad residues (His57, Asp102, Ser195), and Ser189 of KLK3 and Asp189 of KLK5, which determine chymotryptic or tryptic specificity, respectively, are depicted as stick models, as well as the N-terminal residue 16 with Asp194 that form a salt bridge, thereby stabilizing the active site [301]. The long 99 loop of the classical KLK3 is depicted in red.
Overall, the human KLKs can be subdivided into in several tissue-specific groups with distinct physiological substrates and functions. In the 1920s and 1930s, the first kallikrein (KLK1) was discovered and characterized as a proteolytic enzyme, mainly occurring in urine, kidney, and the pancreas, the latter being the inspiration for the protein’s name which is derived from the Greek word for pancreas (καλλικρɛας, Kallikreas) [35], [36], [37]. A major function of KLK1 is the reduction of blood pressure by releasing the peptide hormone Lys-bradykinin (kallidin) from low molecular weight kininogen, which effects muscle relaxation and inflammatory processes [38]. Knockout of the Klk1 gene in mice causes both cardiovascular abnormalities and a defect of efficient renal tubular calcium absorption [39], [40]. Intriguingly, administration of this protease can reduce cardiac and renal injuries, restenosis, and ischemic stroke and promotes angiogenesis and skin wound healing [41]. Similar to the other “classical” KLKs, KLK 2 and 3, which were discovered in the late 1980s, KLK1 features an extended “99-loop” (also called kallikrein loop) of 11 inserted amino residues with respect to chymotrypsin. Among the “new” KLKs, KLK 4–15 that were gradually characterized from the mid-1990s onwards, KLKs 8–13 possess 99-loop insertions from two to eight residues (see alignment in Fig. 1).
In the prostate, KLKs 2, 3, 4, 11, and to some extent KLKs 14 and 15, are produced for secretion into seminal plasma [8], [11]. There, they most likely activate each other in a cascade-like manner resulting in the degradation of semenogelins and fibronectin, mainly by KLK3 and KLK14, for semen liquefaction [42], [43]. Since KLK3 (PSA, “prostate specific antigen”) blood plasma levels correlate with prostate cancer progression, an immunoassay for PSA has become a widespread medical application, despite its moderate reliability as prognostic biomarker for malignant processes. Thus, there still remains the need for additional specific KLK tumor markers [44], [45], [46]. One of these promising markers is KLK4, which is distinctly expressed in the early stages of prostate cancer [47]. Interestingly, KLK4 has the capacity to activate the proform of the urokinase-type plasminogen activator (pro-uPA) and to modulate the activity of its receptor uPAR, both of which play a significant role in prostate and ovarian cancer [48], [49]. In addition, KLKs 2, 4, 5, 6, and 14 seem to be potential players in signal transduction via activation of G-coupled protease receptors, such as PARs 1, 2, and 4, resulting, e.g. in inflammation or in tumor cell proliferation and migration [50], [51], [52], [53], [54], [55], [56].
In addition to its role in the prostate, which is not yet fully understood, KLK4 is imperative for tooth development, particularly in formation of enamel, which also depends on MMP-20 [57]. Under normal circumstances, both proteases degrade the extracellular matrix required for the growth of dentin crystallites. Single mutations, however, result in either the malfunctioning of MMP-20 or KLK4, causing the hereditary disease amelogenesis imperfecta, which is characterized by very fragile teeth [58], [59]. More specifically, KLK4 seems to be crucial for the formation of large coherent enamel crystallites, as seen in Klk4 knockout mice [34].
A larger subset of kallikrein-related peptidases, namely KLKs 5, 7, 11, and 14, is highly expressed in human skin, mainly in the outermost layer, the stratum corneum, while KLKs 6, 8, 10, and 13 are found at medium expression levels [11], [13], [60]. KLKs 5, 7, and 14 are capable of degrading proteins of the corneodesmosomes, leading to desquamation, the shedding of cornified skin cells [61], [62], [63]. In contrast, KLK8 is involved in cellular differentiation and healing of the skin [64], similar to KLK6, which induced rapid wound healing by promoting keratinocyte proliferation and migration in a mouse model based on the shedding of E-cadherin by Klk6 [65]. Also, KLKs 4, 5, and 8 specifically activate the metalloproteinases meprin α and/or β, which are located in separate layers of the epidermis [66]. Tight activity regulation of these KLKs by several types of inhibitors is necessary, otherwise diverse skin diseases will develop [67].
Intriguingly, two KLKs, KLK6 and KLK8 (also termed neurosin and neuropsin, respectively), are expressed at higher levels in human brain [8], [68]. KLK6 accumulates at brain lesions of humans and investigations in mice suggest that excessive KLK6 activity causes inflammation of the central nervous system and promotes multiple sclerosis through demyelinating activity [69], [70]. The physiological role of KLK6 seems to be both de- and remyelination of glia cells, contributing to neurite and axon growth after injuries [71]. In contrast, KLK8, which mostly occurs in the hippocampus, is involved in long term potentiation (LTP) and memory acquisition by restructuring synapses, as shown by mouse models [72], [73], [74], [75]. Furthermore, in human brains with Alzheimer’s disease a more than 10-fold expression of KLK8 was observed [76]. On the other hand, Klk8 knockout mice were shown to be susceptible to epileptic seizure [77]. Furthermore, single nucleotide polymorphisms in the human KLK8 gene are associated with manic-depressive disorder and cognitive impairment [78].
Although the KLK9 protease is present in many tissues and dominates among all KLKs in fetal and adult heart [11], no physiological function has been defined so far, however, it may serve as an ovarian and breast cancer marker [79]. Similarly, KLKs 10, 12, 13 and 15 are associated with distinct cancers without established (patho)physiological roles [17], [80], [81]. Nevertheless, there are some indications of a tumor suppressor role for KLK10 in breast cancer [82], KLK12 may be involved in angiogenesis regulation [83], KLK13 in ovary tissue remodelling and interleukin processing [84], [85], and KLK15 in KLK3/PSA activation [86]. Intriguingly, expression of KLKs in the female reproductive system appears to be complementary to the expression pattern of KLKs in prostate, suggesting an activation cascade that probably involves all KLKs during impregnation [87].
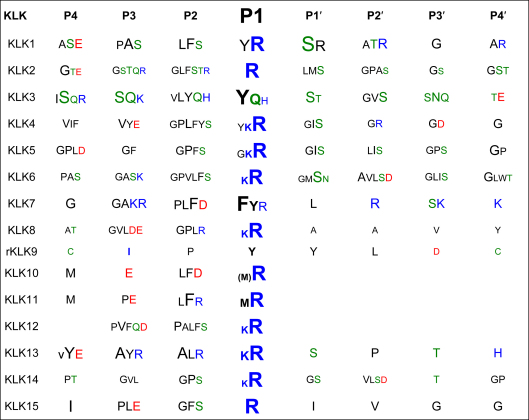
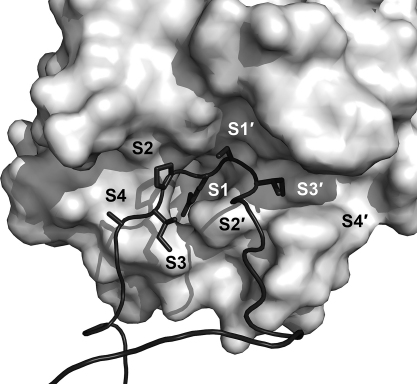
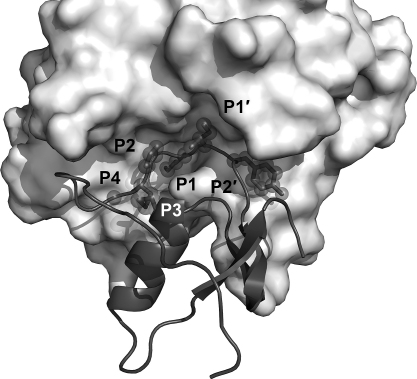
As we will see later for the KLKs, natural inhibitors of proteases often bind directly to the active site, exploiting some degree of complementarity at the interaction surface [88], [89], [90]. Expectedly, the more the components of the inhibitor bind to distinct specificity pockets of the protease, the more specificity and affinity can be gained for inhibition [91], which in some cases may be enhanced by additional binding of the inhibitor to so-called exosites [92], [93], [94]. Also, knowledge of the substrate specificity of the KLKs will be a guideline for the identification of endogenous (and perhaps exogenous) inhibitors, and for the design of synthetic substrates, as well as of highly specific inhibitors, which may eventually yield powerful pharmaceutical compounds. Numerous studies have investigated the specificity for all KLKs, using either individual chromogenic and fluorogenic substrates, such as in the case of KLKs 8, 12, 15 [17], [95], [96], [97] or systematic positional scanning approaches for KLKs 3, 4, 5, 6, 7, 10, 11, 13, 14 [98], [99], [100], [101], phage display for KLKs 1, 2, 3, 4, 6, 14 [102], [103], [104], [105], [106], [107], or peptide libraries for KLKs 1, 2, and 3 [108], [109], [110]. In addition, KLK cleavage sites in natural substrates have also been analyzed to a large extent. Since these studies show many differences, sometimes stark discrepancies, for the single specificity subsites, a general overview is given in Table 1 based on the statistical approach of the MEROPS specificity matrices (http://merops.sanger.ac.uk/). The primary specificity of the kallikrein-related peptidases will allow a basic classification between inhibitors of the twelve tryptic KLKs (1, 2, 4–6, 8, 10–15) and of the three chymotryptic KLKs 3, 7, and presumably 9. The specificity of KLK9 remains still unclear, as it cleaved P1 Arg containing pro-KLKs at very slow rates [20], while enzymatic data are only available for the rat ortholog rKLK9 (Table 1) [111], [112], which possesses as major determinant of P1 specificity an Asp189, in contrast to the unusual Gly189 of human KLK9 (Fig. 1). Furthermore, the preference of KLKs 1, 10, and 11 appears to be mixed tryptic and chymotryptic (Table 1) [99]. It should be noted that the proteolytic activity of recombinant KLK10 is rather slow and only observed in vitro with Leu16 as N-terminal residue, followed by three inserted residues (Fig. 1) [20]. In addition to a problematic KLK10 activation in vivo at Glu16C, if tryptic or chymotryptic proteases were the activators, KLK10 exhibits a Ser193 instead of the extremely conserved Gly193 (Fig. 1). A Ser193 might affect the oxyanion pocket function, similar to various factor XIa mutants of residue 193 with reduced activity and inhibitor binding capacity [113]. Nevertheless, in addition to the proof of enzymatic activity of recombinantly expressed KLK10, it was demonstrated that native KLK10 from human ovarian ascites fluid is associated with two endogenous protease inhibitors and reacts with an activity-based probe [114].
Table 1.
Statistical specificity patterns of human KLK peptidases cleavage sites, according to MEROPS specificity matrices for peptide residue positions P4 to P4′ that bind to protease subsites S4 to S4′ with the scissile bond between P1–P1′ (Schechter–Berger nomenclature [306]). In cases without established matrix, the information on natural and synthetic substrates was used to derive a score from the incidence of a residue, whereby the P1 value was always set to 100%. The font size corresponds to the frequency of a residue in published KLK substrates (an incidence cutoff was individually chosen, in order to limit the number of residues at a given position). Hydrophobic residues are depicted in black, polar ones in green, acidic ones in red, and basic ones in blue. For KLK9 only data of one substrate of the rat ortholog (rKLK9) were available [112], while for KLKs 10, 11, and 13, results from substrate profiling studies [99], [100], [307] have been treated as one substrate per study, employing only the top ranked residues in positions P4 to P1. For KLK12 published substrates from two studies were analyzed [17], [96]. The primary tryptic specificity for Arg and Lys in P1 of many KLKs is conferred by an Asp189 at the bottom of the S1 pocket, which is replaced by a Glu189 in KLK15. The three chymotryptic KLKs with a preference for P1-Tyr, -Phe, or -Gln possess a Ser189 (KLK3), an Asn189 (KLK7), and a Gly189 (KLK9), but rat KLK that shares 40% identical residues with KLK9 (61% homology) exhibits an Asp189, although it has a tendency to cleave chymotryptic substrates. Apparently, KLK1 has a mixed tryptic/chymotryptic specificity, as was also observed for KLKs 10 and 11 [99].
Fine tuning of any protease inhibitor interaction depends on characteristic structural features, including electrostatics, flexibility, and exosites on protein surfaces, which will be investigated in the following together with the most relevant biological, biochemical, and pharmaceutical aspects of KLK inhibition.
2. Natural endogenous KLK inhibitors
To date, a great variety of endogenous inhibitors with physiological significance in the activity regulation of mammalian kallikrein-related peptidases are known. They range from single metal ions to large protein complexes of more than 700 kDa. In fact, natural exogenous inhibitors are widely used for scientific and pharmaceutical studies (see Sections 3, 4.). In general, nature employs the following basic principles of protease inhibition: attenuation by reversible binding of inhibitors, “KO” inhibition by irreversible binding to the inhibitory molecule, which often involves the formation of covalent bonds, and compartmentalization. The latter principle may be achieved by restricted tissue or organelle localization of the enzyme or inhibitor; by homo-oligomerization of the protease itself, which reduces the accessibility to the active site; or by compartment formation of large inhibitors that internalize proteases, for which some rare, but biologically significant examples exist.
2.1. Inhibition of KLKs by metal ions
The activity of several serine proteases is regulated by endogenous cations. Interestingly, alkali and earth alkali ions rather stimulate protease activity at distinct binding sites, as seen for the prominent examples of Na+ with thrombin [115], [116], [117] or Ca2+ with trypsin [118], [119]. Also, for KLKs 1, 3, 4, 6 and 8 activity stimulation by Ca2+, Mg2+, or Na+ and K+ ions has been observed [107], [120], [121], [122], [123]. Zn2+ is a metal ion with manifold functions in living organisms and is present in about 300 human enzymes [124], [125]. It may play a dual role in activity regulation, as it can stimulate serine proteases, e.g. factor XII [126], and inhibit other ones, such as factor VIIa or uPA [127], [128]. To date, Zn2+ inhibition as a significant regulatory mechanism for KLKs can only be excluded for the intensively studied KLK1 and KLK6, while KLKs 9–11, 13, and 15 still require further inhibition studies. Strikingly, for KLKs 2, 3, 4, 5, 7, 8, 12 and 14, inhibition by Zn2+ in the low μM range has been repeatedly reported (Table 2). Thus, Zn2+ should be considered as “attenuator” of KLK activity, which binds in a reversible manner to the targets for fine tuning of their proteolytic action.
Table 2.
KLK affinity matrix for the most relevant endogenous inhibitors. Data for KLKs 9, 10, and 15 were not available. The rating depended on given inhibition constants Ki or IC50 values that are roughly the doubled Ki (○ no inhibition, ● μM, ●● nM, ●●● pM range), or association constants that were also represented (Kass ≥ 105 M−1 s−1 ●●●, ≤ 103 M−1 s−1 ●●), otherwise it was chosen according to the description of the respective publication (“strong inhibition” ●●●, etc., ● slow inhibition). * IC50 ** the protease remains active against small molecule substrates, while protein substrates cannot be cleaved anymore.
| KLK | Zn2+ | α1-AT | α1-ACT | ATIII | α2-AP | PCI | Kallistatin | Other serpins | LEKTI-1 | a2M** |
|---|---|---|---|---|---|---|---|---|---|---|
| KLK1 | ○ | ● [171] |
○ [196] |
○ [196] |
●● [308] |
●●● [196] |
●●● [229] |
|||
| KLK2 | ● (3 μM)* [129] |
○ [172], [309] |
● [205], [172] |
●●● (+heparin) [187], [309] |
●●● [172], [309] |
●●● < 25 pM [129] |
●●● PI-6 [204] ●●● PAI-1 [205] |
●●● [230] [231] |
||
| KLK3 | ● (24 μM) [134] |
●● [175], [176] |
●●● [232], [176] |
● [187] |
●● [192] |
●● MNEI [203] |
●●● [232] |
|||
| KLK4 | ● (15 μM)* [137] |
●●● [178] |
○ [178] |
● [178] |
●● [178] |
● PAI-1 [155] |
● [178] |
|||
| KLK5 | ● (4 μM) [15], [144] |
○/● [173], [170] |
○ [173] |
○ [173], [170] |
●●● [173], [170] |
●●● [170] |
○ [170] |
●● C1I [170] |
●● [218], [219] |
● [173] |
| KLK6 | ○ | ● [174] |
● [182] |
●● [174] |
● [174] |
●● [63] |
○ [174] |
|||
| KLK7 | ● (10 μM) [147] |
●●● [170] |
●●● [170] |
○ [170] |
●● [170] |
●●● [170] |
●●● [170] |
●● [218], [219] |
||
| KLK8 | ● (3.3 μM) [123] |
○ [170] |
○ [170] |
● [170] |
●● [170] |
●●● [170] |
○ [170] |
●●● PI-6 [206] |
||
| KLK11 | ○ [307] |
○ [307] |
○ [307] |
○ [307] |
● [170] |
○ [170] |
||||
| KLK12 | ● (10 μM)* [17] |
●/●● [17], [170] |
○ [170] |
● [17], [170] |
●●● [17] |
●● [17] |
○ [170] |
●● C1I [17] |
○ [17] |
|
| KLK13 | ○ [170] |
○ [170] |
● [170] |
●● [170] |
●●● [170] |
● [170] |
●● PAI-1 [170] |
●● [63] |
● [14] |
|
| KLK14 | ● (2 μM) [15], [31] |
●● (162 nM) [63], [170] |
● (5.6 μm) [63] |
●● (198 nM) [63] |
●● (130 nM) [63], [170] |
●●● [170] |
●● [170] |
● PAI-1 [63] |
●● [63] |
2.1.1. Inhibition of prostatic KLKs 2, 3, and 4 by Zn2+
Human kallikrein-related peptidase 2, also known as glandular kallikrein 2 (KLK2), is a tryptic protease. While recombinant KLK2 was inhibited by various serpins in the picomolar range, Zn2+ attenuated its activity against fluorogenic substrates, e.g. Pro-Phe-Arg-AMC, with a Ki of 3 μM [129]. Lovgren and coworkers describe the inhibition mechanism as a mixture of competitive and non-competitive inhibition, hinting to the binding of more than one Zn2+ per KLK2 molecule and interference with the substrate recognition region of KLK2. Based on the high sequence homology to KLK3 (79% identical residues [130]) and in particular the presence of the conserved His91, His101, and His233, one can assume that the Zn2+ inhibition mechanism of KLK2 may be, at least to some extent, similar to KLK3, which is described in the following.
Zn2+ inhibition of KLK3/PSA, which is mostly activated by KLK2 in seminal fluid, is clearly an important physiological regulatory process, especially because it is here that the highest Zn2+ concentration in the human body is found, reaching up to 9 mM [131]. A first study on the activity of natural KLK3 against a chromogenic substrate, MeO-Suc-Arg-Pro-Tyr-pNA, demonstrated Zn2+ inhibition with an IC50 of 20 μM (Ki 6 μM), whereby a competitive inhibition type was found, involving the binding of at least two Zn2+ [132]. Whereas one group using recombinant KLK3 reported a Kiapp of 45 μM for Zn2+ with non-competitive inhibition, another group found for natural KLK3 an IC50 of 24 μM with competitive inhibition [133], [134]. From a modelled homology structure of human KLK3, a Zn2+ binding site was proposed at His91, His101, and His233, and as a potential fourth ligand an Asp (95, 97, or 98). It was assumed that a Zn2+ bound His101 could sufficiently distort the catalytic triad via a shift of Asp102, resulting in decreased proteolytic activity [135]. Interestingly, in a horse kallikrein 3 (eKLK3) crystal structure an equivalent metal site was occupied by Zn2+: the coordinating atoms were Asp91 Oδ2, His101 Nδ1, and His234 Nɛ2 (Fig. 2A) [136]. In human KLK3, that was crystallized with an antibody required for stabilization of the extended 99 loop but not containing Zn2+, the region around the 75-loop exhibited promising ligands for Zn2+: His25, His70, His75, Glu21, Glu77 and Asp78 [134]. How Zn2+ binding to these ligands could influence the protease activity will be elucidated by the example of KLK4.
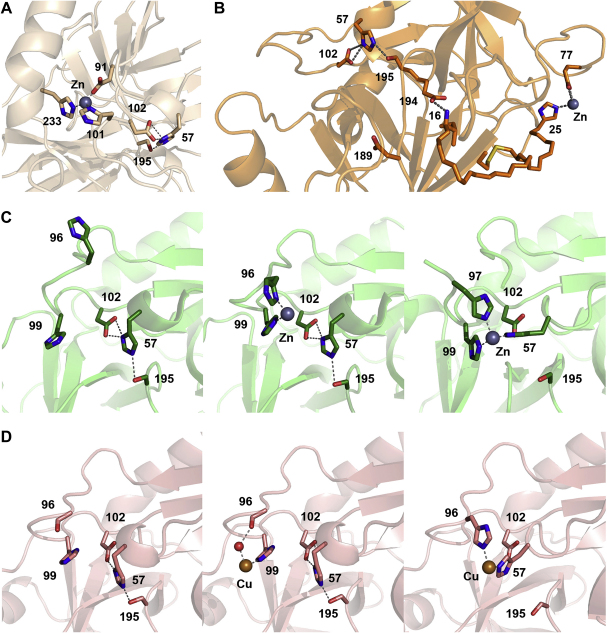
Fig. 2.
