Abstract
A great deal of research into the determinants of bone strength has unequivocally demonstrated that variation in bone strength is highly subject to genetic factors. Increasing attention in skeletal genetic studies is being paid to indicators of bone quality that complement studies of BMD, including studies of the genetic control of bone geometry. The aim of this study is to investigate the degree to which normal population-level variation in femoral midshaft geometry in a population of pedigreed baboons (Papio hamadryas spp.) can be attributed to the additive effect of genes. Using 110 baboons (80 females, 30 males), we 1) characterize normal variation in midshaft geometry of the femur with regard to age and sex, and 2) determine the degree to which the residual variation is attributable to the additive genetic effects. Cross-sectional area (CSA), minimum (IMIN) and maximum (IMAX) principal moments of inertia, and polar moment of inertia (J) were calculated from digitized images of transverse midshaft sections. Maximum likelihood-based variance decomposition methods were used to estimate the mean effects of age, sex, and genes. Together age and sex effects account for ~56% of the variance in each property. In each case the effect of female sex is negative and that of age is positive, although of a lower magnitude than the effect of female sex. Increased age is associated with decreased mean cross-sectional geometry measures in the oldest females. Residual h2 values range from 0.36–0.50, reflecting genetic effects accounting for 15% to 23% of the total phenotypic variance in individual properties. This study establishes the potential of the baboon model for the identification of genes that regulate bone geometric properties in primates. This model is particularly valuable because it allows for experimental designs, environmental consistency, availability of tissues, and comprehensive assessments of multiple integrated bone phenotypes that are not possible in human populations. The baboon is of particular importance in genetic studies, because it provides results that are likely highly relevant to the human condition due to the phylogenetic proximity of baboons to humans.
Keywords: Bone quality, Skeletal aging, Non-human primate model, Skeletal genetics, Bone morphometry
Introduction
Cross-sectional geometry is an important contributor to bone strength. Size and shape of the bone reflect and influence loading patterns during weight bearing. It has been repeatedly demonstrated that cross-sectional morphology in long bones, such as the femur, maximizes efficiency of energy expenditure during load bearing, while minimizing risk of fracture [1–4].
Research into determinants of bone strength has unequivocally demonstrated that variation in bone strength is highly subject to genetic factors. Initially most of this work focused on bone mineral density (BMD) because of the relationship between BMD and clinical evaluation of osteoporosis risk. The importance of genetic effects on BMD has been established repeatedly in studies of both nonhuman animal models, including rodents and primates, and human families and populations [5–11].
Increasing attention in skeletal genetic studies is now being paid to indicators of bone quality that complement studies of BMD, including studies of the genetic control of geometric properties of bone. A number of studies in inbred rodents show that geometric properties contribute significantly to bone strength and are strongly influenced by genetic variation [8, 12–21]. Genetic effects have been demonstrated for vertebral cortical area [16], trabecular bone architecture [16]; trabecular bone volume [17] femoral cross-sectional area (CSA) [8, 16, 19], femoral shape [13, 17, 21], femoral bone fragility [12, 22] and bone structure-function relationships [23–24].
Heritability and association studies conducted in humans indicate that the genetic effect on geometric properties that has been detected in inbred rodent strains may translate into an effect of genes on normal population-level variation in humans [25–26]. Published reviews of the osteoporosis genetics (Ralston [26–27] and Liu [9]) describe a series of successes in the detection of genetic effects and localization of quantitative trait loci (QTLs) for bone geometric properties in twin-, family-, and population-based studies. The results show that genes contribute significantly to variation in femoral CSA [17,28–30] and the shape [29] and structure of the femoral neck [28,31]. However, salient differences in the genetic models needed to detect and localize these effects suggest interactions between genes and other variables (e.g. sex, age, and/or hormonal status). For example, Deng et al. [32] report a total of seven femoral neck geometry-related QTLs European-derived pedigrees, two of which are evident only in women and three of which are specific to men. Similarly, Koller et al. detected a femoral neck axis QTL on 5q only evident in premenopausal women [33]. Investigation of the effects of multiple genes and gene-by-environment interactions on bone geometric properties implicated by these results can be complicated by heterogeneity of diet, physical activity, and other important co-variables in humans.
Populations of biologically relevant, genetically characterized animals, such as the baboon, an established and reliable nonhuman primate model for the genetics of many skeletal traits [30, 34–36], whose environmental exposures may be controlled and/or adequately accounted for, may facilitate more accurate detection of these effects and more precise estimation of their magnitudes. Like humans, baboons naturally undergo skeletal remodeling over a relatively long life-span. Similarity in bone composition and microstructure results in fracture properties of bone tissue that are more similar to those of human bone than is true of other popular animal models [37]. Baboons resemble humans in their patterns of skeletal density changes and bone loss with increasing age, experience a natural menopause late in life [38] and show increased skeletal turnover upon ovariectomy [36, 39–40]. Finally, the captive baboon colony at Southwest National Primate Research Center (SNPRC)/Southwest Foundation for Biomedical Research (SFBR) displays a population-wide level of genetic variation that is absent in inbred rodent models.
The aim of this study is to investigate the degree to which normal variation in femoral midshaft geometry in a population of pedigreed baboons can be attributed to the additive effect of genes with the goal of assessing the potential of this valuable animal model for investigating questions of primate skeletal genetics. Specifically, using a sample of 110 baboons we aim to 1) characterize normal variation in midshaft geometry of the femur with regard to age and sex, and 2) determine the degree to which the residual variation is attributable to the additive effects of genes.
Materials and Methods
Midshaft cross-sectional geometry properties were determined using the right femurs of 110 pedigreed baboons, (Papio hamadryas spp.; 80 females and 30 males) from a breeding colony at the SNPRC/SFBR in San Antonio, TX. All the animals included in this study were adults between the ages of 5 and 33 years and were members of a single large extended pedigree.
