Abstract
Background
Little is known about the degree to which baboons, an important animal model in skeletal research, spontaneously experience age-related osteopenia and osteoporosis.
Methods
We measured bone mineral density (BMD) in 667 baboons, assigned T-scores to older animals based on sex-specific young adult reference groups, and compared reproductive history in older females with low BMD to those with normal BMD.
Results
Approximately 25% of older baboon females were osteopenic. No females or males were osteoporotic. Neither parity nor interbirth interval clearly distinguished low vs. normal BMD groups. Intersite correspondence in low BMD was highest between sites in the same region rather than sites of the same bone type.
Conclusion
As with humans, osteopenia is common among older females. The absence of osteoporotic animals may be due to colony maintenance resulting in truncation of the aged population and selection for healthier animals in the oldest ranges.
Keywords: Bone density, Skeletal Aging, Non-human primate model, Reproductive history, Lactation, Parity, Pregnancy
Introduction
Osteoporosis is a progressive condition involving structural deterioration of bone tissue, leading to skeletal fragility and an increased susceptibility to fractures due to low bone mass and high rates of bone turnover. Areal bone mineral density (aBMD) currently serves as the most reliable single predictor of susceptibility to osteoporotic fracture [6, 7, 25], accounting for 60–70% of bone strength [2], and, depending on skeletal site, 10–44% of fractures in women over the age of 65 [45]. This measure of skeletal mass is most commonly obtained using Dual Energy X-ray Absorptiometry (DXA), which provides an estimate of the amount of bone mineral (g/cm2) in a region of interest. Fracture risk is closely tied to aBMD such that each standard deviation decrease in aBMD results, approximately, in a doubling of fracture risk [25, 40].
Old World monkeys (members of the primate super family Cercopithecoidea), second only to apes in genetic proximity to humans, exhibit many biological similarities with our own species relevant to age-related changes and pathology in bone that may make them superior to non-primate species as models for studies of skeletal structure and maintenance in humans. [3, 34–36, 42, 50] The baboon is one Old World monkey species that has already been shown to exhibit extensive biological similarities to humans.
Baboons show various similarities to humans regarding skeletal biology. Both male and female baboons show bone loss with age, similar to that seen in humans, [3, 28] and females show increased bone turnover after ovariectomy. [27] Baboons also undergo processes of intracortical remodeling that parallel those of humans. Further, in a study of material properties of bone, Wang et al. [50] found that, relative to dogs, cows, and rabbits, baboon bone was more similar to human bone regarding fracture, microstructural, and compositional properties. Specifically, baboon bone was not significantly different from human bone with regard to mineral density, organic density, volume fraction, fracture surface pattern, length of collagen-mineral bundles, and the presence of a secondary osteonal bone structure. These aspects of baboon skeletal biology emphasize their value as a model for processes of skeletal maintenance and repair in humans.
A radiographic study of changes in vertebral shape with age in female baboons showed vertebral body changes similar to those seen in osteoporotic vertebral deformity in humans. [24] These changes include an overall decrease in vertebral body height and the occurrence of vertebral wedging. Both phenomena were most pronounced in older females.
Baboons also resemble humans in reproductive endocrinology, which is known to be important in bone metabolism. Estrogen, for example, strongly influences bone metabolism throughout life and in both sexes. Estrogen deficiency is the major mechanism behind the approximately five year period of rapid bone loss in human postmenopausal females. [17] Low estrogen levels are also reported in men with osteoporosis. [10] The menstrual cycle of baboons and other Old World Monkeys involves timing and phases similar to those of human females [8] and baboons parallel humans with regard to hormonal changes accompanying pregnancy. [23] Further, these primates undergo a natural menopause in their third decade. [13, 38] Peri-menopausal and menopausal phenomena in the Southwest National Primate Research Center/Southwest Foundation for Biomedical Research (SNPRC/SFBR) are characterized in Martin et. al. [38] Onset of peri-menopause, a period of heightened variability in cycle length, decreased fecundity, and altered hormonal profiles, begins at a mean age of 19 years in the baboon. Menopause, defined as an absence of menstrual bleeding and a turgescence (swelling of the perineal skin in response to estrogen) score of zero for one year, occurs in these females at a mean age of approximately 26 years.