Inhibitory metal binding sites in KLK crystal structures as ribbon representation with relevant residues as stick models. Dotted lines indicate hydrogen and coordination bonds, relevant residues are labelled with numbers. A: The Zn2+ ion (grey sphere) bound to horse KLK3 involves ligands Asp91, His101, and His234. The equivalent residues in human KLK3 are His91, His101, and His233. Probably, the Zn2+ binding to His101 causes a backbone shift of Asp102, concomitantly distorting the catalytic triad (Ser195, His57, and Asp102) and resulting in a reduced proteolytic activity. B: In KLK4, the inhibitory Zn2+ site is located far away from the active site, which was confirmed by the single mutations His25Ala and Glu77Ala. Zn2+ binding to KLK4 effects a disruption of the salt bridge Ile16-Asp194 via His25, thereby destabilizing the active site conformation and abolishing its functionality. C: Zn2+ binding to the active site of KLK5 and rKLK2 in standard orientation. On the left, the free active site of KLK5 is shown with both His96 and His99 rotated away from the S2 pocket. The catalytic triad, consisting of Ser195, His57, and Asp102 is connected by hydrogen bonds. In the middle, a Zn2+ ion (grey sphere) has bound to the ligands His96 and His99 with coordination bonds. On the right, the catalytic His57 of rKLK2 has relocated from the catalytic triad to become the third Zn2+ ligand, together with His99 and His97. Most likely, a similar conformation in KLK5 represents the reversible Zn2+ inhibited state. Since the S2 subsite is still accessible for most P2 side chains, the inhibited state allows binding, but not turnover of substrates, as long as the catalytic triad is disrupted. This mechanistic model is in agreement with the observed non-competitive Zn2+ inhibition of KLK5. D: Cu2+ binding to the active sites of KLK7 and the rat trypsin mutant Arg96His in standard orientation. On the left, the free active site of KLK7 is depicted with the catalytic triad connected by hydrogen bonds (dotted lines). In the middle, a Cu2+ ion (orange sphere) has bound to the ligands His99 and Thr96-C=O via a water molecule (red sphere) with coordination bonds (dotted lines). On the right, the catalytic His57 of rat trypsin mutant Arg96His has rotated out from the catalytic triad to become the second Cu2+ ligand, together with the mutated His96. A similar conformation in KLK7 with His99 instead of His96 may represent the reversible Zn2+/Cu2+ inhibited state. As in KLK5 the S2 subsite is still accessible for P2 side chains, allowing binding, but not cleavage of substrates, as long as the catalytic triad is inactive, which agrees with the non-competitive Zn2+ inhibition of KLK7.
Recombinant KLK4 tested with the chromogenic substrate Z-Phe-Val-Arg-pNA was inhibited by Zn2+ with an IC50 value of 16 μM, although a residual activity of about 25% was still observed at 1 mM Zn2+ concentration, hinting to a non-competitive or mixed inhibition type [137]. Intriguingly, in enamel which can only form properly by KLK4 activity, the zinc content is equivalent to 15 μM, which would correspond to the observed in vitro inhibition range [138]. As seen in the crystal structure, KLK4 exhibits a unique metal binding site in the 75 loop with a short helical segment from Glu74-Gln76, which allows the Glu77 Oɛ to adopt an optimal conformation for Zn2+ binding together with the His25 Nɛ2 (Fig. 2B) [137]. In contrast, the 75 loop of trypsin binds an activity enhancing Ca2+ through the Glu70 and Glu80 carboxylate groups [118], which are replaced by Leu70 and Ser80 in KLK4. Three external ligands from a second KLK4 molecule (Glu74 Oɛ, Asp75 Oδ, and Gln76 Oɛ) are additionally coordinating the Zn2+ [137]. Although these ligands could constitute a secondary Zn2+ inhibition site, the single mutations His25Ala or Glu77Ala abolish the Zn2+ inhibition of KLK4 completely. Thus, binding of Zn2+ at the 75 loop has a long-range inhibitory effect on the active site that is probably transmitted through His25 to the N-terminus, causing disruption of the salt bridge between Ile16 and Asp194, which is required for a functional active site conformation (Fig. 2B). This mechanism was confirmed by a strong increase in the accessibility of the Ile16 α-amino of KLK4 for an acetylating agent in the presence of 70 μM Zn2+ [137]. The same Zn2+-dependent phenomenon was previously observed for the low activity form of uncomplexed coagulation factor VIIa (FVIIa) [139]. Interestingly, a Zn2+ mediated cross-talk between the 75 loop and the active site has been proposed for FVIIa as an explanation for the attenuatory effect on its activity, in particular because its S1 subsite displays an intrinsic tendency to disorder [127], [140].
The physiological significance of Zn2+ inhibition of KLKs 2, 3, and 4 is most likely the finely balanced regulation of activation and protease activity [81], [130], [141]. Prostatic fluid contains Zn2+ concentrations up to 9 mM, which keeps the prostatic KLKs in an inactive state [131]. Upon ejaculation, the prostatic fluid mixes with epididymal fluid containing the spermatozoa and with seminal vesicles, which are rich in the structural proteins semenogelin I and II (SgI and II) being responsible for initial sperm immobilization in the seminal coagulum [142]. However, as both SgI and SgII harbor at least ten binding sites for Zn2+, most of the seminal plasma Zn2+ will eventually be chelated leading to the activation the prostatic KLKs. Especially KLK3/PSA, as the major SgI and II degrading enzyme, will then rapidly process these proteins which results in semen liquefaction and allows the initiation of sperm movement [81], [130], [141], [142].
2.1.2. Inhibition of epidermal KLKs 5, 7 and 14 by Zn2+
It has been reported that the tryptic KLK5 activity against tripeptidyl-AMC substrates is inhibited around 12 nM Zn2+ with 25% residual activity [143], whereas comparable studies found an IC50 value of 4 μM and apparent inhibition constants Ki = 8 μM at pH 7.0 and Ki = 2.0 μM at pH 8.0 [15], [144]. Considering the extracellular Zn2+ concentration of roughly 15 μM in skin, a low nanomolar inhibition constant would render its target protease virtually inactive. In contrast, a micromolar inhibitor or attenuator would allow the modulation of proteolytic activity in addition to stronger polypeptidic KLK inhibitors that are also present in the outer layers of the skin (see Section 2.2.2). The studies by Brattsand et al. and Debela et al. confirmed the reversibility of the Zn2+ inhibition by addition of either EDTA or Zn2+-binding substrates, respectively. A comparison of a Zn2+-free KLK5 crystal structure (Fig. 2C, left panel) with the Zn2+-bound KLK5 and rat kallikrein 2 (rKLK2, tonin) structure can explain the inhibition mechanism to a large extent (Fig. 2C) [144], [145]. In KLK5, Zn2+ is coordinated by the His96 Nδ1 and the His99 Nɛ2 and two water molecules. The His96 side chain has to rotate by more than 90° from the Zn2+-free conformation to its position in the Zn2+ coordination sphere, while the rotation of His99 is less than 20°. In the KLK5 Zn2+ structure, the presence of the inhibitor leupeptin (not shown in Fig. 2C, middle panel) bound to the catalytic Ser195 and occupying the S2 pocket with a Leu side chain prevented most likely the formation of a coordination sphere for Zn2+ with a third ligand, namely His57. This conformation, representing the fully inhibited KLK, can be seen in the rKLK2 (tonin) structure, which exhibits the Zn2+ ligands His99, His97 (similarly positioned as the His96 of KLK5), and most significantly, His57, which is relocated from its position in the catalytic triad (Fig. 2C, right panel). The consequence of a disrupted catalytic triad must be inactivation of the enzyme. However, substrate binding should be possible in this inhibited state, due to the largely free S2 subsite, which only has to be unoccupied for the His57 side chain rotation. Also, the observed non-competitive Zn2+ inhibition of KLK5 is in good agreement with this mechanistic model, since Zn2+ does not compete with substrates for active site binding [144]. Interestingly, the substrate H-Gly-His-Arg-AMC yielded a Ki value of about 20 μM for Zn2+, with a residual activity of 25% even at 100 μM [144] which can be explained by a replacement of His57 in the Zn2+ coordination sphere with the P2-His Nɛ2 or by a “substrate-assisted catalysis” mechanism. In the first case, the His57 of KLK5 would be pushed back into the catalytic triad, in the second case, the P2-His substrate would transiently reconstitute a functional catalytic triad and catalyze its own cleavage, as in the classic example of a subtilisin His64Ala mutant [146]. In any case, the employed Phe or Pro-P2 side chains of other fluorogenic or chromogenic substrates could not play the mechanistic role of His which has the capacity either to coordinate Zn2+ or to activate the Ser Oγ nucleophile.
Also, the chymotryptic counterpart of KLK5, KLK7, is inhibited by Zn2+ (Ki(app) = 10 μM) when measured with tetrapeptidyl-AMC substrates, and reveals an even stronger inhibition with Cu2+ (Ki(app) = 0.6 μM) [147]. Crystal structures of KLK7 without bound ions (Fig. 2D, left panel) and in complex with Cu2+ revealed direct metal binding by the His99 Nɛ2 and by the Thr96 carbonyl O via hydrogen bonds to a water molecule (Fig. 2D, middle panel). Since the mutation His99Ala abolished Zn2+ inhibition of KLK7 activity completely, this metal binding site must be the structural basis of the attenuating or inhibitory effect of Zn2+ and Cu2+ [147]. Similar to the coordination of Zn2+ in rKLK2 the catalytic His57 could constitute a more stable coordination sphere for the cation [145]. A striking parallel to the situation in KLK7 is found in a Cu2+ complex structure of the Arg96His mutant of rat trypsin which can also be inhibited by Cu2+ (21 μM) and Zn2+ (128 μM) [148]. In this structure, the metal ion is liganded by His96 and His57 that has rotated out of the catalytic triad, which explains the inactivation of the enzyme [149]. Most likely, the disrupted catalytic triad with His57 bound to Zn2+ (or Cu2+) represents the equivalent inhibited state for KLK7 (Fig. 2D, right panel). Again, the non-competitive Zn2+ inhibition of KLK7 agrees well with this structure based mechanistic model, in which substrate binding is still possible, as long as P2 side chains are not too large [147].
Recently, the group of Diamandis reported that a Zn2+ concentration in the low nanomolar range is sufficient for inhibition of KLK14 (IC50 = 12 nM) [31], similar to their findings on Zn2+ inhibition of KLK5 (IC50 < 12 nM) [143]. However, these values seem to be problematic in that both proteases would basically never be active in the tissues or fluids, e.g. the epidermis, where they are predominantly expressed. Moreover, an earlier study measured an IC50 of 2 μM for KLK14 [15].
Zn2+ modulated activity of epidermal KLKs may be highly relevant under physiological conditions. Because Zn2+ levels in mammalian epidermis are in the higher micromolar range (50–70 μg/g dry weight) with the Zn2+/Ca2+ ratio playing a role in skin maturation [150], [151], this metal ion could be important in the activity regulation of the KLKs 5, 7 and 14, in addition to their natural polypeptide inhibitors, the lympho-epithelial Kazal-type inhibitor (LEKTI) (see Section 2.2.2.). Notably, topical ZnO as contained in the widely used zinc ointments for skin improves wound healing and re-epithelialization [151], which may be based on reduction of an otherwise excessive KLK activity. Although copper regulation of proteases is not established, one should consider this alternative to Zn2+, in particular for tissues that contain relatively high Cu2+ ion levels, such as the brain (0.5 μM), where KLK7 is also expressed [130], [152].
2.1.3. Inhibition of KLKs 8, 10, and 12 by Zn2+
The tissue distribution of the KLKs 8, 10, and 12 is more diverse than the aforementioned prostatic and epidermal KLK groups. Nevertheless, it has been demonstrated that KLKs 8 and 12 are inhibited by Zn2+ as well. For example KLK8, which is thought to have an important function in the hippocampus of the mammalian brain, is stimulated by 10 μM Ca2+, but inhibited by 3 μM Zn2+ [123]. According to the sequence alignment (Fig. 1) it possesses a His99, which could coordinate the inhibiting metal ion together with His57 and a backbone carbonyl, as in KLK7. In the case of KLK10 little is known about activation, natural substrates and regulation, except for the non-prime specificity and its relatively slow cleavage of some pro-KLK peptides [20], [99]. Since KLK10 possesses a Zn2+ binding site involving the catalytic triad with His57 and Asp102, as well as Asp99 in the S2 pocket, it is likely that bound Zn2+ would interfere with substrate binding and catalysis [107]. Similarly, knowledge of the physiological functions of KLK12 is scarce, although a systematic study on its inhibitors showed that its activity against fluorogenic substrates is attenuated by Zn2+ with an IC50 of 10 μM [17].
2.2. Proteinaceous endogenous KLK inhibitors
To date, the most important proteinaceous endogenous KLK inhibitors belong to the serpins (MEROPS class I.04), Kazal-type inhibitors (I.01) and the macroglobulins (I.39) [1]. Only two endogenous Kunitz-type inhibitors (I.02) acting on KLKs are known. The first one, bikunin, binds KLK1 in the picomolar range, which may also play an important role in lung function and diseases [153], [154], while bikunin is also associated with KLKs 6 and 10 in ovarian cancer ascites fluid [114]. The second one is an inhibitor of hepatocyte growth factor activator (HGFA), which is involved in tumor progression and is itself activated by KLKs 4 and 5, which in turn are inhibited by the domains of HGFA inhibitory type 1 (HAI-1) [155]. One member of the whey acidic protein (WAP) type inhibitor family (I.17), the secretory leukocyte peptidase inhibitor (SLPI, also anti-leukoprotease, ALP) was shown to inhibit KLK7, which appears to be significant in protease regulation during desquamation [156].
2.2.1. Serpins – KLK inhibition in blood and prostate
Serpins (derived from SERine Protease INhibitor) are proteins of 33–46 kDa that account for about 10% of human blood plasma [157], [158], [159]. They occur in all kingdoms of life, inhibit (chymo)trypsin-like and subtilisin-like serine proteases, as well as cysteine proteases, and play physiological roles even beyond protease inhibition [160], [161], [162]. In contrast to the substrate-like binding of canonical protease inhibitors, serpins act via a unique “springe suicide” mechanism on their target protease. After cleaving the P1–P1′-bond in the reactive center loop by formation of a covalent bond from the P1 residue to the catalytic Ser195, it is then rapidly translocated by about 70 Å to the opposite part of the inhibitor [163], [164]. Eventually, this reaction causes a massive structural disorder in the protease (up to 40%), mainly because all stabilizing interactions that were formed during enzyme activation are lost now, thus preparing the protease itself for final degradation by other proteases (Fig. 3) [165]. A wealth of mechanistic and structural studies, including several complex structures, have revealed fine details of various steps in serpin protease interaction (Fig. 4) [166], [167], [168], [169]. Although no serpin-KLK complex structure is known to date and the physiological connection of KLK serpin interaction is often unclear, serpins are the best studied KLK inhibitors [12], [25].
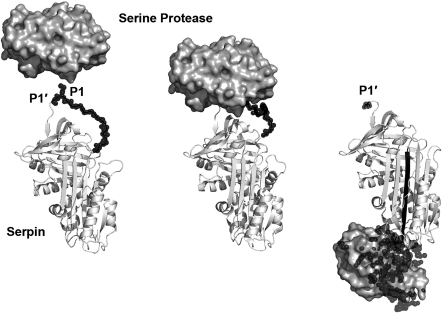
Fig. 3.
Reaction of a serine protease shown with the molecular surface with a serpin as ribbon representation. Left: Protease and unreacted “virgin” serpin with central four-stranded β-sheet (PDB codes of trypsin from 1OPH and α1-AT from 1QLP) [166], [302]. The reactive loop is depicted as black spheres, the P1 and P1′ side chain are labelled. Middle: Michaelis–Menten complex of the protease with serpin corresponding to substrate-like canonical protease inhibitor interaction (PDB code 1OPH). Right: Covalent serpin–protease complex that exhibits now a cleaved reactive center loop with an N-terminal P1′ residue and an inserted fifth strand (black) in the central β-sheet that is covalently linked with the P1 residues to the catalytic Ser195. The activation domain of the protease (black spheres) including a large region of the active site is disordered as seen in several protease zymogen structures (trypsin and α1-AT, PDB code 1EZX) [165].
Fig. 4.
Serpin reactive center loop (RCL) inserted into the active site of a serine protease represented as molecular surface (trypsin and α1-AT, PDB code 1OPH). Canonical (substrate-like) binding before cleavage of the scissile bond between P1–P1′ is mediated by residues P4 to P1′, shown as black stick models. Various serpins exhibit canonical binding extended to P3′ and may overall interact with residues from P6 to P5′ [184].
At first glance it seems surprising, that the serpin α1-antitrypsin (AAT) fails to inhibit the definite tryptic KLKs 1, 2, 5, 6, 8, 11, and 13 in vitro, whereas strong inhibition is observed for the chymotryptic KLK7 with an association constant kass of 3.9 × 106 M−1 s−1 (Table 2) [170], [171], [172], [173], [174], [175]. However, this inhibition pattern can be explained by the substrate-like reactive loop of AAT with the scissile bond between P1 Met and P1′ Ser that should be preferred by chymotryptic proteases, albeit with a low reaction rate of KLK3 [175]. The KLK3-AAT complex is found increased in benign forms of prostate hyperplasia from about 2% to 4% of total KLK3 in serum [176], [177]. Recently, immunological analyses of ovarian cancer ascites fluid indicated that fractions of KLKs 5, 6, 8, and 10 form complexes with ATT [114]. Also, AAT strongly inhibits the tryptic KLKs 4 and 14 and to a lesser degree KLK12 [17], [31], [170], [178].
Contrary to AAT, α1-chymotrypsin (ACT) has been detected in complex with up to 85% of total KLK3 in blood plasma by immunoassays, whereby an increased ratio of free to ACT-complexed KLK3 is correlated with progression of prostate cancer [176], [179]. Despite a significant concentration of ACT in seminal plasma, it is not bound to KLK3, which was explained by an interference of Zn2+ with complex formation [180]. The association constants kass for ACT with the chymotryptic KLK3, which seems to obey a slow inhibition kinetic, and KLK7 were 3.1 × 103 and 3.9 × 106 M−1 s−1, respectively [133], [170]. In line with the presence of a P1 Leu-P1′ Ser scissile bond, ACT does not or only moderately inhibit the tryptic KLKs 1, 2, 4, 5, 8, and 11–14 in vitro (Table 2) [31], [170], [172], [173], [178], [181], [182]. Interestingly, analyses of human milk and ascites showed that about 5% of the tryptic KLK6 are bound to ACT in an ACT-KLK6 complex, which remains stable after HPLC purification [182].
Antithrombin III (AT) is the major inhibitor of fibrin clot generating thrombin and other coagulation factors in blood plasma, which is further enhanced by a ternary complex with heparin that links positively charged exosites of AT and its target protease for optimal binding [183], [184]. The relatively slow AT inhibition seen for thrombin (kass = 104 M−1 s−1) is accelerated up to 1000-fold by heparin [185], [186]. A comparable effect of heparin has been observed for the inhibition of KLK2 by AT, which shows elevated expression and colocalization with KLK2 in tumor cells [187]. In accordance with a reactive center containing a P1 Arg-P1′ Ser, the chymotryptic KLK3 and KLK7 are not inhibited by AT [170], [187]. Among the tryptic KLKs, only KLK6 and KLK14 are moderately inhibited by AT, although no heparin was added [31], [174].
α2-Antiplasmin (AP), the major inhibitor of the fibrinolytic plasmin, is unique in that it binds to the zymogen and the mature protease [188], [189]. Whether AP is significant for the regulation of KLKs is an unsolved question. In agreement with the reactive bond P1-Arg-P1′-Met that seems to be designed for tryptic proteases, AP strongly inhibits KLKs 2, 4, 5, and 12 (kass = 2.2 × 105 M−1 s−1) and moderately inhibits KLKs 7, 8, and 13 [17], [31], [170], [172].
The relatively unspecific heparin-dependent proteinase C inhibitor (PCI) inhibits various coagulation factors, and can probably switch between the P2 Phe-P1 Arg-P1′ Ser residues in the reactive center, so that either Phe or Arg inserts into the S1 pocket of the protease [190], [191]. PCI is a fast binding inhibitor of KLKs 1, 2, 5, 7, 8, 13, 14, and a slow binding inhibitor for KLKs 3, and 12 [17], [170], [192]. Besides a fourfold increased association constant for PCI with KLK2 in the presence of heparin (kass = 8.7 × 105 M−1 s−1), an inhibition constant below 25 pM has been described [129]. Since PCI is present at high molar excess in seminal plasma, roughly 30% of total KLK2 and to a lower extent KLK3 are complexed with PCI, which are both implicated in the fertilization process [193], [194], [195].
Seemingly, one of the serpins, kallistatin, displays high specificity for KLKs, being a strong inhibitor of KLK1 and KLK7, while KLK14 is slowly inhibited [170], [196]. In spite of the apparent dual preference for tryptic and chymotryptic KLKs, it cannot inhibit KLKs 5 and 11–13 [170]. The expression of kallistatin in pancreas and kidney matches that of KLK1 [197]. Interestingly, the specificity of kallistatin for KLK1 is derived from the reactive bond P1 Phe-P1′ Ser, since an exchange to P1-Arg results in a twofold higher inhibition of KLK, but also in similar inhibition of various other serine proteases that are not inhibited by wild type kallistatin [198], [199]. Most likely, kallistatin regulates the KLK1 activity, influencing blood pressure and angiogenesis, which may also depend on the heparin binding capacity of kallistatin [200], [201], [202].
Inhibition of KLKs 5, 7, 8, 12–14 by the C1 inhibitor was only moderate in vitro, and may also not be significant in vivo, as well as the KLK3 inhibition by the monocyte/neutrophil elastase inhibitor (MNEI), which can switch at the reactive center P2 Phe-P1 Cys-P1′ Met to Phe as P1 residue for chymotrypsin-like proteases [170], [203]. The complex of KLK2 with serpin PI-6 that is associated with tissue damage, necrosis or neoplasia and the complex with PAI-1 that seems to promote cancer by inactivation of the primary uPA inhibitor [204], [205] are likely to be of physiological relevance in the prostate. However, serpins may interact with KLKs in other tissues, as exemplified by the human KLK8 (neuropsin) colocalization in keratinocytes with its strong inhibitor PI-6 [206] and by the inhibition of murine KLK8 by the tight binding serine proteinase inhibitor 3, which colocalize in mouse brain [207].