During life all animals were housed out of doors in social group cages and maintained on commercial monkey diet (SWF Primate Diet, Harlan Teklad, Madison, WI) to which they had ad libitum access. Animal care personnel and staff veterinarians provided daily maintenance and health care to all animals in accordance with the Guide for the Care and Use of Laboratory Animals [41]. All procedures related to their treatment the SNPRC/SFBR were approved by the Institutional Animal Care and Use Committee in accordance with established guidelines. Clinical records for each animal were checked and any animals with medical conditions known to affect bone metabolism were omitted from the sample. Femurs were collected opportunistically at routinely performed necropsies (i.e. no animals were sacrificed for the purpose of this study), wrapped in saline-soaked gauze, placed in air tight plastic bags, and frozen until specimen processing.
Specimens were processed as follows: A 10-mm section of bone was removed from the femoral midshaft using a band saw. A section ~300 microns thick was cut from the center of the 10-mm section using an Isomet 1000 Precision Saw (Buehler Ltd. Lake Bluff, IL). The sections were then ground manually to a thickness of ~100 microns according to a standard protocol [42].
These midshaft transverse sections of the right femur were mounted on slides and digitized using a Zeiss Stemi SVII Microscope with an attached color digital camera. Digital images were then segmented using ImageJ (U. S. National Institutes of Health, Bethesda, MD) to extract the bone data using a combination of iterative thresholding and active contours. Segmented images were registered based on nominal anatomic orientation using Matrix Laboratory (MATLAB) (The Mathworks, Inc. Natick, MA). Cross-sectional area (CSA), minimum (IMIN) and maximum (IMAX) principal moments of inertia, and polar moment of inertia (J) were calculated from the segmented/rotated images with the aid of MATLAB to characterize the amount of bone material and its distribution around the neutral axis.