The baboon’s long life span further contributes to its value in bone studies. Many shorter-lived animal models, such as rodents, do not remodel their skeletons the way humans do. [8] In captivity, baboons live approximately 1/3 as long as humans. Their lifespan is short enough to make longitudinal or mixed longitudinal studies possible, yet long enough to provide evidence of reproductive senescence and natural menopause. The maximum longevity of SNPRC/SFBR baboons is 33.68 years for females and 30.40 for males. In analyses of SNPRC/SFBR baboons, Bronikowski et al. [9] report a mean residual life expectancy of 16 years for females. Martin et al. studied baboons that were born into the SNPRC/SFBR colony, were not euthanized as part of an experimental protocol, and died at an age greater than five years. They report a mean age at death of 11.3 for females and 11.1 for males. [39]
Although the baboon clearly serves as a good model for skeletal maintenance and turnover in humans and has been used in a variety of experimental designs [4, 13, 19–22, 27, 31, 32, 41, 42, 46, 49–51], little is known about the degree to which age-related osteopenia and osteoporosis spontaneously occurs with age in these animals. The large SNPRC/SFBR colony of pedigreed baboons provides an excellent opportunity to conduct such an evaluation. The specific aim of our study was to test the hypothesis that older male and female baboons experience bone loss with age severe enough to be termed osteopenia and osteoporosis.
Materials and Methods
The sample (460 females, 207 males) ranging in age from five to 30 years consists of olive baboons (Papio hamadryas anubis), yellow baboons (Papio hamadryas cynocephalus) and their hybrids. All animals from which data were obtained are part of the much larger (n=3700) breeding colony at the SNPRC/SFBR. The colony was established during the late 1950s and early 1960s with wild-caught baboons from Kenya to facilitate the development of the baboon as a model for humans in biomedical research [47]. All animals are housed outdoors in social group cages and maintained on commercial monkey chow to which they have ad libitum access. Animal care personnel and staff veterinarians provide daily maintenance and health care to all animals in accordance with the Guide for the Care and Use of Laboratory Animals [15]. All procedures related to their treatment during the conduct of this study were approved by the Institutional Animal Care and Use Committee in accordance with the established guidelines. Animals with conditions or research history known to affect bone mass were excluded from this study.
After sedation by means of intramuscular Ketamine followed by intravenous “RAAK” (Rompun, Atropine, Acepromazine, and Ketamine), aBMD (g/cm2) was assessed at six different skeletal sites via DXA (Lunar DPX +6529, Lunar Corp., Madison, WI, U.S.A.). All DXA measurements were obtained using the manufacturer’s software for Adults, v. 3.65. Animals were scanned in a standard supine position. The scan width varied for each animal. We used Auto Width (Intelligent Scan Mode) to automatically narrow the path of the scan after locating bone masses. Speed was set manually depending on the thickness of the animal. Medium Mode was used for most animals, but Slow Mode was used for larger ones (> 26cm chest thickness). Both modes involve a scan interval of 1.2 × 1.2 mm. Three axial sites (thoraco-lumbar vertebrae 15 (TL15), 16 (TL16), and 17 (TL17)) were each measured in two different projections, anterior-posterior (AP) and lateral (LAT), resulting in a total of six measurements for each individual for the axial skeleton (AP15, AP16, AP17, LAT15, LAT16, and LAT17). From these measures, a mean lumbar value was calculated for each projection (lumbar LAT and lumbar AP). Three forearm sites were also measured: ultradistal radius (radius UD), diaphyseal radius at a point 33% proximal to the styloid process (radius 1/3), and diaphyseal ulna at a point 33% proximal to the styloid process (ulna 1/3). To determine the coefficients of variation in BMD measurement, five percent of the monkeys were scanned twice. (The first scan was used in our subsequent analyses.) The resulting coefficients of variation for lumbar spine, ultradistal radius, radius 1/3, and ulna 1/3 were 1.9%, 2.3%, 2.5%, and 2.5%, respectively.
Data on additional female reproductive history variables possibly related to aBMD were also collected. Parity, mean interbirth interval (IBI) in months, percent of adult life spent nursing an infant (% life nursing), and days since last birth were collected from extensive clinical records available for each animal.
Osteopenia and Osteoporosis Defined: T-scores
Guidelines provided by the World Health Organization (W.H.O.) for humans classify individuals with aBMD between −1.0 and −2.49 standard deviations below the young adult mean (a T-score between −1.0 and −2.49) as osteopenic. Those with both a T-score less than −2.5 are classified as osteoporotic. Those with a T-score less than −2.5 and an existing fracture are classified as having “severe/established osteoporosis”.
To approximate the criteria for diagnosis of osteopenia and osteoporosis in humans we calculated the young adult mean and standard deviation for females from our sample, then calculated T-scores for the older animals using these values. The human young adult mean referenced in the World Health Organization (W.H.O.) criteria was ascertained from a sample of Caucasian females aged 20–29 years [30]. It is generally accepted that a baboon of a particular age is roughly equivalent, developmentally, to a human three times as old. Using this standard, we calculated the young adult mean for aBMD using female baboons aged 6.67 to 9.67 years (roughly equivalent to 20–29 years in humans). Descriptive statistics (Table 1) were calculated using SPSS v. 12.0. There is disagreement on how to diagnose osteopenia and osteoporosis in males [29]. One option is to calculate their T-scores relative to the same reference sample (young adult females) as for females. Another option is to use a young adult reference sample composed of males. Because of significant sex differences in BMD, we thought it more appropriate to evaluate males based on a reference group of the same sex; therefore, we calculated T-scores for older males using a reference group of young adult males aged 6.67–9.67 years.