2.2.2. LEKTI-1 and 2 – multi-domain Kazal-type KLK inhibitors in skin
Soon after the discovery of the 15 domain, 120 kDa Kazal-type inhibitor, which was called lympho-epithelial Kazal-type inhibitor (LEKTI), it was found that mutations in its gene (SPINK5) cause Netherton syndrome. The syndrome is characterized by a severe autosomal recessive genodermatosis with altered desquamation, impaired keratinization, hair malformation and a skin barrier defect [208], [209]. SPINK5 knockout mice feature a Netherton syndrome-like condition, which is based on the hyperactivity of KLKs 5 and 7 that are not sufficiently controlled by their natural inhibitor, LEKTI-1 [210]. Human mutations in SPINK5 generate LEKTI-variants that have lost different C-terminal domains, such as d1-4 or d1-8, which is accompanied by higher concentrations and activity levels of most epidermal KLKs [211]. Mutations in the SPINK5 gene are also responsible for the widespread atopic dermatitis, which enhances the susceptibility of patients to food allergies and asthma [212], [213], [214]. In addition to the elevated degradation of corneodesmosomes in the stratum corneum, KLK5 causes atopic dermatitis-like lesions via PAR2-mediated thymic stromal lymphopoietin expression [215]. An NMR analysis of LEKTI-1 domains 1 and 6 demonstrated that their structures differ from the classical Kazal-type architecture, especially in the reactive center loops. This may be due to the lack of a third disulfide bond, which is present in the classical canonical serine protease inhibitor scheme. Only two of the 15 LEKTI-1 domains contain three disulfide bonds [216]. Nevertheless, several domains of LEKTI-1 are potent serine protease inhibitors and function according to the substrate-like canonical binding. Canonical Kazal-type inhibitors present the scissile bond between P1–P1′ to the protease, however, its cleavage is very slow compared with substrate turnover, as the hydrolysis constant Khydr is in the range of unity (Khydr = [Inhibitorcleaved]/[Inhibitoruncleaved] = 1) and the cleaved bond may be reconstituted again (Fig. 5) [89], [217]. Full length LEKTI-1 contains mostly potential P1 Arg residues, but also a P1 Gln-P1′ Asp in the reactive center loop of the first domain that might target chymotryptic proteases, in particular KLK7. This suggestion is supported by a study on natural truncated versions of LEKTI-1 that usually comprise the first domain and did not fail to inhibit the chymotryptic activity in the outer layers of skin [211]. Further investigations revealed that domain 6 alone can inhibit both KLK5 and KLK7 [218]. Recombinant LEKTI-1 fragments of domains 6–8 and 9–12 inhibited KLK5 with Ki values between 1.2 and 5.5 nM, while fragment d6-9′ (a truncated domain 9) inhibited KLK7 with a Ki of 11 nM [219]. Additionally, LEKTI-1 d1-6, d6-9′, and d9-12 inhibited KLK5, KLK6, KLK13, and KLK14 with Ki values in the range of 2–416 nM, while only KLK5 was inhibited by d12-d15 (Ki = 22 nM) [63].
Fig. 5.
Kazal-type inhibitor with reactive center loop (RCL) bound to the active site of a serine protease represented as molecular surface (trypsinogen with pancreatic secretory inhibitor, PDB code 1TGS). The 15 LEKTI-1 domains are homologous to the classical Kazal-type inhibitors, but 12 domains may be classified as subtypes. Canonical binding is achieved by residues P4 to P2′, shown as grey stick models with spheres. The bond between P1–P1′ is scissile, but cleavage is extremely slow, since the hydrolysis constant is close to unity and the cleaved bond may reconstituted again [89]. Specificity of LEKTI-1 domains for chymotryptic or tryptic KLK targets depends on the P1 residue, which is in most cases an Arg, but a Gln in the first domain.
In cultured keratinocytes and in the epidermis it was demonstrated that the LEKTI-1 precursor is secreted as several fragments, of which d5, d6, d8-11, and d9-15 specifically inhibit KLK 5, 7, and 14, whereby the strongest inhibition was seen for KLK5 with d8-11, although a shift to acidic pH dissociated the otherwise nearly irreversible, tight binding complex [32].
Recently, a polypeptide of 85 amino acids, expressed in skin and encoded by the SPINK9 gene with high homology to LEKTI-1 was discovered and termed LEKTI-2 [220]. This polypeptide was found colocalized with KLK5 in the epidermis, which possesses the unusual reactive bond P1-His-P1-Met and inhibits recombinant KLK5 (Ki = 65 nM), KLK8, but not KLK7 or 14 [221].
2.2.3. Macroglobulins – large compartments for unspecific protease removal
In a classical paper, Barret and Starkey elaborated a mechanism for α2-macroglobulin (α2M) inhibition, a huge protein of 720 kDa that was able to block the proteolytic activity of nearly all proteases. At that time it was also known that an α2M inhibited protease was still active against small substrates, but no longer cleaved proteins. Thus, it was proposed that α2M is proteolytically attacked by its target protease in a so-called bait region, which causes a conformational change in α2M, resulting in an irreversibly trapped protease [222]. This mechanistic description has remained valid and has been supported and elaborated on by structural information from electron microscopy and X-ray crystallography. The structural arrangement of the protease free α2M comprises two dimers with a large central cavity [223]. Upon protease cleavage in the bait sequence P692QLQQYEMHGPEGLRVGFYESDV-MGRGHARLVHVEEPHT730 and subsequent thiol ester cleavage near the central region, two β-strands of α2M untwist to expose the central cavity to proteases, e.g. to chymotrypsin, of which two molecules were covalently linked inside the central cavity [224], [225]. Since the reactive internal β-cysteinyl-γ-glutamyl-ester of α2M can react with any exposed Lys side chain on a protein surface, any protease can be trapped, and thus α2M has been termed a “panproteinase inhibitor”, which clears the blood from proteases destined for degradation [226], [227]. Typically, the protease is not fixed to a large extent and retains a considerable flexibility inside the α2M cavity after trapping [228].
The ability of α2M to effectively internalize and trap KLK1 has been demonstrated in vitro and it was even used as a tool to remove it from samples, where the KLK1 activity was unwanted [222], [229]. However, α2M complex formation with KLKs is of physiological significance, too, as observed for KLK2 in serum of prostate cancer patients [230], [231]. Also, KLK3 forms readily stable complexes with α2M in blood serum, as well as with the α2M homologue pregnancy zone protein (PZP), which exhibits some interesting mechanistic differences to α2M [232], [233]. Interestingly, KLKs 4, 5, 6, 12, and 13 were not significantly inhibited by α2M [17], [101], [173], [174], [178], although in mouse brain KLK8 (neuropsin) was inhibited by the α2M homologue murinoglobulin 1 and a novel inhibitor, phosphatidyl ethanolamine binding protein (PEBP) [207], [234].
2.2.4. Autoinhibition in KLK4 oligomers – an exceptional case?
Compartmentalization within proteinaceous inhibitors as in the macroglobulins is quite rarely realized, whereas proteases provide more examples of that kind. In the proteasome, bleomycin hydrolase, tricorn protease, or α- and β-tryptases the oligomerization leads to the formation of an internal cavity or blocking of the active sites that are not accessible for most potential protein substrates [235], [236], [237], [238], [239], [240]. This compartmentalization represents some kind of self-inhibition, and can be overcome by activators, which induce a conformational change or transfer substrates to the active site as in the 26S proteasome [241].
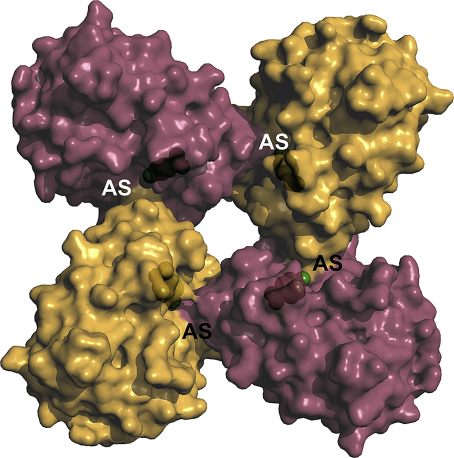
In this context, recombinant, refolded KLK4 exhibited 700 kDa oligomers in solution, corresponding, to 24-mers, which were hardly active against chromogenic and fluorogenic substrates [137]. On the contrary, KLK4 that was refolded in the presence of 2 mM Ca2+ was only monomeric (ca. 25 kDa) and highly active, resulting even in autodegradation within a few hours [107]. Also, these studies revealed that in three known crystal forms KLK4 assembled to oligomers, consisting of stacked cyclic tetramers or octamers, respectively, in which the active sites are partially blocked (Fig. 6). Since binding and turnover of even small tripeptidyl-pNA substrates is sterically not possible, in particular due to a partially blocked S2 subsite, the tetramers and octamers appear to be self-inhibiting protease forms with regulatory potential [137].
Fig. 6.
Potential autoinhibitory homocomplex of KLK4 with monomers depicted as alternating purple and orange surface representations. In solution and in crystals KLK4 forms oligomers, consisting of cyclic tetramers that assemble to octamers, or even higher oligomers, such as dimers and tetramers of octamers. Due to intermolecular contacts, the active site clefts of KLK4 become very narrow at the catalytic centers (labelled AS) with Ser195 shown as green balls. In particular the S1 and S2 subsites are hardly accessible for small chromogenic and fluorogenic substrates, not to mention large protein substrates [137].
3. Exogenous and synthetic inhibitors in KLK research
In biochemical and biophysical studies both exogenous natural inhibitors and synthetic small molecules have been used, mostly to analyze the primary specificity of the respective kallikrein-related peptidase and then for refined studies of extended substrate specificity. This approach can be exemplified by a study on mouse KLK1b27, in which the enzyme was characterized as chymotryptic serine protease, according to its inhibition by diisopropylfluorophosphate (DIFP), phenylmethylsulfonyl fluoride (PMSF), and tosyl-phenyl-chloromethyl ketone (TPCK) and chymostatin, in contrast to the weaker inhibition by the tryptic enzyme inhibitors tosyllysylchloromethyl ketone (TLCK), leupeptin, antipain, soybean trypsin inhibitor (SBTI), and aprotinin (bovine pancreatic trypsin inhibitor, BPTI) [242]. Such knowledge often helps to improve the purification of a KLK by preventing autodegradation. Although the above mentioned inhibitor molecules are rather unspecific and bind to a great variety of proteases, they may serve as starting point for highly specific compounds, as in the recent example of an activity-based compound that was employed for immunofluorimetric measurements of active KLK6: the compound biotin-(PEG)4-Pro-Lys-diphenylphosphonate formed a covalent bond to the nucleophilic Ser195 Oγ [243].
3.1. Small molecules
3.1.1. Sulfonyfluorides and diisopropylfluorophosphate as catalytic serine inhibitors
The unspecific irreversible, but toxic serine protease and esterase inhibitors PMSF (Pubchem CID 4784, http://pubchem.ncbi.nlm.nih.gov/) or DIFP (Pubchem CID 5936) and their derivatives react with nucleophilic serine and cysteine residues, as does the non-toxic trypsin-specific aminoethyl benzenesulfonyl fluoride (AEBSF, Pubchem CID 1701) [244]. For KLK1, inhibition by PMSF and for KLK3, reaction with PMSF and AEBSF has been reported [42], [245]. Due to the high toxicity, the volatile DIFP is not used for purification purposes.
3.1.2. Benzamidines as tryptic S1 inhibitors
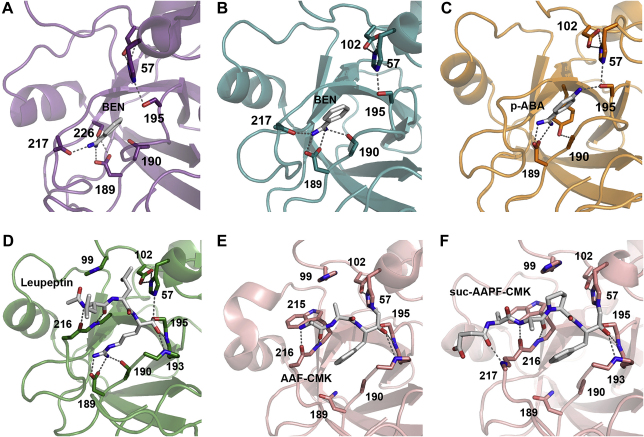
Both benzamidine (BEN) and its derivative para-aminobenzamidine (p-ABA) are specific for tryptic proteases, since they fill the S1 pocket and usually form a salt bridge between their positively charged amidino group and the negatively charged Asp189. Due to a lack of additional interaction with the protease, such as binding to the oxyanion pocket or to the S2–S4 subsites, inhibition constants of benzamidine range from high μM to mM. In case of KLK6 63 μM BEN was required for 20% inhibition, while the inhibitor was applied at 20 mM for crystallization and was well defined in the S1 site of the KLK6 structure [174], [246]. Definitely, BEN and p-ABA are useful tools in protein purification and crystallization of tryptic KLKs, since they inhibit the proteolytic activity and rigidify the active site, as corroborated by more than 100 corresponding crystal structures (Pubchem CIDs 2332 and 1725, http://pubchem.ncbi.nlm.nih.gov/). Thus, crystal structures of pKLK1, KLKs 4 and 6 in complex with benzamidine or para-aminobenzamidine were solved, respectively, explaining subtle differences of the S1 specificity determinants (Table 3, Table 4) (Fig. 7A, B, C) [137], [246], [247].
Table 3.
Exogenous and non-natural inhibitor affinity matrix. The rating (○ no inhibition, etc.) is applied as for Table 2. ∗TPCK related compounds (see Table 4).
| KLK | PMSF/AEBSF/DIPF | BEN/p-ABA | Leupeptin/antipain | Chymostatin | TLCK/PPACK | TPCK | SBTI/LBTI | BPTI |
|---|---|---|---|---|---|---|---|---|
| KLK1 | ● [245] |
● [310] |
● [245] |
○ [245] |
● [245] |
|||
| KLK2 | ● [129] |
● [129] |
●● [129] |
● [129] |
||||
| KLK3 | ● [42], [175] |
○ [42] |
○ [42] |
● [249] |
○ [42] |
○ [42] |
○ [42] |
○ [42] |
| KLK4 | ● [48], [137] |
● [48] |
● [48] |
●● [48] |
||||
| KLK5 | ● [15], [144] |
○ [15] |
● [15] |
● [15] |
||||
| KLK6 | ● [174] |
●● [174] |
||||||
| KLK7 | ● [147]* |
|||||||
| KLK8 | ● [123] |
● [123] |
● [123] |
○ [123] |
||||
| KLK12 | ● [17] |
○ [17] |
● [17] |
|||||
| KLK14 | ● [15] |
● [15] |
● [15] |
● [15] |
Table 4.
Structures of KLK peptidases with PDB accession codes: pKLK, mKLK, rKLK, eKLK: porcine, murine, rat, equine kallikrein-related peptidase. Leupeptin = N-acetyl-l-leucyl-l-leucyl-l-argininal, Suc = succinyl, CMK = chloromethyl ketone, BPTI = bovine pancreatic trypsin inhibitor. *Acyl-intermediate, derived from morpholino-carbonyl-KGISSQY-7-amino-4-(trifluoromethyl)-coumarin. ** Structure not deposited. *** Un-published data.
| KLK | pro | apo | Inhibitor | Ion | Small molecule | Polypeptide |
|---|---|---|---|---|---|---|
| KLK1 | 1SPJ [311] | 1HIA [263] | hirustasin | |||
| pKLK1 | 2KAI [312] | BPTI | ||||
| 2PKA [247] | benzamidine | |||||
| rKLK2 (tonin) | 1TON [145] | Zn2+ | ||||
| KLK3 | 2ZCH [134] | 2ZCK [134] | Mu-KGISSQY- * | |||
| eKLK | 1GVZ [136] | Zn2+[136]** | ||||
| KLK4 | 2BDH [137] | Zn2+ | p-aminobenzamidine | |||
| KLK5 | 2PSX [144] | Ac-LLR- (leupeptin) | ||||
| 2PSY [144] | Zn2+ | Ac-LLR- (leupeptin) | ||||
| KLK6 | 1GVL [313] | 1L2E [246] | benzamidine | |||
| KLK7 | 3BSQ [314] | 2QXG [147] | AAF-CMK | |||
| 2QXH [147] | Suc-AAPF-CMK | |||||
| 2QXJ [147] | Cu2+ | Suc-AAPF-CMK | ||||
| KLK8 | *** [315] | Zn2+ | Ac-LLR- (leupeptin) | |||
| mKLK8 | 1NPM [316] | |||||
| mKLK13 | 1AO5 [317] | |||||
| KLK b3 | 1SGF [295] | Zn2+ |
Fig. 7.
Inhibitory small molecules bound to KLK active sites with important residues depicted as stick models. Hydrogen bonds with a distance shorter than 3.5 Å are shown as grey dotted lines and relevant residues are labelled with numbers. A: Porcine pKLK1 with benzamidine (BEN) occupying the S1 site. The BEN amidino group binds Asp189 only with one amino group, but involves the Ser226 Oγ and the backbone carbonyl O of His217. B: KLK6 binds the two amidino N atoms of benzamidine in the S1-pocket with both Oδ atoms and one with the Oγ of Ser190, as well as with the backbone carbonyl O of Asn217, similar to trypsin [303]. C: The binding mode of para-aminobenzamidine (PABA) to KLK4 is similar to KLK6, but the Ser190 does not participate in stabilizing the amidino groups of the inhibitor, which is additionally bound at the amino group by Ser195. In addition to Asp189, the Ser190 Oγ is enhancing the specificity of the S1 subsite for P1-Lys residues [304], [305]. D: Leupeptin binds with the P1-Arg C covalently to the Ser195 Oγ of KLK5, while the former carbonyl O (now rather a –O−) is accommodated in the oxyanion hole, formed by the backbone NHs of Ser195 and Gly193. The P1 Arg side chain is stabilized by Asp189 and Ser190. For clarity, additional hydrogen bonds of the P2-Leu carbonyl O and P1 Arg Nɛ via a water to Gln192 have been omitted. The relatively small hydrophobic S2 subsite is filled with the P2-Leu side chain. While the P3-Leu side chain extends to the solvent, its carbonyl O and amide NH tightly bound to the corresponding atoms of Gly216. The acetyl group of leupeptin partially occupies the hydrophobic S4 pocket. E: Ala-Ala-Phe-chloromethyl ketone (AAF-CMK) is covalently linked with the P1 methylene group to His57 Nɛ and via the C atom to the Ser195 Oγ of KLK7, whereby the former carbonyl O occupies the oxyanion hole. The P1-Phe side chain is located in the large S1 pocket, but does not interact with the Asn189 at the bottom of the pocket, which is more suited to bind residues with polar tips, such as Tyr or Gln, while Ala190 contributes to the hydrophobic character of this subsite [99]. Also, the S2 pocket is rather mixed hydrophobic polar and not completely filled by the P2 Ala, whereas the P3 Ala extends to the bulk solvent and forms hydrogen bonds from its backbone C O and NH to the backbone of Gly216. F: Covalent bonds of Suc-AAPF-CMK to His57 and Ser195 of KLK7, as well as the orientation of the oxyanion and the P1-Phe side chain are the same as for AAF-CMK (Fig. 7E). The P2 Pro fits equally well as an Ala to the S2 subsite, bordered by His57 and His99 (Fig. 7E). Again, the P3 Ala makes only backbone interactions with Gly216, but contrary to the AAF-CMK, the P4 Ala occupies to some extent the hydrophobic S4 pocket, with the Trp215 side chain as base and His99 and Leu175 as borders, while the succinyl group in P5 position has no interaction partner on the KLK7 surface.
3.1.3. The aldehydes leupeptin, antipain, chymostatin interact with the S1 to S4 pockets
Remarkably, higher organisms utilize exclusively ions or polypeptides as protease activity attenuators or inhibitors, whereas microorganisms produce various small molecule inhibitors, which target host proteases [89]. For example, leupeptin (acetyl-Leu-Leu-Arginal) and antipain (carboxyphenylethyl-carbamoyl-Arg-Val-Argininal) are suitable competitive KLK inhibitors that are produced by Actinobacteria, such as Streptomyces [248]. The aldehyde function of leupeptin and antipain reacts with Ser and Cys nucleophiles to a transition state-like inhibited protease complex, which is reversible. Their P1 Arg is suited to inhibit tryptic KLKs, e.g. KLK2 around 100 μM, while antipain was a much stronger inhibitor of KLK8 (IC50 = 460 nM) than leupeptin (IC50 = 66 μM) [123], [129]. Also, Leupeptin inhibited KLK5 at 1.7 to 10 μM and cocrystallized with this protease, whereby the N-terminal extension occupied the subsites S2–S4 [15], [144] (Fig. 7D). The widely used chymostatin is the counterpart of leupeptin and antipain and is directed against chymotryptic proteases. It corresponds largely to a tetrapeptide with a leucyl-phenylalaninal moiety and reacts with proteases as the above described aldehydes. Chymostatin inhibition was described for the chymotryptic KLK3, but also for the tryptic KLKs 8 and 14 (IC50 = 8 μM and 30 μM) [15], [123], [249].