Maximum likelihood-based variance decomposition methods implemented in the computer software SOLAR [43] were used to simultaneously estimate the mean effects of age, sex, sex-specific age effects, body weight, and the additive effects of genes on baboon midshaft cross-sectional geometric properties. Evaluation of the covariance between relative pairs in geometric properties allows for quantification of the contribution of additive genetic effects, heritability (h2), on these traits. This approach is described in detailed elsewhere [43]. Significance of maximum likelihood estimates for heritability and other parameters was assessed by means of likelihood ratio tests in a manner described in detail elsewhere [44–46].
Age, sex and age-by-sex were selected for inclusion as covariates in the final model by means of a Bayesian model averaging procedure implemented in SOLAR. This procedure evaluates all possible covariates alone and in all possible combinations to identify the best set for inclusion based on a Bayesian Information Criterion for each covariate/combination and a posterior probability assigned to each covariate [47].
Results
Age and Sex Effects
Descriptive statistics by sex are displayed in Table 1. Males show higher means and a wider range in absolute values for all properties than females. The higher means are expected in a sexually dimorphic species [48–49] such as the baboon, in which females have an average body mass that is 52% of the average male body mass [50]. It is important to note that although a specific measure of individual body weight was not selected for inclusion in our final model using our Bayesian model averaging procedure, the effect of body size is most certainly a contributor to the effect of the sex covariate.