Table 1.
Descriptive statistics for age and aBMD (g/cm2) in the young adult reference sample.
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | x̄ | sd | range | n | x̄ | sd | range |
| age (years) | 97 | 7.89 | 0.85 | 6.69–9.64 | 57 | 8.30 | 0.98 | 6.75–9.67 |
| lumbar (AP) | 96 | 1.10 | 0.09 | 0.88–1.43 | 57 | 1.34 | 0.11 | 1.15–1.62 |
| lumbar (LAT) | 97 | 1.05 | 0.13 | 0.74–1.40 | 55 | 1.19 | 0.14 | 0.92–1.58 |
| radius_UD | 96 | 0.64 | 0.06 | 0.51–0.85 | 57 | 0.72 | 0.08 | 0.55–0.90 |
| radius 1/3 | 95 | 0.83 | 0.07 | 0.63–1.01 | 56 | 0.87 | 0.07 | 0.71–1.02 |
| ulna 1/3 | 96 | 0.82 | 0.07 | 0.62–0.97 | 57 | 0.90 | 0.09 | 0.70–1.12 |
Abbreviations in table: AP=anterior-posterior; LAT=lateral; UD=ultradistal; n=number; x̄ = mean; sd= standard deviation.
Older Adult Sample
Because we were interested in spontaneously occurring age-related osteoporosis, we limited our analysis of rates of osteopenia and osteoporosis to the older animals in our sample. This sample included 54 females over the age of 19.0 years (the mean age of onset of perimenopausal phenomena in these baboons [37]). Hormonal profiles are not available for these animals to confirm their reproductive status, but age is a reasonable and commonly used proxy in the absence of hormonal profiles as changes in hormonal profiles known to affect BMD correlate tightly with age. Descriptive statistics for this group are displayed in Table 2. A T-score was calculated for all older females by subtracting the young adult female mean from each older female’s aBMD, then dividing the resulting quantity by the young adult female standard deviation. For males, the age at which an individual is considered “older” is more arbitrary as there is no readily observable process such as perimenopause in females on which to base this category. Because age 50 is often used for reporting of statistics in humans, we included the nine males over the age of 16.67 (roughly equivalent to 50 years of age in humans) in the older adult male category. Their T-scores were calculated in the same way as for females, but using the young adult male mean and standard deviation.
Table 2.
Descriptive statistics for aBMD (g/cm2) and covariates in older animals.
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | x̄ | sd | range | n | x̄ | sd | range |
| age (years) | 54 | 22.94 | 2.26 | 19.57–30.01 | 9 | 19.58 | 2.43 | 17.23–23.82 |
| parity | 54 | 9.22 | 3.01 | 1–15 | -- | -- | -- | -- |
| IBI | 52 | 15.98 | 4.23 | 10.06–31.44 | -- | -- | -- | -- |
| % life nursing | 54 | 19.50 | 8.40 | 3.15–36.42 | -- | -- | -- | -- |
| days since last birth | 54 | 1564.00 | 1259.66 | 196–1564 | -- | -- | -- | -- |
| lumbar (AP) | 51 | 1.32 | 0.19 | 0.98–1.86 | 7 | 1.62 | 0.12 | 1.38–1.73 |
| lumbar (LAT) | 51 | 1.10 | 0.22 | 0.73–1.77 | 7 | 1.40 | 0.25 | 1.13–1.78 |
| radius_UD | 54 | 0.63 | 0.06 | 0.50–0.76 | 9 | 0.71 | 0.06 | 0.66–0.83 |
| radius 1/3 | 54 | 0.83 | 0.08 | 0.68–1.02 | 9 | 0.90 | 0.08 | 0.75–1.00 |
| ulna 1/3 | 54 | 0.82 | 0.08 | 0.69–1.04 | 9 | 0.94 | 0.70 | 0.83–1.05 |
Abbreviations in table: AP=anterior-posterior; LAT=lateral; UD=ultradistal; n=number; x̄ = mean; sd= standard deviation; IBI=mean interbirth interval
As outlined in the W.H.O. guidelines for diagnosis in humans, individuals with a T-score between −1.0 and −2.49 were classified as osteopenic. Those with a T-score less than −2.5 were classified as osteoporotic. Fracture data were not available for these animals, so no category equivalent to the W.H.O. “severe/established osteoporosis” (a T-score less than −2.5 and the presence of a fracture) was included in our analysis.
Post hoc comparisons of osteopenic and osteoporotic and normal aBMD groups
To investigate potential differences between those animals with normal aBMD and those with osteopenia or osteoporosis, groups were compared using Student’s t-tests for differences in age, number of offspring, mean interbirth interval, % life nursing, and days since last birth.