3.1.4. Chloromethyl ketones
Contrary to reversible binding inhibitors, chloromethyl ketones (CKs, CMKs) are widely used irreversible cysteine- and serine protease inhibitors, which, similar to aldehydes, allow the design of specific inhibitors for an individual target. For example, such covalent inhibitors are suitable for co-crystallization with proteases, but often require an iterative process of optimization. For the analysis of the primary KLK specificity some standard compounds have been applied. In case of the tryptic KLKs 1, 4, 8 of tosyl-lysyl chloromethyl ketone (TLCK) was an efficient inhibitor, as well as d-phenylalanyl-l-prolyl-l-arginyl chloromethyl ketone (PPACK), which inhibited KLK2 at 5 μM [48], [123], [129], [245]. Surprisingly, the chymotryptic counterpart tosyl-phenylalanyl chloromethyl ketone (TPCK) does not seem suitable to inhibit KLK3, whereas KLK7 was successfully crystallized with Ala-Ala-Phe-CMK and succinyl-Ala-Ala-Pro-Phe-CMK, resulting in structures with a resolution up to 1.0 Å (Fig. 7E, F) [147].
3.2. Polypeptidic inhibitors
3.2.1. Kunitz- type trypsin inhibitors
Several canonical substrate-like binding Kunitz-type inhibitors have been employed in research for the analysis of KLK enzymatic activity (Table 3) [89]. The most common ones are the soybean trypsin inhibitor (SBTI, I03.001 according to MEROPS), the lima bean trypsin/Bowman-Birk inhibitor (LBTI, I12.001, which occurs as compound inhibitor LI12-001 consisting of I12.001 and I12.008) and aprotinin, the bovine pancreatic trypsin inhibitor (BPTI, I02.001), which is only found in the genus Bos. Interactions of proteases and the 181 amino acid SBTI are well understood on functional and structural level, not least because its reactive P1 Arg-P1′ Ile bond was an early target of bioengineering [250], [251]. The much shorter LBTI comprises 71 residues with a P1 Lys-P1′ Ser reactive bond for trypsin-like and a P1 Leu-P1′ Ser for chymotrypsin-like proteases, but some related Bowman-Birk inhibitors possess a second reactive bond with P1 Arg [252], [253]. Due to its anticarcinogenic effects this inhibitor type from plant nutrients gained much interest in medical sciences [254]. SBTI efficiently inhibited the tryptic KLKs 5 and 14 at an IC50 of 100 nM, while KLK6 required only 16 nM for 82% inhibition and KLK4 about 50 μM for 99% inhibition [15], [48], [174]. It was also demonstrated that SBTI is a good inhibitor of rKlk2 or tonin (Ki = 160 nM) and rKlk1-c9 (Ki = 70 nM) [111].
Mature BPTI is 58 residues long and presents a reactive loop with a scissile P1 Lys-P1′ Ala bond to tryptic proteases. The BPTI/bovine trypsin complex was one of the first refined crystal structures and revealed, in addition to details of canonical binding, that the Ser195 Oγ is covalently bound to the tetrahedral C atom of Lys15, representing the transition state of the cleavage reaction [255], [256]. These findings were consistent with kinetic studies that found for BPTI with trypsin KHydrolysis = [BPTIcleaved]/[BPTIuncleaved] = 0.33, whereby equilibrium is reached in about one year [257]. Applications of BPTI are manifold in laboratories, such as the engineering of its specificity, or its use in cardiopulmonary surgery and organ transplantation [258], [259].
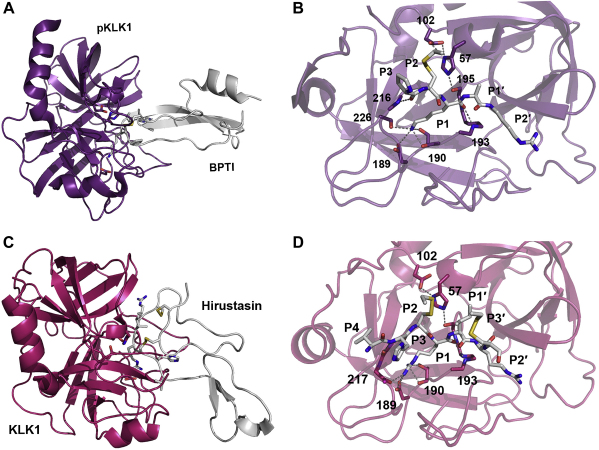
Astonishingly, the pioneers of kallikrein protease research, Kraut, Werle, and Frey discovered as early as 1930 that certain extracts of bovine glands (containing BPTI) inhibited the enzymatic activity of kallikrein (KLK1) [260]. These seminal studies were later confirmed and evidence was found that other tryptic KLKs are well inhibited by BPTI, such as KLK 2 (IC50 = 30 μM), rKlk2 (Ki = 76 μM), KLK4 (100% inhibition with 4 μM BPTI), KLK5 (IC50 = 3 μM), KLK12 (IC50 = 2 μM), and KLK14 (IC50 = 3 μM) [15], [17], [48], [129], [245]. Despite its considerable specificity for tryptic KLKs, to date, only one kallikrein structure in complex with BPTI has been solved, demonstrating canonical inhibitor binding in a transition state-like manner for porcine pKLK1 (Fig. 8A, B) [247].
Fig. 8.
Two complexes of porcine and human KLK1 with the exogenous inhibitors BPTI (Kunitz-type) and hirustasin (antistasin-type), respectively. Proteins are shown as ribbon representation, relevant residues as sticks labelled with numbers, and hydrogen bonds as grey dotted lines. A: pKLK1-BPTI complex displayed with the protease in purple and the inhibitor in white. B: Closer view of the active pKLK1 site, where only the interacting reactive loop residues of BPTI are shown. The P1 Lys is bound with its carbonyl O to the oxyanion hole, while its Nζ-atom is hydrogen bonded to the Thr190 Oγ and carbonyl O, the Ser226 Oγ and virtually not to the Oδ1 of Asp189. The P2 Cys14I-Cys38I (I for inhibitor chain) disulfide bridge occupies the S2 pocket, while the P3 Pro only forms one hydrogen bond with its carbonyl O to the NH of Gly216. In contrast to many other canonical binding inhibitors, no interaction of the P4 residue Gly is observed. On the prime side, P1′ Ala fits to the S1, pocket, but the P2′ Arg exhibits a non-canonical binding around the S2, and S3, subsites. C: KLK1-hirustasin complex depicted with the protease in magenta and the inhibitor in white. D: Closer view of the active KLK1 site, where the inhibitor residues P4 to P3′ are displayed, although canonical binding ends with P1′. The P1 Arg of hirustasin is bound the oxyanion hole and its side chain fills the S1 pocket largely, whereby the Nη1 atom is hydrogen bonded to the Thr190 Oγ and carbonyl O, the Nη1 binds the carbonyl O of His217, whereas the interaction distances with the Oδ atoms of Asp189 are relatively long. Similar to BPTI, the P2 Cys29-Cys48 disulfide bridge is located in the S2 pocket and rigidifies the reactive loop region together with the P3′ Cys33I-Cys50I disulfide, which adopts as the P2′ Arg a not-substrate-like position, in contrast to the P1′ Ile. Typical for canonical P3 and P4 residues, the side chain of His28I extends to the solvent and Val27I occupies the S4 subsite.
3.2.2. Hirustasin
Although produced by the leech Hirudo medicinalis, the canonical 55 residue inhibitor hirustasin with a reactive P1 Arg-P1′ Ile bond apparently inhibits not blood coagulation, as the thrombin inhibitor hirudin or other protease inhibitors from leech [261], [262]. Moreover, hirustasin discriminates strongly between KLK1 (Ki = 13 nM) and plasma kallikrein, which is not inhibited at all [261]. A P2 Cys forming a disulfide characterizes this unusual inhibitor type (I15.001) from the antistasin family, of which a crystal structure was solved in complex with KLK1, exhibiting the typical transition state geometry at the reactive P1-Arg and tight interactions from P4 to P2 with the corresponding specificity pockets of the protease (Fig. 8C, D) [263].
4. Pharmaceutical approaches
Most of the small molecule inhibitors that have been employed in biochemical studies of KLKs are often of insufficient specificity, or cannot be administered to patients. Thus, the synthesis of biotolerable drugs or the engineering of large biomolecules from natural sources is required. The function of distinct KLKs in certain diseases is not always well defined, as shown in an overview disease matrix (Table 5). However, KLKs are attractive diagnostic and therapeutic targets, particularly the members of the family that are involved in prostate cancer and skin diseases [264].
Table 5.
Disease matrix for human kallikrein-related peptidases. The double dots indicate confirmed and the single dots putative roles in the respective disease, also in cases when the KLK is used as a disease marker or acts as a tumor suppressor.
| KLK | Cancer |
Skin disease |
Teeth |
Lung |
Brain |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prostate | Ovarian | Breast | Netherton syndrome | Atopic dermatitis | Amylo-genesis Imperfecta | Asthma | Multiple sclerosis | Alzheimer | Epilepsy | |
| KLK1 | ●● [318] |
|||||||||
| KLK2 | ● [25] |
|||||||||
| KLK3 | ●● [25] |
● [25] |
||||||||
| KLK4 | ●● [47], [319] |
●● [58], [59] |
||||||||
| KLK5 | ●● [25], [114] |
● [25] |
●● [211] |
● [60] |
||||||
| KLK6 | ●● [25], [114] |
● [26] |
● [211] |
● [70] |
● [320] |
|||||
| KLK7 | ●● [211] |
● [60] |
||||||||
| KLK8 | ●● [25], [114] |
● [26] |
● [211] |
● [60] |
● [76] |
● [77] |
||||
| KLK10 | ●● [25], [114] |
● [25] |
||||||||
| KLK11 | ● [25] |
● [25] |
● [26] |
● [211] |
● [60] |
|||||
| KLK13 | ● [25] |
● [211] |
||||||||
| KLK14 | ●● [31] |
● [25] |
● [211] |
|||||||
4.1. Synthetic small compounds directed against KLK3 and KLK7
In order to maximize bioavailability and efficiency some basic principles should hold true for the compound to be designed. A famous and successful guideline for small molecule drugs is to obey Lipinski’s “rule of five” and its recent modifications [265], [266], [267]; nonetheless, a significant part of pharmaceuticals is derived from natural compounds which tend to exceed the initial rule’s suggested molecular weight of 500 Da [268]. It is also of high importance to consider the exact time and tissue localization in an organism for the administration of a given drug, in order to avoid undesirable side effects [269]. Strategies to obtain optimal drugs usually employ combinations of 1) massive screening campaigns; 2) “directed evolution”; 3) “rational design”, often supported by structural data of biomolecules as well as a more sophisticated multiparameter design, yielding extremely tight binding serine protease inhibitors [270], [271], [272], [273]. Common features of small molecule serine protease inhibitors are peptidomimetic groups that bind some of the specificity pockets and an altered “scissile” bond, which may react with the catalytic serine, such as those present in phosphonyl, boronic acid, inverse substrate esters, α-keto heterocycles, isocoumarins, and succinimide derivatives [274].
The major target among the KLKs for medical and pharmaceutical research is definitely KLK3/PSA. Thus, small molecules have been designed by pharmaceutical researchers and companies, stimulated by the recent advancements of structural data on several KLKs (Table 4). Due to the required mimicking of a natural active site binding molecule, such inhibitory compounds often contain amino acid derived groups, as the β-lactam derived 2-azetidinone with a Tyr side chain that inhibited KLK3 with an IC50 of 226 nM and was apparently designed obeying the “rule of five” [275]. In particular, for KLK3 heterocyclic compounds in a structure–activity relationship approach reached an IC50 of 300 nM [276].
The pharmaceutical relevance of KLK7 inhibitors is evidenced by patents for a small molecule KLK7 inhibitor [277] (“Use of cyclic depsipeptides to inhibit Kallikrein 7”, WO2009024528 (A1). Novartis) that was analyzed by X-ray crystallography with the purpose of applying such compounds in treatments for human skin diseases, in particular atopic dermatitis, psoriasis or Netherton’s syndrome [278].
4.2. Peptide-based inhibitors of KLKs
Already in 1981, a very efficient inhibitor of KLK1 was generated, which contained a P3-d-Phe residue and reached an IC50 of 800 nM [279]. Potent analgesic and anti-inflammatory peptidic compounds that contain the non-natural amino acid l-4-aminomethyl-phenylalanine inhibited KLK1 with a Ki of 1.2 μM [280]. Another synthetic peptide-based inhibitor specific for KLK1 (FE999024) was capable to attenuate breast cancer cell invasion in a Matrigel assay [281]. Pure peptide based-inhibitors of KLK2 for targeting prostate cancer were found by screening phage display peptide libraries, resulting in 10–11 amino acid long linear peptides with IC50 values reaching 1.4 μM [282]. This basic approach was extended and improved by introducing cyclic peptides as efficient KLK2 inhibitors [283], [284]. Also, so-called azapeptides that contain one N-atom instead of a Cα, e.g. tBOC-Ser-Phe-aza-Tyr-phenoxy, resulted in KLK3 inhibition with a Ki of 500 nM [285]. Non-hydrolyzable boronic acid inhibitors, such as carbobenzyloxy-Ser-Ser-Lys-P1-Leu-(boro)-P1′-Leu, turned out to be much more specific for the chymotryptic KLK3 with a Ki of 65 nM than for chymotrypsin itself (Ki = 3.9 μM) [286]. This inhibitor type was improved by introducing the non-natural amino acid nor-leucine (Nle) into CBZ-Ser-Ser-Gln-Nle-(boro)-Leu to result for KLK3 in a Ki of 25 nM, which could be employed as imaging agent for prostate cancer by attachment of a metal chelating group to the compound [287]. A detailed overview of peptide based and other small molecule inhibitors of KLK3 by LeBeau and coworkers is focussed on their usage in selective imaging and in targetted treatment of prostate cancer [288]. Recently, the naturally occurring 14 residue long cyclic sunflower trypsin inhibitor (SFTI of the Bowman-Birk family) was re-engineered by a combination of molecular modeling and sparse matrix substrate screening, to block the proteolytic activity of KLK4 (Ki = 3.6 nM) and potentially block stimulation of PAR activity [289]. Fully synthetic domain 6 of LEKTI, which was generated by fragment condensation of residues 1–22 and 23–68, inhibited KLK5 with an IC50 of 120 nM, which is slightly lower than measured for the recombinantly produced peptide (IC50 = 135 nM) [290].
4.3. Endogenous human inhibitors serve as scaffold for therapeutic agents
After determination of the substrate specificity of KLK2 [106], selected substrates sequences were transplanted into the reactive site loop of α1-antichymotrypsin (ACT), yielding a highly specific KLK2 inhibitor with high reactivity, which could be a useful tool to detect and target prostate cancer progression [291]. In a related approach, based on determination of the KLK14 specificity by phage display [104], selected substrates were incorporated as modified reactive site loop into the scaffold of serpins α1-antitrypsin (AAT) or ACT, which formed covalent complexes with KLK14, especially the engineered ACT inhibitors [292].
4.4. Engineered antibodies inhibit the proteolytic activity of KLKs
The antibody scaffold for the design of novel protease inhibitors offers some advantages over common inhibitors that bind in a substrate-like manner, since antibodies can in addition to binding of the often conserved protease active sites exploit binding to more variable individual exosites on the protease surface, which may enhance the selectivity of inhibition extraordinarily [293]. In case of KLK1 an engineered antibody bound specifically to the active site of the tryptic protease, resulting in inhibition of the peptidase activity with an inhibition constant around 130 pM, paving the way for a novel cure of asthma [294]. Also, a monoclonal antibody against KLK4 was capable of reducing its activity in vitro, although this engineered molecule needs further optimization [178]. Similarly, for KLK13, which is overexpressed in ovarian cancer patients, a monoclonal antibody inhibited its activity, leading possibly to a therapeutic application [14].
5. Conclusions and outlook
Kallikrein-related peptidases are a family of serine proteases that has diversified through evolution for physiological tasks that have arisen with the special developments of mammals compared with their ancestors. These novel functions comprise proteolytic processes in fertilization, desquamation, or a sophisticated neuronal plasticity. However, the activity of KLK proteases requires tight regulation by modulators, as activating or attenuating ions or reversible and irreversible proteinaceous inhibitors. In some cases, the failure of endogenous inhibitory systems in humans leads to excessive activity of KLKs with pathophysiological consequences.
Apart from the current scientific and pharmacological key interest, KLK functions may go far beyond the normal protease activity, as seen in some impressive examples depicting the regulatory potential of KLKs. First, in mouse nerve growth factor 7S, the two active mouse KLK b3 proteases or γ subunits are inhibited by Zn2+ mediated complex formation with an inactive β subunit and two inactive α subunits (KLK homologs), whereby the γ active sites are shielded from potential substrates [295], [296], [297]. Secondly, the mysterious localization of KLK4 in the cytoplasm as full length protein and in the nucleus of transfected prostate cancer cells (PC-3) as N-terminally truncated KLK4-205 awaits new functional models for KLKs, in particular due to a likely involvement in cancer [298], [299]. Built according to the rather simple scaffold of (chymo)trypsin-like serine proteases, the kallikrein-related peptidases represent exciting biomolecules with astonishing variations in their structure, function, and physiological regulation. Eventually, our increasing knowledge of the underlying mechanisms of KLK activity control, will help to design very specific compounds directed to individual KLKs in order to cure diseases in which they play an important role.
Acknowledgments
We would like to thank Wolfram Bode and Mekdes Debela for valuable discussions and continuous support of our study, and Sabah Mirza for critical reading of the manuscript, as well as the Austrian Science Fund FWF (project P20582) for financial support.