Table 1.
Descriptive statistics for age and bone geometry measures by sex.
| Variable | N | Range | x̄ | SD | |
|---|---|---|---|---|---|
| Females | Age (years) | 80 | 5.84–33.27 | 18.49 | 7.14 |
| CSA | 80 | 106.73–161.23 | 133.89 | 12.06 | |
| J | 80 | 2862.83–7614.42 | 5195.88 | 978.74 | |
| I_MAX | 80 | 1493.96–3977.33 | 2762.03 | 531.91 | |
| I_MIN | 80 | 1368.87–3723.27 | 2433.85 | 465.76 | |
| Males | Age (years) | 30 | 5.23–25.51 | 15.44 | 6.21 |
| CSA | 30 | 128.54–243.73 | 188.41 | 22.05 | |
| J | 30 | 4760.54–15035.58 | 10498.27 | 2418.02 | |
| I_MAX | 30 | 2525.83–8162.18 | 5644.86 | 1310.20 | |
| I_MIN | 30 | 2234.70–6873.40 | 4853.40 | 1129.84 | |
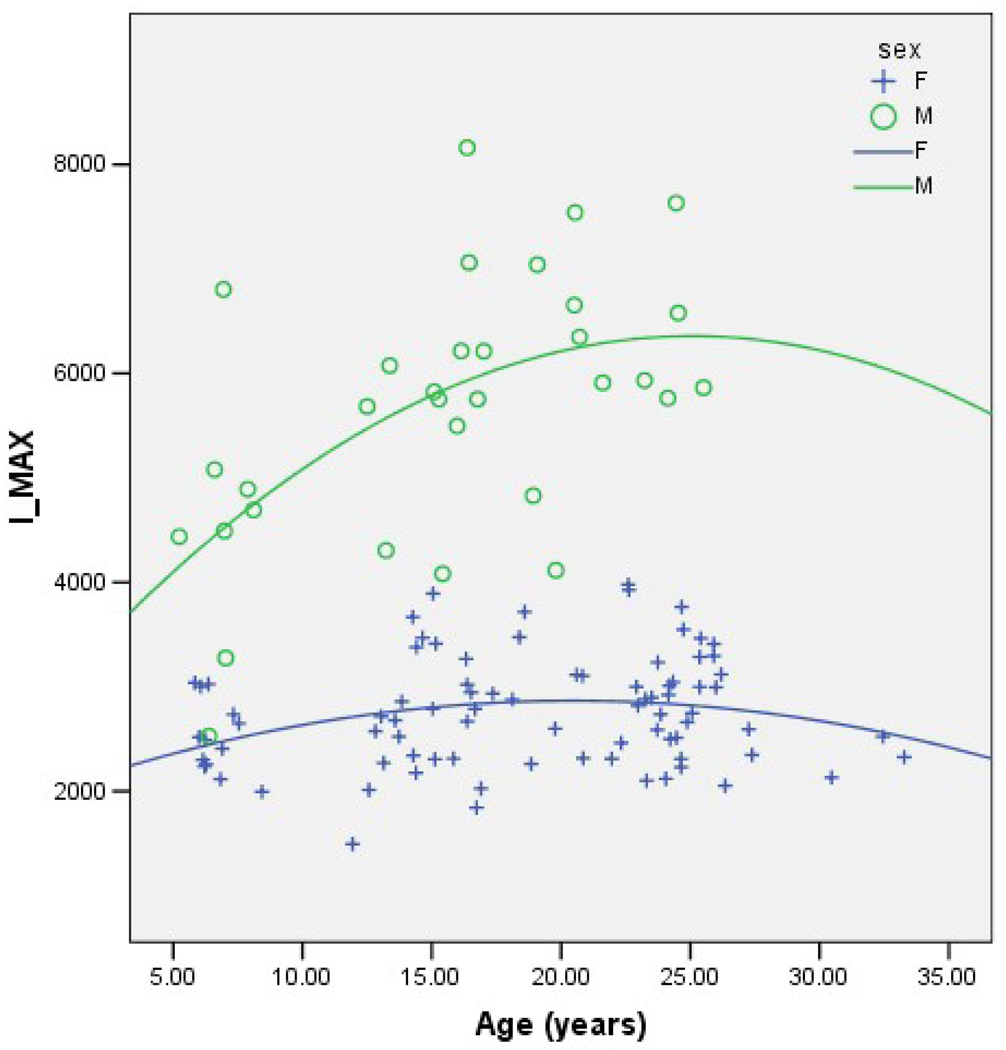
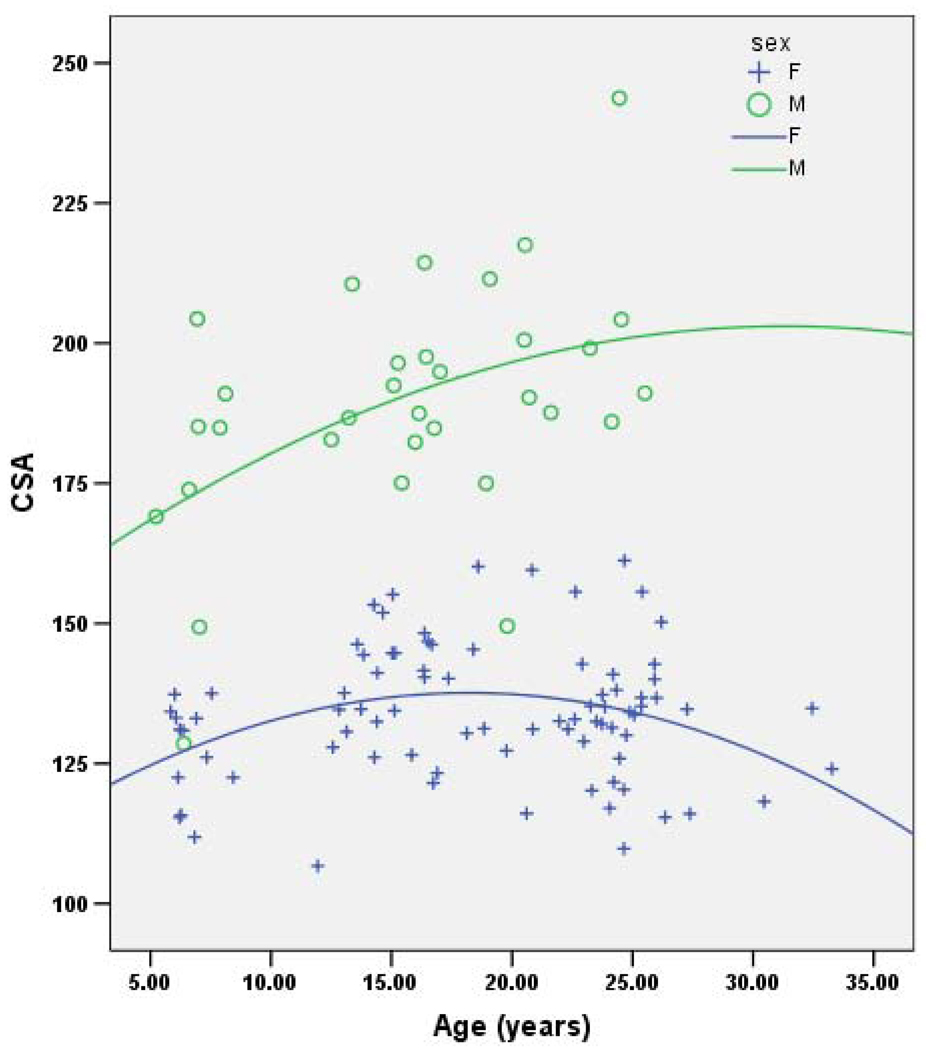
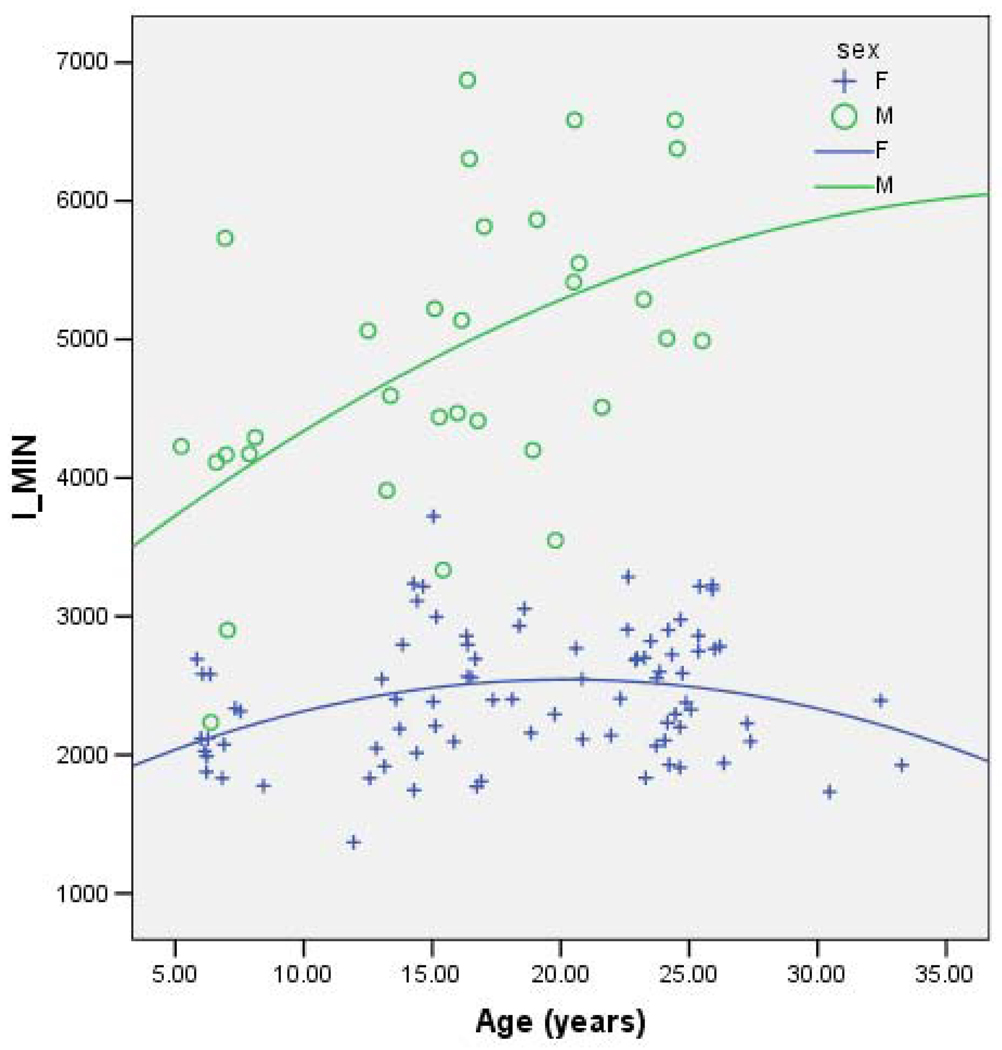
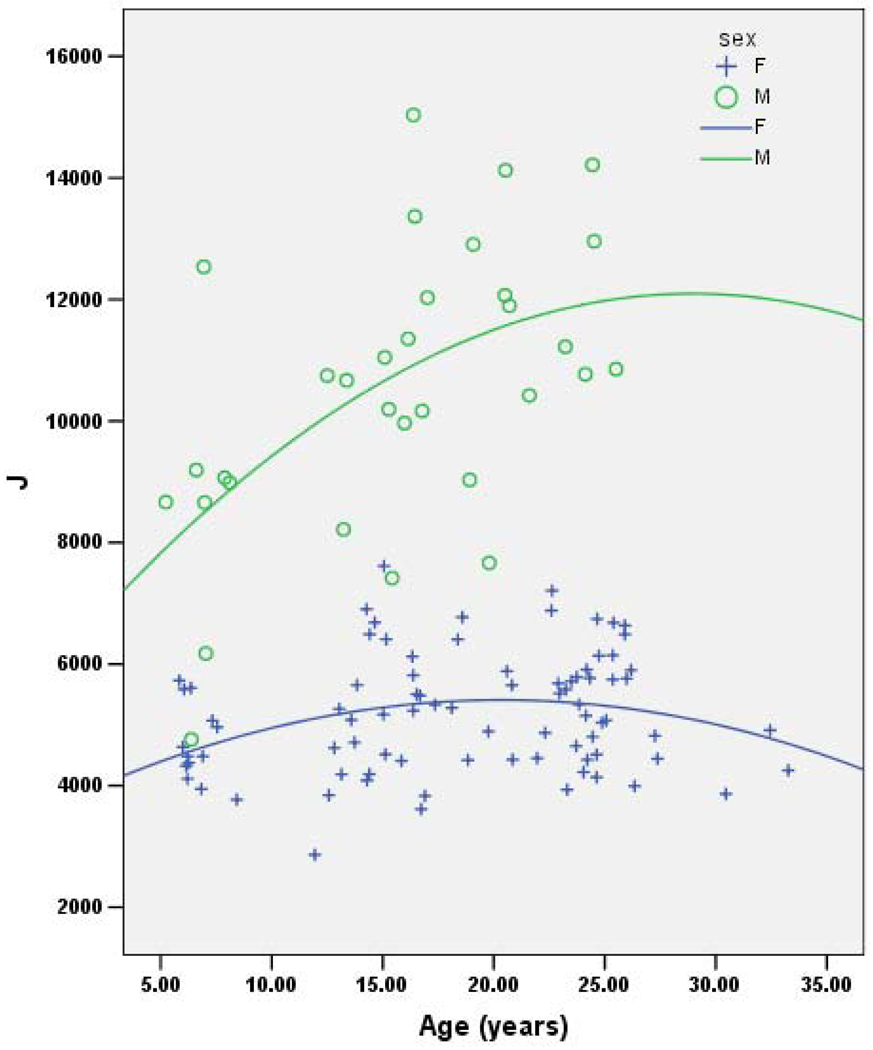
Together, age, sex and age*sex consistently account for ~56% of the variance in each geometric property (Table 2). In each case the effect of female sex is negative and the effect of age is positive, although of a lower magnitude than the effect of female sex. Age-by-sex interaction shows a decrease in the oldest females that is not seen in the oldest males. (Note that this may be due to the lack of older males in the sample.) Figures 1–4 demonstrate the relationship between age by sex for each of the measures of bone geometry and provide the associated r2 values. These values indicate that 8–10% of the variation in midshaft geometry is explained by variation in age in females. This percentage is noticeably higher for males, in which 23–36% of the variation in midshaft geometry is explained by variation in age.