Because the animals from which these data were obtained are related, general regression tests for statistically significant relationships among variables are not entirely appropriate. The requirement of independence for these tests is not met and interpretations of significance may not hold. (Heritability of aBMD in this population of baboons ranges from 0.18 to 0.45 depending on skeletal site [35].) To address this issue, we used a maximum likelihood-based variance decomposition approach to test our hypotheses concerning the mean effects of covariates on aBMD measures while simultaneously accounting for the effects of non-independence due to kinship. [1]
Post hoc analysis of correspondence between sites
To determine the extent to which low bone mass corresponds between skeletal sites according to bone type (cortical vs. trabecular) and according to region (spine vs. forearm) we used qualitative comparisons of inter-site correspondence and bivariate correlations to assess the relationship between T-scores at various sites. Specifically we compared osteopenia at lumbar LAT vs. radius UD (two trabecular bone sites, different regions), radius UD vs. radius 1/3 (trabecular bone vs. cortical bone, same region), and ulna 1/3 vs. radius 1/3 (two cortical bone sites, same region).
Results
Table 1 shows the descriptive statistics for age and aBMD of both the female and the male young adult reference groups. Table 2 shows the descriptive statistics for age, aBMD and, for females, reproductive history covariates for the older animals.
Table 3 shows the percentage of older females showing osteopenia (t-score between −2.49 and −1.0) or osteoporosis (t-score below −2.5) at each skeletal site. Approximately 25% of the older females are osteopenic at each skeletal site excepting the lumbar AP projection which shows a much lower rate of osteopenia (3.9%). No females qualified as osteoporotic. Only one male is osteopenic at any site (a 17.6 year old male with a T-score of −1.71 at the radius 1/3; T-scores were normal at all other sites). No males were osteoporotic. Given this low incidence, no post hoc analyses were carried out for males.
Table 3.
Percentages of older female baboons with osteopenia and osteoporosis.
| Skeletal site | |||||
|---|---|---|---|---|---|
| lumbar (AP) | lumbar (LAT) | radius UD | radius 1/3 | ulna 1/3 | |
| % osteopenic | 3.9 | 23.5 | 27.8 | 31.5 | 18.5 |
| % osteoporotic | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| N | 51 | 51 | 54 | 54 | 54 |
Osteopenic females vs. those with normal aBMD
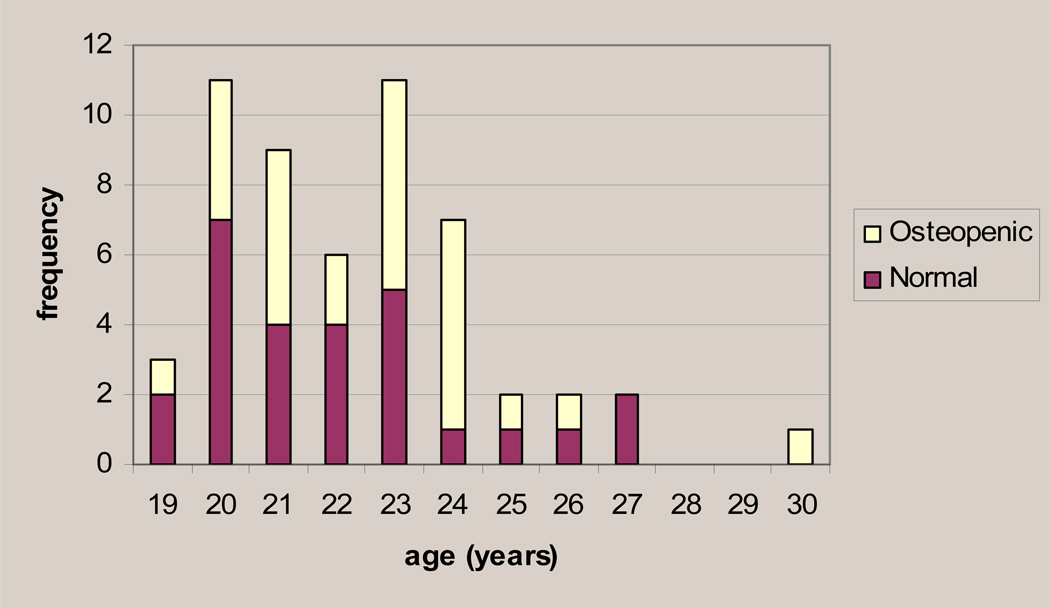
The females with ostoepenia range in age from 19.6–30.0 years. Figure 1 shows the distribution of osteopenic females (those showing a t-score of −2.49 to −1.0 at any of the five skeletal sites) and normal females by age, demonstrating that there is no obvious correlation between affected status and older age. Student’s t-tests indicate that the osteopenic and normal groups did not differ significantly by age. Maximum-likelihood estimates confirm that the effect of age on the population mean T-score is not significant at any site (see Table 4).