References
- 1.Barrett A.J., Tolle D.P., Rawlings N.D. Managing peptidases in the genomic era. Biol. Chem. 2003;384:873–882. doi: 10.1515/BC.2003.098. [DOI] [PubMed] [Google Scholar]
- 2.Lundwall A., Band V., Blaber M., Clements J.A., Courty Y., Diamandis E.P., Fritz H., Lilja H., Malm J., Maltais L.J., Olsson A.Y., Petraki C.D., Scorilas A., Sotiropoulou G., Stenman U.H., Stephan C., Talieri M., Yousef G.M. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biol. Chem. 2006;387:637–641. doi: 10.1515/BC.2006.082. [DOI] [PubMed] [Google Scholar]
- 3.Meyer W. Distribution of tissue kallikrein in the integumental blood vessel system of wild mammals. Cell. Mol. Biol. (Noisy-le-grand) 2001;47:OL125–130. [PubMed] [Google Scholar]
- 4.Elliott M.B., Irwin D.M., Diamandis E.P. In silico identification and Bayesian phylogenetic analysis of multiple new mammalian kallikrein gene families. Genomics. 2006;88:591–599. doi: 10.1016/j.ygeno.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings N.D., Morton F.R., Kok C.Y., Kong J., Barrett A.J. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson A.Y., Lundwall A. Organization and evolution of the glandular kallikrein locus in Mus musculus. Biochem. Biophys. Res. Commun. 2002;299:305–311. doi: 10.1016/s0006-291x(02)02629-3. [DOI] [PubMed] [Google Scholar]
- 7.Yousef G.M., Chang A., Scorilas A., Diamandis E.P. Genomic organization of the human kallikrein gene family on chromosome 19q13.3-q13.4. Biochem. Biophys. Res. Commun. 2000;276:125–133. doi: 10.1006/bbrc.2000.3448. [DOI] [PubMed] [Google Scholar]
- 8.Harvey T.J., Hooper J.D., Myers S.A., Stephenson S.A., Ashworth L.K., Clements J.A. Tissue-specific expression patterns and fine mapping of the human kallikrein (KLK) locus on proximal 19q13.4. J. Biol. Chem. 2000;275:37397–37406. doi: 10.1074/jbc.M004525200. [DOI] [PubMed] [Google Scholar]
- 9.Schmaier A.H., Mccrae K.R. The plasma kallikrein-kinin system: its evolution from contact activation. J. Thromb. Haemost. 2007;5:2323–2329. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 10.Colman R.W. In: Handbook of Proteolytic Enzymes. Barrett A.J., Rawlings N.D., Woessner J.F., editors. Elsevier Academic Press; London: 2004. Plasma prekallikrein and kallikrein; pp. 1644–1651. [Google Scholar]
- 11.Shaw J.L.V., Diamandis E.P. Distribution of 15 human kallikreins in tissues and biological fluids. Clin. Chem. 2007;53:1423–1432. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- 12.Emami N., Diamandis E.P. New insights into the functional mechanisms and clinical applications of the kallikrein-related peptidase family. Mol. Oncol. 2007;1:269–287. doi: 10.1016/j.molonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements J., Hooper J., Dong Y., Harvey T. The expanded human kallikrein (KLK) gene family: genomic organisation, tissue-specific expression and potential functions. Biol. Chem. 2001;382:5–14. doi: 10.1515/BC.2001.002. [DOI] [PubMed] [Google Scholar]
- 14.Kapadia C., Chang A., Sotiropoulou G., Yousef G.M., Grass L., Soosaipillai A., Xing X., Howarth D.H.C., Diamandis E.P. Human kallikrein 13: production and purification of recombinant protein and monoclonal and polyclonal antibodies, and development of a sensitive and specific immunofluorometric assay. Clin. Chem. 2003;49:77–86. doi: 10.1373/49.1.77. [DOI] [PubMed] [Google Scholar]
- 15.Brattsand M., Stefansson K., Lundh C., Haasum Y., Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J. Invest. Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- 16.Denmeade S.R., Lövgren J., Khan S.R., Lilja H., Isaacs J.T. Activation of latent protease function of pro-hK2, but not pro-PSA, involves autoprocessing. Prostate. 2001;48:122–126. doi: 10.1002/pros.1088. [DOI] [PubMed] [Google Scholar]
- 17.Memari N., Jiang W., Diamandis E.P., Luo L.-Y. Enzymatic properties of human kallikrein-related peptidase 12 (KLK12) Biol. Chem. 2007;388:427–435. doi: 10.1515/BC.2007.049. [DOI] [PubMed] [Google Scholar]
- 18.Yousef G.M., Diamandis E.P. Human tissue kallikreins: a new enzymatic cascade pathway? Biol. Chem. 2002;383:1045–1057. doi: 10.1515/BC.2002.113. [DOI] [PubMed] [Google Scholar]
- 19.Yoon H., Laxmikanthan G., Lee J., Blaber S.I., Rodriguez A., Kogot J.M., Scarisbrick I.A., Blaber M. Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J. Biol. Chem. 2007;282:31852–31864. doi: 10.1074/jbc.M705190200. [DOI] [PubMed] [Google Scholar]
- 20.Yoon H., Blaber S.I., Debela M., Goettig P., Scarisbrick I.A., Blaber M. A completed KLK activome profile: investigation of activation profiles of KLK9, 10, and 15. Biol. Chem. 2009;390:373–377. doi: 10.1515/BC.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaber M., Yoon H., Juliano M.A., Scarisbrick I.A., Blaber S.I. Functional intersection of the kallikrein-related peptidases (KLKs) and thrombostasis axis. Biol. Chem. 2010;391:311–320. doi: 10.1515/BC.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu O., Hu J.C., Yamakoshi Y., Villemain J.L., Cao X., Zhang C., Bartlett J.D., Simmer J.P. Porcine kallikrein-4 activation, glycosylation, activity, and expression in prokaryotic and eukaryotic hosts. Eur. J. Oral Sci. 2002;110:358–365. doi: 10.1034/j.1600-0722.2002.21349.x. [DOI] [PubMed] [Google Scholar]
- 23.Tye C.E., Pham C.T., Simmer J.P., Bartlett J.D. DPPI may activate KLK4 during enamel formation. J. Dent. Res. 2009;88:323–327. doi: 10.1177/0022034509334240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaufort N., Plaza K., Utzschneider D., Schwarz A., Burkhart J., Creutzburg S., Debela M., Schmitt M., Ries C., Magdolen V. Interdependence of kallikrein-related peptidases in proteolytic networks. Biol. Chem. 2010;391:581–587. doi: 10.1515/BC.2010.055. [DOI] [PubMed] [Google Scholar]
- 25.Borgoño C.A., Michael I.P., Diamandis E.P. Human tissue kallikreins: physiologic roles and applications in cancer. Mol. Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- 26.Shaw J.L., Diamandis E.P. Regulation of human tissue kallikrein-related peptidase expression by steroid hormones in 32 cell lines. Biol. Chem. 2008;389:1409–1419. doi: 10.1515/bc.2008.158. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence M.G., Lai J., Clements J.A. Kallikreins on steroids: structure, function, and hormonal regulation of prostate-specific antigen and the extended kallikrein locus. Endocr. Rev. 2010 doi: 10.1210/er.2009-0034. er.2009-0034. [DOI] [PubMed] [Google Scholar]
- 28.Blaber S.I., Scarisbrick I.A., Bernett M.J., Dhanarajan P., Seavy M.A., Jin Y., Schwartz M.A., Rodriguez M., Blaber M. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- 29.Blaber S.I., Yoon H., Scarisbrick I.A., Juliano M.A., Blaber M. The autolytic regulation of human kallikrein-related peptidase 6. Biochemistry. 2007;46:5209–5217. doi: 10.1021/bi6025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayés A., Tsetsenis T., Ventura S., Vendrell J., Aviles F.X., Sotiropoulou G. Human kallikrein 6 activity is regulated via an autoproteolytic mechanism of activation/inactivation. Biol. Chem. 2004;385:517–524. doi: 10.1515/BC.2004.061. [DOI] [PubMed] [Google Scholar]
- 31.Borgoño C.A., Michael I.P., Shaw J.L.V., Luo L.-Y., Ghosh M.C., Soosaipillai A., Grass L., Katsaros D., Diamandis E.P. Expression and functional characterization of the cancer-related serine protease, human tissue kallikrein 14. J. Biol. Chem. 2007;282:2405–2422. doi: 10.1074/jbc.M608348200. [DOI] [PubMed] [Google Scholar]
- 32.Deraison C., Bonnart C., Lopez F., Besson C., Robinson R., Jayakumar A., Wagberg F., Brattsand M., Hachem J.P., Leonardsson G., Hovnanian A. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol. Biol. Cell. 2007 doi: 10.1091/mbc.E07-02-0124. E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y., Papagerakis P., Yamakoshi Y., Hu J.C.C., Bartlett J.D., Simmer J.P. Functions of KLK4 and MMP-20 in dental enamel formation. Biol. Chem. 2008;389:695–700. doi: 10.1515/BC.2008.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmer J.P., Hu Y., Lertlam R., Yamakoshi Y., Hu J.C.C. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J. Biol. Chem. 2009;284:19110–19121. doi: 10.1074/jbc.M109.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frey E.K. Zusammenhänge zwischen Herzarbeit und Nierentätigkeit. Arch. Klin. Chir. 1926;142:663. [Google Scholar]
- 36.Kraut H., Frey E.K., Werle E. Der Nachweis eines Kreislaufhormones in der Pankreasdrüse Z. Physiol. Chem. 1930;189:97. [Google Scholar]
- 37.Werle E., Götze W., Kappler A. Über die Wirkung des Kallikreins auf den isolierten Darm und über eine neue darmkontrahierende Substanz. Biochem. Z. 1937;289:217–233. [Google Scholar]
- 38.Chao J. In: Handbook of Proteolytic Enzymes. Barrett A.J., Rawlings N.D., Woessner J.F., editors. Elsevier Academic Press; London: 2004. Human kallikrein 1, tissue kallikrein; pp. 1577–1580. [Google Scholar]
- 39.Meneton P., Bloch-Faure M., Hagege A.A., Ruetten H., Huang W., Bergaya S., Ceiler D., Gehring D., Martins I., Salmon G., Boulanger C.M., Nussberger J., Crozatier B., Gasc J.-M., Heudes D., Bruneval P., Doetschman T., Ménard J., Alhenc-Gelas F. Cardiovascular abnormalities with normal blood pressure in tissue kallikrein-deficient mice. Proc. Natl. Acad. Sci. U S A. 2001;98:2634–2639. doi: 10.1073/pnas.051619598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard N., Van Abel M., Campone C., Seiler M., Bloch-Faure M., Hoenderop J.G.J., Loffing J., Meneton P., Bindels R.J.M., Paillard M., Alhenc-Gelas F., Houillier P. Tissue kallikrein-deficient mice display a defect in renal tubular calcium absorption. J. Am. Soc. Nephrol. 2005;16:3602–3610. doi: 10.1681/ASN.2004110923. [DOI] [PubMed] [Google Scholar]
- 41.Chao J., Shen B., Gao L., Xia C.-F., Bledsoe G., Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol. Chem. 2010;391:345–355. doi: 10.1515/BC.2010.042. [DOI] [PubMed] [Google Scholar]
- 42.Robert M., Gibbs B.F., Jacobson E., Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- 43.Emami N., Deperthes D., Malm J., Diamandis E.P. Major role of human KLK14 in seminal clot liquefaction. J. Biol. Chem. 2008;283:19561–19569. doi: 10.1074/jbc.M801194200. [DOI] [PubMed] [Google Scholar]
- 44.Borgoño C.A., Diamandis E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 45.Stephan C., Jung K., Lein M., Diamandis E.P. PSA and other tissue kallikreins for prostate cancer detection. Eur. J. Cancer. 2007;43:1918–1926. doi: 10.1016/j.ejca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V., Kwiatkowski M., Lujan M., Lilja H., Zappa M., Denis L.J., Recker F., Berenguer A., Maattanen L., Bangma C.H., Aus G., Villers A., Rebillard X., van der Kwast T., Blijenberg B.G., Moss S.M., de Koning H.J., Auvinen A. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 47.Seiz L., Kotzsch M., Grebenchtchikov N.I., Geurts-Moespot A.J., Fuessel S., Goettig P., Gkazepis A., Wirth M.P., Schmitt M., Lossnitzer A., Sweep F.C.G.J., Magdolen V. Polyclonal antibodies against kallikrein-related peptidase 4 (KLK4): immunohistochemical assessment of KLK4 expression in healthy tissues and prostate cancer. Biol. Chem. 2010;391:391–401. doi: 10.1515/BC.2010.033. [DOI] [PubMed] [Google Scholar]
- 48.Takayama T.K., McMullen B.A., Nelson P.S., Matsumura M., Fujikawa K. Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry. 2001;40:15341–15348. doi: 10.1021/bi015775e. [DOI] [PubMed] [Google Scholar]
- 49.Beaufort N., Debela M., Creutzburg S., Kellermann J., Bode W., Schmitt M., Pidard D., Magdolen V. Interplay of human tissue kallikrein 4 (hK4) with the plasminogen activation system: hK4 regulates the structure and functions of the urokinase-type plasminogen activator receptor (uPAR) Biol. Chem. 2006;387:217–222. doi: 10.1515/BC.2006.029. [DOI] [PubMed] [Google Scholar]
- 50.Mize G.J., Wang W., Takayama T.K. Prostate-specific kallikreins-2 and -4 enhance the proliferation of DU-145 prostate cancer cells through protease-activated receptors-1 and -2. Mol. Cancer Res. 2008;6:1043–1051. doi: 10.1158/1541-7786.MCR-08-0096. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay A.J., Dong Y., Hunt M.L., Linn M., Samaratunga H., Clements J.A., Hooper J.D. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs) J. Biol. Chem. 2008;283:12293–12304. doi: 10.1074/jbc.M709493200. [DOI] [PubMed] [Google Scholar]
- 52.Oikonomopoulou K., Hansen K.K., Saifeddine M., Vergnolle N., Tea I., Blaber M., Blaber S.I., Scarisbrick I., Diamandis E.P., Hollenberg M.D. Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs) Biol. Chem. 2006;387:817–824. doi: 10.1515/BC.2006.104. [DOI] [PubMed] [Google Scholar]
- 53.Oikonomopoulou K., Hansen K.K., Saifeddine M., Tea I., Blaber M., Blaber S.I., Scarisbrick I., Andrade-Gordon P., Cottrell G.S., Bunnett N.W., Diamandis E.P., Hollenberg M.D. Proteinase-activated receptors, targets for kallikrein signaling. J. Biol. Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- 54.Stefansson K., Brattsand M., Roosterman D., Kempkes C., Bocheva G., Steinhoff M., Egelrud T. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J. Invest. Dermatol. 2008;128:18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- 55.Gratio V., Beaufort N., Seiz L., Maier J., Virca G.D., Debela M., Grebenchtchikov N., Magdolen V., Darmoul D. Kallikrein-related peptidase 4 (KLK4): a new activator of the aberrantly expressed protease-activated receptor 1 in colon cancer cells. Am. J. Pathol. 2010;176:1452–1461. doi: 10.2353/ajpath.2010.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oikonomopoulou K., Diamandis E.P., Hollenberg M.D. Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol. Chem. 2010;391:299–310. doi: 10.1515/BC.2010.038. [DOI] [PubMed] [Google Scholar]
- 57.Simmer J.P., Hu J.C. Expression, structure, and function of enamel proteinases. Connect. Tissue Res. 2002;43:441–449. doi: 10.1080/03008200290001159. [DOI] [PubMed] [Google Scholar]
- 58.Hart P.S., Hart T.C., Michalec M.D., Ryu O.H., Simmons D., Hong S., Wright J.T. Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis imperfecta. J. Med. Genet. 2004;41:545–549. doi: 10.1136/jmg.2003.017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright J.T., Hart T.C., Hart P.S., Simmons D., Suggs C., Daley B., Simmer J., Hu J., Bartlett J.D., Li Y., Yuan Z.A., Seow W.K., Gibson C.W. Human and mouse enamel phenotypes resulting from mutation or altered expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs. 2009;189:224–229. doi: 10.1159/000151378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komatsu N., Saijoh K., Kuk C., Liu A.C., Khan S., Shirasaki F., Takehara K., Diamandis E.P. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp. Dermatol. 2007;16:513–519. doi: 10.1111/j.1600-0625.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 61.Stefansson K., Brattsand M., Ny A., Glas B., Egelrud T. Kallikrein-related peptidase 14 may be a major contributor to trypsin-like proteolytic activity in human stratum corneum. Biol. Chem. 2006;387:761–768. doi: 10.1515/BC.2006.095. [DOI] [PubMed] [Google Scholar]
- 62.Caubet C., Jonca N., Brattsand M., Guerrin M., Bernard D., Schmidt R., Egelrud T., Simon M., Serre G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 63.Borgoño C.A., Michael I.P., Komatsu N., Jayakumar A., Kapadia R., Clayman G.L., Sotiropoulou G., Diamandis E.P. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J. Biol. Chem. 2007;282:3640–3652. doi: 10.1074/jbc.M607567200. [DOI] [PubMed] [Google Scholar]
- 64.Kirihara T., Matsumoto-Miyai K., Nakamura Y., Sadayama T., Yoshida S., Shiosaka S. Prolonged recovery of ultraviolet B-irradiated skin in neuropsin (KLK8)-deficient mice. Br. J. Dermatol. 2003;149:700–706. doi: 10.1046/j.1365-2133.2003.05484.x. [DOI] [PubMed] [Google Scholar]
- 65.Klucky B., Mueller R., Vogt I., Teurich S., Hartenstein B., Breuhahn K., Flechtenmacher C., Angel P., Hess J. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 66.Ohler A., Debela M., Wagner S., Magdolen V., Becker-Pauly C. Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol. Chem. 2010;391:455–460. doi: 10.1515/BC.2010.023. [DOI] [PubMed] [Google Scholar]
- 67.Ovaere P., Lippens S., Vandenabeele P., Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem. Sci. 2009;34:453–463. doi: 10.1016/j.tibs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida S., Taniguchi M., Hirata A., Shiosaka S. Sequence analysis and expression of human neuropsin cDNA and gene. Gene. 1998;213:9–16. doi: 10.1016/s0378-1119(98)00232-7. [DOI] [PubMed] [Google Scholar]
- 69.Scarisbrick I.A., Blaber S.I., Lucchinetti C.F., Genain C.P., Blaber M., Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- 70.Blaber S.I., Ciric B., Christophi G.P., Bernett M.J., Blaber M., Rodriguez M., Scarisbrick I.A. Targeting kallikrein 6 proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;18:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- 71.Scarisbrick I.A., Sabharwal P., Cruz H., Larsen N., Vandell A.G., Blaber S.I., Ameenuddin S., Papke L.M., Fehlings M.G., Reeves R.K., Blaber M., Windebank A.J., Rodriguez M. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur. J. Neurosci. 2006;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura Y., Tamura H., Horinouchi K., Shiosaka S. Role of neuropsin in formation and maturation of Schaffer-collateral L1cam-immunoreactive synaptic boutons. J. Cell Sci. 2006;119:1341–1349. doi: 10.1242/jcs.02862. [DOI] [PubMed] [Google Scholar]
- 73.Komai S., Matsuyama T., Matsumoto K., Kato K., Kobayashi M., Imamura K., Yoshida S., Ugawa S., Shiosaka S. Neuropsin regulates an early phase of Schaffer-collateral long-term potentiation in the murine hippocampus. Eur. J. Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 74.Tamura H., Ishikawa Y., Hino N., Maeda M., Yoshida S., Kaku S., Shiosaka S. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J. Physiol. 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshida S. Klk8, a multifunctional protease in the brain and skin: analysis of knockout mice. Biol. Chem. 2010;391:375–380. doi: 10.1515/BC.2010.034. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu-Okabe C., Yousef G.M., Diamandis E.P., Yoshida S., Shiosaka S., Fahnestock M. Expression of the kallikrein gene family in normal and Alzheimer’s disease brain. Neuroreport. 2001;12:2747–2751. doi: 10.1097/00001756-200108280-00031. [DOI] [PubMed] [Google Scholar]
- 77.Davies B., Kearns I.R., Ure J., Davies C.H., Lathe R. Loss of hippocampal serine protease BSP1/neuropsin predisposes to global seizure activity. J. Neurosci. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-06993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izumi A., Iijima Y., Noguchi H., Numakawa T., Okada T., Hori H., Kato T., Tatsumi M., Kosuga A., Kamijima K., Asada T., Arima K., Saitoh O., Shiosaka S., Kunugi H. Genetic Variations of human neuropsin gene and psychiatric disorders: polymorphism screening and possible association with bipolar disorder and cognitive functions. Neuropsychopharmacology. 2008;33:3237–3245. doi: 10.1038/npp.2008.29. [DOI] [PubMed] [Google Scholar]
- 79.Memari N., Grass L., Nakamura T., Karakucuk I., Diamandis E.P. Human tissue kallikrein 9: production of recombinant proteins and specific antibodies. Biol. Chem. 2006;387:733–740. doi: 10.1515/BC.2006.092. [DOI] [PubMed] [Google Scholar]
- 80.Darling M.R., Jackson-Boeters L., Daley T.D., Diamandis E.P. Human kallikrein 13 expression in salivary gland tumors. Int. J. Biol. Markers. 2006;21:106–110. doi: 10.1177/172460080602100206. [DOI] [PubMed] [Google Scholar]
- 81.Sotiropoulou G., Pampalakis G., Diamandis E.P. Functional roles of human kallikrein-related peptidases. J. Biol. Chem. 2009;284:32989–32994. doi: 10.1074/jbc.R109.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo L.Y., Meyts E.R.-D., Jung K., Diamandis E.P. Expression of the normal epithelial cell-specific 1 (NES1; KLK10) candidate tumour suppressor gene in normal and malignant testicular tissue. Br. J. Cancer. 2001;85:220–224. doi: 10.1054/bjoc.2001.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giusti B., Serratì S., Margheri F., Papucci L., Rossi L., Poggi F., Magi A., Rosso A.D., Cinelli M., Guiducci S., Kahaleh B., Matucci-Cerinic M., Abbate R., Fibbi G., Rosso M.D. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 2005;52:3618–3628. doi: 10.1002/art.21383. [DOI] [PubMed] [Google Scholar]