Table 2.
Additive genetic effect on bone geometry measures.
| Variable | h2 | p-value | Variance due to covariates |
Total variance due to additive genetic effects |
|---|---|---|---|---|
| CSA | 0.50±0.30 | 0.0218 | 54% | 23% |
| IMAX | 0.36±0.21 | 0.0159 | 59% | 15% |
| IMIN | 0.42±0.23 | 0.0102 | 59% | 17% |
| J | 0.40±0.22 | 0.0096 | 59% | 16% |
h2 = heritability estimate = proportion of variance due to the additive effects of genes.
Covariates: age, sex, age*sex
Fig. 1.
IMAX vs. age for males (r2=0.36) and females (r2=0.08).
IMAX=Maximum Principal Moment of Inertia
Fig. 4.
CSA vs. age for males (r2=0.225) and females r2=0.08).
CSA=Cross-sectional Area
Additive Genetic Effects
A quantifiable additive genetic effect was detected for each of the geometric properties measured. Table 2 displays the heritability estimate for each variable and indicates the proportion of the total phenotypic variance accounted for by the additive genetic effect. CSA, I_MAX, I_MIN, and J show residual h2 values that range from 0.36 to 0.50. Though the standard errors around these estimates are relatively large, all estimates are significant (p=0.02 to 0.01).
Discussion
This study is the first to demonstrate that an additive genetic effect on population-level normal variation in femoral geometry in a non-inbred animal model is detectable and quantifiable, thereby emphasizing that bone geometry is an important potential target of the genetic mediators of bone strength. Residual h2 values from 0.36 to 0.50 reflect genetic effects that account for 15% to 23% of the total phenotypic variance in individual geometric properties in this outbred primate population.
The femoral midshaft, though not a common site of fracture, is highly relevant to the study of contributors to variation in bone strength due to the substantial influence of biomechanical forces of the musculature on this region of the femur [51] Our results clearly identify a substantial genetic effect on variation in midshaft femoral geometry; however, we cannot and do not make any claims as to the nature of or the mechanism(s) of action of the genes responsible for this effect based on this particular study. It may be useful, however, to speculate, based on general knowledge about contributors to variation in midshaft femoral morphology, as to how genes might affect geometric variation in this region. Several possibilities involve interaction between genes and biomechanical loading environment including variation in processes involved in muscle growth, mass, or function. It is also possible that the responsible genes may influence musculoskeletal communication or mechnotransduction in bone, or a host of other processes involved in skeletal response to biomechanical forces exerted by the musculature. It is equally possible that the genetic effect we have detected affects variation through mechanisms that have little or nothing to do with the effects of the muscular component. Unfortunately a detailed treatment of this issue requires data we do not have.
The magnitude of the h2 estimates of both the size and the shape parameters bode well for the success of subsequent studies to localize the observed genetic effects to specific chromosomal regions and, ultimately, to identify the genes responsible. CSA yielded the strongest heritability (though the significant h2 estimates for each of the parameters overlap when the standard errors of the estimates are considered) with an h2 value of 0.50±0.30, accounting for 23% of the total phenotypic variance. This result suggests that the strongest genetic effect observed is with regard to bone size; however, genetic effects on bone shape variables are also substantial.
Our data not only show significant heritability of cross-sectional geometry, but also show that increased age is associated with decreased mean cross-sectional geometry measures of bone fracture resistance in the oldest females. This may be related to decreased bone strength in females with age, since proportionately smaller cross-sectional geometries generally will result in decreased bone bending strength. However, as shown by Tommasini et al. [52], biological co-adaptation of morphological and compositional traits contributes to mechanical functionality and skeletal fragility. Bone tissue properties may compensate for variations in bone shape to maintain bone structural integrity under daily loading conditions. Previous research in these baboons shows age related changes in BMD and substantial occurrence of osteopenia in baboon females of advanced age [34, 39, 53]. Future research should focus on the complex interplay between bone geometry, BMD, and bone strength and to what level these changes are under genetic control. To this end, characterization of bone tissue properties in these animals is currently underway. Tissue property data will be integrated with a comprehensive set of data on BMD, cortical bone microstructure, trabecular bone structure, mechanical properties of cortical and trabecular bone, and gross bone geometric properties from the same animals to investigate shared genetic control of these intimately related indicators of bone health.
Our findings are also interesting with regard to the role of genetics vs. sex in variation in bone quality in inbred rodent models [e.g. 13, 15–16, 19–22]. In a study of the effect of genetic loci on mechanically-stimulated bone formation in three congenic mouse strains, Robling et al. [20] report a sex-specific response in which male congenic mice exhibited a higher response vs. controls than did the females vs. controls, independent of bone size. Our results show that the effect of sex on femoral midshaft geometry is minimal after the effect of genes has been removed. Future studies in these baboons to formally test for genotype-by-sex effects (tests that require a much larger sample size than is currently available) are warranted given the clear evidence for sex-specific genetic effects in inbred mice. The results of the study of Robling et al. [20] and ours, taken together, may indicate that the effect of sex on femoral midshaft geometry is largely the result of sex-specific genetic effects.