Figure 1.
The number of osteopenic and normal females by age in the group of older females.
Table 4.
Maximum likelihood test results, p-values and β for significant covariates.
| covariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skeletal Site | β age | p- value |
β parity | p- value |
β IBI | p- value |
β days since last birth |
p- value |
β % life nursing |
p- value |
| lumbar (AP) | 0.991 | 0.087 | 0.08 | 0.022 | 0.243 | 0.895 | ||||
| lumbar (LAT) | 0.862 | −0.20 | 0.008 | 0.07 | 0.014 | 0.309 | 0.520 | |||
| radius UD | 0.486 | 0.169 | 0.061 | 0.334 | 0.362 | |||||
| radius 1/3 | 0.586 | 0.139 | 0.332 | 0.058 | 0.767 | |||||
| ulna 1/3 | 0.613 | 0.140 | 0.510 | 0.205 | 0.669 | |||||
β=mean effect of significant covariate
Student’s t-tests do not reveal any statistically significant differences between those females of normal aBMD and those with osteopenia for the reproductive history variables tested (see Table 5). Maximum likelihood parameter estimates do, however, indicate that parity and mean interbirth interval have significant mean effects on the lumbar T-scores. The β values associated with the significant covariates indicate that higher parity is associated with lower aBMD, and a longer mean interbirth interval is associated with higher aBMD.
Table 5.
P-values for Student’s t-tests to compare means of osteopenic to normal females.
| skeletal site | ||||||
|---|---|---|---|---|---|---|
| variable | lumbar (AP) |
lumbar (LAT) |
radius UD |
radius 1/3 |
ulna 1/3 |
all sites |
| age | 0.46 | 0.67 | 0.34 | 0.22 | 0.28 | 0.46 |
| parity | 0.13 | 0.06 | 0.29 | 0.76 | 0.38 | 0.13 |
| IBI | 0.61 | 0.17 | 0.60 | 0.62 | 0.51 | 0.61 |
| days since last birth | 0.13 | 0.34 | 0.79 | 0.53 | 0.75 | 0.13 |
| % life nursing | 0.36 | 0.91 | 0.64 | 0.86 | 0.80 | 0.36 |
Correspondence between skeletal sites
Of the 12 females that are osteopenic in the lumbar spine (lateral projection), six (50%) are also osteopenic at the radius UD, a site that, like the lumbar spine (lumbar LAT), is trabecular bone-dominated. Of the 15 females that are osteopenic at the radius UD (trabecular bone), 11 (73%) are also osteopenic at the radius 1/3 (cortical bone), a site that is similar in location, but is dominated by a different type of bone. Of the ten females that are osteopenic at the ulna 1/3 (cortical bone), eight (80%) are also osteopenic at the radius 1/3 (cortical bone), a site that is similar both in location and in bone type. The Pearson’s correlation coefficient (r) between T-scores at the lumbar spine (trabecular bone) and the radius UD (trabecular bone) is 0.38 (p=0.006). That between radius UD (trabecular bone) and radius 1/3 (cortical bone) is 0.67 (p<0.001) and that between radius 1/3 (cortical bone) and ulna 1/3 (cortical bone) is 0.81 (p<0.001). Generally these results show that osteopenia can be site-specific and that there is a higher correspondence between osteopenia incidence between bones of the same anatomical region rather than those of the same bone type.
Discussion
Though it is well-established that the baboon and related species (i.e. macaques) experience bone loss with age [3, 5, 11, 12, 14, 16, 18, 21, 26, 28, 43], particularly in females, the degree to which bone loss of a magnitude substantial enough to be termed osteopenia or osteoporosis using definitions comparable to those applied to humans has only rarely been assessed [11]. In fact, rates of osteopenia and or osteoporosis have never previously been assessed in baboons or in such a large non-human primate population. Our results indicate that in both the forearm and the spine, ~25% of older baboon females are osteopenic using criteria for diagnosis that approximate those established by the W.H.O. for humans. No males qualified as osteopenic or osteoporotic and no females qualified as osteoporotic. This may be biologically meaningful, or could be due to the nature of the maintenance and veterinary care of this captive breeding colony. It may be that both females and males with the most severe bone loss (i.e. that which exceeds the threshold for qualifying as “osteoporotic”) are more likely to be euthanized because of co-morbid conditions associated with a general state of poor health resulting in their under-representation in this sample. With regard to males it must be noted that the number of older males in the population is low (n=9), reflecting both the greater need for females in a breeding colony and the lower mean age at death of males, as is typical of primate species. Nonetheless, in humans, males are at risk of osteopenia and osteoporosis at much older ages than females and our data suggest that the same to be true of baboons.