- 84.Kapadia C., Ghosh M.C., Grass L., Diamandis E.P. Human kallikrein 13 involvement in extracellular matrix degradation. Biochem. Biophys. Res. Commun. 2004;323:1084–1090. doi: 10.1016/j.bbrc.2004.08.206. [DOI] [PubMed] [Google Scholar]
- 85.Sotiropoulou G., Pampalakis G. Kallikrein-related peptidases: bridges between immune functions and extracellular matrix degradation. Biol. Chem. 2010;391:321–331. doi: 10.1515/BC.2010.036. [DOI] [PubMed] [Google Scholar]
- 86.Shaw J.L.V., Grass L., Sotiropoulou G., Diamandis E.P. Development of an immunofluorometric assay for human kallikrein 15 (KLK15) and identification of KLK15 in tissues and biological fluids. Clin. Biochem. 2007;40:104–110. doi: 10.1016/j.clinbiochem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 87.Shaw J.L.V., Diamandis E.P. A potential role for tissue kallikrein-related peptidases in human cervico-vaginal physiology. Biol. Chem. 2008;389:681–688. doi: 10.1515/BC.2008.069. [DOI] [PubMed] [Google Scholar]
- 88.Groll M., Bochtler M., Brandstetter H., Clausen T., Huber R. Molecular machines for protein degradation. Chembiochem. 2005;6:222–256. doi: 10.1002/cbic.200400313. [DOI] [PubMed] [Google Scholar]
- 89.Bode W., Huber R. Natural protein proteinase inhibitors and their interaction with proteinases. Eur. J. Biochem. 1992;204:433–451. doi: 10.1111/j.1432-1033.1992.tb16654.x. [DOI] [PubMed] [Google Scholar]
- 90.Maskos K., Bode W. Structural basis of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Mol. Biotechnol. 2003;25:241–266. doi: 10.1385/MB:25:3:241. [DOI] [PubMed] [Google Scholar]
- 91.Buczek O., Koscielska-Kasprzak K., Krowarsch D., Dadlez M., Otlewski J. Analysis of serine proteinase–inhibitor interaction by alanine shaving. Protein Sci. 2002;11:806–819. doi: 10.1110/ps.3510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maraganore J.M., Bourdon P., Jablonski J., Ramachandran K.L., Fenton J.W., II Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990;29:7095–7101. doi: 10.1021/bi00482a021. [DOI] [PubMed] [Google Scholar]
- 93.Naski M.C., Fenton J.W., Maraganore J.M., Olson S.T., Shafer J.A. The COOH-terminal domain of hirudin. An exosite-directed competitive inhibitor of the action of alpha-thrombin on fibrinogen. J. Biol. Chem. 1990;265:13484–13489. [PubMed] [Google Scholar]
- 94.Rydel T.J., Tulinsky A., Bode W., Huber R. Refined structure of the hirudin-thrombin complex. J. Mol. Biol. 1991;221:583–601. doi: 10.1016/0022-2836(91)80074-5. [DOI] [PubMed] [Google Scholar]
- 95.Shimizu C., Yoshida S., Shibata M., Kato K., Momota Y., Matsumoto K., Shiosaka T., Midorikawa R., Kamachi T., Kawabe A., Shiosaka S. Characterization of recombinant and brain neuropsin, a plasticity-related serine protease. J. Biol. Chem. 1998;273:11189–11196. doi: 10.1074/jbc.273.18.11189. [DOI] [PubMed] [Google Scholar]
- 96.Shinmura K., Tao H., Yamada H., Kataoka H., Sanjar R., Wang J., Yoshimura K., Sugimura H. Splice-site genetic polymorphism of the human kallikrein 12 (KLK12) gene correlates with no substantial expression of KLK12 protein having serine protease activity. Hum. Mutat. 2004;24:273–274. doi: 10.1002/humu.9270. [DOI] [PubMed] [Google Scholar]
- 97.Takayama T.K., Carter C.A., Deng T. Activation of prostate-specific antigen precursor (pro-PSA) by prostin, a novel human prostatic serine protease identified by degenerate PCR. Biochemistry. 2001;40:1679–1687. doi: 10.1021/bi002129r. [DOI] [PubMed] [Google Scholar]
- 98.Matsumura M., Bhatt A.S., Andress D., Clegg N., Takayama T.K., Craik C.S., Nelson P.S. Substrates of the prostate-specific serine protease prostase/KLK4 defined by positional-scanning peptide libraries. Prostate. 2005;62:1–13. doi: 10.1002/pros.20101. [DOI] [PubMed] [Google Scholar]
- 99.Debela M., Magdolen V., Schechter N., Valachova M., Lottspeich F., Craik C.S., Choe Y., Bode W., Goettig P. Specificity profiling of seven human tissue kallikreins reveals individual subsite preferences. J. Biol. Chem. 2006;281:25678–25688. doi: 10.1074/jbc.M602372200. [DOI] [PubMed] [Google Scholar]
- 100.Borgoño C.A., Gavigan J.A., Alves J., Bowles B., Harris J.L., Sotiropoulou G., Diamandis E.P. Defining the extended substrate specificity of kallikrein 1-related peptidases. Biol. Chem. 2007;388:1215–1225. doi: 10.1515/BC.2007.124. [DOI] [PubMed] [Google Scholar]
- 101.Sotiropoulou G., Rogakos V., Tsetsenis T., Pampalakis G., Zafiropoulos N., Simillides G., Yiotakis A., Diamandis E.P. Emerging interest in the kallikrein gene family for understanding and diagnosing cancer. Oncol. Res. 2003;13:381–391. doi: 10.3727/096504003108748393. [DOI] [PubMed] [Google Scholar]
- 102.Coombs G.S., Bergstrom R.C., Pellequer J.L., Baker S.I., Navre M., Smith M.M., Tainer J.A., Madison E.L., Corey D.R. Substrate specificity of prostate-specific antigen (PSA) Chem. Biol. 1998;5:475–488. doi: 10.1016/s1074-5521(98)90004-7. [DOI] [PubMed] [Google Scholar]
- 103.Li H.X., Hwang B.Y., Laxmikanthan G., Blaber S.I., Blaber M., Golubkov P.A., Ren P., Iverson B.L., Georgiou G. Substrate specificity of human kallikreins 1 and 6 determined by phage display. Protein Sci. 2008;17:664–672. doi: 10.1110/ps.073333208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Felber L.M., Borgono C.A., Cloutier S.M., Kundig C., Kishi T., Ribeiro Chagas J., Jichlinski P., Gygi C.M., Leisinger H.J., Diamandis E.P., Deperthes D. Enzymatic profiling of human kallikrein 14 using phage-display substrate technology. Biol. Chem. 2005;386:291–298. doi: 10.1515/BC.2005.035. [DOI] [PubMed] [Google Scholar]
- 105.Sharma N., Oikonomopoulou K., Ito K., Renaux B., Diamandis E.P., Hollenberg M.D., Rancourt D.E. Substrate specificity determination of mouse implantation serine proteinase and human kallikrein-related peptidase 6 by phage display. Biol. Chem. 2008;389:1097–1105. doi: 10.1515/bc.2008.118. [DOI] [PubMed] [Google Scholar]
- 106.Cloutier S.M., Chagas J.R., Mach J.-P., Gygi C.M., Leisinger H.-J., Deperthes D. Substrate specificity of human kallikrein 2 (hK2) as determined by phage display technology. Eur. J. Biochem. 2002;269:2747–2754. doi: 10.1046/j.1432-1033.2002.02960.x. [DOI] [PubMed] [Google Scholar]
- 107.Debela M. Max-Planck-Institute of Biochemistry, TU Munich; Munich: 2007. Crystal Structures of the Human Tissue Kallikreins 4, 5, 7, 10, Characterisation of Their Substrate Specificity and Analysis of Their Various Zinc Inhibition Mechanisms. p. 191. [Google Scholar]
- 108.Bourgeois L., Brillard-Bourdet M., Deperthes D., Juliano M.A., Juliano L., Tremblay R.R., Dubé J.Y., Gauthier F. Serpin-derived peptide substrates for investigating the substrate specificity of human tissue kallikreins hK1 and hK2. J. Biol. Chem. 1997;272:29590–29595. doi: 10.1074/jbc.272.47.29590. [DOI] [PubMed] [Google Scholar]
- 109.Janssen S., Jakobsen C.M., Rosen D.M., Ricklis R.M., Reineke U., Christensen S.B., Lilja H., Denmeade S.R. Screening a combinatorial peptide library to develop a human glandular kallikrein 2-activated prodrug as targeted therapy for prostate cancer. Mol. Cancer Ther. 2004;3:1439–1450. [PubMed] [Google Scholar]
- 110.Yang C.F., Porter E.S., Boths J., Kanyi D., Hsieh M.C., Cooperman B.S. Design of synthetic hexapeptide substrates for prostate-specific antigen using single-position minilibraries. J. Pept. Res. 1999;54:444–448. doi: 10.1034/j.1399-3011.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 111.Moreau T., Brillard-Bourdet M., Bouhnik J., Gauthier F. Protein products of the rat kallikrein gene family. Substrate specificities of kallikrein rK2 (tonin) and kallikrein rK9. J. Biol. Chem. 1992;267:10045–10051. [PubMed] [Google Scholar]
- 112.Yamada A., Nakamura Y., Sugita D., Shirosaki S., Ohkuri T., Katsukawa H., Nonaka K., Imoto T., Ninomiya Y. Induction of salivary kallikreins by the diet containing a sweet-suppressive peptide, gurmarin, in the rat. Biochem. Biophys. Res. Commun. 2006;346:386–392. doi: 10.1016/j.bbrc.2006.05.154. [DOI] [PubMed] [Google Scholar]
- 113.Schmidt A.E., Sun M.-f., Ogawa T., Bajaj S.P., Gailani D. Functional role of residue 193 (chymotrypsin numbering) in serine proteases: influence of side chain length and b-branching on the catalytic activity of blood coagulation factor XIa. Biochemistry. 2008;47:1326–1335. doi: 10.1021/bi701594j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oikonomopoulou K., Batruch I., Smith C.R., Soosaipillai A., Diamandis E.P., Hollenberg M.D. Functional proteomics of kallikrein-related peptidases in ovarian cancer ascites fluid. Biol. Chem. 2010;391:381–390. doi: 10.1515/BC.2010.045. [DOI] [PubMed] [Google Scholar]
- 115.Orthner C.L., Kosow D.P. Evidence that human [alpha]-thrombin is a monovalent cation-activated enzyme. Arch. Biochem. Biophys. 1980;202:63–75. doi: 10.1016/0003-9861(80)90406-3. [DOI] [PubMed] [Google Scholar]
- 116.Wells C.M., Di Cera E. Thrombin is a sodium ion activated enzyme. Biochemistry. 1992;31:11721–11730. doi: 10.1021/bi00162a008. [DOI] [PubMed] [Google Scholar]
- 117.Di Cera E., Guinto E.R., Vindigni A., Dang Q.D., Ayala Y.M., Wuyi M., Tulinsky A. The Na+ binding site of thrombin. J. Biol. Chem. 1995;270:22089–22092. doi: 10.1074/jbc.270.38.22089. [DOI] [PubMed] [Google Scholar]
- 118.Bode W., Schwager P. The single calcium-binding site of crystallin bovin beta-trypsin. FEBS Lett. 1975;56:139–143. doi: 10.1016/0014-5793(75)80128-1. [DOI] [PubMed] [Google Scholar]
- 119.Delaage M., Lazdunski M. The binding of Ca2+ to trypsinogen and its relation to the mechanism of activation. Biochem. Biophys. Res. Commun. 1967;28:390–394. doi: 10.1016/0006-291x(67)90323-3. [DOI] [PubMed] [Google Scholar]
- 120.Lieberthal W., Oza N.B., Bernard D.B., Levinsky N.G. The effect of cations on the activity of human urinary kallikrein. J. Biol. Chem. 1982;257:10827–10830. [PubMed] [Google Scholar]
- 121.Angelo P.F., Lima A.R., Alves F.M., Blaber S.I., Scarisbrick I.A., Blaber M., Juliano L., Juliano M.A. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J. Biol. Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. [DOI] [PubMed] [Google Scholar]
- 122.Hsieh M.-C., Cooperman B.S. Inhibition of prostate-specific antigen (PSA) by alpha1-antichymotrypsin: salt-dependent activation mediated by a conformational change. Biochemistry. 2002;41:2990–2997. doi: 10.1021/bi0117450. [DOI] [PubMed] [Google Scholar]
- 123.Kishi T., Cloutier S.M., Kündig C., Deperthes D., Diamandis E.P. Activation and enzymatic characterization of recombinant human kallikrein 8. Biol. Chem. 2006;387:723–731. doi: 10.1515/BC.2006.091. [DOI] [PubMed] [Google Scholar]
- 124.Coleman J.E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 125.Tapiero H., Tew K.D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 126.Bernardo M.M., Day D.E., Olson S.T., Shore J.D. Surface-independent acceleration of factor XII activation by zinc ions. I. Kinetic characterization of the metal ion rate enhancement. J. Biol. Chem. 1993;268:12468–12476. [PubMed] [Google Scholar]
- 127.Petersen L.C., Olsen O.H., Nielsen L.S., Freskgard P.O., Persson E. Binding of Zn2+ to a Ca2+ loop allosterically attenuates the activity of factor VIIa and reduces its affinity for tissue factor. Protein Sci. 2000;9:859–866. doi: 10.1110/ps.9.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishii K., Usui S., Sugimura Y., Yamamoto H., Yoshikawa K., Hirano K. Inhibition of Aminopeptidase N (AP-N) and urokinase-type plasminogen activator (uPA) by zinc suppresses the invasion activity in human urological cancer cells. Biol. Pharm. Bull. 2001;24:226–230. doi: 10.1248/bpb.24.226. [DOI] [PubMed] [Google Scholar]
- 129.Lovgren J., Airas K., Lilja H. Enzymatic action of human glandular kallikrein 2 (hK2). Substrate specificity and regulation by Zn2+ and extracellular protease inhibitors. Eur. J. Biochem. 1999;262:781–789. doi: 10.1046/j.1432-1327.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 130.Lundwall A., Brattsand M. Kallikrein-related peptidases. Cell. Mol. Life Sci. 2008;65:2019–2038. doi: 10.1007/s00018-008-8024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kavanagh J.P. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J. Reprod. Fertil. 1985;75:35–41. doi: 10.1530/jrf.0.0750035. [DOI] [PubMed] [Google Scholar]
- 132.Malm J., Hellman J., Hogg P., Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn(2+), a tight-binding inhibitor. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 133.Hsieh M.-C., Cooperman B.S. The preparation and catalytic properties of recombinant human prostate-specific antigen (rPSA) Biochim. Biophys. Acta. 2000;1481:75–87. doi: 10.1016/s0167-4838(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 134.Menez R., Michel S., Muller B.H., Bossus M., Ducancel F., Jolivet-Reynaud C., Stura E.A. Crystal structure of a ternary complex between human prostate-specific antigen, its substrate acyl intermediate and an activating antibody. J. Mol. Biol. 2008;376:1021–1033. doi: 10.1016/j.jmb.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 135.Villoutreix B.O., Getzoff E.D., Griffin J.H. A structural model for the prostate disease marker, human prostate-specific antigen. Protein Sci. 1994;3:2033–2044. doi: 10.1002/pro.5560031116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carvalho A.L., Sanz L., Barettino D., Romero A., Calvete J.J., Romao M.J. Crystal structure of a prostate kallikrein isolated from stallion seminal plasma: a homologue of human PSA. J. Mol. Biol. 2002;322:325–337. doi: 10.1016/s0022-2836(02)00705-2. [DOI] [PubMed] [Google Scholar]
- 137.Debela M., Magdolen V., Grimminger V., Sommerhoff C., Messerschmidt A., Huber R., Friedrich R., Bode W., Goettig P. Crystal structures of human tissue kallikrein 4: activity modulation by a specific zinc binding site. J. Mol. Biol. 2006;362:1094–1107. doi: 10.1016/j.jmb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 138.Gerlach R.F., Souza A.P.d., Cury J.A., Line S.R.P. Effect of lead, cadmium and zinc on the activity of enamel matrix proteinases in vitro. Eur. J. Oral Sci. 2000;108:327–334. doi: 10.1034/j.1600-0722.2000.108004327.x. [DOI] [PubMed] [Google Scholar]
- 139.Higashi S., Matsumoto N., Iwanaga S. Molecular mechanism of tissue factor-mediated acceleration of factor VIIa activity. J. Biol. Chem. 1996;271:26569–26574. doi: 10.1074/jbc.271.43.26569. [DOI] [PubMed] [Google Scholar]
- 140.Sichler K., Banner D.W., D’Arcy A., Hopfner K.P., Huber R., Bode W., Kresse G.B., Kopetzki E., Brandstetter H. Crystal structures of uninhibited factor VIIa link its cofactor and substrate-assisted activation to specific interactions. J. Mol. Biol. 2002;322:591–603. doi: 10.1016/s0022-2836(02)00747-7. [DOI] [PubMed] [Google Scholar]
- 141.Pampalakis G., Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim. Biophys. Acta. 2007;1776:22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 142.de Lamirande E. Semenogelin, the main protein of the human semen coagulum, regulates sperm function. Semin. Thromb. Hemost. 2007;33:60–68. doi: 10.1055/s-2006-958463. [DOI] [PubMed] [Google Scholar]
- 143.Michael I.P., Pampalakis G., Mikolajczyk S.D., Malm J., Sotiropoulou G., Diamandis E.P. Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J. Biol. Chem. 2006;281:12743–12750. doi: 10.1074/jbc.M600326200. Epub 12006 Mar 12743. [DOI] [PubMed] [Google Scholar]
- 144.Debela M., Goettig P., Magdolen V., Huber R., Schechter N.M., Bode W. Structural basis of the zinc inhibition of human tissue kallikrein 5. J. Mol. Biol. 2007;373:1017–1031. doi: 10.1016/j.jmb.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 145.Fujinaga M., James M.N. Rat submaxillary gland serine protease, tonin. Structure solution and refinement at 1.8 A resolution. J. Mol. Biol. 1987;195:373–396. doi: 10.1016/0022-2836(87)90658-9. [DOI] [PubMed] [Google Scholar]
- 146.Carter P., Wells J.A. Engineering enzyme specificity by “substrate-assisted catalysis”. Science. 1987;237:394–399. doi: 10.1126/science.3299704. [DOI] [PubMed] [Google Scholar]
- 147.Debela M., Hess P., Magdolen V., Schechter N.M., Steiner T., Huber R., Bode W., Goettig P. Chymotryptic specificity determinants in the 1.0 Å structure of the zinc-inhibited human tissue kallikrein 7. Proc. Natl. Acad. Sci. U S A. 2007;104:16086–16091. doi: 10.1073/pnas.0707811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Higaki J.N., Haymore B.L., Chen S., Fletterick R.J., Craik C.S. Regulation of serine protease activity by an engineered metal switch. Biochemistry. 1990;29:8582–8586. doi: 10.1021/bi00489a012. [DOI] [PubMed] [Google Scholar]
- 149.McGrath M.E., Haymore B.L., Summers N.L., Craik C.S., Fletterick R.J. Structure of an engineered, metal-actuated switch in trypsin. Biochemistry. 1993;32:1914–1919. doi: 10.1021/bi00059a005. [DOI] [PubMed] [Google Scholar]
- 150.Nitzan Y.B., Sekler I., Silverman W.F. Histochemical and histofluorescence tracing of chelatable zinc in the developing mouse. J. Histochem. Cytochem. 2004;52:529–539. doi: 10.1177/002215540405200411. [DOI] [PubMed] [Google Scholar]
- 151.Lansdown A.B.G., Mirastschijski U., Stubbs N., Scanlon E., Ågren M.S. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15:2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 152.Lech T., Sadlik J.K. Copper concentration in body tissues and fluids in normal subjects of southern Poland. Biol. Trace Elem. Res. 2007;118:10–15. doi: 10.1007/s12011-007-0014-z. [DOI] [PubMed] [Google Scholar]
- 153.Forteza R., Casalino-Matsuda S.M., Monzon M.E., Fries E., Rugg M.S., Milner C.M., Day A.J. TSG-6 potentiates the antitissue kallikrein activity of inter-alpha-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 2007;36:20–31. doi: 10.1165/rcmb.2006-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Delaria K.A., Muller D.K., Marlor C.W., Brown J.E., Das R.C., Roczniak S.O., Tamburini P.P. Characterization of placental bikunin, a novel human serine protease inhibitor. J. Biol. Chem. 1997;272:12209–12214. doi: 10.1074/jbc.272.18.12209. [DOI] [PubMed] [Google Scholar]
- 155.Mukai S., Fukushima T., Naka D., Tanaka H., Osada Y., Kataoka H. Activation of hepatocyte growth factor activator zymogen (pro-HGFA) by human kallikrein 1-related peptidases. FEBS J. 2008;275:1003–1017. doi: 10.1111/j.1742-4658.2008.06265.x. [DOI] [PubMed] [Google Scholar]
- 156.Franzke C.-W., Baici A., Bartels J., Christophers E., Wiedow O. Antileukoprotease inhibits stratum corneum chymotryptic enzyme. J. Biol. Chem. 1996;271:21886–21890. doi: 10.1074/jbc.271.36.21886. [DOI] [PubMed] [Google Scholar]
- 157.Carrell R., Travis J. alpha1-Antitrypsin and the serpins: variation and countervariation. Trends Biochem. Sci. 1980;10:20–24. [Google Scholar]
- 158.Hunt L.T., Dayhoff M.O. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha1-proteinase inhibitor. Biochem. Biophys. Res. Commun. 1980;95:864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- 159.Potempa J., Korzus E., Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J. Biol. Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 160.Irving J.A., Steenbakkers P.J.M., Lesk A.M., Op den Camp H.J.M., Pike R.N., Whisstock J.C. Serpins in prokaryotes. Mol. Biol. Evol. 2002;19:1881–1890. doi: 10.1093/oxfordjournals.molbev.a004012. [DOI] [PubMed] [Google Scholar]
- 161.Silverman G.A., Bird P.I., Carrell R.W., Church F.C., Coughlin P.B., Gettins P.G.W., Irving J.A., Lomas D.A., Luke C.J., Moyer R.W., Pemberton P.A., Remold-O’Donnell E., Salvesen G.S., Travis J., Whisstock J.C. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. J. Biol. Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 162.Roberts T.H., Hejgaard J., Saunders N.F.W., Cavicchioli R., Curmi P.M.G. Serpins in unicellular eukarya, archaea, and bacteria: sequence analysis and evolution. J. Mol. Evol. 2004;59:437–447. doi: 10.1007/s00239-004-2635-6. [DOI] [PubMed] [Google Scholar]