Genetic effects on bone geometry are only one part of an exceedingly complex system of genetic regulation of bone strength. Rodent studies consistently reveal that various measures of bone quality are subject to independent genetic effects and also to genetic effects that act pleiotropically on the trait in question and on other bone strength-related traits [15–16; 19–20; 22]. Some of these genetic effects are in common with those that affect BMD, while many others are independent [13; 15–16; 19–21]. Furthermore, it is apparent that genes not only influence the individual bone traits, but also influence the functional relationships between and among traits [24]. Bone geometry components of bone strength are an essential part of a complex suite of traits that result from complex interactions among a number of genetic factors.
Dissecting and characterizing the genetic architecture that ultimately underlies variation in bone strength and its myriad of contributing bone density and quality traits will require an animal model, such as the baboon, in which a comprehensive set of traits can be measured and for which the results can confidently be translated to humans. The baboon model affords the opportunity to assess multiple traits (e.g. BMD [35], material and mechanical properties [54], cortical bone microstructure [55], bone geometry [56], and matrix and mineral properties [57]) for both trabecular and cortical bone in the same animals to capture maximum information on variation in bone density and quality in the study animals. This will, for the first time, allow for thorough investigation of the genetic architecture underlying bone strength in a manner that identifies, then incorporates, pleoitropic genetic effects on networks of bone strength-related traits in an outbred population.
Although limited by practical issues surrounding sample acquisition and complicated by environmental heterogeneity, human studies are yielding results that are consistent with those of the animal studies. In sum, the human studies detect genetic effects on bone shape [28–30, 58–59] that result from multiple genes and that are, to a large degree, independent of genetic effects of BMD [29, 58]. The human studies underscore that genetic influences on bone strength are quite complex, with both independent and pleiotropic genetic effects on all aspects of bone strength, including, but probably not limited to, aspects of geometry and density.
This study establishes the potential of the baboon model for the identification of genes that regulate bone geometry in primates. The baboon model is particularly valuable in that it allows for the testing of hypotheses generated in inbred rodent models and in other animal models more distantly related to humans. It also allows for experimental designs, availability of tissues, and comprehensive assessments of multiple integrated bone phenotypes that are not logistically or ethically possible in human populations. Additionally, the use of a captive population provides an environmental consistency across the sample that is not possible in humans. Finally, this model is of particular importance in genetic studies because it provides results that are likely highly relevant to the human condition due to the phylogenetic proximity of baboons to humans.
Fig. 2.
IMIN vs. age for males (r2=0.32) and females (r2=0.10).
IMIN=Minimum Principal Moment of Inertia
Fig. 3.
J vs. age for males (r2=0.31) and females (r2=0.08).
J=Polar Moment of Inertia
Acknowledgements
The authors wish to acknowledge the technical contributions of Jennifer A.K. Harris, Shayna M. Levine, Don Moravits, and Deborah E. Newman. This research was possible due in part to the NIH NCRR base grant, P51 RR013986, that supports the Southwest National Primate Research Center and a grant from the Southwest Foundation Forum.This investigation was conducted in part in facilities constructed with support from Research Facilities Improvement Program Grants C06 RR017515, C06 RR013556, C06 RR014578 from the National Center for Research Resources (NCRR), NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Currey J. The Mechanical Adaptation of Bones. Princeton: Princeton University Press; 1984. pp. 98–112. [Google Scholar]
- 2.Burr DB, Ruff CB, Johnson C. Structural adaptations of the femur and humerus to terrestrial and arboreal environments in three species of macaque. Am J Phys Anthropol. 1989;79:357–367. doi: 10.1002/ajpa.1330790312. [DOI] [PubMed] [Google Scholar]
- 3.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 4.Stein MS, Thomas CDL, Feik SA, Wark JD, Clement JG. Bone size and mechanics at the femoral diaphysis across age and sex. J Biomech. 1998;31:1101–1110. doi: 10.1016/s0021-9290(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 5.Beamer WG, Donahue LR, Rosen CJ. Genetics and bone: Using the mouse to understand man. J Musculoskel Neuron Interact. 2002;2:225–231. [PubMed] [Google Scholar]
- 6.Klein RF, Mitchell SR, Phillips TJ, Belknap JK, Orwoll ES. Quantitative trait loci affecting peak bone mineral density in mice. J Bone Miner Res. 1998:131648–131656. doi: 10.1359/jbmr.1998.13.11.1648. [DOI] [PubMed] [Google Scholar]
- 7.Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, Baylink DJ, Rosen CJ. Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res. 2001;16:1195–1206. doi: 10.1359/jbmr.2001.16.7.1195. [DOI] [PubMed] [Google Scholar]