The lower rate of osteopenia in the lumbar AP projection is most likely explained by the presence of osteophyte development in these animals that increases with age and that confounds DXA-acquired aBMD measurements as the scans do not discriminate between the bone mineral that is part of the osteophytes vs. that which is in the vertebral body. This bony build-up occurs preferentially along the anterior portions of the vertebral body and can lead to higher aBMD [33]. The lumbar LAT projection is not generally confounded by the osteophytes and is considered to better reflect aBMD in the vertebral body.
In our examination of potential factors (i.e. age and reproductive history variables) that discriminate between those females with normal BMD at older ages and those with osteopenia, we were unable to identify any characteristic that clearly differentiated between the two groups. Our results do show, however, that the mean effect of higher parity on aBMD of the lumbar spine is negative while the mean effect of longer interbirth interval is positive. This effect was not observed in the forearm. This may be due to the predominance of trabecular bone in the lumbar spine relative to the forearm. Trabecular bone is thought to be more susceptible to bone density changes associated with reproduction than is cortical bone (which dominates two of the three forearm sites). It must be noted, however, that the ultradistal radius does contain a substantial amount of trabecular bone and significant effects of parity and IBI are not apparent at this site.
The assessment of consistency of oteopenia diagnoses and T-scores among skeletal sites indicates that, as in humans, this diagnosis can be site specific [44, 48]. Higher correspondence between sites of the same anatomical region vs. those dominated by the same type of bone may reflect locally acting mechanical or endocrinological factors that contribute to low aBMD.
Our results are consistent in many ways with those from related studies of bone loss with age in macaques, another Old World monkey species. The most directly comparable study with regard to methodology (osteopenia and osteoporosis assessed via comparison of BMD in older animals to a young adult reference group) was conducted in a population of free-ranging rhesus monkeys (Macaca mulatta). [11] The females in this population show a steady decline in BMD after the age of 17.2 years. Two of the four females over the age of 20 years qualify as osteopenic, but none of these older females was osteoporotic. As in our study, none of the males were osteopenic or osteoporotic, but Cerroni et al. excluded males over the age of 18.5 years from their analyses due to the presence of osteophytosis.
Our results show that the bone density of a substantial portion of older female baboons is low enough to be diagnosed as osteopenia using criteria that are comparable to those used for diagnosis in humans. Although prevalence figures for osteopenia and osteoporosis in humans vary considerably by populations, the National Osteoporosis Foundation reports that 20% of non-Hispanic white and Asian women aged 50 and older are estimated to have osteoporosis and 52 percent are estimated to have osteopenia. While it is not appropriate to directly compare these percentages to those in our baboon sample, it is clear that osteopenia is prevalent in older females of both species. We observed no animals that would be diagnosed as osteoporotic, but we do not think that this represents a biologically meaningful difference between baboons and humans. It is very likely that by the time the baboons are old enough to display bone loss severe enough that their aBMD falls greater than 2.5 standard deviations below the young adult mean, other health issues have likely resulted in the animals being euthanized. This would result in artificial selection for healthier animals in the oldest age ranges, and could explain our high incidence of osteopenia, but absence of osteoporosis. Ultimately our results validate the use of the baboon as an appropriate and valuable animal model of human bone maintenance and turnover.
Acknowledgements
The authors gratefully acknowledge the technical contributions and support of the following persons: G. Joslyn and J. Lichter (formerly of Sequana Therapeutics, Inc.), W. Hodgson, E. Windhorst, T. Riley, N.J. Whittam, N. Stowell, S. Nair, and S.H. Slifer.
Funding sources: Research reported in this manuscript was made possible in part by support in the form of a collaborative research contact with AxyS Pharmaceuticals, Inc. (formerly Sequana Therapeutics, Inc.) and research grants from the National Institutes of Health (#F32 AR049694, #P01 HL28972, and #P51 RR013986 supporting the SNPRC).