- 163.Engh R.A., Huber R., Bode W., Schulze A.J. Divining the serpin inhibition mechanism: a suicide substrate ’springe’? Trends Biotechnol. 1995;13:503–510. doi: 10.1016/S0167-7799(00)89013-7. [DOI] [PubMed] [Google Scholar]
- 164.Krowarsch D., Cierpicki T., Jelen F., Otlewski J. Canonical protein inhibitors of serine proteases. Cell. Mol. Life Sci. 2003;60:2427–2444. doi: 10.1007/s00018-003-3120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huntington J.A., Read R.J., Carrell R.W. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 166.Dementiev A., Simonovic M., Volz K., Gettins P.G.W. Canonical inhibitor-like interactions explain reactivity of a1-proteinase inhibitor Pittsburgh and antithrombin with proteinases. J. Biol. Chem. 2003;278:37881–37887. doi: 10.1074/jbc.M305195200. [DOI] [PubMed] [Google Scholar]
- 167.Ye S., Cech A.L., Belmares R., Bergstrom R.C., Tong Y., Corey D.R., Kanost M.R., Goldsmith E.J. The structure of a Michaelis serpin–protease complex. Nat. Struct. Biol. 2001;8:979–983. doi: 10.1038/nsb1101-979. [DOI] [PubMed] [Google Scholar]
- 168.Marszal E., Shrake A. Serpin crystal structure and serpin polymer structure. Arch. Biochem. Biophys. 2006;453:123–129. doi: 10.1016/j.abb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 169.Huntington J.A. Shape-shifting serpins – advantages of a mobile mechanism. Trends Biochem. Sci. 2006;31:427–435. doi: 10.1016/j.tibs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 170.Luo L.-Y., Jiang W. Inhibition profiles of human tissue kallikreins by serine protease inhibitors. Biol. Chem. 2006;387:813–816. doi: 10.1515/BC.2006.103. [DOI] [PubMed] [Google Scholar]
- 171.Geiger R., Stuckstedte U., Clausnitzer B., Fritz H. Progressive inhibition of human glandular (urinary) kallikrein by human serum and identification of the progressive antikallikrein as alpha 1-antitrypsin (alpha 1-protease inhibitor) Hoppe Seylers Z. Physiol. Chem. 1981;362:317–325. doi: 10.1515/bchm2.1981.362.1.317. [DOI] [PubMed] [Google Scholar]
- 172.Mikolajczyk S.D., Millar L.S., Kumar A., Saedi M.S. Human glandular kallikrein, hK2, shows arginine-restricted specificity and forms complexes with plasma protease inhibitors. Prostate. 1998;34:44–50. doi: 10.1002/(sici)1097-0045(19980101)34:1<44::aid-pros6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 173.Michael I.P., Sotiropoulou G., Pampalakis G., Magklara A., Ghosh M., Wasney G., Diamandis E.P. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 2005;280:14628–14635. doi: 10.1074/jbc.M408132200. Epub 12005 Feb 14615. [DOI] [PubMed] [Google Scholar]
- 174.Magklara A., Mellati A.A., Wasney G.A., Little S.P., Sotiropoulou G., Becker G.W., Diamandis E.P. Characterization of the enzymatic activity of human kallikrein 6: autoactivation, substrate specificity, and regulation by inhibitors. Biochem. Biophys. Res. Commun. 2003;307:948–955. doi: 10.1016/s0006-291x(03)01271-3. [DOI] [PubMed] [Google Scholar]
- 175.Zhang W.-M., Leinonen J., Kalkkinen N., Stenman U.-H. Prostate-specific antigen forms a complex with and cleaves alpha1-protease inhibitor in vitro. Prostate. 1997;33:87–96. doi: 10.1002/(sici)1097-0045(19971001)33:2<87::aid-pros2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 176.Stenman U.-H., Leinonen J., Alfthan H., Rannikko S., Tuhkanen K., Alfthan O. A complex between prostate-specific antigen and {alpha}1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–226. [PubMed] [Google Scholar]
- 177.Zhang W.-M., Finne P., Leinonen J., Vesalainen S., Nordling S., Stenman U.-H. Measurement of the complex between prostate-specific antigen and {alpha}1-protease inhibitor in serum. Clin. Chem. 1999;45:814–821. [PubMed] [Google Scholar]
- 178.Obiezu C.V., Michael I.P., Levesque M.A., Diamandis E.P. Human kallikrein 4: enzymatic activity, inhibition, and degradation of extracellular matrix proteins. Biol. Chem. 2006;387:749–759. doi: 10.1515/BC.2006.094. [DOI] [PubMed] [Google Scholar]
- 179.Becker C., Lilja H. Individual prostate-specific antigen (PSA) forms as prostate tumor markers. Clin. Chim. Acta. 1997;257:117–132. doi: 10.1016/s0009-8981(96)06437-6. [DOI] [PubMed] [Google Scholar]
- 180.Qian Y., Sensibar J.A., Zelner D.J., Schaeffer A.J., Finlay J.A., Rittenhouse H.G., Lee C. Two-dimensional gel electrophoresis detects prostate-specific antigen-a1-antichymotrypsin complex in serum but not in prostatic fluid. Clin. Chem. 1997;43:352–359. [PubMed] [Google Scholar]
- 181.Mikolajczyk S.D., Catalona W.J., Evans C.L., Linton H.J., Millar L.S., Marker K.M., Katir D., Amirkhan A., Rittenhouse H.G. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin. Chem. 2004;50:1017–1025. doi: 10.1373/clinchem.2003.026823. [DOI] [PubMed] [Google Scholar]
- 182.Hutchinson S., Luo L.-Y., Yousef G.M., Soosaipillai A., Diamandis E.P. Purification of human kallikrein 6 from biological fluids and identification of its complex with {alpha}1-antichymotrypsin. Clin. Chem. 2003;49:746–751. doi: 10.1373/49.5.746. [DOI] [PubMed] [Google Scholar]
- 183.Di Cera E. Thrombin. Mol. Aspects Med. 2008;29:203–254. doi: 10.1016/j.mam.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dementiev A., Petitou M., Herbert J.-M., Gettins P.G.W. The ternary complex of antithrombin-anhydrothrombin-heparin reveals the basis of inhibitor specificity. Nat. Struct. Mol. Biol. 2004;11:863–867. doi: 10.1038/nsmb810. [DOI] [PubMed] [Google Scholar]
- 185.Olson S.T., Björk I., Sheffer R., Craig P.A., Shore J.D., Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J. Biol. Chem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- 186.Bedsted T., Swanson R., Chuang Y.-J., Bock P.E., Bjork I., Olson S.T. Heparin and calcium ions dramatically enhance antithrombin reactivity with factor IXa by generating new interaction exosites. Biochemistry. 2003;42:8143–8152. doi: 10.1021/bi034363y. [DOI] [PubMed] [Google Scholar]
- 187.Cao Y., Lundwall A., Gadaleanu V., Lilja H., Bjartell A. Anti-thrombin is expressed in the benign prostatic epithelium and in prostate cancer and is capable of forming complexes with prostate-specific antigen and human glandular kallikrein 2. Am. J. Pathol. 2002;161:2053–2063. doi: 10.1016/S0002-9440(10)64484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Castellino F.J. vol. 2. Elsevier; London: 2004. (Plasmin). 1692–1699 pp. [Google Scholar]
- 189.Aoki N. The past, present and future of plasmin inhibitor. Thromb. Res. 2005;116:455–464. doi: 10.1016/j.thromres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 190.Geiger M. Protein C inhibitor, a serpin with functions in- and outside vascular biology. Thromb. Haemost. 2007;97:343–347. [PubMed] [Google Scholar]
- 191.Huntington J.A., Li W. Structural insights into the multiple functions of protein C inhibitor. Cell. Mol. Life Sci. 2009;66:113–121. doi: 10.1007/s00018-008-8371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cao Y., Becker C., Lundwall A., Christensson A., Gadaleanu V., Lilja H., Bjartell A. Expression of protein C inhibitor (PCI) in benign and malignant prostatic tissues. Prostate. 2003;57:196–204. doi: 10.1002/pros.10296. [DOI] [PubMed] [Google Scholar]
- 193.España F., Fink E., Sanchez-Cuenca J., Gilabert J., Estellés A., Witzgall K. Complexes of tissue kallikrein with protein C inhibitor in human semen and urine. Eur. J. Biochem. 1995;234:641–649. doi: 10.1111/j.1432-1033.1995.641_b.x. [DOI] [PubMed] [Google Scholar]
- 194.España F., Gilabert J., Estellés A., Romeu A., Aznar J., Cabo A. Functionally active protein C inhibitor/plasminogen activator inhibitor-3 (PCI/PAI-3) is secreted in seminal vesicles, occurs at high concentrations in human seminal plasma and complexes with prostate-specific antigen. Thromb. Res. 1991;64:309–320. doi: 10.1016/0049-3848(91)90002-e. [DOI] [PubMed] [Google Scholar]
- 195.España F., Navarro S., Medina P., Zorio E., Estellés A. The role of protein C inhibitor in human reproduction. Semin. Thromb. Hemost. 2007;33:041–045. doi: 10.1055/s-2006-958460. [DOI] [PubMed] [Google Scholar]
- 196.Zhou G.X., Chao L., Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J. Biol. Chem. 1992;267:25873–25880. [PubMed] [Google Scholar]
- 197.Chao J., Chao L. Biochemistry, regulation and potential function of kallistatin. Biol. Chem. Hoppe-Seyler. 1995;376:705–713. [PubMed] [Google Scholar]
- 198.Chen V.C., Chao L., Chao J. Reactive-site specificity of human kallistatin toward tissue kallikrein probed by site-directed mutagenesis. Biochim. Biophys. Acta. 2000;1479:237–246. doi: 10.1016/s0167-4838(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 199.Chen V.C., Chao L., Chao J. Roles of the P1, P2, and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. J. Biol. Chem. 2000;275:38457–38466. doi: 10.1074/jbc.M005605200. [DOI] [PubMed] [Google Scholar]
- 200.Chen V.C., Chao L., Pimenta D.C., Bledsoe G., Juliano L., Chao J. Identification of a Major heparin-binding site in kallistatin. J. Biol. Chem. 2001;276:1276–1284. doi: 10.1074/jbc.M005791200. [DOI] [PubMed] [Google Scholar]
- 201.Chao J., Miao R.Q., Chen V., Chen L.M., Chao L. Novel roles of kallistatin, a specific tissue kallikrein inhibitor, in vascular remodeling. Biol. Chem. 2001;382:15–21. doi: 10.1515/BC.2001.003. [DOI] [PubMed] [Google Scholar]
- 202.Miao R.Q., Chen V., Chao L., Chao J. Structural elements of kallistatin required for inhibition of angiogenesis. Am. J. Physiol. Cell Physiol. 2003;284:C1604–C1613. doi: 10.1152/ajpcell.00524.2002. [DOI] [PubMed] [Google Scholar]
- 203.Cooley J., Takayama T.K., Shapiro S.D., Schechter N.M., Remold-O’Donnell E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry. 2001;40:15762–15770. doi: 10.1021/bi0113925. [DOI] [PubMed] [Google Scholar]
- 204.Saedi M.S., Zhu Z., Marker K., Liu R.-S., Carpenter P.M., Rittenhouse H., Mikolajczyk S.D. Human kallikrein 2 (hK2), but not prostate-specific antigen (PSA), rapidly complexes with protease inhibitor 6 (PI-6) released from prostate carcinoma cells. Int. J. Cancer. 2001;94:558–563. doi: 10.1002/ijc.1501. [DOI] [PubMed] [Google Scholar]
- 205.Mikolajczyk S.D., Millar L.S., Kumar A., Saedi M.S. Prostatic human kallikrein 2 inactivates and complexes with plasminogen activator inhibitor-1. Int. J. Cancer. 1999;81:438–442. doi: 10.1002/(sici)1097-0215(19990505)81:3<438::aid-ijc18>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 206.Scott F.L., Sun J., Whisstock J.C., Kato K., Bird P.I. SerpinB6 is an inhibitor of kallikrein-8 in keratinocytes. J. Biochem. 2007;142:435–442. doi: 10.1093/jb/mvm156. [DOI] [PubMed] [Google Scholar]
- 207.Kato K., Kishi T., Kamachi T., Akisada M., Oka T., Midorikawa R., Takio K., Dohmae N., Bird P.I., Sun J., Scott F., Miyake Y., Yamamoto K., Machida A., Tanaka T., Matsumoto K., Shibata M., Shiosaka S. Serine proteinase inhibitor 3 and murinoglobulin i are potent inhibitors of neuropsin in adult mouse brain. J. Biol. Chem. 2001;276:14562–14571. doi: 10.1074/jbc.M010725200. [DOI] [PubMed] [Google Scholar]
- 208.Mägert H.-J., Ständker L., Kreutzmann P., Zucht H.-D., Reinecke M., Sommerhoff C.P., Fritz H., Forssmann W.-G. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J. Biol. Chem. 1999;274:21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- 209.Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A.D., Bonafe J.-L., Wilkinson J., Taieb A., Barrandon Y., Harper J.I., de Prost Y., Hovnanian A. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat. Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 210.Descargues P., Deraison C., Prost C., Fraitag S., Mazereeuw-Hautier J., D’Alessio M., Ishida-Yamamoto A., Bodemer C., Zambruno G., Hovnanian A. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J. Invest. Dermatol. 2006;126:1622–1632. doi: 10.1038/sj.jid.5700284. [DOI] [PubMed] [Google Scholar]
- 211.Komatsu N., Saijoh K., Jayakumar A., Clayman G.L., Tohyama M., Suga Y., Mizuno Y., Tsukamoto K., Taniuchi K., Takehara K., Diamandis E.P. Correlation between SPINK5 gene mutations and clinical manifestations in Netherton syndrome patients. J. Invest. Dermatol. 2007;128:1148–1159. doi: 10.1038/sj.jid.5701153. [DOI] [PubMed] [Google Scholar]
- 212.Walley A.J., Chavanas S., Moffatt M.F., Esnouf R.M., Ubhi B., Lawrence R., Wong K., Abecasis G.R., Jones E.Y., Harper J.I., Hovnanian A., Cookson W.O.C.M. Gene polymorphism in Netherton and common atopic disease. Nat. Genet. 2001;29:175–178. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 213.Kabesch M., Carr D., Weiland S.K., von Mutius E. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypes in a large German population sample. Clin. Exp. Allergy. 2004;34:340–345. doi: 10.1111/j.1365-2222.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 214.Meyer-Hoffert U. Reddish, scaly, and itchy: how proteases and their inhibitors contribute to inflammatory skin diseases. Arch. Immunol. Ther. Exp. (Warsz.) 2009;57:345–354. doi: 10.1007/s00005-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 215.Briot A., Deraison C., Lacroix M., Bonnart C., Robin A., Besson C., Dubus P., Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lauber T., Schulz A., Schweimer K., Adermann K., Marx U.C. Homologous proteins with different folds: the three-dimensional structures of domains 1 and 6 of the multiple kazal-type inhibitor LEKTI. J. Mol. Biol. 2003;328:205–219. doi: 10.1016/s0022-2836(03)00245-6. [DOI] [PubMed] [Google Scholar]
- 217.Ardelt W., Laskowski M. Effect of single amino acid replacements on the thermodynamics of the reactive site peptide bond hydrolysis in ovomucoid third domain. J. Mol. Biol. 1991;220:1041–1053. doi: 10.1016/0022-2836(91)90370-l. [DOI] [PubMed] [Google Scholar]
- 218.Egelrud T., Brattsand M., Kreutzmann P., Walden M., Vitzithum K., Marx U.C., Forssmann W.G., Magert H.J. hK5 and hK7, two serine proteinases abundant in human skin, are inhibited by LEKTI domain 6. Br. J. Dermatol. 2005;153:1200–1203. doi: 10.1111/j.1365-2133.2005.06834.x. [DOI] [PubMed] [Google Scholar]
- 219.Schechter N.M., Choi E.J., Wang Z.M., Hanakawa Y., Stanley J.R., Kang Y., Clayman G.L., Jayakumar A. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI) Biol. Chem. 2005;386:1173–1184. doi: 10.1515/BC.2005.134. [DOI] [PubMed] [Google Scholar]
- 220.Meyer-Hoffert U., Wu Z., Schröder J.-M. Identification of lympho-epithelial kazal-type inhibitor 2 in human skin as a kallikrein-related peptidase 5-specific protease inhibitor. PLoS ONE. 2009;4:e4372. doi: 10.1371/journal.pone.0004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brattsand M., Stefansson K., Hubiche T., Nilsson S.K., Egelrud T. SPINK9: a selective, skin-specific kazal-type serine protease inhibitor. J. Invest. Dermatol. 2009;129:1656–1665. doi: 10.1038/jid.2008.448. [DOI] [PubMed] [Google Scholar]
- 222.Barrett A.J., Starkey P.M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem. J. 1973;133:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Andersen G.R., Koch T.J., Dolmer K., Sottrup-Jensen L., Nyborg J. Low resolution X-ray structure of human methylamine-treated alpha(2)-macroglobulin. J. Biol. Chem. 1995;270:25133–25141. doi: 10.1074/jbc.270.42.25133. [DOI] [PubMed] [Google Scholar]
- 224.Sottrup-Jensen L., Sand O., Kristensen L., Fey G.H. The alpha-macroglobulin bait region. Sequence diversity and localization of cleavage sites for proteinases in five mammalian alpha-macroglobulins. J. Biol. Chem. 1989;264:15781–15789. [PubMed] [Google Scholar]
- 225.Kolodziej S.J., Wagenknecht T., Strickland D.K., Stoops J.K. The three-dimensional structure of the human alpha 2-macroglobulin dimer reveals its structural organization in the tetrameric native and chymotrypsin alpha 2-macroglobulin complexes. J. Biol. Chem. 2002;277:28031–28037. doi: 10.1074/jbc.M202714200. [DOI] [PubMed] [Google Scholar]
- 226.Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 227.Christensen U., Sottrup-Jensen L. Mechanism of alpha 2-macroglobulin-proteinase interactions. Studies with trypsin and plasmin. Biochemistry. 1984;23:6619–6626. doi: 10.1021/bi00321a052. [DOI] [PubMed] [Google Scholar]
- 228.Crews B.C., James M.W., Beth A.H., Gettins P., Cunningham L.W. In support of the trap hypothesis. Chymotrypsin is not rigidly held in its complex with human alpha 2-macroglobulin. Biochemistry. 1987;26:5963–5967. doi: 10.1021/bi00393a003. [DOI] [PubMed] [Google Scholar]
- 229.Abe R., Yamamoto K., Sinohara H. Proteinase inhibitory spectrum of mouse murinoglobulin and {alpha}-macroglobulin. J. Biochem. 1989;106:564–568. doi: 10.1093/oxfordjournals.jbchem.a122896. [DOI] [PubMed] [Google Scholar]
- 230.Grauer L.S., Finlay J.A., Mikolajczyk S.D., Pusateri K.D., Wolfert R.L. Detection of human glandular kallikrein, hK2, as its precursor form and in complex with protease inhibitors in prostate carcinoma serum. J. Androl. 1998;19:407–411. [PubMed] [Google Scholar]
- 231.Heeb M.J., España F. a2-Macroglobulin and C1-inactivator are plasma inhibitors of human glandular kallikrein. Blood Cells Mol. Dis. 1998;24:412–419. doi: 10.1006/bcmd.1998.0209. [DOI] [PubMed] [Google Scholar]
- 232.Christensson A., Laurell C.-B., Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur. J. Biochem. 1990;194:755–763. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 233.Jensen P.E., Stigbrand T. Differences in the proteinase inhibition mechanism of human alpha 2-macroglobulin and pregnancy zone protein. Eur. J. Biochem. 1992;210:1071–1077. doi: 10.1111/j.1432-1033.1992.tb17513.x. [DOI] [PubMed] [Google Scholar]
- 234.Hengst U., Albrecht H., Hess D., Monard D. The phosphatidylethanolamine-binding protein is the prototype of a novel family of serine protease inhibitors. J. Biol. Chem. 2001;276:535–540. doi: 10.1074/jbc.M002524200. [DOI] [PubMed] [Google Scholar]
- 235.Brandstetter H., Kim J.-S., Groll M., Huber R. Crystal structure of the tricorn protease reveals a protein disassembly line. Nature. 2001;414:466–470. doi: 10.1038/35106609. [DOI] [PubMed] [Google Scholar]
- 236.Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H.D., Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 237.O’Farrell P.A., Gonzalez F., Zheng W., Johnston S.A., Joshua-Tor L. Crystal structure of human bleomycin hydrolase, a self-compartmentalizing cysteine protease. Structure. 1999;7:619–627. doi: 10.1016/s0969-2126(99)80083-5. [DOI] [PubMed] [Google Scholar]
- 238.Marquardt U., Zettl F., Huber R., Bode W., Sommerhoff C.P. The crystal structure of human a1-tryptase reveals a blocked substrate-binding region. J. Mol. Biol. 2002;321:491–502. doi: 10.1016/s0022-2836(02)00625-3. [DOI] [PubMed] [Google Scholar]
- 239.Pereira P.J.B., Bergner A., Macedo-Ribeiro S., Huber R., Matschiner G., Fritz H., Sommerhoff C.P., Bode W. Human b-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–311. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 240.Brandstetter H., Kim J.S., Groll M., Göttig P., Huber R. Structural basis for the processive protein degradation by tricorn protease. Biol. Chem. 2002;383:1157–1165. doi: 10.1515/BC.2002.127. [DOI] [PubMed] [Google Scholar]
- 241.Voges D., Zwickl P., Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 242.Matsui H., Moriyama A., Takahashi T. Cloning and characterization of mouse Klk27, a novel tissue kallikrein expressed in testicular Leydig cells and exhibiting chymotrypsin-like specificity. Eur. J. Biochem. 2000;267:6858–6865. doi: 10.1046/j.1432-1033.2000.01786.x. [DOI] [PubMed] [Google Scholar]
- 243.Oikonomopoulou K., Hansen K.K., Baruch A., Hollenberg M.D., Diamandis E.P. Immunofluorometric activity-based probe analysis of active KLK6 in biological fluids. Biol. Chem. 2008;389:747–756. doi: 10.1515/BC.2008.086. [DOI] [PubMed] [Google Scholar]
- 244.Dentan C., Tselepis A.D., Chapman M.J., Ninio E. Pefabloc, 4-[2-aminoethyl]benzenesulfonyl fluoride, is a new, potent nontoxic and irreversible inhibitor of PAF-degrading acetylhydrolase. Biochim. Biophys. Acta. 1996;1299:353–357. doi: 10.1016/0005-2760(95)00226-x. [DOI] [PubMed] [Google Scholar]
- 245.Fujimoto Y., Suzuki C., Watanabe Y., Matsuda Y., Akihama S. Purification and characterization of a kallikrein from human submaxillary glands. Biochem. Med. Metab. Biol. 1990;44:218–227. doi: 10.1016/0885-4505(90)90064-8. [DOI] [PubMed] [Google Scholar]