- 8.Turner CH, Sun Q, Schriefer J, Pitner N, Price R, Bouxsein ML, Rosen CJ, Donahue LR, Schultz KL, Beamer WG. Congenic mice reveal sex-specific genetic regulation of femoral structure and strength. Calcif Tissue Int. 2003;73:297–303. doi: 10.1007/s00223-002-1062-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ, Shen H, Xiao P, Xiong DH, Li LH, Recker RR, Deng HW. Molecular genetic studies of gene identification for osteoporosis: A 2004 update. J Bone Miner Res. 2006;21:1511–1535. doi: 10.1359/JBMR.051002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edderkaoui B, Baylink DJ, Beamer WG, Shultz KL, Wergedal JE, Mohan S. Genetic regulation of femoral bone mineral density: Complexity of sex effect in chromosome 1 revealed by congenic sublines of mice. Bone. 2007;41:340–345. doi: 10.1016/j.bone.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Koller DL, Liu LX, Alam I, Sun QW, Econs MJ, Forroud T, Turner CH. Epistatic effects contribute to variation in BMD in Fischer 344 × Lewis F2 rats. J Bone Miner Res. 2008;23:41–47. doi: 10.1359/JBMR.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jepsen KJ, Pennington DE, Lee Y-L, Warman M, Nadeau J. Bone brittleness varies with genetic background in A/J and C57BL/6J inbred mice. J Bone Miner Res. 2001;16:1854–1862. doi: 10.1359/jbmr.2001.16.10.1854. [DOI] [PubMed] [Google Scholar]
- 13.Klein RF, Turner RJ, Skinner LD, Vartanian KA, Serang M, Carlos AS, Shea M, Belknap JK, Orwoll ES. Mapping quantitative trait loci that influence femoral cross-sectional area in mice. J Bone Miner Res. 2002;10:1752–1760. doi: 10.1359/jbmr.2002.17.10.1752. [DOI] [PubMed] [Google Scholar]
- 14.Bouxsein ML, Uchiyama T, Rosen CJ, Schultz KL, Donahue LR, Turner CH, Sen S, Churchill GA, Muller R, Beamer WG. Mapping quantitative trait loci for vertebral trabecular bone volume fraction and microarchitecture in mice. J Bone Miner Res. 2004;19:587–589. doi: 10.1359/JBMR.0301255. [DOI] [PubMed] [Google Scholar]
- 15.Wergedal JE, Sheng MHC, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005;36:111–122. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Alam I, Sun QW, Liu LX, Koller DL, Fishburn T, Carr LG, Econs MJ, Forroud T, Turner CH. Whole-genome scan for linkage to bone strength and structure in Inbred Fischer 344 and Lewis rats. J Bone Miner Res. 2005;20:1589–1596. doi: 10.1359/JBMR.050512. [DOI] [PubMed] [Google Scholar]
- 17.Alam I, Sun Q, Liu L, Koller DL, Fishburn T, Carr LG, Econs MJ, Forroud T, Turner CH. Identification of a quantitative trait locus on rat chromosome 4 that is strongly linked to femoral neck structure and strength. Bone. 2006;39:93–99. doi: 10.1016/j.bone.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Bower AL, Lang DH, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, McClearn GE, Sharkey NA. QTL analysis of trabecular bone in BXD F-2 and RI mice. J Bone Miner Res. 2006;21:1267–1275. doi: 10.1359/jbmr.060501. [DOI] [PubMed] [Google Scholar]
- 19.Wergedal JE, Ackert-Bicknell CL, Tsaih SW, Sheng MHC, Li R, Mohan S, Beamer WG, Churchill GA, Baylink DJ. Femur mechanical properties in the F-2 progeny of an NZB/B1NJ × RF/J cross are regulated predominantly by genetic loci that regulate bone geometry. J Bone Miner Res. 2006;21:1256–1266. doi: 10.1359/jbmr.060510. [DOI] [PubMed] [Google Scholar]
- 20.Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res. 2007;22:984–991. doi: 10.1359/jbmr.070327. [DOI] [PubMed] [Google Scholar]
- 21.Otsuki B, Matsumura T, Shimizu M, Mori M, Okudaira S, Nakanshi R, Higuchi K, Hosokawa M, Tsuboyama T, Nakamura T. Quantitative trait locus that determines the cross-sectional shape of the femur in SAMP6 and SAMP2 mice. J Bone Miner Res. 2007;22:675–685. doi: 10.1359/jbmr.070206. [DOI] [PubMed] [Google Scholar]
- 22.Sun QW, Alam I, Liu LX, Koller DL, Carr LG, Econs MJ, Forroud T, Turner CH. Genetic loci affecting bone structure and strength in inbred COP and DA rats. Bone. 2008;42:547–553. doi: 10.1016/j.bone.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tommasini SM, Morgan TG, van der Meulen MCH, Jepsen KJ. Genetic variation in structure-function relationships for the inbred mouse lumbar vertebral body. J Bone Miner Res. 2005;20:817–827. doi: 10.1359/JBMR.041234. [DOI] [PubMed] [Google Scholar]
- 24.Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Terranova CJ, Nadeau JH. Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome. 2007;18:492–507. doi: 10.1007/s00335-007-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan EL, Cardon LR, Sinsheimer JS, Wass JA, Brown MA. Site and gender specificity of inheritance of bone mineral density. J Bone Miner Res. 2003;18:1531–1538. doi: 10.1359/jbmr.2003.18.8.1531. [DOI] [PubMed] [Google Scholar]
- 26.Ralston SH. Genetic determinants of osteoporosis. Curr Opin Rheumatol. 2005;17:475–479. doi: 10.1097/01.bor.0000166385.62851.92. [DOI] [PubMed] [Google Scholar]
- 27.Ralston SH. Genetic control of susceptibility to osteoporosis. J Clin Endocrinol Metab. 2002;87:2460–2466. doi: 10.1210/jcem.87.6.8621. [DOI] [PubMed] [Google Scholar]
- 28.Xiong DH, Liu YZ, Liu PY, Zhao LJ, Deng HW. Association analysis of estrogen receptor alpha gene polymorphisms with cross-sectional geometry of the femoral neck in Caucasian nuclear families. Osteoporos Int. 2005;16:2113–2122. doi: 10.1007/s00198-005-2011-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang XJ, Kammerer CM, Wheeler VW, Patrick AL, Bunker CH, Zmuda JM. Pleiotropy and heterogeneity in the expression of bone strength-related phenotypes in extended pedigrees. J Bone Miner Res. 2007;22:1766–1772. doi: 10.1359/jbmr.070718. [DOI] [PubMed] [Google Scholar]
- 30.Havill LM, Mahaney MC, Binkley TL, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–746. doi: 10.1359/jbmr.070213. [DOI] [PubMed] [Google Scholar]
- 31.Hui SL, Koller DL, Forroud TM, Econs MJ, Johnston CC, Peacock M. Heritability of changes in bone size and bone mass with age in premenopausal white sisters. J Bone Miner Res. 2006;21:1121–1125. doi: 10.1359/jbmr.060412. [DOI] [PubMed] [Google Scholar]