References
- 1.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14 Suppl 3:S13–S18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 3.Aufdemorte TB, Fox WC, Miller D, Buffum K, Holt GR, Carey KD. A non-human primate model for the study of osteoporosis and oral bone loss. Bone. 1993;14:581–586. doi: 10.1016/8756-3282(93)90197-i. [DOI] [PubMed] [Google Scholar]
- 4.Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D, et al. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993;92:2577–2586. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black A, Tilmont EM, Handy AM, Scott WW, Shapses SA, Ingram DK, Roth GS, Lane MA. A nonhuman primate model of age-related bone loss: a longitudinal study in male and premenopausal female rhesus monkeys. Bone. 2001;28:295–302. doi: 10.1016/s8756-3282(00)00452-x. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Cummings SR, Genant HK, Nevitt MC, Palermo L, Browner W. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res. 1992;7:633–638. doi: 10.1002/jbmr.5650070607. [DOI] [PubMed] [Google Scholar]
- 7.Blank RD. Breaking down bone strength: a perspective on the future of skeletal genetics. J Bone Miner Res. 2001;16:1207–1211. doi: 10.1359/jbmr.2001.16.7.1207. [DOI] [PubMed] [Google Scholar]
- 8.Brommage R. Perspectives on using nonhuman primates to understand the etiology and treatment of postmenopausal osteoporosis. Journal of Musculoskeletal Neuron Interaction. 2001;1:307–325. [PubMed] [Google Scholar]
- 9.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci U S A. 2002;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsen CG, Soerensen TH, Eriksen EF. Prevalence of low serum estradiol levels in male osteoporosis. Osteoporos Int. 2000;11:697–701. doi: 10.1007/s001980070068. [DOI] [PubMed] [Google Scholar]
- 11.Cerroni AM, Tomlinson GA, Turnquist JE, Grynpas MD. Bone mineral density, osteopenia, and osteoporosis in the rhesus macaques of Cayo Santiago. Am J Phys Anthropol. 2000;113:389–410. doi: 10.1002/1096-8644(200011)113:3<389::AID-AJPA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Champ JE, Binkley N, Havighurst T, Colman RJ, Kemnitz JW, Roecker EB. The effect of advancing age on bone mineral content of female rhesus monkeys. Bone. 1996;19:485–492. doi: 10.1016/s8756-3282(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen LD, Kushwaha RS, McGill HC, Jr, Rice KS, Carey KD. Effect of naturally reduced ovarian function on plasma lipoprotein and 27-hydroxycholesterol levels in baboons (Papio sp.) Atherosclerosis. 1998;136:89–98. doi: 10.1016/s0021-9150(97)00190-1. [DOI] [PubMed] [Google Scholar]
- 14.Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24:17–23. doi: 10.1016/s8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 15.Council" NR Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy of Sciences; 1996. [Google Scholar]
- 16.DeRousseau CJ. Aging in the musculoskeletal system of rhesus monkeys: III. Bone loss. Am J Phys Anthropol. 1985;68:157–167. doi: 10.1002/ajpa.1330680203. [DOI] [PubMed] [Google Scholar]
- 17.Eastell R. Pathogenesis of Postmenopausal Osteoporosis. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of bone metabolism. Washington, D.C.: American Society for Bone and Mineral Research; 2006. pp. 259–262. [Google Scholar]
- 18.Grynpas MD, Huckell B, Pritzker KP, Hancock RG, Kessler MJ. Bone mineral and osteoporosis in aging rhesus monkeys. P R Health Sci J. 1989;8:197–204. [PubMed] [Google Scholar]
- 19.Havill LM, Mahaney MC, Cox LA, Morin PA, Joslyn G, Rogers J. A quantitative trait locus for normal variation in forearm bone mineral density in pedigreed baboons maps to the ortholog of human chromosome 11q. J Clin Endocrinol Metab. 2005;90:3638–3645. doi: 10.1210/jc.2004-1618. [DOI] [PubMed] [Google Scholar]
- 20.Havill LM, Mahaney MC, Cox LA, Rogers J. Cross-species replication of a serum osteocalcin QTL on human chromosome 16q in pedigreed baboons. Calcif Tissue Int. 2005;77:250–211. doi: 10.1007/s00223-005-0056-1. [DOI] [PubMed] [Google Scholar]
- 21.Havill LM, Mahaney MC, Czerwinski SA, Carey KD, Rice K, Rogers J. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33:877–888. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 22.Havill LM, Mahaney MC, Rogers J. Genotype-by-sex and environment-by-sex interactions influence variation in serum levels of bone-specific alkaline phosphatase in adult baboons (Papio hamadryas) Bone. 2004;35:198–203. doi: 10.1016/j.bone.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickx A, Dukelow W. Reproductive Biology. In: Bennett BT, C.R. A, Henrickson R, editors. Nonhuman primates in biomedical research: biology and management. San Diego, CA: Academic Press; 1995. pp. 147–191. [Google Scholar]
- 24.Hughes KP, D.B. K, Rogers J, Kammerer CM, Rice KS, Davies KM, Recker RR. A prospective, quantitative study of vertebral body shape in aged female baboons. Journal of bone and mineral research. 1995;10:S365. [Google Scholar]
- 25.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayo MJ, Jerome CP, Lees CJ, Rankin SE, Weaver DS. Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif Tissue Int. 1994;54:231–236. doi: 10.1007/BF00301684. [DOI] [PubMed] [Google Scholar]