- 246.Bernett M.J., Blaber S.I., Scarisbrick I.A., Dhanarajan P., Thompson S.M., Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. J. Biol. Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- 247.Bode W., Chen Z., Bartels K., Kutzbach C., Schmidt-Kastner G., Bartunik H. Refined 2 A X-ray crystal structure of porcine pancreatic kallikrein A, a specific trypsin-like serine proteinase. Crystallization, structure determination, crystallographic refinement, structure and its comparison with bovine trypsin. J. Mol. Biol. 1983;164:237–282. doi: 10.1016/0022-2836(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 248.Fritz H., Förg-Brey B., Umezawa H. Leupeptin and antipain. Strong competitive inhibitors of sperm acrosomal proteinase (boar acrosin) and kallikreins from porcine organs (pancreas, submand. glands, urine) Hoppe Seylers Z. Physiol. Chem. 1973;354:1304–1306. doi: 10.1515/bchm2.1973.354.2.1304. [DOI] [PubMed] [Google Scholar]
- 249.Fielder P.J., Rosenfeld R.G., Graves H.C., Grandbois K., Maack C.A., Sawamura S., Ogawa Y., Sommer A., Cohen P. Biochemical analysis of prostate specific antigen-proteolyzed insulin-like growth factor binding protein-3. Growth Regul. 1994;4:164–172. [PubMed] [Google Scholar]
- 250.Sealock R.W., Laskowski M. Enzymic replacement of the arginyl by a lysyl residue in the reactive site of soybean trypsin inhibitor. Biochemistry. 1969;8:3703–3710. doi: 10.1021/bi00837a032. [DOI] [PubMed] [Google Scholar]
- 251.Song H.K., Suh S.W. Kunitz-type soybean trypsin inhibitor revisited: refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998;275:347–363. doi: 10.1006/jmbi.1997.1469. [DOI] [PubMed] [Google Scholar]
- 252.Koepke J., Ermler U., Warkentin E., Wenzl G., Flecker P. Crystal structure of cancer chemopreventive Bowman-Birk inhibitor in ternary complex with bovine trypsin at 2.3 Å resolution. Structural basis of Janus-faced serine protease inhibitor specificity. J. Mol. Biol. 2000;298:477–491. doi: 10.1006/jmbi.2000.3677. [DOI] [PubMed] [Google Scholar]
- 253.Lin G., Bode W., Huber R., Chi C., Engh R.A. The 0.25-nm X-ray structure of the Bowman-Birk-type inhibitor from mung bean in ternary complex with porcine trypsin. Eur. J. Biochem. 1993;212:549–555. doi: 10.1111/j.1432-1033.1993.tb17692.x. [DOI] [PubMed] [Google Scholar]
- 254.Kennedy A.R. Prevention of carcinogenesis by protease inhibitors. Cancer Res. 1994;54:1999s–2005s. [PubMed] [Google Scholar]
- 255.Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor: II. Crystallographic refinement at 1.9 Å resolution. J. Mol. Biol. 1974;89:73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- 256.Rühlmann A., Kukla D., Schwager P., Bartels K., Huber R. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor: crystal structure determination and stereochemistry of the contact region. J. Mol. Biol. 1973;77:417–436. doi: 10.1016/0022-2836(73)90448-8. [DOI] [PubMed] [Google Scholar]
- 257.Tschesche H., Kupfer S. Hydrolysis-resynthesis equilibrium of the lysine-15-alanine-16 peptide bond in bovine trypsin inhibitor (Kunitz) Hoppe Seylers Z. Physiol. Chem. 1976;357:769–776. doi: 10.1515/bchm2.1976.357.1.769. [DOI] [PubMed] [Google Scholar]
- 258.Krowarsch D., Zakrzewska M., Smalas A.O., Otlewski J. Structure-function relationships in serine protease-bovine pancreatic trypsin inhibitor interaction. Protein Pept. Lett. 2005;12:403–407. doi: 10.2174/0929866054395275. [DOI] [PubMed] [Google Scholar]
- 259.Ascenzi P., Bocedi A., Bolognesi M., Spallarossa A., Coletta M., De Cristofaro R., Menegatti E. The bovine basic pancreatic trypsin inhibitor (Kunitz inhibitor): a milestone protein. Curr. Protein Pept. Sci. 2003;4:231–251. doi: 10.2174/1389203033487180. [DOI] [PubMed] [Google Scholar]
- 260.Kraut H., Frey E.K., Werle E. Über die Inaktivierung des Kallikreins. (VI. Mitteilung Über dieses Kreislaufhormon.) Hoppe Seylers Z. Physiol. Chem. 1930;192:1–21. [Google Scholar]
- 261.Söllner C., Mentele R., Eckerskorn C., Fritz H., Sommerhoff C.P. Isolation and characterization of hirustasin, an antistasin-type serine-proteinase inhibitor from the medical leech Hirudo medicinalis. Eur. J. Biochem. 1994;219:937–943. doi: 10.1111/j.1432-1033.1994.tb18575.x. [DOI] [PubMed] [Google Scholar]
- 262.Ascenzi P., Amiconi G., Bode W., Bolognesi M., Coletta M., Menegatti E. Proteinase inhibitors from the European medicinal leech Hirudo medicinalis: structural, functional and biomedical aspects. Mol. Aspects Med. 1995;16:215–313. doi: 10.1016/0098-2997(95)00002-x. [DOI] [PubMed] [Google Scholar]
- 263.Mittl P.R., Di Marco S., Fendrich G., Pohlig G., Heim J., Sommerhoff C., Fritz H., Priestle J.P., Grutter M.G. A new structural class of serine protease inhibitors revealed by the structure of the hirustasin-kallikrein complex. Structure. 1997;5:253–264. doi: 10.1016/s0969-2126(97)00183-4. [DOI] [PubMed] [Google Scholar]
- 264.Swedberg J.E., de Veer S.J., Harris J.M. Natural and engineered kallikrein inhibitors: an emerging pharmacopoeia. Biol. Chem. 2010;391:357–374. doi: 10.1515/BC.2010.037. [DOI] [PubMed] [Google Scholar]
- 265.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 266.Bhal S.K., Kassam K., Peirson I.G., Pearl G.M. The rule of five revisited: applying log D in place of log P in drug-likeness filters. Mol. Pharm. 2007;4:556–560. doi: 10.1021/mp0700209. [DOI] [PubMed] [Google Scholar]
- 267.Ghose A.K., Viswanadhan V.N., Wendoloski J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1998;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 268.Zhang M.-Q., Wilkinson B. Drug discovery beyond the ’rule-of-five’. Curr. Opin. Biotechnol. 2007;18:478–488. doi: 10.1016/j.copbio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 269.Fear G., Komarnytsky S., Raskin I. Protease inhibitors and their peptidomimetic derivatives as potential drugs. Pharmacol. Ther. 2007;113:354–368. doi: 10.1016/j.pharmthera.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Riester D., Wirsching F., Salinas G., Keller M., Gebinoga M., Kamphausen S., Merkwirth C., Goetz R., Wiesenfeldt M., Stürzebecher J., Bode W., Friedrich R., Thürk M., Schwienhorst A. Thrombin inhibitors identified by computer-assisted multiparameter design. Proc. Natl. Acad. Sci. U S A. 2005;102:8597–8602. doi: 10.1073/pnas.0501983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Fattori D., Squarcia A., Bartoli S. Fragment-based approach to drug lead discovery: overview and advances in various techniques. Drugs R D. 2008;9:217–227. doi: 10.2165/00126839-200809040-00002. [DOI] [PubMed] [Google Scholar]
- 272.Tobin M.B., Gustafsson C., Huisman G.W. Directed evolution: the ’rational’ basis for ’irrational’ design. Curr. Opin. Struct. Biol. 2000;10:421–427. doi: 10.1016/s0959-440x(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 273.Klebe G. Recent developments in structure-based drug design. J. Mol. Med. 2000;78:269–281. doi: 10.1007/s001090000084. [DOI] [PubMed] [Google Scholar]
- 274.Walker B., Lynas J.F. Strategies for the inhibition of serine proteases. Cell. Mol. Life Sci. 2001;58:596–624. doi: 10.1007/PL00000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Adlington R.M., Baldwin J.E., Becker G.W., Chen B., Cheng L., Cooper S.L., Hermann R.B., Howe T.J., McCoull W., McNulty A.M., Neubauer B.L., Pritchard G.J. Design, synthesis, and proposed active site binding analysis of monocyclic 2-azetidinone inhibitors of prostate specific antigen. J. Med. Chem. 2001;44:1491–1508. doi: 10.1021/jm000145g. [DOI] [PubMed] [Google Scholar]
- 276.Koistinen H., Wohlfahrt G., Mattsson J.M., Wu P., Lahdenperä J., Stenman U.-H. Novel small molecule inhibitors for prostate-specific antigen. Prostate. 2008;68:1143–1151. doi: 10.1002/pros.20773. [DOI] [PubMed] [Google Scholar]
- 277.Krastel P., Liechty B.-M., Schmitt E., Schreiner E.P. In: Use of Cyclic Depsipeptides to Inhibit Kallikrein 7. Novartis A.G., editor. 2009. [Google Scholar]
- 278.Bodendorf U., Meingassner J.G., Hassiepen U., Ostermann N., Gerhartz B., Berst F., Randl S.A., Ehrhardt C., Marzinzik A., Flohr S. In: Kallikrein 7 Modulators. Novartis A.G., editor. 2008. Australia. [Google Scholar]
- 279.Fareed J., Messmore H.L., Kindel G., Balis J.U. Inhibition of serine proteases by low molecular weight peptides and their derivatives. Ann. N.Y. Acad. Sci. 1981;370:765–784. doi: 10.1111/j.1749-6632.1981.tb29784.x. [DOI] [PubMed] [Google Scholar]
- 280.Pimenta D., Melo R.L., Caliendo G., Santagada V., Fiorino F., Severino B., de Nucci G., Juliano L., Juliano M.A. Design of inhibitors for human tissue kallikrein using non-natural aromatic and basic amino acids. Biol. Chem. 2002;383:853–857. doi: 10.1515/BC.2002.091. [DOI] [PubMed] [Google Scholar]
- 281.Wolf W.C., Evans D.M., Chao L., Chao J. A synthetic tissue kallikrein inhibitor suppresses cancer cell invasiveness. Am. J. Pathol. 2001;159:1797–1805. doi: 10.1016/S0002-9440(10)63026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hekim C., Leinonen J., Narvanen A., Koistinen H., Zhu L., Koivunen E., Vaisanen V., Stenman U.-H. Novel peptide inhibitors of human kallikrein 2. J. Biol. Chem. 2006;281:12555–12560. doi: 10.1074/jbc.M600014200. [DOI] [PubMed] [Google Scholar]
- 283.Pakkala M., Hekim C., Soininen P., Leinonen J., Koistinen H., Weisell J., Stenman U.-H., Vepsäläinen J., Närvänen A. Activity and stability of human kallikrein-2-specific linear and cyclic peptide inhibitors. J. Pept. Sci. 2007;13:348–353. doi: 10.1002/psc.849. [DOI] [PubMed] [Google Scholar]
- 284.Hekim C., Riipi T., Weisell J., Närvänen A., Koistinen R., Stenman U.-H., Koistinen H. Identification of IGFBP-3 fragments generated by KLK2 and prevention of fragmentation by KLK2-inhibiting peptides. Biol. Chem. 2010;391:475–479. doi: 10.1515/BC.2010.039. [DOI] [PubMed] [Google Scholar]
- 285.Huang X., Knoell C.T., Frey G., Hazegh-Azam M., Tashjian A.H., Hedstrom L., Abeles R.H., Timasheff S.N. Modulation of recombinant human prostate-specific antigen: activation by Hofmeister salts and inhibition by azapeptides. Appendix: thermodynamic interpretation of the activation by concentrated salts. Biochemistry. 2001;40:11734–11741. doi: 10.1021/bi010364j. [DOI] [PubMed] [Google Scholar]
- 286.LeBeau A.M., Singh P., Isaacs J.T., Denmeade S.R. Potent and selective peptidyl boronic acid inhibitors of the serine protease prostate-specific antigen. Chem. Biol. 2008;15:665–674. doi: 10.1016/j.chembiol.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.LeBeau A.M., Banerjee S.R., Pomper M.G., Mease R.C., Denmeade S.R. Optimization of peptide-based inhibitors of prostate-specific antigen (PSA) as targeted imaging agents for prostate cancer. Bioorg. Med. Chem. 2009;17:4888–4893. doi: 10.1016/j.bmc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.LeBeau A.M., Kostova M., Craik C.S., Denmeade S.R. Prostate-specific antigen: an overlooked candidate for the targeted treatment and selective imaging of prostate cancer. Biol. Chem. 2010;391:333–343. doi: 10.1515/BC.2010.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Swedberg J.E., Nigon L.V., Reid J.C., de Veer S.J., Walpole C.M., Stephens C.R., Walsh T.P., Takayama T.K., Hooper J.D., Clements J.A., Buckle A.M., Harris J.M. Substrate-guided design of a potent and selective kallikrein-related peptidase inhibitor for kallikrein 4. Chem. Biol. 2009;16:633–643. doi: 10.1016/j.chembiol.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 290.Vasileiou Z., Barlos K.K., Gatos D., Adermann K., Deraison C., Barlos K. Synthesis of the proteinase inhibitor LEKTI domain 6 by the fragment condensation method and regioselective disulfide bond formation. Biopolymers. 2010;94:339–349. doi: 10.1002/bip.21376. [DOI] [PubMed] [Google Scholar]
- 291.Cloutier S.M., Kundig C., Felber L.M., Fattah O.M., Chagas J.R., Gygi C.M., Jichlinski P., Leisinger H.-J., Deperthes D. Development of recombinant inhibitors specific to human kallikrein 2 using phage-display selected substrates. Eur. J. Biochem. 2004;271:607–613. doi: 10.1111/j.1432-1033.2003.03963.x. [DOI] [PubMed] [Google Scholar]
- 292.Felber L.M., Kündig C., Borgoño C.A., Chagas J.R., Tasinato A., Jichlinski P., Gygi C.M., Leisinger H.-J., Diamandis E.P., Deperthes D., Cloutier S.M. Mutant recombinant serpins as highly specific inhibitors of human kallikrein 14. FEBS J. 2006;273:2505–2514. doi: 10.1111/j.1742-4658.2006.05257.x. [DOI] [PubMed] [Google Scholar]
- 293.Farady C.J., Egea P.F., Schneider E.L., Darragh M.R., Craik C.S. Structure of an Fab-protease complex reveals a highly specific non-canonical mechanism of inhibition. J. Mol. Biol. 2008;380:351–360. doi: 10.1016/j.jmb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sexton D.J., Chen T., Martik D., Kuzmic P., Kuang G., Chen J., Nixon A.E., Zuraw B.L., Forteza R.M., Abraham W.M., Wood C.R. Specific inhibition of tissue kallikrein 1 with a human monoclonal antibody reveals a potential role in airway diseases. Biochem. J. 2009;422:383–392. doi: 10.1042/BJ20090010. [DOI] [PubMed] [Google Scholar]
- 295.Bax B., Blundell T.L., Murray-Rust J., McDonald N.Q. Structure of mouse 7S NGF: a complex of nerve growth factor with four binding proteins. Structure. 1997;5:1275–1285. doi: 10.1016/s0969-2126(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 296.Shooter E.M. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 2003;24:601–629. doi: 10.1146/annurev.neuro.24.1.601. [DOI] [PubMed] [Google Scholar]
- 297.Pattison S.E., Dunn M.F. On the relationship of zinc ion to the structure and function of the 7S nerve growth factor protein. Biochemistry. 1975;14:2733–2739. doi: 10.1021/bi00683a027. [DOI] [PubMed] [Google Scholar]
- 298.Dong Y., Bui L.T., Odorico D.M., Tan O.L., Myers S.A., Samaratunga H., Gardiner R.A., Clements J.A. Compartmentalized expression of kallikrein 4 (KLK4/hK4) isoforms in prostate cancer: nuclear, cytoplasmic and secreted forms. Endocr. Relat. Cancer. 2005;12:875–889. doi: 10.1677/erc.1.01062. [DOI] [PubMed] [Google Scholar]
- 299.Xi Z., Klokk T.I., Korkmaz K., Kurys P., Elbi C., Risberg B., Danielsen H., Loda M., Saatcioglu F. Kallikrein 4 is a predominantly nuclear protein and is overexpressed in prostate cancer. Cancer Res. 2004;64:2365–2370. doi: 10.1158/0008-5472.can-03-2025. [DOI] [PubMed] [Google Scholar]
- 300.Clements J.A., Hooper J.D., Odorico D.M., Dong Y. In: Handbook of Proteolytic Enzymes. Barrett A.J., Rawlings N.D., Woessner J.F., editors. Elsevier Academic Press; London: 2004. The tissue kallikrein gene cluster; pp. 1569–1577. [Google Scholar]
- 301.Bode W., Schwager P., Huber R. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. The refined crystal structures of the bovine trypsinogen-pancreatic trypsin inhibitor complex and of its ternary complex with Ile-Val at 1.9 A resolution. J. Mol. Biol. 1978;118:99–112. doi: 10.1016/0022-2836(78)90246-2. [DOI] [PubMed] [Google Scholar]
- 302.Elliott P.R., Pei X.Y., Dafforn T.R., Lomas D.A. Topography of a 2.0 Å structure of alpha-1-antitrypsin reveals targets for rational drug design to prevent conformational disease. Protein Sci. 2000;9:1274–1281. doi: 10.1110/ps.9.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ota N., Stroupe C., Ferreira-da-Silva J.M.S., Shah S.A., Mares-Guia M., Brunger A.T. Non-Boltzmann thermodynamic integration (NBTI) for macromolecular systems: relative free energy of binding of trypsin to benzamidine and benzylamine. Proteins. 1999;37:641–653. doi: 10.1002/(sici)1097-0134(19991201)37:4<641::aid-prot14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 304.Helland R., Otlewski J., Sundheim O., Dadlez M., Smalås A.O. The crystal structures of the complexes between bovine b-trypsin and ten P1 variants of BPTI.J. Mol. Biol. 1999;287:923–942. doi: 10.1006/jmbi.1999.2654. [DOI] [PubMed] [Google Scholar]
- 305.Sichler K., Hopfner K.-P., Kopetzki E., Huber R., Bode W., Brandstetter H. The influence of residue 190 in the S1 site of trypsin-like serine proteases on substrate selectivity is universally conserved. FEBS Lett. 2002;530:220–224. doi: 10.1016/s0014-5793(02)03495-6. [DOI] [PubMed] [Google Scholar]
- 306.Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 307.Luo L.Y., Shan S.J., Elliott M.B., Soosaipillai A., Diamandis E.P. Purification and characterization of human kallikrein 11, a candidate prostate and ovarian cancer biomarker, from seminal plasma. Clin. Cancer Res. 2006;12:742–750. doi: 10.1158/1078-0432.CCR-05-1696. [DOI] [PubMed] [Google Scholar]
- 308.Ecke S., Geiger M., Resch I., Jerabek I., Sting L., Maier M., Binder B.R. Inhibition of tissue kallikrein by protein C inhibitor. Evidence for identity of protein C inhibitor with the kallikrein binding protein. J. Biol. Chem. 1992;267:7048–7052. [PubMed] [Google Scholar]
- 309.Frenette G., Deperthes D., Tremblay R.R., Lazure C., Dubé J.Y. Purification of enzymatically active kallikrein hK2 from human seminal plasma. Biochim. Biophys. Acta. 1997;1334:109–115. doi: 10.1016/s0304-4165(96)00080-3. [DOI] [PubMed] [Google Scholar]
- 310.Katz B.A., Liu B., Barnes M., Springman E.B. Crystal structure of recombinant human tissue kallikrein at 2.0 A resolution. Protein Sci. 1998;7:875–885. doi: 10.1002/pro.5560070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Laxmikanthan G., Blaber S.I., Bernett M.J., Scarisbrick I.A., Juliano M.A., Blaber M. 1.70 A X-ray structure of human apo kallikrein 1: structural changes upon peptide inhibitor/substrate binding. Proteins. 2005;58:802–814. doi: 10.1002/prot.20368. [DOI] [PubMed] [Google Scholar]
- 312.Chen Z., Bode W. Refined 2.5 A X-ray crystal structure of the complex formed by porcine kallikrein A and the bovine pancreatic trypsin inhibitor. Crystallization, Patterson search, structure determination, refinement, structure and comparison with its components and with the bovine trypsin-pancreatic trypsin inhibitor complex. J. Mol. Biol. 1983;164:283–311. doi: 10.1016/0022-2836(83)90078-5. [DOI] [PubMed] [Google Scholar]
- 313.Gomis-Ruth F.X., Bayes A., Sotiropoulou G., Pampalakis G., Tsetsenis T., Villegas V., Aviles F.X., Coll M. The structure of human prokallikrein 6 reveals a novel activation mechanism for the kallikrein family. J. Biol. Chem. 2002;277:27273–27281. doi: 10.1074/jbc.M201534200. [DOI] [PubMed] [Google Scholar]
- 314.Fernández I.S., Ständker L., Mägert H.-J., Forssmann W.-G., Giménez-Gallego G., Romero A. Crystal structure of human epidermal kallikrein 7 (hK7) synthesized directly in its native state in E. coli: insights into the atomic basis of its inhibition by LEKTI domain 6 (LD6) J. Mol. Biol. 2008;377:1488–1497. doi: 10.1016/j.jmb.2008.01.089. [DOI] [PubMed] [Google Scholar]
- 315.M. Debela, C.S. Craik, E. Schneider, W. Bode, V. Magdolen and P. Goettig, Crystal structure, substrate specificity and zinc/calcium binding of human kallikrein-related peptidase 8 (KLK8). (unpublished) (2010).
- 316.Kishi T., Kato M., Shimizu T., Kato K., Matsumoto K., Yoshida S., Shiosaka S., Hakoshima T. Crystal structure of neuropsin, a hippocampal protease involved in kindling epileptogenesis. J. Biol. Chem. 1999;274:4220–4224. doi: 10.1074/jbc.274.7.4220. [DOI] [PubMed] [Google Scholar]
- 317.Timm D.E. The crystal structure of the mouse glandular kallikrein-13 (prorenin converting enzyme) Protein Sci. 1997;6:1418–1425. doi: 10.1002/pro.5560060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Evans D.M., Jones D.M., Pitt G.R., Ashworth D., De Clerck F., Verheyen F., Szelke M. Synthetic inhibitors of human tissue kallikrein. Immunopharmacology. 1996;32:117–118. doi: 10.1016/0162-3109(95)00069-0. [DOI] [PubMed] [Google Scholar]
- 319.Lawrence M.G., Veveris-Lowe T.L., Whitbread A.K., Nicol D.L., Clements J.A. Epithelial-mesenchymal transition in prostate cancer and the potential role of kallikrein serine proteases. Cells Tissues Organs. 2007;185:111–115. doi: 10.1159/000101311. [DOI] [PubMed] [Google Scholar]
- 320.Yousef G.M., Kishi T., Diamandis E.P. Role of kallikrein enzymes in the central nervous system. Clin. Chim. Acta. 2003;329:1–8. doi: 10.1016/s0009-8981(03)00004-4. [DOI] [PubMed] [Google Scholar]