- 32.Deng F-Y, Xiao P, Lei S-F, Zhang L, Yang Y, Tang Z-H, Liu P-Y, Liu Y-J, Recker RR, Deng HW. Bivariate whole genome linkage analysis for femoral neck geometric parameters and total body lean mass. J Bone Miner Res. 2007;22:808–816. doi: 10.1359/jbmr.070303. [DOI] [PubMed] [Google Scholar]
- 33.Koller DL, Guangda L, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Jr, Foroud T, Peacock M. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 34.Havill LM, Mahaney MC, Czerwinski SA, Carey KD, Rice K, Rogers J. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33:877–888. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 35.Havill LM, Mahaney MC, Cox LA, Morin PA, Joslyn G, Rogers J. A quantitative trait locus for normal variation in forearm bone mineral density in pedigreed baboons maps to the ortholog of human chromosome 11q. J Clin Endocrinol Metab. 2005;90:3638–3645. doi: 10.1210/jc.2004-1618. [DOI] [PubMed] [Google Scholar]
- 36.Mas ID, Biscardi A, Schnitzler CM, Ripamonti U. Bone loss in the ovariectomized baboon Papio ursinus: densitometry, histomorphometry and biochemistry. J Cell Mol Med. 2007;11:852–867. doi: 10.1111/j.1582-4934.2007.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998;8:1–9. [PubMed] [Google Scholar]
- 38.Martin LJ, Carey KD, Comuzzie AG. Variation in menstrual cycle length and cessation of menstruation in captive raised baboons. Mech Ageing Dev. 2003;124:865–871. doi: 10.1016/s0047-6374(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 39.Aufdemorte TB, Fox WC, Miller D, Buffum K, Holt GR, Carey KD. A non-human primate model for the study of osteoporosis and oral bone loss. Bone. 1993;14:581–586. doi: 10.1016/8756-3282(93)90197-i. [DOI] [PubMed] [Google Scholar]
- 40.Kammerer CM, Sparks ML, Rogers J. Effects of age, sex, and heredity on measures of bone mass in baboons (Papio hamadryas) J Med Primatol. 1995;24:236–242. doi: 10.1111/j.1600-0684.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy of Sciences; 1996. [Google Scholar]
- 42.Frost HM. Preparation of thin undecalcified bone sections by rapid manual method. Stain Technol. 1958;33:273–277. doi: 10.3109/10520295809111862. [DOI] [PubMed] [Google Scholar]
- 43.Almasy L, Blangero J. Multipoint quantitative-point linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards AWF. Likelihood. Baltimore: The Johns Hopkins University Press; 1992. [Google Scholar]
- 45.Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982;46:373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 46.Boehnke M, Moll PP, Kottke BA, Weidman WH. Partitioning the variability of fasting plasma glucose levels in pedigrees. Genetic and environmental factors. Am J Epidemiol. 1987;125:679–689. doi: 10.1093/oxfordjournals.aje.a114581. [DOI] [PubMed] [Google Scholar]
- 47.Blangero J, Williams JT, Iturria SJ, Almasy L. Oligogenic model selection using the bayesian information criterion: linkage analysis of the P300 Cz event-related brain potential. Genet Epidemiol. 1999;17:S67–S72. doi: 10.1002/gepi.1370170712. [DOI] [PubMed] [Google Scholar]
- 48.Plavcan JM. Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In: Kappeler P, van Schaik C, editors. Sexual Selection in Primates: New and Comparative perspectives. Cambridge: Cambridge University Press; 2003. pp. 230–252. [Google Scholar]
- 49.Gaulin SJC, Sailer LD. Sexual dimorphism in weight among primates: the realative impact of allometry and sexual selection. Int J Primatol. 1984;5:515–535. [Google Scholar]
- 50.Rowe N. The Pictoral Guide to the Living Primates. East Hampton, New York: Pogonias Press; 1986. p. 136. [Google Scholar]
- 51.Ruff CB. Mechanical Determinants of bone form: Insights from skeletal remains. J Musculoskelet Neuronal Interact. 2005;5(3):202–212. [PubMed] [Google Scholar]
- 52.Tommasini SM, Nasser P, Hu B, Jepsen KJ. Biological Co-adaptation of Morphological and Composition Traits Contributes to Mechanical Functionality and Skeletal Fragility. J Bone Miner Res. 2008;23:236–246. doi: 10.1359/JBMR.071014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Havill LM, Levine SM, Newman DE, Mahaney MC. Osteopenia and osteoporosis in adult baboons (Papio hamadryas) J Med Primatol. 2008;37:146–153. doi: 10.1111/j.1600-0684.2007.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havill LM. Heritability of lumbar trabecular bone mechanical properties in baboons. J Musculoskelet Neuronal Interact. 2007;7:316–317. [PubMed] [Google Scholar]
- 55.Havill LM, Harris JAK, Levine SM, Mahaney MC. Strong and significant genetic effects on osteonal remodeling-associated cortical bone microstructure in pedigreed baboons. Bone. 2008;42:S53–S54. [Google Scholar]
- 56.Bredbenner TL, Havill LM, Mahaney MC, Nicolella DP. Heritability of the principal components of a statistical shape model describing baboon midshaft femur geometry. J Bone Miner Res. 2007;22:S404. [Google Scholar]
- 57.Gourion-Arsiquaud S, Havill LM, Boskey A. Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090201. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Guo YF, Lei SF, Wang YB, Deng HW. Genetic and environmental correlations between bone mineral density and bone size in Caucasians. Human Biol. 2007;79:15–24. doi: 10.1353/hub.2007.0023. [DOI] [PubMed] [Google Scholar]
- 59.Jiang H, Lei S, Xiao S, Chen Y, Sun X, Yang F. Association and linkage analysis of COL1A1 and AHSG gene polymorphisms with femoral neck bone geometric parameters in both Caucasian and Chinese nuclear families. Acta Pharmacol Sin. 2007;28:375–381. doi: 10.1111/j.1745-7254.2007.00522.x. [DOI] [PubMed] [Google Scholar]