- 27.Jerome CP, Kimmel DB, McAlister JA, Weaver DS. Effects of ovariectomy on iliac trabecular bone in baboons (Papio anubis) Calcif Tissue Int. 1986;39:206–208. doi: 10.1007/BF02555119. [DOI] [PubMed] [Google Scholar]
- 28.Kammerer CM, Sparks ML, Rogers J. Effects of age, sex, and heredity on measures of bone mass in baboons (Papio hamadryas) J Med Primatol. 1995;24:236–242. doi: 10.1111/j.1600-0684.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanis JA, Johnell O, Oden A, De Laet C, Mellstrom D. Diagnosis of osteoporosis and fracture threshold in men. Calcif Tissue Int. 2001;69:218–221. doi: 10.1007/s00223-001-1046-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 31.Keller TS, Lovin JD, Spengler DM, Carter DR. Fatigue of immature baboon cortical bone. J Biomech. 1985;18:297–304. doi: 10.1016/0021-9290(85)90847-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Wagner HD, Weiner S. Bending and fracture of compact circumferential and osteonal lamellar bone of the baboon tibia. J Mater Sci Mater Med. 2000;11:49–60. doi: 10.1023/a:1008989719560. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int. 1997;7:564–569. doi: 10.1007/BF02652563. [DOI] [PubMed] [Google Scholar]
- 34.Mahaney MC, Havill LM, Joslyn G, Rogers J. A pedigreed baboon model for age related changes and pathology in bone: genetic correlations between bone mineral density at different anatomical sites. Journal of bone and mineral research submitted [Google Scholar]
- 35.Mahaney MC, Havill LM, Kammerer CM, Joslyn G, Rogers J. A Pedigreed Baboon Model for Age Related Changes and Pathology in Bone: Quantitative Genetics of Normal Variation in Bone Mineral Density. Journal of bone and mineral research submitted [Google Scholar]
- 36.Mahaney MC, Kammerer CM, Whittam H, Hodgson WJ, Dyer T, Lichter JB, Rogers J. Genetic and Environmental Correlations between Bone Mineral Densities at Six Vertebral and Long-bone Sites in Pedigreed Baboons. J Bone Miner Res. 1995;10 S1:364. [Google Scholar]
- 37.Martin LJ, Carey KD, Comuzzie AG. Variation in Menstrual Cycle Length and Cessation of Menstruation in Captive Baboons. Mech Ageing Dev. 2003;124:865–871. doi: 10.1016/s0047-6374(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin LJ, Carey KD, Comuzzie AG. Variation in menstrual cycle length and cessation of menstruation in captive raised baboons. Mech Ageing Dev. doi: 10.1016/s0047-6374(03)00134-9. In press. [DOI] [PubMed] [Google Scholar]
- 39.Martin LJ, Mahaney MC, Bronikowski AM, Dee Carey K, Dyke B, Comuzzie AG. Lifespan in captive baboons is heritable. Mech Ageing Dev. 2002;123:1461–1467. doi: 10.1016/s0047-6374(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 40.Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8:1227–1233. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 41.Nuckley DJ, Eck MP, Carter JW, Ching RP. Spinal maturation affects vertebral compressive mechanics and vBMD with sex dependence. Bone. 2004;35:720–728. doi: 10.1016/j.bone.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Phelps JB, Hubbard GB, Wang X, Agrawal CM. Microstructural heterogeneity and the fracture toughness of bone. J Biomed Mater Res. 2000;51:735–741. doi: 10.1002/1097-4636(20000915)51:4<735::aid-jbm23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Pope NS, Gould KG, Anderson DC, Mann DR. Effects of age and sex on bone density in the rhesus monkey. Bone. 1989;10:109–112. doi: 10.1016/8756-3282(89)90007-0. [DOI] [PubMed] [Google Scholar]
- 44.Stoch SA, Wysong E, Connolly C, Parker RA, Greenspan SL. Classification of osteoporosis and osteopenia in men is dependent on site-specific analysis. J Clin Densitom. 2000;3:311–317. doi: 10.1385/jcd:3:4:311. [DOI] [PubMed] [Google Scholar]
- 45.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 46.Thompson DD, Seedor JG, Quartuccio H, Solomon H, Fioravanti C, Davidson J, Klein H, Jackson R, Clair J, Frankenfield D, et al. The bisphosphonate, alendronate, prevents bone loss in ovariectomized baboons. J Bone Miner Res. 1992;7:951–960. doi: 10.1002/jbmr.5650070812. [DOI] [PubMed] [Google Scholar]
- 47.Vagtborg H. The Story of the Southwest Research Center: A Private, Nonprofit Scientific Research Adventure. Austin: University of Texas Press; 1973. [Google Scholar]
- 48.Varney LF, Parker RA, Vincelette A, Greenspan SL. Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom. 1999;2:275–283. doi: 10.1385/jcd:2:3:275. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Bank RA, TeKoppele JM, Hubbard GB, Athanasiou KA, Agrawal CM. Effect of collagen denaturation on the toughness of bone. Clin Orthop Relat Res. 2000:228–239. doi: 10.1097/00003086-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998;8:1–9. [PubMed] [Google Scholar]
- 51.Wang XD, Masilamani NS, Mabrey JD, Alder ME, Agrawal CM. Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity, and tensile properties. Bone. 1998;23:67–72. doi: 10.1016/s8756-3282(98)00071-4. [DOI] [PubMed] [Google Scholar]